Abstract
Human case-studies have reported an association between green tea-based dietary supplements and hepatotoxicity. Studies have demonstrated the hepatotoxicity of high-dose oral bolus dosing with the tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) in mice and dogs. We examined the effect of pretreatment with dietary EGCG on the hepatotoxicity and bioavailability of acute oral bolus dosing with EGCG in CF-1 mice. EGCG (750 mg/kg, i.g., once daily for 3 days) increased plasma alanine aminotransferase by 80-fold, decreased both reduced (by 59%) and total (by 33%) hepatic glutathione, and increased hepatic levels of phosphorylated histone 2AX. Pretreatment with dietary EGCG (3.2 mg/g diet) for 2 weeks mitigated hepatotoxicity. Acute oral EGCG also decreased mRNA expression of glutathione reductase. Dietary pretreatment prevented this decreases and increased glutathione peroxidase (Gpx)2, Gpx3, Gpx5, and Gpx7 expression. We found that dietary EGCG reduced the plasma (57% reduction) and hepatic (71% reduction) EGCG exposure following oral bolus dosing compared to mice that were not pre-treated. Overall, it appears that EGCG can modulate its own bioavailability and that dietary treatment may reduce the toxic potential of acute high oral bolus doses of EGCG. These data may partly explain the observed variation in hepatotoxic response to green tea-containing dietary supplements.
Keywords: green tea, Camellia sinensis, (−)-epigallocatechin-3-gallate, hepatotoxicity, bioavailability
1. Introduction
(−)-Epigallocatechin-3-gallate (EGCG), is the most abundant polyphenol in green tea (Camellia sinensis), and has been widely-studied for its potential health benefits including modulation of body weight gain (Dulloo et al., 1999; Grove et al., 2012; Wolfram et al., 2005). One result of these reports of positive health effects has been the proliferation of green tea-based dietary supplements for a variety of indications. Whereas historical exposure to EGCG has been as a beverage, green tea-based dietary supplements are generally capsules or pills. The result is the ability to deliver an equivalent dose of green tea components much more quickly and in a much smaller volume. The safety of these alternative formulations is generally based on the historical safety of green tea beverage, however, there is increasing data to suggest that this assumption may not be valid (Mazzanti et al., 2009).
Laboratory studies in mice and dogs have demonstrated the potential hepatotoxicity of acute oral bolus dosing with EGCG or green tea extracts. We have previously reported that EGCG induces dose-dependent hepatotoxicity in CF-1 mice (Lambert et al., 2010). Once daily dosing with EGCG (750 mg/kg, i.g.) induced hepatic necrosis. Similar results were observed in fasted Beagle dogs treated with oral bolus doses of Polyphenon E, a defined tea polyphenol mixture containing 60% EGCG (Kapetanovic et al., 2009). Although the underlying mechanisms of EGCG-induced hepatotoxicity remains understudied, it has been proposed that the acute oral doses of EGCG, depending on dose, can result in oxidative stress leading to liver injury (Lambert et al., 2010; Lambert et al., 2007).
More than 34 case-reports have associated human hepatotoxicity with the use of green tea-containing dietary supplements since 1999 [reviewed in (Mazzanti et al., 2009)]. Although the causative role of these supplement in the hepatotoxicity has not been clearly established, several case-reports have indicated that cessation of supplement use led to resolution of symptoms and re-challenge led to renewed liver injury (Mazzanti et al., 2009). It is interesting to note that hepatotoxicity has not been widely observed in controlled human intervention studies (Bettuzzi et al., 2006; Chow et al., 2003). These inconsistent findings indicate that genetic and/or life-style factors may play a role in susceptibility to EGCG-mediated hepatotoxicity.
