Abstract
The integrin family of cell adhesion receptors regulates a diverse array of cellular functions crucial to the initiation, progression and metastasis of solid tumours. The importance of integrins in several cell types that affect tumour progression has made them an appealing target for cancer therapy. Integrin antagonists, including the αvβ3 and αvβ5 inhibitor cilengitide, have shown encouraging activity in Phase II clinical trials and cilengitide is currently being tested in a Phase III trial in patients with glioblastoma. These exciting clinical developments emphasize the need to identify how integrin antagonists influence the tumour and its microenvironment.
Much of the classic literature regarding cancer and integrins has implicated this family of adhesion receptors in tumour cell proliferation, migration and survival (BOX 1). The role of integrins in cell migration and invasion is one of their most studied functions in tumour biology and has recently been reviewed elsewhere1,2. Integrins directly bind components of the extracellular matrix (ECM) and provide the traction necessary for cell motility and invasion. ECM remodelling is also controlled by integrins, which regulate the localization and activity of proteases. In addition to their well-established role in migration and invasion, integrins can regulate proliferation3. Although adhesion-dependent control of proliferation is deregulated in tumour cells, integrins continue to regulate cell growth in some tumours4,5. Recent studies have shed new light on the crucial, and often contradictory, role integrins have in regulating tumour cell survival. In addition to their ligation-dependent effects, it is now becoming clear that in some cases unligated integrins can positively or negatively influence tumour cell survival, thereby affecting tumour growth and metastasis. How integrins affect tumour cell survival both in the ligated and unligated states could be a crucial determinant of the efficacy of integrin antagonists in cancer.
In addition to their role in tumour cells, integrins on the surface of tumour-associated host cells can profoundly influence the malignant potential of a tumour. The tumour microenvironment is comprised of many host cell types, including endothelial cells, perivascular cells, fibroblasts and inflammatory cells, which contribute to tumour progression by mediating angiogenesis, lymphangiogenesis, desmoplasia and inflammation. The involvement of integrins in angiogenesis is well described, and recent studies have demonstrated that they also influence many other host cell responses to cancer. Therefore, integrin antagonists targeting the tumour microenvironment might significantly curtail tumour progression.
Integrins span the lipid bilayer of cells and promote intracellular signalling, typically in the context of activated cytokine receptors or growth factor receptors. Consequently, tumour growth and invasion probably depend on integrin crosstalk with growth factor receptors or oncogenes in both tumour cells and tumour-associated cells. Recent studies have demonstrated that some growth factors and oncogenes require specific integrins for tumour initiation and progression. These studies highlight the importance of understanding crosstalk mechanisms, as they could influence the tumour response to inhibitors of growth factor or integrin signalling.
In recent years, great progress has been made towards targeting integrins in cancer. Preclinical as well as clinical studies with various integrin antagonists have demonstrated their effectiveness in blocking tumour progression. Phase II clinical trials with cilengitide (developed by Merck KGaA), an avβ3 and avβ5 integrin antagonist, have shown clinical activity and few side effects in patients with glioblastoma. These positive clinical findings have led to the initiation of the first Phase III clinical trial with an integrin antagonist. The advance of integrin antagonists into the clinic highlights the importance of continued research to determine the role integrins have in tumour progression and to identify the factors that influence the effectiveness of these inhibitors.
Integrin biology
The integrin family of cell adhesion receptors
Integrins are heterodimeric cell surface receptors that mediate adhesion to the ECM and immunoglobulin superfamily molecules. At least 24 distinct integrin heterodimers are formed by the combination of 18 α-subunits and 8 β-subunits. Specific integrin heterodimers preferentially bind to distinct ECM proteins. The repertoire of integrins present on a given cell dictates the extent to which that cell will adhere to and migrate on different matrices. αv integrins and integrin a5β1 recognize the RGD sequence on their respective ligands. In fact, these integrins were first identified on the basis of their ability to recognize the RGD sequence6. Other adhesive sequences in ECM proteins have also been observed, including the EILDV and REDV sequences that are recognized by integrin a4β1 in an alternatively spliced form of fibronectin. On ligation to the ECM, integrins cluster in the plane of the membrane and recruit various signalling and adaptor proteins to form structures known as focal adhesions. The composition of these adhesions differs depending on whether the contacts occur in two-dimensional or three-dimensional conditions7. Although integrins lack kinase activity, by clustering they recruit and activate kinases, such as focal adhesion kinases (FAKs) and Src family kinases (SFKs), in addition to scaffolding molecules, such as p130 CRK-associated substrate (p130CAS; also known as BCAR1). Integrins also couple the ECM to the actin cytoskeleton by recruiting proteins, including talin, paxillin, α-actinin, tensin and vinculin. Additionally, a ternary complex consisting of an integrin-linked kinase, PINCH (also known as LIMS1), and parvin regulates many scaffolding and signalling functions required for integrin-mediated effects on cell migration and survival8. Furthermore, integrin recruitment to membrane microdomains by tetraspanins might crucially regulate integrin function in tumour cells9. Regulation of the recruitment and activation of these and other focal adhesion proteins influences cell adhesion and migration on the ECM. In fact, many of these molecules are themselves being investigated as possible targets for cancer therapy. In some cases, the function of an integrin is related to its ligand affinity. Increased affinity or activation can be induced by either ligand-mediated integrin clustering on the cell surface or increased intracellular signalling through molecules, such as the GTPase RAP1A10. Therefore, signalling that is induced by oncogenes or growth factor receptors may outwardly influence integrin affinity and function.
Integrin expression in cancer
A wide variety of integrins contribute to tumour progression. As many solid tumours originate from epithelial cells, the integrins expressed by epithelial cells (including a6β4, a6β1, αvβ5, a2β1 and a3β1) are generally retained in the tumour, though expression levels may be altered. These integrins typically mediate epithelial cell adhesion to the basement membrane, but might contribute to migration, proliferation and survival in tumour cells. However, integrin expression can also vary considerably between normal and tumour tissue. Most notably, integrins αvβ3, a5β1 and αvβ6, are usually expressed at low or undetectable levels in most adult epithelia but can be highly upregulated in some tumours. Expression levels of some integrins, such as a2β1, decrease in tumour cells, potentially increasing tumour cell dissemination u In fact, re-expression of a2β1 in breast cancer cells reversed some of the malignant properties of those cells, suggesting that a2β1 could function as a tumour suppressor12. Studies correlating integrin expression levels in human tumours with pathological outcomes, such as patient survival and metastasis, have identified several integrins that might have an important role in cancer progression. Tumour cell expression of the integrins αvβ3, αvβ5, a5β1, a6β4, a4β1 and αvβ6 is correlated with disease progression in various tumour types (TABLE 1), therefore, these are the most studied integrins in cancer. However, this is by no means a complete list and other integrins certainly contribute to cancer progression, particularly on some of the host cell types in the primary tumour.
Table 1.
Integrins in cancer progression
| Tumour type | Integrins expressed | Associated phenotypes |
|---|---|---|
| Melanoma | ανβ3 and α5β1 | Vertical growth phase 35,172–174 and lymph node metastasis173,175 |
| Breast | α6β4 and ανβ3 | Increased tumour size and grade176, and decreased survival177
(α6β4). Increased bone metastasis36–38,64 (ανβ3) |
| Prostate | ανβ3 | Increased bone metastasis39 |
| Pancreatic | ανβ3 | Lymph node metastasis40 |
| Ovarian | α4β1 and ανβ3 | Increased peritoneal metastasis178 (α4β1) and tumour proliferation179 (ανβ3) |
| Cervical | ανβ3 and ανβ6 | Decreased patient survival41,180 |
| Glioblastoma | ανβ3 and ανβ5 | Both are expressed at the tumour–normal tissue margin and have a possible role in invasion181 |
| Non-small-cell lung carcinoma | α5β1 | Decreased survival in patients with lymph node-negative tumours182 |
| Colon | ανβ6 | Reduced patient survival109 |
Integrin regulation of cell survival and apoptosis
Depending on environmental cues, integrins have the ability to either enhance cell survival or initiate apoptosis (FIG. 1). Integrins constantly interrogate their environment through their capacity to interact with the ECM. Ligated integrins relay survival signals, and unligated integrins can promote pro-apoptotic cascades. The balance of these signals results in cell survival or apoptosis based on the ability of the cell to interact with the surrounding ECM. In this way, integrins maintain the integrity of different organs and tissues by preventing cells from surviving in an improper environment.
Figure 1. Integrin-mediated survival versus apoptotic pathways.
Integrins can paradoxically initiate pro-survival as well as pro-apoptotic signals. Which pathway is more active depends on the ligation status of the surface integrins expressed by a given cell. In a cell in which most of the integrins are ligated, a pro-survival pathway is initiated through increased nuclear factor-κB (NF-κB) or PI3K–AKT activity, decreased p53 activation and increased expression of the pro-survival molecules BCL-2 and FLIP (also known as CFLAR). Cooperative signalling between growth factor receptors and integrins also differentially activates Raf leading to distinct mechanisms of cell survival. Signalling through integrin αvβ3 and the fibroblast growth factor receptor promotes phosphorylation of Ser338 and Ser339 of Raf, protecting cells from the intrinsic pathway of apoptosis, and integrin αvβ5 and vascular endothelial growth factor receptor 2 phosphorylate Tyr340 and Tyr341 of Raf, preventing apoptosis through the extrinsic pathway. In adherent cells in which many of the integrins are unligated, the unligated integrins initiate cleavage of caspase 8, triggering apoptosis through integrin-mediated death (IMD). On complete loss of adhesion, cell death is initiated through a process termed anoikis. Apoptosis induced by anoikis may proceed through either the intrinsic or extrinsic pathways. ECM, extracellular matrix; RTK, receptor tyrosine kinase.
Integrin ligation enhances cell survival through several mechanisms, including increased expression of BCL-2 (REFS 13.14) or FLIP (also known as CFLAR)15, activation of the PI3K-AKT pathway16 or nuclear factor-κB (NF-κB) signalling17·18, and/or p53 inactivation19.These cell survival pathways are differentially regulated by specific integrin-growth factor receptor pairs. For example, in endothelial cells integrin αvβ3 crosstalk with fibroblast growth factor receptor (FGFR) prevents apoptosis through the intrinsic apoptosis pathway, and αvβ5 and the vascular endothelial growth factor receptor 2 (VEGFR2) function together to inhibit extrinsic apoptosis20,21. Although integrin antagonists directed to αvβ3 and αvβ5 promoted endothelial cell death, which led to decreased angiogenesis, genetic deletion of Itgb3 (which encodes integrin β3) or deletion of both Itgb3 and ItgbS in mice did not inhibit angiogenesis. However, mice deficient in these integrins showed increased VEGF-mediated angiogenesis22, reflecting a compensatory increase in VEGFR2 in these mice23. Interestingly, Itgb3−/− mice did show abnormal cardiac endothelial cell morphology associated with increased VEGF signalling24. These results highlight crucial differences between studies involving genetic deletion of an integrin during early development and studies in which integrin antagonists were used to suppress integrin function in adult animals. They also illustrate the important role compensation could have in the interpretation of such knockout studies in mice.
Box 1. Integrins in tumour cells.

Integrins expressed in tumour cells contribute to tumour progression and metastasis by increasing tumour cell migration, invasion, proliferation and survival (see the figure). Integrin adhesion to the extracellular matrix (ECM) provides the traction required for tumour cell invasion. Integrins also contribute to tumour cell invasion by regulating the localization and activity of matrix-degrading proteases, such as matrix metalloprotease 2 (MMP2) and urokinase-type plasminogen activator (uPA). Integrin-mediated migration generally requires focal adhesion kinase (FAK)—Src family kinase (SFK) signalling. However, integrin-specific mechanisms do exist in the signalling pathways that result in cell motility. For example, in neuroblastoma cells although the integrin a5β1 uses the expected FAK-mediated activation of SRC, integrin α4β1 activates SRC through a FAK-independent mechanism168. Some integrins inhibit tumour cell motility, as integrin β1 (Itgb1) deletion increased tumour cell dissemination in a mouse model of spontaneous pancreatic islet cancer11. Regulation of integrin recycling is also crucial for tumour cell invasion. Rab GTPases direct integrins to the leading edge of invading tumour cells79 and coordinately regulate integrin and growth factor receptor recycling, resulting in enhanced growth factor signalling79. Differences in the recycling pathways mediated by particular integrins also influence random rather than persistent cell migration169. In addition to their well-established role in migration and invasion, integrins also regulate proliferation. Integrin ligation controls the expression of key cell cycle proteins, including cyclin D1 (REF. 170) and the cyclin-dependent kinase inhibitor family, which regulate entry into the S phase of the cell cycle171. Adhesion-dependent control of cell proliferation is deregulated in tumour cells, as anchorage-independent growth is a hallmark of malignant transformation. However, integrins also have an important role in tumour growth4,5. Further research is needed to determine the mechanisms by which integrins continue to regulate proliferation in tumour cells. Integrins also control tumour cell survival. Ligated integrins prevent pro-apoptotic signalling cascades initiated by anoikis or integrin-mediated death and increase survival signalling. However, recent evidence also points to a role for unligated integrins in regulating tumour cell survival and malignancy.
