Abstract
Background
The rodent model is commonly used to study facial nerve injury. Because of the exceptional regenerative capacity of the rodent facial nerve, it is essential to consider the timing when studying facial nerve regeneration and functional recovery. Short-term functional recovery data following transection and repair of the facial nerve has been documented by our laboratory. However, because of the limitations of the head fixation device, there is a lack of long-term data following facial nerve injury. The objective of this study was to elucidate the long-term time course and functional deficit following facial nerve transection and repair in a rodent model.
Methods
Adult rats were divided into group 1 (controls) and group 2 (experimental). Group 1 animals underwent head fixation, followed by a facial nerve injury, and functional testing was performed from day 7 to day 70. Group 2 animals underwent facial nerve injury, followed by delayed head fixation, and then underwent functional testing from months 6 to 8.
Results
There was no statistical difference between the average whisking amplitudes in group 1 and group 2 animals.
Conclusion
Functional whisking recovery 6 months after facial nerve injury is comparable to recovery within 1 to 4 months of transection and repair, thus the ideal window for evaluating facial nerve recovery falls within the 4 months after injury.
Keywords: facial nerve transection and repair, facial paralysis, regeneration, whisking
Facial nerve injury can lead to distressing short-term and long-term consequences.1–3 Acutely, patients commonly experience facial asymmetry, incomplete eye closure, nasal valve collapse, and decreased ability to smile. Complications of longstanding facial nerve injury include flaccid paralysis, incomplete recovery of facial function, and aberrant motor axon regeneration leading to inappropriate muscle contraction, also termed synkinesis. In addition to esthetic and functional issues, facial paralysis can impair communication, hinder expression of emotion, and cause disabling psychological complications.4
Animal models are imperative for the study of facial nerve injury, and a large body of experimental work has employed these models.5–8 Many researchers utilize rodent models in the study of peripheral nerve injury, as rodent models provide valid results across species and offer advantages of small size and ease of neurorrhaphy.9,10 However, there are shortcomings to the rodent model, including a neural regenerative capacity which exceeds that of larger animals and humans. The exceptional regeneration after facial nerve injury seen in the rodent model leads to masking of critical differences between experimental groups, especially when groups are compared at later time points.9 Therefore, it is essential to consider timing when studying facial nerve regeneration and recovery.
Our laboratory has developed a rodent facial nerve injury model to examine functional recovery following insult to the facial nerve.11–14 The testing apparatus monitors whisking behavior, an indicator of functional recovery, utilizing laser micrometers that detect vibrissae position across a scan line. During data acquisition, a head-fixation device is crucial to minimize motion artifact and to obtain precise measurements. While the titanium device is easily implantable and has a low complication rate,15 the head fixation devices become loose, precluding long-term facial function assessment. After 4 months, devices sometimes become mobile or partially extrude, rendering the acquisition of data impossible.
In the recent studies using the rodent model, animals underwent head fixation and subsequent facial nerve manipulation. Assays of functional recovery were performed for up to 4 months postoperatively.14,16 As expected, recovery data following crush injury showed near complete recovery. In contrast, recovery after transection and repair injury demonstrated poor function, disorganized whisking, and amplitudes that were scarcely elevated from the baseline.14 Although the posttransection recovery curves are well established up to 4 months after facial nerve injury, current literature lacks data beyond this time frame, secondary to the limitations of the head fixation devices. As recovery curves up to 4 months after injury appears to be on a slope, during this limited time frame it is impossible to determine whether recovery has reached a plateau or whether there is ongoing change. The unresolved issue of long-term recovery following transection and repair requires resolution. Data are needed to determine the precise slope of prolonged regeneration after 6 months, to ensure that there is no change in recovery after the plateaus established within the 4-month time frame. The objective of this study was to elucidate the long-term time course and functional deficit following facial nerve transection and repair in a rodent model, under the hypothesis that long-term facial nerve recovery would not differ significantly after 4 months.
