Abstract
Wolbachia is an obligate intracellular alphaproteobacterium that occurs in arthropod and nematode hosts. Wolbachia presumably provides a fitness benefit to its hosts, but the basis for its retention and spread in host populations remains unclear. Wolbachia genomes retain biosynthetic pathways for some vitamins, and the possibility that these vitamins benefit host cells provides a potential means of selecting for Wolbachia-infected cell lines. To explore whether riboflavin produced by Wolbachia is available to its host cell, we established that growth of uninfected C7–10 mosquito cells decreases in riboflavin-depleted culture medium. A well studied inhibitor of riboflavin uptake, lumiflavin, further inhibits growth of uninfected C7–10 cells with an LC50 of approximately 12 µg/ml. Growth of C/wStr1 mosquito cells, infected with Wolbachia from the planthopper, Laodelphax striatellus, was enhanced in medium containing low levels of lumiflavin, but Wolbachia levels decreased. Lumiflavin-enhanced growth thus resembled the improved growth that accompanies treatment with antibiotics that deplete Wolbachia, rather than a metabolic advantage provided by the Wolbachia infection. We used the polymerase chain reaction to validate the decrease in Wolbachia abundance and evaluated our results in the context of a proteomic analysis in which we detected nearly 800 wStr proteins. Our data indicate that Wolbachia converts riboflavin to FMN and FAD for its own metabolic needs, and does not provide a source of riboflavin for its host cell.
Keywords: Wolbachia, Mosquito cell line, Selection, Riboflavin, Lumiflavin, Proteomics
Introduction
The obligate intracellular bacterium, Wolbachia pipientis occurs in a wide range of arthropods, including some species of mosquitoes, and in filarial worms vectored by mosquitoes and other biting flies. In insects, Wolbachia is typically considered a reproductive parasite, while in filarial worms, Wolbachia is an obligate mutualist thought to provide nutritional supplements such as riboflavin, which cannot be produced by the filarial host (Foster et al. 2005). In mosquitoes, Wolbachia is vertically transmitted in the egg cytoplasm, but its presence in developing testes causes reproductive distortions such as cytoplasmic incompatibility (Beckman and Fallon 2013), which favor Wolbachia invasion of uninfected host populations. Although fitness effects associated with Wolbachia have been difficult to identify in mosquitoes, in which antibiotic-cured populations differ little from infected cohorts (Islam and Dobson 2006), Wolbachia’s ability to invade uninfected populations provides a potential tool for controlling insect pests, including vectors of arthropod-borne disease (Sinkins and Gould 2006).
Implementation of Wolbachia-based control strategies will be facilitated by genetic manipulation and transformation of Wolbachia with genes encoding fluorescent proteins and/or other desirable markers, as has already been achieved with closely related intracellular microbes in the order Rickettsiales (reviewed by Beare et al. 2011). Several strains of Wolbachia can be propagated in insect cell lines, and we have been interested in identifying metabolic interactions that will support recovery of mosquito cells that harbor normal, and eventually transformed, Wolbachia. Here, we explore whether riboflavin (vitamin B2) biosynthesis by Wolbachia provides a selective advantage to infected mosquito cells using the C/wStr1 cell line (Fallon et al. 2013a), which maintains a robust, persistent infection of Wolbachia from the planthopper, Laodelphax striatellus (Noda et al. 2002). Riboflavin is an essential nutrient for mosquito host cells, and despite genomic reduction typical of obligate intracellular bacteria, Wolbachia retains the ability to produce riboflavin (Wu et al. 2004; Dunning Hotopp et al. 2006).
Although provisioning of B vitamins has been implicated in a few insects that have evolved a mutualistic relationship with Wolbachia (Hosokawa et al. 2010), our results indicate that reducing availability of exogenous riboflavin to mosquito host cells compromises survival of Wolbachia. The robust Wolbachia infection in C/wStr1 cells requires adequate host cell access to exogenous riboflavin, which cannot be supplemented by Wolbachia’s biosynthetic activity. In this cell culture system, Wolbachia’s retention of a pathway for riboflavin biosynthesis fails to provide a fitness benefit to its host cell.
Materials and Methods
Cells and culture conditions
We derived C/wStr1 cells (Fallon et al. 2013a) by infecting Aedes albopictus C7–10 cells with wStr from an uncloned mosquito cell line (Noda et al. 2002). C7–10 control cells and C/wStr1-infected cells were maintained in Eagle’s medium containing 5% heat-inactivated fetal bovine serum (E-5 medium; Shih et al. 1998). C7–10 cells were subcultured weekly, at a 10-fold dilution into fresh medium. C/wStr1 cells were subcultured at 2-wk interval by adding 2 ml of cells, resuspended from a 12-ml culture to 5 ml of fresh E-5 medium, in 25-cm2 flasks. On alternate weeks, flasks (containing 7 ml of cells plus medium) were supplemented with an additional 5 ml of fresh E-5 medium to establish a 12 ml culture. Passage number and other details relevant to individual experiments are described in the figure legends. All experiments were replicated with two or more independent populations of cells, and within an experiment, variation between data points from replicate samples was typically less than 5%. Figures show representative experiments.
