Abstract
Objectives
To understand how older adults perceive their risk of Alzheimer’s Disease (AD) and how this may shape their medical care decisions, we examined whether presence of established risk factors of AD is associated with individuals’ perceived risk of AD, and with preference for preventing AD.
Methods
Participants: Data came from the US Health and Retirement Study participants who were asked questions on AD risk perception (N = 778). Measurements: Perceived risk of AD was measured by respondents’ estimate of their percent chance (0–100) developing AD in the next 10 years. Preference for AD prevention was measured with questions eliciting willingness to pay for a drug to prevent AD. Analysis: Multivariate linear regressions were used to estimate correlates of perceived risk and preference for prevention.
Results
Better cognitive functioning and physical activity are associated with decreased perceived risk. Neither age nor cardiovascular disease is associated with perceived risk. African Americans have lower perceived risk than non-Latino whites; the difference is wider among people age 65 and above. Only 4% to 7% of the variation in perceived risk was explained by the model. Preference for prevention is stronger with increased perceived risk, but not with the presence of risk factors. Persons with better cognitive functioning, physical functioning, or wealth status have a stronger preference for prevention.
Conclusion
Some known risk factors appear to inform, but only modestly, individuals’ perceived risk of AD. Furthermore, decisions about AD prevention may not be determined by objective needs alone, suggesting a potential discrepancy between need and demand for AD preventive care.
Keywords: Alzheimer’s disease, prevention preference, risk perception, willingness to pay
Introduction
The prevalence of Alzheimer’s disease (AD) is increasing as the population ages, and in the United States, it is estimated to rise from $4.5 million in 2000 to $13.2 million in 2050 [1]. The growing number of people with AD and the costs associated with the disease put a heavy economic burden on society. In addition to direct and indirect costs to the affected individuals and their caregivers, Medicare costs associated with AD and other types of dementia amounted to $62 billion in 2000, and are projected to be 40% of the Medicare budget by 2050 unless new effective treatments for AD become available [2].
At present, there is no effective treatment for AD, but progression to severe dementia can be slowed and symptoms managed. Growing literature suggests that metabolic changes associated with cardiovascular disease and diabetes may contribute to the development or the severity of AD [3–6]. In addition, clinical and epidemiological studies suggest that development of cognitive impairment could be delayed by addressing modifiable lifestyle factors, such as increasing physical activity [7–11], vegetable consumption [12], and social and mental activity [13]. Because the incidence of AD doubles every 5 years from age 65 and beyond [14], the prevalence and costs of AD might drop by half by delaying the onset of AD by 5 years with preventive interventions.
Understanding how older adults perceive their risk of AD and how they may make decisions regarding AD prevention is a prerequisite for successful implementation of an intervention to prevent or delay onset of AD. The few studies conducted in the United States [15], Australia [16], and Israel [17] consistently demonstrate that people have limited knowledge about risk factors, symptoms, and treatment options of AD. This suggests that individuals’ perceived risk of AD may be inaccurate and thus their ability to make informed decisions regarding AD preventive care may be limited. None of these published studies examined the relationship among risk factors, perceived risk, and stated preference for prevention regarding AD, which is the focus of our study. Studies on cancer suggest a positive association between perceived risk and preference for prevention, although the association was not always consistent [18–23]. Generally, there is positive association between higher perceived risk and increased willingness to take screening tests or pay for preventive programs, but the relationship seems weak or absent when the treatment options involve potential risk or lifestyle changes.
To advance our understanding of older adults’ risk perception for AD and their preferences for AD prevention, the present study examines the relationship among known risk factors and other individual characteristics, perceived risk of AD, and preference for AD prevention, using a nationally representative sample of US older adults. Findings from this study can inform policy on setting priorities for current AD prevention programs as well as on future public health efforts as effective prevention or treatment options become available. Thus, this article seeks to answer the following research questions: First, are known risk factors of AD associated with perceived risk of AD?; Second, is an individual’s preference for AD prevention, measured by willingness to pay (WTP) for a hypothetical drug preventing AD, associated with perceived risk and/or known risk factors of AD?
Methods
Data
We examined data from the Health and Retirement Study (HRS), a nationally representative survey of US residents age 50 and older in 1992, and their spouses of any age (http://hrsonline.isr.umich.edu). Latinos and African Americans were over-sampled. The HRS is the only national survey asking questions about perceived risk for AD and contains information on cognitive functioning and self-reported measures of a variety of health conditions, physical functioning, and demographic and economic status.
Among respondents to the 2002 HRS survey, those who agreed to answer additional questions (81%) were randomly assigned to one of 12 sets of additional questions, one of which focused on risk perception and preference for preventing AD. Proxy interview respondents and individuals who reported having a memory-related disease diagnosed by a physician in the current or the previous interview (conducted 2 years before) were not eligible to answer these questions. The resulting sample of adults is likely to be cognitively healthier and may not be representative of US adults in the age range. Thus, our goal is to understand risk perception and preference for prevention among adults without cognitive impairment. In addition, exclusion of people with a history of memory-related disease might have improved the quality of the data because cognitive functioning is associated with measurement error in older adults [24,25]. Among those who were eligible (n = 778), 95% (n = 740) provided valid answers (neither “do not know” nor “refused”) to the perceived risk question and 98% (n = 760) provided valid answers to the prevention preference questions (Fig. 1).
Figure 1.
