Abstract
Objective
To evaluate glycerol phenylbutyrate (GPB) in the treatment of pediatric patients with urea cycle disorders (UCDs).
Study Design
UCD patients (n=26) ages 2 months through 17 years were treated with GPB and sodium phenylbutyrate (NaPBA) in two short-term, open-label crossover studies, which compared 24-hour ammonia exposure (AUC0–24) and glutamine levels during equivalent steady-state dosing of GPB and sodium phenylbutyrate (NaPBA). These 26 patients plus an additional 23 patients also received GPB in one of three 12-month, open label extension studies, which assessed long-term ammonia control, hyperammonemic (HA) crises, amino acids levels, and patient growth.
Results
Mean ammonia exposure on GPB was non-inferior to NaPBA in each of the individual crossover studies. In the pooled analyses, it was significantly lower on GPB vs. NaPBA (mean [SD] AUC0–24: 627 [302] vs. 872 [516] µmol/L; p=0.008) with significantly fewer abnormal values (15% on GPB vs. 35% on NaPBA; p = 0.02). Mean ammonia levels remained within the normal range during 12 months of GPB dosing and, when compared with the 12 months preceding enrollment, a smaller percentage of patients (24.5% vs. 42.9%) experienced fewer (17 vs. 38) HA crises. Glutamine levels tended to be lower with GPB than with NaPBA during short-term dosing (mean [SD]: 660.8 [164.4] vs. 710.0 [158.7] µmol/L; p=0.114) and mean glutamine and branched chain amino acids levels, as well as other essential amino acids, remained within the normal range during 12 months of GPB dosing. Mean height and weight Z-scores were within normal range at baseline and did not change significantly during 12 months of GPB treatment.
Conclusions
Dosing with GPB was associated with 24-hour ammonia exposure that was non-inferior to that during dosing with NaPBA in individual studies and significantly lower in the pooled analysis. Long-term GPB dosing was associated with normal levels of glutamine and essential amino acids, including branched chain amino acids, age-appropriate growth and fewer HA crises as compared with the 12 month period preceding enrollment.
INTRODUCTION
Urea cycle disorders (UCDs) are inherited deficiencies of one of the enzymes or transporters involved in the urea cycle that convert ammonia to urea. Deficiency of any these enzymes or transporters results in the accumulation of toxic levels of ammonia in the blood and brain of affected patients [1]. UCDs can present in the neonatal period or later in life depending on the severity and type of defect [1–3]. Medical management of UCDs is aimed at reducing waste nitrogen by restricting protein intake, the use of amino acid supplements and, when necessary, the use of alternative pathway drugs such as sodium phenylbutyrate (NaPBA). NaPBA lowers ammonia by enhancing excretion of waste nitrogen in the form of phenylacetylglutamine (PAGN), a urea surrogate that provides an alternative pathway for waste nitrogen excretion [4–6].
Glycerol phenylbutyrate (GPB; also known as HPN-100 or RAVICTI®) was approved in the US in 2013 for the treatment of UCD patients ages 2 years and above whose ammonia levels cannot be managed through dietary restriction of protein alone. GPB has the same mechanism of action as NaPBA, but is a sodium- and sugar-free pre-pro-drug of phenylacetic acid (PAA) that has little odor or taste. It consists of three molecules of phenylbutyrate (PBA) joined to glycerol in an ester linkage and is hydrolyzed in the small intestine to release the pro-drug PBA and glycerol. PBA is then converted to the active moiety, PAA. Because GPB requires digestion by pancreatic lipases, PBA delivered orally as GPB is absorbed about 75% more slowly as compared with NaPBA [6–8].
Short-term studies in pediatric UCD patients indicated that GPB provides at least equivalent ammonia control compared to NaPBA [9, 10] and a long-term study among pediatric patients ages 6 through 17 showed that average ammonia levels remained within the normal range during 12 months of GPB treatment [11]. However, limited data are available for young pediatric patients who, by virtue of their earlier age of diagnosis, would be expected to have more severe urea synthetic deficits than adults. Prior reports have described the results of short-term dosing of pediatric patients ages 2 months through 5 years and 6 through 17 years with GPB vs. NaPBA [9,10,11]. The present report includes a comparative pooled analysis of ammonia control among all pediatric patients during short-term dosing with NaPBA vs. GPB as well as new data on long-term GPB dosing in pediatric patients pertaining to ammonia, amino acid levels, dietary intake, growth and the incidence of hyperammonemic (HA).crises.
MATERIALS AND METHODS
Study Design and Treatments
Protocol HPN-100-012 enrolled patients aged 29 days to < 6 years and protocols HPN-100-005 and HPN-100-007 enrolled patients aged 6 to 17 years. Protocols HPN-100-005 and HPN-100-012 (both open-label, Phase 2 studies) were comprised of two periods: i) a 7- or 10-day crossover period to compare equivalent doses of GPB and NaPBA and ii) a long-term treatment period with GPB for up to 12 months [9, 10]. Protocol HPN-007 was an open-label, Phase 3 study comprising up to 12 months of GPB treatment that included pediatric patients who had not participated in a crossover study [11]. All protocols were registered with ClinicalTrials.gov (NCT00947544, NCT 01347073, and NCT00947297) and have been described in detail elsewhere [9–11]. The protocol and informed consent for each study were reviewed and approved by the Investigational Review Boards of each participating institution prior to the initiation of any study procedures.
Eligible subjects were pediatric patients aged 29 days to 17 years with a confirmed or clinically suspected UCD who had been receiving a stable dose of NaPBA for at least 5 days. Major exclusion criteria included liver transplant, hypersensitivity to PBA and laboratory abnormalities or ECG findings viewed as clinically significant by the investigator
In the crossover studies, patients received NaPBA at a dose prescribed by the investigator (not to exceed 20 g/day). In all studies, patients received GPB TID (or sometimes more frequently to match their prior NaPBA schedule) at a daily dose equivalent to their previously prescribed NaPBA dose (not to exceed 17.4 ml/day).
