Abstract
Parasites can negatively affect the evolutionary fitness of their hosts by eliciting physiological stress responses. Parasite-induced stress can be monitored by measuring changes in the adrenal steroid hormone corticosterone. We examined the effect of an invasive parasite on the corticosterone concentrations of a common species of Darwin’s finch, the medium ground finch (Geospiza fortis). Philornis downsi (Diptera: Muscidae) is a parasitic nest fly recently introduced to the Galapagos Islands, where it feeds on the blood of nestlings and breeding adult female finches. Previous work shows that P. downsi significantly reduces the reproductive success of several species of finches. We predicted that the effect of P. downsi on host reproductive success is mediated by stress responses in breeding female finches. High stress levels could reduce the ability of females to invest in offspring, thus decreasing their reproductive success. To test this hypothesis, we experimentally manipulated the abundance of P. downsi in nests, then measured baseline and acute stress-induced corticosterone levels, body condition, and hematocrit (red blood cell content). Acute stress-induced corticosterone levels increased over baseline levels, but this response did not differ significantly with parasite treatment. There was also no significant difference in the body condition or hematocrit of females from parasitized versus non-parasitized nests. Our results suggest that the lower reproductive success of females from parasitized nests is not mediated by a physiological stress response.
Keywords: Condition, Geospiza fortis, Hematocrit, Invasive, Philornis downsi
1. Introduction
An animal’s ability to cope physiologically with environmental stressors is an important component of its evolutionary fitness (Breuner et al., 2008; Johnstone et al., 2012; Siegel, 1980). One mechanism that mediates this process is the regulation of glucocorticoids, such as corticosterone, through activation of the hypothalamic–pituitary–adrenal axis (HPA-axis) (Sapolsky et al., 2000). Short-term elevations in corticosterone can trigger adaptive responses, such as energy mobilization, activation of the immune system, increased delivery of oxygen to tissues, and night restfulness (Sapolsky et al., 2000; Wingfield et al., 1998). These responses act to help an organism recover from sources of stress and maintain homeostasis (McEwen and Wingfield, 2003; Wingfield and Kitaysky, 2002). However, long-term elevations in corticosterone can have detrimental effects on an organism’s survival and reproductive success by over-depleting fat stores, reducing parental investment in offspring, or compromising host immune responses (Sapolsky et al., 2000; Silverin, 1986; Tsigos and Chrousos, 2002).
Parasites are common stressors faced by most organisms, including birds (Brown et al., 2005; Siegel, 1980). Several studies show that parasitized birds maintain higher levels of both baseline and acute stress-induced corticosterone than non-parasitized birds (Boughton et al., 2006; Brown et al., 2005; Raouf et al., 2006). Nest parasites – those which reside primarily in the nest material – can directly affect both nestlings and breeding adult birds (Clayton and Tompkins, 1994; Marshall, 1981). Indirect effects of parasitism, mediated through the stress response of adult birds, can also exacerbate the direct effects of parasites. For example, increased glucocorticoid activity by parasitized adults may alter parental investment in nestlings, in exchange for self-preservation (Wingfield and Silverin, 1986). Elevated corticosterone levels in breeding birds have been linked to: (1) delays in returning to the breeding grounds (Breuner and Hahn, 2003); (2) less time devoted to guarding nests (Kitaysky et al., 2001; Wingfield and Silverin, 1986); (3) less time feeding nestlings (Silverin, 1986); and (4) an increase in nest abandonment (Love et al., 2004; Silverin, 1986). Thus, while elevations in corticosterone can help to preserve adults, the consequences for nestlings may be severe, reducing adult reproductive success.
In the Galapagos Islands of Ecuador, an introduced parasitic fly, Philornis downsi (Diptera: Muscidae), affects several species of land birds, including Darwin’s finches (Fessl and Tebbich, 2002). Adult flies, which are non-parasitic, feed on decaying, organic matter. Female flies lay their eggs in the nests of birds, or in the nares (nostrils) of nestlings (Fessl et al., 2006). Once the fly eggs hatch, the larvae are hematophagous parasites that feed on the blood of both nestlings and adult female birds when they sit on the nest (Huber et al., 2010). Adult females incubate the eggs and brood the offspring. Adult males do not sit on the nest and, therefore, do not appear to come into contact with the parasites (Huber et al., 2010; Koop et al., 2011). P. downsi was first documented in the Galapagos Islands in 1964 (Causton et al., 2006); however, it was not until 1997 that the fly was observed in large numbers in nests (Fessl et al., 2001; Fessl and Tebbich, 2002). P. downsi has a significant negative effect on the growth rates and fledging success of medium ground finches (Geospiza fortis) (Koop et al., 2011). However, little is known regarding the effect of P. downsi on adult birds, or whether such effects contribute to observed decreases in reproductive success.