Analytical methods
Cells were counted with a Coulter electronic cell counter, and metabolic activity was assayed using methylthiazole tetrazolium (MTT; Fallon et al. 2013b). Infection status was monitored by fluorescence microscopy and by evaluating Wolbachia DNA content using the polymerase chain reaction (PCR) with Wolbachia-specific primers S12F (5′-GCACTAAGGTGTATACTACAACTCC) and S7R (5′-GCCTTATTAGCTTCAGCCAT) as described previously (Fallon et al. 2013b). The concentration of riboflavin in E-5 medium is 0.2 µg/ml. Riboflavin-free medium was prepared by replacing the commercially available 100X vitamin stock (GIBCO, Cat No. 11120) with individually dissolved vitamins (Sigma-Aldrich Cat No. B4639, biotin; P5155, pantothenate; C7527, choline chloride; F8758, folic acid; I7508, myoinositol; N3014, β-NAD; P6155, pyridoxal HCl; R9504, riboflavin; T1270, thiamine HCl) lacking riboflavin. When the riboflavin-free medium was supplemented with riboflavin, growth of cells was identical to that in standard medium with commercial components. Stock concentrations (1.0 mg/ml) of lumiflavin (Sigma-Aldrich, L4879) were prepared in 20-mM NaOH and diluted in culture medium to the final concentrations described in the figure legends.
Mass spectrometry
As detailed in proteomics-based analyses of Wolbachia from filarial nematodes (Darby et al. 2012), we estimated relative abundance levels (RAL) for individual proteins based on counts of MS-matched unique peptide spectra in a series of four MS data sets from a large scale analysis of the wStr proteome. Among a total of 797 proteins, RAL values ranged from 0.2 to 58.2 (Baldridge GD, Witthuhn BA, Higgins LA, Markowski TW, Fallon AM. Proteomic analysis of an insect Wolbachia in mosquito cells reveals robust expression of a constitutive stress response, a complete type IV secretion system and potential supplementation of bacterial amino acid pools by proline, glutamate, glycine and cysteine degradation, unpublished). The subset of these proteins involved in riboflavin metabolism is discussed here.
Results
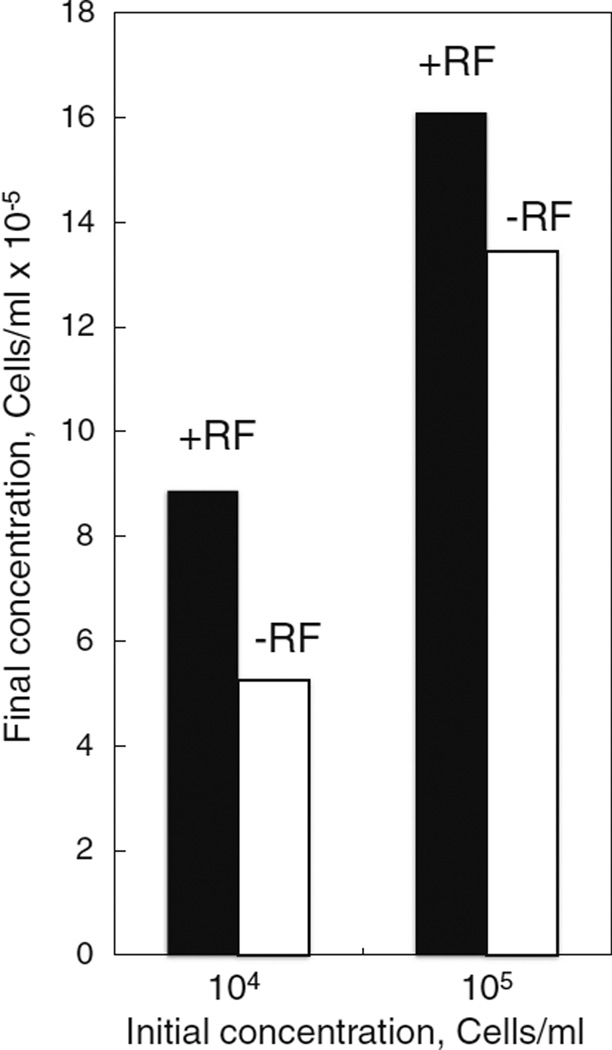
We began this study by evaluating sensitivity of wild type C7–10 cells to culture medium lacking riboflavin, normally present at a final concentration of 0.2 µg/ml (see Shih et al. 1998). Vitamin-free medium was supplemented with fetal bovine serum, at a final concentration of 5% (E-5 medium), which added riboflavin to a concentration of about 8 ng/ml (see Said and Ma 1994). C7–10 cells were plated at 1 × 105 and 1 × 104 cells/ml in 100 mm dishes (12 ml total volume) in complete medium, or in riboflavin-depleted medium, and counted after 7 d (Fig. 1). Cell counts in the absence of riboflavin (−RF) were reduced to 59 and 83% of controls (+RF) for initial cell densities of 104 and 105 cells/ml, respectively.
Figure 1.
Growth of C7–10 cells is sensitive to riboflavin depletion. Cells were plated at 1 × 105 and 1 × 104 cells/ml in 100-mm plates, in 12 ml of complete medium (solid bars) or riboflavin-deficient medium (open bars). After 7 d, cells were resuspended and counted with a Coulter electronic cell counter. Values are averages from duplicate plates; variation between values was typically less than 5%.
