Abstract
Anthracycline-containing regimens (ACRs) are recommended for patients with diffuse large B-cell lymphoma (DLBCL). However, over 40% of elderly patients do not receive ACRs, possibly due to expected toxicities. We characterized treatment choices and compared the 3-year overall survival (OS) rates of 8262 Medicare beneficiaries diagnosed with DLBCL in 2000–2006 identified from the Surveillance, Epidemiology, and End Results (SEER)–Medicare linked database. Of the cohort, 45% had ACR with rituximab (ACR-R), 13% had ACR without R, 6% had non-ACR with R (non-ACR-R), 4% had R monotherapy, 3% had non-ACR and 29% had no systemic therapy. Patients not receiving ACR were older and/or had more comorbidities. The unadjusted OS was highest in ACR-R (65%), followed by ACR without R (55%) and non-ACR-R (44%). After adjusting patient covariates, ACR-R showed the best survival (63%). However, OS was comparable between non-ACR-R (52%) and ACR without R (52%). Non-ACR-R could be considered for patients who are poor candidates for ACR.
Keywords: Diffuse large B-cell lymphoma, immunochemotherapy, survival, elderly
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the leading cause of lymphoma deaths among patients over the age of 65 [1,2]. Prospective studies from research centers have left little debate that anthracycline-containing regimens with rituximab (ACR-R), specifically the R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone), is the best existing therapy for the disease in fit elderly patients with good performance status and uncompromised cardiac function [3,4]. However, population studies demonstrate that ACR delivery in the elderly is limited to about 60% of patients with DLBCL [5–9].
Little is known of the effectiveness of alternative therapy options for elderly patients who by choice or provider recommendation do not receive optimal ACR-R treatment. Prospective clinical trials have evaluated R-CHOP-like regimens with reduced dosing strategies [10–15]. Others have examined using a specific agent as a substitute for anthracyclines among patients who are poor candidates for ACR. These studies suggest that non-anthracycline-containing regimens (non-ACR) are tolerable and active [16–18]. However these studies are small and as a result, the efficacy of non-ACR has not been well-defined for elderly patients with DLBCL.
Without adequately powered prospective trials that include elderly patients with unrestrictive selection criteria, the survival benefits of non-ACR among elderly patients are unclear. Patient registries provide the opportunity to observe treatment patterns and outcomes for many patients who are seldom included in prospective trials. The treatment variation within these registries can be used to make inferences on the relationships between treatment and outcomes for treatments not studied in prospective trials and for patients excluded from prospective trials. This study used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) cancer registry database linked with Medicare data to analyze the effectiveness of chemotherapy combinations for patients with DLBCL on 3-year overall survival.
The objectives of this study were to (1) describe the range of chemotherapy treatment strategies currently being used for elderly patients with DLBCL in real-world settings, and (2) provide clinicians with the most comprehensive information available regarding expected outcomes from chemotherapy strategies other than ACR-R when deciding on the best therapy for their elderly patients with DLBCL.
Materials and methods
Subject selection
The SEER-Medicare linked database was used to establish the study cohort. About 93% of SEER-region cases of cancer aged 65 and older have been linked with the Medicare claims database [19,20]. The addition of Medicare claims to the patient-specific clinical and demographic information collected within the SEER registry enables researchers to examine the treatments received by patients.
The study population included patients (1) newly diagnosed with malignant non-Hodgkin lymphoma, (2) aged 66 or older at the time of diagnosis, (3) diagnosed in 2000–2006 and (4) with pathologically confirmed diffuse large cell lymphoma or DLBCL (International Classification of Diseases for Oncology, 3rd Edition [ICD-O-3] codes 9679, 9680, 9684). Patients were excluded if they (1) had an unknown month of diagnosis (n = 41), (2) had an invalid death date (n = 28) (e.g. had a death date in the SEER registry but not in Medicare claims, or a death date before diagnosis date), (3) lacked continuous Medicare Fee-For-Service coverage from 12 months before diagnosis to the earlier of death or 5 months after diagnosis (n = 4282), (4) had a central nervous system site diagnosis (n = 324), (5) were diagnosed at autopsy or death (n = 22), (6) had unavailable census tract data (n = 5) or (7) had a chemotherapy type that was unknown for all chemotherapy claims (n = 3 68).
Measures
For each patient, initial immunochemotherapy was determined by searching for Medicare claims for chemotherapy and Rituximab (R) within 5 months of diagnosis [6]. Healthcare Common Procedures Classification System (HCPCS) codes were used to identify specific chemotherapy agents, including anthracyclines, non-anthracyclines and R (Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2014.903589). Based on these three treatments, patients were grouped into six mutually exclusive immunochemotherapy combinations: (1) ACR-R, (2) ACR without R (ACR nR), (3) non-ACR with R (non-ACR-R), (4) non-ACR without R (non-ACR nR), (5) R monotherapy (R) and (6) no systemic therapy if patients did not receive any immunochemotherapy.
Covariates in multivariate analyses included demographics (i.e. age at diagnosis, gender and race), tumor stage, radiation treatment, comorbidities and year of diagnosis. Age was categorized into five groups. Tumor stage was measured by the SEER extent of disease. Radiation during an initial immunochemotherapy period [21] was identified from Medicare claims (Supplementary Table II to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2014.903589). We adapted the Charlson comorbidity index [22] to create dummy variables to indicate the presence of non-cancer comorbidities by using Medicare inpatient/outpatient claims in the year prior to DLBCL diagnosis.