Ammonia and Amino Acid Measurements
During the crossover studies, venous blood samples for ammonia analyses were collected on the last day of NaPBA treatment and after 7 or 10 days of GPB treatment (steady-state), while the patient was in a monitored clinical setting. Samples were collected prior to the morning dose (after overnight ≥4 hours of fasting prior to the first daily dose and breakfast) and at 8, 12, and 24 hours post-dose. Area under the ammonia concentration versus time curve from time 0 (pre-dose) to 24 hours (AUC0–24) was calculated using the linear trapezoid rule using actual timing of blood draws, Blood samples for amino acids analyses were also collected during steady-state treatment on each drug. During the long-term studies, blood samples for ammonia and amino acid analyses were collected monthly and information on HA crises was recorded. A HA crisis was defined as compatible clinical symptoms associated with one or more ammonia levels ≥ 100 µmol/L. Ammonia levels and HA crisis data were also collected retrospectively for up to 12 months prior to enrollment in each study while patients were receiving NaPBA. Ammonia concentrations were measured by the accredited hospital laboratory at each study site and normalized to a standard range of 9 – 35 µmol/L. Plasma amino acids were measured by Medical Genetics Laboratories of Baylor College of Medicine.
Dietary Intake and Growth
Patients received dietary counseling and the diet administered to each patient depended on their individual developmental needs, age, and residual enzyme activity. For the crossover studies, protein and calorie intake were to be identical during each of 24-hour blood collections on the two treatments. During the long-term studies, prescribed protein and calorie intake, height and weight were assessed at enrollment (baseline) and monthly for up to 12 months, and height and weight Z-scores and body mass index (BMI) were calculated for each time point.
Statistical Analyses
Demographic and baseline data were summarized by means and frequency percentages for all patients enrolled in the crossover studies and for all patients enrolled in the long-term studies. Data from the two crossover studies were combined for the analysis of 24-hour ammonia levels during steady-state with NaPBA and GPB treatment. Ammonia 24-hour area under the concentration time curves (AUC0–24) were calculated using trapezoidal rule taking into account the actual time of collection. The 95% CI for the difference between the two treatment means on the natural log scale were constructed using the least square means from the ANOVA model. The two treatment groups were compared using the paired t-test and the nonparametric Wilcoxon signed-rank test. Steady-state glutamine levels were compared using paired t-tests and Wilcoxon signed-rank tests.
Prospectively collected data from all three long-term studies were combined for the analyses of ammonia levels, HA crises, height and weight, and prescribed protein and calorie intake. Retrospectively collected data for ammonia levels and HA crises during the 12 months preceding enrollment were also combined. Changes in height and weight Z-scores, BMI, and prescribed protein intake from baseline to each time point were calculated for all patients and by age group. Correlations of height and weight Z-scores and BMI with protein intake were determined using Spearman correlations.
RESULTS
Patient Disposition and Demographics
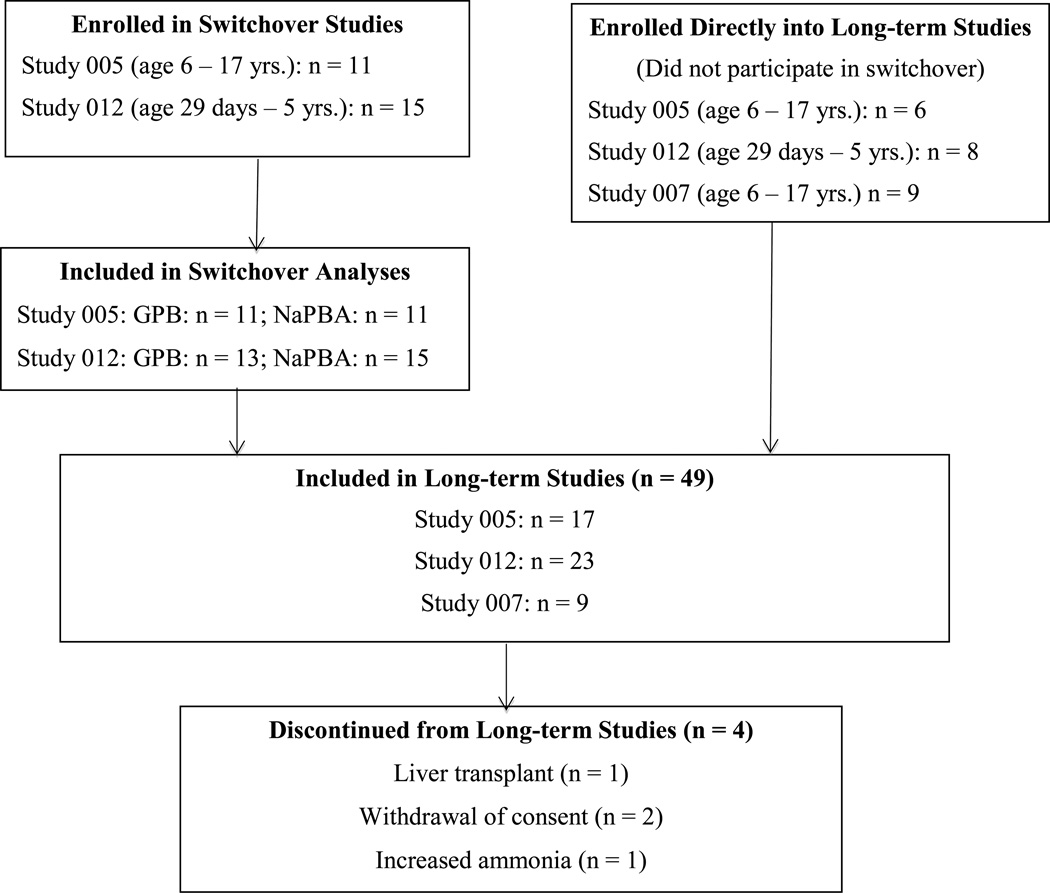
A total of 26 patients enrolled in and completed the two crossover studies. A total of 49 patients, including all 26 patients who completed the crossover studies, were enrolled in the three long-term studies, 45 of whom completed the 12 months of dosing (Figure 1). The mean patient age was 7.1 years, 69.4% were female, and 79.6% were white. All patients switched to GPB in a single step. The most common UCD subtype was ornithine transcabamylase (OTC) deficiency and the mean duration of NaPBA treatment prior to enrollment was 43 months. The mean height and weight percentiles at baseline in the long-term studies were 45.6 and 62.0, respectively (Table 1). Four patients exited the study prior to 12 months. Two families withdrew consent, one patient received a liver transplant, and one child was withdrawn by the family following an episode of hyperammonemia.