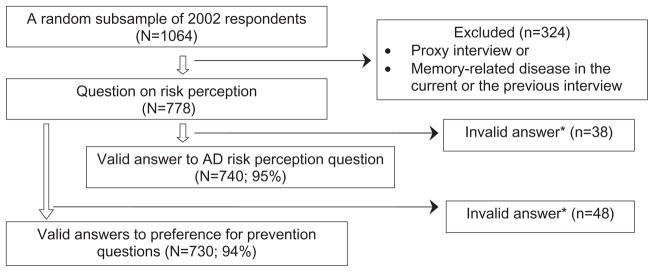
Exclusion criteria and analysis sample.
*Those who answered as “Don’t Know” or who refused answering the question. AD, Alzheimer’s disease.
Measures
Dependent variables
Perceived risk of AD was assessed by asking respondents to give the probability that they would develop AD in the next 10 years. The exact wording is as follows: “Of course, no one can know for sure what will happen in the future, but we would like to know what you think about various health risks. Using a scale of 0–100 where 0 means no chance and 100 means absolutely certain, what are the chances that you will develop Alzheimer’s disease in the next ten years?” Based on the answer for this question, we coded a discrete variable ranging 0–100.
Preference for preventing AD was measured with questions asking about WTP for a hypothetical drug preventing AD. Participants were first asked the following question: “Suppose that a drug were discovered that guaranteed that someone would never develop Alzheimer’s disease, and that the treatment was 100% effective as long as a person took one pill every month for the rest of their life. Suppose further that there are no side effects. Would you be willing and able to pay $100 per month for such a pill, or the same amount in higher insurance payments to cover it?” If the respondent answered yes to this question, a similar question with the amount of $250 was asked again; and if yes to this question, the respondent was further asked a similar question with $1000. If the respondent answered no to the first question (with $100), a similar question with the amount of $25 was asked; and then if no, the respondent was further asked a question with $5. Based on answers to these questions, we created a six-category ordinal variable ranging from 1 to 6, each indicating WTP of: 1) <$5; 2) $5–25; 3) $25–100; 4) $100–250; 5) $250–1000; and 6) $1000.
Known risk factors of AD
Based on our literature review and expertise, we analyzed known risk factors of AD, including age, cognitive functioning, cardiovascular disease and risk factors and level of physical activity. A variety of measures available in the HRS were used to define these risk factors as described below.
Age was used as a continuous variable. Given the fact that the incidence of AD increases drastically after age 65 [14,26], we initially compared estimates from regressions with different functional forms of age, including the quadratic form and various categorical representations. We found that none of these specifications fit the data as determined by the significance level (P > 0.1). Furthermore, to examine the interaction effect of age with the predictors for perceived risk of AD, we conducted analyses with two subgroups—age up to 64, and 65 or older.
We used a multidimensional measure of cognitive functioning, which is the sum of scores on four tests (0–27): immediate word recall (0–10), delayed word recall (0–10), subtraction (0–5), and backward count (0–2). This measure shares questions with two commonly used instruments, the Mini-Mental State Exam (MMSE), a standard geriatric dementia screen [27], and the Telephone Interview for Cognitive Status, a revised version of the MMSE for use over the telephone [28]. Though the cognitive screen in the HRS has not been directly compared with measures used in other surveys or dementia diagnosis, several published studies have examined the screen, demonstrating its internal consistency and construct validity [29–31]. For immediate and delayed word recall tests, respondents were asked to recall as many words as possible from a list of 10 words provided by the interviewer, first immediately after the list of words was administered and again 5 minutes later. For the subtraction test, respondents were asked to subtract 7 from 100 five times. For the backward count test, respondents were asked to count backward from 20 to 10; if respondents failed to count correctly, they could try again. A score of 2, 1, or 0 was given, depending on whether the respondent counted backward correctly the first time, the second time, or not at all. Because these scores are highly correlated with each other, we used a composite score rather than individual scores to avoid high multicollinearity in the regression analyses.
Cardiovascular diseases included were stroke and heart attack. Diabetes, which leads to cardiovascular complications, was included as well. These were measured by dichotomous variables, each indicating whether individuals have experienced any of these problems diagnosed by a physician during the past 2 years. Physical activity was dichotomously coded based on answers to the question: “On average over the last 12 months have you participated in vigorous physical activity or exercise three times a week or more? By vigorous physical activity, we mean things like sports, heavy housework, or a job that involves physical labor.”
Other covariates
Several measures of cognitive and physical functioning that may be associated with people’s perceived risk of AD were also examined. Physical functioning was measured with a dichotomous variable indicating whether an individual has any limitation in activity of daily living (ADL). In sensitivity analyses, we also examined two measures: 1) self-rated memory decline, a dichotomous variable based on the question: “Compared to (last interview date or two years ago), would you say your memory is better now, about the same, or worse now than it was then?;” and 2) longevity expectation, a continuous variable (0–100) based on the question asking the percent chance the respondent would live the next 10 years.
Variables representing sociodemographic characteristics included in all analyses were race/ethnicity, education, gender, and marital status. Four exclusive categories of race/ethnicity: non-Latino white, African American, Latino, and other race were used. For the other race category, further information on national origin is not available in the HRS public use file. Two dummy variables were used for educational attainment, indicating less than high school and college or more, with the referent category of high school graduation. In addition, three variables indicating ability to pay—total household income (continuous variable), total household wealth (continuous variable), and having prescription drug coverage (0/1)—were included for the analysis of preference for preventing AD. We also examined dummy variables indicating each quartile of income and wealth distribution. Except for one missing value in race/ethnicity, valid answers were reported or verified for all other covariates.
