Abstract
Multiple myeloma (MM) is the second most common hematologic malignancy characterized by the clonal expansion of plasma cells. Despite continuing advances, novel biomarkers are needed for diagnosis and prognosis of MM. In this study we characterized the diagnostic and prognostic potential of circulating miRNAs in MM. Serum miRNA levels were analyzed in 108 newly diagnosed symptomatic MM patients and 56 healthy donors (HD). Our analysis identified ninety-five dysregulated miRNAs in newly diagnosed MM patients. Of the ninety-five dysregulated miRNAs, dysregulation of miR-19a, miR-92a, miR-214-3p, miR-135b-5p, miR-4254, miR-3658 and miR-33b were confirmed by RT-qPCR. Receiver operating characteristic analysis revealed that a combination of miR-19a and miR-4254 can distinguish MM from HD with a sensitivity of 91.7% and specificity of 90.5%. Decreased expression of miR-19a was positively correlated with international staging system advancement, del(13q14) and 1q21 amplification. Furthermore, down-regulation of miR-19a resulted in significantly decreased progression free and overall survival. Our analysis indicated that the poor prognostic correlation of miR-19a expression was independent of genetic abnormalities in MM. Multivariate analysis revealed that miR-19a was a significant predictor of shortened PFS and OS. Interestingly, although miR-19a levels portend a poor prognosis, patients with low miR-19a levels had an improved response to bortezomib compared to patients with high miR-19a profile. Patients with down-regulated miR-19a experienced a significantly extended survival upon bortezomib based therapy. These data demonstrate that the expression patterns of serum microRNAs are altered in MM and miR-19a levels are a valuable prognostic marker to identify high-risk MM.
Keywords: Serum miRNAs, miR-19a, Prognostic indicator, Biomarkers, Multiple myeloma
Introduction
Multiple myeloma (MM) is a hematologic malignancy characterized by clonal expansion of plasmas cells associated with paraproteins in the blood, bone lesions, hypercalcemia and renal failure1. MM accounts for approximately 13% of hematological malignancies and is primarily considered a disease within the aging population. Recent advances in the understanding of MM biology, pathology and development of novel therapeutics have significantly increased life expectancy and quality of life however, despite all of our advances MM remains an incurable disease2, 3. Multiple myeloma pathogenesis consists of a multistep process in which plasma cells undergo a series of molecular and cellular changes within the human bone marrow, which supports the growth, survival, and evolution of drug resistant tumor cells4–6. Although there are known diagnostic serum markers for myeloma, recent attention has focused on identifying more powerful markers and has turned to circulating microRNAs as a potential diagnostic and/or prognostic tool7–10.
MiRNAs are small, non-coding RNAs of 20–22 base pairs that repress gene expression by primarily interacting with the 3’ untranslated region of their target transcripts11–13. Following processing and transport out of the nucleus, miRNAs regulate their target mRNAs through Watson-Crick base pairing between the miRNA and a complementary sequence on the target mRNA14. Since their discovery, 1872 miRNAs have been characterized throughout the human genome (www.mirbase.org) with the potential for more to be discovered. Furthermore, each miRNA has the potential to repress the translation of hundreds of mRNA targets13, 15. Recent research has shown that different miRNA expression profiles were described and resulting miRNA levels correlated with patient survival and prognosis16–18. Blood serum or plasma derived miRNA biomarkers offer a potentially significant clinical advantage over conventional tumor cell examination due to the minimally invasive nature of acquiring samples, ease of standardization, and the opportunity for repeated sampling throughout disease progression. Such observations suggest that miRNAs represent a novel clinical marker for detection, classification, prognosis and therapeutic development in patients with MM.
Considering the advancements made in our understanding of aberrantly expressed miRNAs in cancer and their involvement in pathogenesis of MM18, 19,20, we hypothesized that circulating miRNAs could represent an ideal class of blood-based diagnostic and prognostic markers in MM; due to (1) miRNA expression is dysregulated in MM cells; (2) expression patterns of miRNAs in malignant samples appears to be tissue-specific; and (3) miRNAs are stable in circulation21, 22.
In the current study we evaluated serum miRNA expression profiles in MM patient and healthy donor samples to identify differentially expressed miRNAs. Furthermore, diagnostic and prognostic utility of serum miRNAs was analyzed in a 108 patient cohort under differing treatment regimens.
Materials and Methods
Patients and healthy donors
Peripheral blood (PB) serum samples from MM patients and healthy donors (HD) from the Institute of Hematology and Blood Disease Hospital were obtained at the time of diagnosis. All patients signed an informed consent form approved by the Institutional local ethics committee. For 103 newly diagnosed MM patients, bone marrow plasma cells (BMPC) were obtained for routine fluorescence in situ hybridization analysis (FISH), specifically for del(13q14), del(17p),t(11;14), t(4;14), t(14;16) and 1q21 amplification, as described previously23.
According to their request, patients were assigned to either the thalidomide-based (arm A) or bortezomib-based (arm B) treatment. Arm A (n=7) consisted of 4 cycles of induction treatment with thalidomide (TAD) 200 mg/day; intravenous (i.v.) adriamycin 9 mg/m2 on Days 1–4; and oral or i.v. dexamethasone 20 mg/d on Days 1–4 and 9–12; or TCD (i.e. thalidomide 200 mg/day; i.v. cyclophosphamide 300 mg/m2 on Days 1 and 8; and oral or i.v. dexamethasone 20 mg/d on Days 1–4 and 9–12); Arm B (n=9) consisted of 4 cycles of induction treatment with BCD (i.e. i.v. bortezomib 1.3 mg/m2 on Days 1, 4, 8, and 11; i.v. cyclophosphamide 300 mg/m2 on Days 1 and 8; and oral or i.v. dexamethasone 20 mg/d on Days 1, 2, 4, 5, 8, 9, 11 and 12); or PAD (i.v. bortezomib 1.3 mg/m2 on Days 1, 4, 8 and 11; i.v. adriamycin 9 mg/m2 on Days 1–4; and oral or i.v. dexamethasone, 20 mg/d on Days 1, 2, 4, 5, 8, 9, 11 and 12). After at least four cycles of treatment with partial remission or better, patients underwent consolidation therapy. This was either autologous stem cell transplant (ASCT) or chemotherapy with the patient’s original regimen according to patient request. Subsequently, patients were treated with thalidomide (100–150 mg/day) for one year to maintain response. When necessary, some of them also received supportive treatment with zoledronic acid every 1–2 months and erythropoietin or granulocyte colony-stimulating factor. All patients underwent prophylactic acyclovir treatment.
Serum collection and miRNA/RNA extraction
Peripheral blood serum samples were collected by centrifugation at 3000 rpm/10 min at room temperature. Peripheral blood samples were frozen in 0.2 mL aliquots, stored at −80°C and thawed once for RNA extraction. Total RNA (≈1300ng/0.2mL) was harvested and enriched from all serum samples using TRIzol (Invitrogen, Grand Island, NY, USA) and RNeasy mini kits (Qiagen, Valencia, CA) modified for circulating miRNAs according to the manufacturers’ recommendations. RNA quantity was assessed on a NanoDrop ND-1000 Spectrophotometer (Thermo Scientific).