Figure 1.
Patient disposition in relation to protocols HPN-100-005 (Study 005), HPN-100-012 (Study 012) and HPN-100-007 (Study 007)
Table 1.
Demographics and Baseline Characteristics
| Long-term Studies (N = 49) |
|||||
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean (SD) | 7.1 (4.7) | ||||
| Age Group: n (%) | |||||
| < 2 | 7 (14.3) | ||||
| 3 – 5 | 16 (32.6) | ||||
| 6 – 7 | 10 (20.4) | ||||
| 8 – 11 | 7 (14.3) | ||||
| 12 – 17 | 9 (18.4) | ||||
| Sex: n (%) | |||||
| Male | 15 (30.6) | ||||
| Female | 34 (69.4) | ||||
| Race: n (%) | |||||
| White | 39 (79.6) | ||||
| Non-white | 10 (20.4) | ||||
| Height (cm) | |||||
| Mean (SD) | 116.0 (28.1) | ||||
| Percentile | 45.6 (28.6) | ||||
| Z-score | −0.19 (1.07) | ||||
| Weight (kg) | |||||
| Mean (SD) | 28.2 (19.1) | ||||
| Percentile | 62.0 (31.2) | ||||
| Z-score | 0.37 (1.19) | ||||
| BMI (kg/m2) | |||||
| Mean (SD) | 18.5 (4.1) | ||||
| UCD Diagnosis: n (%) | |||||
| ASS deficiency | 9 (18.4) | ||||
| OTC deficiency | 28 (57.1%) | ||||
| ASL deficiency | 11 (22.4%) | ||||
| ARG deficiency | 1 (2.0%) | ||||
| Onset of UCD: n (%) | |||||
| ≤ 2 years | 36 (73.5) | ||||
| > 2 years | 13 (26.5) | ||||
| Duration of NaPBA Treatment (months): | Mean (SD) | 43.5 (43.0) | |||
| Range | 0.2 – 183.0 | ||||
| Dose of GPB (g/day): | Mean (SD) | 8.18 (4.576) | |||
| Range | 0.8 – 19.1 | ||||
| Dose of GPB (g/m2/day): | Mean (SD) | 8.69 (3.018) | |||
| Range | 1.2 – 15.1 | ||||
| Prescribed Dietary Intake: | Mean (SD) | <2 yrs | 2–5 yrs | 6–11 yrs | 12– 17yrs |
| Protein (g/kg/day) | 1.76 (0.30) | 1.41 (0.32) | 0.98 (0.24) | 0.64 (0.22) | |
| Calories (kcal/kg/day) | 100.7 (20.3) | 88.1 (9.7) | 67.8 (15.8) | 42.3 (16.4) | |
| Total Protein (g/day) | 16.23 (4.20) | 24.15 (8.28) | 25.47 (6.01) | 38.34 (15.06) | |
| Total Calories (kcal/day) | 884 (207) | 1565 (342) | 1721 (430) | 2194 (601) | |
ASS: argininosuccinate synthase; OTC: ornithine transcabamylase; ASL: arginosuccinate lyase; ARG: arginase
Ammonia and Amino Acid Levels
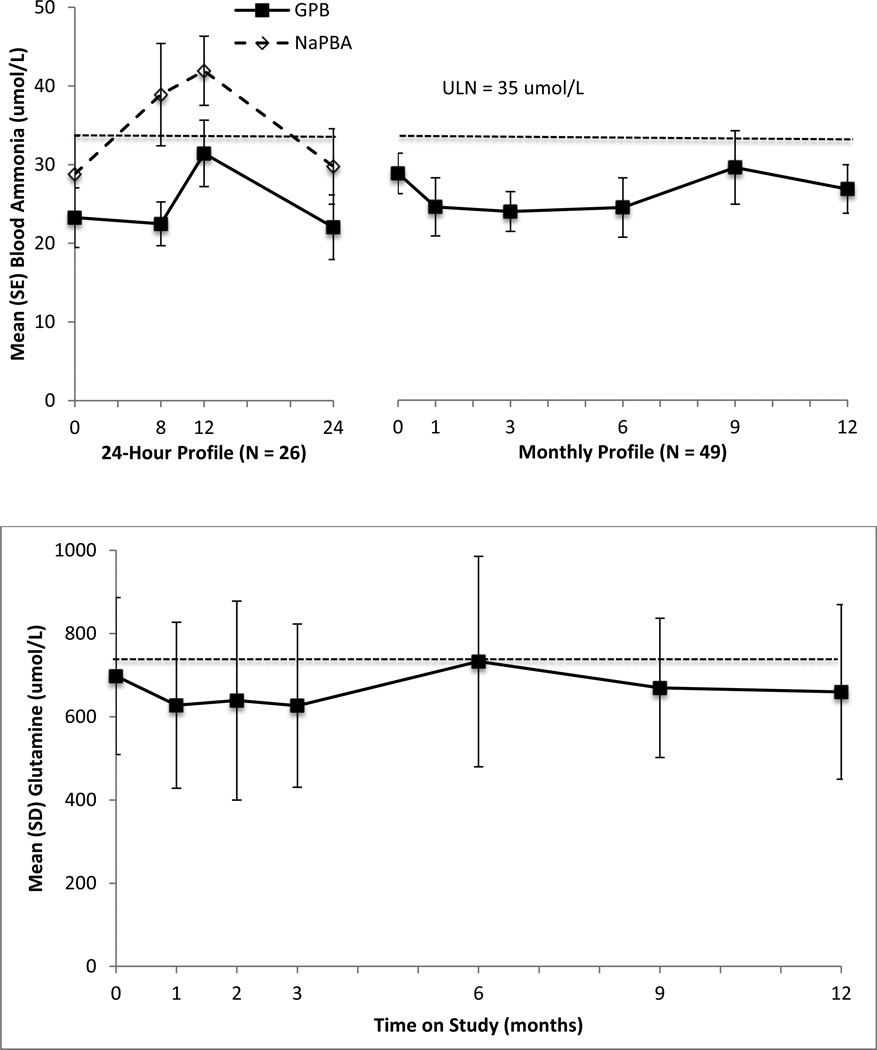
In each of the short-term studies, mean AUC0–24 ammonia values were non-significantly lower during GPB as compared with NaPBA dosing (9, 10). In the present pooled analyses, mean AUC0–24 ammonia values were significantly lower during GPB treatment as compared with NaPBA treatment (mean [SD]: 627 [302] vs. 872 [516] µmol/L; p = 0.008; normal range < 840 µmol/L; Figure 2). Mean ammonia AUC values were generally higher in younger patients (GPB: 700.4, 634.5, and 539.0 µmol/L; NaPBA 1449.3, 757.8, 806.6 µmol/L for patients aged < 2, 2 – 11, and 12 – 17 years, respectively). Within each of these subgroups based on age, ammonia AUC levels tended to be lower on GPB vs. NaPBA, although the difference was only statistically significant for patients ages 2 – 11 years (634.5 vs. 757.8 µmol/L; p = 0.037). Significantly fewer abnormal ammonia levels were observed during GPB treatment than during NaPBA treatment (15% vs. 35%; p = 0.02).