Methods
A total of 20 adult female Wistar Hannover rats (Charles River Laboratories, Wilmington, MA) weighing 200 to 250 g were used. All Massachusetts Eye and Ear Infirmary guidelines for animal care and use were followed. For surgical procedures, animals were anesthetized with an intramuscular injection of ketamine (50 mg/kg) (Fort Dodge Animal Health, Fort Dodge, IA) and medetomidine hydrochloride (0.5 mg/kg) (Orion Corporation, Espoo, Finland).
Animal Conditioning
All the animals received initial conditioning upon arrival to our facility and before the first surgical intervention; conditioning included handling, sack training, and head fixation acclimation for 2 weeks.12 Animals were assigned 1 of the 2 groups. Similar to previous whisking studies, group 1 animals (controls) received initial conditioning, followed by titanium head fixation device implantation, then reconditioning to the whisking apparatus, followed by standard facial nerve manipulation and functional testing (Fig. 1). Animals in group 2 (experimental) underwent nerve manipulation followed by 4 months of recovery and another conditioning cycle before implantation of the head fixation device. Conditioning to the testing apparatus was then initiated, followed by recovery and functional testing (Fig. 1). Our goal was to allow full recovery 4 months after facial nerve manipulation in group 2 animals. The conditioning, implantation of a head fixation device, reconditioning, and recovery took a total of 2 months after full recovery from facial nerve manipulation, so that the functional testing began at month 6. The durations of all condition periods were equal between groups.
Fig. 1.
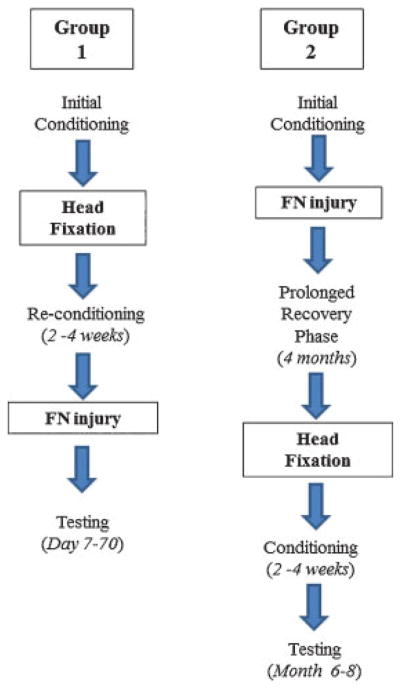
Timing of facial nerve injury and head fixation in group 1 (control) and group 2 (experimental). FN, facial nerve.
Facial Nerve Transection and Repair
Following induction of general anesthesia as described earlier, a preauricular incision was made, the parotid gland was removed, and the distal branches of the facial nerve were exposed. The main trunk of the facial nerve was identified by retrograde dissection from the distal branches. The main trunk was then completely transected and immediately repaired with 2 or 3 10–0 nylon epineural sutures. The 2nd year clinical fellow, who has microsurgical experience, performed all neurorrhaphies. The technique was observed and approved by the senior author (T.A.H.). The incision was closed in a single layer with running absorbable suture. The anesthesia was reversed with a subcutaneous injection of atipamezole hydrochloride (0.05 mg/kg). Rats were allowed to recover on a warming pad and were monitored postoperatively for signs of discomfort, including changes in grooming, social interaction, and maintenance of normal body weight. Food and water were available ad libitum.
Head Fixation
All animals underwent surgical placement of titanium cranial implants in preparation for rigid head fixation during facial movement testing. The procedure for head fixation is detailed by Hadlock et al.15 Briefly, animals were anesthetized as described earlier. A midline incision and two smaller posterior incisions were made in the scalp. A subperiosteal plane was developed over the calvarium. The lightweight titanium implant was secured to the calvarium using screws. The four external attachment points were later used for rigid head fixation.