Analytical Methods
We first examined descriptive statistics for all the variables and then conducted multivariate regression analyses. In order to model preference for preventing AD as an outcome, we estimated ordered probit and linear regressions. As we obtained virtually the same results using both methods, we report the linear regression results for ease of interpretation. Covariates included in all the regression models were known risk factors of AD, functional limitation, and sociodemographic characteristics as described above. We detected heteroskedasticity based on the White test, and used Huber–White robust standard errors [32] for all regression analyses. Sensitivity analyses were conducted to examine the robustness of the results.
Results
Descriptive Statistics
The average age of the respondents (including individuals from the original HRS sample and their spouses) was 68, ranging from 31 years to 96 years. The predominant majority of the sample were in their 50s to 80s (97.2%); a small fraction of the sample was under 50 (1.4%) or over 90 (1.4%), which consisted entirely of spouses. Slightly more than half (53%) were women. The majority was non-Latino white (81%), 11% were African American, and 5% were Latino. The other race/ethnicity group comprised only 1% (n = 10). See Table 1 for statistics on other individual characteristics. Because people with a history of memory-related disease were excluded, the respondents for the current study were more likely to be young and male than the overall HRS participants.
Table 1.
Descriptive statistics of the analysis sample
| Variables*,† | Mean [SD] | Frequency |
|---|---|---|
| Perceived risk of AD in the next 10 years (in % chance: 0–100) | 29.9 [27.1] | |
| Preference for AD prevention: $ willing to pay per month | ||
| <5 | 6.5% | |
| 5–25 | 7.9% | |
| 25–100 | 19.6% | |
| 100–250 | 37.1% | |
| 250–1000 | 22.0% | |
| >1000 | 7.0% | |
| Longevity expectation (0–100) | 48.9 [32.3] | |
| Self-rated memory decline | 19.4% | |
| Known risk factors of AD | ||
| Age | 68.0 [9.66] | |
| Cardiovascular disease/risk factors | ||
| Stroke | 5.8% | |
| Heart attack | 2.7% | |
| Diabetes | 19.0% | |
| Physical activity | 41.5% | |
| Cognitive functioning (0–27)‡ | 15.1 [4.59] | |
| Immediate recall (0–10) | 5.40 [1.79] | |
| Delayed recall (0–10) | 4.42 [2.03] | |
| Subtraction (0–5) | 3.57 [1.68] | |
| Backward count (0–2) | 1.72 [0.70] | |
| Any limitation in ADL | 14.9% | |
| Other sociodemographic characteristics | ||
| Female | 53.2% | |
| Race/ethnicity | ||
| White | 81.2% | |
| Black | 11.2% | |
| Hispanic | 6.0% | |
| Other race | 1.5% | |
| Education | ||
| Less than high school | 23.5% | |
| High school graduate | 35.6% | |
| Some college | 40.7% | |
| Married | 67.4% | |
| Prescription drug coverage | 74.9% | |
| Household wealth (−$130,000–$41,639,999)§ | 391,206 [1,594,994] | |
| Household income (0–$757,000) | 51,769 | |
Range of values for continuous variables is presented in the parentheses.
Total number of participants was 778. For some variables, the number of participants who provided valid answers was as follows: perceived risk of AD (n = 740); preference for AD prevention (n = 760); longevity expectation (n = 714); cognitive functioning (n = 762); and race/ethnicity and education (n = 777).There was no missing value for other variables.
Sum of scores for the four measures in following rows. Nonresponse for each test is excluded (i.e., coded as missing) in the summary statistics.
Some individuals (n = 21; 2.7%) had negative net wealth, largely from mortgage debt. Median wealth was $161,500.
Alzheimer’s disease; ADL, activity of daily living.
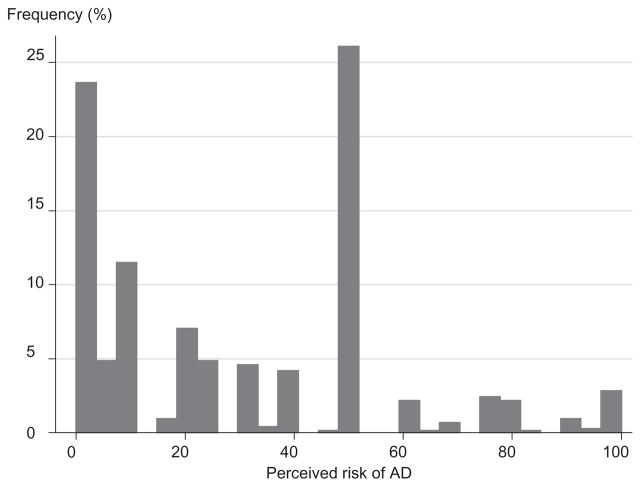
The average perceived risk (percent chance) of AD in 10 years was 29.9 (SD 27.1) and the median preference for AD prevention was in the third category (willing to pay $100 but not $250 per month) (Table 1). The distribution of perceived risk of AD ranged from 0 to 100 with two distinctive peaks at 0 (n = 166; 22.4%) and 50 (n = 193; 26.1%) (Fig. 2). We examined whether the likelihood of providing answers of 0, 50, or 100 was systematic and was predicted by any of the covariates using logit regression. We found no difference (P > 0.1) in respondent characteristics, including cognitive functioning and other risk factors of AD, between those who answered 0, 50, or 100 and those who answered otherwise.
Figure 2.
Distribution of the perceived risk of Alzheimer’s disease (AD). N = 740; mean (SD) = 29.9 (27.1).
