Abstract
Background
Natural IgM antibodies represent a class of innate pattern recognition receptors that recognize danger associated molecular patterns expressed on stressed or dying cells. They play important roles in tissue homeostasis by disposing of pre-necrotic cells and suppressing inflammation. However, ischemic insult leads to a pathogenic level of IgM binding and complement activation, resulting in inflammation and injury. We investigate the role of self-reactive IgM in the unique setting of transplantation, where the donor organ undergoes both cold and warm ischemia, and global ischemic insult.
Methods and Results
By transplanting hearts from wild-type donor mice into antibody-deficient mice reconstituted with specific self-reactive IgM mAbs, we identified neoepitopes expressed post-transplant, and demonstrated a key role for IgM recognition of these epitopes in graft injury. With this information, we developed and characterized a therapeutic strategy that exploited the post-ischemia recognition system of natural antibodies. Based on neoepitope identification, we constructed an anti-annexin-IV single chain antibody (scFv) and an scFv linked to Crry, an inhibitor of C3 activation (scFv-Crry). In an allograft transplant model, in which recipients contain a full natural antibody repertoire, both constructs blocked graft IgM binding and complement activation, and significantly reduced graft inflammation and injury. Furthermore, scFv-Crry specifically targeted to the transplanted heart and, unlike complement deficiency, did not affect immunity to infection, an important consideration for immunosuppressed transplant recipients.
Conclusions
We identified pathophysiologically important epitopes expressed within the heart post-transplant, and describe a novel translatable strategy for targeted complement inhibition that has several advantages over currently available approaches.
Keywords: Ischemia, antibodies, inflammation, transplantation, complement
Introduction
Tissue reperfusion following a period of ischemia triggers a complement-dependent inflammatory reaction that culminates in necrotic and apoptotic cell death. A seminal study by Weiser et al., 1 established that following ischemia and reperfusion (IR) of skeletal muscle, complement is activated by self-reactive natural IgM antibodies (Abs) that bind to post-ischemic tissue. So-called natural IgM Abs are produced mainly by the B-1 subset of B lymphocytes, and the screening of hybridomas generated from an enriched B-1 cell fraction led to the identification of an IgM mAb that recognized non-muscle myosin expressed in reperfused tissues 2, 3. Our recent studies have identified additional targets for self-reactive natural Abs on post-ischemic tissue, namely annexin IV and a subset of phospholipids that are expressed in the post-ischemic intestine and brain 4, 5. Thus, the above studies suggest that ischemia-induced neoepitopes or corresponding self-reactive natural Abs represent targets for therapeutic intervention of ischemia reperfusion injury (IRI). To date, however, studies have not addressed the role of these Abs/epitopes in the unique setting of transplantation, which involves periods of both cold and warm ischemia, and global organ IRI.
The complement system is also a potential target for therapeutic intervention of IRI 6. However, complement inhibitors have met with limited success in the clinic, and there are currently only 2 FDA approved complement inhibitors: an anti-C5 mAb and recombinant C1-inhibitor. These biologics have thus far had minimal health impact since they have only been approved for orphan diseases and are extremely expensive. The two approved inhibitors, as well as most other complement inhibitors that have been investigated, systemically inhibit complement, although it is known that targeting a complement inhibitor to deposited complement fragments can improve bioavailability and significantly improve the efficacy of complement inhibition 7. Here, we demonstrate that natural self-reactive IgM mAbs induce post-transplant complement activation and IRI. Based on IgM target identification, we developed a novel bi-functional reagent that both blocks the binding of pathogenic IgM and targets a complement inhibitor specifically to post-ischemic cardiac endothelium. The reagent is a fusion protein consisting of an annexin IV-specific single chain antibody (scFv) linked to Crry. Annexin IV is a target for natural self-reactive Abs, and Crry is a murine inhibitor of C3 activation that is a structural and functional analogue of human CR1, a soluble untargeted form of which is in clinical trials. With regard to clinical translation, there are significant potential advantages of the therapeutic strategy described here compared to other complement inhibitory approaches, including a previously described CR2-targeted approach 7 (see discussion), and there is also strong evidence that the post-ischemic annexin IV recognition system described here similarly functions in humans 4, 5, 8.
Methods
See also the Methods section in the online-only Data Supplement
Cell lines and antibodies
The B4 and C2 hybridoma cell lines expressing IgM mAbs were isolated following the fusion of spleen cells from unmanipulated wt C57BL/6 mice as described 5. The specificities of these mAbs have been determined; B4 mAb recognizes annexin IV 5 and C2 mAb recognizes a subset of phospholipids 4. As a control Ab, we used IgM mAb F632, expressed by a hybridoma isolated from mice immunized with 4-hydroxy-3-nitrophenylacetyl (NP)-KLH and screened against NP-BSA, as described 5.
Transplantation Studies
Male C57BL/6, Rag1−/− on C57BL/6 background, and BALB/c mice were used in these studies. For isograft studies, we used the following experimental groups: 1. C57BL/6 donors and recipients; 2. Rag1−/− donors and recipients; 3. Rag1−/− donors and recipients with administration of 0.1 mg B4 mAb to recipient; 4. Rag1−/− donors and recipients, with administration of 0.1 mg C2 mAb to recipient; 5. Rag1−/− donors and recipients with administration of 0.1 mg B4 mAb and 0.1 mg C2 mAb to recipient; 6. Rag1−/− donors and recipients with administration of 0.1 mg control F632 mAb to recipient. For allograft studies, Balb/c donor hearts were implanted into C57BL/6 recipients, and recipient groups treated with: 1. Vehicle control (PBS); 2. 0.1 mg B4scFv; 3. 0.2 mg B4scFv-Crry (molar equivalent; MW of B4scFv-Crry and B4scFv is 64 kD and 29 kD, respectively); 4. 0.2 mg B4scFv-Crry and 0.1 mg C2 mAb. All animal procedures were performed according to approved experimental protocols in accordance with institutional animal care guidelines of the Medical University of South Carolina.
Construction and expression of recombinant proteins
For construction of B4 and C2 ScFv expressing plasmids, mRNA was prepared from corresponding hybridomas described above (details in Data Supplement). For construction of the B4 scFv-Crry expression plasmid, the B4scFv sequence was linked to the extracellular region of mouse Crry (residues 1–319 of mature protein, GenBank accession number NM013499) by overlapping PCR with the linker (G4S2)2, and OKT3 light chain signal peptide sequence added to the 5′ end of the VH chain. Recombinant proteins were expressed in CHO cells and purified by anti-His tag affinity chromatography (for B4 and C2 scFv’s) or anti-Crry affinity chromatography (for B4 scFv-Crry) as described 7.
