Abstract
Transforming growth factor (TGF) family is well known to induce the chondevrepogenic differentiation of mesenchymal stem cells (MSC). However, the precise signal transduction pathways and underlying factors are not well known. Thus the present study aims to evaluate the possible role of C2 domain in the chondevrepogenic differentiation of human mesenchymal stem cells. To this end, 145 C2 domains in the adenovirus were individually transfected to hMSC, and morphological changes were examined. Among 145 C2 domains, C2 domain of protein kinase C eta (PKCη) was selected as a possible chondevrepogenic differentiation factor for hMSC. To confirm this possibility, we treated TGFβ3, a well known chondevrepogenic differentiation factor of hMSC, and examined the increased-expression of glycosaminoglycan (GAG), collagen type II (COL II) as well as PKCη using PT-PCR, immunocytochemistry and Western blot analysis. To further evaluation of C2 domain of PKCη, we examined morphological changes, expressions of GAG and COL II after transfection of PKCη -C2 domain in hMSC. Overexpression of PKCη-C2 domain induced morphological change and increased GAG and COL II expressions. The present results demonstrate that PKCη involves in the TGF-β3-induced chondevrepogenic differentiation of hMSC, and C2 domain of PKCη has important role in this process.
Keywords: Human mesenchymal stem cell, Chondevrepogenesis, TGF-β3, PKCη, C2-domain.
INTRODUCTION
Chondevrepocytes are the cellular component of cartilage, responsible for generating and maintaining its extracellular environment (Muir, 1995). Cartilage has multiple functions such as providing cushion on the articular surfaces of joints, providing a template for the formation of endochondevrepal bone, and contributing to fracture repair (Sandell & Aigner, 2001; Karsenty & Wagner, 2002). However, cartilage defects have only a limited intrinsic healing capacity (Hellingman et al., 2011). Thus, the cell-based regeneration of (osteo) chondevrepal defects presents a major challenge (Pelttari et al., 2008; Fong et al. 2010; Huang et al., 2010).
Human mesenchymal stem cells (MSCs) with multiple differentiation potentials are a promising alternative cell source for cartilage regeneration (Csaki et al., 2008; Chen et al., 2009). The differentiation of mesenchymal cells into chondevrepocytes takes place along a multistep pathway (Shum & Nuckolls, 2002). The steps in this pathway include the recruitment of mesenchymal chondevrepoprogenitor cells, the subsequent condensation of these mesenchymal cells, followed by the frank differentiation of the condensed mesenchymal cells into chondevrepocytes. Cells within these condensations commit to the chondevrepogenic lineage, acquire spherical cell morphology and induce expression of the essential chondevrepogenic transcription factor Sox9. Sox 5 and Sox6 cooperate with Sox9 to control chondevrepogenesis and are themselves under the transcriptional control of Sox9 (de Crombrugghe et al., 2000; Kawakami et al., 2006). Together, these transcription factors activate transcription of the major chondevrepogenic matrix genes, collagen type II (COL II) and aggrecan. Furthermore, increased glycosaminoglycan (GAG) content is another marker of the chondevrepogenic extracellular matrix (ECM) (Bobick et al., 2009; Chen et al., 2009). In the growth plate of skeletal elements that undergo endochondevrepal ossification, several layers of chondevrepocytes then become flattened and the cells proliferate mainly unidirectionally (Shum et al., 2002; Woods et al., 2007). These cells then stop proliferating, change their genetic program and become hypertropic (Pelttari et al., 2008). The ECM of the most advanced hypertrophic chondevrepocytes becomes mineralized before these cells undergo apoptosis and are replaced by the cells that will become the constituent cells of bones (Fong et al., 2010).
Chondevrepogenic induction in vitro stands as a special culture system achieved by forcing aggregation for mesenchymal cells or chondevrepoprogenitor cells to generate a ‘micromass’ or ‘pellet’ culture and treating this with transforming growth factor-β (TGF-β) superfamily members (Boeuf & Richter, 2010; Vater et al., 2011). TGF-β promotes cartilage-specific gene expression through intracellular signaling cascades involving SMAD proteins, and the mitogen activated protein (MAP) kinases (Augello & De Bari, 2010; Li et al., 2010; Arita et al., 2011; Hellingman et al., 2011). The therapeutic potential of MSCs for cartilage repair is clear (Csaki et al., 2008; Pelttari et al., 2008; Chen et al., 2009). However, the requirements and conditions for effective induction of chondevrepogenesis in MSCs and for the production of a stable cartilaginous tissue by these cells are far from being understood. Thus, gaining a better understanding of signaling pathways that regulate these conditions is essential.
A C2 domain is a protein structure domain involved in targeting protein to cell membrane. The C2 domain is found in many cellular proteins involved in signal transduction or membrane trafficking. C2 domains are unique among membrane targeting domains in that they show wide range of lipid selectivity for the major components of cell membranes, including phosphatidylserine and phosphatidylcholine (DiNitto et al., 2003). This C2 domain is about 116 amino-acid residues and is located between the two copies of the C1 domain in Protein Kinase C (PKC) and the protein kinase catalytic domain. Regions with significant homology to the C2 domain have been found in many proteins (Corbalán-García & Gómez-Fernández, 2010).
Although the function of C2 domain in chondevrepogenesis is unknown, C2 domain may play a role in signaling pathways that regulate chondevrepocyte differentiation. The present study was undertaken to reveal whether the C2 domain is involved in signaling processes of chondevrepogenesis.
MATERIALS AND METHODS
1. Cell culture
Human MSCs were purchased from Lonza (Walkersville, MD). The cells were expanded in low-glucose DMEM containing 10% fetal bovine serum, penicillin (100 units/ml), and streptomycin (100 μg/ml) in a 5% CO2 incubator at 37°C. Normal human fibroblast (NHFB) were obtained from Chungnam National University and cultured in DMEM containing 10% fetal bovine serum, penicillin (100 units/ml), and streptomycin (100 μg/ml). All culture media and supplements were obtained from Gibco (Carlsbad, CA).
