Abstract
Background:
The aim of the study was to compare postoperative pain relief in patients undergoing an elective thoracotomy with thoracic epidural analgesia using single shot magnesium and clonidine as adjuvants to bupivacaine.
Methods:
In a randomized prospective study, 60 patients of American Society of Anesthesiologists physical status I–III of either sex, between 20 and 60 years undergoing elective unilateral thoracotomy, were allocated to three equal groups of 20 patients. Each patient received thoracic epidural analgesia using bupivacaine alone (Group A) or with magnesium (Group B) or clonidine (Group C) at the end of surgery during skin closure. Postoperatively, pain was measured using a visual analog scale (VAS). Rescue analgesia (50 mg tramadol intravenous) was given at a VAS score of ≥4. Duration of analgesia and total dose of rescue analgesic during 24 h was calculated. Postoperative sedation and other side effects if any were recorded.
Results:
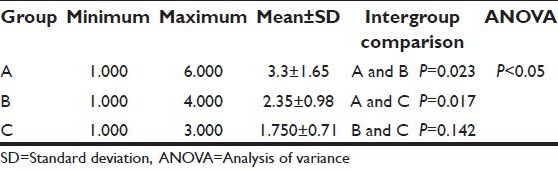
All the groups were homogeneous with respect to their demographics. The 24 h cumulative mean VAS score in Groups A, B, and C was 3.12 ± 0.97, 2.86 ± 0.43, and 1.83 ± 0.59, respectively. The duration of analgesia was prolonged in Group C (165 ± 49.15 min), followed by Group B (138 ± 24.6 min), and Group A (118.5 ± 52.8 min). The duration of analgesia was significantly prolonged in the clonidine group as compared to the control group (P = 0.001). The number of rescue analgesia doses were more in Group A (3.3 ± 1.65) followed by Group B (2.35 ± 0.98) and Group C (1.75 ± 0.71). The sedation scores were significantly higher in Group C. However, shivering was seen in Group A (40%) and Group C (20%) and absent in Group B (P = 0.003).
Conclusion:
Thoracic epidural analgesia using bupivacaine with clonidine is an efficient therapeutic modality for postthoracotomy pain. Magnesium as an adjuvant provided quality postoperative analgesia decreasing the need for postoperative rescue analgesia and incidence of postoperative shivering without causing sedation.
Keywords: Clonidine, epidural, magnesium, thoracotomy
INTRODUCTION
Thoracotomy is widely recognized as one of the most painful surgical procedures.[1] Acute postthoracotomy pain is aggravated by the constant movement of breathing. Pain relief is, therefore, essential to facilitate coughing and deep breathing and to promote early mobilization. Shallow breathing and impaired coughing, resulting from thoracotomy pain are a major cause of atelectasis and retention of secretions, both of which can lead to hypoxemia, hypercapnia, and respiratory failure, especially in patients with preexisting lung disease.[2] It has been demonstrated that poor analgesia is associated with increased intensive care unit admissions and longer hospital stay. For this reason, different analgesic methods such as thoracic epidural analgesia, paravertebral blocks, and systemic analgesics have been tried. However, epidural analgesia is considered to be the gold standard for postthoracotomy pain relief.[3]
The use of neuraxial opioids traditionally as adjuvants to local anesthetics is associated with quite a few side effects, so various options including α-2 agonists such as clonidine, dexmedetomidine, and magnesium are being extensively evaluated as an alternative with emphasis on opioid-related side effects such as respiratory depression, nausea, urinary retention, and pruritus besides improving quality and duration of analgesia.[4] The addition of magnesium to epidurally administered bupivacaine in patients undergoing elective cesarean section with combined spinal-epidural anesthesia helped to improve the quality of postoperative analgesia.[5] To our knowledge, no previous clinical studies with epidural magnesium sulfate for thoracotomy pain have been reported. However, epidural clonidine for postthoracotomy pain has been tried.[6] Therefore, we conducted this clinical study to determine and compare the effects of adding a single dose of magnesium sulfate and clonidine to epidural bupivacaine in patients undergoing an elective thoracotomy.
