Summary
Background
The development of neutralizing antibodies, referred to as inhibitors, against factor VIII (FVIII) is a major complication associated with FVIII infusion therapy for the treatment of hemophilia A (HA). Previous studies have shown that a subset of HA patients and a low percentage of healthy individuals harbor non-neutralizing anti-FVIII antibodies that do not elicit the clinical manifestations associated with inhibitor development.
Objective
Assess HA patients' anti-FVIII antibody profiles as potential predictors of clinical outcomes.
Methods
A fluorescence immunoassay (FLI) was used to detect anti-FVIII antibodies in 491 samples from 371 HA patients.
Results
Assessments of antibody profiles showed that the presence of anti-FVIII IgG1, IgG2, or IgG4 correlated qualitatively and quantitatively with the presence of a FVIII inhibitor as reported by the Nijmegen-Bethesda assay (NBA). Forty-eight patients with a negative inhibitor history contributed serial samples to the study, including seven patients who had negative NBA titers initially and later converted to NBA-positive. The FLI detected anti-FVIII IgG1 in five of those seven patients prior to their conversion to NBA-positive. Five of 15 serial-sample patients who had a negative inhibitor history and a positive anti-FVIII IgG1 later developed an inhibitor, compared to 2 of 33 patients with a negative inhibitor history without anti-FVIII IgG1.
Conclusions
These data provide a rationale for future studies designed both to monitor the dynamics of anti-FVIII antibody profiles in HA patients as a potential predictor of future inhibitor development and to assess the value of the anti-FVIII FLI as a supplement to traditional inhibitor testing.
Keywords: Factor VIII, Factor VIII Deficiency, Hemophilia A, immunoassay, Inherited Blood Coagulation Disorders
Introduction
Hemophilia A (HA) is an X-linked inherited bleeding disorder in which coagulation factor VIII (FVIII) is absent or dysfunctional and is most commonly treated by infusion of plasma-derived or recombinant FVIII. A major complication associated with FVIII infusion therapy is that up to 30% of patients develop antibodies that inhibit the function of and/or induce immune dependent clearance of the infused product(1;2). Anti-FVIII antibodies, referred to as inhibitors, diminish the effectiveness of infusion therapy, and, in the case of high titer inhibitors, necessitate the use of FVIII bypassing agents(3) or immune tolerance induction therapy (ITI)(4;5). Patients who develop FVIII inhibitors face an increased risk of bleeding complications(6) and present substantial financial and patient management challenges to the healthcare system(7).
The Bethesda assay(8) for measurement of FVIII inhibitors was developed in 1975 and modified in 1995 to the Nijmegen-Bethesda assay (NBA)(9), which is the gold standard method in use today. The NBA utilizes the degree to which HA patient plasma inhibits the in vitro clotting reaction of healthy donor plasma as a means to assign FVIII inhibitor titers. More recently, assays utilizing chromogenic substrates(10), enzyme linked immunosorbent assays (ELISA)(11;12), surface plasmon resonance (SPR) (13;14), and fluorescent immunoassays (FLI)(15-19) have been developed to detect anti-FVIII antibodies in HA patients. Many previous studies have observed that there is some discrepancy between the results obtained from functional assays, such as the NBA, and those obtained using other testing methods(11;12;18). Although the assortment of FVIII inhibitor assays all share the common goal of identifying the presence of anti-FVIII antibodies, they have key fundamental differences that contribute to the generation of discrepant results. The NBA and chromogenic inhibitor (CBA) assays attempt to simulate in vivo conditions in order to detect FVIII-specific functional inhibition of the clotting process. For the purpose of these assays, functional inhibition of FVIII-dependent clotting is reflected in decreased extent or kinetics of an in vitro clotting reaction(8;9) or the cleavage of a chromogenic substrate as a surrogate for clotting activity(10), but there is no direct measurement of FVIII-specific immunoreactivity. Alternatively, SPR, ELISA, and anti-FVIII FLI (αFVIII-FLI) inhibitor assays directly detect anti-FVIII antibodies, but do so without any means to assess the detected antibody’s ability to inflict functional inhibition on FVIII. These differences, as well as the lack of uniformity among laboratories used to determine what constitutes a positive reaction, make it difficult to integrate the various test results in order to reach a definitive diagnosis of a clinically significant inhibitor.
