Abstract
Background
Pattern recognition receptor NOD2 (nucleotide binding oligomerization domain 2) is well investigated in immunity, its expression and function in platelets has never been explored.
Method and Results
Using RT-PCR and Western blot we show that both human and mouse platelets express NOD2, and its agonist MDP induced NOD2 activation as evidenced by receptor dimerization. NOD2 activation potentiates platelet aggregation and secretion induced by low concentration of thrombin or collagen, as well as clot retraction. These potentiating effects of MDP were not seen in platelets from NOD2-deficient mice. Plasma from septic patients also potentiates platelet aggregation induced by thrombin or collagen NOD2-dependently. Using intravital microscopy, we found that MDP administration accelerated in vivo thrombosis in FeCl3-injured mesenteric arteriole thrombosis mouse model. Platelet depletion and transfusion experiments confirmed that NOD2 from platelets contributes to the in vivo thrombosis in mice. NOD2 activation also accelerates platelet-dependent hemostasis. We further found that platelets express RIP2 (receptor-interacting protein 2), and provided evidences suggesting that MAPK and NO/sGC/cGMP/PGK pathways downstream of RIP2 mediate the role of NOD2 in platelets. Finally, MDP stimulates proinflammatory cytokine IL-1β maturation and accumulation in human and mouse platelets NOD2-dependently.
Conclusions
NOD2 is expressed in platelets and functions in platelet activation and arterial thrombosis, possibly during infection. To our knowledge, this is the first study on NOD-like receptors in platelets which links thrombotic events to inflammation.
Keywords: NOD2, RIP2, platelet, thrombosis, mitogen-activated protein kinase
Humans are constantly challenged by numerous bacteria and viruses. As the primary barrier defending the humans against the pathogen invasion, innate immune system recognizes the conserved molecular structure in microbes, and initiates inflammatory and antimicrobial response relying on pattern recognition receptors. Among the major families of pattern recognition receptors, Toll-like receptors and NOD-like receptors are the key players in innate immunity1, 2. In contrast to Toll-like receptors, which are found on plasma membrane, NOD-like receptors are cytoplasmic receptors. All NOD-like receptors are structurally similar, containing a central nucleotide-binding oligomerization domain (NOD), C-terminal leucine-rich repeat domain, and a variable N-terminal protein-protein interaction domain which interacts with downstream effectors1. NOD1 and NOD2 are the two important Nod-like receptors fitting the typical structure with NOD1 containing one caspase recruitment domain (CARD), while NOD2 containing two CARD domains1, 2.
NOD1 is broadly distributed, whereas NOD2 is mainly expressed in monocytes, macrophages, dendritic cells, intestinal epithelial cells and Paneth cells2. Although NOD1 and NOD2 have a high degree of similarity, they recognize different bacterial cell wall peptidoglycan components. NOD1 recognizes d‐glutamyl-meso-diaminopimelic acid (iE‐DAP) primarily from Gram-negative bacteria whereas NOD2 detects muramyl dipeptide (MDP), the minimal bioactive motif in peptidoglycan from all bacteria2-4. In addition to their critically important roles in host defending pathogen invasion, they are also crucial to the pathogenesis of a myriad of inflammatory diseases. Homozygous mutations of NOD2 are highly correlated with the incidence of Crohn's disease5, 6.
Though primarily involved in hemostasis and thrombosis, increasing evidences demonstrate that platelets also play key roles in immune and inflammatory responses and the related diseases7. Many pattern recognition receptors, mainly Toll-like receptors, which were originally discovered in classic immune cells, are also expressed in platelets8-10, and play important roles in platelet activation, thrombosis and hemostasis11-13. As the two most important members in NOD-like receptor family, NOD1 and NOD2 were intensively investigated in classic immune cells and in inflammatory diseases2, 5, 14-16, their expression and functions in platelets have never been reported. In this study, we report that NOD2 is also expressed in platelets and functions in platelet activation and thrombosis.
Materials and Methods
Detailed Materials and Methods are described in the online-only Data Supplement.
Platelets and peripheral blood mononuclear cells (PBMC) preparation
All experiments using human subjects were performed in accordance with the Declaration of Helsinki and approved by the Institutional Review Board Fudan University. Platelets and PBMC were prepared as detailed in the online-only Data Supplement.
Preparation of platelet poor plasma from patients with bacterial infection
Blood from patients with bacteremia and clinical sepsis but without DIC and clinical septic shock was obtained from Fudan University Zhongshan Hospital. Ethical permission for all donations was obtained from the Zhongshan Hospital ethics board. Of 14 patients, 7 were infected with Staphylococcus aureus, 4 with Escherichia coli, and 3 with Enterococci. All patients were clinically cured after appropriate treatment. The control blood was obtained from the same donors at least two weeks after cure of infection. Platelet poor plasma was prepared and stored at -80°C before use.
Animal studies
NOD2-deficient mice were purchased from Jackson Laboratory (Bar Harbor, ME). All animal procedures were performed according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23 revised 1985). Mouse platelets were prepared as described previously17.
Platelet functional studies
Platelet aggregation, secretion, spreading and clot retraction were measured as previously described18, 19 and detailed in the online-only Data Supplement.
Bleeding assay
Bleeding assay was measured as described previously20 with more information in the online-only Data Supplement.
cGMP assay
cGMP in platelets was assayed using commercially available cGMP 125I radioimmunoassay kit as reported previously21.
NO detection
Platelet NO level was measured using fluorescent NO sensor DAF-FM DA on a collagen matrix as previously reported22. Briefly, platelets were first preincubated with NO fluorescent indicator DAF-FM DA (1 μM) in Tyrode's buffer at 37°C for 30 min. After stimulation with MDP for 15 min, platelets were immobilized on a collagen matrix slide and fluorescence was observed by confocal microscopy with 495 nm excitation and 515 nm emission filters.
RT-PCR
Total RNA was isolated and 1 μg of total RNA was reversely transcribed to cDNA using RNA isolation kit and RT-PCR kit (TaKaRa, Japan), respectively. PCR reactions were performed using specific primers (Supplementary Table 1).
Intravital microscopy of FeCl3-injured thrombus formation in mouse mesenteric arteriole
Intravital microscopy of FeCl3 thrombus formation in mouse mesenteric arteriole was performed as described previously18, 23. More information is provided in the online-only Data Supplement.
Lethal pulmonary thromboembolism mouse model induced by epinephrine and collagen
C57BL/6 mice were administrated rabbit anti-mouse platelet serum via the tail vein to produce an approximate 90% decrease of the count of circulating platelets. On the second day, the WT and NOD2-/- mice were anesthetized with 10% chloral hydrate and blood was collected from the abdominal aorta. Blood was centrifuged at 200 g for 3 min to obtain platelet rich plasma (PRP), followed by centrifugation at 700 g for 3 min to get platelet pellets, and then platelets were resuspended in 1 mL Tyrode's solution. Then, each 0.5 mL platelet suspension was incubated with MDP (5 μg/mL) or normal saline at 37°C for 30 min, centrifuged, and finally platelet pellets were resuspended in Tyrode's solution. The MDP- or saline-treated platelets were transfused into the platelet-depleted mice (0.125 mL per mouse). After 30 min, the mice were injected with a mixture of recombinant collagen (4 mg/kg) and epinephrine (0.3 mg/kg) dissolved in 0.1 mL saline via the tail vein. The death rate was recorded within 15 min.
IL-1β assay
Platelet IL-1β was assayed by flow cytometry, Western blot and ELISA. More information is available in the online-only Data Supplement.
Statistical analysis
All data are expressed as mean ± SD. Differences between the groups were analyzed by one-way ANOVA, followed by a Tukey test for multiple comparison unless otherwise stated. The Mann-Whitney test was used for comparison between two groups, and the Fisher exact test was used for comparison of death rate. Statistical analysis was performed using Prism 5 (GraphPad Inc, San Diego). P < 0.05 was considered to be statistically significant.
