Abstract
The present study introduces a novel instrumented method to characterize postural movement strategies to maintain balance during stance (ankle and hip strategy), by means of inertial sensors, positioned on the legs and on the trunk.
We evaluated postural strategies in subjects with2 types of parkinsonism: idiopathic Parkinson's disease (PD) and Progressive Supranuclear Palsy (PSP),and inage-matched control subjects standing under perturbed conditions implementedby the Sensory Organization Test (SOT).Coordination between the upper and lower segments of the body during postural sway was measured using a covariance index over time, by a sliding-window algorithm. Afterwards, a postural strategy index was computed. We also measuredthe amount of postural sway, as adjunctive information to characterize balance, by the root mean square of the horizontal trunk acceleration signal (RMS).
Results showed that control subjects were able to change their postural strategy, whilst PSP and PD subjects persisted in use of an ankle strategy in all conditions.PD subjects had RMS values similar to control subjects even without changing postural strategy appropriately, whereas PSP subjects showed much larger RMS values than controls, resulting in several falls during the most challenging SOT conditions (5 and 6). Results are in accordance with the corresponding clinical literature describing postural behavior in the same kind of subjects.
The proposed strategy index, based on the use ofinertial sensors on the upper and lower body segments, isa promising and unobtrusive toolto characterize postural strategies performed to attain balance.
Keywords: Sensory Organization Test, dynamic posture, Parkinson's disease, Progressive Supranuclear Palsy, covariance analysis
1. INTRODUCTION
Idiopathic Parkinson's disease (PD) and Progressive Supranuclear Palsy (PSP) are types of Parkinsonismthat lead to a progressive decline in postural control.Although PSP can start with balance and gait disorders and is characterized by a faster deterioration than idiopathic PD, early symptoms may be so similar that PSP is often misdiagnosed as PD[1,2].Both PD and PSP patients are at high risk for falls related to abnormal use of sensory information and abnormal motor coordination for postural control[3,4].PDpatients can have normal postural sway area in stance, even under altered sensory conditions, although they may show increased muscle co-contraction and falls in response to external perturbations[5].PSP patients experience similar issues [1].However, pathophysiology of postural instability in PSP is not completely understood, although vestibular, as well as visual contributions to stance and posture,have been explored[6].
Postural motor coordination to maintain body equilibrium during stance is organized into two distinct movement patterns: the ankle strategy and the hip strategy[7].In the ankle strategy, the subject rotates the body about the ankle joints, whereas the hip strategy involves corrective movements primarily about the hip joints[8,9]. Subjects can also use a combination of ankle and hip strategies during transitions from one strategy to the other[7], or in response to different sensory conditions, modulating the two co-existing modes[10]. Larger, faster body sway is accompanied by more use of a hip strategy in healthy subjects[9,11].
A quantification of postural movement strategies used by the subjects while keeping their balance in challenging conditions may introduce important insights about their ability to use and integrate sensory information in controlling body equilibrium and in cases of subjects with movement disorders as PD and PSP subjects [5].Direct measurements of body segment motions could quantify postural strategies [9] and wearable sensors can be a good candidate to this aim. Recently developed synchronized, wireless, inertial sensor systems for movement analysis are now available and able to measure acceleration and angular velocity of the body segments [12].
A strategy score based on horizontal ground reaction force has been proposed to characterize hip or ankle strategy[13], but this approach has also been shown to be inaccurate and unreliable since it is based on an indirect method to deduce the relative motion around the ankle and hip[14].
The aim of the present study is to introduce an instrumented easy-to-use method to measure postural strategy. The method is based on body-worn inertial sensors and it is applied on a cohort of 19 subjects, including subjects with PD, subjects with PSP and age-matched control subjects,to evaluate its feasibility and its potentials in clinical practice. In our approach, ankle strategy and hip strategy contributions are quantified both separately and combined using a novel postural strategy index, meant to provide a composite score suitable for clinical practice.The postural strategy index was also integrated with established measures of postural sway (namely the root mean square, RMS), considered as adjunctive information to characterize balance. Possible differences among the three kinds of subjects included in the study were explored and compared with results from clinical literature, to confirmthe appropriateness of the method. To perturb balance for studying postural strategies, the Sensory Organization Test (SOT) of Neurocom's Equitest was used. It consists of a form of dynamic posturography comprising systematic alterations of somatosensory and/or visual information[9,13].