Previous studies utilizing direct antibody detection methods (11-13;20;21) have shown that the Ig subtype and subclass composition of the anti-FVIII antibody response may be critical in assessing the clinical implications of the immune response. These studies implicated IgG1 and IgG4 as the most common anti-FVIII antibody subclasses present in NBA-positive patient samples. The current study investigates the composition of the antibody response in 371 HA patients, the largest group of patients studied to date, using an αVIII-FLI. The study examines the prevalence of anti-FVIII antibodies in HA patient plasma, evaluates the make-up of the antibody response by IgG subclass, and assesses the clinical relevance of antibody subtype by evaluating the extent of correlation between FLI results and those obtained using the NBA.
Materials and Methods
Subjects
The study includes 491 plasma samples from 371HA patients (median/mean age 13/18.5 years) enrolled in the Hemophilia Inhibitor Research Study (22). 20.5% of patients (n=76) and 24.8% of samples (n=122) were NBA positive. Inhibitor measurements were performed using a modified version(23) of the NBA(9). The investigational review boards of the CDC and each participating site approved the protocol, and all participants or parents of minor children gave informed consent. Control samples were obtained from 56 paid healthy donors.
Fluorescence immunoassay
The αVIII-FLI is a modified version of our previously described method(18). Briefly, plasma samples diluted 1:30 in phosphate buffered saline (PBS) containing 1% dried milk (PBSM) were incubated with SeroMAP beads (Luminex Corporation, Austin, TX) coupled to Kogenate FS (Bayer Healthcare, Tarrytown, NY). Anti-FVIII antibodies were detected using serial incubations with biotinylated anti-human Ig (anti-IgG1, A-10650; anti-IgG2, 05-3540; anti-IgG3, MH1532; anti-IgG4, A-10663; anti-IgM, H15015; Life Technologies, Carlsbad, CA) and R-phycoerythrin-conjugated streptavidin (Jackson ImmunoResearch, West Grove, PA) using a Bio-Plex 200 suspension array system (Bio-Rad Laboratories, Hercules, CA). Results are expressed as median fluorescence intensity (MFI). The threshold for positivity was set at two standard deviations above the mean MFI of the results obtained for healthy donors.
Statistical Analyses
Comparisons of FLI and NBA results on individual plasma samples were made using GraphPad Prism (GraphPad Software, San Diego, CA) to generate Spearman’s correlation coefficient and two-tailed p values. Fisher’s exact test was used to evaluate differences in categorical data.
Results and Discussion
Characterization of anti-factor VIII antibodies in the plasma of hemophilia A patients
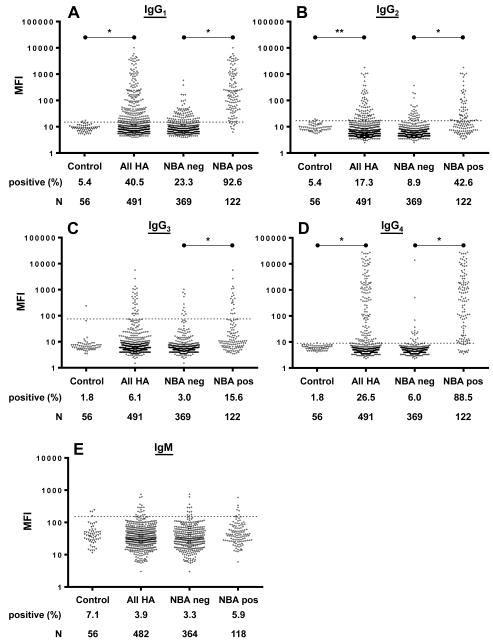
HA patient plasma samples were examined for the presence of anti-FVIII IgGs 1-4 and IgM using an αVIII-FLI (Table 1 and Figure 1). IgG subclass specific analysis of plasma samples showed that 40.5%, 17.3%, 6.1%, and 26.5% of the 491 patient samples were positive for anti-FVIII IgG1, IgG2, IgG3, and IgG4, respectively, compared to 5.4% (IgG1 and IgG2) or 1.8% (IgG3 and IgG4) of healthy donors (IgG1 and IgG4 p<0.0001; IgG2 p=0.02; IgG3 p=0.353). Evaluation of the IgG subclass specific FLI results segregated by NBA status revealed that NBA-positive specimens had significantly higher rates of positivity than NBA-negative samples for anti-FVIII IgG1, IgG2, IgG3, and IgG4 (p<0.0001)(Table 1; Figure 1). Rates of anti-FVIII IgM positivity were not significantly different in patients (3.9%) compared to healthy donors (7.1%) (p=0.285).
Table 1.