MicroRNA profiling and data analysis
To study the differential miRNA expression in MM patients, we performed miRNA expression profiling arrays of serum samples from 7 newly diagnosed symptomatic MM patients and 5 healthy donors using the miRCURY™ LNA Array (version 11.0, Exigon, Denmark) system. The microarray for miRNA profiling was conducted by the China Shanghai Kangcheng Technology Co, Ltd24, 25. Briefly, 3 µg RNA samples were labeled with the Exiqon miRCURY™ Hy3/Hy5 power labeling kit and hybridized to the miRCURY LNA Array station. Scanning was performed using the Axon GenePix 4000B microarray scanner. GenePix pro version 6.0 was used to read image raw intensity. The intensity of the green signal was calculated after background subtraction, and replicated spots on the same slide were averaged to obtain median intensity. The median normalization method was used to acquire normalized data (foreground minus background divided by median)26,27, 28. The median was the 50th percentile of miRNA intensity and was >50 in all samples after background correction. The threshold value for significance used to define up-regulation or down-regulation of miRNAs was a fold change >1.5, with p<0.05 calculated by a student’s t test.
Candidate miRNA confirmation by RT-qPCR
Individual miRNA assays for 10 miRNAs (hsa-miR-19a, hsa-miR-92a, hsa -miR-214-3p, hsa -miR-135b-5p, hsa -miR-4254, hsa –miR-3658, hsa -miR-33b, hsa -miR-132, hsa -miR-574-3p, hsa -miR-376c) were performed using 1µg RNA. The All-in-One™ miRNA First-strand cDNA synthesis kit and miRNA RT-qPCR detection kit was used per the manufactures recommendations (GeneCopoeia, China)29. Quantitative PCR for miRNA was carried out at the following conditions: 95°C for 5 min, 30–50 cycles of 95°C for 5 s and 60°C for 40–60 s depending on different miRNA study followed by a final dissociation analysis with the Ct cutoff determined by a Youden’s index. MiRNA expression for each sample was normalized to expression levels of miR-423-5p, with three biological replicates of comparative RT-qPCR30.
Statistical Analysis
Data was analyzed using SPSS version 17.0 (IBM, Chicago, IL) with the Youden’s Index used to identify optimal cut-off points. Logistic regression analysis was performed to analyze various combinations of miRNA markers. PFS was calculated from the initiation of therapy to progression, date of death or the last follow up. OS was measured from the initiation of treatment to the date of death or last follow up according to the international uniform response criteria.31 Two-sided Fisher’s exact tests were used to assess associations between categorical variables, with a confidence coefficient (confidence interval, CI) of 95%. The survival curves were plotted using the Kaplan-Meier method, with differences assessed by the log rank test. Multivariate analysis was performed using Cox’s regression hazard model with forward stepwise (likelihood ratio). P values <0.05 were considered to be significant. The correlation coefficients (r) were calculated by using the Spearman correlation.
Results
Patients’ characteristics
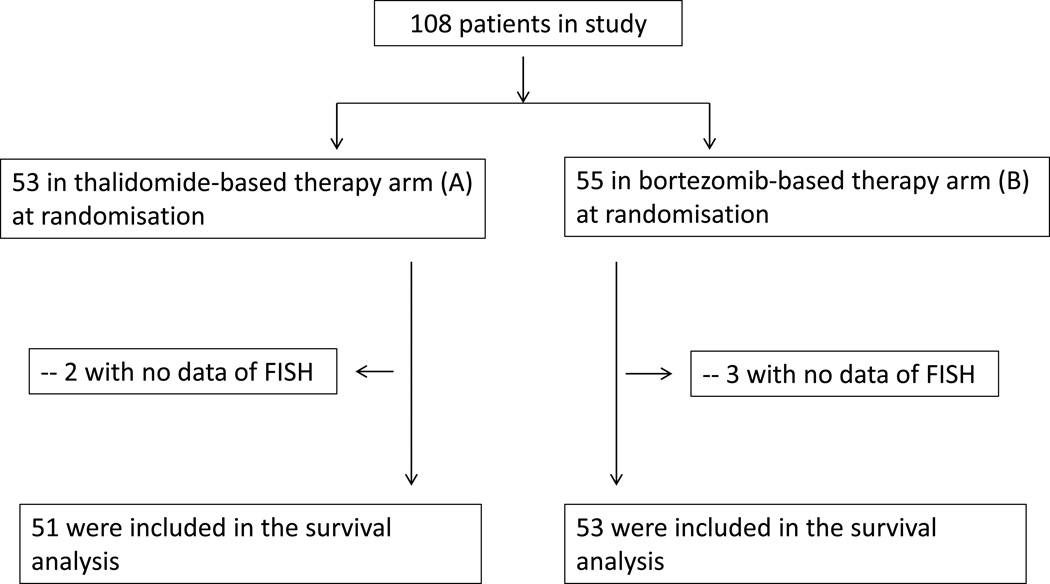
A total of 108 patients with newly diagnosed symptomatic MM were enrolled in the present study between January 2007 and December 2008, with a median follow-up time of 13.5 months from diagnosis. Moreover, 56 healthy donors were chosen at the time of hospital checkups and were chosen based on follow-up studies that determined that indeed they were healthy donors and were also analyzed to determine comparative miRNA expression profiles. Among 108 newly diagnosed symptomatic MM patients, fifty-three patients were included in arm A, fifty-five patients were included in arm B (Figure 1). There were no significant differences in clinical and cytogenetic characteristics between the groups (Table 1). For 16 newly diagnosed patients, their paired serum samples in relapsed and remission were collected as well with 7 patients enrolled in arm A and 9 patients enrolled in arm B..
Figure 1. CONSORT (Consolidated Standards of Reporting Trails) flow diagram.
Among 108 newly diagnosed symptomatic MM patients, fifty-three patients were included in arm A, fifty-five patients were included in arm B. Survival analysis included 103 patients with completed clinical data.
Table 1.
Patients' and healthy donors' base-line characteristics
| Healthy donors | MM patients | |||
|---|---|---|---|---|
| Charateristic | (n=56) | Treatment arm A (n=53) n/N (%) |
Treatment arm B (n=55) n/N (%) |
p value |
| Age(years) | 52 | 56 | 59 | NA |
| Range | 40–78 | 33–83 | 43–76 | NA |
| M component at diagnosis, % | 0.055 | |||
| IgG | ND | 23/53 (43.3) | 28/55(50.9) | |
| IgA | ND | 10/53 (18.9) | 19/55 (34.5) | |
| IgD | ND | 6/53 (11.3) | 2/55 (3.6) | |
| IgM | ND | 0/53 (0) | 0/55 (0) | |
| Light chain | ND | 12/53 (22.6) | 6/55 (10.9) | |
| Non-secretory | ND | 2/53 (3.8) | 0/55 (0) | |
| ISS stage | 0.439 | |||
| I | ND | 9/49 (18.4) | 9/55 (16.14) | |
| II | ND | 18/49 (36.7) | 21/55 (38.1) | |
| III | ND | 24/49 (49.0) | 26/55 (47.3) | |
| β2-microglobulin | 0.070 | |||
| < 5.5mg/dL | ND | 22/49 (44.9) | 32/50 (64) | |
| ≥5.5mg/dL | ND | 27/49 (55.1) | 18/50 (36) | |
| Durie-Salmon stage, % | 0.356 | |||
| I–II | ND | 4/48 (8.3) | 7/49(14.3) | |
| III | ND | 44/48 (91.7) | 40/49 (81.6) | |
| Cytogenetic abnormalities, % | ||||
| del(13q) | ND | 29/50 (58.0) | 23/54 (42.6) | 0.169 |
| del(17p) | ND | 5/50 (10.0) | 3/53 (5.7) | 0.113 |
| 1q21 amplification | ND | 26/48 (54.2) | 25/52 (48.1) | 0.694 |
| t(11;14) | ND | 6/48 (12.5) | 9/53 (17.0) | 0.582 |
| t(4;14) | ND | 11/47 (23.4) | 13/50 (26.0) | 0.817 |
| t(14;16) | ND | 4/45 (8.9) | 1/49 (2.1) | 0.190 |
ISS: International Staging Systerm; Ig: immunoglobulin; DS: Durie Salmon; NS: not significant.