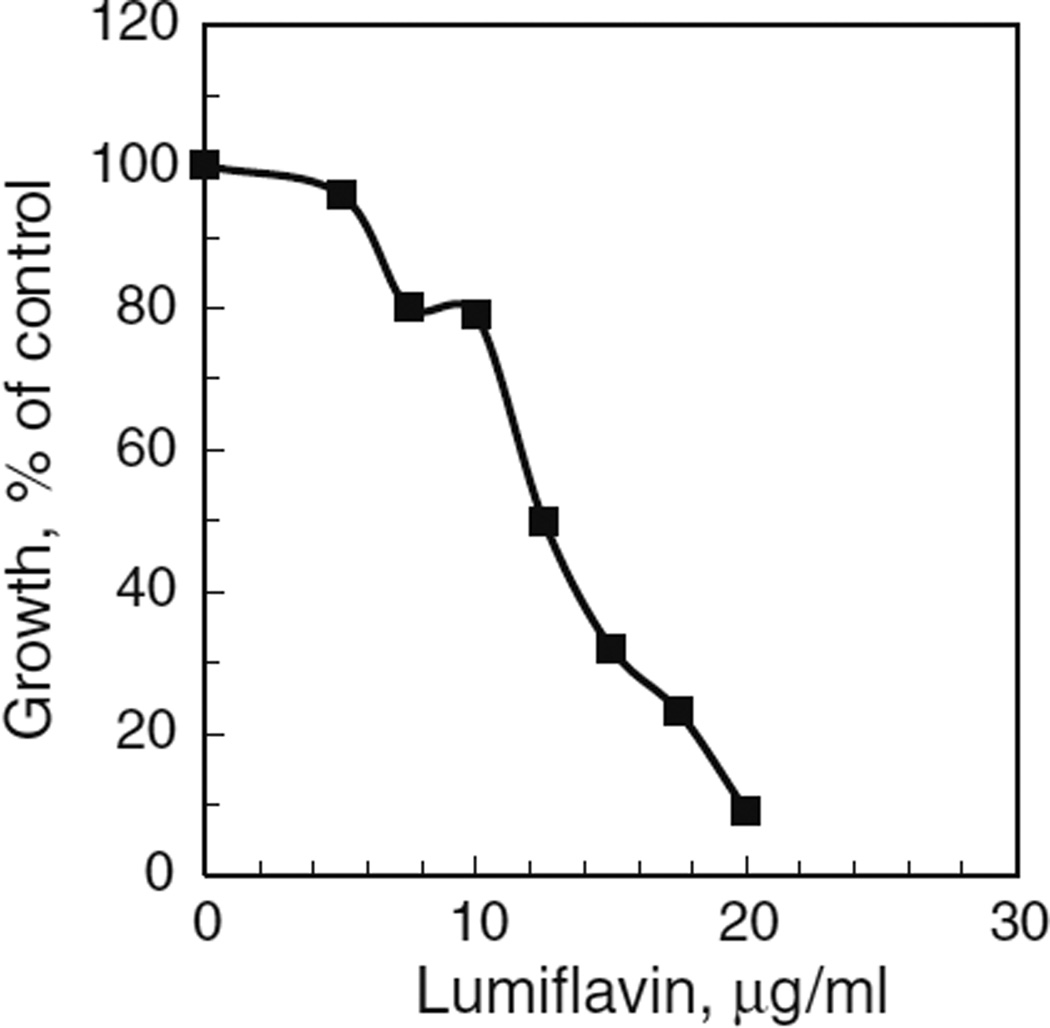
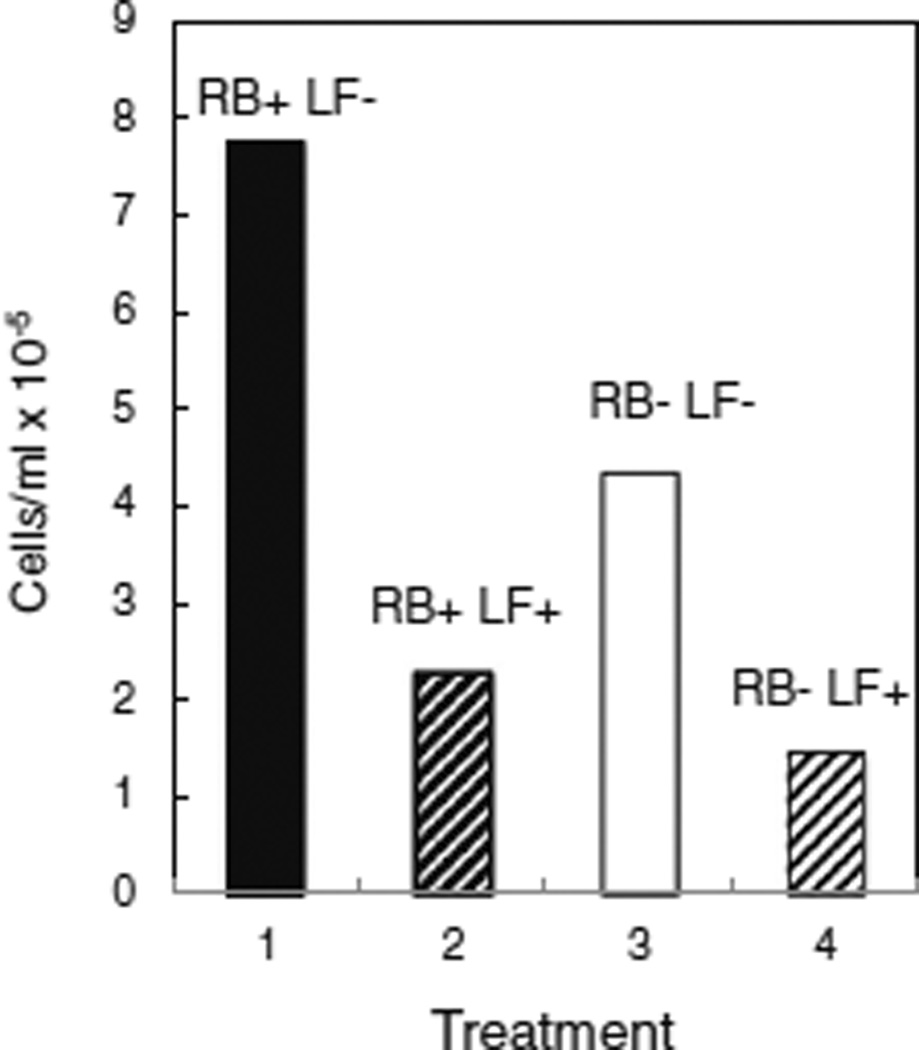
In mammalian cells, riboflavin is acquired from the medium by the transporter proteins, RFT1 and RFT2, recently characterized in rats and humans (Yonezawa et al. 2008; Yamamoto et al. 2009). Homologous hypothetical proteins occur in various mosquito species, with E values ranging from 5e-87 to 2e-71 (Accession Nos. GI : 157104132; GI : 347963328; GI : 170040965; GI : 568255901; GI : 170038973, www.ncbi.nlm.nih.gov/), suggesting that mosquito cells use a similar transport system. Mosquito cells were sensitive to lumiflavin, a strong inhibitor of rat RFT2 (Said and Ma 1994; Yamamoto et al. 2009), with an LC50 of approximately 12 µg/ml (Fig. 2). Consistent with its inhibition of riboflavin uptake, lumiflavin reduced cell growth in complete (RB+) medium to 29% of control levels (Fig. 3, compare columns 1 and 2). In riboflavin-depleted (RB−) medium (Fig. 3, columns 3 and 4), lumiflavin further reduced cell numbers to 19% of controls in complete medium (Fig. 3, compare columns 1 and 4).