The primary end point was the 3-year overall survival after DLBCL diagnosis. Death was defined by Medicare death date. Survival time (in months) was censored at the end of the 3rd year after diagnosis.
Statistical analyses
Descriptive analyses were conducted of patient covariates for the six initial immunochemotherapy combinations. Multivariate logistic regression was used to describe the use of ACR (i.e. ACR-R or ACR nR), controlling for patient covariates. Kaplan–Meier analysis was performed to describe the unadjusted survival rates for each treatment. We then used a multivariate Cox proportional hazards model [23,24] to calculate adjusted survival rates for each treatment, controlling for patient covariates. Non-ACR-R was the reference group. To check for robustness, multivariate logistic regression analysis and propensity score-based matching methods were also conducted to examine 3-year overall survival (details described in Supplementary Material to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2014.903589).
All statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC).
Results
Characteristics of study population
The analysis cohort contained 8262 Medicare beneficiaries. Of these, 71.3% received at least one systemic immunochemotherapy within 5 months of diagnosis: 44.9% ACR-R; 13.2% ACR nR; 6.4% non-ACR-R; 3.6% R; 3.2% non-ACR nR. Nearly 29% of patients did not receive any systemic therapy.
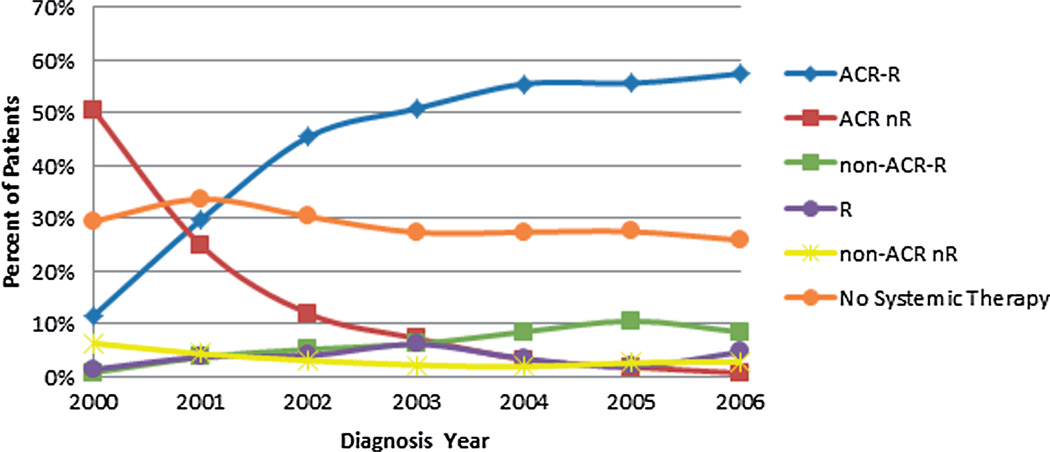
Figure 1 shows the annual percentages of patients using each of the six initial immunochemotherapy combinations in 2000–2006. With R becoming available in 1998, the use of ACR-R quickly increased across the period, which mirrored the reduction in the use of ACR nR. It is also notable that the percentage of patients not receiving any systemic therapy declined slightly in recent years, while use of non-ACR-R increased from 0.8% in 2000 to 8.5% in 2006. Cyclophosphamide, vincristine and etoposide were the most commonly used non-ACR agents. Specifically, more than 65% of patients in the non-ACR-R group were treated by R with cyclophosphamide and/or vincristine.
Figure 1.
Trend of initial immunochemotherapy choices among elderly patients with diffuse large B-cell lymphoma from 2000 to 2006 (n = 8262). ACR, anthracycline-containing regimen; R, rituximab.
Table I shows patient characteristics according to the six immunochemotherapy combinations. Patients not receiving ACR (columns 3–6) were on average older than the patients receiving ACR (columns 1–2). Specifically, 72% of non-ACR versus 48% of ACR treated patients were aged 76 or older (p-value < 0.0001). Compared to ACR users, non-ACR users were less likely to have limited stage (i.e. stage I/II) lymphoma (46% vs. 51%, p-value < 0.0001), and more likely to have one or more comorbidities such as chronic heart failure (CHF) (16% vs. 5%, p-value < 0.0001), chronic obstructive pulmonary disease (COPD) (14% vs. 9%, p-value < 0.0001) or chronic kidney disease (CKD) (5% vs. 2%, p-value < 0.0001). Of note, patients receiving non-ACR-R had similar baseline characteristics to those receiving no systemic therapy with regard to age, tumor stage and the presence of comorbidity. In addition, the R monotherapy group had the highest likelihood of using radiation (22%) across treatment groups, and this group was on average much older than the other treatment groups (39% aged 85+).
Table I.