We tested whether P. downsi causes an increase in the baseline or acute stress responses of breeding adult female medium ground finches. We also measured the effect of the parasite on various aspects of female condition. We experimentally manipulated P. downsi abundance in the nests of medium ground finches to test the effect of the parasite on adult female corticosterone concentration, body condition, and blood loss. We predicted that parasitized females would have higher levels of baseline corticosterone than non-parasitized females. We also predicted that parasitized females would exhibit a higher acute corticosterone response to handling induced stress. Assuming that corticosterone is involved in mobilizing sources of stored energy, such as fat and glucose (Wingfield et al., 1998), we predicted that an increase in glucocorticoid levels would coincide with a reduction in body condition. Finally, we predicted that parasitized females would have lower hematocrit than non-parasitized females. Reduced hematocrit (% red blood cells/total blood volume) is an indicator of blood loss in birds (Olaymeni 2009; Palmer et al., 1979).
2. Methods
2.1. Study species and site
The study was conducted January–April, 2010 at El Garrapatero on Santa Cruz Island, Galapagos. The field site is a 1.5 km × 1.5 km area located in the arid coastal zone. Medium ground finches are abundant at this site (Huber, 2008), where they nest in endemic tree cacti (Opuntia echios gigantea) 1.5–4 m above the ground. Finch clutch sizes range from 2–5 eggs, and the eggs are incubated for approximately 14 days (Grant, 1999). Nestlings normally fledge 10–14 days after hatching.
2.2. Manipulation of parasite abundance
Active nests were checked every other day between 600 and 1100 h throughout the study. When the first nestling hatched, the nest was randomly assigned to either an experimental or control group. Nestlings were temporarily removed while experimental nests (n = 15) were sprayed with a 1% permethrin solution (hereafter, fumigated nests) and control nests (n = 15) were sprayed with water (hereafter, sham-fumigated). After all of the nestlings in a nest had fledged or died, the nest was collected and sealed in a plastic bag; medium ground finches do not reuse nests (Grant 1999). Nests were dissected within 8 h of collection and P. downsi larvae, pupae, and eclosed pupal cases were counted. First instar larvae were not included in tallies of parasite abundance because they are too small to see reliably in the nest material (Koop et al., 2011). Total parasite abundance was calculated as the sum of all second and third instar larvae, pupae, and eclosed pupal cases found in a nest.
2.3. Field monitoring procedures
Adult birds and nests were monitored to determine their reproductive stage. When nestlings were 3–6 days old, a mist net was placed in the nest territory to catch the attending adult female. This time period was chosen to minimize sample loss due to the failure of parasitized nests, which often happens less than a week after hatching of the first egg (Koop et al., 2013). Once opened, nets were under constant surveillance. Netted birds were removed from the net and a blood sample was taken within 3 min of capture to assess baseline corticosterone levels. For each bird, a sterile 28-gauge needle was used to puncture the brachial (alar) vein, and the blood sample (<75 µL) was collected into a heparinized microhematocrit tube. Critoseal-sealed tubes were held on ice until centrifugation, which took place within 6 h of collection. Capillary tubes were spun at 8000 rpm for 10 min in a centrifuge. Hematocrit was measured in the capillary tube from the first blood sample with digital calipers to estimate the volume of packed red blood cells in relation to total blood volume. Blood and plasma were transferred and stored in separate 0.5 mL microcentrifuge vials at −20 °C until the end of the field season. Samples were then transported to the University of Utah and stored at −80 °C until processing for hormones.
Following collection of the first blood sample, each female finch was fitted with a numbered monel metal band and three plastic color bands for identification. Birds were weighed to the nearest 0.1 g and tarsal length was measured in triplicate. Body condition was estimated with a scaled mass index (SMI), calculated using body mass and tarsus length, as described by Peig and Green (2009). Following banding, each bird was placed in an individual cloth bag and another blood sample (<75 µL) was drawn 15 min after capture. The second blood sample was used to monitor changes in corticosterone (stress-induced corticosterone) upon exposure to an acute stressor, following the method of Wingfield et al. (1982). Because of the hot climate at our field site, which poses a danger to the health of captive birds, we released birds within 20 min of capture.