Figure 2.
Effect of lumiflavin on growth of uninfected cells. Cells were plated at 1 × 105 cells/ml, 2 ml/35 mm plate, in E-5 medium containing the indicated concentrations of lumiflavin. After 5 d, cells were counted with a Coulter electronic cell counter. Values are plotted as percentages of controls without lumiflavin (4.8 × 106 cells/plate).
Figure 3.
Lumiflavin reduces cell growth in the presence or absence of riboflavin. C7–10 cells were plated at a density of 104/ml in complete medium (solid bar) and in riboflavin-free medium (open bar) and counted after 10 d. Hatched bars (lanes 2 and 4) represent samples that were supplemented with lumiflavin at a final concentration of 5 µg/ml.
We previously showed that growth of C/wStr1 host cells measured by cell number and metabolic activity measured by reduction of MTT are enhanced by treatment with the antibiotics rifampicin and tetracycline, which are used routinely to eliminate Wolbachia from insects or cell lines. This difference in growth of C/wStr1 cells with and without antibiotics presumably reflects, at least in part, diversion of host resources to support Wolbachia replication, which is reduced, but not completely eliminated, by short-term exposure to antibiotics. In contrast to antibiotics, a test compound that would provide a selective advantage to Wolbachia-infected cells would be expected to increase growth of infected cells, without an accompanying decrease in Wolbachia levels. In a previous study, we noted that improved C/wStr1 cell growth in low concentrations of the oxidizing agent paraquat did not support in vitro selection of Wolbachia-infected cells based on increased expression of host antioxidant activity because Wolbachia itself is more sensitive to paraquat than are the host mosquito cells (Fallon et al. 2013b).
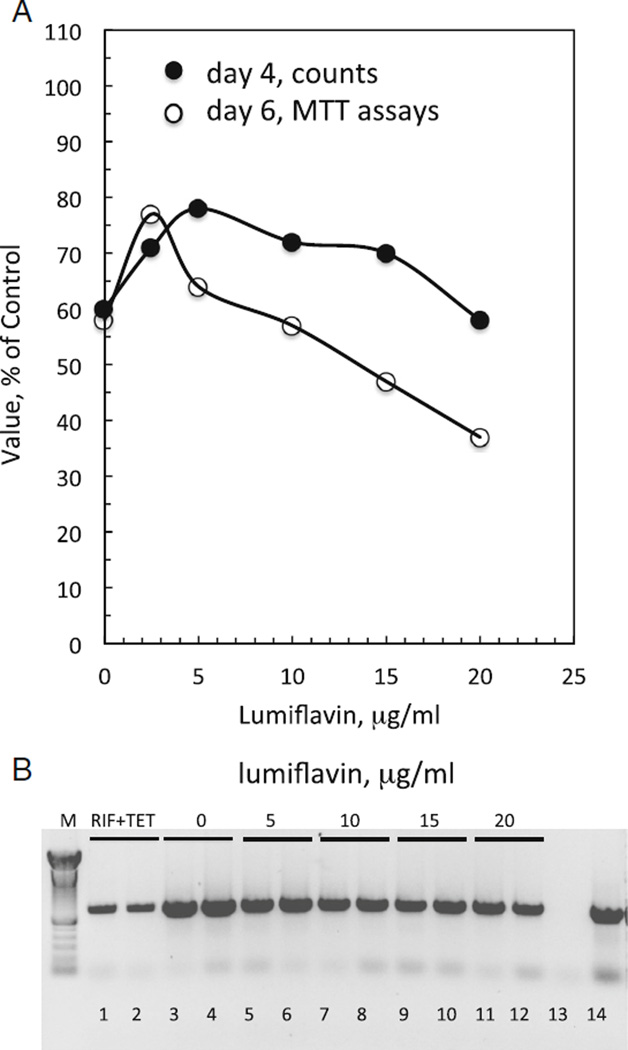
The effect of lumiflavin resembles that of paraquat (Fig. 4A). In the absence of lumiflavin, growth of C/wStr1 cells, or metabolic activity reflected by MTT values, reached about 60% of control levels with tetracycline and rifampicin. At concentrations ranging from 2.5 to 10 µg/ml, lumiflavin increased cell numbers and MTT values to about 80% of values in the presence of rifampicin and tetracycline before dropping back to initial levels for cell counts on day 4, or dropping below initial MTT levels on day 6. Microscopic (data not shown) and biochemical comparisons of samples from the same experiment showed that lumiflavin reduced Wolbachia abundance relative to control cells (Fig. 4B; compare lanes 3 and 4 to lanes 5 to 12), although the effect was weaker than that with antibiotics (Fig. 4B; compare lanes 3 and 4 to lanes 1 and 2). In addition, flow cytometric data (to be described in detail elsewhere) with two additional, independent populations of cells were consistent with the increased cell numbers and reduced Wolbachia numbers in lumiflavin-treated samples depicted in Fig. 4. These results suggest that C/wStr1 host cells do not benefit from riboflavin produced by Wolbachia. Conversely, a BLAST search indicates that the bacterial impX gene from Fusobacterium nucleatum, which encodes a putative riboflavin transporter used as a query in the rat RFT2 study (Yamamoto et al. 2009), has no homologs in Wolbachia or other members of the Rickettsiales, suggesting that Wolbachia is unlikely to have access to metabolic pools of riboflavin acquired from the medium by host cells.