Distribution of patient demographic and clinical characteristics, diff use large B-cell lymphoma, by initial chemotherapy patient received during 2000–2006 (n = 8262).
| 1 | 2 | 3 | 4 | 5 | 6 | |||
|---|---|---|---|---|---|---|---|---|
| Variable | ACR-R (%)† (n = 3706) |
ACR nR (%)† (n = 1090) |
Non-ACR-R (%)† (n = 530) |
Non-ACR nR (%)† (n = 267) |
R (%)† (n = 301) |
No systemic therapy (%)† (n = 2368) |
Total (%)† (n = 8262) |
p-Value |
| Age | <0.0001 | |||||||
| 66–70 | 898 (24) | 253 (23) | 54 (10) | 41 (15) | 27 (9) | 249 (11) | 1522 (18) | |
| 71–75 | 1069 (29) | 290 (27) | 94 (18) | 54 (20) | 39 (13) | 396 (17) | 1942 (24) | |
| 76–80 | 942 (25) | 312 (29) | 125 (24) | 70 (26) | 54 (18) | 514 (22) | 2017 (24) | |
| 81–84 | 521 (14) | 146 (13) | 117 (22) | 52 (19) | 63 (21) | 463 (20) | 1362 (16) | |
| 85+ | 276 (7) | 89 (8) | 140 (26) | 50 (19) | 118 (39) | 746 (32) | 1419 (17) | |
| Gender | 0.0554 | |||||||
| Female | 1992 (54) | 599 (55) | 281 (53) | 128 (48) | 184 (61) | 1280 (54) | 4464 (54) | |
| Male | 1714 (46) | 491(45) | 249 (47) | 139 (52) | 117 (39) | 1088 (46) | 3798 (46) | |
| Race | 0.0016 | |||||||
| White | 3393 (92) | 1012 (93) | 476 (90) | 238 (89) | 283 (94) | 2114 (89) | 7516 (91) | |
| Black | 109 (3) | 38 (3) | 17 (3) | 14 (5) | * | 107 (5) | * | |
| Other | 204 (6) | 40 (4) | 37 (7) | 15 (6) | * | 147 (6) | * | |
| Stage | <0.0001 | |||||||
| Stage I | 1067 (29) | 357 (33) | 144 (27) | 75 (28) | 99 (33) | 716 (30) | 2458 (30) | |
| Stage II | 773 (21) | 229 (21) | 94 (18) | 50 (19) | 56 (19) | 365 (15) | 1567 (19) | |
| Stage III | 635 (17) | 164 (15) | 102 (19) | 32 (12) | 33 (11) | 278 (12) | 1244 (15) | |
| Stage IV | 1057 (29) | 275 (25) | 151 (29) | 93 (35) | 91 (30) | 808 (34) | 2475 (30) | |
| Unknown | 174 (5) | 65 (6) | 39 (7) | 17 (6) | 22 (7) | 201 (9) | 518 (6) | |
| Use of radiation | <0.0001 | |||||||
| Yes | 416 (11) | 210 (19) | 72 (14) | 48 (18) | 65 (22) | 413 (17) | 1224 (15) | |
| Comorbidity | ||||||||
| MI | 86 (2) | 26 (2) | 43 (8) | * | * | 101 (4) | 280 (3) | <0.0001 |
| CHF | 188 (5) | 53 (5) | 106 (20) | 44 (16) | 50 (17) | 346 (15) | 787 (10) | <0.0001 |
| PVD | 111 (3) | 32 (3) | 34 (6) | 16 (6) | 15 (5) | 144 (6) | 352 (4) | <0.0001 |
| CVD | 154 (4) | 54 (5) | 41 (8) | 22 (8) | 20 (7) | 178 (8) | 469 (6) | <0.0001 |
| COPD | 331 (9) | 93 (9) | 71 (13) | 33 (12) | 36 (12) | 329 (14) | 893 (11) | <0.0001 |
| Dementia | * | * | * | * | * | 47 (2) | 77 (1) | <0.0001 |
| Paralysis | * | * | * | * | * | 28 (1) | 41 (0) | <0.0001 |
| Diabetes | 696 (19) | 176 (16) | 132 (25) | 61 (23) | 75 (25) | 518 (22) | 1658 (20) | <0.0001 |
| CKD | 69 (2) | 13 (1) | 28 (5) | 12 (4) | 20 (7) | 126 (5) | 268 (3) | <0.0001 |
| Liver diseases‡ | 13 (0) | * | * | * | * | 16 (1) | 39 (0) | 0.0341 |
| Ulcers | 58 (2) | 23 (2) | * | * | * | 69 (3) | 175 (2) | 0.0158 |
| Rheum disease | 142 (4) | 46 (4) | 15 (3) | 15 (6) | 13 (4) | 75 (3) | 306 (4) | 0.2138 |
| AIDS | * | * | * | * | * | * | * | 0.0506 |
ACR, anthracycline-containing regimen; R, rituximab; MI, myocardial infarction; CHF, chronic heart failure; PVD, peripheral vascular disease; CVD, cerebrovascular disease; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; AIDS, acquired immunodeficiency syndrome.
The cell size is less than 12, or can be used to recalculate in the small cell.
Percentages are column percentages, e.g. 24% of those who received ACR-R were aged 66–70.
Liver diseases: various cirrhodites or moderate–severe liver disease.
Multivariate logistic regression (Supplementary Table III to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2014.903589) predicting the use of ACR yielded estimates consistent with the univariate findings in Table I.