Results
Human and mouse platelets express NOD2
Human platelets express NOD2 receptor both at the RNA and protein levels as detected by RT-PCR (Figure 1A1) and Western blot (Figure 1B), similarly to PBMC (Figure 1). In contrast to PBMC, which expresses both NOD1 and NOD2, only NOD2 was detected in human platelets. The detected NOD2 on platelets is not from the contaminating PBMC, because the PBMC-specific marker CD14, which is robustly expressed in PBMC, is not detectable from platelet sample (Figure 1A2). Similarly, we also detected robust expression of NOD2, but not NOD1, in mouse platelets by RT-PCR (Figure 1C) and Western blot (Figure 1D).
Figure 1.

Both human and mouse platelets express NOD2. A, RT-PCR detection of NOD1, NOD2 (panel A1) and monocyte-specific CD14 (panel A2) in human platelets and peripheral blood mononuclear cells (PBMC). B, Western Blot detection of NOD1 and NOD2 in human platelets and PBMC. CHO cells were used as a negative control. C, RT-PCR detection of NOD1, NOD2 (panel C1), and monocyte-specific CD14 (panel C2) in mouse platelets. 299 bp NOD1 and 273 bp NOD2 are expected. D, Western blot detection of NOD1 and NOD2 in mouse platelets.
NOD2 receptor agonist MDP potentiates platelet aggregation and dense granule release
NOD2 function has been extensively studied in white blood cells, and NOD2 activation elicits proinflammatory responses in white blood cells, playing a critical role in innate immunity. NOD2 functions in platelets have not been studied. NOD2 receptor agonist MDP alone did not induce platelet aggregation, in washed human platelets, even at 100 μg/mL (data not shown). But in the range of 1 - 10 μg/mL, MDP concentration-dependently potentiated human platelet aggregation and ATP release induced by low concentrations of thrombin or collagen (Figure 2A). NOD2 activation induced by MDP was confirmed by the dimerizaton of NOD2 upon treatment of platelets with 10 μg/mL MDP (Figure 2B). Similarly, 10 μg/mL MDP also significantly potentiated mouse platelet aggregation and ATP release induced by low concentration of thrombin or collagen. The potentiating effect of MDP on platelet activation is NOD2 dependent, because it was not observed using platelets from NOD2-/- mice (Figure 2C & D). In an ex vivo study, MDP intraperitoneally given to mice also potentiated platelet aggregation and ATP release induced by thrombin and collagen in a NOD2-dependent manner (data not shown).
Figure 2.
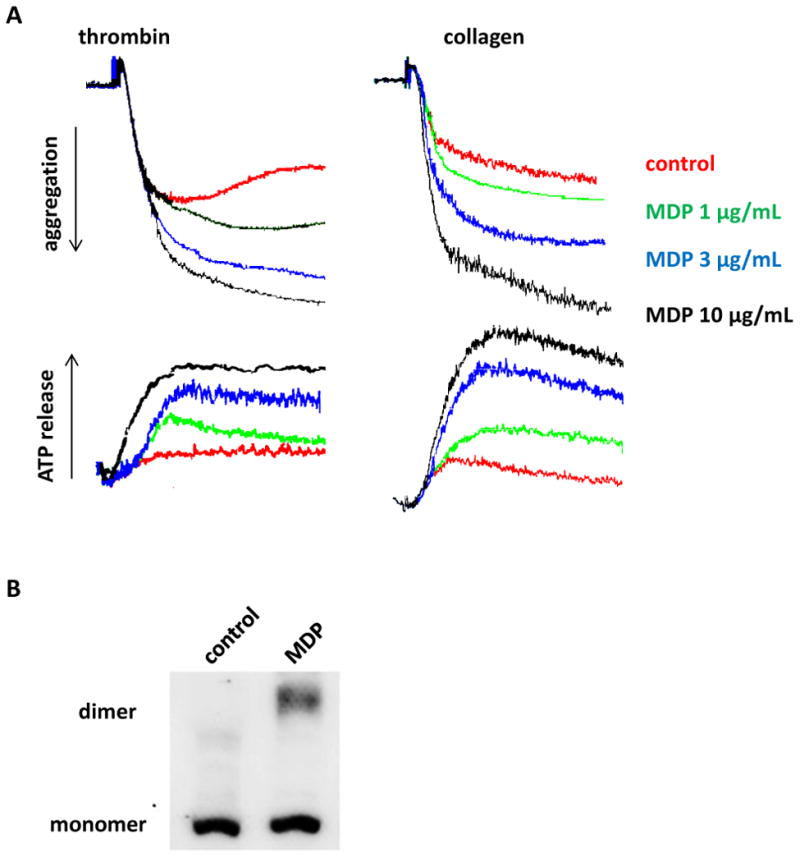
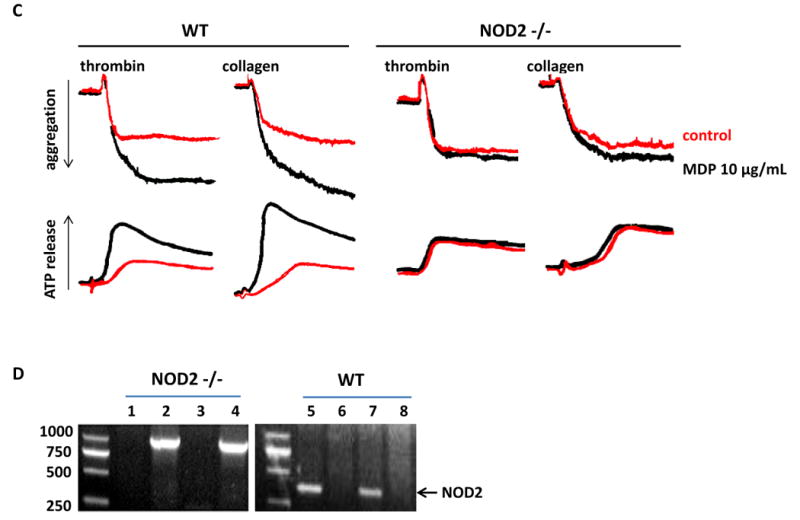
NOD2 activation induced by MDP potentiates platelet activation induced by thrombin and collagen. A, MDP concentration-dependently potentiates human platelet aggregation and ATP release induced by thrombin 0.02 U/mL or collagen 0.3 μg/mL. B, NOD2 dimerization in human platelets stimulated with MDP 10 μg/mL. C, MDP potentiates mouse platelet aggregation and ATP release induced by thrombin 0.02 U/mL and collagen 0.3 μg/mL. Aspirinated washed platelets were incubated with MDP for 15 min, and then stimulated with thrombin or collagen. D, Genotyping of NOD2-/- and WT mice. Even number lanes show 1000 bp PCR products of mutant, while odd number lanes show the 370 bp PCR products of wild type.
NOD2 receptor agonist MDP potentiates platelet clot retraction
Platelet dependent clot retraction is a late phase outside-in signaling event associated with second wave of interaction between talin and integrin β3 intracellular domain during platelet activation24. When clot retraction was examined, we found that NOD2 agonist MDP accelerated clot retraction in human platelet suspension (Figure 3). Similarly, MDP also accelerated clot retraction in mouse platelet suspension (Figure 3). Consistent with the effects of NOD2 activation on platelet aggregation and ATP release, MDP-induced clot retraction acceleration is NOD2-dependent, as it did not occur in NOD2-/- mice (Figure 3). Taken together, these data clearly indicate that NOD2 activation induced by MDP potentiates platelet activation.
Figure 3.