2. METHODS
The present study includes 19 subjects recruited at the Oregon Health and Science University (Portland, OR). All participants provided informed consent according to the Oregon Health & Science University Institutional Review Board.Fivepatients with PD (4 males, 1 female) and 7 patients with PSP (4 males, 3 females)able to stand and walk independently were recruited from the Movement Disorders Clinic and examined by a neurologist specialized in movement disorders.PD patients were tested off medication (after a washout of at least 12 hours), for homogeneity with PSP patients, who do not take levodopa-based medication[15]. The clinical characteristics of the patients were assessed by the Motor subsection of the UPDRS and resulted in a range of 13-53 (mean ± sd: 34±14) for PD subjects and in a range of 22-53 (mean ± sd: 35±11) for PSP subjects. In addition,7healthy subjects(3 males, 4 females) were recruited. The 3 populations were agematched (PD: 62±6 years, PSP: 68±5 years, control subjects: 68±7 years). Cognitive evaluation was performed in the parkinsonian patients using the Montreal Cognitive Assessment (MoCA) [16]resulting in mild cognitive impairment in PSP patients (MoCA >21) and normal values in PD (MoCA>26).
Participants were asked to stand quietly on a moveable plate (Neurocom Balance Master, Neurocom, Clackamas, OR), secured in a safety harness during the SOT. All participants were assessed during 6sensory conditions in 3consecutive trials of 20 seconds each: condition 1 (eyes open), condition2 (eyes closed), condition3 (sway referenced visual surround) with a stable base and condition4 (eyes open), condition5 (eyes closed), condition6 (sway-referenced visual surround) with a moveable base (sway referenced)[9,13]. Their feet were carefully aligned over a defined axis on the force plate.
During the SOT test, tri-axial accelerations were collected with two Opal inertial sensors (ADPM Inc, Portland, OR)placed on the trunk at L5 level and on the right shank with Velcro straps. The knee joint was not included in the model of postural control, in accordance with previous studies[7,9,10].
Data were collected at a sampling frequency of 128 Hz.
2.2 Signal processing and covariance analysis
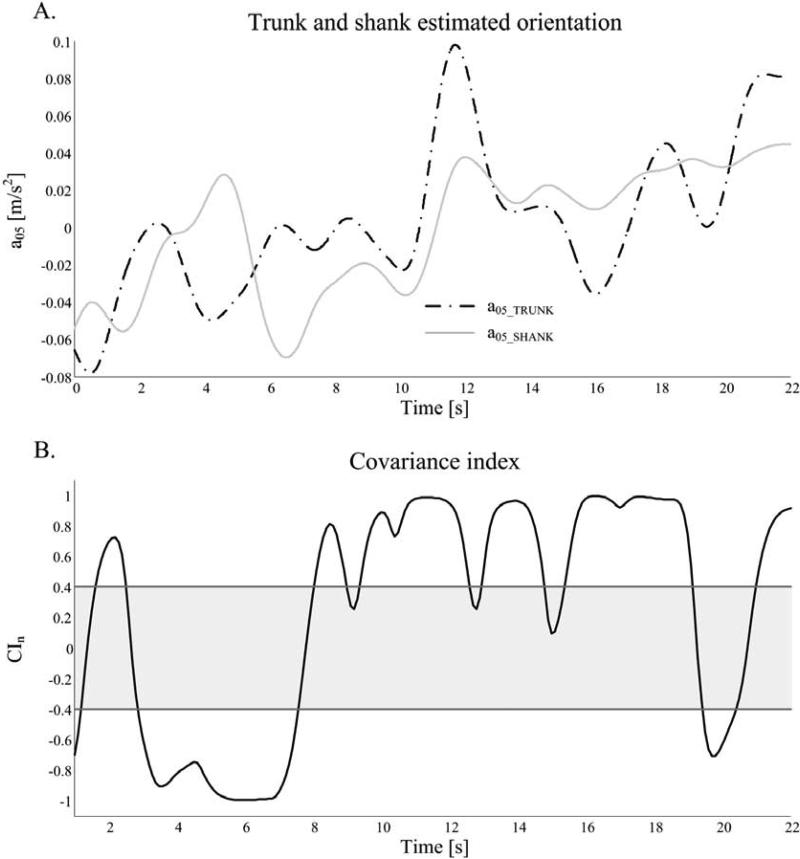
To estimate the orientation of the body segment on which the sensor was mounted, after alignment of axes with respect to gravity, ananthropometric low-pass filter with a cut-off frequency of 0.5 Hz was applied on the antero-posterior (AP) component of the acceleration signal[17]. This approach allowed to obtain an estimation of the AP acceleration that mainly included the gravitational component, thus attaining an information proportional to the body segment orientation in the sagittal plane (with respect to the vertical axis). Figure 1A shows a representative example of the trunk and shank estimated orientations during condition 2 of the SOT, represented by the 0.5 Hz filtered acceleration from the trunk (upper body) a05_TRUNK, and by the filtered acceleration from the shank a05_SHANK.
Figure 1.