Survival patterns
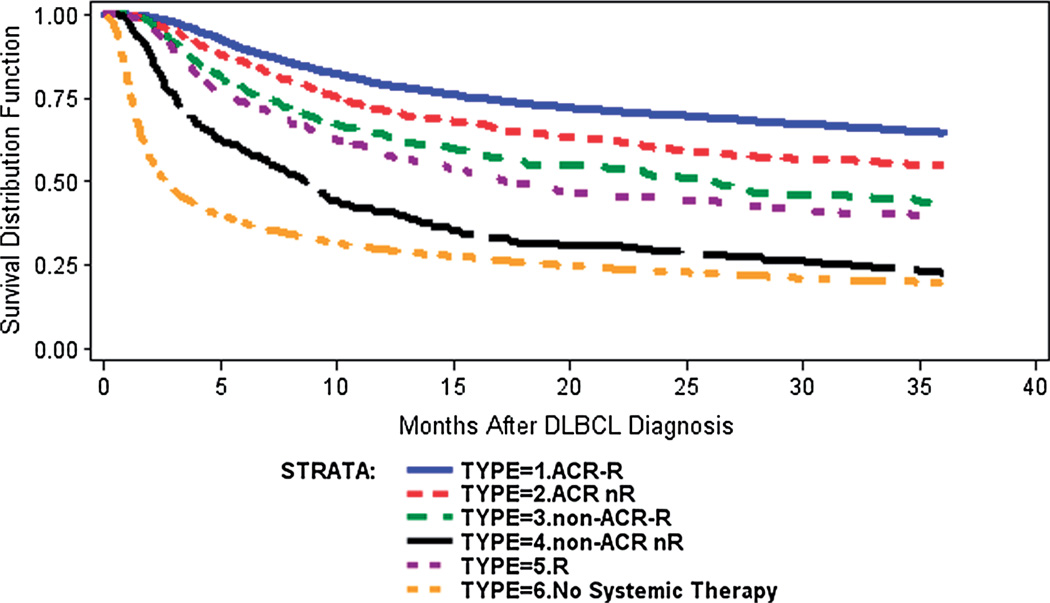
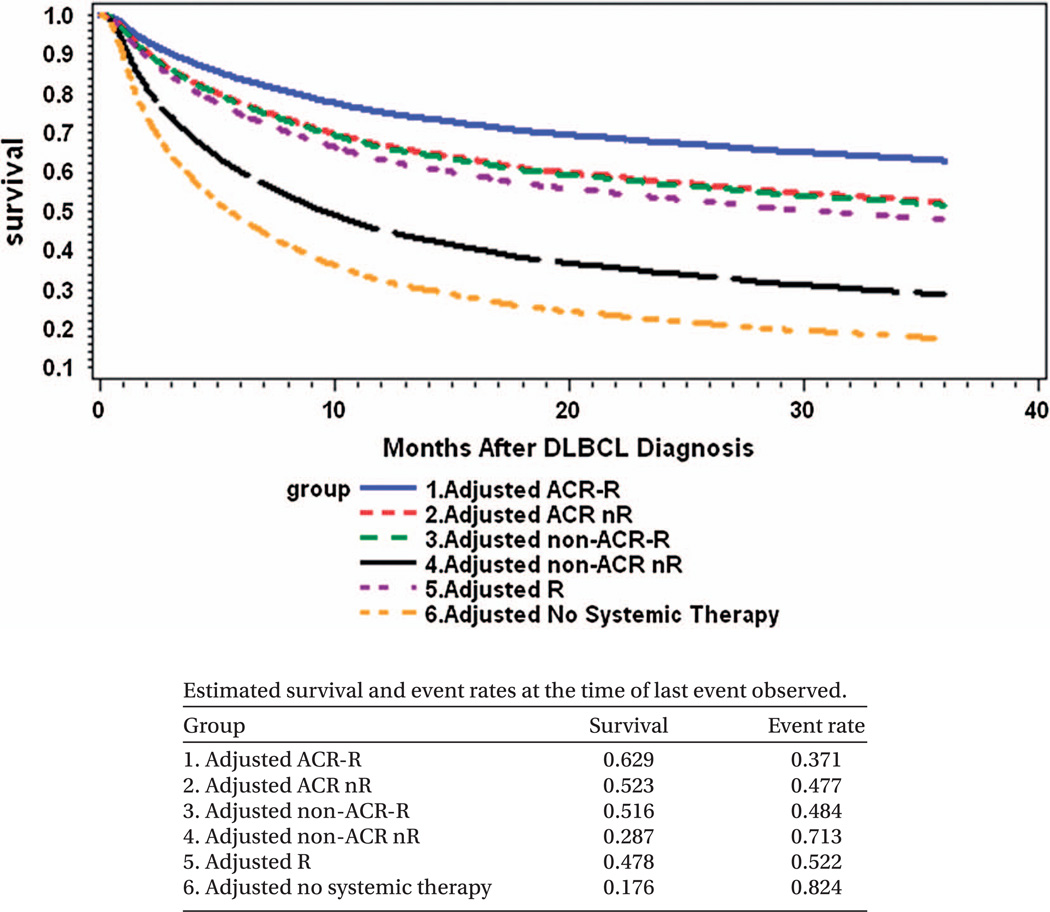
Unadjusted 3-year overall survival improved slightly over time from 43% among those diagnosed in 2000 to 49% for those diagnosed in 2006 (p-value < 0.0001). The mean 3-year overall survival rate was 46.8%, and the median survival time was 27.7 months. Figure 2 shows unadjusted survival curves from Kaplan–Meier analysis. Prior to adjusting for differences in patient characteristics, the ACR-R group had the highest 3-year overall survival (65%), followed by ACR nR (55%), non-ACR-R (44%), R (39%), non-ACR nR (22%) and no systemic therapy (20%). Figure 3 shows adjusted survival curves from the multivariate Cox proportional hazards model. It shows that the ACR-R group had the highest adjusted 3-year overall survival (63%), followed by ACR nR (52%), non-ACR-R (52%), R (48%), non-ACR nR (29%) and no systemic therapy (18%).
