Abstract
Electrophysiologic procedures in the young engender concern about the potential long-term effects of radiation exposure. This concern is manifold if such procedures are contemplated during pregnancy. Catheter ablations in pregnancy are indicated only in the presence of an unstable tachycardia that cannot be controlled by antiarrhythmic agents. This report describes the case of an 18-year-old pregnant woman and our stratagem to minimize irradiation of the mother and the fetus.
Keywords: Catheter ablation/adverse effects; electrophysiologic techniques, cardiac/standards; pregnancy complications, cardiovascular/radiation effects/therapy; puncture, transseptal; radiography/adverse effects; risk factors; tachycardia, supraventricular/therapy
Arrhythmias can occur often in pregnancy. This is predominantly due to neurohormonal effects, autonomic tone, and increased intravascular volume. The treatment of supraventricular tachycardia, apart from sinus tachycardia, is usually conservative; however, radiofrequency ablation is considered for unstable drug-resistant tachycardia. The use of detailed electroanatomic mapping can substantially minimize the fetus's exposure to radiation. This case report describes our stratagem for the ablation of left atrial tachycardia in a young pregnant woman.
Case Report
An 18-year-old woman, who was 33 weeks pregnant, presented at our emergency department with palpitations and shortness of breath of one week's duration. This was associated with declining exercise tolerance and dry cough. She was found to be experiencing supraventricular tachycardia, and treatment was initiated with intravenous diltiazem, intravenous metoprolol, and digoxin. Because of her worsening clinical status, she was urgently referred to our department of cardiac electrophysiology.
On examination, she was uncomfortable at rest. She was tachycardic with a pulse rate of 150 beats/min and a blood pressure of 170/100 mmHg. No jugular venous distention was observed. On auscultation, her heart rate was regular with no added sounds, except for bilateral basal crepitations. The laboratory data were in general unremarkable, but the presence of 2+ proteins in a urine dipstick test suggested preeclampsia. A chest radiograph showed cardiomegaly with bilateral patchy infiltrates. An echocardiogram revealed a left ventricular ejection fraction of 0.35 to 0.40 with no regional wall-motion abnormalities.
The patient's initial medical management included the addition of sotalol for the arrhythmia and of intravenous nitroglycerin, intravenous furosemide, and alpha-methyl-dopa for the heart failure. She then developed severe hypotension, and her continued lack of response to medical therapy prompted us to attempt cardiac ablation.
The patient was taken to the electrophysiology laboratory, with coverage by our anesthesiology, obstetric, and neonatology staff. To shield the fetus, we wrapped the mother's abdomen anteriorly and posteriorly with a lead apron. Her baseline rhythm was noted to be atrial tachycardia, at a rate of 155 beats/min, with 1:1 or 2:1 conduction. Her P-wave morphology was biphasic in leads V1, II, III, and aVF, and it was isoelectric in lead I.
After performing local infiltration with 1% lidocaine, we obtained venous access in the groin. Into the left femoral vein, we inserted both a 7F and an 11F Pinnacle® 25-cm sheath (Terumo Medical Corporation; Somerset, NJ). An 8F Pinnacle 25-cm sheath was inserted into the right femoral vein. The long sheaths were inserted smoothly, with minimal manipulation, to ensure their positioning past the iliac veins.
A SoundStar 3TM intracardiac echocardiography catheter (ICE) (Biosense Webster Inc.; Diamond Bar, Calif) was inserted first to ascertain sheath position on the basis of ICE anatomy and sheath artifacts. Then we smoothly inserted a 7F decapolar CS DF TM curve catheter (Biosense Webster) for coronary sinus (CS) cannulation and a 7.5F EZ Steer® ThermoCool® NAV catheter (Biosense Webster) for mapping and ablation. Catheter positions within the heart were verified by looking at catheter artifacts, with the help of ICE.
Using a Carto® 3 (Biosense Webster) electroanatomic mapping system, we initially created a CartoSound® map of the right atrium to identify and mark all structures, including the venae cavae, the tricuspid annulus, and the CS os.
After seeing the CS catheter and CS os on the CartoSound shell (Fig. 1), we were able—with direct views of the CS os and catheter artifact on ICE—to successfully navigate the CS. Catheter positioning was confirmed by identifying CS signals acquired from this catheter. An electro-anatomic map was generated completely without fluoroscopy. This was achieved by the initial acquisition of a CartoSound map and an electrogram-based activation map. No early activation signals could be identified in the right atrium: hence we decided to map the left atrium.