Previous studies have shown that chronic, dietary administration of green tea can impact the oral bioavailability of the tea polyphenols. Kim et al., have previously reported that long term treatment of rats and mice with dietary green tea resulted in an initial increase in plasma EGCG levels over the first 4 days of treatment (Kim et al., 2000). Over the subsequent 10 days of treatment, plasma levels of EGCG decreased and eventually reached baseline levels again. EGCG has been shown to induce the expression of genes related to antioxidant response and Phase II biotransformation in vivo (Shen et al., 2005). These changes have been shown to alter the metabolic profile of toxicants and carcinogens (Tang et al., 2008). Given that EGCG itself is subject to Phase II metabolism, it is plausible that chronic administration of EGCG or green tea may influence the biotransformation and bioavailability of subsequent doses of EGCG.
In the present study we determined the effect of dietary pretreatment with EGCG on the bioavailability and hepatotoxicity of subsequent oral bolus dosing with EGCG in mice. This study was meant to mimic the potential hepatotoxic effects of high oral bolus dosing with EGCG in chronic tea consumers compared to those in non-consumers, and should aid in identifying factors which predispose subjects to green tea supplement-associated hepatotoxicity.
2. Materials and methods
2.1 Chemicals and reagents
EGCG (93% pure) was purchased from Taiyo Green Power (Jiangsu, China). For dietary exposure, 3.2 mg/g EGCG was incorporated into AIN76A basal diet (Research Diets, Inc., New Brunswick, NJ). Primers for quantitative reverse transcriptase polymerase chain reaction (qPCR) were synthesized by the Genomics Core Facility at The Pennsylvania State University (University Park, PA) and the sequences are shown in Supplemental Table 1. Phosphorylated histone 2AX (γH2AX) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies were purchased from Cell Signaling Technologies (Danvers, MA). All other chemicals were of the highest grade commercially-available.
2.2 Animal studies
Animal studies were approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University (Protocol# 37115). Mice were maintained on a 12 hour light-dark cycle and had ad libitum access to food and water. Male CF-1 mice (6 – 8 weeks old, Charles River Laboratory, Wilmington, MA) were acclimatized for 1 week. The dietary dose of EGCG (3.2 mg/g diet) is equivalent to a total daily dose of 500 mg/kg, body weight. The dietary EGCG dose and the oral bolus EGCG dose (750 mg/kg, body weight) are equivalent to 10 and 16 cups of green tea (2.5 g tea leaves in 250 mL water) (Lambert et al., 2003).
2.1.1 Hepatotoxicity studies
Mice were randomized into three treatment groups: EGCG pretreatment (EP) mice, which were fed AIN76A diet supplemented with EGCG (3.2 mg/g) for 14 days; no pretreatment (NP) mice, which were fed AIN76A diet; and negative control (NC) mice which received AIN76A diet. Following the pretreatment period, EP and NP mice were fasted for 7 hours (07:00 – 14:00 h) and then given a single dose of EGCG (750 mg/kg, i.g.) once daily for 3 days. NC mice were given a single oral bolus dose of sterile saline once daily for 3 days. On day 3, mice were anesthetized and blood was collected by cardiac puncture 1 hour post-gavage. Plasma was prepared by centrifugation at 3200 g for 15 min and frozen at −80°C. The livers were harvested, washed with ice-cold PBS and frozen at −80°for later analysis.
2.1.2 Bioavailability studies
Mice (n = 24 per group) were randomized into EP or NP groups. After a pretreatment period of 14 days, NP and EP mice were fasted for 7 hours and then given a single dose of EGCG (750 mg/kg, i.g.). Mice were anesthetized and blood was collected via cardiac puncture at 0.5 – 6 hours post-gavage. Plasma was prepared as above, combined with 0.1 vol ascorbate preservative (20% ascorbic acid:0.1 % EDTA) and frozen at −80°C. Livers were harvested and frozen as above.