Figure 4.
Low levels of lumiflavin enhance growth of C/wStr1 cells, and decrease Wolbachia levels. (A) C/wStr1 cells at passage 60 were seeded in 35-mm plates at a density of 1 × 105 cells/ml (2 ml/plate) in the absence or presence of lumiflavin at the indicated concentrations. A second set of control plates without lumiflavin was supplemented with rifampicin and tetracycline to suppress Wolbachia growth. Values from these plates were established as 100%. In the absence of antibiotics and lumiflavin, values for C/wStr1 cells reach about 60% of levels with antibiotics, as indicated in the Figure. After 4 d, duplicate plates were resuspended and counted with a Coulter electronic cell counter (closed circles). Values are expressed as percentages of counts in the presence of rifampicin and tetracycline (100% =15.3 × 105 cells/plate). After 6 d, duplicate plates were assayed for metabolic activity by MTT (open circles; 100%=2.32 OD570), and duplicate samples were harvested for isolation of DNA. (B) Relative amounts of Wolbachia determined by the polymerase chain reaction (PCR). PCR reactions were carried out as described previously (Fallon et al. 2013b). Lanes show products from two independent DNA extractions under each experimental condition. Lanes 1 and 2 show suppression of Wolbachia grown in the presence of rifampicin and tetracycline for 6 d after plating. Lanes 3 and 4 show control C/wStr1 cells; lanes 5–12 show cells with lumiflavin at the indicated concentrations. Lane 13 shows no DNA control; lane 14 shows positive control DNA.
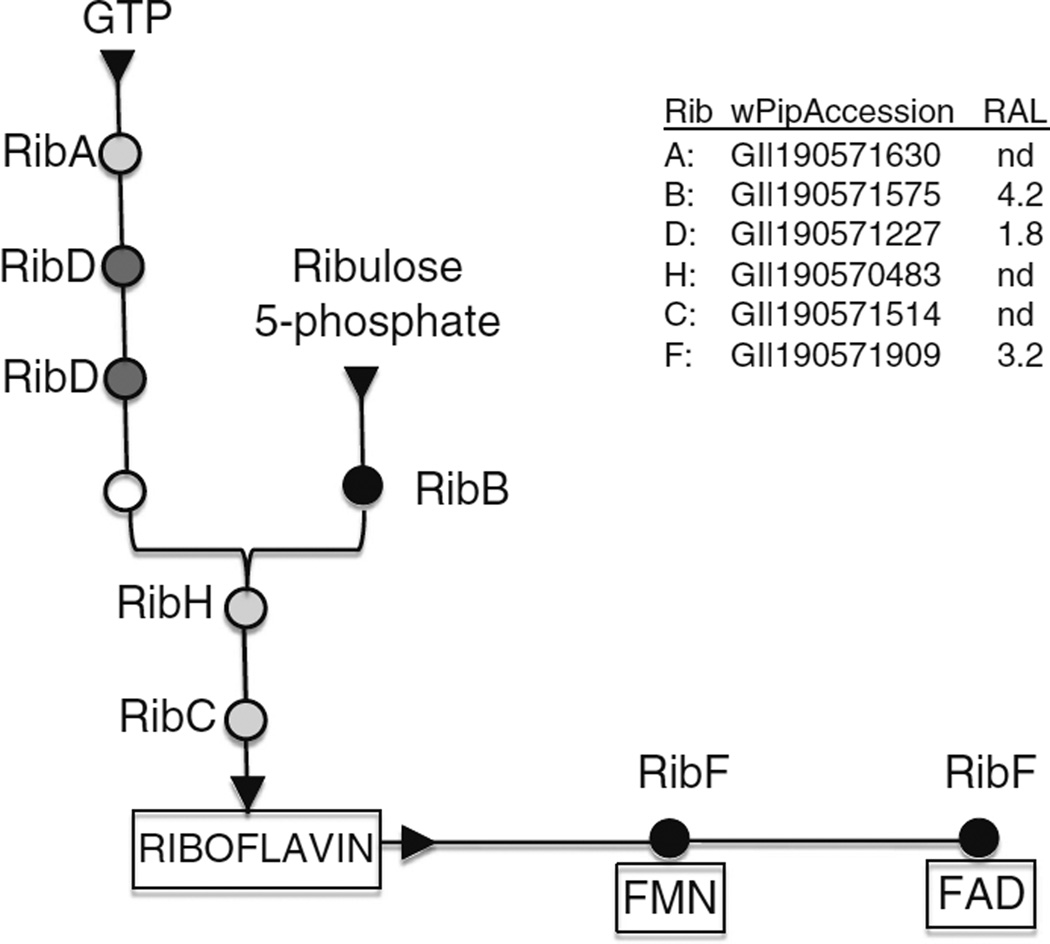
As a final consideration, we compare these experimental observations with a recently completed analysis of the wStr proteome in C/wStr1 cells (Baldridge GD, Witthuhn BA, Higgins LA, Markowski TW, Fallon AM. Proteomic analysis of an insect Wolbachia in mosquito cells reveals robust expression of a constitutive stress response, a complete type IV secretion system and potential supplementation of bacterial amino acid pools by proline, glutamate, glycine and cysteine degradation, unpublished). The present data suggest that Wolbachia and their host cells are unlikely to share riboflavin pools. Figure 5 summarizes the riboflavin biosynthetic pathway in Wolbachia, with accession numbers for gene homologs in wPip, the annotated Wolbachia genome that most closely matches the majority of wStr-encoded proteins. For those proteins detected in our proteomic analysis, relative abundance levels (RAL) are given at right (nd, not detected in the proteomic analysis), and in the schematic at left, more intense shading designates the more abundant enzymes. Riboflavin is synthesized from one molecule of GTP, a product of the purine synthesis pathways, condensed with 3,4-dihydroxy-2-butanone 4-phosphate derived from two molecules of ribulose 5-phosphate provided by the pentose phosphate pathway (Bacher et al. 2000). Of the enzymes that participate in riboflavin biosynthesis, RibB (3,4-dihydroxy-2-butanone 4-phosphate synthase), was the most abundant in our proteomic analysis of wStr. The RAL score for RibF, which acts downstream of riboflavin biosynthesis, converting riboflavin to FAD and FMN cofactors, approaches that for RibB, whose high RAL score potentially reflects functions outside the riboflavin pathway (Jin et al. 2003; Knegt et al. 2008; McDonagh et al. 2011). The high RAL score for RibF, relative to RibA, RibD, RibH, and RibC, is consistent with Wolbachia’s production of riboflavin solely for its own metabolic needs, rather than to provision its host cell.