Statistics
GraphPad Prism version 5.0 for Mac OS X (GraphPad, San Diego, CA) and SAS version 9.2 (SAS Institute Inc, Cary, NC) were used for statistical analysis. Differences between various groups were compared by non-parametric Wilcoxon rank-sum or Mann-Whitney test, due to small sample sizes for some experiments. For complement inhibitor and scFv analyses, 4 specific pairwise comparisons were made to analyze the key questions of the effect of B4scFv and B4scFv-Crry on IRI outcome; specifically comparisons between control and B4scFv, control and B4scFv-Crry, control and B4scFv-Crry plus C2 mAb, and B4scFv and B4scFv-Crry. For analysis of survival, SAS Proc LIFETEST was used to conduct log-rank tests for comparing survival across the 3 groups. All analyses were two-sided and a p<0.05 was deemed significant.
Results
IgM mAbs restore cardiac IRI in Rag1−/− recipient mice
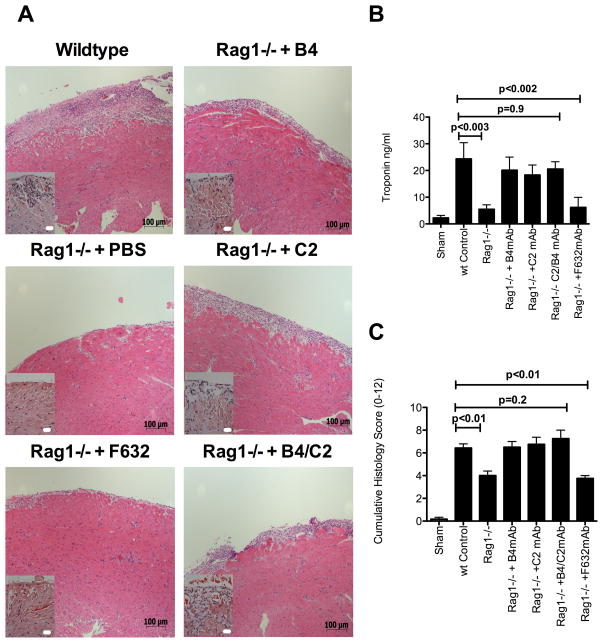
To investigate the role of self-reactive natural IgM in post-transplant cardiac IRI, we assessed IRI in isografts after transplantation from C57BL/6 wt donors to either Ab deficient Rag1−/− recipients or Rag1−/− recipients reconstituted with a specific IgM mAb. Following transplantation, Rag1−/− recipients were reconstituted with either B4 or C2 mAbs, or a combination of both mAbs. These mAbs have been shown previously to recognize post-ischemic neoepitopes in the intestine and brain 4, 5. B4 recognizes annexin IV 5 and C2 recognizes a subset of phospholipids 4. Anti-NP mAb F632 (see methods) was used as a control Ab. Compared to grafts in wild type (wt) recipients, grafts in Rag1−/− recipients were protected from post-transplant cardiac IRI as evidenced by reduced histologic injury and inflammation, and decreased serum levels of cardiac troponin I (an index of cardiac cell damage). However, reconstitution of Rag1−/− recipients with either B4 or C2 mAbs, but not F632 mAb, restored graft IRI to that seen in wt recipients. Furthermore, compared to administration of B4 and C2 mAbs individually, there was no increase in injury when B4 and C2 mAbs were co-administered (Figure 1)
Figure 1.
Administration of IgM mAbs in Rag1−/− recipients of cardiac isografts. Hearts from Rag1−/− donor mice were transplanted into wt recipients, or into Rag1−/− recipients with or without administration of indicated IgM mAb. Isografts were analyzed 48 hours post-transplantation. A, Representative Hematoxylin and Eosin stained cardiac sections from grafts, showing that epi- and endocardial inflammation and damage is restored by B4, C2, or B4+C2 combined mAbs, but not F632 mAb treatment of otherwise protected Rag1−/− recipients. Scale bars = 20 μm high power insert and 100 μm low power. B, Serum cardiac troponin I levels and C. Semi-quantitative histology injury scores. Pairwise comparisons between wt control vs. Rag1−/−, wt control vs. F632 mAb, wt Control vs B4, C2, B4+C2 mAbs combined were made using non-parametric analysis. Mean ± SEM, n = 4–8.
Analysis of IgM binding and complement activation
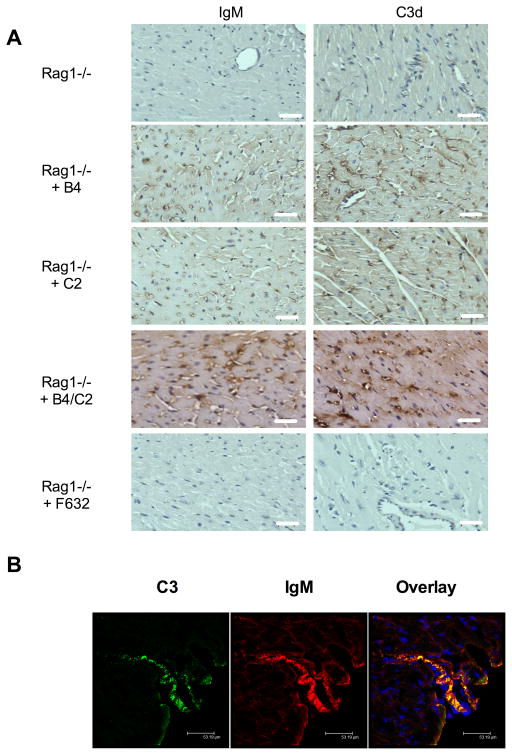
The graft specificity and binding characteristics of B4 and C2 mAbs in Rag1−/− recipients was investigated by immunohistochemical analysis of graft sections. Both B4 and C2 mAbs bound to endothelial cells of arterioles, capillaries, and microvessels within the myocardium of the transplanted heart (Figure 2A), but not the native heart (not shown). Additional Ab binding in grafts was seen on myocytes, with localization to injured myocytes in the epicardium. The staining pattern in grafts was similar whether recipients were treated individually with B4 or C2 mAbs or with combined B4 + C2 mAbs. No IgM immunostaining was detected in grafts from Rag1−/− recipients treated with F623 control mAb (Figure 2A) or in native hearts (not shown). The above data together demonstrate that annexin IV and a previously identified subset of phospholipids are post-ischemically expressed in the transplanted heart, and that IgM recognition of these epitopes is associated with graft IRI. Additionally, IgM deposition in grafts from recipients treated with B4, C2 or a combination of B4 + C2 mAbs, was associated with intra-graft complement activation as determined by the presence of C3d in the microvasculature in a similar staining pattern to that for IgM (Figure 2A). C3d was absent or barely detectable in grafts from recipients treated with PBS or control F632 mAb.
Figure 2.