2.3 Determination of hepatotoxicity and oxidative stress
Plasma alanine aminotransferase (ALT) levels were determined spectrophotometrically (λmax = 340 nm) using a commercially-available assay (CATACHEM, Inc, Bridgeport, CT) according the manufacturer’s instructions. Hepatic γH2AX was determined by western blot. Protein was extracted from liver samples using Tissue Protein Extraction Reagent containing 1% (v/v) phosphatase and protease inhibitor cocktails (Thermo-Scientific, Rockford, IL). Protein samples (30 μg) were resolved by SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Membranes were probed with γH2AX primary antibody and a fluorescent-conjugated secondary antibody (LI-COR Co., Lincoln, NE). Bands were visualized using an Odyssey Imaging System (LI-COR Co.). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a protein loading control. Total and reduced glutathione levels were determined using the GSH-Glo™ Glutathione assay kit (Promega, Madison, WI).
2.4 Gene expression studies
Hepatic gene expression was examined by qPCR. RNA was isolated from liver tissue using Tri-reagent (Sigma Chemical Co. St. Louis, MO) and quantified using the NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE). cDNA was reverse transcribed and amplified using SYBR Green PCR Master Mix and an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA). Standard curves were prepared using serial dilutions from pooled cDNA samples. mRNA levels were normalized to Gapdh.
2.5 HPLC analysis of EGCG
Plasma and liver EGCG levels were determined as previously described by high performance liquid chromatography with electrochemical detection (HPLC-ECD) (Lambert et al., 2003). In brief, plasma samples were sequentially extracted with dichloromethane and ethyl acetate. The ethyl acetate fraction was dried under vacuum and resuspended in 10% aqueous acetonitrile for HPLC analysis. Liver samples were homogenized in a 1:1 mixture of hydrosulfite buffer (0.4 M sodium phosphate monobasic containing 0.3 M sodium hydrosulfite and 0.1% disodium EDTA) and methanol:ethyl acetate (2:1, v:v). The supernatant was collected after centrifugation for 4 min at 16 000 g. After removing the methanol and ethyl acetate under vacuum, samples were treated in a manner analogous to plasma. All samples were analyzed using a Shimadzu HPLC system equipped with two LC-20AD pumps (Shimadzu Co, Columbia, MD), an ESA 5600A Coularray detector (Chelmsford, MA), and a Supelcosil LC18 column (4.6 × 150 mm, 5 μm particle size, Supelco, Bellefonte, PA).
2.6 Statistical Analysis
All experiments were repeated twice. Maximum plasma concentrations (Cmax) and exposure (AUC0→6h) were determined using GraphPad Prism 5.0 (San Diego, CA). One-way ANOVA with Dunnett’s post-test was used to compare differences in biochemical markers. Pharmacokinetic parameters were compared using the Student’s t test. Statistical analysis was performed using GraphPad Prism 5.0. Data are expressed as mean ± SEM and significance was reached at P<0.05.
3. Results and Discussion
In the present study we investigated the effect of dietary pretreatment with EGCG on hepatotoxicity induced by subsequent high oral bolus dosing with EGCG in male CF-1 mice. These experiments were meant to model the potential hepatotoxic effects of a high oral bolus dose of EGCG, as might be expected with green tea-based dietary supplements, in regular tea consumers (EP mice in this study) compared to naïve subjects (NP mice in this study).
Treatment of naïve mice (NP) with oral bolus EGCG (750 mg/kg, i.g., once daily) for 3 days resulted in an 80-fold increase in plasma ALT levels compared to vehicletreated control mice (NC) (Fig. 1 A). These effects are consistent with our previous studies (Lambert et al. 2010). By contrast, dietary pretreatment with EGCG for 2 weeks (EP) reduced this elevation in plasma ALT by 75% indicating a reduction in hepatotoxicity induced by oral bolus EGCG. This bolus dose of EGCG is equivalent to a human dose of 30 mg/kg body weight based on allometric scaling (Lambert et al., 2010). Although somewhat higher than the typical recommended dose of commerciallyavailable supplements (2 – 12 mg/kg/day), such a dose is well within the range achievable by human subjects who exceed the recommended dose either inadvertently or due to a belief that higher doses of EGCG will provide additional benefit.
Figure 1.