A: accelerometer signals, filtered at 0.5 Hz, of a control subject in condition 1 of the SOT. B: normalized covariance index, CIn, computed by the sliding window algorithm. CIn thresholds are represented (grey line, ±0.4). Both in-phase and counter-phase local patterns of the signals are present in the same trial.
Afterwards, the coordination between the upper and lower segments of the body was quantified by a covariance indexbetween the trunk and shank (CIn), defined as the covariance of the signals a05_TRUNK and a05_SHANKnormalized by the standard deviations of the two signals. A positive CIn value close to 1 indicates that the two signals are in-phase, while a CIn toward -1 indicates that the two signals are in counter-phase. Sincethe two a05 signals estimate segments orientation in the sagittal plane, in-phase pattern can be associatedto a postural ankle strategy and counter-phase pattern to ahip strategy.
To be able to detect changes of CInin time during the 20 secondstrial length, CIn was computed using a sliding-window algorithm (window width: 2 seconds, taking into account the frequency components of the signals; time-shift between consecutive windows: 0.1 seconds, mainly for the sake of smoothness of the output signal).
An example of CIncalculated on a sliding window base is represented in Figure 1B. During the time-frames for whichCInwas higherrespect to a specific threshold, the postural behavior corresponded to in-phase pattern, while when CInwas lowerthan a specific threshold, the postural behavior corresponded to counter-phase pattern.This specific thresholdsused to distinguish between in-phase or counter-phase patterns were identified as +0.4 and −0.4 respectively, representing a medium correlation between the two variables (or signals), with significant interaction but no complete overlapping of the information in the variables[18]. The percentages of time, with respect to trial duration, corresponding to in-phase or counter-phase patterns (respectively TIP and TCP)were also considered.CIn values in between (−0.4< CIn<+0.4) were not considered for analysis since theyrepresent an undefined, transitional behavior.
2.3 Postural Strategy Index
An overall summary Strategy Index (SI) is also proposed in this study. Based onthe calculation of a symmetry index[19], SI was defined as a function of strategy time ratesto provide a more synthetic description of each trial.
Being TIP the percentage of time spent in in-phasepattern and TCP the percentage of time spent in counter-phase pattern, the SI is expressed as follows:
where W is a weight factor to balance the value of SI depending on the percentage of time during which a clearly identified pattern is present: W=(TIP+TCP)/100 (with 100 representing the total trial duration).
The SI ranges from −1 to 1, reaching the value of 1 when pure in-phase pattern (ankle strategy) ispredominant during the trial, and the value of -1 when pure counter-phase pattern (hip strategy) is predominant during the trial duration. Values close to 0 indicate that none of the strategies is the leading or that the rate of classified points isn't enough to provide a clear description of the trial.