miRNA profiling and analyzing
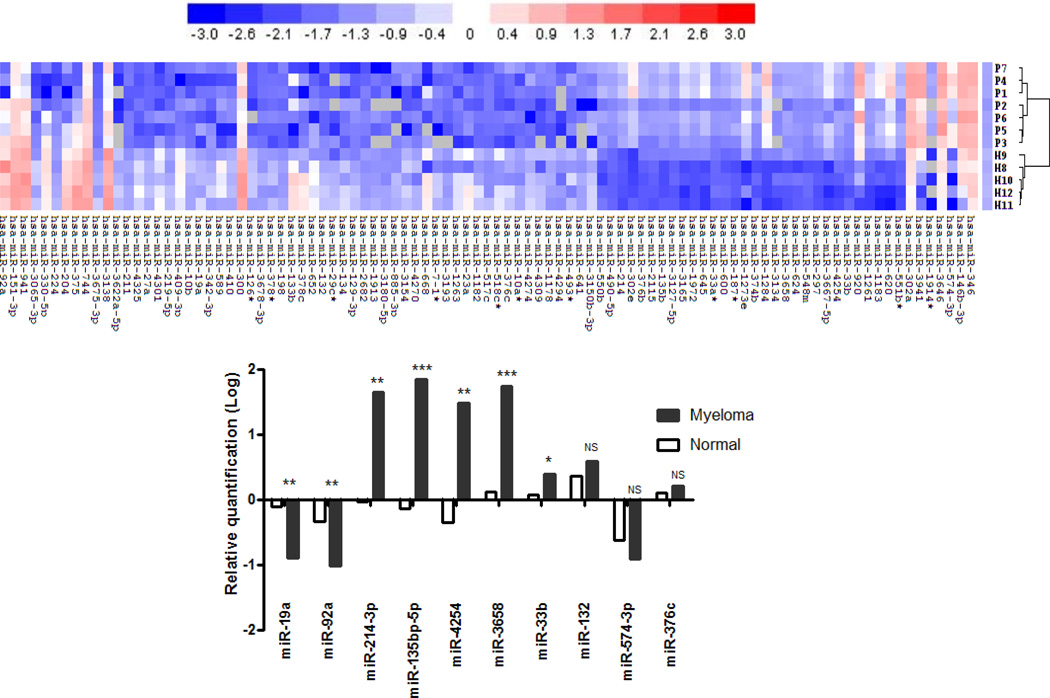
To perform the miRNA screen on 1891 miRNAs, we utilized the 6th generation of the miRCURY™ LNA Array. We analyzed samples from 7 newly diagnosed MM patient and 5 HD sample (Suppl. Table 1) to identify differentially expressed circulating miRNAs that could serve as putative biomarkers. Ninety-five miRNAs were significantly dysregulated (fold change ≥ 3.0, all p<0.01) between MM patients and HD: 37 (38.9%) miRNAs were up-regulated and 58 miRNAs (61.1%) were down-regulated in patients with MM (Figure 2A). Of the dysregulated miRNA, miR-19a, miR-92a, miR-214-3p, miR-135b-5p, miR-4254, miR-3658, miR-33b, miR-132, miR-574-3p and miR-376c were chosen for further validation, based on their chromosomal location, fold change and p-value (Suppl. Table 2).
Figure 2. Hierarchical clustering analysis of miRNA expression and Validation of candidate miRNAs using RT-qPCR.
(A) The expression of miRNA is hierarchically clustered on the x axis, and MM serum samples or healthy control serum samples are hierarchically clustered on the y axis. The legend indicates the miRNA represented in the corresponding row. Relative miRNA expression is depicted according to the color scale shown: Red indicates up-regulation and blue, down-regulation. Numbers with P indicate MM samples; numbers with H, healthy control samples. (B) Relative expression of 10 miRNAs on a large cohort of 108 newly diagnosed MM patients and 56 HD samples. The significant dysregulation of miRNAs was observed in miR-19a, miR-92a, miR-214-3p, miR-135b-5p, miR-4254, miR-3658 and miR-33b. Statistical significance was determined by a student’s t-test (*p<0.05, **p<0.01, ***p<0.0001, NS: not significant).
Validation of candidate miRNAs using RT-qPCR
Validation of miRNAs was performed using RT-qPCR over microarray validation due to its increased sensitivity and dynamic range32. We employed miRNA specific assays (miR-19a, miR-92a, miR-214-3p, miR-135b-5p, miR-4254, miR-3658, miR-33b, miR-132, miR-574-3p and miR-376c) in a large cohort of 108 newly diagnosed MM patients and 56 HD to confirm the pattern of candidate miRNA expression profiles between MM patients and HD samples (Figure 2B). The RNA yield from 0.2mL serum was 1360 ±214 ng. The amount of total RNA used for the reverse transcription reaction was 1µg according to the manufacturers’ instruction. The mean Ct values (95% confidence interval (CI)) of miR-423-5p were 28.5 (27.6–28.6) in healthy donors and 28.7 (27.7–28.8) in patients with MM, demonstrating that miR-423-5p expression in serum is stable and can be used as an internal control to normalize sampling variations in our RT-qPCR analysis.
The RQ value (2−ΔΔCt) significantly decreased in miR-19a (−0.91 vs. 0.03, p=0.0016) and miR-92a (−1.03 vs. −0.19, p=0.0023) in the MM group compared with healthy donors. However, miR-214-3p (1.65 vs. −0.04, p=0.0007), miR-135b-5p (1.84 vs. −0.13, p=0.0001), miR-4254 (1.38 vs. −0.13, p=0.007), miR-3658 (1.74 vs. 0.12, p=0.025) and miR-33b (0.38 vs. 0.07, p=0.026) values significantly increased compared to HD group. Since our analysis did not identify significant dysregulation in miR-132, miR-574-3p and miR-376c we chose to excluded them from further analysis.
miRNA expression pattern correlation analysis
Serum miRNA levels were further analyzed by Pearson-moment correlation coefficient calculations utilizing CoExpress version 1.5 (P.V. Nazarov, Luxembourg), a bioinformatics tool for co-expression analysis of microarray data. Three miRNA clusters (miR-33b, miR-92a and miR-3658) were identified with correlation coefficients > 0.600 and corresponding p <0.0001 (highlighted in Suppl. Table 3). Furthermore, a strong correlation was observed between miR-19a and miR-33b (r=0.866, p<0.001). However, the correlation between these two miRNAs remains unlikely due to their distinct chromosomal locations. Similarly, correlations were identified for miR-19a and miR-92a, and for miR-3658 and miR-214-3p, with each correlative group sharing similar chromosomal locations.
Correlation of miRNAs with clinical parameters and chromosome aberrations in plasma cells
To determine the correlation of miRNA expression levels with clinical parameters, a two-sided Fisher’s exact test was performed. The lower level of miR-19a revealed a significant positive correlation with advancement of ISS staging (r=0.214, p=0.026). However, no correlation was observed with age, gender, beta 2-microglobulin, percentage of BMPC infiltration and M component when compared to healthy controls.