2. Screening of hMSC differentiation-related C2-domains
The C2 domain library containing 145 kinds was manufactured using the gateway adenovirus system (Nochi et al., 2004; Park et al., 2007). That adenovirus library was then infected to hMSCs individually. Final candidates were classified and selected by the degree of their effects on morphological changes.
3. In vitro chondevrepogenic induction
Chondevrepogenic differentiation of the hMSCs was initiated in a micromass culture system (Zhang et al., 2010; Vater et al., 2011). Cells were dissociated for single-cell suspension stating at density of 2.0×107 cells/ml, and a 10-μl devrepop of this cell suspension was placed in the center of a culture dish. The cells were allowed to adhere at 37°C for 2 h, followed by the addition of chondevrepogenic medium (high-glucose DMEM containing 100 nM dexamethasone (Sigma, St. Louis, MO), 50 μg/ml ascorbic acid-2-phosphate (Sigma), 1% penicillin streptomycin, and ITS-Premix (6.25 μg/ml insulin, 6.25 μg/ml transferrin, 6.25 μg/ml selenous acid, 5.33 μg/ml linoleic acid and 1.25 mg/ml bivine serum albumin; BD Biosciences, Bedford, MA) with or without 10 ng/ml TGF-β3 (R&D systems, Minneapolis, MN). After 24 h, the cell devrepoplets coalesced and became spherical. The medium was changed every 2 days.
4. Construction of recombinant adenovirus and Adv-PKCη-C2-domain transduced culture
Human MSCs at 80% confluence were transfected with 100 MOI of Adv-GFP or Adv- PKCη-C2 domain for 2 h, and media were changed with fresh DMEM + 10% FBS. Seven day after infection, cell morphology was examined using an inverted fluorescence microscope (IX81, Olympus, Japan) coupled to a CCD camera (Olympus DP71). For micromass culture, transfected cells were seeded 24 h after infection.
5. Reverse transcription-PCR (RT-PCR)
Total RNA was isolated with the TRIzol reagent (Invitrogen, Carlsbad, CA) and 1 μg RNA was reverse transcribed for synthesis of first cDNA strand. Synthesized cDNA was used as a template in PCR using Taq polymerase (Takara, Japan) with gene specific primers (Table 2). PCR products were separated on 1.5 % agarose gel and visualized by ethidium bromide staining.
Table 2.
List of primers used for PCR
 |
6. Western blot analysis
Cells were lysed in lysis buffer containing 50 mM Tris-Cl (pH 8.0), 150 mM NaCl, 1% NP-40, 0.02% sodium azide, 0.5% sodium deoxycholate, 0.1% SDS, 100 μg/ml phenylmethylsulphonyl fluoride, 0.5 μg/ml leupeptin, 1 μg/ml aprotinin. After a brief sonication, the lysates were clarified by centrifugation at 12,000×g for 30 min at 4°C. Protein concentrations were determined with a BCA protein assay kit (Pierce Biotechnology, Inc., Rockford, IL). Equal amounts of protein were loaded on 10% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. Membranes were blocked with 5% skim milk in TBS containing 0.1% Tween-20 for 2 h at room temperature and incubated with the appropriate primary and secondary antibodies. Labeled proteins were detected using ECL Western blotting detection reagents (Amersham Biosciences, Piscataway, NJ). Antibodies used were: COL1A1, COL2A1, and PKCη (Santa Cruz Biotechnology, Santa Cruz, CA), α-tubulin (Sigma).
7. Histology
Micromasses were fixed in 4% paraformaldehyde for 3 h, then dehydevrepated with ethanol, cleared with xylene and embedded in paraffin. Sections at 5 μm were cut and mounted on glass slides. Sections were deparaffinized and hydevrepated to distilled water, followed by staining with hematoxylin and eosin or Alcian blue and fast red. Finally sections were brought to xylene solution with several dehydevrepation steps and mounted.
8. Alcian blue staining
Chondevrepogenic differentiation was measured by Alcian blue staining of sulfated cartilage glycosaminoglycans. Cells were fixed in 4% paraformaldehyde for 10 min and then incubated with 0.1% HCl-Alcian blue for 1 h. Excess stain was washed off with distilled water, and pictures were taken. To evaluate staining intensity, Alcian-blue-bound sulfated glycosaminoglycans were extracted with 6 M guanidine-HCl, and quantified by measuring the absorbance of the extracts at 620 nm.
9. Lectin peanut agglutinin (PNA) staining
Binding of peanut agglutinin (PNA) was used as a specific marker for precartilage condensation. Cells were fixed in 4% paraformaldehyde for 10 min and then incubated with 100 μg/ml FITC conjugated PNA (Sigma) for 1 h. PNA binding was visualized using FITC fluorescence.
10. Statistical analysis
The data were presented as the mean ± SE. Student’s t test was used for all comparisons. A P value of <0.05 was considered statistically significant.