Functional Testing
In control animals, facial nerve testing was performed pre-operatively to document normal baseline movement. Testing began on postoperative day 7 and continued weekly for 10 weeks. In experimental animals, testing began 6 months after facial nerve transection and repair and concluded 10 weeks later. The laser micrometer testing apparatus has been previously described.12 Briefly, polymide tubes weighing 0.0030 g were placed on the C1 whisker, on each side of the head and whisking was tracked independently using laser micrometers (MetraLight, San Mateo, CA). Whisking was recorded in 5-minute sessions.
Histomorphometric Analysis of Axons
To confirm the presence of axons distal to the repair, animals were euthanized 8 months after facial nerve injury in group 2. Nerves were fixed in a cold, buffered 3% glutaraldehyde solution for 24 hours, postfixed with osmium tetroxide, and embedded in Araldite 502 (Polysciences Inc., Warrington, PA). Next, 1-μm thick cross-sections were cut with an LKB III Ultramicrotome (LKB-Produkter A.B., Bromma, Sweden) and stained with 1% toluidine blue. Under light microscopy, these stained cross-sections were evaluated for overall nerve architecture, quality and quantity of regenerated nerve fibers, extent of myelination, and presence of Wallerian degeneration.
Data Analysis
Whisking Analysis
Data were analyzed using whisking software developed by Bermejo et al.17 All whisks greater than 3 degrees were analyzed in an automated fashion. The three largest amplitude whisks were identified and averaged. The amplitude, velocity, and acceleration of the three largest amplitudes, accelerations, and velocities were calculated. The mean whisk amplitudes of group 1 were compared with group 2 using two-tailed t tests, with p < 0.05 considered statistically significant.
Histomorphometric Analysis
Using an automated digital image-analysis system linked to morphometry macros developed for peripheral nerve analysis (Leco Instruments, St. Joseph, MI), the microscope image was digitized and displayed on a video monitor with a calibration of 0.125 μm/pixel. Binary histomorphometry analysis of the digitized information based on gray and white scales allowed measurements of total fascicular area and the total fiber number in the recipient nerves. At ×1,000 magnification, 5 to 7 randomly selected fields per nerve, or a minimum of 500 myelinated fibers, were evaluated for myelin width, axon width, and fiber width. From these, calculations of nerve fiber density (fibers/mm2), total number of myelinated fibers, myelin width, percentage of neural tissue (100 × neural area/intrafascicular area), and fibrin debris were made. An observer blinded to the experimental groups performed all measurements. For the histomorphometry, the differences between group means were calculated using two-tailed t tests, with p < 0.05 considered statistically significant.
Results
All the 20 animals underwent uncomplicated conditioning, head fixation, and nerve transection and repair. Two animals from each group were excluded from the study because of head fixation failures.
Whisking Recovery
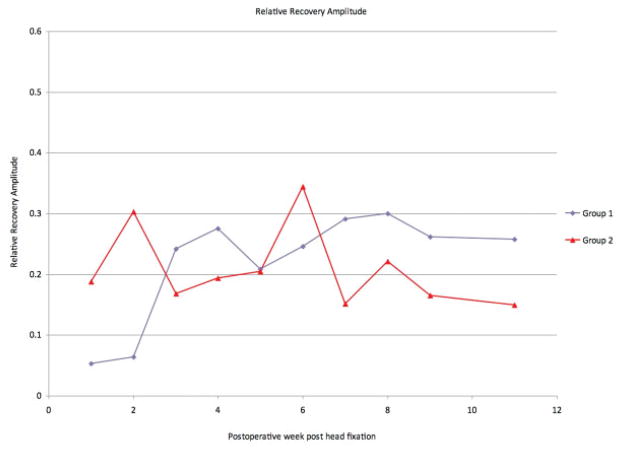
Group 1 animals demonstrated complete, unilateral absence of whisking on postoperative day 1. Initial recovery was apparent by postoperative day 21(Fig. 2). The average whisking amplitude was 16.36 degrees (± 6.29 degrees). Group 2 had an average whisking amplitude of 12.89 degrees (± 3.03 degrees) during the 10-week testing period (Fig. 3). There was no statistically significant difference in mean whisking amplitudes between group 1 and group 2 (p = 0.13). The relative recovery between the uninjured side and the injured side was calculated, demonstrating a large overlap between group 1 and group 2 (Fig. 4). Complete whisking kinematics are presented in Table 1.