Correlates of Perceived Risk of AD
Multivariate regression results show that several risk factors of AD are associated with perceived risk of AD (Table 2). For example, a five-point increase in the cognitive functioning score is associated with a 3.8 percentage points (P = 0.0063) lower perceived risk of AD. Likewise, people who engage in regular physical activity are likely to have 4.6 percentage points (P = 0.025) lower perceived risk of AD. Nevertheless, age and cardiovascular disease indicators are not related to perceived risk. African Americans are associated with 8.4 percentage points (P = 0.053) lower perceived risk of AD as compared with non-Latino whites.
Table 2.
Perceived risk of AD: multivariate linear regression with varying specifications
| Dependent variable: probability of developing AD in the next 10 years (0–100)
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (A) Overall sample N = 725 |
(B) Age 64 or under N = 300 |
(C) Age 65 or older N = 425 |
(D) Including “self-rated memory decline” N = 725 |
(E) Including “self-rated longevity expectation” N = 679 |
||||||
| Coefficient (SE) | P-value | Coefficient (SE) | P-value | Coefficient (SE) | P-value | Coefficient (SE) | P-value | Coefficient (SE) | P-value | |
| Self-rated memory decline | 11.16‡ (2.63) | <0.001 | ||||||||
| Longevity expectation (0–100) | −0.10‡ (0.04) | 0.006 | ||||||||
| Known risk factors of AD | ||||||||||
| Age | −0.02 (0.12) | 0.85 | 0.57* (0.32) | 0.081 | −0.64‡ (0.21) | 0.0031 | −0.01 (0.12) | 0.93 | 0.04 (0.13) | 0.75 |
| Cognitive functioning (0–27) | −0.75‡ (0.28) | 0.0063 | −0.58 (0.42) | 0.17 | −0.96† (0.38) | 0.012 | −0.70† (0.27) | 0.010 | −0.70† (0.29) | 0.014 |
| Physical activity | −4.57† (2.03) | 0.025 | −4.21 (3.02) | 0.16 | −5.57* (2.86) | 0.053 | −3.99† (2.02) | 0.048 | −4.07† (2.06) | 0.049 |
| Cardiovascular disease | ||||||||||
| Stroke | 4.09 (4.81) | 0.40 | −2.32 (8.49) | 0.79 | 9.01 (6.06) | 0.14 | 3.33 (4.84) | 0.49 | 3.37 (5.05) | 0.51 |
| Heart attack | −8.02 (6.95) | 0.25 | −11.44 (10.30) | 0.27 | −4.71 (9.54) | 0.62 | −8.28 (7.12) | 0.25 | −8.88 (8.17) | 0.28 |
| Diabetes | 3.19 (2.74) | 0.25 | 1.73 (3.72) | 0.65 | 3.60 (3.76) | 0.34 | 2.88 (2.70) | 0.29 | 2.90 (2.84) | 0.31 |
| Any ADL limitation | 0.57 (3.31) | 0.86 | 6.77 (5.10) | 0.19 | −1.73 (4.35) | 0.69 | −1.46 (3.25) | 0.65 | 1.78 (3.62) | 0.62 |
| Other sociodemographic factors | ||||||||||
| Female | 2.26 (2.16) | 0.30 | 4.65 (3.05) | 0.11 | −0.51 (2.99) | 0.85 | 2.54 (2.12) | 0.21 | 2.09 (2.19) | 0.32 |
| Race/ethnicity (ref: non-Latino white) | ||||||||||
| Black | −8.35* (4.31) | 0.053 | 1.92 (6.54) | 0.77 | −16.86‡ (5.59) | 0.0027 | −7.93* (4.31) | 0.066 | −6.71 (4.75) | 0.16 |
| Latino | −6.71 (4.32) | 0.12 | −6.42 (5.01) | 0.20 | −7.58 (7.47) | 0.31 | −6.37 (4.14) | 0.12 | −7.91* (4.48) | 0.078 |
| Other race | 8.64 (8.96) | 0.34 | −1.99 (11.44) | 0.86 | 9.30 (11.74) | 0.43 | 7.39 (9.60) | 0.44 | 8.54 (8.69) | 0.33 |
| Education (ref: high school graduate) | ||||||||||
| Less than high school | 1.44 (3.01) | 0.63 | −2.72 (4.97) | 0.59 | 4.14 (3.82) | 0.28 | 1.78 (2.96) | 0.55 | 1.44 (3.16) | 0.65 |
| Some college | −1.36 (2.20) | 0.54 | −4.97 (3.18) | 0.12 | 1.57 (3.08) | 0.61 | −1.38 (2.17) | 0.53 | −0.67 (2.29) | 0.77 |
| Married | 1.18 (2.55) | 0.64 | 4.56 (3.96) | −2.02 (3.41) | 0.55 | 1.22 (2.49) | 0.63 | −0.19 (2.64) | 0.94 | |
| Constant | 43.56‡ (11.25) | <0.001 | −1.03 (21.60) | 0.96 | 95.96‡ (19.84) | <0.001 | 39.48‡ (11.07) | <0.001 | 43.97‡ (11.76) | <0.001 |
| R-squared | 0.04 | 0.07 | 0.06 | 0.07 | 0.06 | |||||
P < 0.1,
P < 0.05,
P < 0.01.
AD, Alzheimer’s disease; ADL, activity of daily living; SE, standard error.