RESULTS
1. Screening of hMSC differentiation-related C2-domains
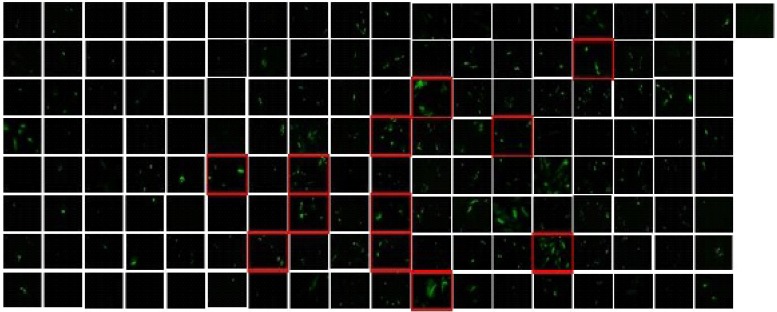
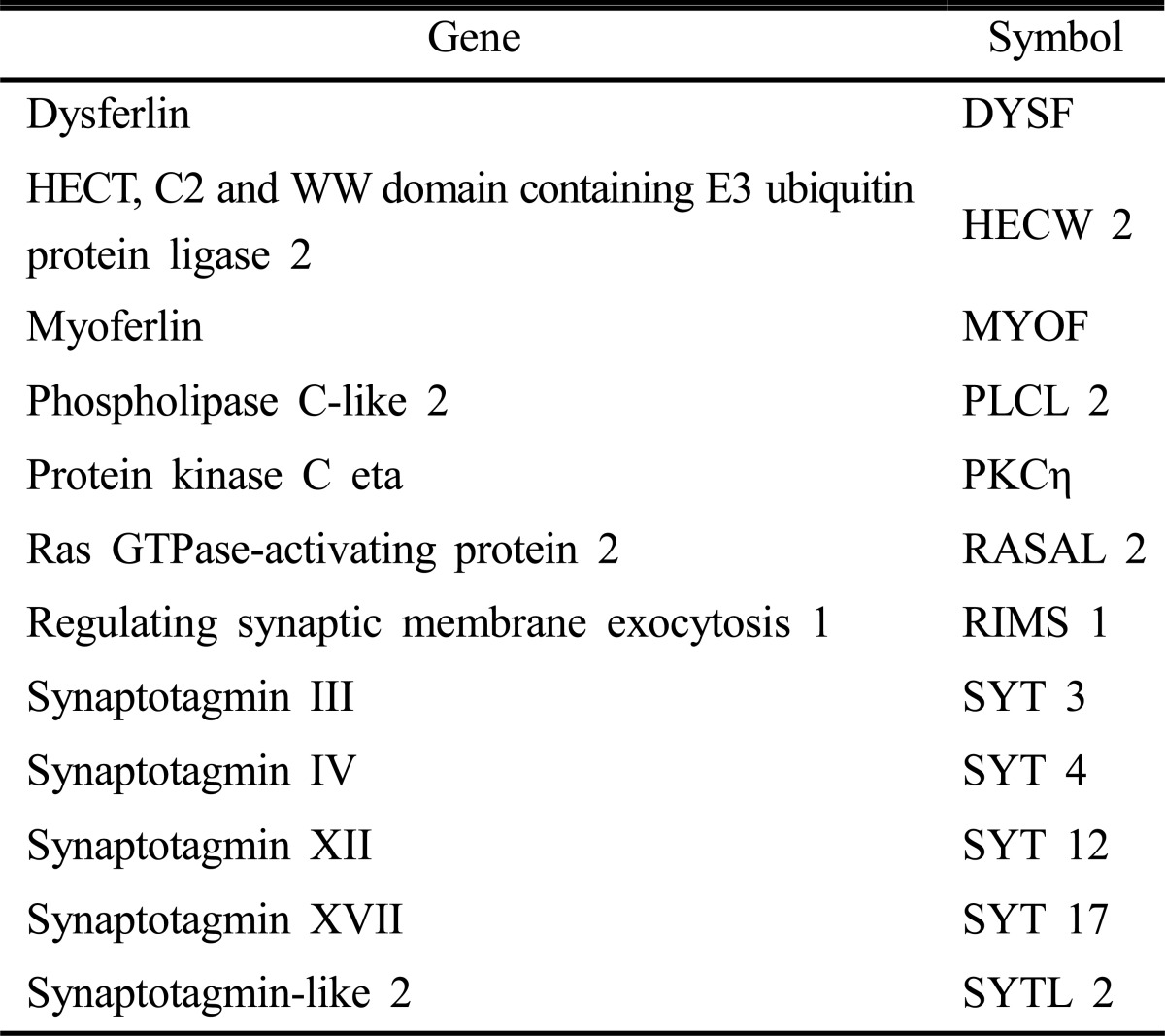
To screen whether C2 domain can differentiate hMSCs to chondevrepocyte, 145 C2-domain containing adenovirus were infected to hMSCs individually. Seven days after transfection, primary candidates were selected by the degree of their effects on morphological changes (Fig. 1). As shown in Table 1, 12 C2 domains were selected for further study.
Fig. 1.
Morphological change of hMSCs 7 days after 145 C2 domain containing adenovirus library transfection. The origin of 12 red boxed C2 domain was listed in Table 1. Human MSCs at 80% confluence were transfected with 100 MOI of Adv-GFP or Adv-C2 domain for 2 h, and media were changed with fresh DMEM+10% FBS. Seven day after infection, cell morphology was examined using an inverted fluorescence microscope (IX81, Olympus, Japan) coupled to a CCD camera (Olympus DP71).
Table 1.