Summary of positive fluorescence immunoassay (FLI) results for anti-FVIII antibodies segregated by Ig subclass
| % Positive for Anti-Factor VIII by FLI |
||||||
|---|---|---|---|---|---|---|
| n | IgG1 | IgG2 | IgG3 | IgG4 | IgM* | |
| Healthy donors | 56 | 5.4 | 5.4 | 1.8 | 1.8 | 7.1 |
| All HA specimens | 491 | 40.5 | 17.3 | 6.1 | 26.5 | 3.9 |
| NBA-negative HA specimens |
369 | 23.3 | 8.9 | 3 | 6 | 3.3 |
| NBA-positive HA specimens |
122 | 92.6 | 42.6 | 15.6 | 88.5 | 5.9 |
|
| ||||||
| Correlation of FLI and NBA |
0.5438 p<0.0001 |
0.3411 p<0.0001 |
0.2829 p<0.0001 |
0.5766 p<0.0001 |
0.0643 p=0.1589 |
|
n=482 HA specimens, 364 NBA-negative, and 118 NBA-positive
Figure 1.
Fluorescence immunoassay (FLI) results for anti-FVIII antibodies in plasma from hemophilia A (HA) patients and healthy controls. Individual data points represent plasma samples assayed for anti-FVIII IgG1 (A), IgG2 (B), IgG3 (C), or IgG4 (D), or IgM (E). Results are displayed on a log-scale for control plasmas from healthy donors, all HA patient samples, and the subsets of HA patient samples with negative or positive Nijmegen-Bethesda (NBA) results for each Ig measured. The dashed line, which represents the assay’s positive threshold, is two standard deviations above the mean median fluorescence intensity (MFI) of 56 control samples from healthy donors. The number of samples (n) and the percentage of the samples that tested positive are as indicated. * p<0.0001; **p=0.02
In order to assess the relative importance of each subclass of anti-FVIII IgG in patients with FVIII inhibitors, we analyzed the IgG subclass specific FLI results to determine the composition of the FVIII antibody response in NBA positive samples. The results show that 98.4% of the NBA positive samples had positive FLI titers for one or more subclasses of anti-FVIII IgG, including 13.9% that were positive for a single subclass of anti-FVIII IgG, 84.4% that contained multiple subclasses of anti-FVIII IgG, and the remaining 1.6% had no FLI detectable anti-FVIII antibodies (Table 2). All of the 120 NBA-positive samples that also tested positive by FLI contained anti-FVIII IgG1and/or IgG4, and 101 (84.2%) were positive for both IgG1 and IgG4. Both of the NBA positive/FLI negative results were on samples with low titer inhibitors (0.7 and 0.8 NBU), and one of these samples was previously reported to a be a false positive due to its negative result by CBA(18).
Table 2.
Fluorescence immunoassay (FLI) results on 122 NBA-positive specimens
| NBA-Positive Samples |
Number of FLI-Positive Samples | ||||
|---|---|---|---|---|---|
| FLI Result | Percent (n) | IgG1 | IgG2 | IgG3 | IgG4 |
| Negative | 1.6 (2) | 0 | 0 | 0 | 0 |
| Positive for one subclass of IgG |
13.9 (17) | 10 | 0 | 0 | 7 |
| Positive for two subclasses of IgG |
40.2 (49) | 49 | 1 | 1 | 47 |
| Positive for three subclasses of IgG |
32.0 (39) | 39 | 37 | 2 | 39 |
| Positive for four subclasses of IgG |
12.3 (15) | 15 | 15 | 15 | 15 |
Linear correlations were calculated according to Spearman to evaluate the relationship between titers obtained from the αFVIII-FLIs and NBA. The αFVIII-FLIs for IgG1 and IgG4, which were positive in 92.6% and 88.5% of samples, respectively, exhibited a strong positive correlation with NBA titers (r(IgG1)=0.5438, r(IgG4)=0.5766; p<0.0001). Correlations between FLI and NBA results were weak, yet significant for anti-FVIII IgG2 (r=0.3411; p<0.0001) and IgG3 (r=0.2829; p<0.0001), while anti-FVIII IgM did not exhibit a quantitative correlation with NBA results (Table 1).