NOD2 agonist MDP accelerates clot retraction NOD2-dependently. Clot retraction in human or mouse washed platelets was assayed as described in Materials and Methods. A, Results shown are representative of four experiments using platelets from different donors or mice. MDP 10 μg/mL was used. B, Quantification of clot retraction at 60 min expressed as mean ± SD, n = 4.
NOD2 agonist MDP does not affect platelet spreading
Platelet spreading is an early phase outside-in signaling event downstream of platelet αIIbβ3 integrin activation24. In contrast to enhancing clot retraction, NOD2 agonist MDP had no effect on human platelet spreading on fibrinogen at 10 μg/mL (Figure S1), the concentration which activates NOD2 receptor as evidenced by receptor dimerization (Figure 2B). Similarly, mouse platelet spreading was also unaffected by MDP stimulation (Figure S2). Moreover, NOD2 deficiency also did not affect mouse platelet spreading (Figure S2), contrasting to the impaired clot retraction in NOD2-/- mice (Figure 3).
NOD2 deficiency impairs thrombus formation and hemostasis in vivo
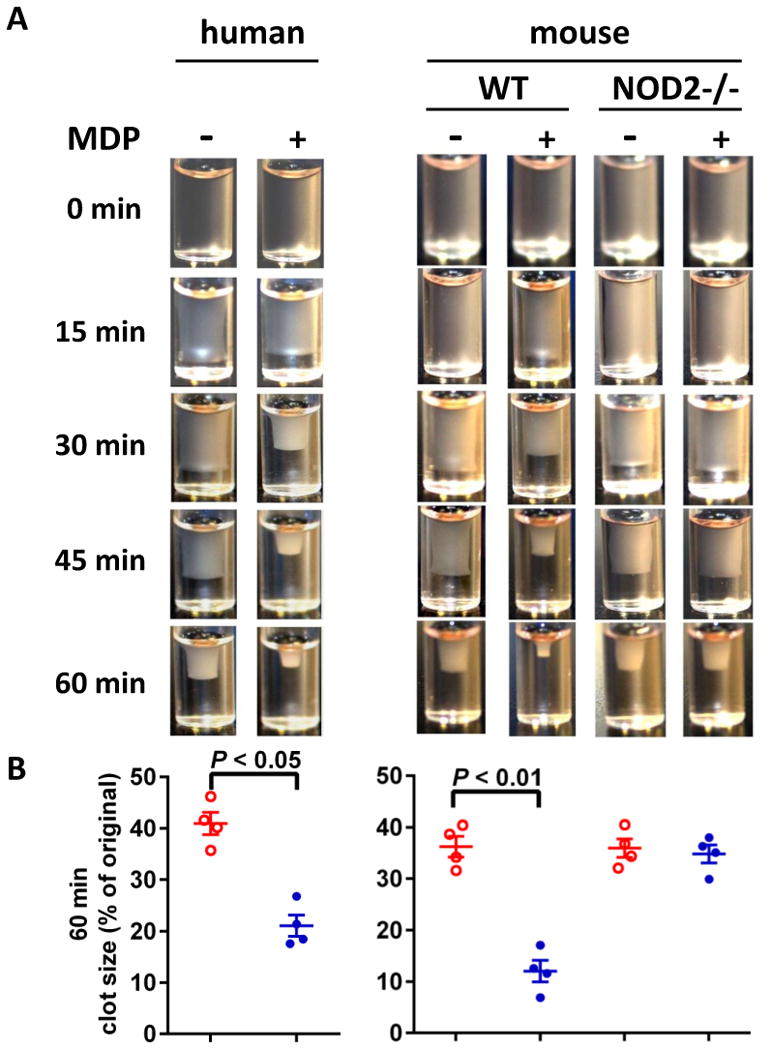
To explore the role of NOD2 in thrombus formation in vivo, we examined the FeCl3-injured thrombus formation in mesenteric arteriole in WT and NOD2-/- mice using intravital microscopy. As shown in Figure 4A & B, WT mice receiving MDP exhibit increased thrombus formation in mesenteric arterioles whereas NOD2-/- mice do not, indicating the enhancing role of NOD2 activation in thrombosis. Consistently, decreased blood loss after tail snip was observed in WT mice receiving MDP but not NOD2-deficient mice receiving the same dose of MDP (Figure 4C), suggesting that NOD2 contributes to hemostasis as well as thrombosis under MDP stimulation. These results are consistent with the potentiating effects of MDP on platelet aggregation and secretion.
Figure 4.
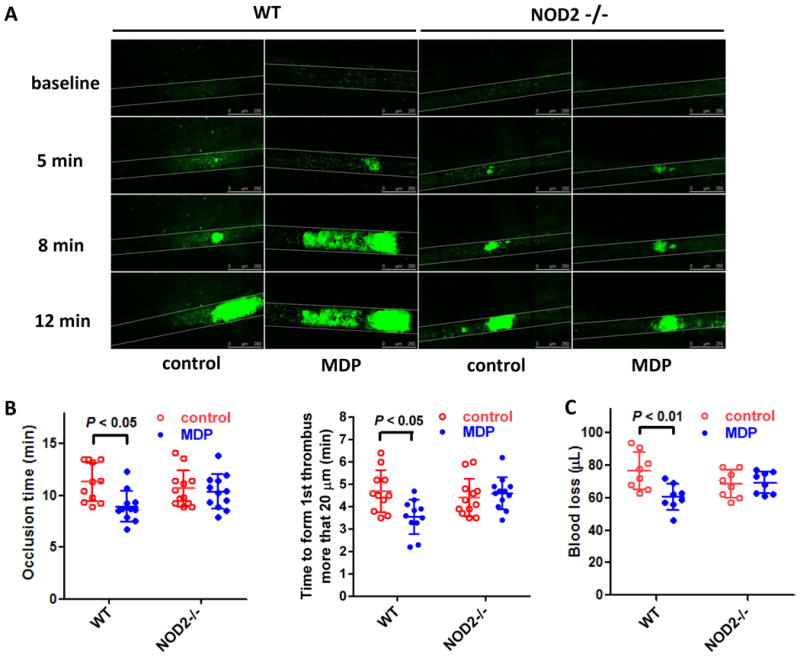
Impaired thrombus formation and hemostasis in NOD2-deficient mice. A, Impaired thrombus formation in mesenteric arterioles of NOD2-/- mice as compared with WT mice. Thrombosis was induced by FeCl3 injury, and recorded with intravital microscopy. Calcein was used to label platelets. MDP (100 μg/100 μL per mouse) or saline were injected intraperitoneally 12 hours before FeCl3 injury. B, Statistical analysis of FeCl3-induced thrombus formation. Occlusion time and time to first thrombus (larger than 20 μm and stable for more than 2 min) were analyzed. Data are expressed as mean ± SD with 11 WT and 12 NOD2-/- mice. C, MDP injection causes less blood loss in WT but not in NOD2-/- mice. Data are expressed as mean ± SD, n = 8.
NOD2 is also expressed in endothelial cells and macrophages15, which may also contribute to thrombosis and hemostasis upon MDP stimulation in septic settings. To corroborate that the NOD2 expressed in platelets prompts thrombosis and hemostasis in vivo after MDP dosing, we preincubated platelets with normal saline or MDP in vitro and transfused into platelet-depleted mice. We then challenged the mice with epinephrine and collagen, and examined the mortality using the lethal lung thromboembolism mouse model25. The death rate in mice receiving MDP-treated platelets is 69.2% (n = 13), significantly higher than 28.6% in the mice receiving saline-treated platelets (n = 14; P < 0.05, one tailed Fisher's exact test). In contrast, MDP-treated platelets from NOD2-deficient mice did not significantly increase death rate in platelet-depleted WT mice challenged with epinephrine and collagen compared with the mice receiving saline-treated NOD2-/- platelets (30.8% vs 25%, n = 13 and 12, respectively; P > 0.05, one tailed Fisher's exact test). We also examined the FeCl3-injured thrombus in mesenteric arteriole in platelet-depleted mice. Mice receiving MDP-treated platelets exhibited increased thrombus formation than the mice receiving saline-treated platelets. In contrast, MDP-treated platelets from NOD2-deficient mice did not significantly increase thrombus formation in platelet-depleted WT mice compared with the mice receiving saline-treated NOD2-/- platelets (Figure S3). These data clearly indicates that platelet-expressed NOD2 plays a significant role in thrombosis and hemostasis, correlating well with NOD2 potentiating effect on platelet activation (Figure 2).