METHODS
After Institutional Ethical Committee approval, 60 patients with physical status American Society of Anesthesiologists (ASA) I-III between 20 and 60 years admitted for elective unilateral thoracic surgery were enrolled for the study. Sealed envelope method was used for randomization and the patients undergoing an elective unilateral thoracotomy were divided into three groups (Group A, Group B, and Group C). Patients with ASA score of intravenous (IV) or more, body mass index >30 kg/m2, hypersensitivity to drugs in the study, severe renal, hepatic, or neurologic disease and those using opioid or systemic analgesic preoperatively were excluded from the study. At the preoperative visit, patients were instructed about pain evaluation using visual analog scale (VAS) of 0-10 cm (0 cm = no pain and 10 cm = the worst pain). On arrival to the operating room, all patients were preloaded with 500 ml of crystalloid. In the operating room, electrocardiography, oxygen saturation, and continuous arterial blood pressure monitoring were started. Epidural catheter (20G) was inserted through 18G Tuohy needle at T5-T8 intervertebral space in lateral decubitus position depending on the type of thoracotomy. The epidural space was identified using loss of resistance to saline technique. Epidural catheter was inserted and fixed at 4 cm inside the space. A test dose of 3 ml of 1.5% lidocaine with epinephrine 1:200,000 was given to rule out intravascular or intrathecal placement of the catheter. Catheter was covered with a sterile transparent dressing.
Propofol (1.5-2.5 mg/kg) and fentanyl (2 μg/kg) were used intravenously for induction of anesthesia. After the administration of 0.5 mg/kg of rocuronium, patients were intubated with a double-lumen tube. Anesthesia was maintained with equal volumes of oxygen and nitrous oxide in isoflurane (0.5-1.5%). Intraoperative rescue analgesia with fentanyl 1 μg/kg was used. Toward the end of the surgery, approximately 20 min prior to anticipated extubation, patients were divided into three groups to receive following drug mixtures epidurally:
Group A: Bupivacaine 0.25% 8 ml + saline 0.9% 1 ml (control group)
Group B: Bupivacaine 0.25% 8 ml + magnesium sulfate 50 mg in 1 ml 0.9% saline
Group C: Bupivacaine 0.25% 8 ml + clonidine 150 mcg 1 ml 0.9% saline.
Patients received ondansetron hydrochloride 0.1 mg/kg toward the end of the surgery. Epidural infusion with 5 ml/h of 0.1% bupivacaine was started 15 min after the bolus dose, and it was continued during the postoperative period via epidural catheter using an infusion pump. After extubation, patients were shifted to the post anesthesia care unit for 2 h and then shifted to the postoperative ward for 24 h. The intensity of postoperative pain was measured using VAS. Pain scores and sensory level was assessed every 30 min for 2 h and then every 2 h for 12 h and every 4 h, thereafter till 24 h. Patients complaining of pain in the postoperative period with VAS score ≥4, received tramadol 50 mg IV as rescue analgesia and time to the first request for analgesia was noted. The total number of rescue analgesic doses were noted in the 24 h period.
Postoperative monitoring included heart rate, blood pressure, pulse oxymetry, respiratory rate, and sensory block using pin prick sensation (T2-T12 dermatome). A decrease in mean arterial pressure of >20% below the baseline preanesthetic value or <60 mmHg was treated by incremental doses of 6 mg ephedrine IV. A decrease in heart rate of >15% below the baseline or 50 beats/min, whichever was lesser, was defined as bradycardia and was treated by atropine 0.5 mg IV. The degree of sedation was assessed after admission to the recovery room every 30 min for 2 h using modified Ramsay sedation scale [Table 1]. Side effects such as hypotension, nausea, vomiting, pruritus, and shivering were recorded.
Table 1.