Anti-FVIII IgG composition in serial samples from individual hemophilia A patients
Sixteen patients exhibited a change in NBA inhibitor status over the course of specimen collection. Seven of those patients (patients 1-7) had negative NBA titers on their initial study specimen, but later developed a positive NBA reaction following FVIII infusion therapy for the indicated exposure days (Table 3). Examination of FLI results on plasma samples from these seven patients revealed that five of them harbored one or more classes of anti-FVIII Igs in samples prior to developing an inhibitor detectable by the NBA (Table 3, patients 1-5). All of these five patients were positive for anti-FVIII IgG1 prior to their conversion from NBA negative to NBA positive; one was also positive for anti-FVIII IgG4 (patient 5) and one for IgM (patient 4). Analysis of the FLI results on 201 samples from all 81 patients who contributed multiple specimens (data not shown) showed that 5 of 15 (33.3%) patients with a negative inhibitor history and a positive IgG1result later developed an inhibitor, compared to 2 of 33 (6.1%) patients with a negative inhibitor history without anti-FVIII IgG1 antibodies (p=0.0239). Patients 8-16 (Table 3) all have a history of inhibitors and are of interest due to the transitory nature of their NBA positivity. It is important to note that while overall the FLI results for anti-FVIII IgG1, IgG2, IgG3, and IgG4 displayed significant positive correlations with the NBA, FLI and NBA results on serial samples from individual patients did not necessarily change proportionally with time. The lack of intra-patient consistency is probably attributable to the differing role of kinetics in the two assays, and may also reflect changes in the patient’s immune response over time.
Table 3.
Anti-factor VIII fluorescence immunoassay (FLI) results on serial plasma draws from hemophilia A patients who exhibited a change in Nijmegen-Bethesda assay (NBA) status over the course of sample collection. A grey background highlights positive results.
| Pt. | severity | Draw date | Median fluorescence intensity units (MFI) | NBU | Exposure days |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgG1 | IgG2 | IgG3 | IgG4 | IgM | ||||||
| No history of inhibitor |
12/5/07 | 5.5 | 4.5 | 5 | 6 | 11.5 | 0.1 | 0-20 | ||
| 9/10/08 | 11.5 | 7 | 7 | 6 | 84.3 | 0 | ||||
| 1 | mild | 9/16/09 | 25.5 | 5.5 | 6 | 5 | 17 | 0 | ||
| 4/21/10 | 1093.3 | 7 | 191.5 | 8 | 38 | 1.7 | 0-20 | |||
| 6/9/10 | 4646.8 | 46 | 332.3 | 85 | 60.8 | 1.3 | ||||
| 9/21/10 | 386.5 | 7.5 | 20.5 | 96.8 | 29 | 1.8 | ||||
|
| ||||||||||
| 8/9/10 | 22 | 5 | 4.5 | 3.3 | 39.5 | 0.1 | 0-20 | |||
| 10/11/10 | 4111.8 | 42.3 | 612 | 1921 | 34 | 3.2 | 21-50 | |||
| 2 | severe | 11/18/10 | 827 | 10 | 28.8 | 1109 | 83.5 | 18.7 | ||
| 2/8/11 | 3352 | 43.5 | 44 | 1277 | 25.5 | 7.2 | ||||
| 3/9/11 | 234.5 | 7.5 | 10.3 | 262 | 25 | 1.4 | ||||
|
| ||||||||||
| 3 | severe | 10/1/08 | 75.3 | 5 | 8 | 4.5 | 90.5 | 0 | 0-20 | |
| 9/22/09 | 441.3 | 15.5 | 8.3 | 1592 | 85.3 | 13.6 | 0-20 | |||
|
| ||||||||||
| 7/23/08 | 37.5 | 8.3 | 5.8 | 6.5 | 746.5 | 0.2 | 0-20 | |||
| 4 | severe | 7/8/09 | 16.8 | 5.8 | 5.8 | 4 | 69 | 0 | ||
| 6/2/10 | 240.5 | 9 | 8 | 792.3 | 173.8 | 3.9 | 21-50 | |||
|
| ||||||||||
| 8/6/08 | 33 | 6 | 3.5 | 9 | 25.5 | 0.3 | 21-100 | |||
| 5 | severe | 8/12/09 | 48.5 | 12.8 | 6.5 | 14.5 | 53.8 | 1.4 | >150 | |
| 8/14/09 | 11 | 6 | 3.5 | 10.5 | 46 | 1.4 | ||||
| 6/30/10 | 6 | 6.8 | 4 | 3.5 | 58.8 | 0 | ||||
|
| ||||||||||
| 6 | mild | 3/3/10 | 10.5 | 4.3 | 6 | 4.5 | 109.3 | 0.1 | 0-20 | |