Recent studies have highlighted the role integrins have in modulating apoptosis. Early work hinted at a possible dual role for integrins in both promoting cell survival and inducing cell death. Studies have shown that although some integrins, such as αvβ3 and a6β4, enhance tumour progression25, paradoxically, others such as a5β1 inhibit oncogene-induced transformation.26,27. Further experiments showed that the pro-tumorigenic integrin αvβ3 could inhibit tumour progression in some mouse models of glioblastoma28 and melanoma29. This apparent discrepancy might be explained by the discovery that unligated integrins can induce apoptosis30,31. In a process termed integrin-mediated death (IMD), unligated integrins on adherent cells recruit and activate caspase 8, resulting in apoptotic cell death30 IMD is distinct from anoikis, which is apoptosis that occurs in response to cellular detachment from the ECM32. Further studies demonstrated that the loss of caspase 8 is one mechanism by which tumour cells can avoid IMD, allowing increased metastatic dissemination33. It is still unclear what part IMD plays in the therapeutic effects of integrin antagonists. However, it is thought that by inhibiting adhesion to the ECM, integrin antagonists can induce IMD and therefore have a greater effect in IMD-sensitive tumours.
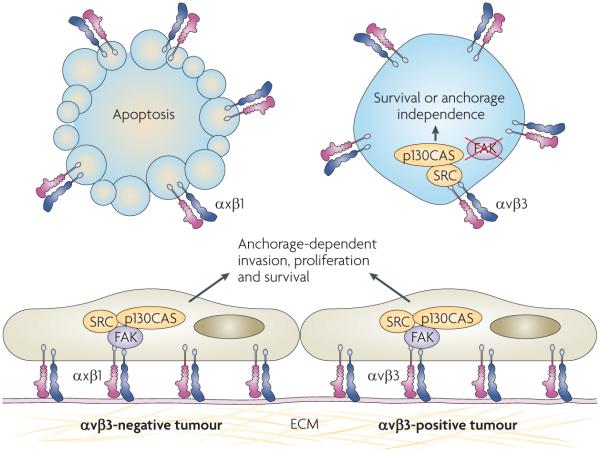
In recent work, we have shown that in IMD-resistant tumour cells the unligated integrin αvβ3 substantially increases anchorage-independent tumour cell survival in vitro and metastasis in vivo34 (FIG. 2). These effects specifically required integrin αvβ3 recruitment and the activation of the non-receptor tyrosine kinase SRC, which leads to a FAK-independent survival pathway. This anchorage-independent integrin αvβ3-SRC signalling module might explain the association between integrin αvβ3 and tumour progression, as observed in various clinical studies35–41, and could have important clinical ramifications. First, it suggests that αvβ3 antagonists that function by blocking ligand binding to tumour cells might be ineffective in treating some αvβ3-positive tumours. However, it remains possible that such antagonists could still function as anti-angiogenic agents. Second, our study shows that integrin αvβ3-expressing tumours that recruit and activate SRC in this manner may be particularly sensitive to SFK inhibitors such as dasatinib.
Figure 2. An integrin αvβ3–SRC oncogenic unit promotes anchorage independence.
In tumour cells, both β1 integrins (that is, αxβ1) and integrin αvβ3 induce adhesion-dependent activation of focal adhesion kinase (FAK) and SRC, in addition to phosphorylation of the adaptor protein p130 CRK-associated substrate (p130CAS). These signalling events result in invasion, proliferation and survival of tumour cells bound to the extracellular matrix (ECM). In suspended tumour cells unligated integrin αvβ3 signals directly through SRC and p130CAS to increase cell survival independently of FAK. This effect occurs in tumour cells that are already resistant to integrin-mediated death.
Integrin regulation of cancer stem cells
Cancer stem cells represent a highly tumorigenic subset of cells in the primary tumour. Recent evidence has implicated integrins as markers of both normal progenitor and stem cell populations and cancer stem cells. In particular, the integrin αvβ3 represents a marker of luminal progenitor cells in the mammary ductal epithelium42. In a mouse model of spontaneous mammary tumorigenesis, expression of the proto-oncogene WNT1 caused the expansion of the luminal progenitor cell population, among which the luminal marker integrin β3 (also known as CD61) represented a highly tumorigenic cancer stem cell population43. Integrin β3 expression identified cancer stem cells in around 50% of Trp53+/− tumours, but interestingly an integrin β3-positive cancer stem cell population was not found in the more homogeneous ERBB2 (also known as Neu)-positive tumours43. This finding may explain the lack of any observed effect when ERBB2 was used to drive tumour initiation in Itgb3−/− mice44. Integrin signalling seems to maintain the cancer stem cell population in tumours, as ablation of Ptk2 (which encodes FAK) decreased the pool of cancer stem cells in spontaneously forming mouse mammary tumours45. Additionally, integrins may regulate the expression of cancer stem cell markers, such as CD44 (REF. 46). As cancer stem cells are thought to represent the most tumorigenic and aggressive subset of a particular tumour, it is tempting to speculate that the expression of specific integrins could enhance cancer stem cell properties through cooperation with tumour-initiating oncogenes or growth factor receptors.
The host cellular response to cancer
In addition to their role in tumour cells, integrins are also important for the host cellular response to cancer. Endothelial cells, fibroblasts, pericytes, bone marrow-derived cells, inflammatory cells and platelets all use integrins for various functions, including angiogenesis, desmoplasia and the immune response (FIG. 3). In addition to integrins expressed on tumour cells, integrins present on many of these cell types might be potential therapeutic targets.
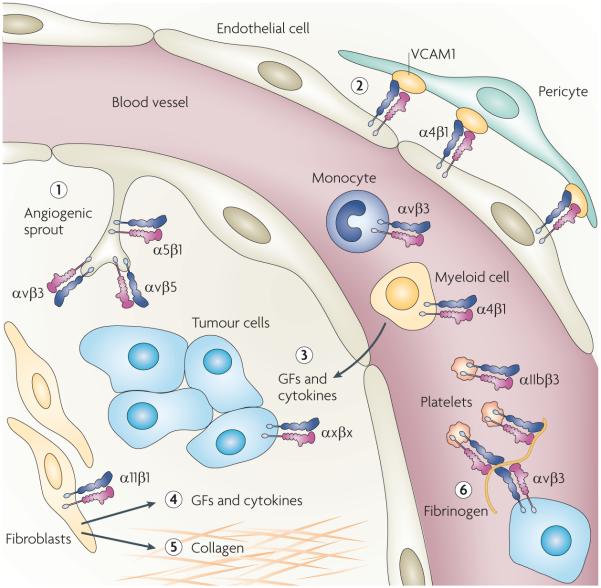
Figure 3. Integrins in the host response to cancer.
Integrins expressed in many tumour-associated cell types have crucial roles in increasing tumour progression and metastasis. In endothelial cells, integrins regulate the migration, proliferation and survival necessary for angiogenesis (step 1). The interaction between pericytes and endothelial cells is crucial for the stabilization of newly formed vessels during angiogenesis. Binding of integrin α4β1 on endothelial cells to vascular cell adhesion molecule 1 (VCAM1) on pericytes plays an important part in pericyte recruitment to the neovasculature (step 2). Myeloid cells and monocytes in primary tumours contribute to disease progression by secreting cytokines and growth factors (GFs) that initiate angiogenesis and tumour cell migration (step 3). Several studies have shown that integrins have an essential role in the homing of myeloid cells and monocytes to tumours. Fibroblast infiltration into the primary tumour, known as desmoplasia, also contributes to tumour progression through increased growth factor secretion (step 4). In addition, the invading fibroblasts deposit large amounts of collagen that might result in resistance to therapy in some tumours (step 5). A recent study showed that integrins, such as α11β1, are crucial regulators of growth factor secretion by these fibroblasts. Platelet expression of αIIbβ3 may be important for interacting with tumour cells through a fibrinogen bridge, possibly aiding in metastatic dissemination (step 6).
Angiogenesis
The contribution of angiogenesis to tumour progression is well established and the role of integrins has recently been reviewed47. Tumour-associated blood vessels are structurally and biologically distinct from quiescent vessels, and according to Harold Dvorak “tumours make bad blood vessels” (REF. 48). Their tortuous and leaky characteristics compromise blood flow, impair drug delivery, promote fibrosis and facilitate tumour cell intravasation leading to haematogenous or lymphatic metastasis. We have established that, unlike quiescent endothelium, tumour-associated vessels express integrin αvβ3 (REF. 49). It is possible that increased expression of integrins αvβ3 and αvβ5 allow angiogenic endothelial cells to bind provisional matrix proteins such as vitronectin, fibrinogen, von Willebrand factor, osteopontin and fibronectin that are deposited in the tumour microenvironment. In addition, proteolyzed, but not native, collagen is a ligand for integrin αvβ3 owing to the exposure of RGD sites made available by proteolysis50. These adhesive interactions could provide survival cues and/or traction for invading endothelial cells.
Through genetic deletion, or treatment with integrin antagonists, several additional integrins have been identified as crucial for angiogenesis, including α1β1, α2β1, α4β1, α5β1, α6β1, α9β1 and α6β4 (REF. 47). Integrin cooperation with particular growth factor receptors seems to confer responsiveness to specific angiogenic growth factors that are highly expressed in tumours. For example, αvβ3 and FGFR interaction induces angiogenesis downstream of FGF binding, and αvβs and VEGFR2 promote VEGF-induced angiogenesis51. The development of cilengitide as an anti-tumour and antiangiogenic agent directed to both integrins αvβ3 and αvβ5 was partly based on these findings. These distinct pathways of angiogenesis highlight the fact that integrins can integrate cues from the ECM and growth factors to drive specific intracellular signalling events.
Perivascular cells
Angiogenesis not only depends on the invading endothelium but also requires perivascular cells, such as pericytes and vascular smooth muscle cells, which associate with the developing endothelium and promote blood vessel maturation. Tumours typically express immature blood vessels with reduced perivascular coverage52. This leads to tortuous and leaky vessels that account for much of the hypoxia and poor perfusion typically observed in tumours. Integrins regulate the interaction between endothelial cells and the vascular basement membrane, and recent studies suggest that the endothelial cell integrin α4β1 is necessary for an interaction with vascular cell adhesion molecule 1 (VCAM1) on pericytes, resulting in endothelial cell–pericyte interaction and vessel stabilization53. Recent studies have described an important role for blood vessel recruitment of pericytes in regulating blood vessel branching and patency in tumours through vascular normalization54, the manipulation of which may improve the delivery of chemotherapeutics. According to Jain and colleagues54 normalizing the tumour vasculature with agents such as bevacizumab or other VEGF pathway inhibitors should make it possible to increase drug delivery to the tumour and gain an improved therapeutic index for a wide range of anti-tumour agents. In fact, crosstalk between growth factor receptors, such as VEGFR2 and platelet-derived growth factor receptor (PDGFR), regulates pericyte recruitment to tumour-associated blood vessels55. Integrin cooperation with these growth factor receptors may be vital for regulating blood vessel normalization in tumours.
Desmoplasia
Abundant collagen deposition is a hallmark of the desmoplastic reaction in both primary tumours and their metastases. Through integrin signalling, the deposited collagen increases tumour cell proliferation, survival and chemoresistance, possibly contributing to the establishment and progression of metastatic lesions56. Integrins that are expressed on stromal fibroblasts also contribute to enhanced tumour growth. Integrin α11 is commonly overexpressed in stromal fibroblasts that are associated with non-small-cell lung carcinoma (NSCLC). Expression of α11β1 on fibroblasts increased tumour growth by stimulating the release of insulin-like growth factor 2 (IGF2)57. This study highlights the importance of the regulation of growth factor signalling by integrins for the tumour-promoting effects of the host stroma. Targeting the tumour stroma with integrin antagonists could represent a new avenue for tumour therapy.
Bone marrow-derived cells
Circulating bone marrow-derived cells are recruited to solid tumours, in which they can suppress tumour growth and also secrete pro-angiogenic growth factors and cytokines that contribute to tumour progression. Immune cells, including macrophages and natural killer cells, are crucial for tumour suppression. For example, macrophage tumour infiltration is decreased in Itgb3−/− mice and contributes to increased tumour burden, demonstrating that the expression of integrin αvβ3 on macrophages is important for their tumour suppressive function58. Alternatively, the tumour-homing of bone marrow-derived cells can result in increased tumour progression by increasing angiogenesis. Bone marrow cells expressing a functionally inactive integrin β3 mutant failed to be recruited to sites of neovascularization, resulting in decreased pathological angiogenesis59. Homing of endothelial and monocyte precursors to tumours also requires integrin α4β1 (REF. 60). Expression of integrin α4β1 on bone marrow-derived cells promotes adhesion to the tumour-associated endothelium, and blockade of integrin α4β1 reduced blood vessel density60,61. It is not clear whether blocking tumour homing of bone marrow-derived cells represents a viable therapeutic strategy as their tumour-suppressive effects might outweigh their pro-angiogenic potential.