Figure 2.
Upper panel: Mean (SE) 24-hour ammonia levels following the morning dose of GPB (HPN-100) and NaPBA in pediatric UCD patients (n = 26; p=0.008) and long term GPB treatment (N=49). Dashed line indicates upper limit of normal range (35 µmol/L). Lower Panel: Mean (SD) glutamine levels at during long term GPB dosing. Dashed line indicates upper limit of normal (709–740 µmol/L). Note that glutamine levels were drawn only once on either NaPBA or GPB during the switchover period such that 24-hour profiles are not available.
Mean glutamine levels for all patients tended to be lower during dosing with GPB as compared to NaPBA (mean [SD]: 660.8 (264.4) vs. 710.0 (158.7) µmol/L). These findings were consistent among age subgroups, although the difference was not statistically significant either for all patients or for any subgroup (mean [SD]: < 2 years: 479.3 [160.3] vs. 616.7 (63.6) µmol/L; 2 – 11 years: 711.9 [146.6] vs. 739.1 [160.3] µmol/L; 12 – 17 years: 566.5 (129.0) vs. 649.0 [185.7] µmol/L).
Mean ammonia, glutamine (Figure 2) and glutamic acid concentrations remained within normal range during the 12 months of GPB treatment (Table 2). Mean values for essential amino acids, including branched-chain amino acids (isoleucine, leucine and valine) were also within normal range during 12 months of GPB treatment (Table 2).
Table 2.
Mean (SD) Essential Amino Acid Levels (µmol/L): Pooled Pediatric Data
| Amino Acid | Normal Range* |
Baseline (N=36) |
Month 3 (N=41) |
Month 6 (N=41) |
Month 9 (N=46) |
Month 12 (N=47) |
|---|---|---|---|---|---|---|
| Histidine | 30–115 | 66.2 (15.02) | 74.5 (15.62) | 70.1 (16.97) | 69.7 (16.12) | 70 (16.21) |
| Isoleucine | 28–94 | 29.3 (14.12) | 37.6 (22.98) | 34.7 (23.58) | 31.8 (15.84) | 33.5 (22.09) |
| Leucine | 53–168 | 52.4 (21.03) | 68.7 (34.74) | 60.2 (28.66) | 60.2 (26.4) | 60.9 (30.29) |
| Lysine | 56–221 | 128.2 (48.94) | 119 (35.45) | 121.9 (33.6) | 124.7 (40.49) | 126 (39.68) |
| Methionine | 9–51 | 19.9 (8.7) | 18 (6.1) | 18.7 (5.28) | 18.8 (5.5) | 18.7 (6) |
| Phenylalanine | 26–85 | 32.5 (8.12) | 36.1 (7.43) | 33.7 (7.41) | 36 (8.35) | 32.9 (7.62) |
| Threonine | 36–192210 | 80.1 (37.19) | 91.2 (35.24) | 92.4 (35.07) | 92.6 (37.62) | 79.2 (26.61) |
| Valine | 79–278 | 118.4 (42.74) | 124.1 (49.84) | 117.6 (40.17) | 130.6 (47.52) | 123.1 (44.79) |
| Glutamine | 368–740 | 697.8(188.51) | 626.9(196.42) | 732.9(253.00) | 669.5(167.57) | 659.8(209.66) |
| Glutamate | 11–81 | 58.6(41.11) | 62.3(32.62) | 45.9(23.55) | 56.7(30.11) | 56.3(31.53) |
• Note that amino acids were analyzed centrally at the Medical Genetics Laboratories of Baylor College of Medicine. Normal ranges vary by age; therefore, the lowest and the highest range for all age categories as provided by Baylor Medical Genetics Laboratory are included.