| 5/27/10 | 504.8 | 11.8 | 73.5 | 12.3 | 597.5 | 1.4 | 0-20 | |||
| 6/14/10 | 3914.5 | 111.3 | 746.8 | 114.5 | 103 | 1.7 | ||||
| 11/14/12 | 7.5 | 5.5 | 5 | 4.5 | 70.8 | 0.1 | ||||
|
| ||||||||||
| 2/5/07 | 7 | 4.5 | 3.5 | 4 | ND | 0 | 101-150 | |||
| 7 | severe | 6/18/08 | 34 | 6.8 | 4.8 | 1193.8 | 39 | 6.5 | 101-150 | |
| 6/17/09 | 51.5 | 7 | 6.8 | 1276.5 | 248.8 | 3.8 | ||||
|
| ||||||||||
| Previous history of inhibitor |
8 | severe | 7/5/06 | 249.5 | 12 | 13 | 8548.3 | 32 | 19.3 | ND |
| 7/23/08 | 7 | 5.5 | 5 | 5 | 42 | 0.2 | ||||
|
| ||||||||||
| 3/15/06 | 10 | 5.3 | 4 | 11.5 | 231 | 0.5 | ND | |||
| 9 | severe | 5/7/08 | 14.5 | 6.3 | 6.5 | 18.5 | 110.3 | 0 | ||
| 5/6/09 | 9 | 8 | 5.8 | 7 | 93.8 | 0 | ||||
|
| ||||||||||
| 10 | severe | 9/5/07 | 157.3 | 11 | 6.8 | 27 | 26.5 | 1.1 | ND | |
| 9/5/12 | 41 | 6.3 | 5.5 | 27.3 | 16 | 0.4 | ||||
|
| ||||||||||
| 6/17/08 | 35.8 | 5.8 | 6.5 | 39.5 | 42.5 | 0.5 | ND | |||
| 11 | severe | 6/17/09 | 38.5 | 10.5 | 8.5 | 21 | 15.8 | 0.3 | ||
| 6/16/10 | 19.5 | 4.5 | 5.5 | 22.5 | 25 | 0.3 | ||||
|
| ||||||||||
| 12 | 4/12/06 | 15.5 | 4 | 4.5 | 4 | 54.8 | 0.5 | ND | ||
| severe | 4/23/08 | 16.5 | 6 | 4 | 6 | 15.5 | 0.4 | |||
| 4/29/09 | 8 | 8.5 | 5 | 4 | 46.5 | 0 | ||||
|
| ||||||||||
| 13 | mild | 12/15/08 | 66.3 | 96.5 | 12 | 542 | 37 | 0.8 | ND | |
| 3/4/09 | 10.8 | 58.3 | 6.5 | 14 | 40.5 | 0 | ||||
|
| ||||||||||
| 11/16/07 | 85.5 | 10 | 5.8 | 1527 | 15 | 24.6 | ND | |||
| 14 | severe | 9/25/09 | 14.5 | 5 | 4 | 9.8 | 37 | 0.3 | ||
| 6/2/10 | 337.5 | 92.3 | 398.5 | 145.8 | 95.5 | 3.3 | ||||
|
| ||||||||||
| 15 | severe | 2/6/08 | 240.8 | 55.5 | 1341.5 | 85.3 | 41.5 | 3.9 | ND | |
| 4/8/09 | 16 | 6.5 | 14.5 | 69 | 23.5 | 0.2 | ||||
|
| ||||||||||
| 16 | severe | 10/10/07 | 48 | 25 | 31 | 510 | 20.8 | 0.3 | ND | |
| 12/5/08 | 13.3 | 9 | 7 | 207.5 | 26.5 | 0.6 | ||||
|
|
||||||||||
| Threshold for Positivity |
14.6* | 16.1* | 75.5* | 8.3* | 153.6* | 0.5 | ||||
Mean + 2sd of 56 healthy donors
ND- No data collected
Positive FLI results on samples with a corresponding negative NBA result were present in a low percentage of samples tested for anti-FVIII IgG2-4, occurring in 3-9%, while disparities for anti-FVIII IgG1 were more common, with positive FLI results occurring in 23.3% of NBA negative samples. These discrepant results may be caused by the presence of anti-FVIII antibodies that are of insufficient titer to render an inhibitory effect on coagulation in the NBA, the presence of anti-FVIII antibodies that recognize epitopes that are insignificant to the functional integrity of the FVIII molecule, or non-specific or indirect antibody binding to the FVIII coupled beads. Our data on serial samples drawn from 81 patients support the first hypothesis. While it is important to note that patients harboring non-neutralizing antibodies may never progress to developing an inhibitor, one-third of 15 patients who had a negative inhibitor history and were positive for IgG1converted from NBA negative to NBA positive over the course of the sample collection, compared to only 6.1% of patients with a negative inhibitor history without anti–FVIII IgG1. These findings, although preliminary, suggest that NBA-negative patients with IgG1 anti-FVIII antibodies are more likely to develop inhibitors detectable by the NBA than patients without such antibodies, and that these patients may merit closer scrutiny (e.g., patients undergoing surgical procedures) or more frequent follow-up testing (e.g., patients receiving initial FVIII infusions) to facilitate prompt clinical intervention.