2.4 Postural measures characterizing sway
The present study also measured postural stability from accelerometric signals,based on recently published approaches[17,20]. Specifically, signals from the raw accelerations on the trunk, after correction of possible misalignment with respect to vertical axis, were used. Raw signals were filtered at 3.5 Hz (zero-phase, low-pass Butterworth filter),to exclude possible influence of tremor as suggested in [20]. The root mean square of the signal (RMS) was computed as measure describing the amount of sway[20]. This measure wascalculated only from the AP component to allow more immediate comparison with the SI, computed from the AP signals as well. Only the AP direction was used since the surface rotational perturbations during the SOT were in the sagittal plane.
All the analyses mentioned in the previous sections were performed using Matlab R2012b. To evaluate the differences between conditions and populations a repeated measure ANOVA followed by Tukey Kramer test for multiple comparison was performed (NCSS software).
3. RESULTS
Representative a05_TRUNK and a05_SHANK traces are illustrated for a control subject(Figure 2A)and for a PD subject (Figure 2B) during condition 4of SOT. Whilethe trunk and shank signals of the control subject are mainly counter-phase, (CIn<-0.4 for 80% of trial), suggesting a prevalent hip strategy to attain balance,the PD subject showstrunk and shank sway that are mainly in-phase (CIn>0.4)during the entire trial, suggesting predominant adoption by the subject of ankle strategy.
Figure 2.
A: filtered accelerometer signals, a05, of a control subject (condition 4 of the SOT) showing a predominant counter-phase pattern (TCP= 80.0%) suggesting the principal use of hip strategy during the trial to attain balance. B: filtered accelerometer signals, a05, of a PD subject (same condition) showing a predominant in-phase pattern (TIP= 90.5%) suggesting that the subject preferred to use ankle strategy.
Overall, the percentage of time spent in in-phase pattern is larger than the percentage of time spent in counter-phase pattern. Mean and standard deviation valuesof TIP and TCPare reported in Table 1. Table 1 also shows that the undefined/transitional area,in which subjects do not show a predominant pattern, is quite limitedin all the subjects.
Table 1.
Mean values and standard deviations of percentages of time, with respect to trials duration, characterized by counter-phase (TCP) and in-phase pattern (TIP) for the control, PD and PSP subjects in the different SOT conditions. The remaining percentage of time corresponds to undefined behavior.
| SOT Conditions | ||||||
|---|---|---|---|---|---|---|
| Cond. 1 | Cond. 2 | Cond. 3 | Cond 4 | Cond 5 | Cond 6 | |
| In-phase behavior (TIP) [% of time w.r.t trial duration] | ||||||
| CTR | 62(21)% | 83(17)% | 67(16) % | 49(21) % | 71(16) % | 75(12) % |
| PD | 80(10) % | 89(8) % | 88(6) % | 85(13) % | 86(9)% | 85(12)% |
| PSP | 75(25) % | 80(22) % | 83(17) % | 77(15) % | 83(12) % | 81(11) % |
| Counter-phase behavior (TCP) [% of time w.r.t trial duration] | ||||||
| CTR | 19(13) % | 8(10) % | 15(9) % | 32(20) % | 15(14) % | 11(7) % |
| PD | 7(6) % | 3(3) % | 4(3) % | 7(8) % | 5(4) % | 6(7) % |
| PSP | 14(20) % | 9(14) % | 8(12) % | 11(10) % | 8(7) % | 9(6) % |
| Undefined behavior [% of time w.r.t trial duration] | ||||||
| CTR | 19(9) % | 9(8) % | 17(9) % | 19(6) % | 13(5) % | 13(6) % |
| PD | 13(6) % | 8(5) % | 8(4) % | 8(6) % | 9(6) | 9(7) % |
| PSP | 11(9) % | 11(8)% | 9(7) % | 12(8) % | 8(6) % | 10(6) % |
CTR: control subjects; PD: subjects with Parkinson's Disease, PSP: subjects with Progressive Supranuclear Palsy
Out ofthe 7 PSP subjects included in this study, only 3 were able to complete all 6 SOT conditions, and some trials in conditions 4-6 were shortened by falls (all the PSP subjects experienced at least 1 fall in the last 2 conditions).