Deposition of IgM and C3d in cardiac isografts following IgM mAb reconstitution of Rag1−/− recipient mice. A, IgM and C3d binding was assessed by immunohistochemistry in transplanted hearts 6 hours post-transplantation. IgM and C3d deposition is seen in B4, C2, and B4 and C2 combined mAb treated recipients, with staining localized predominantly to endothelial cells of arterioles, capillaries, and microvessels within the graft myocardium. Little to no staining for IgM or C3d seen in grafts from PBS treated Rag1−/− recipients or Rag1−/− recipients treated with F623 control mAb. Representative images, n = 3. Scale bars, 20 μm. B, Colocalization of IgM and C3d in isografts from B4 mAb treated Rag1−/− recipient mice. Binding was assessed by immunofluorescence microscopy of sections from epicardial surface of transplanted hearts isolated 6 hours post-transplantation. IgM and C3d binding colocalized on the vessel endothelium and myocardial tissue. Representative image, n = 3. Scale bars, 53 μm.
Based on the above data, we investigated whether a post-ischemic cardiac neoepitope may represent a specific therapeutic target for complement inhibition. Since there was no detectable difference between B4 and C2 mAbs in terms of their graft binding characteristics and their ability to restore graft IRI in Rag1−/− recipients, we chose B4 mAb as the focus for further studies since it is known that annexin IV is expressed on human hypoxic endothelial cells 4, and IgM reactivity to annexin IV is present in human serum 5. To additionally confirm that the B4 epitope represents an appropriate target for complement inhibition, we demonstrated that B4 mAb co-localizes with C3d in hearts transplanted into Rag1−/− recipients (Figure 2B). We also demonstrated that IgM co-localizes with C3d in grafts in wt mice (data not shown). Natural IgM Abs are known to activate the lectin pathway following myocardial ischemia and reperfusion in a non-transplant setting 9. However, by immunohistochemistry we were unable to detect MBL-A, MBL-C (or C1q) in grafts 6 hours post-transplantation. Whereas C3d is a long-lived opsonin at sites of complement activation, it is possible that the 6 hour time point (chosen because this is when we see maximal injury and inflammation post-transplant) misses the window of MBL binding/detection. In any case, while we cannot confirm that the lectin pathway is activated by IgM in this transplant setting, the targeted complement inhibitor we develop in this study inhibits all complement pathways at the downstream C3 activation step.
Preparation and initial characterization of neoepitope-targeted B4scFv
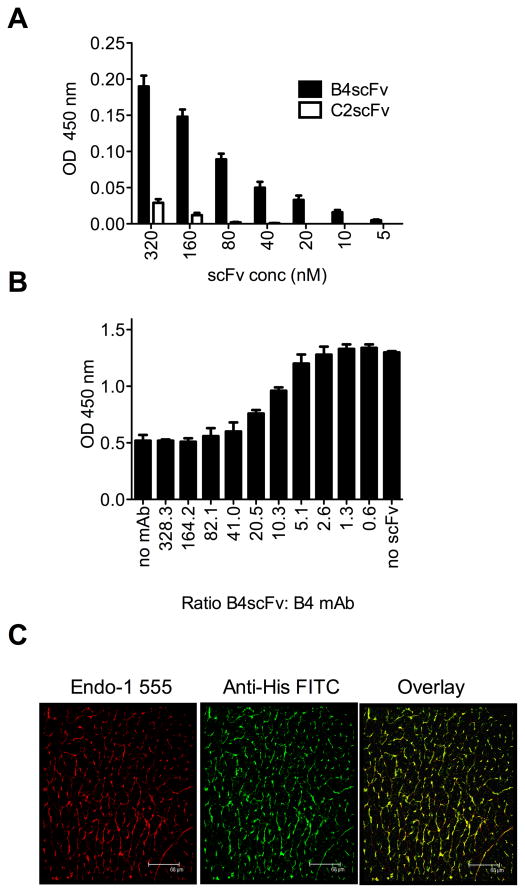
Our planned strategy for neoepitope-targeted complement inhibition involves linking a complement inhibitor to a B4-derived scFv. Such a construct has the potential to not only target complement inhibition to the post-ischemic graft, but also to inhibit the binding of specific self-reactive pathogenic IgM to the reperfused graft. The VH and VL sequences from the B4 mAb hybridoma were cloned, linked (VH-VL) and expressed as an scFv with an N-terminal His-tag. Purified B4scFv had the expected MW by SDS-PAGE (30 kD, not shown), and the construct retained parent IgM binding specificity as demonstrated by its ability to bind directly to recombinant annexin IV, as well as to competitively inhibit the binding of B4 mAb to annexin IV (Figure 3A & B). A similarly prepared C2scFv was prepared and used as a control scFv for these in vitro experiments.
Figure 3.
Characterization of neoepitope-targeted B4scFv. A, Direct binding of His-tagged B4scFv to annexin IV, as demonstrated by ELISA using anti-His tag detection Ab. C2scFv was used as specificity control. Mean +/− SEM, n = 3. B, Competitive inhibition of B4 mAb binding to annexin IV by B4scFv, as determined by competition ELISA using anti-IgM detection Ab. Mean +/− SEM, n = 3. C, B4scFv targets cardiac allografts post reperfusion. Hearts from Balb/c donors were transplanted into C57BL/6 recipients with administration of B4scFv, and allografts analyzed 6 hours post-transplantation. Images show immunofluorescence staining of section from intra-myocardial space for endothelial cells (red) and His-tagged B4scFv (green), with colocalization of B4scFv and endothelial cells indicated by yellow (composite). B4scFv localized predominantly to the microvasculature of the cardiac allograft. Representative image, n = 3. Scale bars, 68 μm.
To confirm that B4scFv recognizes a post-ischemic graft, His-tagged B4scFv was administered after reperfusion, and confocal analysis of graft sections subsequently performed. In this and in subsequent experiments, a wt allograft model (Balb/c to C57BL/6) was used to address targeting and therapeutic questions in a more clinically relevant setting, and in the context of an entire natural Ab repertoire. Immunostaining of grafts isolated 6 hours after transplantation demonstrated B4scFv binding colocalized with a pan-endothelial marker in the post-ischemic cardiac vasculature (Figure 3C).
Preparation and in vitro characterization of neoepitope-targeted complement inhibitor, B4scFv-Crry
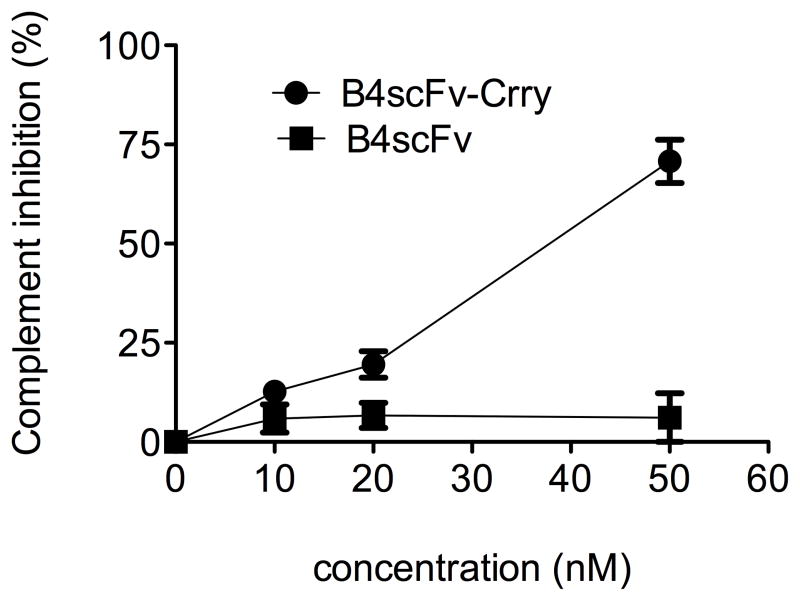
To prepare a targeted complement inhibitor, the extracellular region of Crry was linked to B4scFv. The resulting B4scFv-Crry fusion protein had the expected MW by SDS-PAGE (64 kD, not shown), and the activity of the complement inhibitor was retained (Figure 4). Note that this is an untargeted system since the B4 epitope is not expressed on zymosan particles, and that while B4scFv-Crry exhibits complement inhibitory activity, B4scFv does not.