The identification of anti-FVIII antibodies in HA patients is an important clinical development, but results presented here and by others have shown that the mere presence of antibodies does not always correlate with the clinical manifestations of FVIII inhibition (11;12;16-19;24;25). Identifying the underlying features that distinguish cases of benign and/or transient anti-FVIII antibodies from those that are clinically relevant anti-FVIII inhibitors is an important area of research. Although it remains unclear why the presence of certain antibody subclasses may be predictive of a worse clinical outcome, the data presented herein support those from a recently published study by Whelan et al.(12) in which the authors used an ELISA to show that anti FVIII IgG1 and IgG4 were present in 19 of 20 inhibitor positive HA patients. They also found that IgG4 was completely absent in 77 non-inhibitor patients and 600 healthy individuals, and that anti–FVIII IgG1 was present in 19% and 6% of non-inhibitor HA patients and healthy individuals, respectively(12). Whelan et al. hypothesized that their data could indicate the presence of variations in immune regulatory pathways in the different study cohorts. Previous studies that examined the potential link between SNPs in immune response genes and a predisposition to inhibitor development(26-30), and the results from the current study with a larger patient population using different methodology support this hypothesis. In addition, our data illustrate that IgG4 may be present in a low percentage of patients lacking inhibitors as measured by the NBA including 2.5% (7 of 283) of patients with a negative inhibitor history(data not shown) and that anti-FVIII IgG1 production may be an early checkpoint in inhibitor development. Taken together, these data provide a rationale for future clinical studies designed to monitor the dynamics of HA patients’ anti-FVIII antibody profiles in order to assess their value as predictors of future development of clinically relevant inhibitors and to determine the usefulness of the αFVIII FLI as a supplement to traditional inhibitor testing methods.
Acknowledgments
Thanks to the patients who participated and the study coordinators and administrators at the study sites: Jan Kuhn, Gail Long, Pam Bryant, Margaret Geary, Rosanne Lamoreaux, Mindy Nolte, Jamie Leonard, Julie Thomas, Betsy Wilson, Beverly Yandell, Leslie Morse, Neelam Thukral, Michael Lammer, Debbie Nelson, Holly Davidson, Megan Lemanczyk, Madeline Cantini, Aroub Khleif, Carol Dekernion, Jeanettte Buehler, Amanda Hollatz, Brenda Riske, Wendy Mitsuyama, David Waters, Angie Riedel, Michiyo Tomita, Young Chong, Ann Forsberg, Deirdre Cooper-Blacketer, and Rebecca Hauke.
Disclosures
This work was supported by the CDC Foundation through a grant from Pfizer Pharmaceuticals. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Reference List
- 1.Kempton CL, Soucie JM, Abshire TC. Incidence of inhibitors in a cohort of 838 males with hemophilia A previously treated with factor VIII concentrates. J Thromb Haemost. 2006 Dec;4:2576–81. doi: 10.1111/j.1538-7836.2006.02233.x. [DOI] [PubMed] [Google Scholar]
- 2.Kruse-Jarres R. Inhibitors: our greatest challenge. Can we minimize the incidence? Haemophilia. 2013 Jan;19(Suppl 1):2–7. doi: 10.1111/hae.12049. [DOI] [PubMed] [Google Scholar]
- 3.Leissinger CA. Prevention of bleeds in hemophilia patients with inhibitors: emerging data and clinical direction. Am J Hematol. 2004 Oct;77:187–93. doi: 10.1002/ajh.20162. [DOI] [PubMed] [Google Scholar]
- 4.Waters B, Lillicrap D. The molecular mechanisms of immunomodulation and tolerance induction to factor VIII. J Thromb Haemost. 2009 Sep;7:1446–56. doi: 10.1111/j.1538-7836.2009.03538.x. [DOI] [PubMed] [Google Scholar]
- 5.Dimichele DM. Immune tolerance in haemophilia: the long journey to the fork in the road. Br J Haematol. 2012 Oct;159:123–34. doi: 10.1111/bjh.12028. [DOI] [PubMed] [Google Scholar]