2.4. Radioimmunoassay protocol
Plasma samples were assayed in duplicate for corticosterone (antibody from Fitzgerald #20-CR45) using a previously described protocol (French et al., 2010). Briefly, samples were extracted using a 30% ethyl acetate/isooctane mixture. Corticosterone was separated from the sample using column chromatography (50% ethyl acetate/isooctane elution). The ethyl acetate/isooctane phase was separated, dried, and re-suspended in PBS buffer. For each sample we used an aliquot of the re-suspended fractions to measure individual recoveries following extraction and chromatography. These recoveries were used to adjust the final sample concentration values to account for any losses during these procedures. The coefficient of variation for corticosterone was 13.5% and the average minimum detectable value was 0.3 ng/mL. Two females (both from sham-fumigated nests) had baseline corticosterone values that fell below the standard curve for the assay (outside the range of detectable values); these birds were excluded from further analyses. Because plasma volumes were limited, these samples could not be re-run at different dilutions.
2.5. Statistical analysis
Statistical analyses were done in Prism® v.5.0b (GraphPad Software, Inc.). All relevant parameters were tested for normality using a D’Agostino and Pearson omnibus normality test. Parasite abundance was compared between treatments with a Mann–Whitney U test. A two-way ANOVA was used to compare baseline and stress-induced corticosterone concentrations between parasite treatments. Body condition and hematocrit were compared between parasite treatments with separate two-tailed t-tests.
3. Results
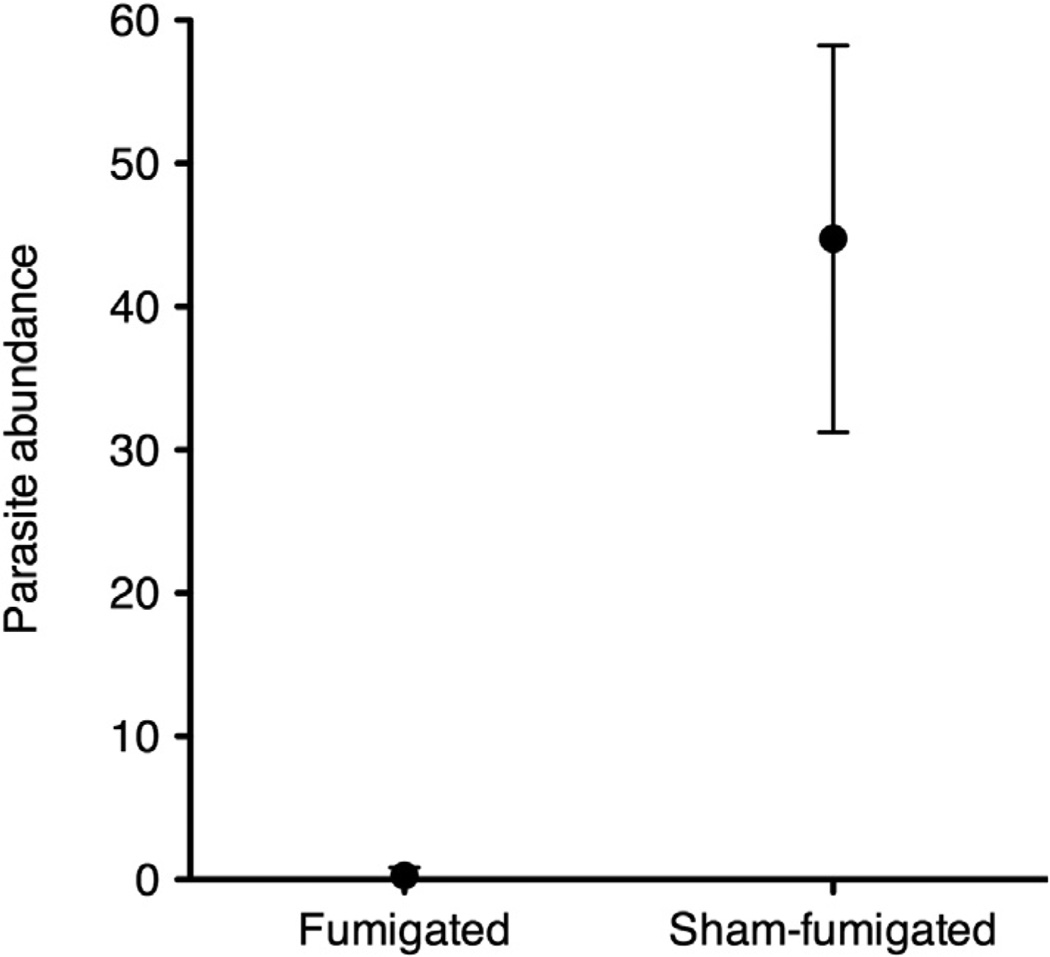
The experimental manipulation of nests was successful. Sham-fumigated nests had a mean ± SE of 44.73 ± 6.30 parasites/nest (lower and upper 95% confidence intervals of the mean (95% CI: 31.23–58.24), whereas fumigated nests had a mean of 0.27 ± 0.27 parasites/nest (95% CI: −0.31 to 0.84) (Fig. 1; Mann–Whitney, U = 0.00, P < 0.0001). Fourteen of the 15 fumigated nests were parasite free; the remaining nest, which experienced heavy rain soon after being treated, had four fly larvae. The female finch from this nest was included in all analyses. In the fifteen sham-fumigated nests, parasite abundance ranged from 5 to 79 flies per nest.