NOD2 deficiency attenuated platelet hyperreactivity induced by bacterial infection
During bacterial infection, MDP is shed from bacteria and released into the blood, resulting in a strong increase in MDP concentration and activation of NOD2 signal cascade in vivo. To check whether NOD2 activation affects platelet activity during bacterial infection, we treated PRP from WT or NOD2-/- mice with plasma from patients with bacteremia, and then detected platelet aggregation. NOD2 deficiency attenuated the potentiating effects of septic plasma on platelet aggregation induced by thrombin and collagen (Figure 5). Our results demonstrated a critical role of NOD2 in platelet hyperreactivity caused by bacterial infection.
Figure 5.
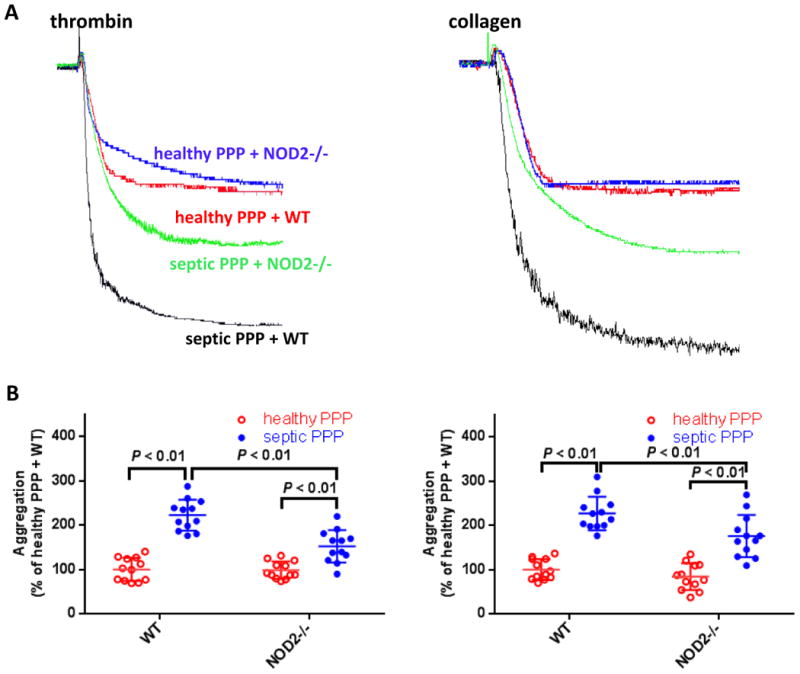
Plasma from patients with bacterial infection enhances platelet aggregation NOD2-dependently. A, Representative platelet aggregation tracings. B, Summary of aggregation experiments expressed as mean ± SD of 12 experiments. Platelet poor plasma (PPP) prepared from 14 patients with proven bacteremia was pooled and used as septic PPP. PPP prepared from the same patients after cure of infection was used as healthy PPP. For each experiment, PRP prepared from WT or NOD2-/- mice was preincubated with patient PPP (1:1 vol/vol) for 10 min, then platelet aggregation was induced by 0.02 U/mL thrombin or 0.3 μg/mL collagen. Please note that though septic plasma also enhances platelet aggregation of NOD2-/- mice, the potentiating effects are significantly weakened compared with that on WT mice.
Platelets express RIP2, RIP2/MAPK pathway is activated downstream of NOD2
Our results thus far indicate that NOD2 activation enhances platelet activation, and thus prompts thrombosis and hemostasis. Next, we sought to elucidate the mechanism of NOD2-mediated platelet activation. Mitogen activated protein kinase (MAPK) is well documented in platelet activation and thrombosis. RIP2/MAPK pathway activation has been reported to mediate NOD2-induced inflammation and anti-infection response in white blood cells1, 2. We therefore examined whether RIP2/MAPK pathway is involved in NOD2-mediated platelet activation.
Though a variety of cells express RIP2, it is not clear whether platelets express RIP2. Using RT-PCR and Western blot, we found that both human and mouse platelets express robust RIP2, similarly to PBMC (Figure 6A). To our knowledge, this is the first report that platelets express RIP2. Furthermore, upon MDP (10 μg/mL) stimulation, platelet RIP2 was phosphorylated at 5 min lasting for more than 10 min (Figure 6B). Erk, p38, and JNK were also phosphorylated with the peak phosphorylation apparently later than RIP2 (Figure 6B). These results agree with previous study with white blood cells and indicate that MAPK pathway is activated downstream of RIP2 in platelets upon NOD2 activation triggered by MDP.
Figure 6.
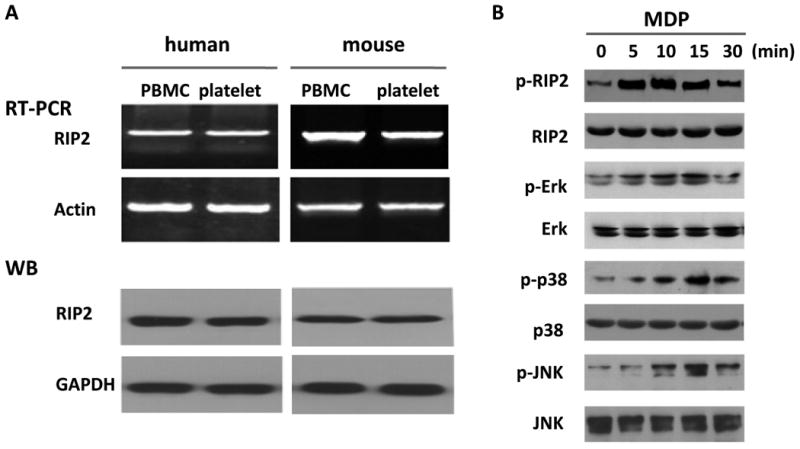
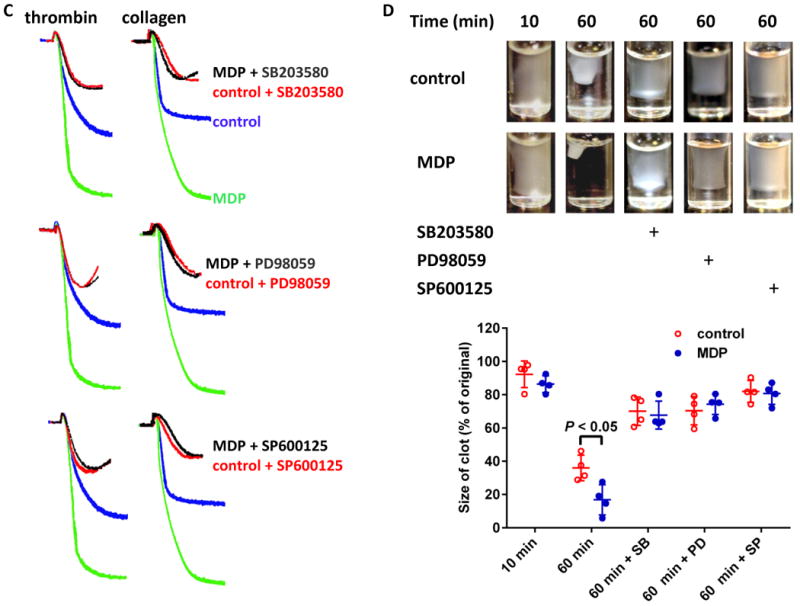
RIP2/MAPK mediates the potentiating effects of MDP on platelet activation. A, Platelets express RIP2. B, MDP phosphorylates RIP2, Erk, p38, and JNK in human platelets. Results shown are representative of at least three experiments using platelets from different donors or mice. C, Enhanced platelet aggregation is abolished by MAPK inhibitors. Human platelets (aspirinated washed platelets) were pretreated with 10 μM PD98059 (ERK1/2 inhibitor), 10 μM SB203580 (p38 MAPK inhibitor), or 10 μM SP600125 (JNK inhibitor) for 10 min, then treated with 10 μg/mL MDP or vehicle as control before stimulation by 0.02 U/mL thrombin or 0.3 μg/mL collagen. D, Enhanced clot retraction in human washed platelets is abolished by MAPK inhibitors. Clot retraction was assayed as in Figure 3. The upper panel is the representative of 4 experiments using platelets from different donors, the lower panel is the summary expressed as mean ± SD. MAPK inhibitors and MDP were used at the same concentration as in panel C.