Platelets
Multiple studies have linked tumour cell–platelet interactions with increased tumour metastasis. The ECM protein fibrinogen functions as a bridge between integrins αIIbβ3 on platelets and αvβ3 on tumour cells. This interaction facilitates tumour cell arrest in the vasculature, leading to metastasis to various sites, including the bone marrow62-64. Combined blockade of both tumour integrin αvβ3 and platelet integrin αIIbβ3 increased the antiangiogenic and anti-tumour effects compared with blocking tumour integrin αvβ3 alone65, suggesting that antagonists that target both integrins on platelets and endothelial cells could have greater clinical efficacy in patients.
Integrin cooperation with oncogenes
Although integrins lack the ability to transform cells, and therefore do not function as oncogenes, several integrins cooperate with oncogenes or receptor tyrosine kinases to enhance tumorigenesis. In spontaneous mouse models of tumorigenesis, integrins such as α6β4 cooperate with ERBB2 to increase breast tumour onset and invasion66. Integrin β1 mediates breast cancer that is driven by the polyoma middle T oncoprotein67, and integrin α1 is required for KRAS-G12D-induced tumours in the lung68. There seems to be some specificity to the ability of integrins to crosstalk with particular oncogenes, as integrin signalling through FAK is required for oncogenesis through Ras and PI3K69,70, whereas tumorigenesis induced by ERBB2 required integrin α6β4 (REF. 70). In another example, in vitro and in vivo experiments showed that integrin αvβ3 synergizeswith the SRC oncogene to increase tumorigenic potential71. Interestingly, this effect enhanced only the oncogenic effects of SRC, and not morphological transformation72. These studies suggest that some oncogenes are dependent on integrin signalling, a property that could potentially be exploited therapeutically.
Integrin crosstalk with growth factor cytokines
Growing evidence supports a central role for cooperative signalling between integrins, growth factor receptors and cytokine receptors in many aspects of tumour progression (FIG. 4). Integrin crosstalk not only regulates tumour cell adhesion, migration, invasion and survival, but also affects many aspects of the host response to cancer, particularly in the angiogenic endothelium. However, not all crosstalk is pro-tumorigenic, as some integrins can inhibit tumorigenesis that is induced by certain oncogenes73. From the numerous examples of crosstalk that have been described, several themes have emerged regarding the underlying mechanisms involved. In some instances cooperative signalling, possibly mediated by the formation of an integrin–growth factor receptor complex74–77, potentiates activation of downstream kinases such as MAPK78 or AKT79 and therefore enhances cell migration and survival. In other examples, integrins and growth factor or cytokine receptors reciprocally regulate the surface expression of one another80–89, or the release of their respective ligands90,91. In addition to these general mechanisms, recent studies have elucidated other models of crosstalk (described below) that could have important implications for tumour metastasis and the acquisition of drug resistance.
Figure 4. Integrin–growth factor and integrin–cytokine receptor crosstalk.
Cooperation between integrin and growth factor signalling or integrin and cytokine signalling is crucial to tumour progression. Several crosstalk mechanisms have so far been elucidated. Integrin ligation may lead directly to the increased secretion of growth factors and/or cytokines, which can then bind to their receptors in an autocrine or paracrine manner to further induce signalling. In addition, signalling induced by either integrin ligation or growth factor binding may activate common downstream pathways resulting in enhanced signalling overall compared with the activation of either receptor alone. This signalling seems to most commonly converge on kinases such as Src family kinases (SFKs), scaffolding proteins such as p130 CRK-associated substrate (p130CAS), and GTPases, such as the Ras family. Alternatively, both chemokine and growth factor signalling may regulate integrin function by directly controlling integrin expression levels. ECM, extracellular matrix; EGF, epidermal growth factor; FAK, focal adhesion kinase; SDF1, stromal cell-derived factor 1; RTK, receptor tyrosine kinase.
EGF and its receptors
Members of the epidermal growth factor (EGF) receptor family, including EGFR and ERBB2, contribute to tumour formation and metastasis in many tumour types, including breast and pancreatic cancer. Increased expression and hyperactivation of EGF receptors occurs in many cancers, and overexpression of ERBB2 is oncogenic. In tumour cells, cooperation between integrins and members of the EGF receptor family affect many aspects of tumour progression, including tumour initiation, proliferation, migration and invasion. The integrin α6β4 may be particularly vital to tumour formation in the subset of patients with breast cancers that express high levels of ERBB2 as it cooperates with integrin α6β4 to induce spontaneous mammary tumour formation and tumour cell invasion66. This cooperative effect could be due to the formation of an integrin α6β4-ERBB2 complex that enhances the activation of signal transducer and activator of transcription 3 (STAT3) and JUN, leading to the loss of cell polarity and hyperproliferation, respectively66. Furthermore, these studies found that the deletion of Itgb4 increased the efficacy of targeted ERBB2-specific therapy66, highlighting the potential importance of combination therapy using antagonists targeting integrin and EGF receptor family members.
In pancreatic cancer, the EGF pathway is often hyperactivated, which potentiates the tumour cell migration and metastasis of this highly aggressive disease. EGF stimulates pancreatic tumour cell migration on vitronectin in vitro92–94 and metastasis in vivo.93,94 and these effects require integrin αvβ5 (REF. 94). Interestingly, before activation in non-stimulated cells integrin αvβ5 is unable to cluster and form focal adhesions on its own95, which may be a prerequisite for integrin-mediated cell migration. Instead, αvβ5 requires EGF-dependent activation of SRC for its ability to mediate cell migration94. Further studies revealed a requirement for SRC phosphorylation of the p130CAS substrate domain and subsequent activation of the GTPase RAP1A (REF. 94), a known mediator of integrin activation. EGF–integrin crosstalk is not limited to pancreatic cancer, and it also increases the migration of colon cancer cells through integrins α3β1 and α6β4 (REF. 96), and hepatocellular carcinoma through integrins α1β1 and α2β1 (REF. 97). Therefore, EGF signalling in tumour cells may increase the ability of particular integrins to mediate cell migration and survival, resulting in increased metastatic potential.
Other studies have demonstrated that integrin ligation itself regulates EGF signalling, crucially influencing tumour cell susceptibility to treatment. In fact, integrin ligation can induce EGFR phosphorylation independently of EGF, resulting in increased MAPK activation, tumour cell proliferation and survival98 through a SRC-pl 30CAS pathway99. The ability of integrins to increase EGFR signalling may be particularly important in breast cancers expressing high levels of ERBB2. A recent study found that integrin signalling increased EGF secretion and ERBB2 clustering in breast cancer cells, resulting in resistance to the ERBB2 inhibitor trastuzumab90. Inhibition of integrin signalling reversed trastuzumab resistance, suggesting that this combined approach may prove therapeutically efficacious in ERBB2-expressing breast cancers.
HGF and receptors
The hepatocyte growth factor (HGF) receptor MET is implicated in tumour initiation and the metastasis of various cancers, and integrin cooperation with MET results in enhanced tumour progression. In particular, integrin β4 synergizes with MET to enhance the transformation of fibroblasts and increase tumorigenic potential100. In breast cancer cells, HGF binding to MET increases anchorage-independent growth by inducing phosphorylation of integrin β4, resulting in the recruitment of proteins such as SHP2 (also known as PTPN11) and the subsequent activation of SRC and ERK101. Complex formation between MET and integrin α6β4 enhances HGF-induced signals, including tumour cell invasion77. This effect may be due to the potentiation of HGF-induced Ras and PI3K signalling by α6β4-mediated recruitment of proteins such as SHC1 and PI3K77. However, integrin β4 is not required for HGF-induced tumour cell invasion102. Additionally, MET cooperates with other integrins, as integrin αvβs contributes to MET signalling by controlling the expression of HGF-induced genes required for cell migration103. Integrin-MET crosstalk is also indirectly regulated by other molecules. For example, the tetraspanin KAI1 (also known as CD82) suppresses integrin-mediated activation of MET resulting in reduced tumour cell invasion104. Therefore, the role of MET in cancer seems to depend on crosstalk with tumour cell-associated integrins.
TGFβ3 and receptors
Although generally known for its anti-proliferative effects, transforming growth factor-β (TGFβ) is a well-characterized inducer of epithelial-mesenchymal transformation (EMT) in tumour cells, resulting in enhanced cell migration and invasion. Integrins are instrumental in the activation of TGFβ signalling. TGFβ ligands are secreted as inactive complexes with a latency-associated peptide (LAP). The TGFβ1 LAP was first identified as a ligand for integrin αvβ6, and expression of integrin αvβ6 regulates TGFβ1 activation105. It is now known that multiple αv integrins can bind the RGD motif in the LAP of TGFβ1, but only binding of integrin αvβ6 and αvβ8 results in TGFβ1 activation106. In cancers such as basal cell carcinoma, increased integrin αvβ6 expression correlates with aggressive disease, possibly owing to increased TGFβ1 activation forming a dense tumour stroma107. Further studies have shown that integrin αvβ6 activates TGFβ1 in vivo, contributing to tumour growth108, and that upregulation of integrin αvβ6 in tumour cells is associated with EMT, TGFβ1 activation and increased migration109
Integrin signalling can also directly modulate TGFβ responses. Integrin αvβ3and SRC cooperate with TGFβ to induce EMT of mammary epithelial cells110, and this requires SRC-dependent phosphorylation of TGFβ receptor type 2 (TGFBR2)111. Additionally, TGFβ stimulation induces phosphorylation of the cytoplasmic domain of integrin β1, resulting in integrin activation and tumour cell invasion112. As TGFβ is predominantly secreted by tumour stromal cells, crosstalk between integrins and TGFβ may have an important role in the contribution of the tumour stroma to cancer progression.
VEGF, FGF and their receptors
Integrin-growth factor crosstalk not only occurs on tumour cells, but also has a role in various host cell types, including endothelial cells, in which it contributes to tumour angiogenesis. During angiogenesis, both integrins and growth factors are vital to endothelial cell migration, proliferation and survival. Therefore, considerable effort has gone into identifying specific functional interactions between individual integrins and growth factor receptors. In fact, distinct integrin-growth factor pairs have been described that contribute to angiogenesis through different signalling pathways.
During tumour angiogenesis endothelial cells in the tumour microenvironment must resist cell death that is induced by stresses such as hypoxia and nutrient deprivation (intrinsic apoptosis) or inflammatory mediators (extrinsic apoptosis). We have previously described distinct pathways of angiogenesis that are mediated by specific integrin-growth factor receptor pairs49. These pathways signal to protect endothelial cells from distinct apoptotic stimuli through differential activation of Raf19,20 (FIG. 2). FGFR cooperates with integrin αvβ3 to increase the phosphorylation of Raf Ser338 and Ser339 through PAK19,20, resulting in Raf–ASKl (also known as MAP3K5) complex formation in the mitochondria, inhibiting the intrinsic pathway of apoptosis113. By contrast, VEGFR2 cooperates with integrin αvβs, leading to SRC-dependent phosphorylation of Raf Tyr340 and Tyr341 and resistance to extrinsic apoptosis that is induced by inflammatory mediators such as tumour necrosis factor (TNF)19,20. This underscores the importance of Raf during tumour angiogenesis and documents how distinct integrin-growth factor pairs can differentially influence downstream endothelial survival pathways. A central role for Raf in angiogenesis was established by targeting mutationally inactive Raf to the tumour vasculature, which potently inhibited angiogenesis and tumour growth in mice114.
Additional examples of crosstalk between VEGFR2 and αv integrins have also been described in vascular endothelial cells. For example, SRC recruitment to VEGFR2 promotes crosstalk with integrin αvβ3 and SRC-dependent tyrosine phosphorylation of the cytoplasmic domain of integrin β3 (REF. 113). This may be related to the increased levels of VEGFR2 in Itgb3−/− mice leading to a compensatory increase in angiogenesis22. Another mechanism by which VEGF influences integrin αvβ3 signalling is through regulating the affinity state, or activation of the integrin115. Activation of integrin αvβ3 can in turn increase tumour cell secretion of VEGF, providing a feedback loop resulting in increased tumour growth91. Specific integrin-growth factor pairs have also been identified in FGF-mediated angiogenesis. Integrin β4 contributes to FGF-mediated angiogenesis, as a targeted deletion of the signalling portion of the cytoplasmic domain of integrin β4 resulted in decreased FGF-induced angiogenesis and reduced tumour size116. Therefore, signalling through distinct integrin-growth factor receptor pairs has crucial roles in tumour angiogenesis.