Fig. 1.
Comparison of the mean (95% CI) number of P. downsi in fumigated (n = 15) and sham-fumigated (n = 15) nests.
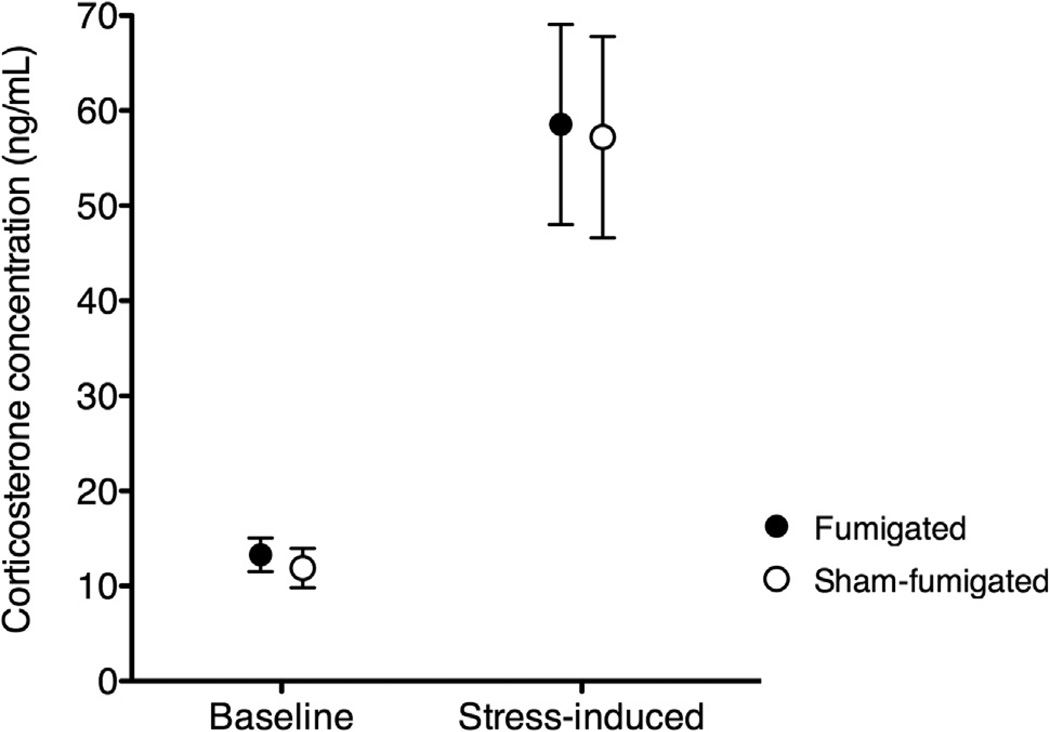
There was a significant effect of stress on corticosterone concentration: stress-induced corticosterone was significantly higher than baseline corticosterone (Fig. 2; two-way ANOVA, F1,50 = 131.5, P < 0.0001). However, there was no significant effect of parasite treatment on corticosterone (F1,50 = 0.0001, P = 0.99), nor was there a significant interaction between treatment and time (F1,50 = 0.12, P = 0.73). Baseline corticosterone values were as follows: fumigated: n = 13, mean ± SE = 13.29 ± 0.82 ng/mL, 95% CI: 11.51–15.08; sham-fumigated: n = 11, mean ± SE = 11.89 ± 0.93 ng/mL, 95% CI: 9.81–13.96. Stress-induced corticosterone values were as follows: fumigated n = 15, mean ± SE = 58.54 ± 4.90 ng/mL, 95% CI: 48.03–69.05; sham-fumigated n = 15, mean ± SE = 57.22 ± 4.94 ng/mL, 95% CI: 46.62–67.81.