Modified Ramsay sedation scale*

Statistical analysis
All statistical analyses were performed using All statistical analyses were performed using SPSS forwindows versions 15.0, Chicago, SPSS Inc. Continuous variables were tested for a normal distribution by the Kolmogorov–Smirnov test. Parametric data were compared using analysis of variance. Comparison between groups at different time intervals was assessed using paired t-test. All the categorical data were compared by using the Chi-square test. A power analysis based on 95% confidence interval and β error of 20% revealed a sample size of 60 subjects (20 subjects in each group). A sample size of 20 patients in each group was needed to detect an intergroup difference of at least 20% with two-sample t-test. Data were collected by a blinded observer and presented as mean ± standard deviation (SD) or number (%). A P < 0.05 was considered to be significant.
RESULTS
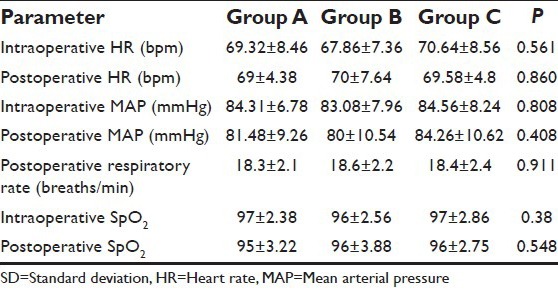
All the three groups were homogenous with reference to age, sex, body weight, duration of surgery, and surgical procedures [Table 2]. The hemodynamic variables (mean ± SD) between the groups at different study intervals were comparable [Table 3]. All subjects in our study groups assessed at various intervals during the study period had a good sensory block extending from T2 to T12 dermatomes, depending on the level of epidural.
Table 2.
Demographic data of three groups
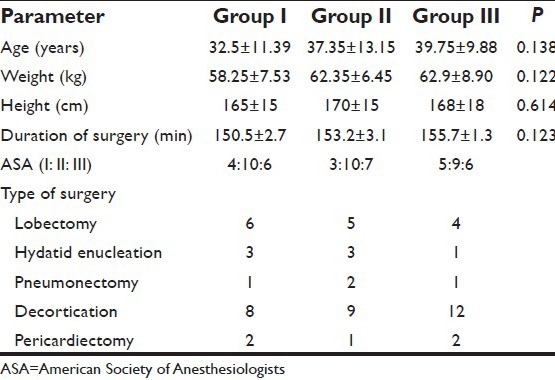
Table 3.
Comparison of vital parameters (mean±SD) between three groups
On comparing, the VAS scores between the three groups at various intervals, a statistically significant difference was found between magnesium versus clonidine and clonidine versus control group. The VAS scores in Group B were lower than in Group A, but the association was statistically insignificant [Figure 1].
Figure 1.
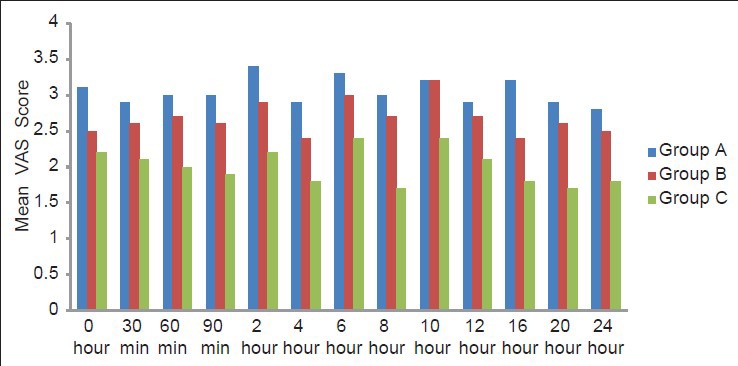
Comparison of postoperative visual analog scale at different intervals in three study groups
The mean cumulative 24 h VAS scores recorded throughout first 24 h after surgery were significantly lower in Group C (1.83 ± 0.59) compared with the Group A (3.12 ± 0.97) (P = 0.001) and Group B (2.86 ± 0.43) (P = 0.003). However, the association between Group A and Group B was insignificant.