Figure 4.
Crry activity is retained in the B4scFv-Crry construct. Increasing concentrations of B4scFv-Crry or B4scFv were incubated with activated zymosan particles in mouse serum and C3 deposition assayed by flow cytometry. Mean +/− SD, n = 3.
Effect of B4 scFv and B4 scFv-Crry on graft IRI and inflammation
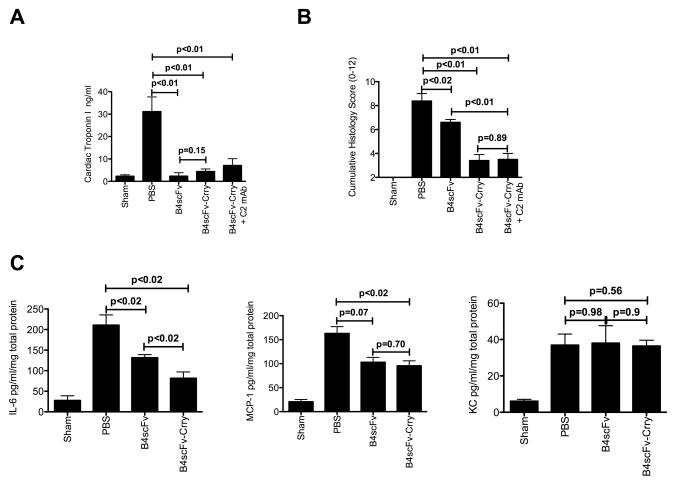
Allograft recipients were treated with B4 scFv, B4 scFv-Crry or PBS vehicle immediately post-transplantation, and serum levels of cardiac troponin I determined 48 hours post-transplant. Compared to vehicle treated controls, recipients treated with either B4scFv or B4scFv-Crry had significantly and similarly reduced levels of serum cardiac troponin I (Figure 5A). Grafts were also assessed for histological evidence of injury and inflammatory cell infiltration 48 hours after transplantation. Consistent with cardiac troponin I levels, grafts from B4scFv-Crry and B4scFv treated recipients also had significantly lower injury/inflammation scores than grafts from vehicle treated recipients (Figure 5B). Natural antibody repertoires are likely to vary significantly in humans and therefore we sought to investigate whether B4scFv-Crry would be efficacious in the presence of high serum levels of an antibody that does not recognize the B4 epitope. Therefore, using the same allograft model which has a full repertoire of natural antibodies, we supplemented the natural antibody response of the transplant recipient with 0.1 mg of C2 mAb prior to transplant, and then treated with B4scFv-Crry. We demonstrate that even in the presence of a high concentration of C2 mAb, B4scFv-Crry significantly reduced injury compared to controls (Figure 5A and B), and the difference between the two B4scFv-Crry treated groups was not significantly different.
Figure 5.
Effect of B4scFv and B4scFvCrry on allograft ischemia reperfusion injury and inflammation. Recipient mice were treated with PBS, B4scFv, B4scFvCrry, or B4scFvCrry and C2mAb combined, and allografts and serum isolated 48 hours post-transplantation for analysis. A, Serum cardiac troponin I levels. Pairwise comparisons between wt control vs. B4scFv (p<0.01), wt control vs. B4scFv-Crry (p<0.001), wt control vs B4scFv-Crry + C2 mAb (p<0.001), and B4scFv vs. B4scFv-Crry was not significant (p=0.15). Mean ± SEM, n = 6–8. B, Histological assessment of injury from hematoxylin and eosin stained cardiac sections. Non-parametric two tailed analysis demonstrated that wt PBS control vs. B4scFv (p=0.02), wt PBS control vs. B4scFv-Crry (p<0.01), wt control vs B4scFv-Crry + C2 mAb (p<0.01), and B4scFv vs. B4scFv-Crry (p<0.01) were noted. Mean ± SEM, n = 6–8. C, Intragraft expression of IL-6, MCP-1 and KC as determined by by ELISA. Pairwise two tailed non=parametric analysis between wt PBS control vs. B4scFv, wt PBS control vs. B4scFv-Crry, and B4scFv vs. B4scFv-Crry were made for each analyzed cytokine. Mean ± SEM, n = 6–8.
To further investigate how B4scFv and B4scFv-Crry modulate the inflammatory environment, we determined the effect of each molecule on graft levels of the cardiotoxic cytokine IL-6, and the macrophage and neutrophil chemokines MCP-1 and KC, all of which are associated reperfusion injury and the onset of rejection 10. At 48 hours post-transplant, B4scFv-Crry treated recipients had significantly reduced graft levels of IL-6 and MCP-1 compared to PBS treated controls (Figure 5C). IL-6, but not MCP-1 levels were significantly lower in grafts from B4scFv treated recipients compared to untreated control recipients. Graft levels of KC at 48 hours post-transplant were not altered by treatment with either molecule, but it has been shown that KC levels peak within the first 24 hours after transplantation 10.