To further examine the interaction effect of age, we conducted subgroup analyses in two age groups—64 or younger and 65 or older—and found a substantial difference between the two groups. The most notable difference is the effect of age: in the younger group, perceived risk increases with age (P = 0.081), consistent with the increasing risk of AD with aging, but in the older group, perceived risk decreases with age (P = 0.0031). Better cognitive functioning (P = 0.012) and regular physical activity (P = 0.053) are associated with decreased perceived risk of AD only for the older group. Racial/ethnic difference exists only among the elderly group in that African Americans have a lower perceived risk of AD by 16.9 percentage points (P = 0.0027) than non-Latino whites. The overall coefficients of each subgroup are statistically different from the coefficients from the combined sample.
Sensitivity Analysis
We explored the possibility that individual’s perceived risk of AD may have been based on their perception of decline in memory, which is accepted as a critical precursor of dementia [24,33]. People who reported that their memory declined over the last 2 years were likely to report 11 percentage points higher perceived risk of AD (P < 0.001). The inclusion of self-rated memory decline improved the overall explanatory power of the model, indicated by the R-square, by 0.03, although the R-square of the model is still very small, 0.07.
A potential confounding factor in the relationship between risk factors and perceived risk of AD might be longevity expectation. That is, people who have longer life expectancy may have higher perceived risk of AD; conversely, people with multiple comorbidities may have a shorter longevity expectation, and thus, may have a low perceived risk of AD. To examine this hypothesis, we included an additional covariate, subjective longevity expectation. The results, however, are similar to prior models. Furthermore, high longevity expectation is associated with decreased level of perceived risk of AD (P = 0.06), confirming that people do not relate aging directly to risk of AD.
Preference for Preventing AD
Perceived risk is associated with a stronger preference for preventing AD, which is measured by WTP for a hypothetical drug preventing AD (Table 3). Individuals with better cognitive functioning (P = 0.010) and those without ADL limitations (P = 0.019) have a stronger preference for AD prevention. As expected, individuals with higher levels of wealth are willing to pay more (P = 0.0048). An alternative specification of wealth and income, using dummy variables reflecting each quartile, produced similar results (i.e., increasing WTP with wealth; no income effect). None of the known risk factors for AD is significant. The results are similar in the regression, without perceived risk of AD, as a covariate (Table 3).
Table 3.
Preference for preventing AD: multivariate linear regression
| Dependent variable: preference for AD prevention with six categories (1–6), each indicating WTP (per month) of <$5, $5–25, $25–100, $100–250, $250–1000, and $1000 for a drug preventing AD
| ||||
|---|---|---|---|---|
| (A) With perceived risk of AD N = 715 |
(B) Without perceived risk of AD N = 743 |
|||
| Coefficient (SE) | P-value | Coefficient (SE) | P-value | |
| Perceived risk of AD (0–100) | 0.01‡ (0.002) | <0.001 | ||
| Known risk factors of AD | ||||
| Age | −0.01* (0.01) | 0.10 | −0.01 (0.01) | 0.14 |
| Cognitive functioning (0–27) | 0.03‡ (0.01) | 0.01 | 0.03† (0.01) | 0.01 |
| Physical activity | 0.02 (0.09) | 0.85 | 0.02 (0.09) | 0.79 |
| Stroke | 0.07 (0.18) | 0.70 | 0.16 (0.18) | 0.39 |
| Heart attack | −0.08 (0.36) | 0.83 | −0.27 (0.39) | 0.49 |
| Diabetes | 0.16 (0.12) | 0.18 | 0.19 (0.12) | 0.10 |
| Any ADL limitation | −0.34† (0.15) | 0.02 | −0.28* (0.15) | 0.06 |
| Other sociodemographic factors | ||||
| Female | −0.07 (0.10) | 0.46 | −0.01 (0.10) | 0.93 |
| Race/ethnicity (ref: non-Hispanic white) | ||||
| Black | 0.14 (0.16) | 0.40 | 0.02 (0.16) | 0.93 |
| Hispanic | −0.33 (0.21) | 0.11 | −0.39* (0.22) | 0.08 |
| Other race | 0.70 (0.44) | 0.11 | 0.55 (0.40) | 0.18 |
| Education (ref: high school graduate) | ||||
| Less than high school | −0.08 (0.13) | 0.54 | −0.13 (0.12) | 0.27 |
| Some college | −0.07 (0.11) | 0.49 | −0.05 (0.11) | 0.60 |
| Married | 0.13 (0.11) | 0.26 | 0.16 (0.11) | 0.13 |
| Household wealth (/100 k) | 0.005‡ (0.002) | 0.005 | 0.005† (0.002) | 0.02 |
| Household income (/100 k) | 0.08 (0.07) | 0.24 | 0.06 (0.06) | 0.38 |
| Prescription drug coverage | 0.15 (0.11) | 0.18 | 0.14 (0.11) | 0.22 |
| Constant | 3.64‡ (0.53) | <0.001 | 3.75‡ (0.52) | <0.001 |
| R-squared | 0.10 | 0.08 | ||
P < 0.1,
P < 0.05,
P < 0.01.
Coefficient (robust standard errors).
AD, Alzheimer’s disease; ADL, activity of daily living; SE, standard error; WTP, willingness to pay.
Discussion
Older adults appear to formulate their perceived risk of AD in relation to some known risk factors for AD, such as cognitive functioning, perceived memory decline, and level of physical activity. Nevertheless, we found that age is not associated with perceived risk of AD in the overall sample and is inversely related to perceived risk of AD among people aged 65 or older, suggesting that individuals may not link aging to an increased risk of AD. Consistently, people with higher longevity expectation are likely to report lower levels of perceived risk for AD. Given the fact that age is one of the most important risk factors for AD and the actual risk of AD increases exponentially with age [14,26], our findings suggest a lack of knowledge about risk factors for AD among older adults. Similarly, perceived risk is not related to the presence of cardiovascular disease or diabetes which is potentially modifiable risk factors for AD. Taken together, our findings suggest that individuals’ perceived risk of AD may not be associated with objective risk of AD, and thus, may not be accurate.