MAPK inhibition abolishes the potentiating effects of NOD2 on platelet activation
After showing the activation of RIP2/MAPK pathway in platelets downstream of MDP-induced NOD2 activation, we further examined whether MAPK signaling mediates the potentiating effects of NOD2 on platelet activation. As shown in Figure 6C, inhibition of Erk, p38, and JNK with PD98059, SB203580, and SP600125, respectively, abolished the potentiating effect of MDP on platelet aggregation induced by thrombin or collagen. Similarly, the accelerated platelet-dependent clot retraction elicited by MDP was also prevented by inhibitors for Erk, p38, and JNK (Figure 6D). These data confirmed that MAPK phosphorylation downstream of NOD2/RIP2 mediates the potentiating effects of MDP on platelet activation.
MDP elevates cGMP and NO in platelets in a NOD2 dependent manner
cGMP elevation has been observed in platelets stimulated with collagen, thrombin, and vWF26, 27. cGMP-PKG-MAPK pathway has been reported to mediate platelet activation downstream of GPIb-IX28-30. After showing MAPK phosphorylation downstream of NOD2 activation upon MDP stimulation, we measured cGMP levels in platelets stimulated with MDP. As shown in Figure 7A, NOD2 agonist MDP concentration-dependently increased cGMP in human platelets up to 3.4 times in the range of 1 - 10 μg/mL, the concentrations which activated NOD2 and potentiated platelet activation concentration-dependently (Figure 2). MDP also increased cGMP in mouse platelets. At 10 μg/mL, MDP increased intracellular cGMP 3.7 times in mouse platelets, similarly to human platelets (Figure 7B). The cGMP-enhancing effect of MDP is mediated by NOD2, as it was abolished by NOD2 deficiency (Figure 7B). These results show that cGMP is downstream of NOD2, and may contribute to platelet activation as in the case of GPIb-IX-mediated platelet activation, which was confirmed by that PKG inhibitor KT5823 abolished the potentiating effect of MDP on platelet aggregation (Figure 7C). However, we did not observe the inhibition of KT5823 on MAPK phosphorylation elicited by MDP (data not shown), suggesting a MAPK-independent mechanism for the stimulatory role for cGMP/PKG in platelet activation29. Platelets express functional iNOS and NO/sGC which medicate cGMP elevation during platelet activation31. As expected, MDP-treated human platelets showed elevated intracellular NO levels, as detected by fluorescent NO sensor DAF-FM DA (Figure 7D). NOD2 deficiency abolished MDP-induced elevation of intracellular NO levels, showing NOD2 dependency (Figure 7E). Thus, we propose that cGMP elevation in platelets elicited by NOD2 activation is mediated by RIP2/iNOS/NO/sGC signaling32 (Figure 7F).
Figure 7.
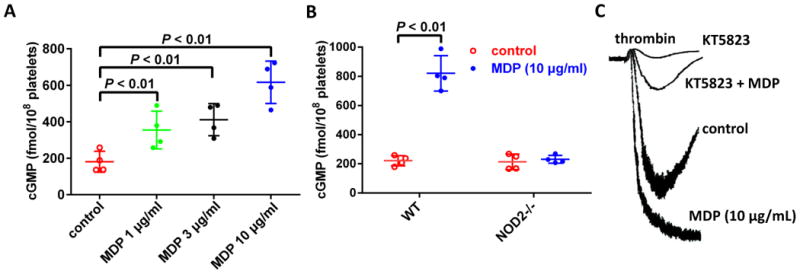
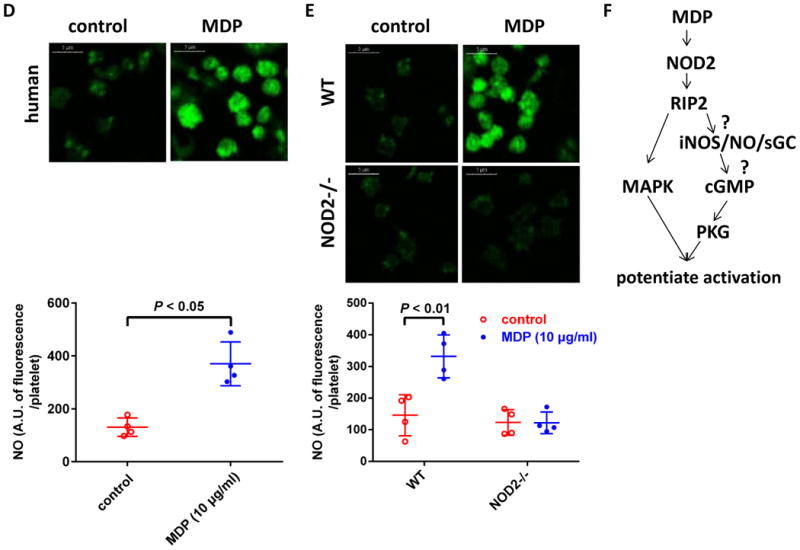
MDP elevates platelet cGMP and NO levels NOD2-dependently. A, MDP concentration-dependently increases cGMP in human platelets. Human platelets were treated with MDP at 37°C for 15 min, the cGMP in platelets was detected by 125I radioimmunoassay. B, MDP elevates cGMP in platelets from WT but not in NOD2-/- mice. Data are expressed as mean ± SD using platelets from different donors or mice (n = 4). C, MDP potentiation of platelet aggregation induced by low concentration of thrombin was abolished by PKG inhibitor KT5823. Human washed platelets were used. Tracing shown are representative of 3 experiments using platelets from different donors. D, MDP elevates NO in human platelets. Platelets preincubated with fluorescent NO sensor DAF-FM DA were stimulated with MDP. Upper panel shows platelet fluorescence under confocal microscope (scale bar = 5 μm), bottom panel is the quantification of NO expressed as fluorescence per platelet in arbitrary unit (mean ± SD of 4 experiments). E, MDP elevates platelet NO in WT but not in NOD2-/- mice. NO was assayed as in panel D. Upper panel is representative of 4 experiments using platelets from different mice, bottom panel is the quantification of NO expressed as mean ± SD, n = 4. The concentrations for MDP, thrombin and KT5823 were 10 μg/mL, 0.02 U/mL and 8 μM, respectively. F, MAPK and NO/cGMP/PKG pathways downstream of RIP2 mediate the role of NOD2 in platelets.