Figure 2.
Kaplan–Meier curve for overall survival among elderly patients with diffuse large B-cell lymphoma from 2000 to 2006 (n = 8262). Note: all patients were censored at 36 months of follow-up. ACR, anthracycline-containing regimen; R, rituximab.
Figure 3.
Cox proportional hazards model for overall survival adjusted by demographics, year of diagnosis and clinical factors among elderly patients with diffuse large B-cell lymphoma from 2000 to 2006 (n = 8262). Note: all patients were censored at 36 months of follow-up. ACR, anthracycline-containing regimen; R, rituximab.
Table II compares mortality rates across the six immunochemotherapy combinations by using the multivariate Cox proportional hazards model. As expected, the mortality rate for patients receiving non-ACR-R was significantly greater than that for patients receiving ACR-R (hazard ratio = 0.680, 95% confidence interval [CI]: [0.598, 0.773]). However, the mortality rate for non-ACR-R was not significantly different from patients receiving ACR nR (hazard ratio = 0.980, 95% CI: [0.838, 1.145]). We further examined whether non-ACR-R yielded a survival advantage compared to no systemic therapy. The non-ACR-R or no systemic therapy groups were very similar in measured characteristics (Table I). However, patients not receiving any systemic therapy died at about three times the rate of those treated with non-ACR-R (hazard ratio = 3.055, 95% CI: [2.697, 3.461]). In addition, we noted that the mortality rate was not significantly different between patients receiving non-ACR-R and those receiving R (hazard ratio = 1.130, 95% CI: [0.938, 1.361]) after adjusting for patient characteristics.
Table II.
Multivariate Cox proportional hazards model for 3-year mortality among elderly patients with diff use large B-cell lymphoma from 2000 to 2006 (n = 8262).
| Variable | Adjusted hazard ratio (95% CI) |
p-Value |
|---|---|---|
| Type of initial chemotherapy (reference = non-ACR-R) | ||
| ACR-R | 0.680 (0.598–0.773) | <0.0001 |
| ACR nR | 0.980 (0.838–1.145) | 0.7949 |
| R | 1.130 (0.938–1.361) | 0.1985 |
| Non-ACR nR | 2.052 (1.712–2.458) | <0.0001 |
| No systemic therapy | 3.055 (2.697–3.461) | <0.0001 |
| Age (reference = 66–70) | ||
| 71–75 | 1.228 (1.102–1.367) | 0.0002 |
| 76–80 | 1.593 (1.435–1.768) | <0.0001 |
| 81–84 | 1.777 (1.592–1.982) | <0.0001 |
| 85+ | 2.393 (2.146–2.668) | <0.0001 |
| Gender (reference = female) | ||
| Male | 1.054 (0.992–1.120) | 0.0885 |
| Race (reference = white) | ||
| Black | 1.010 (0.867–1.177) | 0.8974 |
| Other | 0.980 (0.861–1.116) | 0.7590 |
| Tumor stage (reference = stage I) | ||
| Stage II | 1.565 (1.423–1.720) | <0.0001 |
| Stage III | 1.930 (1.744–2.135) | <0.0001 |
| Stage IV | 2.473 (2.280–2.682) | <0.0001 |
| Unknown | 1.422 (1.245–1.625) | <0.0001 |
| Use of radiation in initial treatment (reference = no) | ||
| Yes | 0.681 (0.622 –0.745) | <0.0001 |
| Comorbidity (reference = no comorbidity) | ||
| Myocardial infarction (MI) | 1.130 (0.971–1.315) | 0.1132 |
| Chronic heart failure (CHF) | 1.276 (1.161–1.403) | <0.0001 |
| Peripheral vascular disease (PVD) | 1.057 (0.923–1.210) | 0.4213 |
| Cerebrovascular disease (CVD) | 1.114 (0.983–1.263) | 0.0897 |
| Chronic obstructive pulmonary disease (COPD) | 1.262 (1.154–1.381) | <0.0001 |
| Dementia | 1.608 (1.259–2.054) | 0.0001 |
| Paralysis | 1.039 (0.716–1.507) | 0.8404 |
| Diabetes | 1.205 (1.119–1.297) | <0.0001 |
| Chronic kidney disease (CKD) | 1.560 (1.350–1.802) | <0.0001 |
| Liver diseases* | 2.655 (1.882–3.746) | <0.0001 |
| Ulcers | 1.061 (0.876–1.285) | 0.5467 |
| Rheum disease | 1.092 (0.933–1.277) | 0.2723 |
| Acquired immunodeficiency syndrome (AIDS) | 1.089 (0.450–2.637) | 0.8493 |
| Year of diagnosis (reference = 2000) | ||
| 2001 | 1.029 (0.917–1.154) | 0.6316 |
| 2002 | 0.967 (0.859–1.089) | 0.5829 |
| 2003 | 0.954 (0.845–1.076) | 0.4428 |
| 2004 | 0.999 (0.885–1.127) | 0.9842 |
| 2005 | 0.909 (0.804–1.027) | 0.1253 |
| 2006 | 0.965 (0.853–1.091) | 0.5653 |
ACR, anthracycline-containing regimen; R, rituximab; CI, confidence interval.