CXCR4
Although best characterized for its role in the recruitment of haematopoietic cells to sites of injury or infection, the chemokine receptor CXCR4 is also expressed on tumour cells and various tumour-associated cell types. Binding of CXCR4 to its cognate ligand stromal cell-derived factor 1 (SDFL; also known as CXCL12) induces tumour cell migration and contributes to metastasis. SDF1 stimulation of CXCR4 on tumour cells increases the expression of integrins, such as α5β1 and αvβ3, increasing cell adhesion and invasion in vitro87,88 and experimental metastasis in vivo117. In addition to increasing integrin surface expression, SDF1 controls adhesion by augmenting integrin activation118. In another example, integrin ligation reciprocally regulates CXCR4 expression levels89. Further studies are needed to determine the relevance of integrin-CXCR4 crosstalk to tumour growth and spontaneous metastasis.
Integrins as targets for cancer therapy
The expression of integrins in various cell types that are involved in tumour progression and their ability to crosstalk with growth factor receptors has made them appealing therapeutic targets. Preclinical studies showed that integrin antagonists inhibit tumour growth by affecting both tumour cells and tumour-associated host cells, most notably the angiogenic endothelium. Integrin antagonists currently in clinical trials include monoclonal antibodies and RGD peptide mimetics (see Avraamides et al.47 for a complete review). Years of preclinical studies and early clinical trials have now culminated in the initiation of a Phase III clinical trial in glioblastoma with the RGD peptide mimetic cilengitide. In this section, we discuss the current status of integrin antagonists in cancer therapy, including the studies supporting the design of the first Phase III clinical trial of an integrin inhibitor in cancer.
Targeting αvβ3 and αvβ5
Integrin αvβ3 is upregulated in both tumour cells and angiogenic endothelial cells, making it an attractive therapeutic target. Function-blocking monoclonal antibodies, such as LM609, were among the first integrin antagonists developed, and showed considerable anti-angiogenic activity in preclinical models119. As a result of these studies, etaracizumab (MEDI-522), a humanized version of LM609, was developed. In addition to its anti-angiogenic effects, etaracizumab inhibited tumour growth by directly affecting tumour cells120, and impaired bone resorption by inhibiting osteoclast attachment, suggesting possible efficacy in reducing bone metastasis121. As a result of its efficacy in preclinical studies, etaracizumab was one of the first integrin antagonists introduced into clinical trials. Phase I trials with vitaxin, the precursor of etaracizumab, showed antiangiogenic activity122, low toxicity and disease stabilization in some patients with advanced solid tumours123 and renal cell cancer124. A Phase II study showed some efficacy in metastatic melanoma125. The human avintegrin-specific monoclonal antibody CNTO 95, which targets both αvβ3 and αvβs integrins, also had anti-tumour and anti-angiogenic effects in xenograft tumour models126,127. In a Phase I trial, CNTO 95 was non-toxic128, localized to tumours and showed signs of anti-tumour activity129. Both CNTO 95 and etaracizumab are being further evaluated in additional clinical trials.
Cilengitide is an inhibitor of both αvβ3 and αvβ5 integrins. We had shown that αvβ3 and αvβ5 integrins were important regulators of angiogenesis and tumour growth49,51,191,192 and developed a cell-free receptor assay to select for antagonists of integrins αvβ3 and αvβ5 that did not effect integrin αIIbβ3 (REF. 130). This assay was used to screen a library of integrin binding cyclic RGD peptides designed and synthesized by H. Kessler and colleagues for αvβ3 activity and selectivity193–195 from which cilengitide was developed196. Cilengitide is currently being tested in Phase II trials in patients with lung and prostate cancer131, and Phase II and Phase III trials are currently underway in glioblastoma. So far, cilengitide has shown significant promise in patients with late-stage glioblastoma by extending patient survival with minimal side effects (discussed below)132–135. Nevertheless, in mouse studies, Reynolds et al.22 found that the continuous infusion of very low concentrations of RGD peptides paradoxically stimulates tumour growth and angiogenesis by promoting VEGF-induced endothelial cell migration22. These results are consistent with published accounts from other groups showing that low concentrations of soluble integrin antagonists can function as integrin agonists in some cases136–138. Such studies might be relevant to recent studies in which inhibitors of VEGF increased tumour perfusion resulting in enhanced tumour progression139,140. However, the increased tumour perfusion associated with antiangiogenic therapy might be exploited to increase the delivery of chemotherapeutic agents, potentially explaining why anti-angiogenic agents such as cilengitide are most effective when used in combination with chemotherapy. It is also important to consider that cilengitide and other anti-angiogenic therapies might target multiple cell types in the tumour microenvironment, including the tumour cells themselves, and therefore their anti-tumour effects may not be entirely due to anti-angiogenic activity.
Glioblastomas are aggressive, highly vascularized brain tumours for which patient survival is only marginally increased by current therapies. Once diagnosed, patients typically have a short life expectancy of only a few months. Consequently, the development of therapeutic options that control this disease is crucial. These highly vascularized tumours express integrin αvβ3 on angiogenic blood vessels, as well as the tumour cells themselves, suggesting that antagonists to this integrin might be therapeutically beneficial in patients with glioblastoma. In preclinical studies, cilengitide effectively inhibited angiogenesis and the growth of orthotopic glioblastoma141,142. Importantly, the brain microenvironment was a crucial determinant of the susceptibility of these tumours to cilengitide, as tumours that formed in the flank of these same mice were unaffected by treatment with this drug141. In addition, high-grade glioblastomas abundantly express the ECM protein vitronectin, an integrin αvβ3 ligand, and this interaction affects tumour cell survival14 and invasion143. Therefore, the relatively large quantity of vitronectin present in the brain microenvironment surrounding glioblastomas might explain why these tumours are susceptible to cilengitide treatment.
These promising preclinical studies were the basis for clinical trials in patients with glioblastoma. Phase I studies with cilengitide in patients with recurrent glioblastoma showed that it was well tolerated and produced durable responses that seemed to be related to changes in relative cerebral blood flow132. Another Phase I study in children with refractory brain tumours determined that it was well tolerated and produced stable disease, with a full response in some patients133. A Phase II trial with cilengitide showed anti-tumour efficacy and minimal toxicity in patients with recurrent glioblastoma134. A different Phase I/II trial examined cilengitide in patients with newly diagnosed glioblastoma and met its primary end point with 69% of patients progression-free after 6 months135. Importantly, this trial made the observation that patients with lowered expression of O-6-methylguanine-DNA methyltransferase (MGMT), owing to promoter methylation, exhibited a higher rate (91%) of progression-free survival at 6 months. The MGMT promoter is a prognostic marker in patients with glioblastoma: tumours with unmethylated MGMT promoters indicate a lower probability of patient survival, as MGMT is thought to increase resistance to drugs such as temozolomide, which is a current standard therapy.
The favourable results obtained from these early clinical trials provided the impetus for a Phase III trial with cilengitide that began in October 2008. The CENTRIC trial will enroll approximately 500 patients and measure the effect of cilengitide on the survival of patients with MGMT promoter methylation in combination with temozolomide and radiotherapy. This is the first Phase III oncology trial carried out with any integrin antagonist. As a companion to the CENTRIC trial, the Phase II CORE trial will assess the efficacy of cilengitide in a large number of patients whose tumours have unmethylated MGMT promoters.
Targeting β1 integrins
Strategies that target β1 integrins, particularly αsβ1, have also shown efficacy in reducing tumour burden in preclinical models. An integrin β1 inhibitory antibody significantly affected in vitro and in vivo growth of human breast cancer tumour cells144. Volociximab, a function-blocking monoclonal antibody against integrin α5β1, inhibits angiogenesis and impedes tumour growth145,146. A Phase I trial in patients with advanced solid malignancies showed that volociximab was well tolerated and may have clinical efficacy147. Volociximab is currently in Phase II clinical trials for solid tumours148. ATN-161 is a non-RGD-based peptide inhibitor of integrin α5β1 that blocks breast cancer growth and metastasis in vivo149. In mouse models of colon cancer metastasis to the liver, combination therapy with ATN-161 and fluorouracil significantly reduced tumour burden and liver metastases compared with either treatment alone150. ATN-161 was tested in patients with advanced solid tumours and was well tolerated and prolonged stable disease in one-third of the patients151.
Additional integrin antagonists
Several additional integrin antagonists have shown efficacy in preclinical studies, but have not yet made it to the clinic. In xenograft tumour models, the integrin αvβ3 small-molecule antagonist S247 inhibited breast cancer bone metastases152, decreased colon cancer metastasis and angiogenesis, and increased survival153. A non-peptide antagonist of integrin αvβ3, PSK1404, inhibited breast and ovarian cancer bone metastases without affecting osteoclast activity154. Administration of the RGD peptidomimetics S137 and S247 produced anti-metastatic effects155. Preclinical studies showed that antisense to either ITGAV or ITGB3 suppressed the growth of subcutaneously injected human hepatocellular carcinoma cells156. A monoclonal antibody against integrin αvβ6, 6.3G9, blocked the growth of human pharyngeal carcinoma cells both in vitro and in vivo108. Interestingly, treatment of cells with this antibody also inhibited TGFβ signalling, suggesting that at least some of its efficacy might require crosstalk with the TGFβ receptors. It will be interesting to observe the performance of these new agents in clinical trials, and study how their efficacy may be optimized in combination with additional therapeutic strategies.
Targets for cancer imaging and drug delivery
Imaging
Currently, there are no validated biomarkers for clinically assessing the efficacy of anti-angiogenic therapies, including cilengitide. Although candidate markers are being investigated, including serum levels of VEGF, FGF and placental growth factor, as well as the abundance of circulating endothelial cells and their precursors, these markers have not yet consistently predicted tumour response. As a result, better vascular imaging techniques are being developed to monitor responsiveness to treatment. In particular, considerable effort has been expended on characterizing integrin antagonists for their ability to specifically deliver diagnostic agents to tumour cells and associated blood vessels. Coupling of the integrin αvβ3 antibody LM609 or other antagonists to a paramagnetic contrast agent157 or radionuclides158 has allowed the detection of angiogenic vessels in rabbit and mouse tumour models. An integrin αvβ3-targeted magnetic resonance imaging nanoparticle has also been used to detect the neovasculature of minute solid tumours in a xenograft tumour model159. Additionally, angiogenic vessels can be detected by contrast enhanced ultrasound with microbubbles targeting αv integrins160. RGD peptides labelled with64Cu,18F and ultrasmall superparamagnetic iron oxide particles have also been used to detect αv integrins in xenograft models of breast161 brain162 and lung cancer163 respectively. Some of these imaging agents have recently undergone evaluation in cancer patients. Scintigraphic imaging using a radiolabelled integrin αvβ3-targeted peptide (99mTc-NC100692) detected a high proportion of malignancies in patients with breast cancer164,165. In another study, delivery of18F-galacto-RGD in combination with positron emission tomography (PET) provided non-invasive quantitative assessment of integrin αvβ3 expression in human tumours166. These studies suggest that labelled integrin antagonists could provide important diagnostic tools for assessing the efficacy of anti-angiogenic and anti-tumour therapies.
Targeted delivery of therapy
Integrin-targeted therapeutics have recently proved beneficial in delivering chemotherapeutics, oncolytic viruses, proapoptotic peptides (such as TNF and TNF-related apoptosis-inducing ligand (TRAIL)) and radionucleotides to both tumour cells and the supporting vasculature (TABLE 2). The use of integrin targeting to deliver therapeutics to the tumour vasculature was first shown by Hood et al.114, who used an integrin αvβ3-targeted nanoparticle to selectively deliver a mutant RAF1 gene to the tumour vasculature, resulting in apoptosis of endothelial cells and tumour regression. More recent studies showed that delivery of targeted nanoparticles loaded with doxorubicin to integrin αvβ3-positive tumour vasculature inhibited the growth of metastases while eliminating the toxicity and weight loss associated with systemic administration of this drug167. This delivery method resulted in a 15-fold improvement in tumour and anti-metastatic activity when compared with administration of the free drug. The preferential activity of these nanoparticles on metastases suggests that growing metastatic tumours may have a greater dependence on angiogenic vessels and so could be more susceptible to integrin αvβ3-targeted therapy.
Table 2.
Integrin targeting methods
| Therapeutic agent | Targeting moiety | Tumour model(s) | Results | Refs |
|---|---|---|---|---|
| Mutant RAF1 | Organic ανβ3 ligand |
Subcutaneous human melanoma cells |
Regression of established primary and metastatic tumours and apoptosis of the tumour-associated vasculature |
113 |
| Nanoparticle loaded with doxorubicin |
cRGD | Orthotopic mouse pancreatic tumour cells and human melanoma and renal tumour cells |
Suppressed spontaneous metastases by disrupting the associated vasculature at very low doses |
167 |
| Nanoparticle loaded with fumagillin |
cRGD | Vx-2 rabbit adenocarcinoma | Suppressed angiogenesis and tumour development at low doses |
183 |
| Oncolytic measles virus |
cRGD | Subcutaneous human myeloma cells |
Targeted delivery of virus to tumour neovessels |
184 |
| TRAIL | RGD | Subcutaneous human colon cancer cells |
Inhibited primary tumour burden to a greater extent than TRAIL alone |
185 |
| p53 | RGDK-lipopeptide | Orthotopic mouse melanoma cells |
Targeted tumour vasculature and inhibited tumour volume |
186 |
| Radionucleotide | cRGD | Subcutaneous human ovarian cancer cells |
Increased survival compared with untreated mice |
187, 188 |
| Radionucleotide | Etaracizumab | Orthotopic human glioblastoma cells and subcutaneous human colon cancer cells |
Decreased angiogenesis and reduced tumour volume better than delivery of the antibody alone |
189 |
| Cytotoxic immunoconjugates |
CNTO 95 | Subcutaneous human colon and lung cancer cells |
Reduced primary tumour burden above the level observed with antibody alone |
190 |
cRGD, cyclic arginine-glycine-aspartic acid; RGDK, arginine-glycine-aspartic acid-lysine; TRAIL, tumour necrosis factor-related apoptosis-inducing ligand.