Figure 5.
Pathways for riboflavin biosynthesis in Wolbachia. Circles indicate enzymes that participate in riboflavin biosynthesis from two substrates, GTP derived from purine biosynthesis, and ribulose 5-phosphate derived from the pentose phosphate pathway. RibA GTP cyclohydrolase II; RibD bifunctional deaminase and reductase; open circle unidentified enzyme (see Bacher et al. 2000; Abbas and Sibirny 2011); RibB 3,4-dihydroxy-2-butanone 4-phosphate synthase; RibH lumazine synthase; RibC riboflavin synthase; RibF riboflavin kinase and FAD synthase. At the left, shading represents relative expression level for these pathways, based on data in a related proteomics study (Baldridge GD, Witthuhn BA, Higgins LA, Markowski TW, Fallon AM. Proteomic analysis of an insect Wolbachia in mosquito cells reveals robust expression of a constitutive stress response, a complete type IV secretion system and potential supplementation of bacterial amino acid pools by proline, glutamate, glycine and cysteine degradation, unpublished; see the legend at the right). Black represents highly expressed enzyme; dark gray represents moderately expressed; light gray represents not detected (nd) in the proteomics study. Accession numbers are from the annotated wPip (Pel) genome.
Discussion
Despite considerable research, it has been difficult to identify metabolic processes that underlie Wolbachia’s interactions with its diverse hosts and presumably contribute a fitness advantage that accompanies its invasion of host populations (see Harcombe and Hoffmann 2004; Islam and Dobson 2006). In insects, Wolbachia is typically a reproductive parasite, but it may be symbiotic in the wasp, Asobara tabida. In A. tabida, Drosophila simulans, and Aedes aegypti RML12 cell line, Wolbachia has been implicated in iron homeostasis (Kremer et al. 2009), but the dynamics of iron partitioning between insect hosts and Wolbachia have been difficult to quantify by manipulation of dietary iron (Brownlie et al. 2009). Wolbachia that infect filarial worms are obligate symbionts that have coevolved with their nematode hosts. Annotation of the genome of wBm, a symbiont of Brugia malayi, initially suggested that Wolbachia might provide heme, nucleotides, riboflavin, and FAD to its nematode host (Foster et al. 2005). Moreover, Li and Carlow (2012) reported that in RPMI medium, doxycycline-induced inhibition of worm motility and reproduction were partially restored by supplementing the medium with riboflavin. Aside from these data, however, Darby et al. (2012) recently reported that Wolbachia strain wOo, which infects Onchocerca ochengi, a filarial parasite of cattle, lacks pathways for both riboflavin and heme provisioning, and suggest that in this system, Wolbachia may generate ATP for its host. Moreover, secondary loss of Wolbachia in members of the Onchocercidae (McNulty et al. 2010) implies that the symbiotic relationship is not universal in filarial worms.