Liver diseases: various cirrhodites or moderate–severe liver disease.
Multivariate logistic regression analysis and propensity score methods conducted to verify the robustness of our multivariate survival analyses yielded similar estimates in magnitude and statistical significance.
Discussion
This population-based analysis of treatment patterns and outcomes for elderly patients with DLBCL gives the most comprehensive evaluation to date of how such patients are treated in the United States, and notably identifies a common (nearly 40%) avoidance of ACRs. As expected, patients treated with ACR-R have the highest survival rates when all available clinical features are accounted for. Notably, non-ACR-R had indistinguishable 3-year survival outcomes compared to ACR nR when accounting for clinical features.
Anthracycline avoidance is consistently described in substantial observational studies among elderly patients with DLBCL. Grann et al. first reported that only 33% of patients with advanced stage DLBCL received doxorubicin-based chemotherapy [5]. Two Dutch series had similar results. Maartense et al. found 26% and van de Schans et al. found 46% of elderly patients with DLBCL receiving ACRs [7,9]. Keating et al. found that 71% of male patients with DLBCL over 65 years old in the Veterans Health Administration received ACRs compared to 59% of a SEER–Medicare cohort [8].
In contrast, minimal evidence is available to guide our understanding of the survival outcomes following non-ACRs in the upfront treatment of elderly patients with DLBCL [7,16,18]. Moccia et al. conducted a retrospective study among just 81 patients in whom etoposide was substituted for doxorubicin, and found it to be well tolerated among patients with numerous comorbidities, resulting in an unadjusted 5-year overall survival of 49% [18]. A Dutch registry study by van de Schans et al. reported unadjusted 3-year survival rates of less than 20% for 66 patients treated with non-ACRs [7]. One prospective trial observed a 50% 3-year overall survival when anthracycline was omitted from R-CHOP (i.e. R-CVP) followed by R maintenance in 14 patients greater than 70 years old considered poor candidates for a full course of R-CHOP [16]. Our population-based study found that non-anthracycline treatments were commonly used in elderly patients in current practice. Moreover, the 3-year overall survival for over 500 people treated with non-ACR-R was 44%. Similar to findings in the smaller studies, we suggest that non-anthracycline treatments are not futile.
The 3-year overall survival remained highest for those treated with optimal therapy (ACR-R) even after adjusting for patient characteristics, and thus ACR-R (e.g. R-CHOP) should be the treatment of choice whenever feasible in elderly DLBCL. Less expected was the comparable adjusted survival observed between patients receiving ACR nR and non-ACR-R. Because ACR nR was the treatment standard for so many years, this finding is interesting. Patients receiving non-ACR-R were more likely to be older or have comorbidities (such as CHF, COPD or CKD) compared to those receiving ACR nR. Like other non-randomized studies that use healthcare claims data to measure treatments and comorbidities, unmeasured factors (e.g. albumin rate, or International Prognostic Index) might confound our estimates. However, based on the observed channeling of non-ACR-R toward older patients and those with comorbidities, we suspect that any residual confounding would tend to lead to an underestimation of the survival benefits for non-ACR-R. This implies that the survival benefit of non-ACR-R compared to anthracycline agents might be even greater than our estimation. In addition, both non-ACR-R and no systemic therapy groups were similar in measured characteristics. Unmeasured confounders such as frailty might lead physicians to recommend against any therapy. If so, our estimates might provide the upper bound of survival benefits for patients receiving non-ACR-R compared to those receiving no systemic therapy.
Our results also showed that the survival outcomes were not different between non-ACR-R and R monotherapy. Although this is beyond the scope of the present study, future studies should examine whether administration of a newer non-ACR under development decreases the rate of relapse or death from disease in this population when added to monoclonal antibody.
Although our data along with previous studies [5–9] demonstrated that anthracycline avoidance was common, there is no evidence to support that this degree of avoidance is optimal. Strategies are emerging that might help clinicians predict who among the elderly are at the highest risk for severe toxicity, so that non-ACR-R or R therapies might be discussed. For example, the comprehensive geriatric assessment has been proposed to assess prognosis and predict toxicity to enhance chemotherapy tolerance [17,25–29]. Specific strategies for administering ACR in elderly patients with DLBCL have been suggested, and should be utilized whenever possible [30,31].