NOD2 receptor agonist MDP stimulates platelet processing of IL-1β
Platelets represent an important linkage between thrombosis and inflammation. To assess the role of platelet NOD2 in inflammation, we measured proinflammatory cytokine IL-1β production in human platelets stimulated with MDP. In the range of 10 - 100 ng/mL, MDP concentration-dependently stimulates IL-1β accumulation at protein level in human platelets (Figure 8A). The effect of MDP is time-dependently from 1 to 4 hours (Figure 8A). This effect appeared to be mediated by caspase-1 activation, because caspase-1 inhibitor FMK002 abolished MDP-stimulated IL-1β maturation (Figure 8B) and accumulation (Figure 8C) in human platelets. MDP also stimulated IL-1β accumulation in mouse platelets, which is NOD2-dependent (Figure 8D). MDP NOD2-dependently stimulates platelets IL-1β maturation provided a direct evidence that NOD2 links the prothrombotic and proinflammatory roles of platelets.
Figure 8.
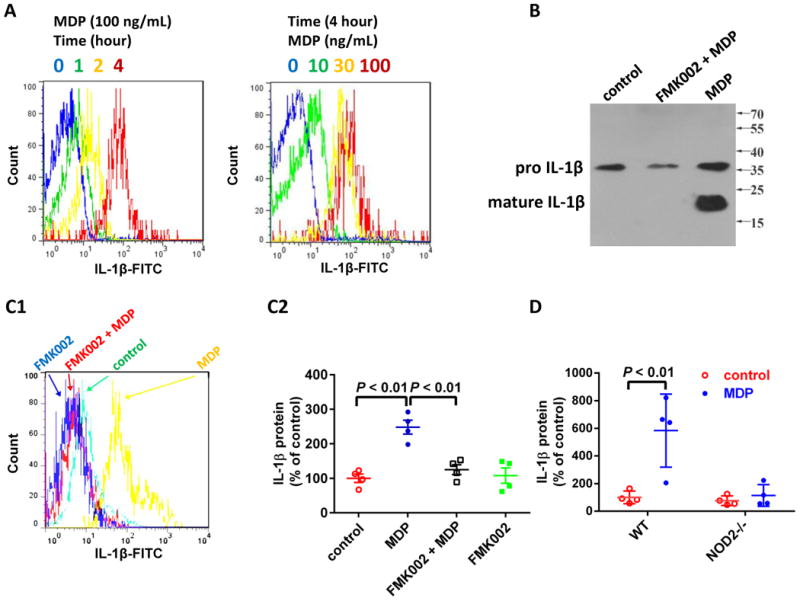
MDP stimulates platelet IL-1β maturation. A, MDP time- and concentration-dependently stimulates IL-1β accumulation in human platelets. IL-1β was assayed by flow cytometry as described under Materials and Methods. Results shown are representative of 3 experiments using platelets from different donors. B & C, Caspase-1 inhibitor FMK002 abolishes MDP-induced IL-1β maturation in human platelets. Platelets were treated with MDP (100 μg/mL) overnight, in the presence or absence of caspase-1 inhibitor FMK002 (25 μM), then platelets were resuspended in reducing SDS sample buffer and probed for IL-1β by Western blot (B) or flow cytometry (C). Results shown are representative of 3 (panel B) and 4 experiments (panel C1) using platelets from different donors, respectively. Panel C2 is the summary of panel C1 expressed as mean ± SD (n = 4). D, MDP stimulates IL-1β accumulation in platelets from WT, but not NOD2-/- mice. Platelets were treated with MDP (100 ng/mL) overnight, mature IL-1β protein in platelet lysates was measured by ELISA. Data are expressed as mean ± SD with n = 4.
Discussion
Beyond the well-recognized pivotal role in thrombosis and hemostasis, platelets also play a critical role in inflammatory and infectious diseases33, and increasing evidences indicate that bacterial infection predisposes atherosclerosis and thrombotic events34-36. Among the mechanisms underlying the infection-associated prothrombotic state, Toll-like receptors have been reported to medicate platelet activation in response to bacterial challenge12. As a pattern recognition receptor, although NOD2 is well investigated in immunity, its expression and function in platelets have never been reported. The present study demonstrates that (1) both human and mouse platelets express robust NOD2; (2) NOD2 receptor activation potentiates platelet activation and enhances in vivo thrombosis; (3) Plasma from septic patients potentiates platelet aggregation NOD2-dependently; 4) MAPK and iNOS/NO/sGC/cGMP/PKG pathways downstream of RIP2 mediate the role of NOD2 in platelet activation; (5) NOD2 receptor activation triggers platelet processing of proinflammatory cytokine IL-1β. To our knowledge, this is the first report on NOD-like receptor in platelets, our findings provided novel insight into platelet activation mechanism, NOD2 function, and further highlighted platelet NOD2 as a critical receptor bridging thrombosis/hemostasis and immunity.
MAPK is activated during platelet stimulation and play critical roles in platelet activation. Previously MAPK activation has been attributed to NOD2 signaling downstream of RIP2 in immune cells1, 2. Therefore, we checked MAPK pathway activation downstream of NOD2 in platelets using MDP as an agonist. We found that Erk, p38, and JNK were phosphorylated in MDP-stimulated platelets. Importantly, we found that platelets expressed robust RIP2, and RIP2 was phosphorylated earlier than Erk, p38, and JNK upon MDP stimulation, indicating that MAPK is activated downstream of RIP2 activation. In addition, we found that MAPK inhibition abolished the potentiating effects of MDP on platelet aggregation, further confirming the critical roles of MAPK in NOD2-mediated platelet activation. These results also suggest that p38, Erk or JNK may be sequentially activated or coactivation of them is needed for platelet aggregation induced by low concentration of agonists. This hypothesis is supported by the previous studies showing that p38 sequentially activates Erk, JNK1 deficiency impairs Erk2 phosphorylation37, 38, and p38 or JNK inhibition abolished platelet aggregation under low concentration of agonist stimulation39, 40.
Biphasic role of cGMP has been reported in agonist-induced platelet activation, low concentration of cGMP stimulates platelet activation during the early process41 via the cGMP/PKG/MAPK pathway29. After showing that NOD2 medicates platelet activation via MAPK pathway downstream of RIP2, we next studied the role of cGMP in NOD2-mediated platelet activation. We found that NOD2 activation dramatically enhanced platelet cGMP. In line with our findings, the activation of Toll like receptor, another pattern recognition receptor which potentiates platelet activation, also increases platelet cGMP levels and activates platelets via cGMP-dependent protein kinase (PKG) pathway11. Consistently, we also found that NOD2 agonist MDP potentiates platelet aggregation PKG-dependently.
Previously, Shimada et al ever reported that macrophages from NOD2-deficient mice exhibited impaired NO production iNOS-dependently in response to Chlamydophila pneumoniae16. NO production from RIP2-deficient mice was also decreased in response to Chlamydophila pneumonia, suggesting the RIP2-dependent NO production16. Platelets express functional iNOS and NO/sGC medicates cGMP elevation during platelet activation31; thus, we propose that cGMP elevation in platelets elicited by NOD2 activation is mediated by RIP2/iNOS/NO/sGC signaling32, which is supported by our findings that MDP increases platelet NO level NOD2-dependently. Generally, RIP2 activation is regarded as the result of its phosphorylation via direct interaction with dimerization form of NOD2 after NOD2 activation42. Therefore, we propose that NOD2 promotes platelet activation via MAPK and iNOS/NO/sGC/cGMP pathways downstream of RIP2. However, how RIP2 mediates iNOS activation in platelets is still not clear and deserves further investigation.