- 6.Colowick AB, Bohn RL, Avorn J, Ewenstein BM. Immune tolerance induction in hemophilia patients with inhibitors: costly can be cheaper. Blood. 2000 Sep 1;96:1698–702. [PubMed] [Google Scholar]
- 7.Guh S, Grosse SD, McAlister S, Kessler CM, Soucie JM. Healthcare expenditures for males with haemophilia and employer-sponsored insurance in the United States, 2008. Haemophilia. 2012 Mar;18:268–75. doi: 10.1111/j.1365-2516.2011.02692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasper CK, Aledort L, Aronson D, Counts R, Edson JR, van Eys J, Fratantoni J, Green D, Hampton J, Hilgartner M, Levine P, Lazerson J, McMillan C, Penner J, Shapiro S, Shulman NR. Proceedings: A more uniform measurement of factor VIII inhibitors. Thromb Diath Haemorrh. 1975 Nov 15;34:612. [PubMed] [Google Scholar]
- 9.Verbruggen B, Novakova I, Wessels H, Boezeman J, van den Berg M, Mauser-Bunschoten E. The Nijmegen modification of the Bethesda assay for factor VIII:C inhibitors: improved specificity and reliability. Thromb Haemost. 1995 Feb;73:247–51. [PubMed] [Google Scholar]
- 10.Blanco AN, Alcira PA, Grosso SH, Gennari LC, Perez BR, Lazzari MA. A chromogenic substrate method for detecting and titrating anti-factor VIII antibodies in the presence of lupus anticoagulant. Haematologica. 2002 Mar;87:271–8. [PubMed] [Google Scholar]
- 11.Gilles JG, Arnout J, Vermylen J, Saint-Remy JM. Anti-factor VIII antibodies of hemophiliac patients are frequently directed towards nonfunctional determinants and do not exhibit isotypic restriction. Blood. 1993 Oct 15;82:2452–61. [PubMed] [Google Scholar]
- 12.Whelan SF, Hofbauer CJ, Horling FM, Allacher P, Wolfsegger MJ, Oldenburg J, Male C, Windyga J, Tiede A, Schwarz HP, Scheiflinger F, Reipert BM. Distinct characteristics of antibody responses against factor VIII in healthy individuals and in different cohorts of hemophilia A patients. Blood. 2013 Feb 7;121:1039–48. doi: 10.1182/blood-2012-07-444877. [DOI] [PubMed] [Google Scholar]
- 13.Lewis KB, Hughes RJ, Epstein MS, Josephson NC, Kempton CL, Kessler CM, Key NS, Howard TE, Kruse-Jarres R, Lusher JM, Walsh CE, Watts RG, Ettinger RA, Pratt KP. Phenotypes of allo- and autoimmune antibody responses to FVIII characterized by surface plasmon resonance. PLoS One. 2013;8:e61120. doi: 10.1371/journal.pone.0061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen PC, Lewis KB, Ettinger RA, Schuman JT, Lin JC, Healey JF, Meeks SL, Lollar P, Pratt KP. High-resolution mapping of epitopes on the C2 domain of factor VIII by analysis of point mutants using surface plasmon resonance. Blood. 2014 Apr 24;123:2732–9. doi: 10.1182/blood-2013-09-527275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavigne-Lissalde G, Tarrade C, Lapalud P, Chtourou S, Schved JF, Granier C, Villard-Saussine S. Simultaneous detection and epitope mapping of anti-factor VIII antibodies. Thromb Haemost. 2008 Jun;99:1090–6. doi: 10.1160/TH07-08-0497. [DOI] [PubMed] [Google Scholar]
- 16.Krudysz-Amblo J, Parhami-Seren B, Butenas S, Brummel-Ziedins KE, Gomperts ED, Rivard GE, Mann KG. Quantitation of anti-factor VIII antibodies in human plasma. Blood. 2009 Mar 12;113:2587–94. doi: 10.1182/blood-2008-08-174987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zakarija A, Harris S, Rademaker AW, Brewer J, Krudysz-Amblo J, Butenas S, Mann KG, Green D. Alloantibodies to factor VIII in haemophilia. Haemophilia. 2011 Jul;17:636–40. doi: 10.1111/j.1365-2516.2010.02468.x. [DOI] [PubMed] [Google Scholar]
- 18.Miller CH, Rice AS, Boylan B, Shapiro AD, Lentz SR, Wicklund BM, Kelly FM, Soucie JM. Comparison of clot-based, chromogenic and fluorescence assays for measurement of factor VIII inhibitors in the US Hemophilia Inhibitor Research Study. J Thromb Haemost. 2013 Jul;11:1300–9. doi: 10.1111/jth.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lebreton A, Lapalud P, Chambost H, Biron-Andreani C, Morange PE, Combescure C, Marques-Verdier A, Berger C, Schved JF, Granier C, Lavigne-Lissalde G. Prevalence and epitope specificity of non-neutralising antibodies in a large cohort of haemophilia A patients without inhibitors. Thromb Haemost. 2011 Jun;105:954–61. doi: 10.1160/TH10-10-0668. [DOI] [PubMed] [Google Scholar]