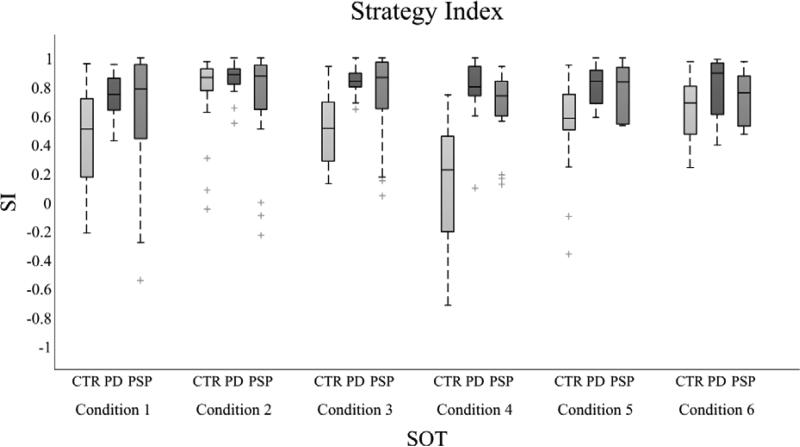
In contrast, all the PD and control subjects were able to perform all 6 conditions. The values of the postural strategy index, SI, are reported in Figure 3, with boxplots.The control subjectschanged their strategy index across conditions, with more variability in conditions 1 and 4 than in other conditions. In addition,theeyes open sway-referenced surface condition(condition 4)was characterized by high inter-subject variability and the SI resulted significantly lower compared to all the other SOT conditions (p<0.05). In contrast, the PD group didn't show a marked change in the use of postural strategies across conditions, with a SI close to 1 in all the SOT conditions. The PSP group revealed a trend similar to the PD group, except for a larger variability. Group differences in terms of SI were significant in condition 4, where both PSP and PD subjects showed a SI value higherthan control subjects (p<0.05).
Figure 3.
Postural strategy index (SI) values for control, PD and PSP subjects in each SOT condition, represented using boxplots (central line is the median values, the box includes from the 25th to 75th percentiles and the whiskers extend to the most extreme data-points, with outliers plotted individually). In control subjects, in condition 4 the SI resulted significantly lower compared to all the other SOT conditions (p<0.05). Group differences in terms of SI were significant in condition 4, where both PSP and PD subjects showed a SI value higher than control subjects (p<0.05).
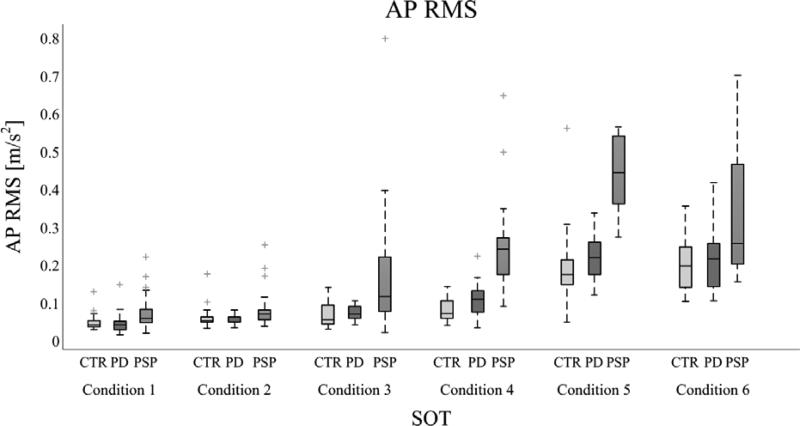
AP RMS values are represented in Figure 4. This measure, which quantifies the amount of postural oscillation, is influenced both by conditions and kind of populations. AP RMS increased with the difficulty of the conditions,reaching the highest values in conditions 5-6 (movable support base) compared to conditions 1-3 (fix support base)in all the groups (p<0.05).AP RMS values were similar between PD and control subjects in all the SOT conditions. In contrast, the PSP subjects who were able to perform all the SOT conditionsshowedamuch larger AP RMS compared to control and PD subjects in conditions 4 and 5 of SOT(p<0.05). Condition 6 did not present any significant difference, probably because of the frequent falls in the PSP group and subsequent reduced number of data (only 4 PSP subjects performed at least one trial in condition 6).