As a key proinflammatory cytokine, IL-1β is linked to the pathogenesis of a variety of thromboinflammatory diseases including sepsis, Crohn's disease, ulcerative colitis, type 2 diabetes and atherosclerosis. To exploit the proinflammatory role of platelet NOD2 and its connecting role in thrombosis and inflammation/immunity during bacterial infection, we provided data showing that MDP stimulates IL-1β accumulation in human and mouse platelets NOD2-dependently; we also found that plasma from patients with bacteremia enhances platelet activation NOD2-dependendly. In line with our findings, P. gingivalis challenge has been reported to promote atherogenesis via IL-1 signaling43, S. pneumonia infection worsens atherosclerosis and exacerbates ischemic brain injury platelet- and IL-1-dependently35, while IL-1β deletion decreases the severity of atherosclerosis44.
It's well-known that platelets participate in the pathogenesis of atherosclerosis45. The role of NOD2 in atherosclerosis is limited and inconsistent. Though two groups found that NOD2 prompt atherosclerosis15, 46, Yuan et al recently reported that MDP/NOD2 axis activation reduces atherosclerosis47. The role of platelet NOD2 in the growth and destabilization of atherosclerosis is not clear. The prothrombotic and proinflammatory roles of platelet NOD2 demonstrated by our present study suggest that platelet NOD2 may facilitate the initiation and development of atherosclerosis.
The different MDP concentrations needed for NOD2-mediated prothrombotic and proinflammatory response attracted our attention. At low MDP concentrations (10 - 100 ng/mL), platelet NOD2 triggers IL-1β maturation; while at high MDP concentrations (1 - 10 μg/mL), platelet NOD2 potentiates platelets activation, but does not stimulate IL-1β maturation (data not shown). Although the underlying mechanism accounting for this different concentration dependence remains to be elucidated, the present study implies that platelet NOD2 is likely to switch its roles at different stages of infection. At the initial stage of infection, in response to low concentrations of MDP from pathogens, platelet NOD2 appears to mainly exert proinflammatory effects. With the pathogen accumulation as the infection progresses, high concentration of MDP from bacteria triggers the prothrombotic response of platelet NOD2.
In conclusion, NOD2, which is mainly expressed in white blood cells and plays critical roles in innate immune against infection, is also expressed in platelets and potentiates platelet activation, thrombosis, and prompts proinflammatory response. Our study also proposed a novel signaling pathway, MDP-NOD2-RIP2-MAPK/iNOS-NO-sGC-cGMP-PKG (Figure 7F), for platelet activation, which may explain the prothrombotic state in infectious diseases. Considering the critical roles of NOD2 in innate immune against infection and the crucial role of platelets in thrombosis, hemostasis, and immune response, further studies could shed new insight into the pathogenesis and treatment of inflammatory and thrombotic diseases.
Supplementary Material
Acknowledgments
Funding Sources: This work was partially supported by National Natural Science of Foundation of China (Grant No. 81173053, 81373411, 81100344), Drug Innovative Program from Shanghai Municipal Science and Technology Commission (Grant No. 11431920103), Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning to ZD and SZ, and grants HL93231 and HL118593 from National Institutes of Health to SPK.
Footnotes
Disclosures: None.
References
- 1.Chen G, Shaw MH, Kim YG, Nunez G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev Pathol. 2009;4:365–98. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- 2.Philpott DJ, Sorbara MT, Roberston SJ, Croitoru K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 2014;14:9–23. doi: 10.1038/nri3565. [DOI] [PubMed] [Google Scholar]
- 3.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–72. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 4.Mo J, Boyle JP, Howard CB, Monie TP, Davis BK, Duncan JA. Pathogen sensing by nucleotide-binding oligomerization domain-containing protein 2 (NOD2) is mediated by direct binding to muramyl dipeptide and ATP. J Biol Chem. 2012;287:23057–67. doi: 10.1074/jbc.M112.344283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–4. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Li J, Li Y, Lang S, Yougbare I, Zhu G, Chen P, Ni H. Crosstalk between Platelets and the Immune System: Old Systems with New Discoveries. Adv Hematol. 2012;2012 doi: 10.1155/2012/384685. 384685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiraki R, Inoue N, Kawasaki S, Takei A, Kadotani M, Ohnishi Y, Ejiri J, Kobayashi S, Hirata K, Kawashima S, Yokoyama M. Expression of Toll-like receptors on human platelets. Thromb Res. 2004;113:379–85. doi: 10.1016/j.thromres.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Aslam R, Speck ER, Kim M, Crow AR, Bang KW, Nestel FP, Ni H, Lazarus AH, Freedman J, Semple JW. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood. 2006;107:637–41. doi: 10.1182/blood-2005-06-2202. [DOI] [PubMed] [Google Scholar]
- 10.Andonegui G, Kerfoot SM, McNagny K, Ebbert KV, Patel KD, Kubes P. Platelets express functional Toll-like receptor-4. Blood. 2005;106:2417–23. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 11.Zhang G, Han J, Welch EJ, Ye RD, Voyno-Yasenetskaya TA, Malik AB, Du X, Li Z. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J Immunol. 2009;182:7997–8004. doi: 10.4049/jimmunol.0802884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blair P, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S, Hayashi C, Genco CA, Iafrati M, Freedman JE. Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ Res. 2009;104:346–54. doi: 10.1161/CIRCRESAHA.108.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panigrahi S, Ma Y, Hong L, Gao D, West XZ, Salomon RG, Byzova TV, Podrez EA. Engagement of platelet toll-like receptor 9 by novel endogenous ligands promotes platelet hyperreactivity and thrombosis. Circ Res. 2013;112:103–12. doi: 10.1161/CIRCRESAHA.112.274241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeda S, Hsu LC, Liu H, Bankston LA, Iimura M, Kagnoff MF, Eckmann L, Karin M. Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–8. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 15.Liu HQ, Zhang XY, Edfeldt K, Nijhuis MO, Idborg H, Back M, Roy J, Hedin U, Jakobsson PJ, Laman JD, de Kleijn DP, Pasterkamp G, Hansson GK, Yan ZQ. NOD2-mediated innate immune signaling regulates the eicosanoids in atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33:2193–201. doi: 10.1161/ATVBAHA.113.301715. [DOI] [PubMed] [Google Scholar]
- 16.Shimada K, Chen S, Dempsey PW, Sorrentino R, Alsabeh R, Slepenkin AV, Peterson E, Doherty TM, Underhill D, Crother TR, Arditi M. The NOD/RIP2 pathway is essential for host defenses against Chlamydophila pneumoniae lung infection. PLoS Pathog. 2009;5:e1000379. doi: 10.1371/journal.ppat.1000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y, Ahmad SS, Zhou J, Wang L, Cully MP, Essex DW. The disulfide isomerase ERp57 mediates platelet aggregation, hemostasis, and thrombosis. Blood. 2012;119:1737–46. doi: 10.1182/blood-2011-06-360685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang SH, Zhang Y, Shen J, Zhang S, Chen L, Gu J, Mruk JS, Cheng G, Zhu L, Kunapuli SP, Ding Z. Tumor vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid inhibits platelet activation and thrombosis via inhibition of thromboxane A2 signaling and phosphodiesterase. J Thromb Haemost. 2013;11:1855–66. doi: 10.1111/jth.12362. [DOI] [PubMed] [Google Scholar]