Fig. 2.
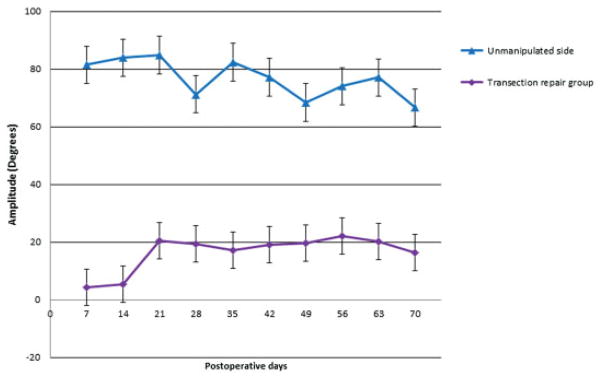
Recovery of whisking amplitude following transection and epineural repair in group 1 (control group). The bottom line represents the manipulated side. The top line represents the unmanipulated side. Error bars represent 1 standard deviation from the mean.
Fig. 3.
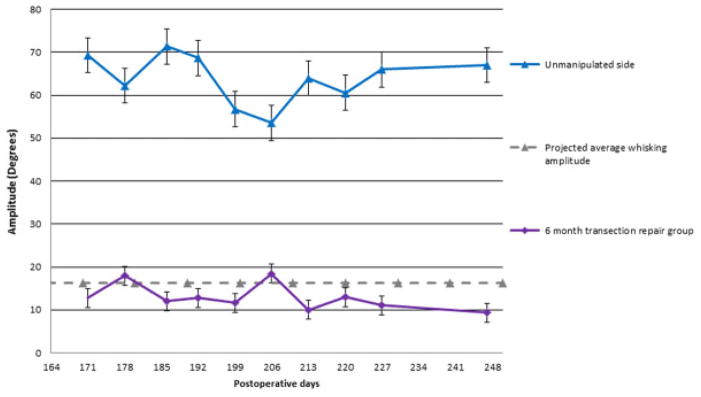
Recovery of whisking amplitude 6 months after transection and epineural repair in the experimental group (group 2). The gray line represents the projected whisking amplitude, based on the average whisking from the control group (group 1). The bottom line represents the manipulated side. The top line represents the unmanipulated side. Error bars represent 1 standard deviation from the mean.
Fig. 4.
Relative recovery of whisking amplitude in the control group (group 1) and the experimental group (group 2), calculated by comparing the manipulated side to the unmanipulated side.
Table 1.
Kinematics of whisking in group 2 (experimental) animals
| Unmanipulated side (right) | Transection and repair (left) | |
|---|---|---|
| Amplitude (degrees) | 63.58 ± 5.94 | 13.29 ± 2.92 |
| Velocity (degree/s) | 1,830.56 ± 249.55 | 550.85 ± 101.73 |
| Acceleration (degree/s2) | 292.37 ± 67.18 | 89.70 ± 11.55 |
Histomorphic Evaluation
In group 2, photomicrographs of the injured (left side) and uninjured (right side) facial nerves were compared (Fig. 5). Mean fiber counts in the uninjured facial nerve were 2,454.36 ± 675.87. The transection and repair groups had the mean fiber counts of 6,561.64 ± 2,562.39. The average nerve density of the right nerve was 15,882.55 ± 2,552.112 fibers/mm2, whereas the nerve density on the left was 34,705.73 ± 9,256.97. Differences of mean fiber count and nerve density of the two groups were statistically significant (p < 0.05).