Fig. 1.
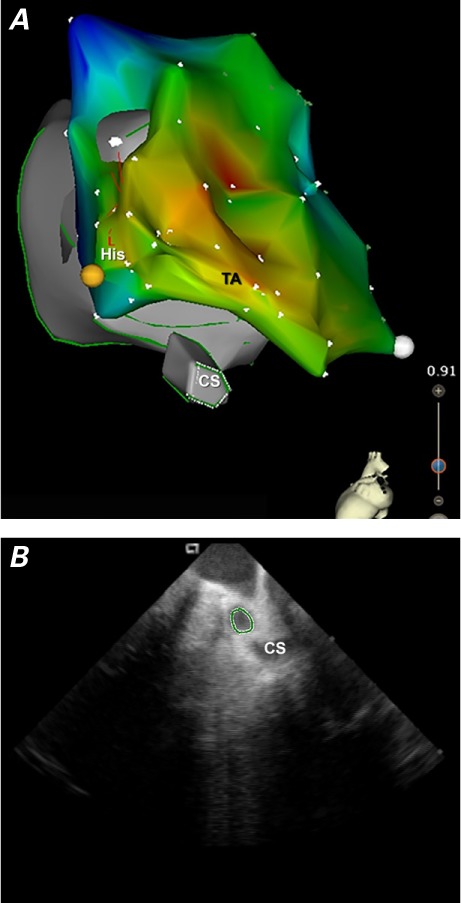
A) Composite electroanatomic map of the CartoSound shell and activation map and B) intracardiac echocardiographic image of the coronary sinus.
CS = coronary sinus; His = Bundle of His; TA = tricuspid annulus area
Intravenous heparin was administered before transseptal puncture, to achieve an activated clotting time of greater than 300 s. Transseptal puncture was performed in the usual fashion, with use of a Fast-CathTM guiding introducer RAMPTM (St. Jude Medical, Inc.; St. Paul, Minn) and a BRK-1TM 71-cm transseptal needle (St. Jude Medical). We used ICE, on the basis of artifacts, to ascertain our introduction of the needle within the heart. During the entire case, fluoroscopy was used only at the time of transseptal puncture to confirm the transseptal needle position and “tenting” before the actual puncture. The introducer was placed in the left atrium.
Three-dimensional mapping of the left atrium was performed. The earliest point of activation was observed at the anteroseptal aspect of the mitral annulus (Figs. 2 and 3). Radiofrequency ablation, performed at this spot with 40 W, terminated the tachycardia in 5 s.
Fig. 2.
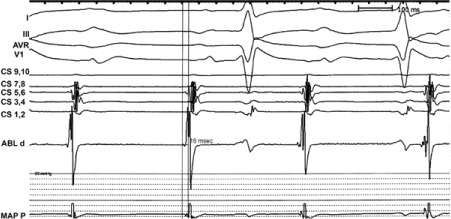
Atrial electrogram in the distal ablation electrode shows site of the earliest atrial potential 18 ms before the P wave.
ABL d = distal ablation electrogram; CS = coronary sinus electrograms 1–10; MAP P = proximal mapping electrogram
Fig. 3.
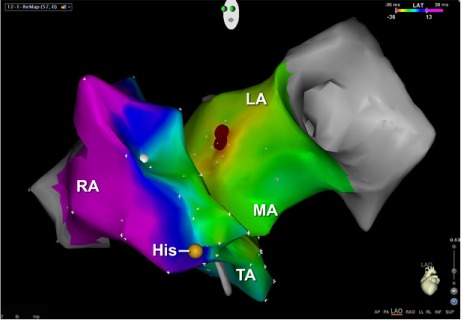
Electroanatomic map shows the ablation lesion (red) in the left anterior oblique position.
His = bundle of His; LA = left atrium; MA = mitral annulus area; RA = right atrium; TA = tricuspid annulus area
Tachycardia could not be induced by pacing maneuvers. We deferred isoproterenol infusion for fear of fetal hypoperfusion. Our patient was transferred to the operating room for a caesarian section.
During the case, pulsed fluoroscopy was set at the low rate of 7.5 pulses per second, and we used only 1.3 min of fluoroscopy during the entire procedure. We recorded 5.21 mGys of radiation in the dosimeter placed beneath the lead apron. A healthy male child was delivered by cesarian section. Both the mother and the child had an uneventful postoperative course. At 6 months after ablation, the mother was in sinus rhythm and had no symptoms or signs of heart failure, and the baby was doing well.