Figure 4.
The values of the AP RMS measure for control, PD and PSP subjects are represented in each SOT condition with boxplots (central line is the median values, the box includes from the 25th to 75th percentiles and the whiskers extend to the most extreme data-points, with outliers plotted individually).RMS reached the highest values in conditions 5-6 (movable support base) compared to conditions 1-3 (fix support base) in all the groups (p<0.05). The PSP subjects showed a much larger AP RMS compared to control and PD subjects in conditions 4-5(p<0.05). PSP subjects fell frequently: all the PSP subjects experienced at least 1 fall in the conditions 5-6. In contrast, all the PD and control subjects were able to perform all 6 conditions.
4. DISCUSSION
This study introduces, for the first time, a method to characterize postural movement strategies with easy-to-use, body-worn, inertial sensors.Our results are consistent with previous studies about postural strategy in the kind of subjects included in the present study, and this confirms the feasibility of the approach and its potentials in studies about postural strategies. In fact, postural strategy quantification showed that control subjectsmodified theirpostural strategies with changes in sensory conditions. Specifically, control subjectsprimarily used an ankle strategy,rather than a hip strategy, in all 6 sensory conditions. However, when proprioception was altered by sway- referencingthe support surface, the use of hip strategy increased, especially when vision was not disrupted (condition 4 for which significant statistical difference was shown with respect to the other conditions).This behavior is consistent with previous findings, which show that hip strategy in healthy subjects may occur when somatosensory information from the surface is impaired [21]. The adaptability of postural responses to external perturbation or sensory altered conditions is interpretable as an effective method to maintain balance [5,22,23].PSP and PD subjects persisted in use of an ankle strategy even when proprioception was altered, although with a large variability across subjects within each group. The lack of use of a hip strategy by patients with PD is consistent with previous studies suggesting that PD patients have small postural responses[24], stiff postural coordination[9,24] and impaired proprioception[25,26]. Postural strategies have not previously been described in patients with PSP, but the lack of a hip strategy may have contributed to the high frequency of falls in challenging sensory conditions, consistent with the clinical literature describing falls in PSP [4,15,27].
The same experimental approach that allowed to quantitatively characterize postural strategies,also allowed the assessment of the postural swayfrom the accelerometer on the trunk[17,20,28].The postural sway, measured with RMS,was smaller than normal in PD, tested in the OFF state, inagreementwith previous studies[19,24]. PSP subjects experienced several falls in the last SOT conditions, whereas PD patients did not, although the two groups had similar severity of symptoms. PSP subjects who did not fall showed larger postural sway than PD and control subjects, confirming severe balance impairment in PSP subjects[1,2]. This difference between parkinsonian groups is emphasized in condition 4, in which both PD and PSP subjects showed a predominant ankle strategy,unlike the control group. This may suggest that PD patients were able to overcome this specific sensory challenge just using an ankle strategy, probably by allowing very little sway as compensation, whereas PSP patients were not able to switch to hip strategy nor to compensate by reducing sway area, resulting in falls.
Further evaluation about PSP and PD populations are a desirable development of the present study, and our SI may be an interesting tool for such investigation. In addition, other symptoms of parkinsonsims may be evaluated with the present approach, such as tremor[29] or anomalous posture.
Our results suggest that a postural strategy index basedon covariance of estimated inclination of upper and lower body segments in challenging sensory conditions during stancecould add important insights into balance control in patients with movement disorders. In addition, the simple and accessible experimental set-up can easily be performed even in a clinical setting and it also allows the computation of adjunctive measures describing balance maintenance [17,20,30].
Research Highlights.