- 20.Towfighi F, Gharagozlou S, Sharifian RA, Kazemnejad A, Esmailzadeh K, Managhchi MR, Shokri F. Comparative measurement of anti-factor VIII antibody by Bethesda assay and ELISA reveals restricted isotype profile and epitope specificity. Acta Haematol. 2005;114:84–90. doi: 10.1159/000086580. [DOI] [PubMed] [Google Scholar]
- 21.van Helden PM, Van Den Berg HM, Gouw SC, Kaijen PH, Zuurveld MG, Mauser-Bunschoten EP, Aalberse RC, Vidarsson G, Voorberg J. IgG subclasses of anti-FVIII antibodies during immune tolerance induction in patients with hemophilia A. Br J Haematol. 2008 Aug;142:644–52. doi: 10.1111/j.1365-2141.2008.07232.x. [DOI] [PubMed] [Google Scholar]
- 22.Soucie JM, Miller CH, Kelly FM, Payne AB, Creary M, Bockenstedt PL, Kempton CL, Manco-Johnson MJ, Neff AT. A study of prospective surveillance for inhibitors among persons with haemophilia in the United States. Haemophilia. 2014 Mar;20:230–7. doi: 10.1111/hae.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller CH, Platt SJ, Rice AS, Kelly F, Soucie JM. Validation of Nijmegen-Bethesda assay modifications to allow inhibitor measurement during replacement therapy and facilitate inhibitor surveillance. J Thromb Haemost. 2012 Jun;10:1055–61. doi: 10.1111/j.1538-7836.2012.04705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klintman J, Hillarp A, Berntorp E, Astermark J. Long-term anti-FVIII antibody response in Bethesda-negative haemophilia A patients receiving continuous replacement therapy. Br J Haematol. 2013 Nov;163:385–92. doi: 10.1111/bjh.12540. [DOI] [PubMed] [Google Scholar]
- 25.Ling M, Duncan EM, Rodgers SE, Street AM, Lloyd JV. Low detection rate of antibodies to non-functional epitopes on factor VIII in patients with hemophilia A and negative for inhibitors by Bethesda assay. J Thromb Haemost. 2003 Dec;1:2548–53. doi: 10.1046/j.1538-7836.2003.00477.x. [DOI] [PubMed] [Google Scholar]
- 26.Hay CR, Ollier W, Pepper L, Cumming A, Keeney S, Goodeve AC, Colvin BT, Hill FG, Preston FE, Peake IR. HLA class II profile: a weak determinant of factor VIII inhibitor development in severe haemophilia A. UKHCDO Inhibitor Working Party. Thromb Haemost. 1997 Feb;77:234–7. [PubMed] [Google Scholar]
- 27.DE Barros MF, Herrero JC, Sell AM, De Melo FC, Braga MA, Pelissari CB, Machado J, De Souza SS, De Souza HL, Visentainer JE. Influence of class I and II HLA alleles on inhibitor development in severe haemophilia A patients from the south of Brazil. Haemophilia. 2012 May;18:e236–e240. doi: 10.1111/j.1365-2516.2011.02604.x. [DOI] [PubMed] [Google Scholar]
- 28.Astermark J, Oldenburg J, Pavlova A, Berntorp E, Lefvert AK. Polymorphisms in the IL10 but not in the IL1beta and IL4 genes are associated with inhibitor development in patients with hemophilia A. Blood. 2006 Apr 15;107:3167–72. doi: 10.1182/blood-2005-09-3918. [DOI] [PubMed] [Google Scholar]
- 29.Pavlova A, Delev D, Lacroix-Desmazes S, Schwaab R, Mende M, Fimmers R, Astermark J, Oldenburg J. Impact of polymorphisms of the major histocompatibility complex class II, interleukin-10, tumor necrosis factor-alpha and cytotoxic T-lymphocyte antigen-4 genes on inhibitor development in severe hemophilia A. J Thromb Haemost. 2009 Dec;7:2006–15. doi: 10.1111/j.1538-7836.2009.03636.x. [DOI] [PubMed] [Google Scholar]
- 30.Astermark J, Oldenburg J, Carlson J, Pavlova A, Kavakli K, Berntorp E, Lefvert AK. Polymorphisms in the TNFA gene and the risk of inhibitor development in patients with hemophilia A. Blood. 2006 Dec 1;108:3739–45. doi: 10.1182/blood-2006-05-024711. [DOI] [PubMed] [Google Scholar]