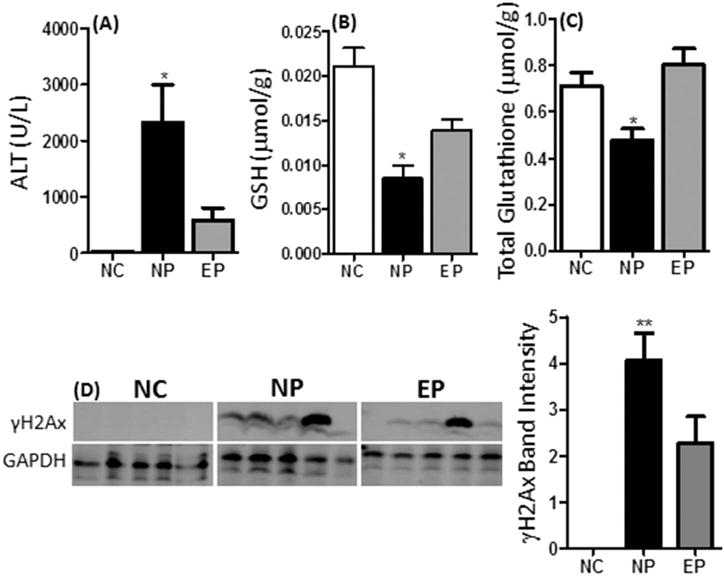
Hepatotoxic effects of acute oral bolus EGCG in naïve or dietary EGCG-pretreated mice. (A) Plasma ALT levels were determined following administration of 3 daily doses of 750 mg/kg, i.g. EGCG to male CF-1 mice pretreated with dietary EGCG [3.2 mg/g diet] for 14 days [EP] or not [NP] using spectrophotometric methods. (B) Hepatic levels of reduced glutathione [GSH] and (C) total glutathione in EP and NP mice following 3 daily doses of 750 mg/kg, i.g. EGCG using a luminescence-based assay. N = 8 – 16. (D) Hepatic levels of γH2AX were determined by western blot analysis in EP and NP mice following 3 daily doses of 750 mg/kg, i.g. EGCG. Expression was determined by densitometry and normalized to GAPDH. The blot shows representative expression patterns. The histogram shows quantification of all samples analyzed (n = 10 per treatment group). Error bars represent SEM. Data were analyzed by one-way ANOVA with Dunnett’s post-test [* = P < 0.05; ** = P < 0.01]. All biomarkers were compared to vehicle-treated control mice [NC].
EGCG has been shown to exhibit pro-oxidant activity both in vitro and in vivo (reviewed in (Forester and Lambert, 2011)), and previous studies have shown that EGCG-mediated liver toxicity was associated with increased hepatic oxidative stress (Galati et al., 2006; Lambert et al., 2010). Here, we found that treatment with oral bolus EGCG decreased both reduced (57% decrease) and total (33% decrease) glutathione in NP mice compared to vehicle-treated controls (Fig. 1B and 1C). In addition, oral bolus EGCG increased phosphorylation of hepatic histone 2AX (γH2AX), marker of oxidative stress and double strand DNA breaks (Fig. 1D). These pro-oxidant effects were partially ameliorated in EP mice indicating a reduction in oxidative stress (Fig. 1B – 1D).
In addition to blunting the biomarkers of oxidative stress, dietary pretreatment with EGCG also preserved or enhanced the hepatic expression of several genes related to antioxidant response in mice following treatment with a single high dose oral bolus of EGCG. A panel of genes related to antioxidant response was examined in the hepatic tissue of mice treated with daily oral bolus EGCG (Fig. 2). Following oral bolus dosing with EGCG, hepatic expression of glutathione reductase (Gsr), glutathione-S-transferase ζ1 (Gstz1), and glutathione peroxidase (Gpx)2, Gpx3, Gpx5, and Gpx7 was higher in mice that had been pretreated with dietary EGCG compared to those that did not receive EGCG pretreatment (Fig. 2), This was particularly true at the at 0.5 hour time-point. No significant changes in the expression of Gpx1, Gpx4, superoxide dismutase (Sod)2 was observed (Fig. 2).
Figure 2.