Little is known about the origin of circulating miRNAs and their relationship with BMPCs. To expand our understanding of this species of miRNAs, we explored the correlation of miRNA levels with chromosome aberrations in PCs. The level of miR-19a exhibited a significant positive correlation with 1q21 amplification (r=0.228, p=0.023) and 13q14 deletion (r=0.195, p=0.048). We discovered that the presence of 1q21 amplification in MM exhibited a significant correlation with low levels of miR-19a. Furthermore, we observed a positive correlation with low levels of miR-19a and del(13q14). To address the correlation of miR-19a with other common cytogenetic abnormalities in myeloma, we examined the correlation between miR-19a and del(17p), t(4;14), t(11;14) and t(14;16). Our analysis did not discover a correlation between miR-19a expression levels and the common myeloma genetic abnormalities (Data not shown).
The miR-19a/miR-4254 combination offers a powerful diagnostic tool for identification of myeloma
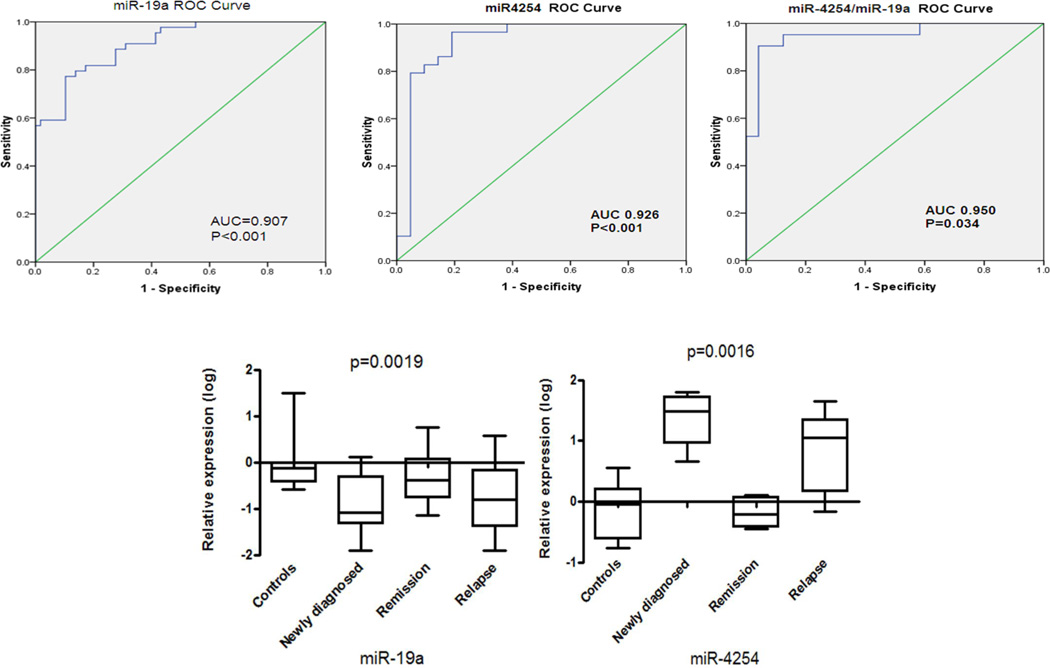
Receiver operating characteristic analysis (ROC) of 108 MM samples revealed that serum levels of miR-19a, miR-92a, miR-135b-5p and miR-4254 can be used to distinguish MM from HD with area under the curve (AUC)>0.8 compared to healthy donors (Suppl. Table 4). Moreover, multivariate logistical regression analysis showed that the combination of miR-19a and miR-4254 could improve the stratification power characterized with AUC of 0.95, sensitivity of 91.7% and specificity 90.5% for MM (Figure 3A). Furthermore, to assess the dynamics of miRNA levels during disease progression of 16 MM patients, serum samples at the time of diagnosis, in remission and relapse were collected. Our data indicated that miR-19a and miR-4254 expression levels were closer to HD levels when in remission phase after 4 cycle treatment. However, down-regulated miR-19a and up-regulated miR-4254 levels return to diagnostic levels upon relapse (Figure 3B). These results demonstrate that miR-19a and miR-4254 varied with myeloma progression and have utility in monitoring myeloma progression.
Figure 3. The miR-19a combined with miR-4254 offers a powerful diagnostic tool for identification of MM.
(A) Receiver operating characteristic analysis was performed for miR-19a and miR-4254, and area under the ROC curve (AUC) was performed for miR-19a or miR-4254 to test the prognostic validity of each miRNA. Furthermore, ROC and AUC were performed for both miR-19a and miR-4254 to determine the sensitivity of a combination of miRNAs as a biomarker. (B) Expression profiling of miR-19a and miR-4254 was performed at various stages (newly diagnosed, remission and relapse) of MM progression. Statistical significance for miRNA expression profiles was determined by a one-way ANOVA (p<0.05).
Prognostic value of miR-19a in MM patients
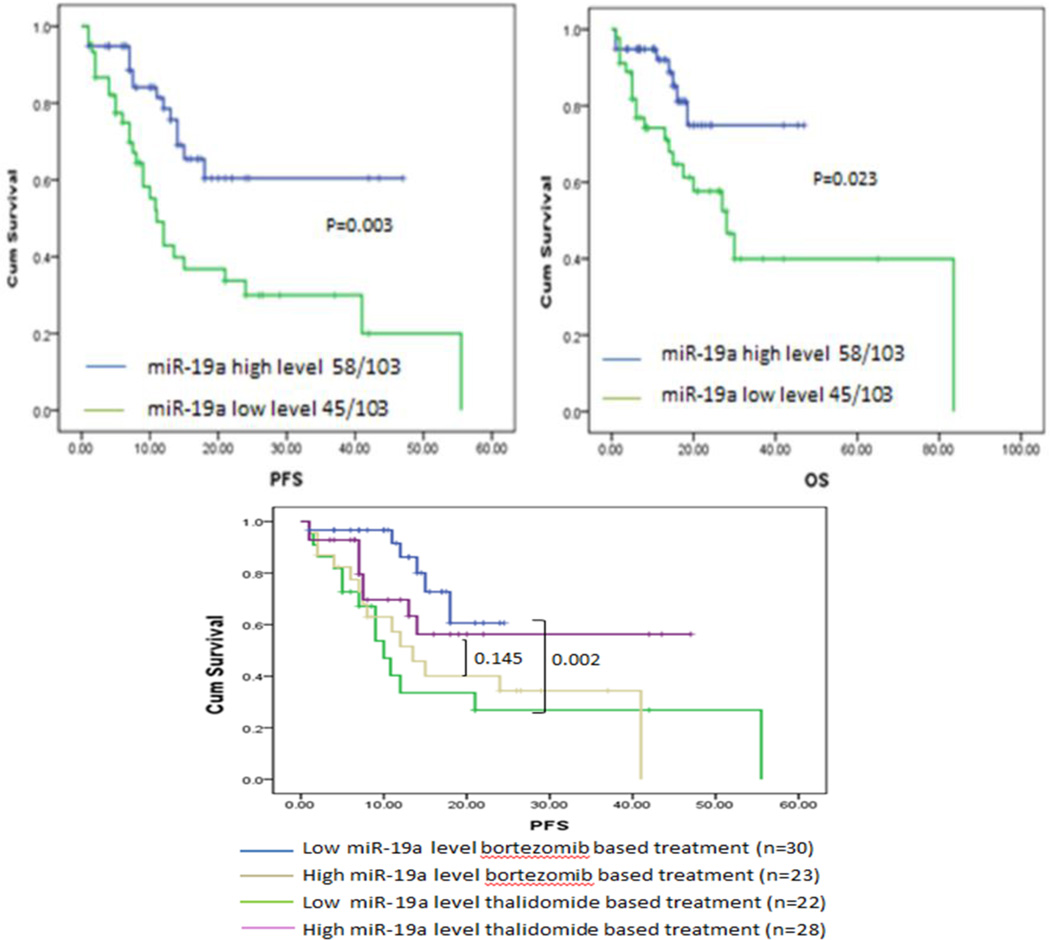
We have shown that miR-19a can serve as a diagnostic tool for identifying MM, we next queried the impact of miRNA expression on survival in newly diagnosed MM patients. The 108 newly diagnosed patients with completed clinical data were grouped into a high or low miR-19a group with the Youden’s Index used to identify the optimal cut-off point for miR-19a expression grouping. We discovered that expression of miR-19a exhibited a profoundly negative impact on survival. Furthermore, patients with down-regulated miR-19a expression had a significantly shortened PFS (11.0 months, p=0.002) and shortened OS (28.1 months, p=0.020) than patients with increased miR-19a expression (Figure 4A). Interestingly, a positive correlation was drawn between miR-19a and 1q21 amplification (r=0.228, p=0.023).Patients exhibiting the 1q21 amplification correlation had a poorer clinical outcome, which is consistent with previous studies23. Therefore, we then analyzed the survival of patients with miR-19a in the absence of 1q21 amplification and found that the median PFS among these cases were 12.5 months (p=0.026). We determined that the inferior prognostic outlook for patients with low miR-19a levels was independent of 1q21 amplification. A multivariate analysis was performed building on our univariate analysis to determine the correlation between miR-19a expression and 1q21 amplification (Table 2). ISS stage (HR 1.948, [95%CI 1.012–3.752]; p=0.046) and miR-19a (HR 2.787, [95% CI 1.421–5.468], p=0.003) were statistically independent predictors of PFS. A similar analysis for prediction of OS identified that miR-19a (HR 2.995, [95% CI 1.167–7.690], p=0.023) was statistically independent and a valuable predictor of overall survival.
Figure 4. Prognostic value of miR-19a level in MM patients.
(A) Progression free survival and overall survival was determined for MM patients according to expression of miR-19a. Survival analysis was determined via Kaplan–Meier survival analysis, with differences between curves analyzed via a log-rank test. Significance was defined as p<0.05. (B) Patients with low miR-19a expression had significantly greater response to bortezomib than patients with high miR-19a expression. Progression free survival was determined for MM patients treated with a thalidomide-based or a bortezomib-based therapy according to their miR-19a expression profile. Survival analysis was determined using Kaplan–Meier survival analysis, with the differences between curves analyzed via a log-rank test. Significance was defined as p<0.05.