Several new non-anthracycline agents are under development for the treatment of lymphoma [32–35]. Targeting elderly patients who are not candidates for aggressive therapy at time of DLBCL relapse is a commonly considered developmental strategy for these agents. Our findings demonstrate that a substantial fraction of elderly patients with newly diagnosed DLBCL are apparently not considered candidates for ACRs, and represent another unmet need that could be addressed by studying these new active agents.
Despite standard comparative effectiveness research techniques, our study has limitations. We used a first treatment carried forward analysis, and thus our results pertain to the question only of comparative results of the initial treatment chosen. This is a very relevant question clinically; however, it must be recognized that some patients will later switch therapies, for instance from ACR to non-ACR therapy or vice-versa. The misclassification of treatment received would have dampened any differences that would have been observed had the original treatment selection been maintained. Similarly, these estimates do not take into consideration any variation in dose intensity of the therapies because the dose is not available in the claims data. Also, we were unable to track all oral agents used during this period as they were not subject to Medicare claims. As a result, some of those categorized as receiving no systemic therapy may have been taking chlorambucil, oral etoposide or methotrexate. Finally, although we measured comorbidity, we were unable to distinguish the severity of the described comorbidities.
Most resources in the study of patients with DLBCL are historically devoted to evaluating the best therapy for the fittest patients. Few resources are devoted to understanding the potential value of “suboptimal” therapy for those who are not candidates for “the best” therapy. This population-based study provides information on how non-ACR with R or R monotherapy could improve survival outcomes for a substantial number of elderly patients with DLBCL who are otherwise not receiving systemic therapy. Future research should examine treatment outcomes in terms of quality of life in this patient population. In addition, there is a need to explore why one-third of patients did not receive any therapy. Finally, future studies could investigate how providers choose to use alternative/non-guideline therapies.
In conclusion, only a slight majority of elderly patients with DLBCL receive optimal anthracycline-containing immunochemotherapy, and a large proportion receive no systemic therapy at all. The difference in survival between these extremes is stark, with an adjusted 3-year survival of 63% and 18%, respectively. In between these extremes lies non-anthracycline immunochemotherapy, with a 3-year adjusted survival of 52%. With survival outcomes virtually equivalent to that of anthracycline chemotherapy in the pre-rituximab era, non-anthracycline immunochemotherapy or rituximab monotherapy should be strongly considered for patients who are poor candidates for anthracycline-containing regimens, especially for those not receiving systemic therapy. Knowledge of these treatment patterns and outcomes will also more effectively inform clinical research agendas for elderly patients with DLBCL in the future.
Supplementary Material
Acknowledgements
This work was made possible by the University of Iowa Holden Comprehensive Cancer Center Population Research Core which is supported in part by P30 CA086862. This study used the linked SEER–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute (NCI); the Office of Research, Development and Information, Centers for Medicare & Medicaid Services (CMS); Information Management Services (IMS), Inc.; and the SEER Program tumor registries in creation of the SEER Medicare database.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
Supplementary material available online
Methods and tables showing codes and further data.
References
- 1.Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the united states, 1992–2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 4.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 5.Grann VR, Hershman D, Jacobson JS, et al. Outcomes and diffusion of doxorubicin-based chemotherapy among elderly patients with aggressive non-hodgkin lymphoma. Cancer. 2006;107:1530–1541. doi: 10.1002/cncr.22188. [DOI] [PubMed] [Google Scholar]
- 6.Link BK, Brooks J, Wright K, et al. Diffuse large B-cell lymphoma in the elderly: diffusion of treatment with rituximab and survival advances with and without anthracyclines. Leuk Lymphoma. 2011;52:994–1002. doi: 10.3109/10428194.2011.557167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van de Schans SA, Wymenga AN, van Spronsen DJ, et al. Two sides of the medallion: poor treatment tolerance but better survival by standard chemotherapy in elderly patients with advanced-stage diffuse large B-cell lymphoma. Ann Oncol. 2012;23:1280–1286. doi: 10.1093/annonc/mdr411. [DOI] [PubMed] [Google Scholar]
- 8.Keating NL, Landrum MB, Lamont EB, et al. Quality of care for older patients with cancer in the veterans health administration versus the private sector: a cohort study. Ann Intern Med. 2011;154:727–736. doi: 10.7326/0003-4819-154-11-201106070-00004. [DOI] [PubMed] [Google Scholar]
- 9.Maartense E, Hermans J, Kluin-Nelemans JC, et al. Elderly patients with non-Hodgkin’s lymphoma: population-based results in the Netherlands. Ann Oncol. 1998;9:1219–1227. doi: 10.1023/a:1008485722472. [DOI] [PubMed] [Google Scholar]