Mean duration of analgesia in Groups A, B, and C was 118.5 ± 52.84 min, 138 ± 24.62 min, and 165 ± 49.15 min, respectively [Table 4]. The duration of analgesia was significantly prolonged in the clonidine group as compared to the control group (P = 0.001). However, there was no statistical significance in duration of analgesia between clonidine and magnesium groups (P = 0.057) and magnesium and control groups (P = 0.167). Throughout the first 24 h postoperative period, all patients requested rescue analgesia. About 17 patients (28.3%) requested it once, 24 patients (40%) requested it twice, 13 patients (21.7%) requested it thrice, and six patients (10%) requested it >3 times [Figure 2]. The mean number of rescue analgesia doses in Group A, Group B, and Group C were 3.3 ± 1.6, 2.35 ± 0.98, and 1.750 ± 0.71, respectively. The association was significant between magnesium (P = 0.023) and clonidine (P = 0.017) versus control group [Table 5]. The mean rescue analgesia was significantly reduced in Group C (86.7 ± 44.2 mg) and Group B (100 ± 46.3 mg) as compared to control the group (133.3 ± 55.6 mg). Two patients in Group A, one patient each in group B and Group C developed bradycardia (heart rate <60/min) and hypotension which was treated as per protocol. No significant respiratory depression was reported in any patient in this study, and none of the patients had a SpO2 value of <95% on pulse oximetry.
Table 4.
Comparison of duration of analgesia in the three groups
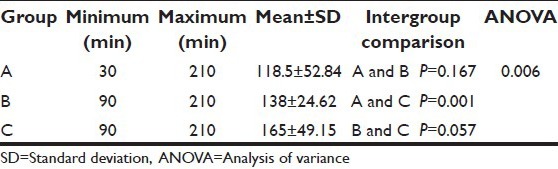
Figure 2.
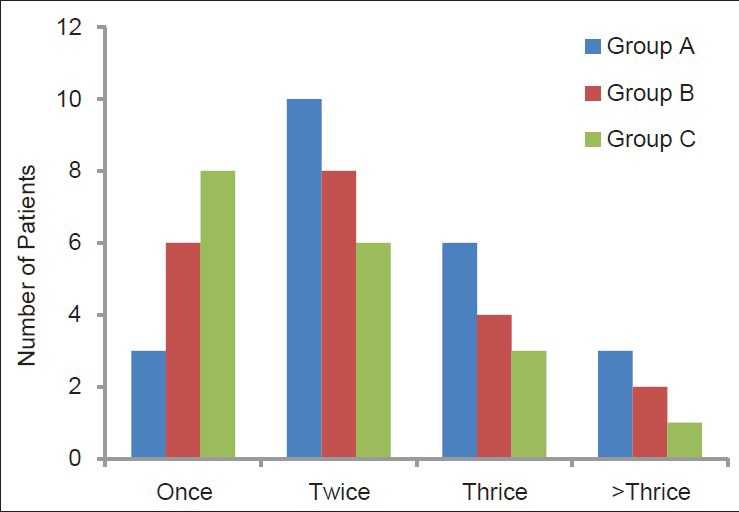
Patients’ distribution according to the number of requests of rescue analgesia
Table 5.
Comparison of number of rescue analgesia doses in three groups
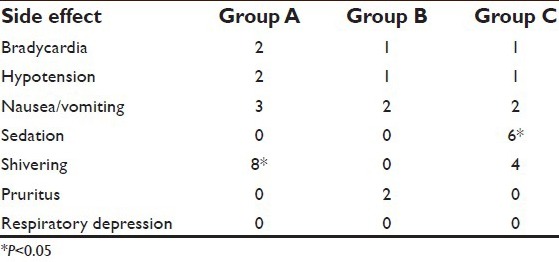
Shivering occurred in eight patients (40%) in the control group and four patients (20%) belonging to the clonidine group but none in the magnesium group. The difference among groups was statistically significant (P < 0.001). In this study, a dose of 150 μg clonidine caused sedation (Ramsay sedation score >3) in eight patients (40%) of Group C, while no patients in Group A or B developed sedation, the observation was found to be statistically significant (P < 0.05).