We evaluated postural strategies in PD, PSP and CTR subjects during SOT.
Covariance index and a new strategy index were based on inertial sensors signals.
We measured amount of sway as RMS of filtered signal from 1 inertial sensor.
CTR subjects changed postural strategies during the test. PD and PSP did not.
PSP had RMS values greater than CTR and PD. CTR and PD had similar RMS values.
Acknowledgments
The research leading to these results has been partially supported by the European Union - Seventh Framework Programme (FP7/2007-2013) under grant agreement n°288516 (CuPiD project) and by NIA Merit Award (AG006457).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
OSHU and Dr Horak have a significant financial interest in APDM, a company that may have a commercial interest in the results of this research and technology. This potential institutional and individual conflict has been reviewed and managed by OSHU.
REFERENCES
- 1.Egerton T, Williams DR, Iansek R. Comparison of gait in progressive supranuclear palsy, Parkinson's disease and healthy older adults. BMC Neurol. 2012;12:116. doi: 10.1186/1471-2377-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanska DJ. Chapter 33: the history of movement disorders. Handb Clin Neurol. 2010;95:501–46. doi: 10.1016/S0072-9752(08)02133-7. [DOI] [PubMed] [Google Scholar]
- 3.Johnson L, James I, Rodrigues J, Stell R, Thickbroom G, Mastaglia F. Clinical and posturographic correlates of falling in Parkinson's disease. Mov Disord. 2013 doi: 10.1002/mds.25449. [DOI] [PubMed] [Google Scholar]
- 4.Zwergal A, la Fougère C, Lorenzl S, Rominger A, Xiong G, Deutschenbaur L, et al. Postural imbalance and falls in PSP correlate with functional pathology of the thalamus. Neurology. 2011;77:101–9. doi: 10.1212/WNL.0b013e318223c79d. [DOI] [PubMed] [Google Scholar]
- 5.Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. J Neurophysiol. 1996;75:2380–96. doi: 10.1152/jn.1996.75.6.2380. [DOI] [PubMed] [Google Scholar]
- 6.Ondo W, Warrior D, Averell O, Calmes J, Hendersen N, Olson S, et al. Computerized posturography analysis of progressive supranuclear palsy: a case-control comparison with Parkinson's disease and healthy controls. Arch Neurol. 2000;57:1464–9. doi: 10.1001/archneur.57.10.1464. [DOI] [PubMed] [Google Scholar]
- 7.Horak FB, Nashner LM. Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol. 1986;55:1369–81. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- 8.Horak FB, Kuo A. Postural adaptation for altered environments, tasks, and intentions. In: Winters JM, Crago PE, editors. Biomech. Neural Control Posture Mov. Springer New York; New York, NY: 2000. pp. 267–81. [Google Scholar]
- 9.Kuo AD, Speers RA, Peterka RJ, Horak FB. Effect of altered sensory conditions on multivariate descriptors of human postural sway. Exp Brain Res. 1998;122:185–95. doi: 10.1007/s002210050506. [DOI] [PubMed] [Google Scholar]
- 10.Creath R, Kiemel T, Horak FB, Peterka R, Jeka J. A unified view of quiet and perturbed stance: simultaneous co-existing excitable modes. Neurosci Lett. 2005;377:75–80. doi: 10.1016/j.neulet.2004.11.071. [DOI] [PubMed] [Google Scholar]
- 11.Park S, Horak FB, Kuo AD. Postural feedback responses scale with biomechanical constraints in human standing. Exp Brain Res. 2004;154:417–27. doi: 10.1007/s00221-003-1674-3. [DOI] [PubMed] [Google Scholar]
- 12.Cuesta-Vargas AI, Galán-Mercant A, Williams JM. The use of inertial sensors system for human motion analysis. Phys Ther Rev. 2010;15:462–73. doi: 10.1179/1743288X11Y.0000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nashner LM, Peters JF. Dynamic posturography in the diagnosis and management of dizziness and balance disorders. Neurol Clin. 1990;8:331–49. [PubMed] [Google Scholar]