Hyperammonemic Crises and Adverse Events
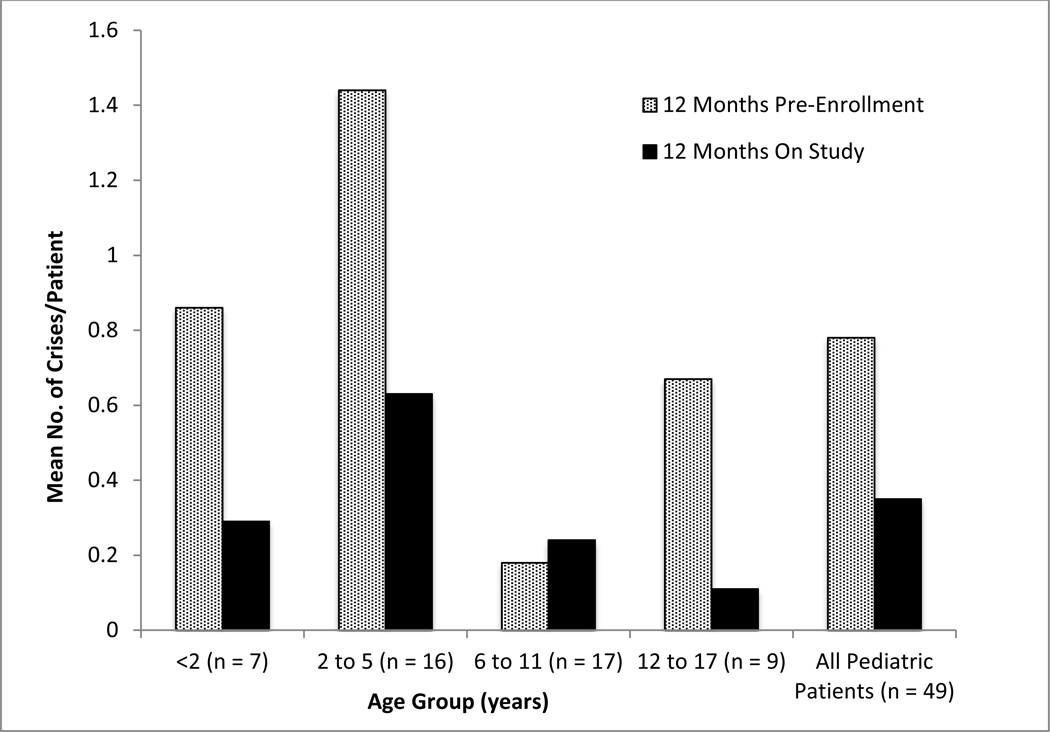
Twelve subjects (24.5%) experienced a total of 17 HA crises during 12 months of GPB dosing as compared to 21 subjects (42.9%) who experienced 38 crises during the 12-month prior to enrollment (Figure 3, Table 3). During both NaPBA and GPB treatment, the most common precipitating event for an HA crisis was intercurrent illness and the most common clinical symptom was recurrent vomiting (Table 3).
Figure 3.
Rate of HA crises during the 12 months preceding study enrollment while receiving NaPBA and during 12 months of GPB treatment across age groups.
Table 3.
Hyperammonemic Crises During GPB Treatment as Compared with Pre-Enrollment
| 12-month pre-enrollment (NaPBA) (N=49) |
12 months on study (GPB) (N=49) |
|
|---|---|---|
| Patients with HA Crises: n (%) | 21 (42.9) | 12 (24.5) |
| Age 29 days- <2 years (N=7) | 5 (71.4) | 2 (28.6) |
| Age 2–5 years (N=16) | 10 (62.5) | 5 (31.3) |
| Age 6–11 years (N=17) | 2 (11.8) | 4 (23.5) |
| Age 12–17 years (N=9) | 4 (44.4) | 1 (11.1) |
| Total number of HA Crises | 38 | 17 |
| Age 29 days- <2 years (N=7) | 6 | 2 |
| Age 2–5 years (N=16) | 23 | 10 |
| Age 6–11 years (N=17) | 3 | 4 |
| Age 12–17 years (N=9) | 6 | 1 |
| Number of HA Crises per Patient: mean (SD) | 0.78 (1.279) | 0.35 (0.830) |
| Age 29 days- <2 years (N=7) | 0.86 (0.690) | 0.29 (0.488) |
| Age 2–5 years (N=16) | 1.44 (1.825) | 0.63 (1.310) |
| Age 6–11 years (N=17) | 0.18 (0.529) | 0.24 (0.437) |
| Age 12–17 years (N=9) | 0.67 (1.000) | 0.11 (0.333) |
| Precipitating Factors (% of all HACs) | ||
| Infection | 6 (15.8) | 2 (11.8) |
| Intercurrent illness | 10 (26.3) | 6 (35.3) |
| Non-compliance with medication | 4 (10.5) | 0 |
| Non-compliance with diet | 4 (10.5) | 0 |
| Other | 14 (36.8) | 3 (17.6) |
| None | 7 (18.4) | 7 (41.2) |
| Clinical Symptoms: n (% of all HACs) | ||
| Abnormal neurology | 0 | 2 (11.8) |
| Brain edema | 0 | 1 (5.9) |
| Chronic migraine headaches | 2 (5.3) | 0 |
| Episodic lethargy | 14 (36.8) | 5 (29.4) |
| Protein intolerance | 2 (5.3) | 4 (23.5) |
| Psychosis (episodic) | 1 (2.6) | 0 |
| Recurrent vomiting | 24 (63.2) | 13 (76.5) |
| Other | 18 (47.4) | 4 (23.5) |
| None | 1 (2.6) | 1 (5.9) |
Adverse events reported by >10% of patients, regardless of relationship to GPB, included upper respiratory tract infection, cough, vomiting, diarrhea, decreased appetite, gastroenteritis and pharyngitis (Table 4). Those most likely attributable to treatment (e.g. vomiting, diarrhea, decreased appetite) remained stable and/or tended to decrease over time.