Table2.
Details of multivariate Cox regression analysis for miR-19a.
| PFS | OS | |||||
|---|---|---|---|---|---|---|
| Parameters | Exp(B) | 95% CI | P value | Exp (B) | 95% CI | P value |
| ISS stage | 1.948 | 1.012–3.752 | 0.046 | 1.742 | 0.738–4.114 | 0.206 |
| del(13q14) | 0.821 | 0.418–1.612 | 0.567 | 0.613 | 0.246–1.526 | 0.293 |
| 1q21 gain | 1.992 | 0.980–4.047 | 0.057 | 2.335 | 0.898–6.072 | 0.082 |
| miR-19a | 2.787 | 1.421–5.468 | 0.003 | 2.995 | 1.167–7.690 | 0.023 |
Significant value p< 0.05 are marked with bold and italics.
Prognostic value of miR-19a in MM patients treated with bortezomib based therapy
We have shown that the serum concentration of miR-19a can serve as a prognostic indicator of survival in MM patients. We further queried whether levels of miR-19a factored in a patients’ response to different therapy. We consolidated the patients into 4 subgroups with therapeutic reagent (thalidomide and bortezomib) and the level of miR-19a. The data showed the different PFS in those 4 subgroups, p= 0.012. The therapeutic response in patients with up-regulated miR-19a serum levels was modest: median PFS was not significantly prolonged in the treatment with either bortezomib or thalidomide (13.5 month vs. NR, p=0.145). Strikingly, bortezomib based treatment significantly extended PFS of patients with down-regulated miR-19a (NR vs. 10.0 months, p=0.002, Figure 4B). Therefore, bortezomib was able to reverse the poor prognosis of MM patients with a low miR-19a level and significantly improve the survival within these patients.
Discussion
Interest in serum miRNAs as potential biomarkers is a rapidly growing research area32–34. To date, multiple reports describe altered serum miRNA profiles in metastatic neoplasms of various origins21, 35, 36. In association with other biomarkers, changes in circulating miRNAs levels offers the potential for a highly sensitive and specific tumor detection and classification system37, 38. Furthermore, circulating markers are more accessible, which further increase their potential utility. The recent introduction of new therapeutic regimens has greatly improved the response and survival of patients with MM39 however, some MM patients respond poorly due to adverse genetic features and the development of refractory disease. Since there has been a lag in the development of novel therapeutics, identification of new diagnostic and prognostic markers are urgently needed to improve patient survival and quality of life40–42.
In this study, we queried a constellation of serum miRNAs of newly diagnosed MM patients and healthy donors to identify novel biomarkers for detection, classification of disease progression and therapeutic design. MiRCURY™ LNA arrays were utilized to identify circulating miRNAs that are differentially expressed in MM serum samples. Our results for the global miRNA analysis identified ninety-five differentially expressed miRNAs in MM samples when compared to HD serum samples. Interestingly, the results from our miRNA analysis closely mirrored results from a previous study examing dysregulated circulating miRNAs from MM plasma and serum samples10, 43. Similar to our results, Yoshizawa et al. identified miR-92a as down-regulated in MM patient samples43. Furthermore, Kubiczkova et al. identified miR-744 and let-7e as dysregulated in MM patient serum samples10, however, we did not see dysregulation of let-7e in our serum miRNA analysis and could be due to different sensitivities of the array platforms used by.
Of the ninety-five dysregulated miRNAs identified seven miRNAs (miR-19a, miR-92a, miR-214-3p, miR-135b-5p, miR-3658, miR-4254 and miR-33b) were confirmed as significantly dysregulated in an independent cohort of MM patient samples via RT-qPCR. Previous work has shown that miRNAs are present in circulation and present as a new class of powerful and minimally invasive biomarkers for MM detection and prognosis7, 9, 10. However, researchers have failed to reach a consensus regarding miRNAs as biomarkers, proper array platforms and analytical approaches to take in the analysis of the data. So far no miRNA is accepted as a standard for serum samples however, miR-16 has worked as a normalizer in many research studies exploring prognostic markers in patients with carcinoma44, 45. Interestingly, miR-16 was not suitable to work as a normalizer in serum samples of MM due to its tumor suppressor function, which was identified in our previous studies46, 47. According to microarray profiling results, the level of RNU6, hsa-miR-423-5p, hsa-miR-130a and hsa-miR-4288 exhibited no significant different between MM patient samples and HD. Furthermore, RT-qPCR validation revealed that the mean Ct value (95% confidence interval (CI)) of miR-423-5p were 28.5 (27.6–28.6) in HD and 28.7 (27.7–28.8) in patient samples, identifying miR-423-5p as a suitable internal control for the normalization of sample variations in RT-qPCR analysis.