- 14.Barin K. Dynamic posturography: analysis of error in force plate measurement of postural sway. IEEE Eng Med Biol Mag. 1992;11:52–6. [Google Scholar]
- 15.Bohlhalter S, Kaegi G. Parkinsonism: heterogeneity of a common neurological syndrome. Swiss Med Wkly. 2011;141:w13293. doi: 10.4414/smw.2011.13293. [DOI] [PubMed] [Google Scholar]
- 16.Dalrymple-Alford JC, MacAskill MR, Nakas CT, Livingston L, Graham C, Crucian GP, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75:1717–25. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- 17.Palmerini L, Rocchi L, Mellone S, Valzania F, Chiari L. Feature selection for accelerometer- based posture analysis in Parkinson's disease. IEEE Trans Inf Technol Biomed. 2011;15:481–90. doi: 10.1109/TITB.2011.2107916. [DOI] [PubMed] [Google Scholar]
- 18.Coehn J. Statistical Power Analysis for the Behavioral Science. I. Academic Press; New York: 1969. [Google Scholar]
- 19.Rocchi L, Chiari L, Horak FB. Effects of deep brain stimulation and levodopa on postural sway in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002;73:267–74. doi: 10.1136/jnnp.73.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancini M, Salarian A, Carlson-Kuhta P, Zampieri C, King L, Chiari L, et al. ISway: a sensitive, valid and reliable measure of postural control. J Neuroeng Rehabil. 2012;9:59. doi: 10.1186/1743-0003-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horak FB, Nashner LM, Diener HC. Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res. 1990;82:167–77. doi: 10.1007/BF00230848. [DOI] [PubMed] [Google Scholar]
- 22.Welch TDJ, Ting LH. A Feedback Model Explains the Differential Scaling of Human Postural Responses to Perturbation Acceleration and Velocity. J Neurophysiol. 2009:3294–309. doi: 10.1152/jn.90775.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai Y-C, Hsieh L-F, Yang S. Age-related changes in posture response under a continuous and unexpected perturbation. J Biomech. 2013:1–9. doi: 10.1016/j.jbiomech.2013.10.047. [DOI] [PubMed] [Google Scholar]
- 24.Horak FB, Nutt JG, Nashner LM. Postural inflexibility in parkinsonian subjects. J Neurol Sci. 1992;111:46–58. doi: 10.1016/0022-510x(92)90111-w. [DOI] [PubMed] [Google Scholar]
- 25.Vaugoyeau M, Viallet F, Mesure S, Massion J. Coordination of axial rotation and step execution: deficits in Parkinson's disease. Gait Posture. 2003;18:150–7. doi: 10.1016/s0966-6362(03)00034-1. [DOI] [PubMed] [Google Scholar]
- 26.Konczak J, Corcos DM, Horak FB, Poizner H, Shapiro M, Tuite P, et al. Proprioception and motor control in Parkinson's disease. J Mot Behav. 2009;41:543–52. doi: 10.3200/35-09-002. [DOI] [PubMed] [Google Scholar]
- 27.Gerstenecker A, Duff K, Mast B, Litvan I. Behavioral abnormalities in progressive supranuclear palsy. Psychiatry Res. 2013;210:1205–10. doi: 10.1016/j.psychres.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitney SL, Roche JL, Marchetti GF, Lin C-C, Steed DP, Furman GR, et al. A comparison of accelerometry and center of pressure measures during computerized dynamic posturography: a measure of balance. Gait Posture. 2011;33:594–9. doi: 10.1016/j.gaitpost.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scanlon BK, Levin BE, Nation DA, Katzen HL, Guevara-Salcedo A, Singer C, et al. An accelerometry-based study of lower and upper limb tremor in Parkinson's disease. J Clin Neurosci. 2013;20:827–30. doi: 10.1016/j.jocn.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Halická Z, Lobotková J, Bučková K, Hlavačka F. Effectiveness of different visual biofeedback signals for human balance improvement. Gait Posture. 2013;39:410–4. doi: 10.1016/j.gaitpost.2013.08.005. [DOI] [PubMed] [Google Scholar]