IgM and complement deposition in grafts
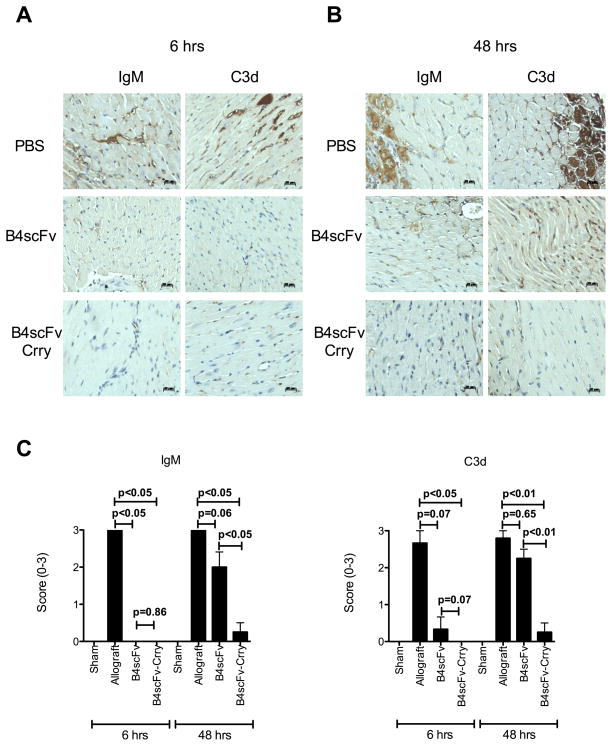
The impact of B4scFv and B4scFv-Crry on IgM binding and C3d deposition in grafts was assessed at 6 hours and 48 hours post-transplantation. As expected, IgM binding and C3d deposition occurred in grafts from recipients treated with PBS, with staining predominantly in the microvasculature at 6 hours post-transplantation, and in the microvasculature and on myocytes at 48 hours post-transplantation (Figure 6). Treatment of recipients with B4scFv-Crry eliminated IgM and C3 deposition when analyzed at 6 hours post-transplantation; B4scFv treatment also eliminated IgM deposition, but while C3 deposition appeared to be reduced, the difference with PBS treated controls did not reach significance. At 48 hours post-transplantation, however, significant reductions in both IgM binding and C3d deposition was seen only in grafts from B4scFv-Crry treated recipients (Figure 6).
Figure 6.
Effect of B4scFv and B4scFvCrry treatment on IgM and C3d deposition in transplanted allografts. Recipient mice were treated with PBS, B4scFv or B4scFvCrry and allografts isolated at 6 hours (A) or 48 hours (B) post-transplantation for analysis. Representative images of IgM and C3d deposition in grafts are shown. Scale bars, 20 μm. C, Semi-quantitative histological scoring of IgM and C3d deposition at 6 and 48 hours post-transplantation. Mean ± SEM, n=6–8.
In vivo kinetics and biodistribution of B4scFv-Crry and B4scFv
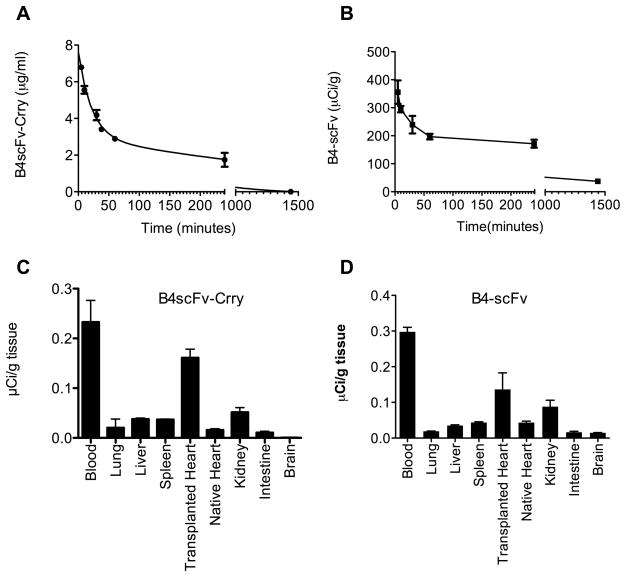
To determine the circulatory half-life (t1/2) of B4scFv-Crry and B4scFv, the proteins were injected i.v. into C57BL/6 (recipient) mice, and plasma levels of each protein determined at different times post-injection. There was a similar two-phase elimination profile for both proteins, with the bulk of injected protein eliminated in an initial rapid clearance phase. For B4scFv-Crry there was a fast phase t1/2 of 9.7 mins and a slow phase t1/2 of 5.5 hours, and for B4scFv there was a fast phase t1/2 of 6.9 mins and a slow phase t1/2 of 7.0 hours (Figure 7A). A similar two-phase elimination profile has been shown for other biologics, including untargeted Crry (Crry-Ig), although the second phase t1/2 for Crry-Ig was considerably longer (40 hrs) 11.
Figure 7.
Serum circulatory half-life and biodistribution of B4scFv-Crry and B4-scFv. A, Plasma levels of B4scFv-Crry and B4-scFv in blood collected at indicated times following i.v. injection were determined by ELISA or radiometry (see methods). Mean ± SEM, n = 3. B, Biodistribution of 125I-labeled B4scFv-Crry and B4-scFv determined 6 hours post-injection and transplantation. Mean ± SEM, n = 3.
Previous studies have shown that for untargeted Crry, systemic complement inhibition and a high dose (up to 3 mg) is required for therapeutic benefit in various mouse models of disease 7, 11–14. In the current study, significant therapeutic efficacy is seen with a 0.2 mg dose of B4scFv-Crry and, at an early time point, with a molar equivalent dose of B4scFv (0.1 mg). Since preferential accumulation at the target site would be expected to translate to a lower dose requirement for therapeutic benefit, we performed a biodistribution study with the two proteins. 125I-labeled B4scFv-Crry or B4scFv was injected into recipient mice immediately after heart transplantation, and tissue distribution of radiolabel determined 6 hours later. In addition to being present in the circulation, both 125I-B4scFv-Crry and 125I-B4scFv localized primarily to the transplanted graft (Figure 7B). There was a higher localization to the kidneys compared to other organs, and this is likely a consequence of the kidney being a site of clearance. It is also possible that the kidneys suffered some ischemic injury induced by graft implantation and the clamping of the infra-vena cava and descending aorta proximal to the kidneys. Importantly no significant binding was noted in the native heart, supporting the concept that B4scFv binds to epitopes exposed by ischemia/injury and not merely epitopes expressed normally in the heart. This biodistribution data is also consistent with the immunostaining data for IgM and B4scFv binding in grafts (above).
Data shown above in Figure 6 demonstrate that B4scFv-Crry and B4scFv similarly protect against graft IgM and C3d deposition at 6 hours post-transplantation, but that only grafts from B4scFv-Crry treated animals display reduced IgM and C3d deposition at 48 hours post-transplantation. These differences cannot be attributed to differences in circulatory half-life of the proteins (see above), so we performed an additional kinetic study to determine whether there are differences in the amount of each protein that localizes to the graft, or whether there are differences in retention time of the proteins in the graft. B4scFv-Crry or B4scFv was spiked with corresponding 125I-labeled protein and injected into recipient mice immediately after heart transplantation. The amount of labeled protein bound by the graft was determined at 6 and 48 hours post-transplantation. At 6 hours after transplantation, 24.2 ng 125I-B4scFv-Crry (+/− 1.86 ng, n = 5) and 14.1 ng 125I-B4scFv (+/− 0.62 ng, n = 5) were bound in grafts, which are close to molar equivalents (1:1.18). At 48 hours after transplantation, 4.6 ng 125I-B4scFv-Crry (+/− 1.34 ng, n = 5) and 1.8 ng 125I-B4scFv (+/− 0.23 ng, n = 5) were bound in grafts. These differences between 6 and 48 hours calculate to a tissue half-life of 17.5 hours for B4scFv-Crry and 12.8 hours for B4scFv. Thus, while the differences are not great, it is possible that the shorter retention time of B4scFv in the graft compared to B4scFv-Crry may contribute to the reduced efficacy of B4scFv at 48 hours post-transplantation.