In insects, obligate endosymbionts such as Buchnera aphidicola in aphids and Wigglesworthia glossinida in tsetse flies occur in distinct symbiotic organs known as bacteriomes; in contrast, Wolbachia typically occurs in non-specialized cells and tissues and is generally considered facultative or parasitic. An exception is the bedbug, Cimex lectularius, wherein Wolbachia occurs in bacteriocytes and functions as an obligate nutritional mutualist (Hosokawa et al. 2010). Disruption of bedbug development by rifampicin treatment was ameliorated when the blood meal was supplemented with a mixture of B vitamins, including the four vitamins (riboflavin, folic acid, pantothenate, and pyridoxine) for which Wolbachia retains complete biosynthetic pathways (Dunning Hotopp et al. 2006), but investigation of individual vitamins was not reported.
The possibility that Wolbachia provisions mosquito cells with one or more vitamins is consistent with early nutritional studies indicating that mosquitoes require riboflavin and pantothenic acid for larval growth, and pyridoxine and folic acid for pupation (Lichtenstein 1948; Singh and Brown 1957). If riboflavin produced by Wolbachia was available to its host cell, it might provide a convenient method to select for infected cells, for example, after Wolbachia transformation. Riboflavin provides a particularly attractive candidate because mosquito genomes encode a homolog of rat and human riboflavin transporter proteins, which can be inhibited by lumiflavin, a water-soluble compound produced by photolysis of riboflavin. Although the present study demonstrates that uninfected mosquito cells respond to riboflavin depletion and to inhibition of riboflavin transport, we find no evidence for a bacterial contribution to the host riboflavin pool in Wolbachia-infected cells. Moreover, Wolbachia growth is diminished when availability of exogenous riboflavin to the host cell is reduced, and proteomics data suggest that Wolbachia requires the riboflavin it produces for synthesis of FAD and NAD cofactors. Nevertheless, we cannot eliminate the possibility that the metabolic costs of the Wolbachia infection obscure any small benefit that might be derived from riboflavin production. Once transformation of Wolbachia is achieved, direct manipulation of the Wolbachia genome may allow us to exploit the many biotechnological implications that have emerged from investigation of riboflavin biosynthesis and transport (Long et al. 2010; Abbas and Sibirny 2011). For example, Coquard et al. (1997) described a mutation in the RibC gene in Bacillus subtilis that causes riboflavin overproduction.
Insects provide complex and diverse relationships with Wolbachia, and our own observations indicate that strains of Wolbachia from various sources differ in their adaptability to clonal A. albopictus cell lines (Fallon 2008; Fallon and Witthuhn 2009). Host cell factors that regulate infection and replication of Wolbachia are largely unexplored, and many strains of Wolbachia have not yet been established in cell lines. Using the wStr system, we anticipate that further exploration of Wolbachia metabolism in the context of proteomics data will facilitate manipulation of Wolbachia-infected cells, provide a framework for Wolbachia transformation and genetic manipulation, and improve establishment of recalcitrant Wolbachia strains in insect cell lines.
Acknowledgments
This work was supported by the NIH grant AI081322 and by the University of Minnesota Agricultural Experiment Station, St. Paul, MN. We thank John Beckmann for comments and suggestions.
References
- Abbas CA, Sibirny AA. Genetic control of biosynthesis and transport of riboflavin and flavin nucleotides and construction of robust biotechnological producers. Microbiol Mol Biol Rev. 2011;75:321–360. doi: 10.1128/MMBR.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacher A, Eberhardt S, Fischer M, Kis K, Richter G. Biosynthesis of vitamin B2 (riboflavin) Annu Rev Nutr. 2000;20:153–167. doi: 10.1146/annurev.nutr.20.1.153. [DOI] [PubMed] [Google Scholar]
- Beare PA, Sandoz KM, Omsland A, Rockey DD, Henzein RA. Advances in genetic manipulation of obligate intracellular bacterial pathogens. Front Microbiol. 2011;2:1–13. doi: 10.3389/fmicb.2011.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JF, Fallon AM. Detection of the Wolbachia protein WPIP0282 in mosquito spermathecae: implications for cytoplasmic incompatibility. Insect Biochem Mol Biol. 2013;43:867–878. doi: 10.1016/j.ibmb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlie JC, Cass BN, Riegler M, Witsenburg JJ, Iturbe-Ormaetxe I, McGraw EA, O’Neill SL. Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog. 2009;5(4):e1000368. doi: 10.1371/journal.ppat.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquard D, Huecas M, Ott M, van Dijl JM, van Loon APGM, Hohmann HP. Molecular cloning and characterization of the ribC gene from Bacillus subtilis: a point mutation in ribC results in riboflavin overproduction. Mol Gen Genet. 1997;254:81–84. doi: 10.1007/s004380050393. [DOI] [PubMed] [Google Scholar]