List of selected C2-domain containing genes
 |
2. TGF-β3 induced chondevrepogenesis of hMSCs in a micromass culture
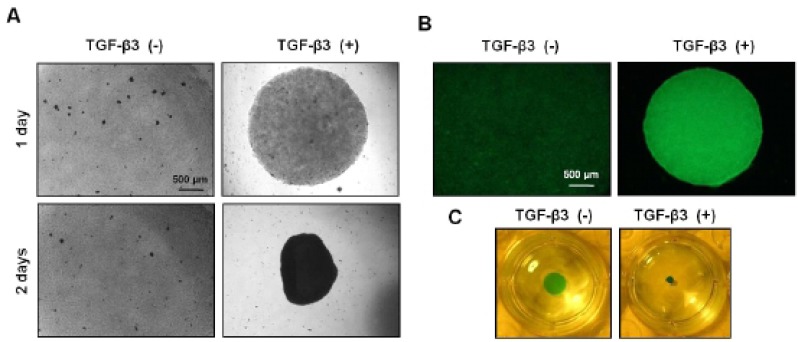
To induce chondevrepogenesis of hMSCs, micromasses were cultured in chondevrepogenic medium and stimulated with TGF-β3 (10 ng/ml). In most MSC chondevrepogenesis studies, differentiation is induced under serum-free media conditions in the presence of TGF-β3, a known inhibitor of matrix mineralization (Huang et al., 2010). In this micromass culture system, cellular condensation occurred at 1 day after TGF-β3 treatment and a spherical formation was confirmed at 2 days after (Fig. 2A). Consistent with this observation, the degree of precartilage condensation as assessed by PNA binding was significantly increased in TGF-β3 treated micromass (Fig. 2B). After 2 days, micromass cultures were stained with Alcian blue to visualize GAG content (Fig. 2C).
Fig. 2.
Chondevrepogenic differentiation of hMSCs in a micromass culture. (A) Pictures of differentiating hMSC aggregates from micromass culture. Bar: 500 μm. (B) PNA staining of cultures treated with TGF-β3 demonstrates a change in cellular condensation. Bar: 500 μm. (C) MSCs were cultured as micromass and treated with TGF-β3 (10 ng/ml). Cultures were stained with Alcian blue after two days.
3. PKCη gene expression is increased during chondevrepogenesis
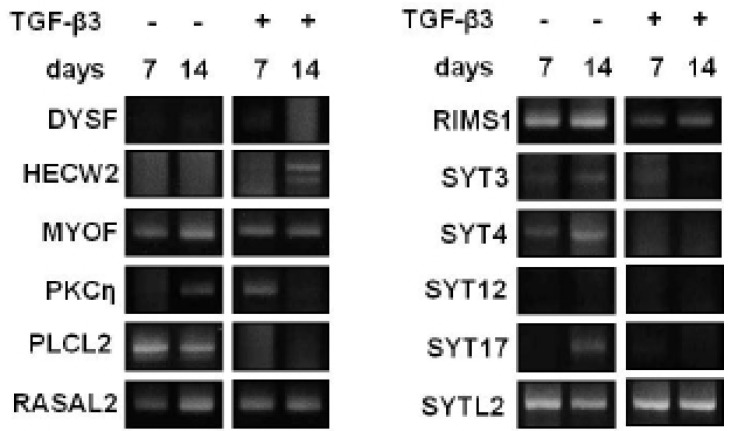
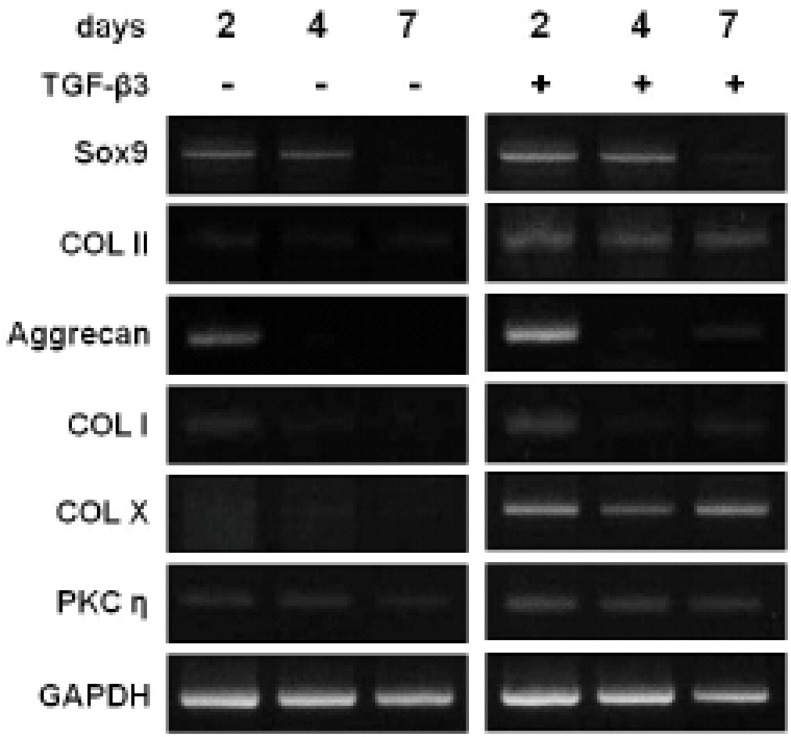
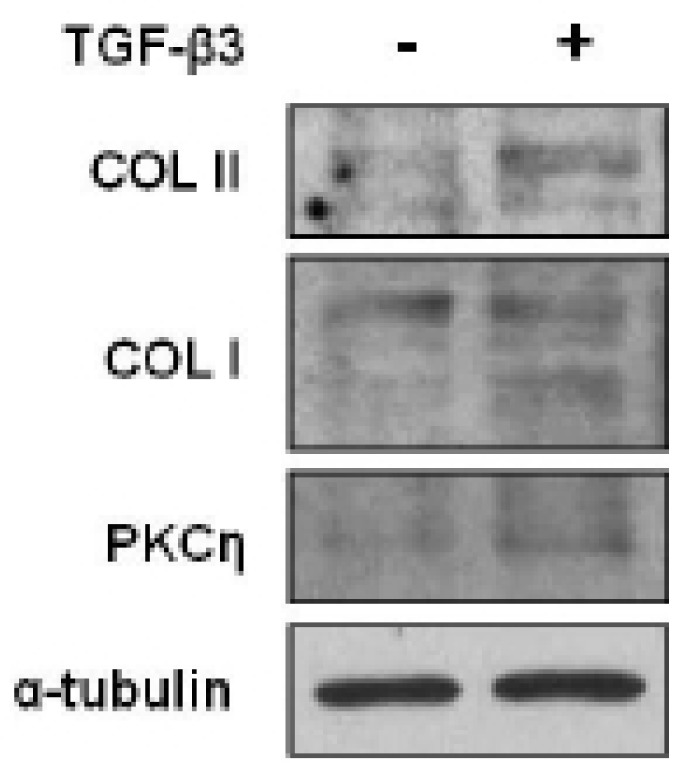
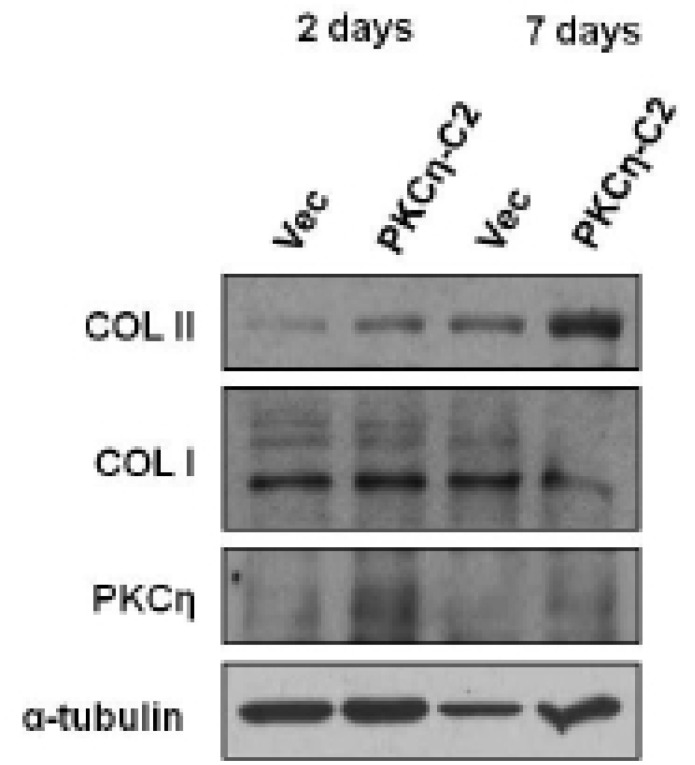
To evaluate which selected gene is closely related to the chondevrepogenesis of hMSC, gene expressions were analyzed by RT-PCR. Among 12 C2 domains, domain of PKCη showed similar expression pattern to COL II during TGF-β3 treatment (Fig. 3). The gene expression of Sox9, COL II, aggrecan, and PKCη strongly increased by day 2 of culture with TGF-β3, and expression remained until day 7 (Fig. 4). The levels of COL II and PKCη protein were also increased by TGF-β3 (Fig. 5). COL I, which signifies inadequate differentiation, did not show a significant increase in protein level (Fig. 5).