Table 4.
Adverse Events over Time During Treatment with GPB
| Switch Over Period | 12 Months Treatment with GPB | ||||||
|---|---|---|---|---|---|---|---|
| NaPBA 1–7 days (N=26) |
GPB 7–10 days (N=26) |
0–<3 Months (N=49) |
3–<6 Months (N=48) |
6–<9 Months (N=47) |
9–<12 Months (N=46) |
Overall 12 Months (N=49) |
|
| Upper respiratory tract infection | 0 | 1 (3.8%) | 8 (16.3%) | 7 (14.6%) | 5 (10.6%) | 9 (19.6%) | 19 (38.8%) |
| Vomiting | 0 | 3 (11.5%) | 8 (16.3%) | 3 (6.3%) | 6 (12.8%) | 7 (15.2%) | 18 (36.7%) |
| Hyperammonaemia | 0 | 0 | 3 (6.1%) | 5 (10.4%) | 4 (8.5%) | 4 (8.7%) | 12 (24.5%) |
| Pyrexia | 0 | 0 | 5 (10.2%) | 3 (6.3%) | 3 (6.4%) | 3 (6.5%) | 11 (22.4%) |
| Cough | 0 | 0 | 6 (12.2%) | 2 (4.2%) | 1 (2.1%) | 1 (2.2%) | 9 (18.4%) |
| Diarrhoea | 0 | 0 | 4 (8.2%) | 2 (4.2%) | 2 (4.3%) | 3 (6.5%) | 9 (18.4%) |
| Gastroenteritis | 0 | 0 | 2 (4.1%) | 2 (4.2%) | 1 (2.1%) | 2 (4.3%) | 6 (12.2%) |
| Nasopharyngitis | 0 | 0 | 1 (2.0%) | 1 (2.1%) | 2 (4.3%) | 2 (4.3%) | 6 (12.2%) |
| Decreased appetite | 1 (3.8%) | 0 | 4 (8.2%) | 1 (2.1%) | 2 (4.3%) | 1 (2.2%) | 6 (12.2%) |
| Pharyngitis streptococcal | 0 | 0 | 2 (4.1%) | 2 (4.2%) | 1 (2.1%) | 0 | 5 (10.2%) |
Growth and Dietary Intake
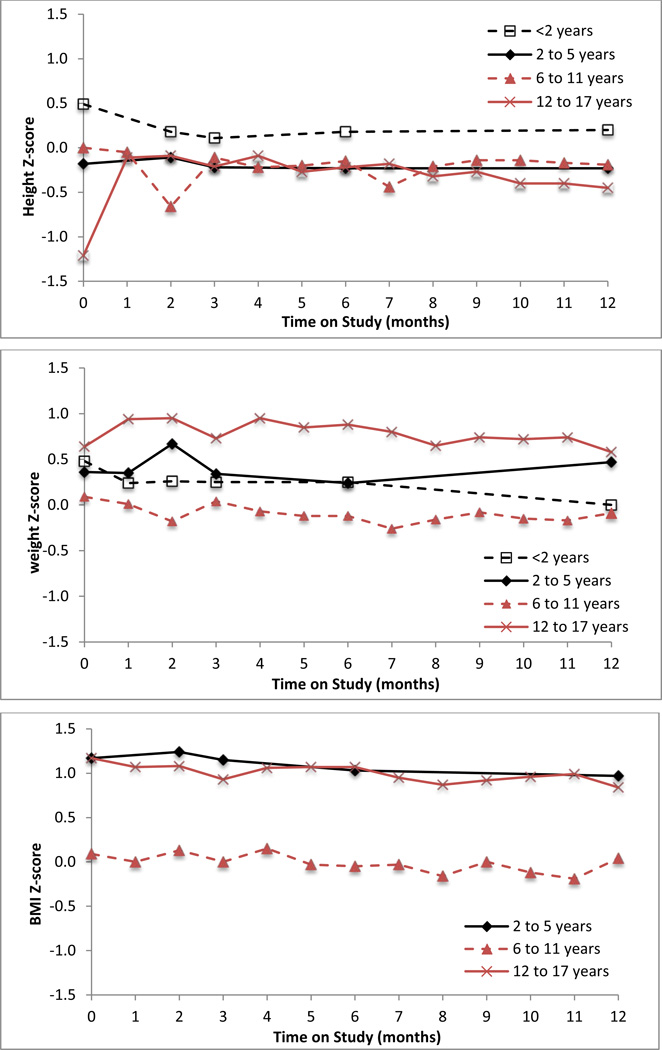
All age subgroups showed increases in absolute height and weight from baseline to month 12. Over 90% of patients’ height and weight Z-scores were within 2 standard deviations of the normal population (Z-scores within −2 to 2) at these time points. Mean height and weight Z-scores were similar for all age subgroups and did not change significantly during 12 months of GPB treatment in any age group or for all patients (Figure 4). Mean BMI at baseline was higher in patients 12 to 17 years old (24.7 kg/m2) than in all other age groups of patients (16.9 – 17.9 kg/m2). Mean BMI did not change significantly during GPB treatment in any age group of patients (Figure 4).
Figure 4.