What would be the reasons for the lack of concordance between known risk factors and perceived risk of AD? One possibility is that people may not have an appropriate level of knowledge about specific risk factors of AD [15,16] and may not process dementia risk factor information based on scientific evidence [16]. Another reason might be that measures of risk factors in the data are crude. For example, if well controlled, cardiovascular disease risk factors may not be perceived as a disease or risk factors for AD at all. In fact, in our data, 92% of people who had diabetes reported that their diabetes was well controlled. Likewise, people who had a memory-related disease because of stroke were not included in the study sample.
The overall variation in perceived risk explained by known risk factors and demographic factors in our model is very small (R-squared = 0.04), suggesting that only a small amount of variation in risk perception was explained by the observed factors. There are many potential explanations. First, the available data used in the analysis are limited in their ability to capture the full range of information people use in formulating risk perception of AD. Second, the measure of risk perception may not perform well in capturing the true level of perceived risk of AD. People may have problems answering risk perception questions which involve probability with a 0–100 scale [34,35]. Third, perceived risk of AD may not be in fact based on either observed or unobserved objective risk factors. To better understand the process and type of information individuals use to formulate risk perception on AD, a more in-depth study may be needed.
Given the lack of explanatory power of the known risk factors on perceived risk of AD, it is not surprising to find that none of the known risk factors explain preference for AD prevention. Furthermore, both better cognitive test performance and better physical functioning are strong positive predictors of preferences for AD prevention. There are several possible explanations for the discrepancy. First, more cognitively active persons may have strong preference for avoiding cognitive impairment and hence are willing to pay more to prevent it. Second, cognitive status may be a proxy for other unobserved factors that are associated with greater awareness about AD and its consequences. Third, people with higher cognitive functioning may have a greater capacity to weight multiple factors when estimating the benefit of treatment. Fourth, people with a lower level of cognitive functioning may not value cognitive health as much as those who have better cognitive functioning because of other impending issues such as other physical health problems or financial hardships.
While these potential pathways merit further investigation in future studies, our findings indicate that demands for AD prevention may be inversely related to objective needs and suggest a potential discrepancy between need and demand for preventive care for AD. Although it is yet unknown whether WTP for AD prevention would confirm to the realized preventive behavior when the actual preventive option is available, evidence from cancer suggests a strong correlation between stated preferences for and actual use of certain preventive screening [36,37]. Thus, our findings suggest that, when effective preventive treatments for AD are developed in the future, the treatments will be sought by people who are more cognitively intact and are less likely to develop AD than those who are more vulnerable to the development of AD. This finding has significant public health implications, and needs to be addressed in health education and intervention programs to achieve better efficiency and equity of health-care resource use.
The difference in perceived risk by race/ethnicity also suggests a potential discordance between needs and demands for AD care. The lower level of perceived risk of AD among African Americans, as compared with non-Latino whites, is alarming given that the actual risk of AD among African Americans is not lower than among non-Latino whites [38–41]. This may be because of lower levels of knowledge or sources of information regarding AD among African Americans [15]. Lower level of perceived risk, despite of equal or higher level of objective risk among African Americans, may lead to inadequate levels of preventive behavior, such as seeking professional help in an early stage of the disease. Not surprisingly, African Americans are underserved with regard to dementia care, often receiving services later in the disease course than non-Latino whites [42].
In interpreting our results about preferences for AD prevention, it is important not to interpret the WTP value as the actual amount respondents were willing to pay or to attempt to compare WTP values across studies. First, the values may reflect “starting point bias,” in which individuals answer differently depending on which dollar amount is presented first. In our study, everyone was given the value $100 first in the questions asking about WTP for AD prevention and $100 was the most common response. To address potential starting point bias, we treated WTP as an ordinal variable (0–6) reflecting relative preference instead of coding the value as a cardinal variable reflecting the amount of money willing to paid. Analyzed this way, use of a different starting point might not have changed the results of our study. Second, the hypothetical treatment used to measure preference for AD prevention was not a feasible AD prevention option under current technology. The value individuals are willing to pay when available prevention options, which are likely to involve risks of adverse side effects, are used might be different from the amount willing to pay for an imaginary drug.
It is important to note what this study does not do. Some questions of interest could not be addressed because relevant data are not available in the HRS. First, we were not able to examine whether disease awareness through personal experience with relatives or friends with AD would affect individuals’ perceived risk. Second, we were not able to evaluate individuals’ knowledge about prevention and treatment options on risk perception and prevention preference. Knowledge about AD may influence how accurately individuals translate known risk factors into personal risk and thus would affect individuals’ perception of the risk and prevention preference. Third, we were not able to validate how well individuals’ perceived risk predicts actual risk because actual risk is unknown. Although validation of subjective perception of AD risk is an important question to be answered by collecting more extensive data in another study, here we used available data to examine this link in an indirect way by exploring whether perceived risk of AD is explained by known risk factors of AD and other measurable individual characteristics.