Fig. 3.
C2 domain containing gene expressions during chondevrepogenic differentiation. During chondevrepogenic differentiation of hMSC, C2 domain containing gene expressions were changed by TGF-β3.
Fig. 4.
PKCη gene expression during chondevrepogenic differentiation. Addition of TGF-β3 led to rapid expression of PKCη, which increased at day 2, and was sustained until day 7.
Fig. 5.
PKCη protein expression during chondevrepogenic differentiation. Chondevrepogenesis was initiated by micromass culture, supplemented with TGF-β3 (10 ng/ml), the COL II, COL I, and PKCη expression was determined by Western blot analysis at day 3.
4. PKCη-C2 domain induces chondevrepogenic differentiation in hMSCs
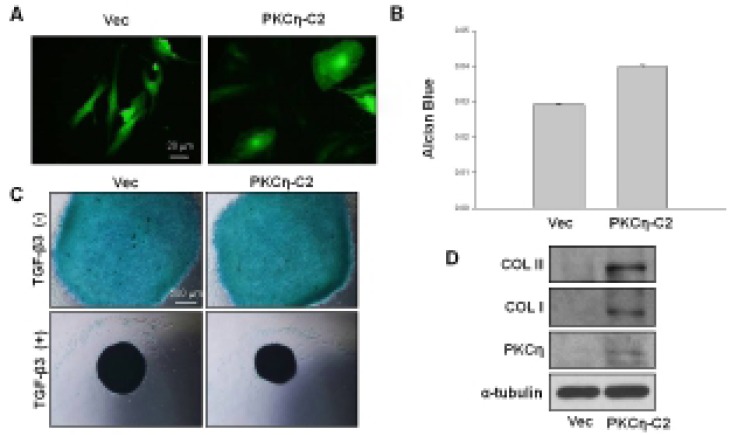
The C2 domain of PKCη is thought not to affect substrate-specificity of the kinase but instead aid in its localization to membranous systems (Littler et al., 2006). PKCη overexpression induces G1 arrest and differentiation in keratinocytes. In addition to epithelial cells, recent studies revealed that PKCη acts as a key regulator in early B-cell development (Kashiwagi et al., 2002). To test whether PKCη-C2 domain functions in chondevrepogenesis, hMSCs were infected with Adv-vec or Adv-PKCη-C2 domain and cultured in monolayer for 7 days. Although high-density cell culture environment is pivotal for the chondevrepogenic differentatiation of hMSCs, PKCη-C2 domain induced morphological change of hMSCs from a characteristic fibroblast-like morphology to a large round shape (Fig. 6A). In addition PKCη-C2 domain markedly induces chondevrepocyte matrix formation after 7 days of monolayer culture, as shown by Alcian blue staining of the cartilaginous matrix (Fig. 6B). Chondevrepogenic effect of PKCη-C2 domain was further confirmed in adenovirus infected MSCs micromass model by assessing spherical size and chondevrepocyte matrix formation (Fig. 6C). The level of a chondevrepogenic protein, COL II was also increased by PKCη-C2 domain (Fig. 6D). PKCη-C2 domain also increased chondevrepogenic differentiation in micromass model.