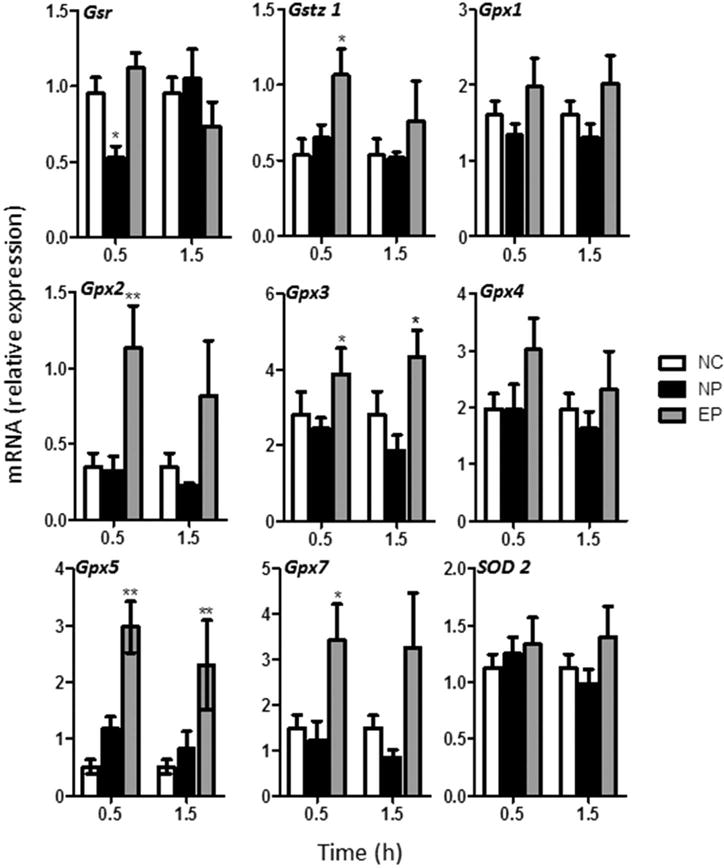
Effect of dietary pretreatment with EGCG on the expression of antioxidant response genes in mice following subsequent acute oral bolus dosing with EGCG. Hepatic gene expression of the genes of interest were determined following administration of 3 daily doses of 750 mg/kg, i.g. EGCG to male CF-1 mice pretreated with 3.2 mg/g dietary EGCG for 14 days [EP] or not [NP]. Levels were determined 0.5 and 1.5 hours after the last EGCG treatment and normalized to Gapdh expression. Each bar represents the mean of N= 4–8. Error bars represent SEM. Data were analyzed by one-way ANOVA with Dunnett’s post-test (* = P < 0.05, ** = P < 0.01).
Previous studies which have shown that oral dosing with EGCG can modulate hepatic antioxidant gene expression and function. For example, oral bolus administration of non-toxic doses of EGCG (200 mg/kg once daily) has been shown to enhance the expression of a number of antioxidant-related genes in mice in a Nuclear factor (erythroid-derived 2)-like 2-dependent manner (Shen et al. 2005). Another study found that dietary green tea extract could enhance glutathione-mediated detoxification of aflatoxin in piglets (Tulayakul et al., 2007). Taken together, the present results and these previous studies provide a putative mechanism by which dietary EGCG can mitigate the pro-oxidant effects of toxic oral bolus doses of EGCG.
Previous studies in mice and rats have indicated that longer-term treatment with dietary green tea can reduce the overall plasma and tissue bioavailability of EGCG and the other green tea polyphenols (Kim et al., 2000). These authors reported that in A/J mice given 0.6% green tea solids as the sole source drinking fluid, plasma and liver levels of EGCG increased from day 0 to day 4 and then declined over the subsequent 10 days of treatment. Here, we examined the effect of dietary pretreatment with EGCG on the plasma and liver concentrations of unconjugated EGCG following oral bolus dosing with EGCG (Fig. 3). Following a single oral bolus dose of 750 mg/kg, i.g. EGCG, the plasma Cmax and AUC0→6h of unconjugated EGCG was 1.3-fold higher in NP mice compared to EP mice (Table 1). Similarly, the hepatic EGCG Cmax and AUC0→6h were higher in NP mice than EP mice (Table 1). These results indicate that EGCG can impact its own bioavailability and that chronic exposure can result in lower plasma and tissue levels of unconjugated EGCG.