ROC analysis revealed that miR-19a and miR-4254 can discriminate MM from HD. However, the combined detection of miR-19a and miR-4254 (highest specificity 90.5% and sensitivity 91.7% for MM) proved to be an even more powerful diagnostic tool in distinguishing MM patients from healthy controls. Different miRNA expression profiles were confirmed in 16 paired MM samples taken at diagnosis, relapse and in remission however, lower levels of miR-19a were only seen at diagnosis and at relapse, suggesting that dysregulated levels of miRNAs reflects on the patients’ condition and disease progression. This observation is further supported by the relationship of higher miR-4254 levels at diagnosis and at relapse.
In the newly diagnosed MM patients, dysregulation of miR-19a was observed to be associated with clinical parameters and advanced ISS stages. Rocci, et al48 and us both described serum miR-19 family members correlated with del(13q14). Based on our miRNA profiling data, the level of serum miR-17–92 cluster has no significant difference between MM patients and healthy donors except miR-19a and miR-92a. Serum miR-19a and miR-92a were significantly decreased in MM patients. Furthermore, low level of miR-19a exhibited a positive correlation with 13q14 deletion. MiR-19a located on chromosome 13q31.3 where it was very close to 13q14. The correlation between low miR-19a level and del(13q14) probably due to their chromosome location. Moreover, lower levels of miR-19a were associated with 1q21 amplification. However, our data did not support a direct correlation between low levels of miR-19a and common cytogenetic changes in myeloma; t(4;14), t(11;14), t(14;16) and del(17p).
We then investigated the possibility of a prognostic serum miRNAs marker in MM, our results indicated that patients with down-regulated miR-19a expression had a significantly shortened PFS (12.0 vs. NR, p=0.002) and OS (26.5 vs. NR, p=0.020) than patients with an increase in miR-19a expression. A multivariate analysis showed miR-19a (HR 2.787, [95% CI 1.421–5.468], p=0.003) and ISS stage (HR 1.948, [95% CI 1.012–3.752], p=0.046) were statistically independent predictors of PFS. MiR-19a (HR=2.995, 95% CI 1.167–7.690, p=0.023) was statistically independent predictor of OS as well. This observation could be partially explained by the fact that the gene for miR-19a lies within the 13q31.3 region, close to the RB tumor suppressor gene (13q14). Also, decreased expression of miR-19a was highly correlated with 1q21 amplification, which is associated with a decreased PFS and OS23.
Interestingly, miR-19a expression was shown to have a profound effect on patient response to bortezomib based therapeutic regimens. Patients with a low miR-19a expression profiles responded very well to bortezomib therapy, with an improved PFS and OS, whereas patients with increased miR-19a levels did not respond as dramatically to treatment with bortezomib. These results identify bortezomib as an effective first line treatment for patients with low miR-19a expression and further support the validity of miR-19a as an important prognostic marker. Our results therefore suggest that miRNAs can serve as accurate and reliable biomarkers for detection and monitoring disease progression.
MiR-19a is one of six precursors of the oncogenic miR-17–92 cluster, which is transcribed in a common primary transcript (miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1, and miR-92a)49, 50. Members of the miR-17–92 cluster are known for their pleotropic functions in cell survival, differentiation, proliferation and angiogenesis. The first study of circulating miRNAs in MM revealed down-regulated plasma miR-92a levels in patients with MM43. Moreover, the down-regulation of miR-92a in plasma also correlated with response to treatment. Unfortunately, limited information is available describing the function of miR-19a, the only available information identifies it as up-regulated in many cancers51–53. Interestingly, Yu et al. showed that over-expression of miR-19a suppressed CD142 expression and inhibited cell migration and invasion in colon cancer53. Our results closely mirror results from the Yu et al. study however, we go further to show that MM patients with a high miR-19a serum expression have better outcomes than patients with low serum miR-19a expression, suggesting that miR-19a may be targeting genes essential to cellular progression. Interestingly, the dysregulated circulating miRNAs are different from miRNAs detected within myeloma cells, as Yoshizawa et al. reported that miR-92a is down-regulated in plasma, but is part of the up-regulated miR-17–92 cluster in MM cells43. This trend is further supported by our present study, miR-19a was down-regulated in serum of MM patients, but up-regulated in MM cells (data not shown).
It has been shown that miR-19a expression directly correlates with patient outcome however, little is known about how miR-19a functions in MM. Ye et al. demonstrated that miR-19a can suppress CLYD translation in acute lymphoblastic leukemia54. Moreover, a decrease in CLYD signaling results in the over-activation of NF-κB signaling, a hallmark of MM55. Of particular interest, studies by Grillari et al., Olive et al. and Wang et al. have identified miR-19a as a key mediator of PTEN expression. Their data suggests that over-expression of miR-19a results in the suppression of PTEN and activation of the AKT/mTOR pathway56–58. Wang et al. further identified miR-19a as a mediator of drug resistance in gastric cell cancer, demonstrating that miR-19a accelerates drug efflux through up-regulation of MDR158. Interestingly, patients in our study with high miR-19a expression responded poorly to bortezomib therapy. A potential explanation relates to high miR-19a expression leading to increase in drug efflux pumps. In aggregate, these findings suggest that inhibitors to miR-19a may enhance response to bortezomib in patients with high miR-19a.
In summary, our data offers novel insights into the miR-17–92 cluster in MM. We demonstrate that the expression of miRNAs in serum of MM patients varies significantly, depending on stage of disease. These results suggest that miR-19a and miR-4254 may serve as important prognostic markers for detection of MM and determination of disease progression. Furthermore, low serum level of miR-19a was identified as a poor prognostic indicator. Interestingly, although miR-19a levels portend a poor prognosis, patients with low miR-19a levels had a better response to bortezomib than patients with high miR-19a expression. The results of this study show the importance of miR-19a expression in myeloma detection and prognosis, suggesting that clinical applications are appropriate.
Supplementary Material
Novelty & Impact Statements.
With the successful detection of stable miRNAs in bodily fluids of humans and animals, the use of miRNAs as potential biomarkers is a reality. These discoveries play an important role in the way we diagnose and treat MM and will have a positive impact on human health. Our findings indicated the miRNA profile identified for MM has potential benefits to help improve the diagnosis and prognosis of MM and predict therapeutic efficacy and disease recurrence for patients.
Acknowledgements
The authors are really grateful to Dr. Kenneth C. Anderson for editing manuscript. This work was supported by grants from the National Natural Science Foundation of China (No.81400174, 81172255 & 81370632). Tianjin Science and Technology Supporting Program (13JCQNJC12800) and Foundation of MOE of China (20111106120037).
Footnotes
Authors’ Contributions
MH provided the concept and design of the study, acquisition of data, analysis and interpretation of data and drafting of the manuscript; MRZ performed the experiment; EW assisted with manuscript preparation; YX and GA collected clinical data from myeloma patients; YY and FL assisted in RT-qPCR; DSG assisted in microarray data analysis; FQ and YRZ assisted in data analysis; FHZ and LGQ revised the manuscript and gave final approval of the submitted manuscript. All authors have read and approved the final manuscript.
Conflict of Interest Statement
None declared.