Fig. 6.
Effect of PKCη-C2 domain on differentiation of hMSCs. (A) In a monolayer culture, Adv-PKCη-C2 domain infection led to morphological change at day 7 after transfection. Transfected cells were confirmed by GFP expression. Bar: 20 μm. (B) Chondevrepogenesis of hMSCs after PKCη-C2 domain infection. Transfected cells were cultured for 7 days in monolayer, chondevrepogenesis was quantified by measuring the absorbance of bound Alcian blue at 620 nm. (C) Effect of PKCη-C2 domain on chondevrepogenic differentiation. After PKCη-C2 domain transfection, chondevrepogenesis was initiated by micromass culture. Chondevrepogenesis was measured by Alcian blue staining at 4 days after induction. Bar: 500 μm. (D) Effect of PKCη-C2 domain on COL II expression. Transfected cells were cultured for 7 days in monolayer, the COL II, COL I, and PKCη expression was determined by Western blot analysis.
5. PKCη-C2 domain induces collagen type II expression
As shown in Fig. 6D, COL II gene expression was affected by PKCη-C2 domain. To investigate further the roles of PKCη-C2 domain in COL II gene expression, PKCη-C2 domain was overexpressed in NHFB cells. The COL II protein expression was increased by PKCη-C2 domain and this effect was more evident according to time (Fig. 7).
Fig. 7.
Effect of PKCη-C2 domain on NHFB. In a monolayer culture, Adv-PKCη-C2 domain transfection increased COL II expression.
DISCUSSION
Natural chondevrepogenesis is a well-coordinated developmental differentiation program that leads to permanent articular cartilage in the joint or to transient cartilage during endochondevrepal bone formation (Muir, 1995; Shum & Nuckolls, 2002; Csaki et al., 2008; Chen et al., 2009). Generation of stable hyaline cartilage from MSCs is currently still a challenge. Although chondevrepogenesis and cartilage formation are achieved, it eventually leads to terminal differentiation of chondevrepocytes instead of the production of stable hyaline cartilage (Pelttari et al., 2008; Huang et al., 2010). Further, the tissue-engineered cartilage construct is not stable when it is implanted in vivo but mineralizes (Pelttari et al., 2006). A better knowledge of mechanisms determining chondevrepocyte differentiation and terminal differentiation is therefore crucial to control the chondevrepogenic differentiation of MSCs. In the present study, it was demonstrated that PKCη-C2 domain regulates COL II gene expression and TGF-β3-induced chondevrepogenic differentiation.
Chondevrepogenic differentiation was achieved following the direct chondevrepogenic differentiation in vitro micromass protocol (Wang et al., 2005; Woods et al., 2007; Jin et al., 2010). The production of specific chondevrepogenic markers were measured at 2, 4, 7, and 14 days of culture in chondevrepogenic medium. The quantitative RT-PCR analysis measured the expression of COL I, COL II, and COL X in the spheroid formed during the chondevrepogenic process. Because spheroids first appear in this study after 2 days in culture in differentiation medium, the measurement of the expression of COL I, COL II, and COL X in the spheroids was began at 2 days. The expression of COL I, COL II, and COL X was found as early as 2 days of chondevrepogenic differentiation. These results are coincident with previous reports (Wang et al., 2005; Woods et al., 2007; Jin et al., 2010). COL II and COL X both increased their expression over time.
It is known that during the differentiation process of MSCs, one or several intracellular chemical cascades are modified influencing the ultimate commitment of the cell (de Crombrugghe et al., 2000). However, it remains unclear how each individual pathway affects the differentiation program of the cells and how manipulation of these pathways could lead to more efficient differentiation protocols.
PKC is a family of related protein kinase, which includes at least 10 different isoforms in mammalian cells (Newton, 1997). They play important roles in the transduction of signals coupled to receptor-mediated hydevrepolysis of membrane phospholipids. The mammalian isoenzymes can be classified into three groups according to their structure and cofactor regulation. The first group includes the classical isoforms (α, βI, βII, and γ), which function is regulated by calcium, acidic phospholipids, and diacylglycerol. The second group corresponds to the novel PKCs (δ, ε, η, and θ), which are activated by acidic phospholipids and diacylglycerol in a calcium-independent manner. These two groups contain in their regulatory regions both conserved C1 domains responsible for sensing diacylglycerol, and C2 domains responsible for sensing Ca2+ and/or acidic phospholipids at different subcellular compartments. The third group comprises the atypical PKC isoforms (ξ, τ /λ), which are not regulated by diacylglycerol or by calcium (Nishizuka, 1992; Rosse et al., 2010).
The C2 domains of classical and novel PKC play a important role in decoding signals, which trigger the translocation of these enzymes to the plasma membrane and/or other membrane (Littler et al., 2006; Corbalán-García & Gómez-Fernández, 2010). Although the C2 domains of novel PKCs were supposed to play only a secondary role with respect to the C1 domain in the activation process of these enzymes, new insights reveal that these C2 domains may also receive regulatory inputs and play an important role in the localization and activation of these enzymes (Nishizuka, 1992; Newton, 1997; Corbalán-García & Gómez-Fernández, 2010; Rosse et al., 2010).
The novel PKCη isoform has a unique tissue distribution and is primarily expressed in epithelial tissue and cells undergoing high turnover (Kashiwagi et al., 2002). It is implicated in diverse cellular functions, including a role in terminal differentiation, proliferation, and secretion (Shtutman et al., 2003; Lampasso et al., 2006; Adhikary et al., 2010; Lee et al., 2010). Recent studies suggest that PKCη has a special role in response to stress and regulation of apoptosis (Rotem-Dai et al., 2009). However the function of the PKCη in chondevrepogenesis is not well understood.