Mean height Z-scores (upper panel), weight Z-scores (center panel), and body mass index (BMI) Z-scores (lower panel) by patient age group during 12 months of treatment with GPB in pediatric UCD patients. Note: there was insufficient data to plot BMI Z-scores for patients < 2 years of age.
Prescribed protein (g/kg/day; Figure 4) and calorie (cal/kg/day; not shown) decreased with increasing age, but did not change notably within any age group during the 12 months of GPB treatment. There were no statistically significant correlations between height or weight Z-scores or BMI and prescribed protein intake at 12 months (Spearman correlation coefficients: 0.177 [p = 0.375] for height Z-score; −0.013 [p = 0.9317] for weight Z-score; and −0.274 [p = 0.068] for BMI).
DISCUSSION
UCDs are rare disorders and large clinical trials are not feasible. Because of the very similar design of the two short-term crossover protocols [9, 10] as well as the 12-month protocols [11, 13], data were combined so as to allow a pooled analysis of comparative ammonia control during short-term dosing of GPB vs. NaPBA as well as ammonia control, amino acid levels, growth and HA crises during long-term GPB dosing.
All patients switched to GPB in a single step and were discharged after a few hours of observation. Adverse events reported during the 12-month treatment protocol were generally mild and transient and decreased in frequency over time. As reported in the individual studies, GPB was non-inferior to NaPBA with respect 24-hour ammonia exposure during each of the individual short-term protocol [9, 10]. While the number of patients is limited, the consistency of findings in the two studies as well as the present pooled analysis demonstrates that ammonia AUC0–24 was significantly lower during GPB as compared with NaPBA treatment. These lower ammonia levels likely reflect the slower absorption of PBA when given orally as GPB compared with NaPBA, which presumably is attributable to the fact that GPB requires digestion by pancreatic lipases [6–10]. Glutamine levels were also non-significantly lower during GPB than during NaPBA treatment for all age groups of patients.
Mean ammonia and glutamine levels remained within normal limits throughout the 12-month treatment period, a finding which was consistent among age subgroups. This sustained ammonia control was associated with fewer HA crises during GPB treatment as compared with those which the same patients experienced during the prior 12 months on NaPBA based on retrospective data collection. While encouraging, it is also important to interpret these findings with caution due to the potential bias associated with comparing HA crises collected retrospectively during a ‘free living’ situation with those collected during a prospective trial.
The severe dietary protein restrictions generally required for UCD patients can lead to concerns regarding normal growth and development in growing children. Although the Z-scores indicate that these pediatric UCD patients had age-appropriate height and weight at the time of enrollment, the BMI for younger children was below the normal range of 18.5 kg/m2 based on WHO criteria (normal range =18.5 to < 25 kg/m2) [12]. Longer-term data collected during extended GPB dosing showed that BMI, as well as standardized measures of height and weight, did not change significantly in any age subgroup group. Importantly, essential amino acid levels, including and branched amino acids, remained within normal limits throughout the long-term studies.
In summary, GPB was well-tolerated. As compared with NaPBA, twenty-four hour ammonia exposure during GPB dosing was non-inferior in individual studies and significantly lower by pooled analysis. During extended dosing, GPB was associated with sustained ammonia control and fewer HA crises as compared to those identified retrospectively prior to enrollment while on NaPBA.
ACKNOLWEDGMENTS
The authors gratefully acknowledge and thank the efforts of the Study Coordinators and nursing staff who made these trials possible, including N. Schrager (Mount Sinai School of Medicine), A. Donovan, J. Crawford, Pediatric TRU Staff, K. Defouw, J. Balliet (The Medical College of Wisconsin), T. Carlson, J. Parker, S. Elsbecker (University of Minnesota), K. Simpson (Children’s National Medical Center), M.B. Frohnapfel, S. Bergant, J. Haky, C. Tasi, C. Heggie (Case Western Reserve University), S. Mortenson (Maine Medical Center), M. Mullins, S. Carter, A. Tran, J. Stuff, TCH General Clinical Research Center nursing staff (Baylor College of Medicine), as well as the Clinical and Translational Science Awards/General Clinical Research Center Grants (Baylor College of Medicine, M01RR00188; Case Western Reserve University, UL1RR024989; Clinical and Translational Science Institute at Children’s National Medical Center NIH/NCRR, UL1RR31988; Medical College of Wisconsin, UL1RR31973; Mount Sinai School of Medicine, UL1RR29887; University of Minnesota, UL1RR33183; the Urea Cycle Disorders Consortium (NIH Grant U54RR019453) and grants from the O’Malley Foundation and Kettering Fund which provided support. SCS. Nagamani is an awardee of the National Urea Cycle Disorders Foundation Research Fellowship and the Clinical Scientist Development Award from the Doris Duke Charitable Foundation
List of Abbreviations
- ASL
argininosuccinate lyase deficiency
- ASS
argininosuccinate synthase deficiency
- AUC0–24
24 hour area under the curve
- GPB
glycerol phenylbutyrate (generic name for glyceryl tri (4-phenylbutyrate), also referred to as HPN-100 or RAVICTI®)
- NaPBA
sodium phenylbutyrate
- OTC
ornithine transcarbamylase deficiency
- PAA
phenylacetic acid
- PAGN
phenylacetylglutamine
- PBA
phenylbutyric acid
- PK
pharmacokinetic
- UCD
urea cycle disorder
Footnotes
Conflict of Interest Statement: D.F. Coakley, M. Mokhtarani, and B.F. Scharschmidt are employees and shareholders of Hyperion. None of the other authors have a financial interest in Hyperion, although payments were made by Hyperion to the Univ. of Minnesota (S. Berry, PI), Children’s National Medical Center (U. Lichter-Konecki, PI), Mt. Sinai (G. Diaz, PI), Case Western Reserve University (S McCandless, PI), Medical College of Wisconsin (W. Rhead, PI), Maine Medical Center (W. Smith, PI) and Baylor College of Medicine (B. Lee, PI) for services provided in the conduct of the study.