References
- 1.Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374:324–339. doi: 10.1016/S0140-6736(09)60221-X. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KC, Shaughnessy JD, Jr, Barlogie B, Harousseau JL, Roodman GD. Multiple myeloma. Hematology Am Soc Hematol Educ Program. 2002:214–240. doi: 10.1182/asheducation-2002.1.214. [DOI] [PubMed] [Google Scholar]
- 3.Vicente-Duenas C, Romero-Camarero I, Garcia-Criado FJ, Cobaleda C, Sanchez-Garcia I. The cellular architecture of multiple myeloma. Cell cycle. 2012;11:3715–3717. doi: 10.4161/cc.22178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Shi J, Tolomelli G, Xu H, Xia J, Wang H, Zhou W, Zhou Y, Das S, Gu Z, Levasseur D, Zhan F, et al. RARalpha2 expression confers myeloma stem cell features. Blood. 2013;122:1437–1447. doi: 10.1182/blood-2013-02-482919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou W, Yang Y, Xia J, Wang H, Salama ME, Xiong W, Xu H, Shetty S, Chen T, Zeng Z, Shi L, Zangari M, et al. NEK2 induces drug resistance mainly through activation of efflux drug pumps and is associated with poor prognosis in myeloma and other cancers. Cancer cell. 2013;23:48–62. doi: 10.1016/j.ccr.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou W, Yang Y, Gu Z, Wang H, Xia J, Wu X, Zhan X, Levasseur D, Zhou Y, Janz S, Tricot G, Shi J, et al. ALDH1 activity identifies tumor-initiating cells and links to chromosomal instability signatures in multiple myeloma. Leukemia. 2014;28:1155–1158. doi: 10.1038/leu.2013.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones CI, Zabolotskaya MV, King AJ, Stewart HJ, Horne GA, Chevassut TJ, Newbury SF. Identification of circulating microRNAs as diagnostic biomarkers for use in multiple myeloma. British journal of cancer. 2012;107:1987–1996. doi: 10.1038/bjc.2012.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yyusnita, Norsiah, Zakiah I, Chang KM, Purushotaman VS, Zubaidah Z, Jamal R. MicroRNA (miRNA) expression profiling of peripheral blood samples in multiple myeloma patients using microarray. The Malaysian journal of pathology. 2012;34:133–143. [PubMed] [Google Scholar]
- 9.Sevcikova S, Kubiczkova L, Sedlarikova L, Slaby O, Hajek R. Serum miR-29a as a marker of multiple myeloma. Leukemia & lymphoma. 2013;54:189–191. doi: 10.3109/10428194.2012.704030. [DOI] [PubMed] [Google Scholar]
- 10.Kubiczkova L, Kryukov F, Slaby O, Dementyeva E, Jarkovsky J, Nekvindova J, Radova L, Greslikova H, Kuglik P, Vetesnikova E, Pour L, Adam Z, et al. Circulating serum microRNAs as novel diagnostic and prognostic biomarkers for multiple myeloma and monoclonal gammopathy of undetermined significance. Haematologica. 2014;99:511–518. doi: 10.3324/haematol.2013.093500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosshans H, Filipowicz W. Molecular biology: the expanding world of small RNAs. Nature. 2008;451:414–416. doi: 10.1038/451414a. [DOI] [PubMed] [Google Scholar]
- 13.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 14.Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nature genetics. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 15.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 16.Tian Z, Zhao JJ, Tai YT, Amin SB, Hu Y, Berger AJ, Richardson P, Chauhan D, Anderson KC. Investigational agent MLN9708/2238 targets tumor-suppressor miR33b in MM cells. Blood. 2012;120:3958–3967. doi: 10.1182/blood-2012-01-401794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu P, Agnelli L, Walker BA, Todoerti K, Lionetti M, Johnson DC, Kaiser M, Mirabella F, Wardell C, Gregory WM, Davies FE, Brewer D, et al. Improved risk stratification in myeloma using a microRNA-based classifier. British journal of haematology. 2013;162:348–359. doi: 10.1111/bjh.12394. [DOI] [PubMed] [Google Scholar]
- 18.Dimopoulos K, Gimsing P, Gronbaek K. Aberrant microRNA expression in multiple myeloma. European journal of haematology. 2013;91:95–105. doi: 10.1111/ejh.12124. [DOI] [PubMed] [Google Scholar]
- 19.Lionetti M, Biasiolo M, Agnelli L, Todoerti K, Mosca L, Fabris S, Sales G, Deliliers GL, Bicciato S, Lombardi L, Bortoluzzi S, Neri A. Identification of microRNA expression patterns and definition of a microRNA/mRNA regulatory network in distinct molecular groups of multiple myeloma. Blood. 2009;114:e20–e6. doi: 10.1182/blood-2009-08-237495. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Chen L, Barlogie B, Stephens O, Wu X, Williams DR, Cartron MA, van Rhee F, Nair B, Waheed S, Pineda-Roman M, Alsayed Y, et al. High-risk myeloma is associated with global elevation of miRNAs and overexpression of EIF2C2/AGO2. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7904–7909. doi: 10.1073/pnas.0908441107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, Lin DW, Urban N, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nature reviews. Cancer. 2011;11:426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 23.An G, Xu Y, Shi L, Shizhen Z, Deng S, Xie Z, Sui W, Zhan F, Qiu L. Chromosome 1q21 gains confer inferior outcomes in multiple myeloma treated with bortezomib but copy number variation and percentage of plasma cells involved have no additional prognostic value. Haematologica. 2014;99:353–359. doi: 10.3324/haematol.2013.088211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu W, Yang L, Shan H, Zhang Y, Zhou R, Su Z, Du Z. MicroRNA expression analysis: clinical advantage of propranolol reveals key microRNAs in myocardial infarction. PloS one. 2011;6:e14736. doi: 10.1371/journal.pone.0014736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, Zhu J, Zhang W, Chen Y, Zhang K, Popescu LM, Ma X, Lau WB, Rong R, Yu X, Wang B, Li Y, et al. Signature microRNA expression profile of essential hypertension and its novel link to human cytomegalovirus infection. Circulation. 2011;124:175–184. doi: 10.1161/CIRCULATIONAHA.110.012237. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Lai L, Chen Q, Song Y, Xu S, Ma F, Wang X, Wang J, Yu H, Cao X, Wang Q. MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressor cells via targeting of PTEN. J Immunol. 2012;188:5500–5510. doi: 10.4049/jimmunol.1103505. [DOI] [PubMed] [Google Scholar]
- 27.Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S, Guo X, Wang B, Gang Y, Zhang Y, Li Q, Qiao T, et al. MiR-218 inhibits invasion and metastasis of gastric cancer by targeting the Robo1 receptor. PLoS genetics. 2010;6:e1000879. doi: 10.1371/journal.pgen.1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan HL, Xue G, Mei Q, Wang YZ, Ding FX, Liu MF, Lu MH, Tang Y, Yu HY, Sun SH. Repression of the miR-17–92 cluster by p53 has an important function in hypoxia-induced apoptosis. The EMBO journal. 2009;28:2719–2732. doi: 10.1038/emboj.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin ZY, Huang YQ, Zhang YQ, Han ZD, He HC, Ling XH, Fu X, Dai QS, Cai C, Chen JH, Liang YX, Jiang FN, et al. MicroRNA-224 inhibits progression of human prostate cancer by downregulating TRIB1. International journal of cancer. Journal international du cancer. 2013 doi: 10.1002/ijc.28707. [DOI] [PubMed] [Google Scholar]