Conclusions
Integrins expressed by tumour and tumour-associated host cells mediate a diverse array of cellular effects resulting in tumour progression and metastasis. Important among these is the role integrins have in determining tumour cell survival. In the past few years studies have revealed new roles for unligated integrins in this process. Under some circumstances unligated integrins can induce tumour cell apoptosis through IMD by recruiting and activating caspase cleavage. Tumour cells that are resistant to IMD gain the ability to metastasize. In this case unligated integrins may promote cell survival, resulting in increased anchorage-independence and metastasis. These effects of unligated integrins may be clinically relevant and probably represent important factors in determining tumour cell sensitivity to integrin antagonists.
Crosstalk with growth factor receptors is required for many of the cancer-promoting effects of integrins. Recent studies have shown that certain growth factor receptors and oncogenes require specific integrins for their effects on tumorigenesis and metastasis. This suggests that it may be plausible to tailor the use of integrin antagonists in individual patients whose tumours are responsive to particular growth factors or oncogenes. Alternatively, the ECM composition of the tumour microenvironment may have a vital role in determining the sensitivity of a tumour to integrin antagonists. This may partly explain the clinical responsiveness of patients with glioblastoma treated with cilengitide. Future studies will have to elucidate the factors responsible for tumour susceptibility to these inhibitors and these will ultimately influence how effective these agents are as cancer therapeutics.
At a glance.
Integrin signalling regulates diverse functions in tumour cells, including migration, invasion, proliferation and survival. In several tumour types, the expression of particular integrins correlates with increased disease progression and decreased patient survival.
In addition to tumour cells, integrins are also found on tumour-associated host cells, such as the vascular endothelium, perivascular cells, fibroblasts, bone marrow-derived cells and platelets. Integrin signalling crucially regulates the contribution of these cell types to cancer progression. Therefore, integrin antagonists may inhibit tumour progression by blocking crucial signalling events in both the tumour microenvironment and the tumour cells themselves.
Integrins have a profound influence on tumour cells, both in the ligated and unligated states, in which they regulate tumour cell survival and malignancy.
Although integrins alone are not oncogenic, recent data have found that some oncogenes may require integrin signalling for their ability to initiate tumour growth and invasion. These effects may be due to the important contribution of integrin signalling in maintaining the cancer stem cell population in a given tumour.
Crosstalk between integrins and growth factor or cytokine receptors on both tumour and host cell types is vital for many aspects of tumour progression. Mechanisms of crosstalk include both direct and indirect association of integrins with growth factor or cytokine receptors, which affects the expression, ligand affinity and signalling of the receptors.
The important contribution of integrins to the biology of both tumour cells and tumour-associated cell types has made them appealing targets for the design of specific therapeutics. Of particular interest, the integrin αv inhibitor cilengitide is now in a Phase III clinical trial in glioblastoma, and because this is the first integrin antagonist to achieve this milestone it places anti-integrin therapy on the doorstep of clinical availability.
In addition to their use as therapeutic targets, integrins can be imaging biomarkers for assessing the efficacy of anti-angiogenic and anti-tumour agents. Integrin-targeted nanoparticles with a diverse array of anti-tumour payloads also represent a particularly promising area of research that may decrease the toxicities associated with systemic delivery of radiation or chemotherapy.
Acknowledgements
D.A.C. was supported by grants CA50286 and CA45726 from the US National Institutes of Health.
Glossary
- Desmoplasia
The growth of fibrous or connective tissue.
- Cilengitide
An RGD-containing cyclic pentapeptide that inhibits ligand binding to αV integrins.
- Glioblastoma
The most common human brain tumour, it originates from glial cells and has no known cure.
- Focal adhesions
Dynamic, macromolecular protein complexes that link the ECM to the actin cytoskeleton through integrins.
- Intrinsic apoptosis
Cell death initiated by cell stress or DNA damage and induced by mitochondrial release of cytochrome c and activation of pro-apoptotic caspases.
- Extrinsic apoptosis
Cell death induced by ligand binding to transmembrane death receptors and the activation of caspases, including caspase 8.
- Integrin-mediated death (IMD)
Apoptotic cell death resulting from the recruitment and activation of caspase 8 by unligated integrins on otherwise adherent cells.
- Dasatinib
A dual ABL 1 and SFK inhibitor manufactured by Bristol-Meyers Squibb and approved for treatment of patients with chronic myelogenous leukaemia.
- Vascular normalization
The process of restoring the integrity and function of the vasculature through ‘pruning’ immature vessels and increasing pericyte and basement membrane coverage of the remaining vessels.
- Tumour stroma
The fibroblasts, immune cells, pericytes, endothelial cells and inflammatory cells that surround a tumour and have a major role in tumour growth and progression.
- Polyoma middle T
Derived from the polyomavirus, the middle T antigen is commonly used to induce spontaneous tumorigenesis in mouse mammary epithelial cells as a model of breast cancer.
- Trastuzumab
A humanized monoclonal antibody that binds ERBB2 on tumour cells and prevents uncontrolled proliferation caused by aberrant ERBB2 signalling.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nature Rev. Mol. Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 3.Assoian RK, Klein EA. Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol. 2008;18:347–352. doi: 10.1016/j.tcb.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han S, Khuri FR, Roman J. Fibronectin stimulates non-small cell lung carcinoma cell growth through activation of Akt/mammalian target of rapamycin/S6 kinase and inactivation of LKB1/AMP-activated protein kinase signal pathways. Cancer Res. 2006;66:315–323. doi: 10.1158/0008-5472.CAN-05-2367. [DOI] [PubMed] [Google Scholar]
- 5.Vellon L, Menendez JA, Lupu R. αVβ3 integrin regulates heregulin (HRG)-induced cell proliferation and survival in breast cancer. Oncogene. 2005;24:3759–3773. doi: 10.1038/sj.onc.1208452. [DOI] [PubMed] [Google Scholar]
- 6.Pytela R, Pierschbacher MD, Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985;40:191–198. doi: 10.1016/0092-8674(85)90322-8. This seminal work provides the first description of an RGD-mediated cell adhesion mechanism. [DOI] [PubMed] [Google Scholar]
- 7.Berrier AL, Yamada KM. Cell-matrix adhesion. J. Cell Physiol. 2007;213:565–573. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- 8.Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nature Rev. Mol. Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 9.Zoller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nature Rev. Cancer. 2009;9:40–55. doi: 10.1038/nrc2543. [DOI] [PubMed] [Google Scholar]
- 10.Han J, et al. Reconstructing and deconstructing agonist-induced activation of integrin αIIbβ3. Curr. Biol. 2006;16:1796–1806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 11.Kren A, et al. Increased tumor cell dissemination and cellular senescence in the absence of β1-integrin function. EMBO J. 2007;26:2832–2842. doi: 10.1038/sj.emboj.7601738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zutter MM, Santoro SA, Staatz WD, Tsung YL. Re-expression of the alpha 2 beta 1 integrin abrogates the malignant phenotype of breast carcinoma cells. Proc. Natl Acad. Sci. USA. 1995;92:7411–7415. doi: 10.1073/pnas.92.16.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matter ML, Ruoslahti E. A signaling pathway from the α5β1 and αvβ3 integrins that elevates bcl-2 transcription. J. Biol. Chem. 2001;276:27757–27763. doi: 10.1074/jbc.M102014200. [DOI] [PubMed] [Google Scholar]
- 14.Uhm JH, Dooley NP, Kyritsis AP, Rao JS, Gladson CL. Vitronectin, a glioma-derived extracellular matrix protein, protects tumor cells from apoptotic death. Clin. Cancer Res. 1999;5:1587–1594. [PubMed] [Google Scholar]
- 15.Aoudjit F, Vuori K. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-flip and implications for anoikis. J. Cell Biol. 2001;152:633–643. doi: 10.1083/jcb.152.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene. 2001;20:4995–5004. doi: 10.1038/sj.onc.1204554. [DOI] [PubMed] [Google Scholar]
- 17.Scatena M, et al. NF-κB mediates αvβ3 integrininduced endothelial cell survival. J. Cell Biol. 1998;141:1083–1093. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Courter DL, Lomas L, Scatena M, Giachelli CM. Src kinase activity is required for integrin αVβ3-mediated activation of nuclear factor-κB. J. Biol. Chem. 2005;280:12145–12151. doi: 10.1074/jbc.M412555200. [DOI] [PubMed] [Google Scholar]
- 19.Bao W, Stromblad S. Integrin αv-mediated inactivation of p53 controls a MEK1-dependent melanoma cell survival pathway in three-dimensional collagen. J. Cell Biol. 2004;167:745–756. doi: 10.1083/jcb.200404018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hood JD, Frausto R, Kiosses WB, Schwartz MA, Cheresh DA. Differential αv integrin-mediated Ras-ERK signaling during two pathways of angiogenesis. J. Cell Biol. 2003;162:933–943. doi: 10.1083/jcb.200304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alavi A, Hood JD, Frausto R, Stupack DG, Cheresh DA. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 2003;301:94–96. doi: 10.1126/science.1082015. This paper identifies a central role for Raf in protecting endothelial cells from distinct mediators of apoptosis. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds LE, et al. Enhanced pathological angiogenesis in mice lacking β3 integrin or β3 and β5 integrins. Nature Med. 2002;8:27–34. doi: 10.1038/nm0102-27. This paper demonstrates that the genetic deletion of Itgb3 in mice failed to inhibit, and actually potentiated, angiogenesis. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds AR, et al. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in β3-integrin-deficient mice. Cancer Res. 2004;64:8643–8650. doi: 10.1158/0008-5472.CAN-04-2760. [DOI] [PubMed] [Google Scholar]
- 24.Weis SM, et al. Cooperation between VEGF and β3 integrin during cardiac vascular development. Blood. 2007;109:1962–1970. doi: 10.1182/blood-2005-10-038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petitclerc E, et al. Integrin αvβ3 promotes M21 melanoma growth in human skin by regulating tumor cell survival. Cancer Res. 1999;59:2724–2730. [PubMed] [Google Scholar]
- 26.Varner JA, Emerson DA, Juliano RL. Integrin alpha 5 beta 1 expression negatively regulates cell growth: reversal by attachment to fibronectin. Mol. Biol. Cell. 1995;6:725–740. doi: 10.1091/mbc.6.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giancotti FG, Ruoslahti E. Elevated levels of the α5 β1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990;60:849–859. doi: 10.1016/0092-8674(90)90098-y. This study shows that in addition to their pro-tumorigenic effects, integrins can suppress transformation under some conditions. [DOI] [PubMed] [Google Scholar]
- 28.Kanamori M, Vanden Berg SR, Bergers G, Berger MS, Pieper RO. Integrin β3 overexpression suppresses tumor growth in a human model of gliomagenesis: implications for the role of β3 overexpression in glioblastoma multiforme. Cancer Res. 2004;64:2751–2758. doi: 10.1158/0008-5472.can-03-3354. [DOI] [PubMed] [Google Scholar]
- 29.Danen EH, van Kraats AA, Cornelissen IM, Ruiter DJ, van Muijen GN. Integrin β3 cDNA transfection into a highly metastatic αv β3-negative human melanoma cell line inhibits invasion and experimental metastasis. Biochem. Biophys. Res. Commun. 1996;226:75–81. doi: 10.1006/bbrc.1996.1313. [DOI] [PubMed] [Google Scholar]
- 30.Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J. Cell Biol. 2001;155:459–470. doi: 10.1083/jcb.200106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao H, Ross FP, Teitelbaum SL. Unoccupied αvβ3 integrin regulates osteoclast apoptosis by transmitting a positive death signal. Mol. Endocrinol. 2005;19:771–780. doi: 10.1210/me.2004-0161. [DOI] [PubMed] [Google Scholar]