Figure 3.
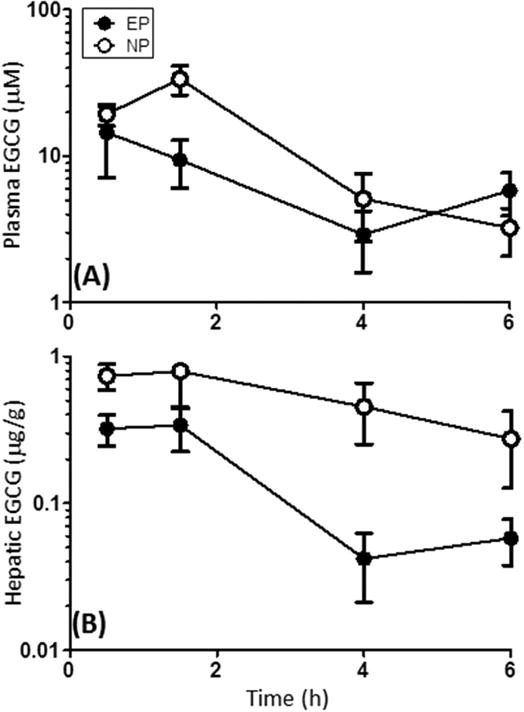
Effect of dietary pretreatment with EGCG on the oral bioavailability of a single oral bolus EGCG in mice. (A) Plasma and (B) liver levels of unconjugated EGCG were determined following a single oral bolus dose of 750 mg/kg, i.g. EGCG to male CF-1 mice pretreated with 3.2 mg/g dietary EGCG for 14 days [EP] or not [NP]. EGCG levels were determined by HPLC with electrochemical detection. Data represent the mean of N = 5 – 6 mice. Error bars represent SEM.
Table 1.
Comparative EGCG maximum concentration (Cmax) and exposure (AUC0→6h) in mice pretreated (EP) and not (NP) with dietary EGCG.1
| Parameter | Units | EP | NP | |
|---|---|---|---|---|
| Plasma | ||||
| Cmax | μM | 14.5 ± 7.4 | 33.7 ± 7.6 | |
| AUC0→6h | μM●h | 36.2 ± 8.4* | 83.3 ± 12.5 | |
|
| ||||
| Liver | ||||
| Cmax | μg/g | 0.34 ± 0.11 | 0.79 ± 0.35 | |
| AUC0→6h | μg/g●h | 0.9 ± 0.2† | 3.1 ± 0.7 | |
Data represent the mean ± SEM.
indicates p < 0.05,
indicates p = 0.0637
Unconjugated EGCG has been reported to have greater biological activity than glucuronidated and methylated conjugates of EGCG (Landis-Piwowar et al., 2008; Lu et al., 2003a), therefore this observed decrease in bioavailability, coupled with increased expression of hepatic antioxidant genes, provides an mechanism by which dietary pretreatment with EGCG can reduce acute oral bolus-related hepatotoxicity.
Further studies are needed to determine the molecular mechanism by which EGCG can modulate its own bioavailability however it is reasonable to speculate that EGCG may induce Phase II metabolic enzymes as well as active efflux transporters. We and others have previously shown that the bioavailability of EGCG is modulated by uridine diphosphate glucuronyltransferases, catechol-O-methyltransferase, and the multidrug resistance-associated proteins in the liver or small intestine (Hong et al., 2003; Lambert et al., 2003; Lu et al., 2003a; Lu et al., 2003b; Zhang et al., 2004). Previous studies have shown that these systems are inducible by other compounds (Badolo et al., 2014; Kensler et al., 2000). Further studies are needed to determine if chronic oral EGCG can induce such changes and the extent to which such induction may influence the oral bioavailability of acute oral bolus doses of EGCG.