- 30.Eguchi T, Watanabe K, Hara ES, Ono M, Kuboki T, Calderwood SK. OstemiR: a novel panel of microRNA biomarkers in osteoblastic and osteocytic differentiation from mesencymal stem cells. PloS one. 2013;8:e58796. doi: 10.1371/journal.pone.0058796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson KC, Kyle RA, Rajkumar SV, Stewart AK, Weber D, Richardson P. Clinically relevant end points and new drug approvals for myeloma. Leukemia. 2008;22:231–239. doi: 10.1038/sj.leu.2405016. [DOI] [PubMed] [Google Scholar]
- 32.Resnick KE, Alder H, Hagan JP, Richardson DL, Croce CM, Cohn DE. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecologic oncology. 2009;112:55–59. doi: 10.1016/j.ygyno.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell research. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 34.Gilad S, Meiri E, Yogev Y, Benjamin S, Lebanony D, Yerushalmi N, Benjamin H, Kushnir M, Cholakh H, Melamed N, Bentwich Z, Hod M, et al. Serum microRNAs are promising novel biomarkers. PloS one. 2008;3:e3148. doi: 10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecologic oncology. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 36.Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T, Konishi H, Morimura R, Deguchi K, Fujiwara H, Okamoto K, Otsuji E. Circulating microRNAs in plasma of patients with gastric cancers. British journal of cancer. 2010;102:1174–1179. doi: 10.1038/sj.bjc.6605608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garzon R, Volinia S, Liu CG, Fernandez-Cymering C, Palumbo T, Pichiorri F, Fabbri M, Coombes K, Alder H, Nakamura T, Flomenberg N, Marcucci G, et al. MicroRNA signatures associated with cytogenetics and prognosis in acute myeloid leukemia. Blood. 2008;111:3183–3189. doi: 10.1182/blood-2007-07-098749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zen K, Zhang CY. Circulating microRNAs: a novel class of biomarkers to diagnose and monitor human cancers. Medicinal research reviews. 2012;32:326–348. doi: 10.1002/med.20215. [DOI] [PubMed] [Google Scholar]
- 39.Palumbo A, Anderson K. Multiple myeloma. The New England journal of medicine. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 40.Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK, Morgan G, Van Ness B, Chesi M, Minvielle S, Neri A, Barlogie B, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23:2210–2221. doi: 10.1038/leu.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, Kroger N, Einsele H, Vesole DH, Dimopoulos M, San Miguel J, Avet-Loiseau H, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–1127. doi: 10.1038/leu.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munshi NC, Anderson KC, Bergsagel PL, Shaughnessy J, Palumbo A, Durie B, Fonseca R, Stewart AK, Harousseau JL, Dimopoulos M, Jagannath S, Hajek R, et al. Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. Blood. 2011;117:4696–4700. doi: 10.1182/blood-2010-10-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshizawa S, Ohyashiki JH, Ohyashiki M, Umezu T, Suzuki K, Inagaki A, Iida S, Ohyashiki K. Downregulated plasma miR-92a levels have clinical impact on multiple myeloma and related disorders. Blood cancer journal. 2012;2:e53. doi: 10.1038/bcj.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halimi M, Parsian H, Asghari SM, Sariri R, Moslemi D, Yeganeh F, Zabihi E. Clinical translation of human microRNA 21 as a potential biomarker for exposure to ionizing radiation. Translational research : the journal of laboratory and clinical medicine. 2014 doi: 10.1016/j.trsl.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Koberle V, Kronenberger B, Pleli T, Trojan J, Imelmann E, Peveling-Oberhag J, Welker MW, Elhendawy M, Zeuzem S, Piiper A, Waidmann O. Serum microRNA-1 and microRNA-122 are prognostic markers in patients with hepatocellular carcinoma. Eur J Cancer. 2013;49:3442–3449. doi: 10.1016/j.ejca.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Hao M, Zhang L, An G, Meng H, Han Y, Xie Z, Xu Y, Li C, Yu Z, Chang H, Qiu L. Bone marrow stromal cells protect myeloma cells from bortezomib induced apoptosis by suppressing microRNA-15a expression. Leukemia & lymphoma. 2011;52:1787–1794. doi: 10.3109/10428194.2011.576791. [DOI] [PubMed] [Google Scholar]
- 47.Hao M, Zhang L, An G, Sui W, Yu Z, Zou D, Xu Y, Chang H, Qiu L. Suppressing miRNA-15a/-16 expression by interleukin-6 enhances drug-resistance in myeloma cells. Journal of hematology & oncology. 2011;4:37. doi: 10.1186/1756-8722-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rocci A, Hofmeister CC, Geyer S, Stiff A, Gambella M, Cascione L, Guan J, Benson DM, Efebera YA, Talabere T, Dirisala V, Smith EM, et al. Circulating miRNA markers show promise as new prognosticators for multiple myeloma. Leukemia. 2014 doi: 10.1038/leu.2014.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 50.Roth C, Kasimir-Bauer S, Pantel K, Schwarzenbach H. Screening for circulating nucleic acids and caspase activity in the peripheral blood as potential diagnostic tools in lung cancer. Molecular oncology. 2011;5:281–291. doi: 10.1016/j.molonc.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ouchida M, Kanzaki H, Ito S, Hanafusa H, Jitsumori Y, Tamaru S, Shimizu K. Novel direct targets of miR-19a identified in breast cancer cells by a quantitative proteomic approach. PloS one. 2012;7:e44095. doi: 10.1371/journal.pone.0044095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin TL, Wang QH, Brown P, Peacock C, Merchant AA, Brennan S, Jones E, McGovern K, Watkins DN, Sakamoto KM, Matsui W. Self-renewal of acute lymphocytic leukemia cells is limited by the Hedgehog pathway inhibitors cyclopamine and IPI-926. PloS one. 2010;5:e15262. doi: 10.1371/journal.pone.0015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu L, Simonson OE, Mohamed AJ, Smith CI. NF-kappaB regulates the transcription of protein tyrosine kinase Tec. FEBS J. 2009;276:6714–6724. doi: 10.1111/j.1742-4658.2009.07385.x. [DOI] [PubMed] [Google Scholar]
- 54.Ye H, Liu X, Lv M, Wu Y, Kuang S, Gong J, Yuan P, Zhong Z, Li Q, Jia H, Sun J, Chen Z, et al. MicroRNA and transcription factor co-regulatory network analysis reveals miR-19 inhibits CYLD in T-cell acute lymphoblastic leukemia. Nucleic acids research. 2012;40:5201–5214. doi: 10.1093/nar/gks175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li ZW, Chen H, Campbell RA, Bonavida B, Berenson JR. NF-kappaB in the pathogenesis and treatment of multiple myeloma. Current opinion in hematology. 2008;15:391–399. doi: 10.1097/MOH.0b013e328302c7f4. [DOI] [PubMed] [Google Scholar]
- 56.Grillari J, Hackl M, Grillari-Voglauer R. miR-17–92 cluster: ups and downs in cancer and aging. Biogerontology. 2010;11:501–506. doi: 10.1007/s10522-010-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olive V, Bennett MJ, Walker JC, Ma C, Jiang I, Cordon-Cardo C, Li QJ, Lowe SW, Hannon GJ, He L. miR-19 is a key oncogenic component of mir-17–92. Genes & development. 2009;23:2839–2849. doi: 10.1101/gad.1861409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang F, Li T, Zhang B, Li H, Wu Q, Yang L, Nie Y, Wu K, Shi Y, Fan D. MicroRNA-19a/b regulates multidrug resistance in human gastric cancer cells by targeting PTEN. Biochemical and biophysical research communications. 2013;434:688–694. doi: 10.1016/j.bbrc.2013.04.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.