DISCUSSION
Epidural analgesia with local anesthetics is one of the most effective techniques used for postoperative pain relief and may improve patient outcome.[7] Adding other drugs to the neuraxial block to improve the quality of anesthesia and prolong the duration of analgesia without any added side effects is a common practice. The faster onset of both sensory and motor blockade, prolonged duration of analgesia, dose-sparing action of local anesthetics and stable cardiovascular parameters makes these agents a very effective adjuvant in regional anesthesia.[8]
The present study was undertaken to compare the postoperative analgesic efficacy and safety of clonidine and magnesium as adjuvants to bupivacaine in thoracic epidurals in patients undergoing an elective thoracotomy. Three main results emerged from this clinical study. First, magnesium and clonidine had a beneficial effect on postoperative pain intensity and analgesic requirements in the postoperative period. Second, magnesium treatment decreased the incidence of postoperative shivering. Third, epidural clonidine 2 μg/kg causes postoperative sedation without respiratory depression.
In concordance with our study, Ghatak et al.[9] also observed nonsignificant association in the hemodynamic parameters after epidural administration of clonidine and magnesium for lower abdomen surgery. However, Gupta et al.[10] in their study, however, experienced significant fall in mean arterial pressure at 30 min postepidural block in the clonidine group. Similar results were obtained by Nishikawa[11] who concluded that epidural administration of clonidine induces hypotension and bradycardia secondary to decreased sympathetic nerve activity.
Nociceptive transmission in thoracotomy is via C and Aδ fibers and can be considered in three discrete routes. Intercostal nerves carry impulses from the skin and intercostal muscles. Stimuli from lung and mediastinum are carried by the vagus nerve. Thoracotomy for lung resection, usually, involves a skin incision at the 5th intercostal space, a variable degree of muscle cutting and either excision or division of a rib. A posterolateral thoracotomy incision usually traverses around six dermatomal levels, starting at the 3rd thoracic dermatome posteriorly extending to the 7th or 8th thoracic dermatome anteriorly. Chest wall muscles involved are latissimus dorsi, serratus anterior, pectoralis major, and the intercostal muscles.[12] Furthermore, patients may well be extremely anxious after major thoracic surgery, exacerbating the perception of postoperative pain.[13]
Noxious stimulation leads to the release of neurotransmitters, which bind to various subclasses of excitatory amino acid receptors, including N-methyl-D-aspartate (NMDA) receptors. Activation of these receptors leads to calcium entry into the cell and initiates a series of central sensitization such as wind-up and long-term potentiation in the spinal cord. NMDA receptor signaling may be important in determining the duration of acute pain.[14] Magnesium, a major cation in the human body, blocks calcium influx and noncompetitively antagonizes NMDA receptor channels. Magnesium ions and NMDA receptors are involved in the modulation of pain. Magnesium is also a physiological antagonist at various voltage-gated channels which may be important in the mechanism of antinociception.[15] Clonidine is a centrally acting α2 agonist which inhibits which inhibits voltage-gated sodium channels. It induces dose-dependent spinal cord antinociception, mainly through stimulation of α2-adrenoceptors in the dorsal horn, mimicking the activation of descending inhibitory pathways.[16]
In this study, the time gap between the initial epidural dose and the first dose of rescue analgesia was the longest in the clonidine group followed by the magnesium group, and it was the shortest in the control group. The association in duration of analgesia was statistically significant between clonidine and control group (P = 0.001). Our results are also in concordance with Ghatak et al. (180.3 ± 29.97 min) and Gupta et al. (334.2 ± 38.6) who observed a maximum prolongation of analgesia with epidural clonidine. We observed both epidural magnesium and clonidine reduced the need of postoperative rescue analgesia. The results of our study correlate with Bilir et al.[17] who observed that co-administration of magnesium in lumbar epidural analgesia results in a reduction in fentanyl consumption without any side effects in patients undergoing hip surgery. Ehab et al[6] observed similar results of decrease in postoperative meperidine requirements in patients receiving epidural clonidine (1 μg/kg) for postthoracotomy pain relief.