- 19.Su X, Mi J, Yan J, Flevaris P, Lu Y, Liu H, Ruan Z, Wang X, Kieffer N, Chen S, Du X, Xi X. RGT, a synthetic peptide corresponding to the integrin beta 3 cytoplasmic C-terminal sequence, selectively inhibits outside-in signaling in human platelets by disrupting the interaction of integrin alpha IIb beta 3 with Src kinase. Blood. 2008;112:592–602. doi: 10.1182/blood-2007-09-110437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fay WP, Parker AC, Ansari MN, Zheng X, Ginsburg D. Vitronectin inhibits the thrombotic response to arterial injury in mice. Blood. 1999;93:1825–30. [PubMed] [Google Scholar]
- 21.Zhang S, Hu L, Du H, Guo Y, Zhang Y, Niu H, Jin J, Zhang J, Liu J, Zhang X, Kunapuli SP, Ding Z. BF0801, a novel adenine derivative, inhibits platelet activation via phosphodiesterase inhibition and P2Y12 antagonism. Thromb Haemost. 2010;104:845–57. doi: 10.1160/TH10-05-0285. [DOI] [PubMed] [Google Scholar]
- 22.Fang C, Stavrou E, Schmaier AA, Grobe N, Morris M, Chen A, Nieman MT, Adams GN, LaRusch G, Zhou Y, Bilodeau ML, Mahdi F, Warnock M, Schmaier AH. Angiotensin 1-7 and Mas decrease thrombosis in Bdkrb2-/- mice by increasing NO and prostacyclin to reduce platelet spreading and glycoprotein VI activation. Blood. 2013;121:3023–32. doi: 10.1182/blood-2012-09-459156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Ye J, Hu L, Zhang S, Zhang SH, Li Y, Kunapuli SP, Ding Z. Increased platelet activation and thrombosis in transgenic mice expressing constitutively active P2Y12. J Thromb Haemost. 2012;10:2149–57. doi: 10.1111/j.1538-7836.2012.04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen B, Zhao X, O'Brien KA, Stojanovic-Terpo A, Delaney MK, Kim K, Cho J, Lam SC, Du X. A directional switch of integrin signalling and a new anti-thrombotic strategy. Nature. 2013;503:131–5. doi: 10.1038/nature12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nieswandt B, Schulte V, Bergmeier W, Mokhtari-Nejad R, Rackebrandt K, Cazenave JP, Ohlmann P, Gachet C, Zirngibl H. Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice. J Exp Med. 2001;193:459–69. doi: 10.1084/jem.193.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riba R, Sharifi M, Farndale RW, Naseem KM. Regulation of platelet guanylyl cyclase by collagen: evidence that Glycoprotein VI mediates platelet nitric oxide synthesis in response to collagen. Thromb Haemost. 2005;94:395–403. doi: 10.1160/TH05-01-0027. [DOI] [PubMed] [Google Scholar]
- 27.Gambaryan S, Kobsar A, Hartmann S, Birschmann I, Kuhlencordt PJ, Muller-Esterl W, Lohmann SM, Walter U. NO-synthase-/NO-independent regulation of human and murine platelet soluble guanylyl cyclase activity. J Thromb Haemost. 2008;6:1376–84. doi: 10.1111/j.1538-7836.2008.03014.x. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Xi X, Du X. A mitogen-activated protein kinase-dependent signaling pathway in the activation of platelet integrin alpha IIbbeta3. J Biol Chem. 2001;276:42226–32. doi: 10.1074/jbc.M106129200. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Delaney MK, O'Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. 2010;30:2341–9. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin H, Liu J, Li Z, Berndt MC, Lowell CA, Du X. Src family tyrosine kinase Lyn mediates VWF/GPIb-IX-induced platelet activation via the cGMP signaling pathway. Blood. 2008;112:1139–46. doi: 10.1182/blood-2008-02-140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marjanovic JA, Stojanovic A, Brovkovych VM, Skidgel RA, Du X. Signaling-mediated functional activation of inducible nitric-oxide synthase and its role in stimulating platelet activation. J Biol Chem. 2008;283:28827–34. doi: 10.1074/jbc.M801646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stojanovic A, Marjanovic JA, Brovkovych VM, Peng X, Hay N, Skidgel RA, Du X. A phosphoinositide 3-kinase-AKT-nitric oxide-cGMP signaling pathway in stimulating platelet secretion and aggregation. J Biol Chem. 2006;281:16333–9. doi: 10.1074/jbc.M512378200. [DOI] [PubMed] [Google Scholar]
- 33.Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123:2759–67. doi: 10.1182/blood-2013-11-462432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–8. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 35.Denes A, Pradillo JM, Drake C, Sharp A, Warn P, Murray KN, Rohit B, Dockrell DH, Chamberlain J, Casbolt H, Francis S, Martinecz B, Nieswandt B, Rothwell NJ, Allan SM. Streptococcus pneumoniae worsens cerebral ischemia via interleukin 1 and platelet glycoprotein Ibalpha. Ann Neurol. 2014;75:670–83. doi: 10.1002/ana.24146. [DOI] [PubMed] [Google Scholar]
- 36.Dalager-Pedersen M, Sogaard M, Schonheyder HC, Nielsen H, Thomsen RW. Risk for myocardial infarction and stroke after community-acquired bacteremia: a 20-year population-based cohort study. Circulation. 2014;129:1387–96. doi: 10.1161/CIRCULATIONAHA.113.006699. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, Zhang G, Feil R, Han J, Du X. Sequential activation of p38 and ERK pathways by cGMP-dependent protein kinase leading to activation of the platelet integrin alphaIIb beta3. Blood. 2006;107:965–72. doi: 10.1182/blood-2005-03-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adam F, Kauskot A, Nurden P, Sulpice E, Hoylaerts MF, Davis RJ, Rosa JP, Bryckaert M. Platelet JNK1 is involved in secretion and thrombus formation. Blood. 2010;115:4083–92. doi: 10.1182/blood-2009-07-233932. [DOI] [PubMed] [Google Scholar]
- 39.Saklatvala J, Rawlinson L, Waller RJ, Sarsfield S, Lee JC, Morton LF, Barnes MJ, Farndale RW. Role for p38 mitogen-activated protein kinase in platelet aggregation caused by collagen or a thromboxane analogue. J Biol Chem. 1996;271:6586–9. doi: 10.1074/jbc.271.12.6586. [DOI] [PubMed] [Google Scholar]
- 40.Kauskot A, Adam F, Mazharian A, Ajzenberg N, Berrou E, Bonnefoy A, Rosa JP, Hoylaerts MF, Bryckaert M. Involvement of the mitogen-activated protein kinase c-Jun NH2-terminal kinase 1 in thrombus formation. J Biol Chem. 2007;282:31990–9. doi: 10.1074/jbc.M701596200. [DOI] [PubMed] [Google Scholar]
- 41.Li Z, Xi X, Gu M, Feil R, Ye RD, Eigenthaler M, Hofmann F, Du X. A stimulatory role for cGMP-dependent protein kinase in platelet activation. Cell. 2003;112:77–86. doi: 10.1016/s0092-8674(02)01254-0. [DOI] [PubMed] [Google Scholar]
- 42.Franchi L, Warner N, Viani K, Nunez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–28. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chi H, Messas E, Levine RA, Graves DT, Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation. 2004;110:1678–85. doi: 10.1161/01.CIR.0000142085.39015.31. [DOI] [PubMed] [Google Scholar]
- 44.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–60. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 45.Lievens D, von Hundelshausen P. Platelets in atherosclerosis. Thromb Haemost. 2011;106:827–38. doi: 10.1160/TH11-08-0592. [DOI] [PubMed] [Google Scholar]
- 46.Johansson ME, Zhang XY, Edfeldt K, Lundberg AM, Levin MC, Boren J, Li W, Yuan XM, Folkersen L, Eriksson P, Hedin U, Low H, Sviridov D, Rios FJ, Hansson GK, Yan ZQ. Innate immune receptor NOD2 promotes vascular inflammation and formation of lipid-rich necrotic cores in hypercholesterolemic mice. Eur J Immunol. 2014;44:3081–92. doi: 10.1002/eji.201444755. Epub 2014 Aug 25. [DOI] [PubMed] [Google Scholar]
- 47.Yuan H, Zelka S, Burkatovskaya M, Gupte R, Leeman SE, Amar S. Pivotal role of NOD2 in inflammatory processes affecting atherosclerosis and periodontal bone loss. Proc Natl Acad Sci U S A. 2013;110:E5059–68. doi: 10.1073/pnas.1320862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