Discussion
Electrophysiologic procedures in the young engender concern about the potential long-term effects of irradiation. This concern is manifold if such procedures are contemplated during pregnancy. Catheter ablations in pregnancy are indicated only in the presence of an unstable tachycardia that cannot be controlled by use of antiarrhythmic agents.1
Radiation exposures of less than 5 rads to the fetus are not associated with miscarriages, congenital malformations, or growth restriction;2 however, in utero radiation exposure has been associated with an increased risk of cancers, especially leukemia.3 According to the revised 2013 American College of Radiology and Society of Pediatric Radiology guidelines,4 a fetal radiation dose of 50mGy, administered 15 weeks or more after conception, was associated with an attributable cancer incidence of about 2%. During this later period of gestation, the fetus is less radiosensitive than in early pregnancy.
Lead shielding of the abdomen and pelvis is controversial. In the past, lead shielding of the pelvis has not proved adequate to protect the fetus, because the irradiation is predominantly by internal scatter.5 Therefore, the shielding provides more of an emotional barrier for the mother than a physical barrier for the fetus.
In accordance with the ALARA (“as low as reasonably achievable”) policy to limit the irradiation of physicians and patients,6 the significance of which has been embraced by many interventional societies, we designed a stratagem to minimize irradiation of the fetus. No fluoroscopy was used for vascular access; catheter placements were performed with the help of long sheaths. Placement of the CS catheter was achieved with the help of the electroanatomic map and the ICE catheter alone.
It was decided to use fluoroscopy very briefly, after tenting had been confirmed with ICE. This was done given the unstable nature of the tachycardia and the need to perform a rapid procedure. The median fluoroscopy time for supraventricular tachycardia ablation is 24.5 minutes,7 but during this ablation we used only 1.3 minutes of fluoroscopy.
We conclude—tentatively and subject to confirmation by the experience of other investigators—that catheter ablation of arrhythmia can be successfully performed with very low risk to the mother and fetus during pregnancy. The mother and baby had an uneventful postoperative course, and both were doing well at the 6-month follow-up appointment. To our knowledge, no one else has yet attempted similar work.
Footnotes
From: Departments of Cardiology (Dr. Sundara Raman) and Cardiac Electrophysiology (Drs. Hariharan and Sharma), University of Texas Health Science Center at Houston, Houston, Texas 77030
References
- 1.Blomstrom-Lundqvist C, Scheinman MM, Aliot EM, Alpert JS, Calkins H, Camm AJ et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary. J Am Coll Cardiol. 2003;42(8):1493–531. doi: 10.1016/j.jacc.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Brent RL. The effect of embryonic and fetal exposure to x-ray, microwaves, and ultrasound: counseling the pregnant and nonpregnant patient about these risks. Semin Oncol. 1989;16(5):347–68. [PubMed] [Google Scholar]
- 3.Guidelines for diagnostic imaging during pregnancy. The American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 1995;51(3):288–91. [PubMed] [Google Scholar]
- 4.American College of Radiology. ACR-SPR practice parameter for imaging pregnant or potentially pregnant adolescents and women with ionizing radiation. Available from: http://www.acr.org/media/ACR/Documents/PGTS/guidelines/Pregnant_Patients.pdf [cited 2013 Aug 15]
- 5.Protection of the patient in diagnostic radiology. A report of Committee 3 of the International Commission on Radiological Protection. Ann ICRP. 1982;9(2–3):1–82. [PubMed] [Google Scholar]
- 6.Klein LW, Miller DL, Balter S, Laskey W, Haines D, Norbash A et al. Occupational health hazards in the interventional laboratory: time for a safer environment. Radiology. 2009;250(2):538–44. doi: 10.1148/radiol.2502082558. [DOI] [PubMed] [Google Scholar]
- 7.Efstathopoulous EP, Katritsis DG, Kottou S, Kalivas N, Tzanalaridou E, Giazitzoglou E et al. Patient and staff radiation dosimetry during cardiac electrophysiology studies and catheter ablation procedures: a comprehensive analysis. Europace. 2006;8(6):443–8. doi: 10.1093/europace/eul041. [DOI] [PubMed] [Google Scholar]