Of note, there has been a limited number of studies that have examined the impact repeating dosing on the bioavailability of EGCG in human subjects. Chow et al., have reported that daily dosing with 800 mg EGCG for 4 weeks increased both the plasma Cmax and AUC (Chow et al., 2003). No such increase was observed at a lower dose (400 mg per day). The underlying mechanism for these results remains unclear, and no further studies have reported similar findings. It is possible that the differences between this study in humans and our present study in mice are related to species differences in the response of inducible Phase II and active transport systems. Alternatively, the differences could result for the dosage form for the chronically administered EGCG. In our study, it was incorporated into the diet, and mice are to be exposed continuously to lower concentrations throughout the feeding period. In the human study, EGCG was administered in pill form once or twice daily. The result would be higher peak plasma concentrations and more frequent periods of very low levels. To date there have been no studies to determine the impact of such differences on bioavailability of EGCG over long periods of time.
4. Conclusions
In summary, our results show that dietary pretreatment with EGCG can reduce the bioavailability and hepatotoxic potential of subsequent acute high dose oral bolus EGCG. In terms of translation to human consumption patterns, our results are analogous to comparing exposure of a non-tea consumer (NP) to exposure of a regular tea consumer (EP), and suggest that regular consumers of green tea beverage may be at decreased risk of hepatotoxicity associated with the use of green tea-based dietary supplements. Further studies in human subjects are required to compare the bioavailability of EGCG in regular tea consumers to non-consumers, and to establish the overall translational application of our present studies.
Supplementary Material
Highlights.
Pretreatment with dietary (−)-epigallocatechin-3-gallate (EGCG) mitigated the hepatotoxicity of oral bolus EGCG in mice
Pretreatment with dietary EGCG prevented a reduction in hepatic antioxidant gene expression caused by oral bolus EGCG in mice
Dietary pretreatment with EGCG reduced the plasma and hepatic bioavailability of subsequent oral bolus EGCG in mice
Acknowledgments
This study was supported by a grant from the National Institutes of Health (AT004678) to JDL
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: ALT, alanine aminotransferase; AUC0→6h, exposure over 6 h; Cmax, maximum concentration; EGCG, (−)-epigallocatechin-3-gallate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GPX, glutathione peroxidase; GSR, glutatione reductase; GST, glutathione-S-transferase; λH2AX, phosphorylated histone 2AX; SOD, superoxide dismutase; qPCR, quantitative reverse transcriptase polymerase chain reaction
References
- Badolo L, Jensen B, Sall C, Norinder U, Kallunki P, Montanari D. Evaluation of 309 molecules as inducers of CYP3A4, CYP2B6, CYP1A2, OATP1B1, OCT1, MDR1, MRP2, MRP3 and BCRP in cryopreserved human hepatocytes in sandwich culture. Xenobiotica. 2014:1–11. doi: 10.3109/00498254.2014.955831. [DOI] [PubMed] [Google Scholar]
- Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, Dorr RT, Hara Y, Alberts DS. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–3319. [PubMed] [Google Scholar]
- Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, Chantre P, Vandermander J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr. 1999;70:1040–1045. doi: 10.1093/ajcn/70.6.1040. [DOI] [PubMed] [Google Scholar]
- Forester SC, Lambert JD. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol Nutr Food Res. 2011;55:844–854. doi: 10.1002/mnfr.201000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galati G, Lin A, Sultan AM, O’Brien PJ. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic Biol Med. 2006;40:570–580. doi: 10.1016/j.freeradbiomed.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Grove KA, Sae-tan S, Kennett MJ, Lambert JD. (−)-Epigallocatechin-3-gallate inhibits pancreatic lipase and reduces body weight gain in high fat-fed obese mice. Obesity (Silver Spring) 2012;20:2311–2313. doi: 10.1038/oby.2011.139. [DOI] [PubMed] [Google Scholar]