Fig. 5.
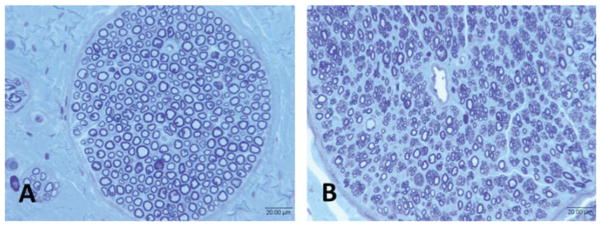
Representative photomicrographs of group 2 facial nerves from (A) uninjured side and (B) injured side.
Discussion
It has been established in the literature that facial nerve transection and repair leads to poor whisking recovery.16,18,19 Studies have determined recovery curves for facial nerve injury in the acute and intermediate setting; however, little is known about the long-term patterns of facial nerve recovery in the rat model. Our present investigation demonstrates that functional recovery 6 months after the injury is unchanged from recovery seen within 1 to 4 months following facial nerve manipulation.
Selection of the time points for analysis is critical when studying facial nerve regeneration in the rodent model. In their study of rodent transection and repair, Brenner et al provided evidence for a “blow-through” effect whereby, if given enough time, the rodent nervous system will exhibit exceptional regeneration not seen in larger animal models or humans.9 The results of their study showed that by 40 days, the animals who received the neuroregenerative agent tacrolimus had statistically significant improvement in acceleration of nerve regeneration and functional recovery. However, by 70 days, there was no difference between the experimental groups. The findings of Brenner et al highlight a limitation of the rodent model and suggest that there is a narrow time frame available to evaluate nerve repair, after which the model is insensitive to differences between experiment groups.
While the precise time course of facial nerve regeneration in the rodent model is not completely elucidated, there is a large body of research focusing on peripheral nerve recovery.20 After nerve transection, the distal nerve stump undergoes Wallerian degeneration within 3 days.21 After 10 days, the Schwann cells have realigned,22 and within 4 weeks, axons outgrow from the proximal nerve stump and cross the surgical repair site.23 Growth of axons into the distal nerve stump continues at a rate of 1 to 3 mm/d.24 Long-term recovery of peripheral nerves varies, and studies have found that myelination of regenerating nerves can take months to years to return to baseline.25,26 While there is variability in functional recovery, Hare et al found that rodents reached near-optimal functional recovery by 12 weeks after transection and repair of the sciatic, tibial, and peroneal nerves.26 Our data support the finding that optimal functional recovery of the facial nerve is completed within the first several months after injury, and that no additional recovery is seen after 6 months.
Poor functional recovery after transection and repair is attributed to the misguided regeneration of axons, loss of regenerating fibers, and poor axonal penetration.27 Our standard practice is to perform the neurorrhaphies of the main trunk with two or three epineural sutures. Histomorphic evaluation of the repaired nerve is needed to determine which of the earlier-mentioned causes are responsible for insufficient recovery and to confirm that our suture technique is sufficient to prevent loss of regenerating fibers. Fox et al established patterns of sciatic nerve regeneration and motor neuron survival over 2 years following the transection and repair.28 They determined that nerve fiber count and density rapidly increased initially, peaked at 3 months, and then plateaued through 24 months. Determining the long-term recovery of the rodent facial nerve is equally as important as establishing recovery curves after sciatic nerve transection and repair. In this study, fiber counts and nerve density in the delayed group were significantly elevated in the nerves that were transected and repaired compared with those on the contralateral, uninjured side. This robust axonal regeneration suggests that the deficient recovery seen at 6 months was caused by misrouting of axons rather than decreased axon count, and provides a benchmark for future studies to establish whether facial nerve axons fluctuate over time.