B4scFv-Crry treatment and immunity to infection
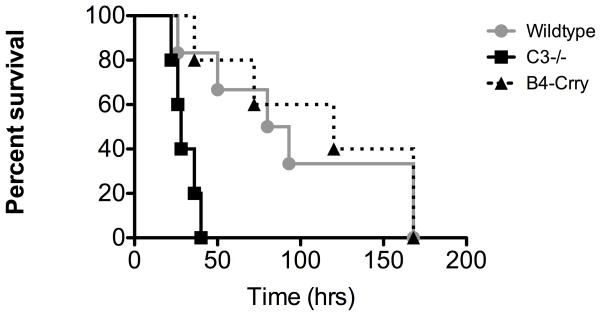
A major potential benefit of targeted vs. systemic complement inhibition is the minimization of side effects associated with the normal physiological functions of complement. An important function of complement is to provide host defense against infection, a function of particular significance for a transplant recipient. We therefore investigated the effect of a therapeutic dose of B4scFv-Crry on immunity to infection using a well characterized model of polymicrobial sepsis. Cecal ligation and puncture was performed in C3 deficient mice, or in wt mice treated with either B4scFv-Crry or PBS, and survival monitored. All C3 deficient mice succumbed to infection within 48 hours of CLP, and a similar result was reported previously for wt mice treated with a minimum therapeutic dose of the systemic inhibitor, Crry-Ig 7. However, B4scFv-Crry treated mice survived significantly longer following CLP, and survival was not significantly different compared to PBS treated wt mice (Figure 8).
Figure 8.
Survival analysis in model of polymicrobial sepsis. Survival of C3-deficient mice and wt mice treated with either PBS or 0.2 mg B4scFv-Crry immediately following cecal ligation and puncture. Log-rank tests comparing survival across the three groups demonstrated that survival times were significantly different between groups (p<0.001). All pairwise comparisons demonstrated that survival was significantly greater in PBS and B4scFv-Crry as compared to C3−/− (p<0.01 for all), even after Bonferroni adjustment for multiple comparisons. No significant difference was seen between PBS and B4scFv-Crry (p>0.05). n = 5–6.
Discussion
Naturally occurring self-reactive IgM Abs are germline encoded and arise without exogenous antigen stimulation. They are constitutively secreted by B1 cells, and may represent up to 80% of circulating IgM 15. Natural IgM Abs represent a class of innate pattern recognition receptors that can recognize danger associated molecular patterns that become exposed on stressed or dying (apoptotic) cells. Via this recognition system and the subsequent activation of complement, natural IgM Abs play an important role in tissue homeostasis by disposing of injured or dying cells, and in health this is an anti-inflammatory process 16. However, the expression of danger associated molecular patterns following a significant insult, such as organ ischemia, leads to pathogenic levels of IgM binding and complement activation, resulting in host tissue injury. Here, we demonstrate a key role for natural IgM in post-transplant cardiac IRI, and identify pathophysiologically important neoepitopes expressed in a transplanted heart. Although most natural IgM Abs that have been characterized are polyreactive, they nevertheless recognize a specific set of epitopes, and we demonstrate that two mAbs characterized here are specific for stressed or injured cells. The two mAbs, B4 and C2, were similar in terms of their cardiac graft binding characteristics and ability to restore cardiac graft IRI in Rag1−/− recipients. We subsequently focused on B4 mAb for therapeutic development since it is known that annexin IV is expressed on hypoxic human endothelial cells 4, and IgM reactivity for annexin IV is present in human serum 5.
It is nevertheless important to note that available evidence indicates that self-reactive Abs of multiple specificities exist, and while these Abs which are pathogenic when engaged are injury specific, it is less clear whether there are differences in tissue specificity. For example, B4 and C2 mAb neopitopes are also expressed in post-ischemic mouse intestine 5 and brain 4. A previously described natural self-reactive IgM mAb that recognizes nonmuscle myosin restores intestinal 3, myocardial 17 and skeletal muscle 2 IRI in Rag1−/− mice, and the same Ab specificity is present in human serum 18. Anti-phospholipid and anti-β2 glycoprotein I Abs have also been shown to be pathogenic following ischemia and reperfusion 19. It is also likely, therefore, that neoepitopes other than annexin-IV (and phospholipid recognized by C2 mAb) are involved in ischemia-related cardiac allograft injury. It is not clear, however, how recognition of different epitopes by natural Abs relate to each other pathophysiologically. For example, a peptide mimic of nonmuscle myosin 18 and recombinant annexin IV 4, 5 can each protect against IRI, and there is currently no explanation of how blockade of a single Ab specificity in wild type mice that contain a full natural Ab repertoire is protective. Regardless, there are drawbacks with a therapeutic approach directed at neutralizing a pathogenic Ab. Such a strategy would require a systemic effect and, potentially, a high dose of blocking agent. It is also not known if there are significant variations in the natural Ab repertoire, particularly in humans, and the redundancy in this recognition system may make an approach that blocks a single Ab specificity suboptimal. Preventing the binding of pathogenic IgM by targeting neoepitopes expressed on the post-ischemic organ/tissue thus appears a preferable approach. Nevertheless, because of the concern of redundancy, blocking a single neoepitope may still be suboptimal, especially in a clinical setting. We therefore linked B4scFv, which we demonstrated targeted to post-ischemic endothelium, to Crry, an inhibitor of complement that functions at the central C3 activation step of all pathways. Thus, in the event of incomplete blockade of pathogenic IgM binding by scFv, inhibition of complement activation would be maintained, as demonstrated in our B4scFv-Crry and C2 mAb co-administration studies. Note that complement inhibitors display species selective activity, and it is appropriate and relevant to use mouse complement inhibitors in mouse models. Mouse Crry used in these studies is the structural and functional analogue of human CR1 20.
While both B4scFv and B4scFv-Crry provided protection from IRI, B4scFv-Crry was overall more effective. Interestingly, both constructs eliminated IgM deposition in grafts at 6 hours post-transplantation, but by 48 hours only grafts from B4scFv-Crry treated animals had significantly reduced IgM and C3d deposition compared to controls. Thus, B4scFv blocks the binding of pathogenic IgM early after reperfusion, but its function as a targeting vehicle with co-delivery of a complement inhibitor provides continued protection from IgM deposition, complement activation and injury. A likely explanation for the difference in IgM and complement deposition between the two time points is redundancy in the IgM recognition system, and there may be differences in the temporal expression profiles of different neoepitopes. It is also possible that delayed complement activation, by incomplete blockade of IgM-triggered classical/lectin pathways, by subsequent activation of the alternative pathway, or by graft recognition by alloreactive Abs, quantitatively or qualitatively alters the epitope expression profile on the cell surface. In the current allograft model, as well as clinically, low levels of pre-existing alloreactive Abs are likely present. Thus, although the scFv may block post-ischemic natural Ab recognition, the co-delivery of a complement inhibitor will also block complement activation by alloreactive Abs.