- Hong J, Lambert JD, Lee SH, Sinko PJ, Yang CS. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (−)-epigallocatechin-3-gallate and its methyl metabolites. Biochem Biophys Res Commun. 2003;310:222–227. doi: 10.1016/j.bbrc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Kapetanovic IM, Crowell JA, Krishnaraj R, Zakharov A, Lindeblad M, Lyubimov A. Exposure and toxicity of green tea polyphenols in fasted and non-fasted dogs. Toxicology. 2009;260:28–36. doi: 10.1016/j.tox.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Curphey TJ, Maxiutenko Y, Roebuck BD. Chemoprotection by organosulfur inducers of phase 2 enzymes: dithiolethiones and dithiins. Drug Metabol Drug Interact. 2000;17:3–22. doi: 10.1515/dmdi.2000.17.1-4.3. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee MJ, Hong J, Li C, Smith TJ, Yang GY, Seril DN, Yang CS. Plasma and tissue levels of tea catechins in rats and mice during chronic consumption of green tea polyphenols. Nutr Cancer. 2000;37:41–48. doi: 10.1207/S15327914NC3701_5. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Kennett MJ, Sang S, Reuhl KR, Ju J, Yang CS. Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food Chem Toxicol. 2010;48:409–416. doi: 10.1016/j.fct.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JD, Lee MJ, Lu H, Meng X, Hong JJ, Seril DN, Sturgill MG, Yang CS. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J Nutr. 2003;133:4172–4177. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Sang S, Yang CS. Possible controversy over dietary polyphenols: benefits vs risks. Chem Res Toxicol. 2007;20:583–585. doi: 10.1021/tx7000515. [DOI] [PubMed] [Google Scholar]
- Landis-Piwowar KR, Milacic V, Dou QP. Relationship between the methylation status of dietary flavonoids and their growth-inhibitory and apoptosis-inducing activities in human cancer cells. J Cell Biochem. 2008;105:514–523. doi: 10.1002/jcb.21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Meng X, Li C, Sang S, Patten C, Sheng S, Hong J, Bai N, Winnik B, Ho CT, Yang CS. Glucuronides of tea catechins: enzymology of biosynthesis and biological activities. Drug Metab Dispos. 2003a;31:452–461. doi: 10.1124/dmd.31.4.452. [DOI] [PubMed] [Google Scholar]
- Lu H, Meng X, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate. Drug Metab Dispos. 2003b;31:572–579. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, Mastrangelo S. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol. 2009;65:331–341. doi: 10.1007/s00228-008-0610-7. [DOI] [PubMed] [Google Scholar]
- Shen G, Xu C, Hu R, Jain MR, Nair S, Lin W, Yang CS, Chan JY, Kong AN. Comparison of (−)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Pharm Res. 2005;22:1805–1820. doi: 10.1007/s11095-005-7546-8. [DOI] [PubMed] [Google Scholar]
- Tang L, Tang M, Xu L, Luo H, Huang T, Yu J, Zhang L, Gao W, Cox SB, Wang JS. Modulation of aflatoxin biomarkers in human blood and urine by green tea polyphenols intervention. Carcinogenesis. 2008;29:411–417. doi: 10.1093/carcin/bgn008. [DOI] [PubMed] [Google Scholar]
- Tulayakul P, Dong KS, Li JY, Manabe N, Kumagai S. The effect of feeding piglets with the diet containing green tea extracts or coumarin on in vitro metabolism of aflatoxin B1 by their tissues. Toxicon. 2007;50:339–348. doi: 10.1016/j.toxicon.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Wolfram S, Raederstorff D, Wang Y, Teixeira SR, Elste V, Weber P. TEAVIGO (epigallocatechin gallate) supplementation prevents obesity in rodents by reducing adipose tissue mass. Ann Nutr Metab. 2005;49:54–63. doi: 10.1159/000084178. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zheng Y, Chow MS, Zuo Z. Investigation of intestinal absorption and disposition of green tea catechins by Caco-2 monolayer model. Int J Pharm. 2004;287:1–12. doi: 10.1016/j.ijpharm.2004.08.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