Our study has several limitations, largely from the limited information available in the data, as described above. First, we did not have information about family background or genetic risk factors, such as the apolipoprotein E-e4 allele. Genetic predisposition is known to explain a significant proportion of variations in AD incidence [43]. A large part of the unexplained variation in perceived risk may have been based on family background that individuals may take into account, but was not captured by the survey questions. Second, we did not investigate the pathways of how people formulate their own risk of AD. The way known risk factors of AD, let alone other personalized information, may be linked to the perceived AD risk is complex, and we could not tease apart the potential pathways in the present study. Third, the 0 to 100 probability scale used to measure perceived risk may not be the best way to capture true perceived risk among older adults. Nevertheless, we found that the likelihood of providing simplified answers (e.g., 0, 50, and 100) were not associated with risk factors of AD included in the model, and thus, measurement error of this type does not seem to have influenced our estimates. Fourth, the WTP approach considers only preference, not an individual’s actual preventive behavior. Tests of how closely stated preference translates into actual behavior, particularly in the context of AD, requires further information not available in the data.
Findings from our study, and related previous studies, suggest some issues to be further addressed. First, future research needs to investigate the process and type of information people use to formulate perceived risk of AD, particularly the link between knowledge about AD risk factors and perceived personal risk for AD. One way to investigate this would be comparing perceived risk of AD before and after providing information on AD (e.g., risk factors and prevention options). Awareness of the disease through personal experience could be measured by asking if the respondent knows someone personally and whether the person is genetically related or not; further, this information could be linked with perceived risk of AD to assess whether individuals process personal information correctly. Second, perceived risk of AD measure can be validated against actual incidence using prospective data. Observable risk factors for AD in our data are limited; people may use other information in estimating their risk for AD. Perhaps individuals’ perceived risk does actually conform to their actual risk more closely than available data would suggest. Third, the underlying reasons for the discrepancy between objective risk factors and perceived risk of AD need to be better understood.
In conclusion, older adults’ perceived risk of AD is related to some risk factors of AD and their preference for AD prevention corresponds to the perceived risk of AD. A large part of the variation in perceived risk is unexplained by known risk factors and individual characteristics, suggesting that more information about disease awareness through the personal experiences is needed to understand how people formulate personal risk of AD. On the other hand, preference for preventive care for AD does not seem to reflect the objective need for prevention. The sources of discrepancy between need and demand for AD preventive care should be better understood to help people who are at higher risk of developing AD engage in preventive behavior at earlier stages of cognitive decline. Similarly, lower level of perceived risk of AD among African Americans suggests that the existing disparity in AD treatment by race/ethnicity [44,45] might be exacerbated because of lower preference for prevention or less engagement in preventive behavior that could help delay onset of AD. Thus, it is important to address the racial/ethnic disparity in the perceived risk of AD by designing and implementing prevention and education program relevant to varied racial/ethnic groups.
Supplementary Material
Acknowledgments
This study was supported by a pilot grant from the Center for Aging in Diverse Communities funded by grant No. P30-AG15272 under the Resource Centers for Minority Aging Research program by the National Institute on Aging, National Institute of Nursing Research, and the National Center for Minority Health and Health Disparities.
We thank participants at the fifth Bay Area Health Care Quality and Outcomes Conference, the 14th Annual Conference of the International Society for Quality of Life Research, and the 60th Annual Scientific Meeting of the Gerontological Society of America.
Footnotes
Supporting information for this article can be found at: http://www.ispor.org/publications/value/ViHsupplementary.asp
References
- 1.Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–22. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Lewin Group. A report commissioned by the Alzheimer’s Association. Washington, DC: The Lewin Group; 2004. Saving Lives, Saving Money: Dividends for American Investing in Alzheimer Research. [Google Scholar]
- 3.Luchsinger JA, Reitz C, Honig LS, et al. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–51. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman AB, Fitzpatrick AL, Lopez O, et al. Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J Am Geriatr Soc. 2005;53:1101–7. doi: 10.1111/j.1532-5415.2005.53360.x. [DOI] [PubMed] [Google Scholar]
- 5.Petrovitch H, Ross GW, Steinhorn SC, et al. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol. 2005;57:98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- 6.Launer L. Diabetes and brain aging: epidemiologic evidence. Curr Diab Rep. 2005;5:59–63. doi: 10.1007/s11892-005-0069-1. [DOI] [PubMed] [Google Scholar]
- 7.Abbott RD, White LR, Ross GW, et al. Walking and dementia in physically capable elderly men. JAMA. 2004;292:1447–53. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 8.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 9.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–30. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 10.Weuve J, Kang JH, Manson JE, et al. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–61. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 11.Podewils LJ, Guallar E, Kuller KH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–51. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 12.Kang JH, Ascherio A, Grodstein F. Fruit and vegetable consumption and cognitive decline in aging women. Ann Neurol. 2005;57:713–20. doi: 10.1002/ana.20476. [DOI] [PubMed] [Google Scholar]