In the magnesium group, no patient had shivering during this study, whereas shivering occurred in four patients (20%) belonging to clonidine group and eight patients (40%) in the control group. The difference among groups was statistically highly significant (P < 0.001). Perioperative magnesium supplementation has been seen to prevent the postoperative hypomagnesaemia and decrease the incidence of postoperative shivering.[18] Jeon et al. observed that intrathecal administration of clonidine 150 mcg fails to prevent post spinal shivering.[19] Sedation is a side effect frequently associated with the use of clonidine for postoperative analgesia, often in conjunction with opioids.[20] In this study, a dose of 150 μg clonidine caused sedation (Ramsay sedation score >3) in eight patients (40%) of Group C. While no patients in Group A or B developed sedation. The observation was found to be statistically significant (P < 0.05). The results of our study were contradictory to Gupta et al. who did not observe sedation in their study subjects. This is attributed to lower the dose of epidural clonidine (1 μg/kg) in their study. Studies conducted in women in labor have shown that boluses of epidural clonidine at doses larger than 100 μg may cause significant sedation.[21] In this study, we used a small dose of magnesium sulfate that would not cause any side-effects. Additional side effects such as pruritus, hypotension, bradycardia, and nausea were statistically insignificant between the three groups [Table 6].
Table 6.
Comparison of side effects in the three groups
Our study concluded that thoracic epidural analgesia using bupivacaine with clonidine is an efficient therapeutic modality for postthoracotomy pain. Magnesium as an adjuvant provided quality postoperative analgesia decreasing the need for postoperative rescue analgesia and incidence of postoperative shivering without causing sedation. However, larger studies are required before establishing magnesium as a strong adjuvant to local anesthetics for thoracic epidural analgesia.
Limitation
Our study is limited by the absence of dose-response of epidural magnesium for postoperative analgesia. In the absence of the literature regarding body weight-based dosage of epidural magnesium, we had used a fixed dose of epidural magnesium in our study. The result of present randomized controlled trial raises the need for more clinical studies regarding the dosage regimen of epidural magnesium.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bong CL, Samuel M, Ng JM, Ip-Yam C. Effects of preemptive epidural analgesia on post-thoracotomy pain. J Cardiothorac Vasc Anesth. 2005;19:786–93. doi: 10.1053/j.jvca.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 2.McGovern I, Walker C, Cox F. Pain relief after thoracotomy. Br J Anaesth. 2007;98:844. doi: 10.1093/bja/aem112. [DOI] [PubMed] [Google Scholar]
- 3.Ng A, Swanevelder J. Pain relief after thoracotomy: Is epidural analgesia the optimal technique? Br J Anaesth. 2007;98:159–62. doi: 10.1093/bja/ael360. [DOI] [PubMed] [Google Scholar]
- 4.Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004;98:153–8. doi: 10.1213/01.ANE.0000093225.39866.75. [DOI] [PubMed] [Google Scholar]
- 5.Ghrab BE, Maatoug M, Kallel N, Khemakhem K, Chaari M, Kolsi K, et al. Does combination of intrathecal magnesium sulfate and morphine improve postcaesarean section analgesia? Ann Fr Anesth Reanim. 2009;28:454–9. doi: 10.1016/j.annfar.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Ehab S, Ehab ES, Mohammed A. Dexamethasone as adjuvant to thoracic epidural provided more prolonged analgesia for post thoracotomy pain than clonidine and fentanyl. Ain Shamas J Anesthesiol. 2012;5:307–12. [Google Scholar]