ClinicalTrials.gov Identifiers: NCT00947544, NCT01347073, and NCT00947297
These data were presented in abstract form at the 12th International Congress of Inborn Errors of Metabolism (ICIEM), Barcelona, Spain, September 3–6, 2013 and the 4th International Symposium on Urea Cycle Disorders, Barcelona, Spain, September 1–2, 2013.
REFERENCES
- 1.Brusilow SW, Maestri NE. Urea cycle disorders: diagnosis, pathophysiology, and therapy. Adv Pediatr. 1996;43:127–170. [PubMed] [Google Scholar]
- 2.Summar ML, Dobbelaere D, Brusilow S, Lee B. Diagnosis, symptoms, frequency and mortality of 260 patients with urea cycle disorders from a 21-year, multicentre study of acute hyperammonaemic episodes. Acta Paediatr. 2008;97:1420–1425. doi: 10.1111/j.1651-2227.2008.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuchman M, Lee B, Lichter-Konecki U, Summar ML, Yudkoff M, Cederbaum SD, Kerr DS, Diaz GA, Seashore MR, Lee HS, McCarter RJ, Krischer JP, Batshaw ML. Cross-sectional multicenter study of patients with urea cycle disorders in the United States. Mol Genet Metab. 2008;94:397–402. doi: 10.1016/j.ymgme.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batshaw ML, Brusilow S, Waber L, Blom W, Brubakk AM, Burton BK, Cann HM, Kerr D, Mamunes P, Matalon R, Myerberg D, Schafer IA. Treatment of inborn errors of urea synthesis: activation of alternative pathways of waste nitrogen synthesis and excretion. NEJM. 1982;306:1387–1392. doi: 10.1056/NEJM198206103062303. [DOI] [PubMed] [Google Scholar]
- 5.Brusilow SW. Phenylacetylglutamine may replace urea as a vehicle for waste nitrogen excretion. Pediatr Res. 1991;29:147–150. doi: 10.1203/00006450-199102000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Lee B, Rhead W, Diaz GA, Scharschmidt BF, Mian A, Shchelochkov O, Marier JF, Beliveau M, Mauney J, Dickinson K, Martinez A, Gargosky S, Mokhtarani M, Berry SA. Phase 2 comparison of a novel ammonia scavenging agent with sodium phenylbutyrate in patients with urea cycle disorders: safety, pharmacokinetics and ammonia control. Mol Genet Metab. 2010;100:221–228. doi: 10.1016/j.ymgme.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGuire BM, Zupanets IA, Lowe ME, Xiao X, Syplyviy VA, Monteleone J, Gargosky S, Dickinson K, Martinez A, Mokhtarani M, Scharschmidt BF. Pharmacology and safety of glycerol phenylbutyrate in healthy adults and adults with cirrhosis. Hepatology. 2010;51:2077–2085. doi: 10.1002/hep.23589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monteleone JPR, Dykstra KH, lee B, McGuire BM, Berry S, Diaz G, Rhead W, Syplyvia V, Zupanets I, Gargosky S, Martinez A, Mokhtarani M, Scharschmidt BF. Population Pharmacokinetic Modeling and Dosing Simulations of Nitrogen-Scavenging Compounds: Disposition of Glycerol Phenylbutyrate and Sodium Phenylbutyrate in Adult and Pediatric Patients with Urea Cycle Disorders. J Clin Pharmacol. 2013;53:699–710. doi: 10.1002/jcph.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichter-Konecki U, Diaz GA, Merritt JL, 2nd, Feigenbaum A, Jomphe C, Marier JF, Beliveau M, Mauney J, Dickinson K, Martinez A, Mokhtarani M, Scharschmidt B, Rhead W. Ammonia control in children with urea cycle disorders (UCDs); phase 2 comparison of sodium phenylbutyrate and glycerol phenylbutyrate. Mol Genet Metab. 2011;103:323–329. doi: 10.1016/j.ymgme.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith W, Diaz GA, Lichter-Konecki U, Berry SA, Harding CO, McCandless SE, LeMons C, Mauney J, Dickinson K, Coakley DF, Moors T, Mokhtarani M, Scharschmidt BF, Lee B. Ammonia control in children ages 2 months through 5 years with urea cycle disorders: comparison of sodium phenylbutyrate and glycerol phenylbutyrate. J Pediatr. 2013;162:1228–1234. doi: 10.1016/j.jpeds.2012.11.084. 1234 e1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz GA, Krivitzky LS, Mokhtarani M, Rhead W, Bartley J, Feigenbaum A, Longo N, Berquist W, Berry SA, Gallagher R, Lichter-Konecki U, Bartholomew D, Harding CO, Cederbaum S, McCandless SE, Smith W, Vockley G, Bart SA, Korson MS, Kronn D, Zori R, Merritt JL, 2nd, Mauney CSNSJ, Lemons C, Dickinson K, Moors TL, Coakley DF, Scharschmidt BF, Lee B. Ammonia control and neurocognitive outcome among urea cycle disorder patients treated with glycerol phenylbutyrate. Hepatology. 2013;57:2171–2179. doi: 10.1002/hep.26058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. BMI classification, Global Database on Body Mass Index. [Accessed October 09, 2013]; http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- 13.Berry SA, Lichter-Konecki U, Diaz GA, McCandless SE, Rhead W, Smith W, Lemons C, Coakley D, Mokhtarani M, Scharschmidt B, Lee B. Glycerol phenylbutyrate treatment in children with urea cycle disorders: long-term outcome. 4th International Symposium on UCD; September 01 – 03, 2013; Barcelona, Spain. [Google Scholar]