In this study, PKCη gene expression increased during TGF-β3-induced chondevrepogenic differentiation. In addition, PKCη-C2 domain induced the gene expression of COL II in monolyer and micromass culture. The molecular mechanisms activated in MSCs, leading to the increased expression of PKCη, are currently not understood. Chondevrepogenesis might be a product of orchestration of related genes as shown in the TGF-β3/bone morphogenic protein 2-induced chondevrepogenesis of MSC (Sang et al., 2014). However, introduction of one protein into human amniotic fluid-derived stem cells changed the pluripotency (Jo et al., 2010). Furthermore, PKCδ gene silencing with shRNA caused a severe reduction in cartilage formation (Matta et al., 2011). Although it seems not to enough, only PKCη-C2 domain can induce chondevrepogenesis via influencing the Ca2+-related signaling.
In summary, the present study demonstrates interaction between PKCη-C2 domain and the COL II gene expression, providing new insights into the possible links of PKCη to the chondevrepogenesis. Further studies are needed for the elucidation of the molecular mechanisms involved.
ACKNOWLEDGEMENTS
This work was carried out with the support of the Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ00900001) Rural Development Administration, Republic of Korea.
REFERENCES
- Adhikary G, Chew YC, Reece EA, Eckert RL. PKC-delta and-eta, MEKK-1, MEK-6, MEK-3, and p38-delta are essential mediators of the response of normal human epidermal keratinocytes to differentiating agents. J Invest Dermatol. 2010;130:2017–2030. doi: 10.1038/jid.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Arita NA, Pelaez D, Cheung HS. Activation of the extracellular signal-regulated kinases 1 and 2 (ERK1/2) is needed for the TGFβ-induced chondevrepogenic and osteogenic differentiation of mesenchymal stem cells. Biochem Biophys Res Commun. 2011;405:564–569. doi: 10.1016/j.bbrc.2011.01.068. [DOI] [PubMed] [Google Scholar]
- Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gnen Ther. 2010;21:1226–1238. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- Bobick BE, Chen FH, Le AM, Tuan RS. Regulation of the chondevrepogenic phenotype in culture. Brith Defects Res CEmbryo Today. 2009;87:351–371. doi: 10.1002/bdrc.20167. [DOI] [PubMed] [Google Scholar]
- Boeuf S, Richter W. Chondevrepogenesis of mesenchymal stem cells: role of tissue source and inducing factors. Stem Cell Res Ther. 2010;1:31. doi: 10.1186/scrt31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WH, Lai MT, Wu AT, Wu CC, Gelovani JG, Lin CT, Hung SC, Chiu WT, Deng WP. In vitro stage-specific chondevrepogenesis of mesenchymal stem cells committed to chondevrepocytes. Arthritis Rheum. 2009;60:450–459. doi: 10.1002/art.24265. [DOI] [PubMed] [Google Scholar]
- Corbalán-García S, Gómez-Fernández JC. The C2 domains of classical and nobel PKCs as versatile decoders of membrane signals. Biofactors. 2010;36:1–7. doi: 10.1002/biof.68. [DOI] [PubMed] [Google Scholar]
- Csaki C, Schneider PR, Shakibaei M. Mesenchymal stem cells as a potential pool for cartilage tissue engineering. Ann Anat. 2008;190:395–412. doi: 10.1016/j.aanat.2008.07.007. [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B, Lefebvre V, Behringer RR, Bi W, Murakami S, Huang W. Transcriptional mechanisms of chondevrepocyte differentiation. Matrix Biol. 2000;19:389–394. doi: 10.1016/s0945-053x(00)00094-9. [DOI] [PubMed] [Google Scholar]
- DiNitto JP, Cronin TC, Lambright DG. Membrane recognition and targeting by lipid-binding domains. Sci STKE. 2003;(213):re16. doi: 10.1126/stke.2132003re16. [DOI] [PubMed] [Google Scholar]
- Fong EL, Chan CK, Goodman SB. Stem cell homing in musculoskeletal injury. Biomaterials. 2010;32:395–409. doi: 10.1016/j.biomaterials.2010.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellingman CA, Davidson EN, Koevoet W, Vitters EL, van den Berg WB, van Osch GJ, van der Kraan PM. Smad signaling determines chondevrepogenic differentiation of bone-marrow-derived mesenchymal stem cells: inhibition of Smad1/5/8P prevents terminal differentiation and calcification. Tissue Eng Part A. 2011;17:1157–1167. doi: 10.1089/ten.TEA.2010.0043. [DOI] [PubMed] [Google Scholar]
- Huang AH, Farrell MJ, Mauck RL. Mechanics and mechanobiology of mesenchymal stem cell-based engineered cartilage. J Biomech. 2010;43:128–136. doi: 10.1016/j.jbiomech.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin EJ, Park KS, Kim D, Lee YS, Sonn JK, Jung JC, Bang OS, Kang SS. TGF-beta3 inhibits chondevrepogenesis by suppressing precartilage condensation through stimulation of N-cadherin shedding and reduction of cRREB-1 expression. Mol Cells. 2010;29:425–432. doi: 10.1007/s10059-010-0078-z. [DOI] [PubMed] [Google Scholar]
- Jo J, Lee Y, Oh MH, Ko JJ, Cheon Y-P, Lee devrep. Up-regulation of pluripotency-related genes in human amniotic fluid-derived stem cells by ESRRB conjugated with cell-penetrating peptide. Dev Reprod. 2010;14:243–251. [Google Scholar]