A potential problem in the translation of a complement inhibitory strategy to the clinic, especially in the transplant setting where the patient is heavily immunocompromised, is the additional immunosuppressive effect of systemic complement inhibition. One approach to alleviate such concerns is to target the terminal complement pathway with anti-C5 mAb therapy. Although still systemic, anti-C5 mAb inhibits C5a and MAC generation without blocking the generation of C3 complement activation products that are important for host defense. However, in clinical trials, C5 blockade provided limited benefit for the treatment of ischemic heart disease 21. Also, although anti-C5 mAb treatment can synergize with CTLA-4 to prolong graft survival 22, C3 activation in the graft still occurs 23, which may prime an alloimmune response. In this context, recent studies demonstrated an important role for graft endothelial C3a (and C5a) in priming allogeneic T cell responses 24, 25. Two other strategies to overcome the problem of systemic inhibition have been investigated in the context of transplantation. One involved organ perfusion ex-vivo with a complement inhibitor linked to a membrane-inserting fatty acid component. Using this approach, a lipid-tagged fragment of CR1 localized within rat kidneys and provided protection from IRI 26. The second approach specifically targeted complement inhibition to sites of C3 deposition 10, 27. In this approach, complement receptor 2 (CR2) that binds C3d was linked to a complement inhibitor, and such constructs protect against complement-dependent injury in various mouse models (reviewed in 28). One such construct, CR2-fH (TT30) is currently in clinical trials. While CR2-mediated targeting of complement inhibition is effective, there are a number of advantages with the targeting strategy described here for ameliorating post-ischemic injury. First, it will target the proximal event in complement activation. Second, the targeting vehicle itself contributes to therapeutic activity by blocking the binding of complement activating pathogenic IgM, which will in turn reduce the binding of C1q and MBL, which can impact inflammation, endothelial activation and cell trafficking 29. Third, B4scFv-Crry has a considerably shorter circulatory half-life than CR2-Crry (at least in mice), which is a beneficial characteristic since it reduces systemic effects. Fourth, the current strategy may be more specific for sites of stress/injury since CR2 also binds other ligands, such as IFNα 30, CD23 31, DNA containing complexes 32, Epstein-Barr virus 33, as well as sites of spontaneous complement activation such as kidney tubules. Fifth, both functional arms of this kind of construct can be modified in terms of target specificity as well as the complement pathway/product inhibited. Finally, neoepitope targeting may be less immunosuppressive since not all sites of infection and C3 deposition will be targeted with complement inhibition. In this regard, we demonstrated that B4scFv-Crry did not affect host immune resistance to infection in a model of polymicrobial sepsis.
In summary, we have identified different neoepitopes expressed in the cardiac vasculature post-transplantation, and demonstrated that recognition of these neoepitopes by IgM promotes global transplant induced myocardial IRI. Based on these analyses, we developed and characterized a novel therapeutic strategy that exploits the post-ischemia immune recognition system to target a complement regulatory protein to a site of ischemic stress. Since post-ischemic neoepitopes and the IgM specificities that bind to them are conserved across species, there is the potential for direct translational development.
Acknowledgments
Funding Sources: This study was supported by grants from the NIH (RO1 HL 86576 to ST, RO1 HL091944 to CA), the Veteran’s Affairs (1BX001218 and 1RX001141 to ST), and the American Heart Association (SDG AHA 065100 to CA). Statistical support was provided through the South Carolina Clinical & Translational Research Institute, Medical University of South Carolina’s CTSA, National Institutes of Health/National Center for Advancing Translational Sciences grant UL1TR000062.
Footnotes
Disclosures: None.
References
- 1.Weiser MR, Williams JP, Moore FD, Jr, Kobzik L, Ma M, Hechtman HB, Carroll MC. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med. 1996;183:2343–2348. doi: 10.1084/jem.183.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, Kessler B, Shimaoka M, Chan R, Friend D, Mahmood U, Weissleder R, Moore FD, Carroll MC. Identification of the target self-antigens in reperfusion injury. J Exp Med. 2006;203:141–152. doi: 10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang M, Austen WG, Jr, Chiu I, Alicot EM, Hung R, Ma M, Verna N, Xu M, Hechtman HB, Moore FD, Jr, Carroll MC. Identification of a specific self-reactive igm antibody that initiates intestinal ischemia/reperfusion injury. PNAS. 2004;101:3886–3891. doi: 10.1073/pnas.0400347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elvington A, Atkinson C, Kulik L, Zhu H, Yu J, Kindy MS, Holers VM, Tomlinson S. Pathogenic natural antibodies propagate cerebral injury following ischemic stroke in mice. J Immunol. 2012;188:1460–1468. doi: 10.4049/jimmunol.1102132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulik L, Fleming SD, Moratz C, Reuter JW, Novikov A, Chen K, Andrews KA, Markaryan A, Quigg RJ, Silverman GJ, Tsokos GC, Holers VM. Pathogenic natural antibodies recognizing annexin iv are required to develop intestinal ischemia-reperfusion injury. J Immunol. 2009;182:5363–5373. doi: 10.4049/jimmunol.0803980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorsuch WB, Chrysanthou E, Schwaeble WJ, Stahl GL. The complement system in ischemia-reperfusion injuries. Immunobiol. 2012;217:1026–1033. doi: 10.1016/j.imbio.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkinson C, Song H, Lu B, Qiao F, Burns TA, Holers VM, Tsokos GC, Tomlinson S. Targeted complement inhibition by c3d recognition ameliorates tissue injury without apparent increase in susceptibility to infection. J Clin Invest. 2005;115:2444–2453. doi: 10.1172/JCI25208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rescher U, Gerke V. Annexins--unique membrane binding proteins with diverse functions. J Cell Sci. 2004;117:2631–2639. doi: 10.1242/jcs.01245. [DOI] [PubMed] [Google Scholar]
- 9.Walsh MC, Bourcier T, Takahashi K, Shi L, Busche MN, Rother RP, Solomon SD, Ezekowitz RA, Stahl GL. Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J Immunol. 2005;175:541–546. doi: 10.4049/jimmunol.175.1.541. [DOI] [PubMed] [Google Scholar]