- 32.Frisch SM, Screaton RA. Anoikis mechanisms. Curr. Opin. Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 33.Stupack DG, et al. Potentiation of neuroblastoma metastasis by loss of caspase-8. Nature. 2006;439:95–99. doi: 10.1038/nature04323. This paper demonstrates that tumour invasion and metastasis occurs following resistance to IMD owing to the loss of caspase 8. [DOI] [PubMed] [Google Scholar]
- 34.Desgrosellier JS, et al. Integrin αvβ3-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nature Med. 2009;15:1163–1169. doi: 10.1038/nm.2009. This paper describes a role for unligated integrin αvβ3 in increasing anchorage-independent survival and metastasis through an integrin αvβ3–SRC signalling module. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albelda SM, et al. Integrin distribution in malignant melanoma: association of the β3 subunit with tumor progression. Cancer Res. 1990;50:6757–6764. [PubMed] [Google Scholar]
- 36.Takayama S, et al. The relationship between bone metastasis from human breast cancer and integrin αvβ3 expression. Anticancer Res. 2005;25:79–83. [PubMed] [Google Scholar]
- 37.Liapis H, Flath A, Kitazawa S. Integrin αVβ3 expression by bone-residing breast cancer metastases. Diagn. Mol. Pathol. 1996;5:127–135. doi: 10.1097/00019606-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Sloan EK, et al. Tumor-specific expression of αvβ3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006;8:R20. doi: 10.1186/bcr1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCabe NP, De S, Vasanji A, Brainard J, Byzova TV. Prostate cancer specific integrin αvβ3 modulates bone metastatic growth and tissue remodeling. Oncogene. 2007;26:6238–6243. doi: 10.1038/sj.onc.1210429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosotani R, et al. 2002;25:e30–35. [Google Scholar]
- 41.Gruber G, et al. Correlation between the tumoral expression of β3-integrin and outcome in cervical cancer patients who had undergone radiotherapy. Br. J. Cancer. 2005;92:41–46. doi: 10.1038/sj.bjc.6602278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asselin-Labat ML, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nature Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 43.Vaillant F, et al. The mammary progenitor marker CD61/β3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res. 2008;68:7711–7717. doi: 10.1158/0008-5472.CAN-08-1949. [DOI] [PubMed] [Google Scholar]
- 44.Taverna D, Crowley D, Connolly M, Bronson RT, Hynes RO. A direct test of potential roles for β3 and β5 integrins in growth and metastasis of murine mammary carcinomas. Cancer Res. 2005;65:10324–10329. doi: 10.1158/0008-5472.CAN-04-4098. [DOI] [PubMed] [Google Scholar]
- 45.Luo M, et al. Mammary epithelial-specific ablation of the focal adhesion kinase suppresses mammary tumorigenesis by affecting mammary cancer stem/progenitor cells. Cancer Res. 2009;69:466–474. doi: 10.1158/0008-5472.CAN-08-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samanna V, Wei H, Ego-Osuala D, Chellaiah MA. Alpha-V-dependent outside-in signaling is required for the regulation of CD44 surface expression, MMP-2 secretion, and cell migration by osteopontin in human melanoma cells. Exp. Cell Res. 2006;312:2214–2230. doi: 10.1016/j.yexcr.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 47.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nature Rev. Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ribatti D. The contribution of Harold F. Dvorak to the study of tumor angiogenesis and stroma generation mechanisms. Endothelium. 2007;14:131–135. doi: 10.1080/10623320701421651. [DOI] [PubMed] [Google Scholar]
- 49.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. This paper provides the first demonstration that an antagonist to integrin αvβ3 blocks angiogenesis. [DOI] [PubMed] [Google Scholar]
- 50.Davis GE. Affinity of integrins for damaged extracellular matrix: αvβ3 binds to denatured collagen type I through RGD sites. Biochem. Biophys. Res. Commun. 1992;182:1025–1031. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- 51.Friedlander M, et al. Definition of two angiogenic pathways by distinct αv integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. This paper defines two distinct pathways of angiogenesis that require specific integrin–growth factor receptor pairs. [DOI] [PubMed] [Google Scholar]
- 52.Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr. Opin. Genet. Dev. 2005;15:102–111. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 53.Garmy-Susini B, et al. Integrin α4β1-VCAM-1- mediated adhesion between endothelial and mural cells is required for blood vessel maturation. J. Clin. Invest. 2005;115:1542–1551. doi: 10.1172/JCI23445. This paper describes the importance of integrin-mediated interactions between endothelial cells and pericytes for vascular maturation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc. Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greenberg JI, et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conti JA, et al. The desmoplastic reaction surrounding hepatic colorectal adenocarcinoma metastases aids tumor growth and survival via αv integrin ligation. Clin. Cancer Res. 2008;14:6405–6413. doi: 10.1158/1078-0432.CCR-08-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu CQ, et al. Integrin α11 regulates IGF2 expression in fibroblasts to enhance tumorigenicity of human non-small-cell lung cancer cells. Proc. Natl Acad. Sci. USA. 2007;104:11754–11759. doi: 10.1073/pnas.0703040104. This paper demonstrates a crucial role for integrin α11β1 that is expressed on tumour-associated fibroblasts in tumour progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taverna D, et al. Increased primary tumor growth in mice null for β3- or β3/β5-integrins or selectins. Proc. Natl Acad. Sci. USA. 2004;101:763–768. doi: 10.1073/pnas.0307289101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feng W, et al. The angiogenic response is dictated by β3 integrin on bone marrow-derived cells. J. Cell Biol. 2008;183:1145–1157. doi: 10.1083/jcb.200802179. This paper describes the novel observation that integrin αvβ3 expression on bone marrow-derived cells is crucial for angiogenesis that occurs during wound healing and tumour growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin H, et al. A homing mechanism for bone marrowderived progenitor cell recruitment to the neovasculature. J. Clin. Invest. 2006;116:652–662. doi: 10.1172/JCI24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin H, Su J, Garmy-Susini B, Kleeman J, Varner J. Integrin α4β1 promotes monocyte trafficking and angiogenesis in tumors. Cancer Res. 2006;66:2146–2152. doi: 10.1158/0008-5472.CAN-05-2704. [DOI] [PubMed] [Google Scholar]
- 62.Bakewell SJ, et al. Platelet and osteoclast β3 integrins are critical for bone metastasis. Proc. Natl Acad. Sci. USA. 2003;100:14205–14210. doi: 10.1073/pnas.2234372100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Felding-Habermann B, Habermann R, Saldivar E, Ruggeri ZM. Role of β3 integrins in melanoma cell adhesion to activated platelets under flow. J. Biol. Chem. 1996;271:5892–5900. doi: 10.1074/jbc.271.10.5892. [DOI] [PubMed] [Google Scholar]
- 64.Felding-Habermann B, et al. Integrin activation controls metastasis in human breast cancer. Proc. Natl Acad. Sci. USA. 2001;98:1853–1858. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trikha M, et al. Multiple roles for platelet GPIIb/IIIa and αvβ3 integrins in tumor growth, angiogenesis, and metastasis. Cancer Res. 2002;62:2824–2833. [PubMed] [Google Scholar]
- 66.Guo W, et al. β4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126:489–502. doi: 10.1016/j.cell.2006.05.047. This paper provides the first demonstration that oncogenes may cooperate with integrins to initiate tumorigenesis. [DOI] [PubMed] [Google Scholar]
- 67.White DE, et al. Targeted disruption of β1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 68.Macias-Perez I, et al. Loss of integrin α1β1 ameliorates Kras-induced lung cancer. Cancer Res. 2008;68:6127–6135. doi: 10.1158/0008-5472.CAN-08-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lahlou H, et al. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc. Natl Acad. Sci. USA. 2007;104:20302–20307. doi: 10.1073/pnas.0710091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pylayeva Y, et al. Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J. Clin. Invest. 2009;119:252–266. doi: 10.1172/JCI37160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huveneers S, et al. Integrin αvβ3 controls activity and oncogenic potential of primed c-Src. Cancer Res. 2007;67:2693–2700. doi: 10.1158/0008-5472.CAN-06-3654. [DOI] [PubMed] [Google Scholar]
- 72.Huveneers S, Arslan S, van de Water B, Sonnenberg A, Danen EH. Integrins uncouple Srcinduced morphological and oncogenic transformation. J. Biol. Chem. 2008;283:13243–13251. doi: 10.1074/jbc.M800927200. [DOI] [PubMed] [Google Scholar]
- 73.Shimizu H, Seiki T, Asada M, Yoshimatsu K, Koyama N. α6β1 integrin induces proteasomemediated cleavage of erbB2 in breast cancer cells. Oncogene. 2003;22:831–839. doi: 10.1038/sj.onc.1206203. [DOI] [PubMed] [Google Scholar]
- 74.Borges E, Jan Y, Ruoslahti E. Platelet-derived growth factor receptor β and vascular endothelial growth factor receptor 2 bind to the β3 integrin through its extracellular domain. J. Biol. Chem. 2000;275:39867–39873. doi: 10.1074/jbc.M007040200. [DOI] [PubMed] [Google Scholar]
- 75.Schneller M, Vuori K, Ruoslahti E. αvβ3 integrin associates with activated insulin and PDGFβ receptors and potentiates the biological activity of PDGF. EMBO J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soldi R, et al. Role of αvβ3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trusolino L, Bertotti A, Comoglio PM. A signaling adapter function for α6β4 integrin in the control of HGFdependent invasive growth. Cell. 2001;107:643–654. doi: 10.1016/s0092-8674(01)00567-0. This paper identifies a crucial crosstalk mechanism between integrin α6β4 and MeT that increases HGF-induced tumour cell invasion. [DOI] [PubMed] [Google Scholar]
- 78.Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J. Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. This paper describes the important collaboration between growth factors and integrins for increased activation of growth factor receptors and potentiation of downstream signalling through MAPK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Caswell PT, et al. Rab-coupling protein coordinates recycling of α5β1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J. Cell Biol. 2008;183:143–55. doi: 10.1083/jcb.200804140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spangenberg C, et al. ERBB2-mediated transcriptional up-regulation of the α5β1 integrin fibronectin receptor promotes tumor cell survival under adverse conditions. Cancer Res. 2006;66:3715–3725. doi: 10.1158/0008-5472.CAN-05-2823. [DOI] [PubMed] [Google Scholar]
- 81.Yoon SO, Shin S, Lipscomb EA. A novel mechanism for integrin-mediated ras activation in breast carcinoma cells: the α6β4 integrin regulates ErbB2 translation and transactivates epidermal growth factor receptor/ErbB2 signaling. Cancer Res. 2006;66:2732–2739. doi: 10.1158/0008-5472.CAN-05-2941. [DOI] [PubMed] [Google Scholar]
- 82.Ning Y, Buranda T, Hudson LG. Activated epidermal growth factor receptor induces integrin α2 internalization via caveolae/raft-dependent endocytic pathway. J. Biol. Chem. 2007;282:6380–6387. doi: 10.1074/jbc.M610915200. [DOI] [PubMed] [Google Scholar]
- 83.Ning Y, et al. Down-regulation of integrin α2 surface expression by mutant epidermal growth factor receptor (EGFRvIII) induces aberrant cell spreading and focal adhesion formation. Cancer Res. 2005;65:9280–9286. doi: 10.1158/0008-5472.CAN-05-0407. [DOI] [PubMed] [Google Scholar]
- 84.Ignotz RA, Massague J. Cell adhesion protein receptors as targets for transforming growth factor-β action. Cell. 1987;51:189–197. doi: 10.1016/0092-8674(87)90146-2. [DOI] [PubMed] [Google Scholar]
- 85.Wei YY, et al. Osteoblasts-derived TGF-β1 enhance motility and integrin upregulation through Akt, ERK, and NF-κB-dependent pathway in human breast cancer cells. Mol. Carcinog. 2008;47:526–537. doi: 10.1002/mc.20411. [DOI] [PubMed] [Google Scholar]
- 86.Wang D, et al. Control of type II transforming growth factor-β receptor expression by integrin ligation. J. Biol. Chem. 1999;274:12840–12847. doi: 10.1074/jbc.274.18.12840. [DOI] [PubMed] [Google Scholar]