- 13.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–53. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 14.Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51:728–33. doi: 10.1212/wnl.51.3.728. [DOI] [PubMed] [Google Scholar]
- 15.Roberts JS, Connell CM, Cisewski D, et al. Differences between African Americans and whites in their perceptions of Alzheimer disease. Alzheimer Dis Assoc Disord. 2003;17:19–26. doi: 10.1097/00002093-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Low LF, Anstey KJ. The public’s perception of the plausibility of dementia risk factors is not influenced by scientific evidence. Dement Geriatr Cogn Disord. 2007;23:202–6. doi: 10.1159/000099038. [DOI] [PubMed] [Google Scholar]
- 17.Werner P. Knowledge about symptoms of Alzheimer’s disease: correlates and relationship to help-seeking behavior. Int J Geriatr Psychiatry. 2003;18:1029–36. doi: 10.1002/gps.1011. [DOI] [PubMed] [Google Scholar]
- 18.Katapodi MC, Lee KA, Facione NC, et al. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: a meta-analytic review. Prev Med. 2004;38:388–402. doi: 10.1016/j.ypmed.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Peters E, McCaul KD, Stefanek M, et al. A heuristics approach to understanding cancer risk perception: contributions from judgment and decision-making research. Ann Behav Med. 2006;31:45–52. doi: 10.1207/s15324796abm3101_8. [DOI] [PubMed] [Google Scholar]
- 20.Johnson FR, Manjunath R, Mansfield CA, et al. High-risk individuals’ willingness to pay for diabetes risk-reduction programs. Diabetes Care. 2006;29:1351–6. doi: 10.2337/dc05-2221. [DOI] [PubMed] [Google Scholar]
- 21.Codori AM, Petersen GM, Miglioretti DL, et al. Attitudes toward colon cancer gene testing: factors predicting test uptake. Cancer Epidemiol Biomarkers Prev. 1999;8:345–51. [PubMed] [Google Scholar]
- 22.Bowen DJ, Alfano CM, McGregor BA, et al. The relationship between perceived risk, affect, and health behaviors. Cancer Detect Prev. 2004;28:409–17. doi: 10.1016/j.cdp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Coyle D, Wells G, Graham I, et al. The impact of risk on preference values: implications for evaluations of postmenopausal osteoporosis therapy. Value Health. 2001;4:385–91. doi: 10.1046/j.1524-4733.2001.45038.x. [DOI] [PubMed] [Google Scholar]
- 24.Park DC. Cognitive aging, processing resources, and self-report. In: Schwarz N, Park DC, Knauper B, Sudman S, editors. Cognition, Aging, and Self-reports. Philadelphia, PA: Psychology Press; 1998. [Google Scholar]
- 25.Teresi JA, Golden RR, Cross P, et al. Item bias in cognitive screening measures: comparisons of elderly white, Afro-American, Hispanic and high and low education subgroups. J Clin Epidemiol. 1995;48:473–83. doi: 10.1016/0895-4356(94)00159-n. [DOI] [PubMed] [Google Scholar]
- 26.Kawas C, Gray S, Brookmeyer R, et al. Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–7. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, Mchugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–17. [Google Scholar]
- 29.Ofstedal MB, McAuley GF, Herzog AR. Documentation of Cognitive Functioning Measures in the Health and Retirement Study: Survey Research Center. Ann Arbor, MI: University of Michigan; 2001. [Google Scholar]
- 30.Herzog AR, Rodgers WL. Cognitive performance measures in survey research on older adults. In: Schwarz N, Park DC, Knauper B, Sudman S, editors. Cognition, Aging, and Self-reports. Philadelphia, PA: Psychology Press; 1998. [Google Scholar]
- 31.Herzog AR, Wallace RB. Measures of cognitive functioning in the AHEAD Study. J Gerontol B Psychol Sci Soc Sci. 1997;52:37–48. doi: 10.1093/geronb/52b.special_issue.37. [DOI] [PubMed] [Google Scholar]
- 32.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–38. [Google Scholar]
- 33.Jorm AF, Jacomb PA. The Information Questionnaire on Cognitive Decline in the Elderly (IQCODE): sociodemographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–22. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz LM, Woloshin S, Black WC, Welch HG. The role of numeracy in understanding the benefit of screening mammography. Ann Intern Med. 1997;127:966–72. doi: 10.7326/0003-4819-127-11-199712010-00003. [DOI] [PubMed] [Google Scholar]
- 35.Weinstein ND. What does it mean to unders tand a risk? Evaluating risk comprehension. J Natl Cancer Inst Monogr. 1999:15–20. doi: 10.1093/oxfordjournals.jncimonographs.a024192. [DOI] [PubMed] [Google Scholar]
- 36.Wolf RL, Basch CE, Brouse CH, et al. Patient preferences and adherence to colorectal cancer screening in an urban population. Am J Public Health. 2006;96:809–11. doi: 10.2105/AJPH.2004.049684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kee F, Telford AM, Donaghy P, O’Doherty A. Attitude or access: reasons for not attending mammography in Northern Ireland. Eur J Cancer Prev. 1992;1:311–15. [PubMed] [Google Scholar]
- 38.Froehlich TE, Bogardus ST, Jr, Inouye SK. Dementia and race: are there differences between African Americans and Caucasians? J Am Geriatr Soc. 2001;49:477–84. doi: 10.1046/j.1532-5415.2001.49096.x. [DOI] [PubMed] [Google Scholar]
- 39.Green RC, Cupples LA, Go R, et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287:329–36. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- 40.Fillenbaum GG, Heyman A, Huber MS, et al. The prevalence and 3-year incidence of dementia in older Black and White community residents. J Clin Epidemiol. 1998;51:587–95. doi: 10.1016/s0895-4356(98)00024-9. [DOI] [PubMed] [Google Scholar]
- 41.Gurland BJ, Wilder DE, Lantigua R, et al. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry. 1999;14:481–93. [PubMed] [Google Scholar]
- 42.Manly JJ, Jacobs D, Mayeux R. Alzheimer’s disease among different ethnic and racial groups. In: Terry RD, Katzman R, Bick KL, et al., editors. Alzheimer’s Disease. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 43.Plassman BL, Khachaturian AS, Townsend JJ, et al. Comparison of clinical and neuropathological diagnoses of AD in three epidemiological samples. Alzheimers Dement. 2006;2:2–11. doi: 10.1016/j.jalz.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Gurland BJ, Wilder DE, Lantigua R, et al. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry. 1999;14:481–93. [PubMed] [Google Scholar]
- 45.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.