- Darby AC, Armstrong SD, Bah GS, Kaur G, Hughes MA, Kay SM, et al. Analysis of gene expression from the Wolbachia genome of a filarial nematode supports both metabolic and defensive roles within the symbiosis. Genome Res. 2012;22:2467–2477. doi: 10.1101/gr.138420.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning Hotopp JC, Lin M, Madupu R, Crabtree J, Angiuoli SV, Eisen JA, et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2006;2(2):e21. doi: 10.1371/journal.pgen.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon AM. Cytological properties of an Aedes albopictus mosquito cell line infected with Wolbachia strain wAlbB. In Vitro Cell Dev Biol Anim. 2008;44:154–161. doi: 10.1007/s11626-008-9090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon AM, Baldridge GD, Higgins LA, Witthuhn BA. Wolbachia from the planthopper Laodelphax striatellus establishes a robust, persistent, streptomycin-resistant infection in clonal mosquito cells. In Vitro Cell Dev Biol Anim. 2013a;49:66–73. doi: 10.1007/s11626-012-9571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon AM, Kurtz CM, Carroll EM. The oxidizing agent, paraquat, is more toxic to Wolbachia than to mosquito host cells. In Vitro Cell Dev Biol Anim. 2013b;49:501–507. doi: 10.1007/s11626-013-9634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon AM, Witthuhn BA. Proteasome activity in a naivemosquito cell line infected with Wolbachia pipientis wAlbB. In Vitro Cell Dev Biol Anim. 2009;45:460–466. doi: 10.1007/s11626-009-9193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J, Ganatra M, Kamal I, Ware J, Makarova K, Ivanova N, et al. The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 2005;3(4):e121. doi: 10.1371/journal.pbio.0030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcombe W, Hoffmann AA. Wolbachia effects in Drosophila melanogaster: in search of fitness benefits. J Invertebr Pathol. 2004;87:45–50. doi: 10.1016/j.jip.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Hosokawa T, Koga R, Kikuchi Y, Meng X-Y, Fukatsu T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci U S A. 2010;107:769–774. doi: 10.1073/pnas.0911476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS, Dobson SL. Wolbachia effects on Aedes albopictus (Diptera: Culicidae) immature survivorship and development. J Med Entomol. 2006;43:689–695. doi: 10.1603/0022-2585(2006)43[689:weoaad]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Jin C, Barrientos A, Tzagoloff A. Yeast dihydroxybutanone phosphate synthase, an enzyme of the riboflavin biosynthetic pathway, has a second unrelated function in expression of mitochondrial respiration. J Biol Chem. 2003;278:14698–14703. doi: 10.1074/jbc.M300593200. [DOI] [PubMed] [Google Scholar]
- Knegt FHP, Mello LV, Reis FC, Santos MT, Vicentini R, Ferraz LFC, Ottoboni LMM. ribB and ribBA genes from Acidithiobacillus ferrooxidans: expression levels under different growth conditions and phylogenetic analysis. Res Microbiol. 2008;159:423–431. doi: 10.1016/j.resmic.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Kremer N, Voronin D, Charif D, Mavingui P, Mollereau B, Vavre F. Wolbachia interferes with ferritin expression and iron metabolism in insects. PLoS Pathog. 2009;5(10):e1000630. doi: 10.1371/journal.ppat.1000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Carlow CKS. Characterization of transcription factors that regulate the type IV secretion system and riboflavin biosynthesis in Wolbachia of Brugia malayi. PLoS ONE. 2012;7(12):e51597. doi: 10.1371/journal.pone.0051597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein EP. Growth of Culex molestus under sterile conditions. Nature. 1948;162:227. doi: 10.1038/162227b0. [DOI] [PubMed] [Google Scholar]
- Long Q, Ji L, Wang H, Xie J. Riboflavin biosynthetic and regulatory factors as potential novel anti-infective drug targets. Chem Biol Drug Des. 2010;75:339–347. doi: 10.1111/j.1747-0285.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- McDonagh B, Reguejo R, Fuentes-Almagro CA, Ogueta S, Bárcena JA, Padilla CA. Thiol redox proteomics identifies differential targets of cytosolic and mitochondrial glutaredoxin-2 isoforms in Saccharomyces cerevisiae. Reversible S-glutathionylation of DHBP synthase (RIB3) J Proteome. 2011;74(1):2487–2497. doi: 10.1016/j.jprot.2011.04.018. [DOI] [PubMed] [Google Scholar]
- McNulty SN, Foster JM, Mitreva M, Dunning Hotopp JC, Martin J, Fischer K, et al. Endosymbiont DNA in endobacteria-free filarial nematodes indicates ancient horizontal genetic transfer. PLoS ONE. 2010;5(6):e1029. doi: 10.1371/journal.pone.0011029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H, Miyoshi T, Koizumi Y. In vitro cultivation of Wolbachia in insect and mammalian cell lines. In Vitro Cell Dev Biol Anim. 2002;38:423–427. doi: 10.1290/1071-2690(2002)038<0423:IVCOWI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Said HM, Ma TY. Mechanism of riboflavine uptake by Caco-2 human intestinal epithelial cells. Am J Physiol. 1994;266:G15–G21. doi: 10.1152/ajpgi.1994.266.1.G15. [DOI] [PubMed] [Google Scholar]
- Shih KM, Gerenday A, Fallon AM. Culture of mosquito cells in Eagle’s medium. In Vitro Cell Dev Biol Anim. 1998;34:629–630. doi: 10.1007/s11626-996-0010-1. [DOI] [PubMed] [Google Scholar]
- Singh KRP, Brown AWA. Nutritional requirements of Aedes aegypti L. J Insect Physiol. 1957;1:199–220. [Google Scholar]
- Sinkins SP, Gould F. Gene drive systems for insect disease vectors. Nat Rev Genet. 2006;7:427–435. doi: 10.1038/nrg1870. [DOI] [PubMed] [Google Scholar]
- Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R, Brownlie JC, et al. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol. 2004;2:E69. doi: 10.1371/journal.pbio.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Inoue K, Ohta K, Fukatsu R, Maeda J, Yoshida Y, Yuasa H. Identification and functional characterization of rat riboflavin transporter 2. J Biochem. 2009;145:437–443. doi: 10.1093/jb/mvn181. [DOI] [PubMed] [Google Scholar]
- Yonezawa A, Masuda S, Katsura T, Inui K. Identification and functional characterization of a novel human and rat riboflavin transporter, RFT1. Am J Physiol Cell Physiol. 2008;295:632–641. doi: 10.1152/ajpcell.00019.2008. [DOI] [PubMed] [Google Scholar]