- 87.Engl T, et al. CXCR4 chemokine receptor mediates prostate tumor cell adhesion through α5 and β3 integrins. Neoplasia. 2006;8:290–301. doi: 10.1593/neo.05694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun YX, et al. Expression and activation of αv β3 integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate. 2007;67:61–73. doi: 10.1002/pros.20500. [DOI] [PubMed] [Google Scholar]
- 89.Grzesiak JJ, Smith KC, Burton DW, Deftos LJ, Bouvet M. Integrin-mediated laminin-1 adhesion upregulates CXCR4 and IL-8 expression in pancreatic cancer cells. Surgery. 2007;141:804–814. doi: 10.1016/j.surg.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang SE, et al. Transforming growth factor beta induces clustering of HER2 and integrins by activating Src-focal adhesion kinase and receptor association to the cytoskeleton. Cancer Res. 2009;69:475–482. doi: 10.1158/0008-5472.CAN-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De S, et al. VEGF-integrin interplay controls tumor growth and vascularization. Proc. Natl Acad. Sci. USA. 2005;102:7589–7594. doi: 10.1073/pnas.0502935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Klemke RL, Yebra M, Bayna EM, Cheresh DA. Receptor tyrosine kinase signaling required for integrin αvβ5-directed cell motility but not adhesion on vitronectin. J. Cell Biol. 1994;127:859–866. doi: 10.1083/jcb.127.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brooks PC, et al. Insulin-like growth factor receptor cooperates with integrin αvβ5 to promote tumor cell dissemination in vivo. J. Clin. Invest. 1997;99:1390–1398. doi: 10.1172/JCI119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ricono JM, et al. Specific cross-talk between epidermal growth factor receptor and integrin αvβ5 promotes carcinoma cell invasion and metastasis. Cancer Res. 2009;69:1383–1391. doi: 10.1158/0008-5472.CAN-08-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wayner EA, Orlando RA, Cheresh DA. Integrins αvβ3 and αvβ5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J. Cell Biol. 1991;113:919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pouliot N, Nice EC, Burgess AW. Laminin-10 mediates basal and EGF-stimulated motility of human colon carcinoma cells via α3β1 and α6β4 integrins. Exp. Cell Res. 2001;266:1–10. doi: 10.1006/excr.2001.5197. [DOI] [PubMed] [Google Scholar]
- 97.Yang C, et al. Integrin α1β1 and α2β1 are the key regulators of hepatocarcinoma cell invasion across the fibrotic matrix microenvironment. Cancer Res. 2003;63:8312–8317. [PubMed] [Google Scholar]
- 98.Moro L, et al. Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesiondependent cell survival. EMBO J. 1998;17:6622–6632. doi: 10.1093/emboj/17.22.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moro L, et al. Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J. Biol. Chem. 2002;277:9405–9414. doi: 10.1074/jbc.M109101200. [DOI] [PubMed] [Google Scholar]
- 100.Bertotti A, Comoglio PM, Trusolino L. β4 integrin is a transforming molecule that unleashes Met tyrosine kinase tumorigenesis. Cancer Res. 2005;65:10674–10679. doi: 10.1158/0008-5472.CAN-05-2827. [DOI] [PubMed] [Google Scholar]
- 101.Bertotti A, Comoglio PM, Trusolino L. β4 integrin activates a Shp2-Src signaling pathway that sustains HGF-induced anchorage-independent growth. J. Cell Biol. 2006;175:993–1003. doi: 10.1083/jcb.200605114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chung J, Yoon SO, Lipscomb EA, Mercurio AM. The Met receptor and α6β4 integrin can function independently to promote carcinoma invasion. J. Biol. Chem. 2004;279:32287–32293. doi: 10.1074/jbc.M403809200. [DOI] [PubMed] [Google Scholar]
- 103.Crouch S, Spidel CS, Lindsey JS. HGF and ligation of αvβ5 integrin induce a novel, cancer cellspecific gene expression required for cell scattering. Exp. Cell Res. 2004;292:274–287. doi: 10.1016/j.yexcr.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 104.Sridhar SC, Miranti CK. Tetraspanin KAI1/CD82 suppresses invasion by inhibiting integrin-dependent crosstalk with c-Met receptor and Src kinases. Oncogene. 2006;25:2367–2378. doi: 10.1038/sj.onc.1209269. [DOI] [PubMed] [Google Scholar]
- 105.Munger JS, et al. The integrin αvβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 106.Ludbrook SB, Barry ST, Delves CJ, Horgan CM. The integrin αvβ3 is a receptor for the latency-associated peptides of transforming growth factors β1 and β3. Biochem. J. 2003;369:311–318. doi: 10.1042/BJ20020809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marsh D, et al. αvβ6 Integrin promotes the invasion of morphoeic basal cell carcinoma through stromal modulation. Cancer Res. 2008;68:3295–3303. doi: 10.1158/0008-5472.CAN-08-0174. [DOI] [PubMed] [Google Scholar]
- 108.Van Aarsen LA, et al. Antibody-mediated blockade of integrin αvβ6 inhibits tumor progression in vivo by a transforming growth factor-β-regulated mechanism. Cancer Res. 2008;68:561–570. doi: 10.1158/0008-5472.CAN-07-2307. [DOI] [PubMed] [Google Scholar]
- 109.Bates RC, et al. Transcriptional activation of integrin β6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J. Clin. Invest. 2005;115:339–347. doi: 10.1172/JCI23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Galliher AJ, Schiemann WP. β3 integrin and Src facilitate transforming growth factor-beta mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2006;8:R42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Galliher AJ, Schiemann WP. Src phosphorylates Tyr284 in TGF-β type II receptor and regulates TGF-β stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res. 2007;67:3752–3758. doi: 10.1158/0008-5472.CAN-06-3851. [DOI] [PubMed] [Google Scholar]
- 112.Fransvea E, Mazzocca A, Antonaci S, Giannelli G. Targeting transforming growth factor (TGF)-βRI inhibits activation of β1 integrin and blocks vascular invasion in hepatocellular carcinoma. Hepatology. 2009;49:839–850. doi: 10.1002/hep.22731. [DOI] [PubMed] [Google Scholar]
- 113.Mahabeleshwar GH, Feng W, Reddy K, Plow EF, Byzova TV. Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ. Res. 2007;101:570–580. doi: 10.1161/CIRCRESAHA.107.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hood JD, et al. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296:2404–2407. doi: 10.1126/science.1070200. This paper demonstrates an essential role for RAF1 in angiogenesis and provides the first demonstration of the effectiveness of integrin-targeted therapeutic delivery to the tumour vasculature. [DOI] [PubMed] [Google Scholar]
- 115.Byzova TV, et al. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol. Cell. 2000;6:851–860. [PubMed] [Google Scholar]
- 116.Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Giancotti FG. Integrin β4 signaling promotes tumor angiogenesis. Cancer Cell. 2004;6:471–483. doi: 10.1016/j.ccr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 117.Cardones AR, Murakami T, Hwang ST. CXCR4 enhances adhesion of B16 tumor cells to endothelial cells in vitro and in vivo via β1 integrin. Cancer Res. 2003;63:6751–6757. [PubMed] [Google Scholar]
- 118.Hartmann TN, Burger JA, Glodek A, Fujii N, Burger M. CXCR4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (SCLC) cells. Oncogene. 2005;24:4462–4471. doi: 10.1038/sj.onc.1208621. [DOI] [PubMed] [Google Scholar]
- 119.Brooks PC, et al. Antiintegrin αvβ3 blocks human breast cancer growth and angiogenesis in human skin. J. Clin. Invest. 1995;96:1815–1822. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mulgrew K, et al. Direct targeting of αvβ3 integrin on tumor cells with a monoclonal antibody, Abegrin. Mol. Cancer Ther. 2006;5:3122–3129. doi: 10.1158/1535-7163.MCT-06-0356. [DOI] [PubMed] [Google Scholar]
- 121.Gramoun A, et al. Effects of Vitaxin, a novel therapeutic in trial for metastatic bone tumors, on osteoclast functions in vitro. J. Cell Biochem. 2007;102:341–352. doi: 10.1002/jcb.21296. [DOI] [PubMed] [Google Scholar]
- 122.Gutheil JC, et al. Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin αvβ3. Clin. Cancer Res. 2000;6:3056–3061. [PubMed] [Google Scholar]
- 123.Delbaldo C, et al. Phase I and pharmacokinetic study of etaracizumab (Abegrin), a humanized monoclonal antibody against αvβ3 integrin receptor, in patients with advanced solid tumors. Invest. New Drugs. 2008;26:35–43. doi: 10.1007/s10637-007-9077-0. [DOI] [PubMed] [Google Scholar]
- 124.McNeel DG, et al. Phase I trial of a monoclonal antibody specific for αvβ3 integrin (MEDI-522) in patients with advanced malignancies, including an assessment of effect on tumor perfusion. Clin. Cancer Res. 2005;11:7851–7860. doi: 10.1158/1078-0432.CCR-05-0262. [DOI] [PubMed] [Google Scholar]
- 125.Hersey PS, et al. A phase II, randomized, open-label study evaluating the antitumor activity of MEDI-522, a humanized monoclonal antibody directed against the human alpha v beta 3 (avb3) integrin, ± dacarbazine (DTIC) in patients with metastatic melanoma (MM). J. Clin. Oncol; 2005 ASCO Annual Meeting Proceedings; 2005. p. 7507. Part I of II (June 1 Supplement) [Google Scholar]
- 126.Trikha M, et al. CNTO 95, a fully human monoclonal antibody that inhibits αv integrins, has antitumor and antiangiogenic activity in vivo. Int. J. Cancer. 2004;110:326–335. doi: 10.1002/ijc.20116. [DOI] [PubMed] [Google Scholar]
- 127.Chen Q, et al. CNTO 95, a fully human anti αv integrin antibody, inhibits cell signaling, migration, invasion, and spontaneous metastasis of human breast cancer cells. Clin. Exp. Metastasis. 2008;25:139–148. doi: 10.1007/s10585-007-9132-4. [DOI] [PubMed] [Google Scholar]
- 128.Martin PL, et al. Absence of adverse effects in cynomolgus macaques treated with CNTO 95, a fully human anti-alphav integrin monoclonal antibody, despite widespread tissue binding. Clin. Cancer Res. 2005;11:6959–6965. doi: 10.1158/1078-0432.CCR-04-2623. [DOI] [PubMed] [Google Scholar]
- 129.Mullamitha SA, et al. Phase I evaluation of a fully human anti-alphav integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumors. Clin. Cancer Res. 2007;13:2128–2135. doi: 10.1158/1078-0432.CCR-06-2779. [DOI] [PubMed] [Google Scholar]
- 130.Smith JW, Ruggeri ZM, Kunicki TJ, Cheresh DA. Interaction of integrins αvβ3 and glycoprotein IIb-IIIa with fibrinogen. Differential peptide recognition accounts for distinct binding sites. J. Biol. Chem. 1990;265:12267–12271. [PubMed] [Google Scholar]
- 131.Beekman KW, et al. Phase II evaluations of cilengitide in asymptomatic patients with androgenindependent prostate cancer: scientific rationale and study design. Clin. Genitourin Cancer. 2006;4:299–302. doi: 10.3816/CGC.2006.n.012. [DOI] [PubMed] [Google Scholar]
- 132.Nabors LB, et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J. Clin. Oncol. 2007;25:1651–1657. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.MacDonald TJ, et al. Phase I clinical trial of cilengitide in children with refractory brain tumors: pediatric brain tumor consortium study PBTC-012. J. Clin. Oncol. 2008;26:919–924. doi: 10.1200/JCO.2007.14.1812. [DOI] [PubMed] [Google Scholar]
- 134.Reardon DA, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J. Clin. Oncol. 2008;26:5610–5617. doi: 10.1200/JCO.2008.16.7510. This study shows the efficacy of cilengitide in a Phase II study of recurrent glioblastoma and provides the basis for the ongoing Phase III trial with this agent. [DOI] [PubMed] [Google Scholar]
- 135.Stupp R, et al. Phase I/IIa trial of cilengitide (EMD121974) and temozolomide with concomitant radiotherapy, followed by temozolomide and cilengitide maintenance therapy in patients (pts) with newly diagnosed glioblastoma (GBM). J. Clin, Oncol; 2007 ASCO Annual Meeting Proceedings; 2007. p. 2000. Part 1 (June 20 Supplement) [DOI] [PubMed] [Google Scholar]
- 136.Odekon LE, Frewin MB, Del Vecchio P, Saba TM, Gudewicz PW. Fibronectin fragments released from phorbol ester-stimulated pulmonary artery endothelial cell monolayers promote neutrophil chemotaxis. Immunology. 1991;74:114–120. [PMC free article] [PubMed] [Google Scholar]
- 137.Legler DF, Wiedle G, Ross FP, Imhof BA. Superactivation of integrin αvβ3 by low antagonist concentrations. J. Cell Sci. 2001;114:1545–1553. doi: 10.1242/jcs.114.8.1545. [DOI] [PubMed] [Google Scholar]
- 138.Aznavoorian S, Stracke ML, Krutzsch H, Schiffmann E, Liotta LA. Signal transduction for chemotaxis and haptotaxis by matrix molecules in tumor cells. J. Cell Biol. 1990;110:1427–1438. doi: 10.1083/jcb.110.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ebos JM, et al. Accelerated metastasis after shortterm treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Paez-Ribes M, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.MacDonald TJ, et al. Preferential susceptibility of brain tumors to the antiangiogenic effects of an αv integrin antagonist. Neurosurgery. 2001;48:151–157. doi: 10.1097/00006123-200101000-00026. [DOI] [PubMed] [Google Scholar]