- 10.Atkinson C, He S, Morris K, Qiao F, Casey S, Goddard M, Tomlinson S. Targeted complement inhibitors protect against posttransplant cardiac ischemia and reperfusion injury and reveal an important role for the alternative pathway of complement activation. J Immunol. 2010;185:7007–7013. doi: 10.4049/jimmunol.1001504. [DOI] [PubMed] [Google Scholar]
- 11.Quigg RA, Kozono Y, Berthiaume D, Lim A, Salant J, Weinfeld A, Griffin P, Kremmer E, Holers VM. Blockade of antibody-induced glomerulonephritis with crry-ig, a soluble murine complement inhibitor. J Immunol. 1998;160:4553–4560. [PubMed] [Google Scholar]
- 12.Leinhase I, Schmidt OI, Thurman JM, Hossini AM, Rozanski M, Taha ME, Scheffler A, John T, Smith WR, Holers VM, Stahel PF. Pharmacological complement inhibition at the c3 convertase level promotes neuronal survival, neuroprotective intracerebral gene expression, and neurological outcome after traumatic brain injury. Exp Neurol. 2006;199:454–464. doi: 10.1016/j.expneurol.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 13.Bao L, Haas M, Kraus DM, Hack BK, Rakstang JK, Holers VM, Quigg RJ. Administration of a soluble recombinant complement c3 inhibitor protects against renal disease in mrl/lpr mice. J Am Soc Nephrol. 2003;14:670–679. doi: 10.1097/01.asn.0000051597.27127.a1. [DOI] [PubMed] [Google Scholar]
- 14.Banda NK, Kraus D, Vondracek A, Huynh LH, Bendele A, Holers VM, Arend WP. Mechanisms of effects of complement inhibition in murine collagen-induced arthritis. Arthritis Rheum. 2002;46:3065–3075. doi: 10.1002/art.10591. [DOI] [PubMed] [Google Scholar]
- 15.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. PNAS. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silverman GJ, Gronwall C, Vas J, Chen Y. Natural autoantibodies to apoptotic cell membranes regulate fundamental innate immune functions and suppress inflammation. Discov Med. 2009;8:151–156. [PubMed] [Google Scholar]
- 17.Zhang M, Michael LH, Grosjean SA, Kelly RA, Carroll MC, Entman ML. The role of natural igm in myocardial ischemia-reperfusion injury. J Mol Cell Cardiol. 2006;41:62–67. doi: 10.1016/j.yjmcc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Haas MS, Alicot EM, Schuerpf F, Chiu I, Li J, Moore FD, Carroll MC. Blockade of self-reactive igm significantly reduces injury in a murine model of acute myocardial infarction. Cardiovasc Res. 2010;87:618–627. doi: 10.1093/cvr/cvq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleming SD, Egan RP, Chai C, Girardi G, Holers VM, Salmon J, Monestier M, Tsokos GC. Anti-phospholipid antibodies restore mesenteric ischemia/reperfusion-induced injury in complement receptor 2/complement receptor 1-deficient mice. J Immunol. 2004;173:7055–7061. doi: 10.4049/jimmunol.173.11.7055. [DOI] [PubMed] [Google Scholar]
- 20.Molina H, Wong W, Kinoshita T, Brenner C, Foley S, Holers VM. Distinct receptor and regulatory properties of recombinant mouse complement receptor 1 (cr1) and crry, the two genetic homologues of human cr1. J Exp Med. 1992;175:121–129. doi: 10.1084/jem.175.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Testa L, Van Gaal WJ, Bhindi R, Biondi-Zoccai GG, Abbate A, Agostoni P, Porto I, Andreotti F, Crea F, Banning AP. Pexelizumab in ischemic heart disease: A systematic review and meta-analysis on 15,196 patients. J Thorac Cardiovasc Surg. 2008;136:884–893. doi: 10.1016/j.jtcvs.2007.12.062. [DOI] [PubMed] [Google Scholar]
- 22.Raedler H, Vieyra MB, Leisman S, Lakhani P, Kwan W, Yang M, Johnson K, Faas SJ, Tamburini P, Heeger PS. Anti-complement component c5 mab synergizes with ctla4ig to inhibit alloreactive t cells and prolong cardiac allograft survival in mice. Am J Transplant. 2011;11:1397–1406. doi: 10.1111/j.1600-6143.2011.03561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferraresso M, Macor P, Valente M, Della Barbera M, D’Amelio F, Borghi O, Raschi E, Durigutto P, Meroni P, Tedesco F. Posttransplant ischemia-reperfusion injury in transplanted heart is prevented by a minibody to the fifth component of complement. Transplantation. 2008;86:1445–1451. doi: 10.1097/TP.0b013e31818a68e2. [DOI] [PubMed] [Google Scholar]
- 24.Raedler H, Yang M, Lalli PN, Medof ME, Heeger PS. Primed cd8(+) t-cell responses to allogeneic endothelial cells are controlled by local complement activation. Am J Transplant. 2009;9:1784–1795. doi: 10.1111/j.1600-6143.2009.02723.x. [DOI] [PubMed] [Google Scholar]
- 25.Kwan WH, Hashimoto D, Paz-Artal E, Ostrow K, Greter M, Raedler H, Medof ME, Merad M, Heeger PS. Antigen-presenting cell-derived complement modulates graft-versus-host disease. J Clin Invest. 2012;122:2234–2238. doi: 10.1172/JCI61019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel H, Smith RA, Sacks SH, Zhou W. Therapeutic strategy with a membrane-localizing complement regulator to increase the number of usable donor organs after prolonged cold storage. J Am Soc Nephrol. 2006;17:1102–1111. doi: 10.1681/ASN.2005101116. [DOI] [PubMed] [Google Scholar]
- 27.Atkinson C, Floerchinger B, Qiao F, Casey S, Williamson T, Moseley E, Stoica S, Goddard M, Ge X, Tullius SG, Tomlinson S. Donor brain death exacerbates complement-dependent ischemia reperfusion injury in transplanted hearts. Circulation. 2013;127:1290–1299. doi: 10.1161/CIRCULATIONAHA.112.000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holers VM, Rohrer B, Tomlinson S. Cr2-mediated targeting of complement inhibitors: Bench-to-bedside using a novel strategy for site-specific complement modulation. Adv Exp Med Biol. 2013;734a:137–154. doi: 10.1007/978-1-4614-4118-2_9. [DOI] [PubMed] [Google Scholar]
- 29.Oroszlan M, Daha MR, Cervenak L, Prohaszka Z, Fust G, Roos A. Mbl and c1q compete for interaction with human endothelial cells. Mol Immunol. 2007;44:1150–1158. doi: 10.1016/j.molimm.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Delcayre AX, Salas F, Mathur S, Kovats K, Lotz M, Lernhardt W. Epstein barr virus/complement c3d receptor is an interferon alpha receptor. Embo J. 1991;10:919–926. doi: 10.1002/j.1460-2075.1991.tb08025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aubry JP, Pochon S, Graber P, Jansen KU, Bonnefoy JY. Cd21 is a ligand for cd23 and regulates ige production. Nature. 1992;358:505–507. doi: 10.1038/358505a0. [DOI] [PubMed] [Google Scholar]
- 32.Holers VM, Kulik L. Complement receptor 2, natural antibodies and innate immunity: Interrelationships in b cell selection and activation. Mol Immunol. 2007;44:64–72. doi: 10.1016/j.molimm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Lowell CA, Klickstein LB, Carter RH, Mitchell JA, Fearon DT, Ahearn JM. Mapping of the epstein-barr virus and c3dg binding sites to a common domain on complement receptor type 2. J Exp Med. 1989;170:1931–1946. doi: 10.1084/jem.170.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]