- 10.Hasselblom S, Stenson M, Werlenius O, et al. Improved outcome for very elderly patients with diffuse large B-cell lymphoma in the immunochemotherapy era. Leuk Lymphoma. 2012;53:394–399. doi: 10.3109/10428194.2011.616612. [DOI] [PubMed] [Google Scholar]
- 11.Mori M, Niitsu N, Takagi T, et al. Reduced-dose chop therapy for elderly patients with non-Hodgkin’s lymphoma. Leuk Lymphoma. 2001;41:359–366. doi: 10.3109/10428190109057991. [DOI] [PubMed] [Google Scholar]
- 12.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 13.Spina M, Balzarotti M, Uziel L, et al. Modulated chemotherapy according to modified comprehensive geriatric assessment in 100 consecutive elderly patients with diffuse large B-cell lymphoma. Oncologist. 2012;17:838–846. doi: 10.1634/theoncologist.2011-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011;12:460–468. doi: 10.1016/S1470-2045(11)70069-9. [DOI] [PubMed] [Google Scholar]
- 15.Lyman GH, Dale DC, Friedberg J, et al. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin’s lymphoma: a nationwide study. J Clin Oncol. 2004;22:4302–4311. doi: 10.1200/JCO.2004.03.213. [DOI] [PubMed] [Google Scholar]
- 16.Hainsworth JD, Flinn IW, Spigel DR, et al. Brief-duration rituximab/chemotherapy followed by maintenance rituximab in patients with diffuse large B-cell lymphoma who are poor candidates for R-CHOP chemotherapy: a phase II trial of the Sarah Cannon Oncology Research Consortium. Clin Lymphoma Myeloma Leuk. 2010;10:44–50. doi: 10.3816/CLML.2010.n.004. [DOI] [PubMed] [Google Scholar]
- 17.Fields PA, Linch DC. Treatment of the elderly patient with diffuse large B cell lymphoma. Br J Haematol. 2012;157:159–170. doi: 10.1111/j.1365-2141.2011.09011.x. [DOI] [PubMed] [Google Scholar]
- 18.Moccia A, Schaff K, Hoskins P, et al. R-CHOP with etoposide substituted for doxorubicin (R-CEOP): excellent outcome in diffuse large B cell lymphoma for patients with a contraindication to anthracyclines. Blood. 2009;114(Suppl. 1) Abstract 408. [Google Scholar]
- 19.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl.):IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute: SEER-Medicare: Brief description of the SEER-Medicare database. Available from: http://healthservices.cancer.gov/seermedicare/overview/brief.html.
- 21.Wirth A. The rationale and role of radiation therapy in the treatment of patients with diffuse large B-cell lymphoma in the rituximab era. Leuk Lymphoma. 2007;48:2121–2136. doi: 10.1080/10428190701636468. [DOI] [PubMed] [Google Scholar]
- 22.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 23.Nieto FJ, Coresh J. Adjusting survival curves for confounders: a review and a new method. Am J Epidemiol. 1996;143:1059–1068. doi: 10.1093/oxfordjournals.aje.a008670. [DOI] [PubMed] [Google Scholar]
- 24.Ghali WA, Quan H, Brant R, et al. Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA. 2001;286:1494–1497. doi: 10.1001/jama.286.12.1494. [DOI] [PubMed] [Google Scholar]
- 25.Soubeyran P, Khaled H, MacKenzie M, et al. Diffuse large B-cell and peripheral T-cell non-hodgkin’s lymphoma in the frail elderly: a phase II EORTC trial with a progressive and cautious treatment emphasizing geriatric assessment. J Geriatr Oncol. 2011;2:36–44. [Google Scholar]
- 26.Balducci L, Beghe C. The application of the principles of geriatrics to the management of the older person with cancer. Crit Rev Oncol Hematol. 2000;35:147–154. doi: 10.1016/s1040-8428(00)00089-5. [DOI] [PubMed] [Google Scholar]
- 27.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to eastern cooperative oncology group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology study. J Clin Oncol. 2002;20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 28.Soubeyran P, Fonck M, Blanc-Bisson C, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol. 2012;30:1829–1834. doi: 10.1200/JCO.2011.35.7442. [DOI] [PubMed] [Google Scholar]
- 29.Monfardini S, Basso U. Oncological causes of frailty in older cancer patients. Eur J Cancer. 2007;43:1230–1231. doi: 10.1016/j.ejca.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera international trial (MInT) group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 31.Pfreundschuh M. How I treat elderly patients with diffuse large B-cell lymphoma. Blood. 2010;116:5103–5110. doi: 10.1182/blood-2010-07-259333. [DOI] [PubMed] [Google Scholar]
- 32.Czuczman M, Vose J, Zinzani P, et al. Confirmation of the efficacy and safety of lenalidomide oral monotherapy in patients with relapsed or refractory diffuse large B-cell lymphoma: results of an international study (NHL-003) Blood. 2008;112(Suppl. 1) Abstract 268. [Google Scholar]
- 33.Hainsworth J, Arrowsmith E, Mccleod M, et al. Randomized phase II study of R-CHOP plus enzastaurin versus R-CHOP in the first line treatment of patients with intermediate and high-risk diffuse large B cell lymphoma (DLBCL)-preliminary analysis. Ann Oncol. 2011;22(Suppl. 4) Abstract 074. [Google Scholar]
- 34.Ruan J, Martin P, Furman RR, et al. Bortezomib plus CHOP-rituximab for previously untreated diffuse large B-cell lymphoma and mantle cell lymphoma. J Clin Oncol. 2011;29:690–697. doi: 10.1200/JCO.2010.31.1142. [DOI] [PubMed] [Google Scholar]
- 35.Wong BY, Dang NH. Inotuzumab ozogamicin as novel therapy in lymphomas. Expert Opin Biol Ther. 2010;10:1251–1258. doi: 10.1517/14712598.2010.498418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.