Conclusion
Determining the appropriate time frame for evaluation of facial nerve recovery is critical for future studies targeting both acceleration of facial nerve recovery and improvement of recovery. In this study, we demonstrate that functional whisking recovery 6 months after facial nerve injury is comparable to recovery within 1 to 4 months of transection and repair; thus, the ideal window for evaluating facial nerve recovery falls within the 4 months after injury. Our long-term data add to well-established short-term facial nerve recovery curves of the rodent model and confirms that studies are adequate to end in 4 months.
References
- 1.Ryzenman JM, Pensak ML, Tew JM., Jr Facial paralysis and surgical rehabilitation: a quality of life analysis in a cohort of 1,595 patients after acoustic neuroma surgery. Otol Neurotol. 2005;26(3):516–521. doi: 10.1097/01.mao.0000169786.22707.12. discussion 521. [DOI] [PubMed] [Google Scholar]
- 2.Lee J, Fung K, Lownie SP, Parnes LS. Assessing impairment and disability of facial paralysis in patients with vestibular schwannoma. Arch Otolaryngol Head Neck Surg. 2007;133(1):56–60. doi: 10.1001/archotol.133.1.56. [DOI] [PubMed] [Google Scholar]
- 3.Coulson SE, O’dwyer NJ, Adams RD, Croxson GR. Expression of emotion and quality of life after facial nerve paralysis. Otol Neurotol. 2004;25(6):1014–1019. doi: 10.1097/00129492-200411000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Ishii L, Godoy A, Encarnacion CO, Byrne PJ, Boahene KD, Ishii M. Not just another face in the crowd: society’s perceptions of facial paralysis. Laryngoscope. 2012;122(3):533–538. doi: 10.1002/lary.22481. [DOI] [PubMed] [Google Scholar]
- 5.Kumagami H. Experimental facial nerve paralysis. Arch Otolaryngol. 1972;95(4):305–312. doi: 10.1001/archotol.1972.00770080499003. [DOI] [PubMed] [Google Scholar]
- 6.Burgette RC, Benscoter BJ, Monaco GN, et al. A rat model for intracranial facial nerve crush injuries. Otolaryngol Head Neck Surg. 2012;146(2):326–330. doi: 10.1177/0194599811427531. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Han SJ, Shin DH, Lee WS, Choi JY. Subthreshold continuous electrical stimulation facilitates functional recovery of facial nerve after crush injury in rabbit. Muscle Nerve. 2011;43(2):251–258. doi: 10.1002/mus.21840. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Sun B, Yu Z, An J, Liu Q, Ren T. High dose erythropoietin promotes functional recovery of rats following facial nerve crush. J Clin Neurosci. 2009;16(4):554–556. doi: 10.1016/j.jocn.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Brenner MJ, Moradzadeh A, Myckatyn TM, et al. Role of timing in assessment of nerve regeneration. Microsurgery. 2008;28(4):265–272. doi: 10.1002/micr.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moran LB, Graeber MB. The facial nerve axotomy model. Brain Res Brain Res Rev. 2004;44(2–3):154–178. doi: 10.1016/j.brainresrev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Hadlock TA, Heaton J, Cheney M, Mackinnon SE. Functional recovery after facial and sciatic nerve crush injury in the rat. Arch Facial Plast Surg. 2005;7(1):17–20. doi: 10.1001/archfaci.7.1.17. [DOI] [PubMed] [Google Scholar]
- 12.Heaton JT, Kowaleski JM, Bermejo R, Zeigler HP, Ahlgren DJ, Hadlock TA. A system for studying facial nerve function in rats through simultaneous bilateral monitoring of eyelid and whisker movements. J Neurosci Methods. 2008;171(2):197–206. doi: 10.1016/j.jneumeth.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadlock T, Kowaleski J, Lo D, et al. Functional assessments of the rodent facial nerve: a synkinesis model. Laryngoscope. 2008;118(10):1744–1749. doi: 10.1097/MLG.0b013e31817f5255. [DOI] [PubMed] [Google Scholar]