- 142.Yamada S, et al. Effect of the angiogenesis inhibitor Cilengitide (EMD 121974) on glioblastoma growth in nude mice. Neurosurgery. 2006;59:1304–1312. doi: 10.1227/01.NEU.0000245622.70344.BE. [DOI] [PubMed] [Google Scholar]
- 143.Gladson CL, Cheresh DA. Glioblastoma expression of vitronectin and the αvβ 3 integrin. Adhesion mechanism for transformed glial cells. J. Clin. Invest. 1991;88:1924–1932. doi: 10.1172/JCI115516. Reveals a crucial role for integrin αvβ3 and its ligand vitronectin for adhesion and the malignant progression of glioblastoma and may help explain the role of cilengitide in patients with brain cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Park CC, et al. β1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66:1526–1535. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bhaskar V, et al. A function blocking anti-mouse integrin α5β1 antibody inhibits angiogenesis and impedes tumor growth in vivo. J. Transl. Med. 2007;5:61. doi: 10.1186/1479-5876-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bhaskar V, et al. Volociximab, a chimeric integrin α5β1 antibody, inhibits the growth of VX2 tumors in rabbits. Invest. New Drugs. 2008;26:7–12. doi: 10.1007/s10637-007-9078-z. [DOI] [PubMed] [Google Scholar]
- 147.Ricart AD, et al. Volociximab, a chimeric monoclonal antibody that specifically binds α5β1 integrin: a phase I, pharmacokinetic, and biological correlative study. Clin. Cancer Res. 2008;14:7924–7929. doi: 10.1158/1078-0432.CCR-08-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kuwada SK. Drug evaluation: Volociximab, an angiogenesis-inhibiting chimeric monoclonal antibody. Curr. Opin. Mol. Ther. 2007;9:92–98. [PubMed] [Google Scholar]
- 149.Khalili P, et al. A non-RGD-based integrin binding peptide (ATN-161) blocks breast cancer growth and metastasis in vivo. Mol. Cancer Ther. 2006;5:2271–2280. doi: 10.1158/1535-7163.MCT-06-0100. [DOI] [PubMed] [Google Scholar]
- 150.Stoeltzing O, et al. Inhibition of integrin α5β1 function with a small peptide (ATN-161) plus continuous 5-FU infusion reduces colorectal liver metastases and improves survival in mice. Int. J. Cancer. 2003;104:496–503. doi: 10.1002/ijc.10958. [DOI] [PubMed] [Google Scholar]
- 151.Cianfrocca ME, et al. Phase 1 trial of the antiangiogenic peptide ATN-161 (Ac-PHSCN-NH2), a beta integrin antagonist, in patients with solid tumours. Br. J. Cancer. 2006;94:1621–1626. doi: 10.1038/sj.bjc.6603171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Harms JF, et al. A small molecule antagonist of the αvβ3 integrin suppresses MDA-MB-435 skeletal metastasis. Clin. Exp. Metastasis. 2004;21:119–128. doi: 10.1023/b:clin.0000024763.69809.64. [DOI] [PubMed] [Google Scholar]
- 153.Reinmuth N, et al. αvβ3 integrin antagonist S247 decreases colon cancer metastasis and angiogenesis and improves survival in mice. Cancer Res. 2003;63:2079–2087. [PubMed] [Google Scholar]
- 154.Zhao Y, et al. Tumor αvβ3 integrin is a therapeutic target for breast cancer bone metastases. Cancer Res. 2007;67:5821–5830. doi: 10.1158/0008-5472.CAN-06-4499. [DOI] [PubMed] [Google Scholar]
- 155.Shannon KE, et al. Anti-metastatic properties of RGD-peptidomimetic agents S137 and S247. Clin. Exp. Metastasis. 2004;21:129–138. doi: 10.1023/b:clin.0000024764.93092.5f. [DOI] [PubMed] [Google Scholar]
- 156.Li J, et al. Antisense integrin αV and β3 gene therapy suppresses subcutaneously implanted hepatocellular carcinomas. Dig. Liver Dis. 2007;39:557–565. doi: 10.1016/j.dld.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 157.Sipkins DA, et al. Detection of tumor angiogenesis in vivo by αVβ3-targeted magnetic resonance imaging. Nature Med. 1998;4:623–626. doi: 10.1038/nm0598-623. [DOI] [PubMed] [Google Scholar]
- 158.Cai W, et al. In vitro and in vivo characterization of 64Cu-labeled Abegrin, a humanized monoclonal antibody against integrin αvβ3. Cancer Res. 2006;66:9673–9681. doi: 10.1158/0008-5472.CAN-06-1480. [DOI] [PubMed] [Google Scholar]
- 159.Winter PM, et al. Molecular imaging of angiogenesis in nascent Vx-2 rabbit tumors using a novel ανβ3- targeted nanoparticle and 1.5 tesla magnetic resonance imaging. Cancer Res. 2003;63:5838–5843. [PubMed] [Google Scholar]
- 160.Leong-Poi H, Christiansen J, Klibanov AL, Kaul S, Lindner JR. Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to αv-integrins. Circulation. 2003;107:455–460. doi: 10.1161/01.cir.0000044916.05919.8b. [DOI] [PubMed] [Google Scholar]
- 161.Chen X, et al. MicroPET imaging of breast cancer αv-integrin expression with 64Cu-labeled dimeric RGD peptides. Mol. Imaging Biol. 2004;6:350–359. doi: 10.1016/j.mibio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 162.Chen X, et al. MicroPET imaging of brain tumor angiogenesis with 18F-labeled PEGylated RGD peptide. Eur. J. Nucl. Med. Mol. Imaging. 2004;31:1081–1089. doi: 10.1007/s00259-003-1452-2. [DOI] [PubMed] [Google Scholar]
- 163.Zhang C, et al. Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Res. 2007;67:1555–1562. doi: 10.1158/0008-5472.CAN-06-1668. [DOI] [PubMed] [Google Scholar]
- 164.Bach-Gansmo T, Bogsrud TV, Skretting A. Integrin scintimammography using a dedicated breast imaging, solid-state gamma-camera and (99m) Tc-labelled NC100692. Clin. Physiol. Funct. Imaging. 2008;28:235–239. doi: 10.1111/j.1475-097X.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 165.Bach-Gansmo T, et al. Integrin receptor imaging of breast cancer: a proof-of-concept study to evaluate 99mTc-NC100692. J. Nucl. Med. 2006;47:1434–1439. [PubMed] [Google Scholar]
- 166.Haubner R, et al. Noninvasive visualization of the activated αvβ3 integrin in cancer patients by positron emission tomography and [18F]Galacto-RGD. PLoS Med. 2005;2:e70. doi: 10.1371/journal.pmed.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Murphy EA, et al. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc. Natl Acad. Sci. USA. 2008;105:9343–9348. doi: 10.1073/pnas.0803728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wu L, et al. Distinct FAK-Src activation events promote α5β1 and α4β1 integrin-stimulated neuroblastoma cell motility. Oncogene. 2008;27:1439–1448. doi: 10.1038/sj.onc.1210770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.White DP, Caswell PT, Norman JC. αvβ3 and α5β1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J. Cell Biol. 2007;177:515–525. doi: 10.1083/jcb.200609004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fournier AK, et al. Rac-dependent cyclin D1 gene expression regulated by cadherin- and integrinmediated adhesion. J. Cell Sci. 2008;121:226–233. doi: 10.1242/jcs.017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Carrano AC, Pagano M. Role of the F-box protein Skp2 in adhesion-dependent cell cycle progression. J. Cell Biol. 2001;153:1381–1390. doi: 10.1083/jcb.153.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hsu MY, et al. Adenoviral gene transfer of β3 integrin subunit induces conversion from radial to vertical growth phase in primary human melanoma. Am. J. Pathol. 1998;153:1435–1442. doi: 10.1016/s0002-9440(10)65730-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hieken TJ, Ronan SG, Farolan M, Shilkaitis AL, Das Gupta TK. Molecular prognostic markers in intermediate-thickness cutaneous malignant melanoma. Cancer. 1999;85:375–382. doi: 10.1002/(sici)1097-0142(19990115)85:2<375::aid-cncr15>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 174.Danen EH, et al. Emergence of α5β1 fibronectin- and αvβ3 vitronectin-receptor expression in melanocytic tumour progression. Histopathology. 1994;24:249–256. doi: 10.1111/j.1365-2559.1994.tb00517.x. [DOI] [PubMed] [Google Scholar]
- 175.Nip J, Shibata H, Loskutoff DJ, Cheresh DA, Brodt P. Human melanoma cells derived from lymphatic metastases use integrin αvβ3 to adhere to lymph node vitronectin. J. Clin. Invest. 1992;90:1406–1413. doi: 10.1172/JCI116007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Diaz LK, et al. β4 integrin subunit gene expression correlates with tumor size and nuclear grade in early breast cancer. Mod. Pathol. 2005;18:1165–1175. doi: 10.1038/modpathol.3800411. [DOI] [PubMed] [Google Scholar]
- 177.Friedrichs K, et al. High expression level of α6 integrin in human breast carcinoma is correlated with reduced survival. Cancer Res. 1995;55:901–906. [PubMed] [Google Scholar]
- 178.Slack-Davis JK, Atkins KA, Harrer C, Hershey ED, Conaway M. Vascular cell adhesion molecule-1 is a regulator of ovarian cancer peritoneal metastasis. Cancer Res. 2009;69:1469–1476. doi: 10.1158/0008-5472.CAN-08-2678. [DOI] [PubMed] [Google Scholar]
- 179.Landen CN, et al. Tumor-selective response to antibody-mediated targeting of αvβ3 integrin in ovarian cancer. Neoplasia. 2008;10:1259–1267. doi: 10.1593/neo.08740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hazelbag S, et al. Overexpression of the αvβ6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J. Pathol. 2007;212:316–324. doi: 10.1002/path.2168. [DOI] [PubMed] [Google Scholar]
- 181.Bello L, et al. αvβ3 and αvβ5 integrin expression in glioma periphery. Neurosurgery. 2001;49:380–389. doi: 10.1097/00006123-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 182.Adachi M, et al. Significance of integrin α5 gene expression as a prognostic factor in node-negative non-small cell lung cancer. Clin. Cancer Res. 2000;6:96–101. [PubMed] [Google Scholar]
- 183.Winter PM, et al. Minute dosages of ανβ3-targeted fumagillin nanoparticles impair Vx-2 tumor angiogenesis and development in rabbits. FASEB J. 2008;22:2758–2767. doi: 10.1096/fj.07-103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ong HT, et al. Intravascularly administered RGDdisplaying measles viruses bind to and infect neovessel endothelial cells in vivo. Mol. Ther. 2009;17:1012–1021. doi: 10.1038/mt.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cao L, et al. Enhancement of antitumor properties of TRAIL by targeted delivery to the tumor neovasculature. Mol. Cancer Ther. 2008;7:851–861. doi: 10.1158/1535-7163.MCT-07-0533. [DOI] [PubMed] [Google Scholar]
- 186.Pramanik D, et al. Lipopeptide with a RGDK tetrapeptide sequence can selectively target genes to proangiogenic α5β1 integrin receptor and mouse tumor vasculature. J. Med. Chem. 2008;51:7298–7302. doi: 10.1021/jm800915y. [DOI] [PubMed] [Google Scholar]
- 187.Dijkgraaf I, et al. αvβ3 integrin-targeting of intraperitoneally growing tumors with a radiolabeled RGD peptide. Int. J. Cancer. 2007;120:605–610. doi: 10.1002/ijc.22297. [DOI] [PubMed] [Google Scholar]
- 188.Janssen ML, et al. Tumor targeting with radiolabeled αvβ3 integrin binding peptides in a nude mouse model. Cancer Res. 2002;62:6146–6151. [PubMed] [Google Scholar]
- 189.Veeravagu A, et al. Integrin αvβ3-targeted radioimmunotherapy of glioblastoma multiforme. Clin. Cancer Res. 2008;14:7330–7339. doi: 10.1158/1078-0432.CCR-08-0797. [DOI] [PubMed] [Google Scholar]
- 190.Chen Q, et al. αv integrin-targeted immunoconjugates regress established human tumors in xenograft models. Clin. Cancer Res. 2007;13:3689–3695. doi: 10.1158/1078-0432.CCR-07-0026. [DOI] [PubMed] [Google Scholar]
- 191.Cheresh DA. Human endothelial cells synthesize and express an Arg Gly Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc. Natl. Acad. Sci. USA. 1987;84:6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Brooks PC, et al. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 193.Aumailley M, et al. Arg Gly Asp constrained within cyclic pentapeptides.Strong and selective inhibitors of cell adhesion to vitronectin and laminin fragment P1. FEBS Lett. 1991;291:50–54. doi: 10.1016/0014-5793(91)81101-d. [DOI] [PubMed] [Google Scholar]
- 194.Gurrath M, et al. Conformation/activity studies of rationally designed potent anti-adhesive RGD peptides. Eur. J. Biochem. 1992;210:911–921. doi: 10.1111/j.1432-1033.1992.tb17495.x. [DOI] [PubMed] [Google Scholar]
- 195.Pfaff M, et al. Selective Recognition of Cyclic RGD Peptides of NMR Defined Conformation by αIIbβ3, αVβ3 and α5β1 Integrins. J. Biol. Chem. 1994;269:20233–20238. [PubMed] [Google Scholar]
- 196.Dechantsreiter MA, et al. N-Methylated cyclic RGD peptides as highly active and selective αVβ3 integrin antagonists. J. Med. Chem. 1999;42:3033–3040. doi: 10.1021/jm970832g. [DOI] [PubMed] [Google Scholar]