- 7.Rodgers A, Walker N, Schug S, McKee A, Kehlet H, van Zundert A, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: Results from overview of randomised trials. BMJ. 2000;321:1493. doi: 10.1136/bmj.321.7275.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajwa SJ, Bajwa SK, Kaur J, Singh G, Arora V, Gupta S, et al. Dexmedetomidine and clonidine in epidural anaesthesia: A comparative evaluation. Indian J Anaesth. 2011;55:116–21. doi: 10.4103/0019-5049.79883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghatak T, Chandra G, Malik A, Singh D, Bhatia VK. Evaluation of the effect of magnesium sulphate vs. clonidine as adjunct to epidural bupivacaine. Indian J Anaesth. 2010;54:308–13. doi: 10.4103/0019-5049.68373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S, Raval D, Patel M, Patel N, Shah N. Addition of epidural clonidine enhances post operative analgesia: A double blind study in total knee replacement surgeries. Anaesth Essays Res. 2010;4:70–4. doi: 10.4103/0259-1162.73510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishikawa T. Extradural clonidine for postoperative pain relief. Br J Anaesth. 1996;76:171. doi: 10.1093/bja/76.1.171. [DOI] [PubMed] [Google Scholar]
- 12.Rogers ML, Henderson L, Mahajan RP, Duffy JP. Preliminary findings in the neurophysiological assessment of intercostal nerve injury during thoracotomy. Eur J Cardiothorac Surg. 2002;21:298–301. doi: 10.1016/s1010-7940(01)01104-6. [DOI] [PubMed] [Google Scholar]
- 13.Bachiocco V, Morselli-Labate AM, Rusticali AG, Bragaglia R, Mastrorilli M, Carli G. Intensity, latency and duration of post. thoracotomy pain: Relationship to personality traits. Funct Neurol. 1990;5:321–32. [PubMed] [Google Scholar]
- 14.Pockett S. Spinal cord synaptic plasticity and chronic pain. Anesth Analg. 1995;80:173–9. doi: 10.1097/00000539-199501000-00026. [DOI] [PubMed] [Google Scholar]
- 15.Fawcett WJ, Haxby EJ, Male DA. Magnesium: Physiology and pharmacology. Br J Anaesth. 1999;83:302–20. doi: 10.1093/bja/83.2.302. [DOI] [PubMed] [Google Scholar]
- 16.Ossipov MH, Suarez LJ, Spaulding TC. Antinociceptive interactions between alpha 2-adrenergic and opiate agonists at the spinal level in rodents. Anesth Analg. 1989;68:194–200. [PubMed] [Google Scholar]
- 17.Bilir A, Gulec S, Erkan A, Ozcelik A. Epidural magnesium reduces postoperative analgesic requirement. Br J Anaesth. 2007;98:519–23. doi: 10.1093/bja/aem029. [DOI] [PubMed] [Google Scholar]
- 18.Lysakowski C, Dumont L, Czarnetzki C, Tramèr MR. Magnesium as an adjuvant to postoperative analgesia: A systematic review of randomized trials. Anesth Analg. 2007;104:1532–9. doi: 10.1213/01.ane.0000261250.59984.cd. [DOI] [PubMed] [Google Scholar]
- 19.Jeon YT, Jeon YS, Kim YC, Bahk JH, Do SH, Lim YJ. Intrathecal clonidine does not reduce post-spinal shivering. Acta Anaesthesiol Scand. 2005;49:1509–13. doi: 10.1111/j.1399-6576.2005.00783.x. [DOI] [PubMed] [Google Scholar]
- 20.Eisenach JC, De Kock M, Klimscha W. alpha (2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984-1995) Anesthesiology. 1996;85:655–74. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- 21.Roelants F, Lavandhomme PM, Mercier-Fuzier V. Epidural administration of neostigmine and clonidine to induce labor analgesia: Evaluation of efficacy and local anesthetic-sparing effect. Anesthesiology. 2005;102:1205–10. doi: 10.1097/00000542-200506000-00021. [DOI] [PubMed] [Google Scholar]