- 14.Hadlock TA, Kowaleski J, Lo D, Mackinnon SE, Heaton JT. Rodent facial nerve recovery after selected lesions and repair techniques. Plast Reconstr Surg. 2010;125(1):99–109. doi: 10.1097/PRS.0b013e3181c2a5ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadlock T, Kowaleski J, Mackinnon S, Heaton JT. A novel method of head fixation for the study of rodent facial function. Exp Neurol. 2007;205(1):279–282. doi: 10.1016/j.expneurol.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsay RW, Heaton JT, Edwards C, Smitson C, Vakharia K, Hadlock TA. Daily facial stimulation to improve recovery after facial nerve repair in rats. Arch Facial Plast Surg. 2010;12(3):180–185. doi: 10.1001/archfacial.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bermejo R, Szwed M, Friedman W, Ahissar E, Zeigler HP. One whisker whisking: unit recording during conditioned whisking in rats. Somatosens Mot Res. 2004;21(3–4):183–187. doi: 10.1080/08990220400012430. [DOI] [PubMed] [Google Scholar]
- 18.Angelov DN, Ceynowa M, Guntinas-Lichius O, et al. Mechanical stimulation of paralyzed vibrissal muscles following facial nerve injury in adult rat promotes full recovery of whisking. Neurobiol Dis. 2007;26(1):229–242. doi: 10.1016/j.nbd.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Skouras E, Merkel D, Grosheva M, et al. Manual stimulation, but not acute electrical stimulation prior to reconstructive surgery, improves functional recovery after facial nerve injury in rats. Restor Neurol Neurosci. 2009;27(3):237–251. doi: 10.3233/RNN-2009-0474. [DOI] [PubMed] [Google Scholar]
- 20.Wood MD, Kemp SW, Weber C, Borschel GH, Gordon T. Outcome measures of peripheral nerve regeneration. Ann Anat. 2011;193(4):321–333. doi: 10.1016/j.aanat.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14(1–2):67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 22.Witzel C, Rohde C, Brushart TM. Pathway sampling by regenerating peripheral axons. J Comp Neurol. 2005;485(3):183–190. doi: 10.1002/cne.20436. [DOI] [PubMed] [Google Scholar]
- 23.Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002;22(15):6631–6638. doi: 10.1523/JNEUROSCI.22-15-06631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sunderland S. Rate of regeneration in human peripheral nerves; analysis of the interval between injury and onset of recovery. Arch Neurol Psychiatry. 1947;58(3):251–295. doi: 10.1001/archneurpsyc.1947.02300320002001. [DOI] [PubMed] [Google Scholar]
- 25.Mackinnon SE, Dellon AL, O’Brien JP. Changes in nerve fiber numbers distal to a nerve repair in the rat sciatic nerve model. Muscle Nerve. 1991;14(11):1116–1122. doi: 10.1002/mus.880141113. [DOI] [PubMed] [Google Scholar]
- 26.Hare GM, Evans PJ, Mackinnon SE, et al. Walking track analysis: a long-term assessment of peripheral nerve recovery. Plast Reconstr Surg. 1992;89(2):251–258. [PubMed] [Google Scholar]
- 27.Guntinas-Lichius O, Irintchev A, Streppel M, et al. Factors limiting motor recovery after facial nerve transection in the rat: combined structural and functional analyses. Eur J Neurosci. 2005;21(2):391–402. doi: 10.1111/j.1460-9568.2005.03877.x. [DOI] [PubMed] [Google Scholar]
- 28.Fox IK, Brenner MJ, Johnson PJ, Hunter DA, Mackinnon SE. Axonal regeneration and motor neuron survival after microsurgical nerve reconstruction. Microsurgery. 2012;32(7):552–562. doi: 10.1002/micr.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]