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Ohba M, Chida K, Kuroki T. Protein kinase C eta (PKC eta): its involvement in keratinocyte differentiation. J Biochem. 2002;132:853–857. doi: 10.1093/oxfordjournals.jbchem.a003297. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Rodevrepiquez-Leόn J, Izpisúa Belmonte JC. The role of TGFbetas and Sox9 during limb chondevrepogenesis. Curr Opin Cell Biol. 2006;18:723–729. doi: 10.1016/j.ceb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Lampasso JD, Chen W, Marzec N. The expression profile of PKC isoforms during MC3T3-E1 differentiation. Int J Mol Med. 2006;17:1125–1131. [PubMed] [Google Scholar]
- Lee HK, Yeo S, Kim JS, Lee JG, Bae YS, Lee C, Baek SH. Protein kinase C-eta and phospholipase D2 pathway regualates foam cell formation via regulator of G protein signaling 2. Mol Pharmacol. 2010;78:478–485. doi: 10.1124/mol.110.064394. [DOI] [PubMed] [Google Scholar]
- Li J, Zhao Z, Liu J, Huang N, Long D, Wang J, Li X, Liu Y. MEK/ERK and p38 MAPK regulate chondevrepogenesis of rat bone marrow mesenchymal stem cells through delicate interaction with TGF-beta1/Smads pathway. Cell Prolif. 2010;43:333–343. doi: 10.1111/j.1365-2184.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littler devrep, Walker JR, She YM, Finerty PJ, Jr, Newman EM, Dhe-Paganon S. Structure of human protein kinase C eta (PKCeta) C2 domain and identification of phosphorylation sites. Biochem Biophys Res Commun. 2006;349:1182–1189. doi: 10.1016/j.bbrc.2006.08.160. [DOI] [PubMed] [Google Scholar]
- Matta C, Juhász T, Szíjgyártó Z, Kolozsvári B, Somogyi C, Nagy G, Gergely P, Zákány R. PKCdelta is a positive regulator of chondevrepogenesis in chicken high density micromass cell cultures. Biochimie. 2011;93:149–159. doi: 10.1016/j.biochi.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Muir H. The chondevrepocyte, architect of cartilage. Biomechanics, structure, function and molecular biology of cartilage matrix macromolecules. Bioessays. 1995;17:1039–1048. doi: 10.1002/bies.950171208. [DOI] [PubMed] [Google Scholar]
- Newton AC. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydevrepolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nochi H, Sung JH, Lou J, Adkisson HD, Maloney WJ, Hruska KA. Adenovirus mediated BMP-13 gene transfer induces chondevrepogenic differentiation of murine mesenchymal progenitor cells. J Bone Miner Res. 2004;19:111–122. doi: 10.1359/jbmr.2004.19.1.111. [DOI] [PubMed] [Google Scholar]
- Park JY, Hwang EM, Park N, Kim E, Kim DG, Kang D, Han J, Choi WS, Ryu PD, Hong SG. Gateway RFP-fusion vectors for high throughput functional analysis of genes. Mol Cells. 2007;23:357–362. [PubMed] [Google Scholar]
- Pelttari K, Steck E, Richter W. The use of mesenchymal stem cells for chondevrepogenesis. Injury. 2008;39(Suppl 1):S58–65. doi: 10.1016/j.injury.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W. Premature induction of hypertrophy during in vitro chondevrepogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- Rosse C, Linch M, Kermorgant S, Cameron AJ, Boeckeler K, Parker PJ. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol. 2010;11:103–112. doi: 10.1038/nrm2847. [DOI] [PubMed] [Google Scholar]
- Rotem-Dai N, Oberkovitz G, Abu-Ghanem S, Livneh E. PKCeta confers protection against apoptosis by inhibiting the pro-apoptotic JNK activity in MCF-7 cells. Exp Cell Res. 2009;315:2616–2623. doi: 10.1016/j.yexcr.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3:107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Y, Zang W, Yan Y, Liu Y, Fu Q, Wang K, Chen Y, Qi N. Study of differential effects of TGF-beta3/BMP2 on chondevrepogenesis in MSC cells by gene microarray data analysis. Mol Cell Biochem. 2014;385:191–198. doi: 10.1007/s11010-013-1827-z. [DOI] [PubMed] [Google Scholar]
- Shtutman M, Hershko T, Maissel A, Fima E, Livneh E. PKCeta associates with cyclin E/Cdk2 complex in serum-starved MCR-7 and NIH-3T3 cells. Exp Cell Res. 2003;286:22–29. doi: 10.1016/s0014-4827(03)00087-9. [DOI] [PubMed] [Google Scholar]
- Shum L, Nuckolls G. The life cycle of chondevrepocytes in the developing skeleton. Arthritis Res. 2002;4:94–106. doi: 10.1186/ar396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater C, Kasten P, Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011;7:463–377. doi: 10.1016/j.actbio.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Wang Y, Belflower RM, Dong YF, Schwarz EM, O’Keefe RJ, devrepissi H. Runx1/AML1/Cbfa2 mediates onset of mesenchymal cell differentiation toward chondevrepogenesis. J Bone Miner Res. 2005;20:1624–1636. doi: 10.1359/JBMR.050516. [DOI] [PubMed] [Google Scholar]
- Woods A, Wang G, Beier F. Regulation of chondevrepocyte differentiation by the actin cytoskeleton and adhesive interactions. J Cell Physiol. 2007;213:1–8. doi: 10.1002/jcp.21110. [DOI] [PubMed] [Google Scholar]
- Woods A, Wang G, Dupuis H, Shao Z, Beier F. Rac1 signaling stimulated N-cadherin expression, mesenchymal condensation, and chondevrepogenesis. J Biol Chem. 2007;282:23500–23508. doi: 10.1074/jbc.M700680200. [DOI] [PubMed] [Google Scholar]
- Zhang L, Su P, Xu C, Yang J, Yu W, Huang D. Chondevrepogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotechnol Lett. 2010;32(9):1339–1346. doi: 10.1007/s10529-010-0293-x. [DOI] [PubMed] [Google Scholar]