Fig. 2.
Mean plasma corticosterone levels (95% CI) in female medium ground finches from fumigated and sham-fumigated nests. Baseline measurements were taken within 3 min of capture; stress-induced measurements were taken 15 min after capture.
Body condition (estimated by SMI) did not differ significantly between females from fumigated (n = 14, mean ± SE = 21.8 ± 0.9 g, 95% CI: 19.9–23.7) and sham-fumigated nests (n = 12, mean ± SE = 23.3 ± 1.0 g, 95% CI: 21.1–25.5; two-tailed t-test, t = 1.15, df = 24, P = 0.26). Likewise, hematocrit did not differ significantly between treatments (fumigated: n = 13, mean ± SE = 47.08, 95% CI: 44.74–49.41 ± 1.10; sham-fumigated: n = 14, mean ± SE = 47.57, 95% CI: 45.78–49.36 ± 0.83; two-tailed t-test, t = 0.37, df = 25, P = 0.72). Across both treatments, baseline corticosterone did not correlate significantly either with body condition (Spearman, r = −0.20, P = 0.38) or hematocrit (r = −0.16, P = 0.49).
4. Discussion
To our knowledge, this is the first study to examine the stress response of Darwin’s finches in relation to parasites. We found that female finches are capable of a functional stress response, as indicated by significantly higher stress-induced corticosterone levels than baseline levels. We predicted that females at parasitized nests would have higher levels of baseline and stress-induced corticosterone than females at non-parasitized nests. However, there was no significant difference between parasite treatments, indicating that parasitism by P. downsi does not alter corticosterone levels in adult female finches, at least over the time interval of our study.
We also investigated whether P. downsi affects female body condition and hematocrit, which are known correlates of corticosterone response (Kitaysky et al., 1999; Schoech et al., 1997; Sockman and Schwabl, 2001). P. downsi had a significant negative effect on the reproductive success of birds in this study; see Koop et al. (2013) for details. However, neither the body condition nor hematocrit of adult females differed significantly between parasite treatments. These results suggest that female medium ground finches did not suffer direct adverse effects of parasitism by P. downsi.
There are several possible explanations for why we did not observe a relationship between parasitism and corticosterone. The simplest explanation is that P. downsi is not a significant stressor for adult female birds. While previous studies suggest that female finches are bitten by P. downsi (Huber et al., 2010; Koop et al., 2013), the frequency with which this occurs is unknown. Our study did not find a significant difference in hematocrit values between females sitting on parasitized and non-parasitized nests. This result suggests that females are not losing much blood to P. downsi larvae. Hence, there may be little stimulus for increased corticosterone levels.
It is important to note the short time frame of our study. P. downsi can cause nests to fail within a week of the eggs hatching (Koop et al., 2013). We therefore sampled adult female birds for blood relatively quickly (within 4–6 days of the eggs hatching). Since we did not sample females for blood after their nests had failed, we were unable to test whether nest failure itself is a stressor for female finches. Logan and Wingfield (1995) found significant increases in the corticosterone levels of female northern mockingbirds (Mimus polyglottos) during incubation of replacement clutches. It is possible that female medium ground finches show an increase in corticosterone levels if and when they re-nest. Further work is needed to determine whether females experiencing prolonged exposure to P. downsi exhibit a corresponding stress response.
It is also possible that significant changes in corticosterone in response to parasitism may be apparent only during “bad” years when birds experience other intense sources of stress. For example, Raouf et al. (2006) showed that adult cliff swallows parasitized by hematophagous swallow bugs have higher baseline corticosterone than non-parasitized birds; however, this was true only for birds nesting in large colonies, which are thought to increase levels of social stress. Cliff swallows in smaller colonies did not show higher levels of corticosterone, perhaps because of reduced competition for food. This result suggests that a combination of stressors can increase corticosterone levels. Our study took place in a year of high rainfall, during which birds were presumably able to find sufficient food and breed readily. Finches do not breed well in years of low rainfall and scarce food supplies (Koop et al., in press). Changes in corticosterone induced by P. downsi may be more apparent in dry years, when birds are under greater nutritional stress. It would be interesting to repeat our study in a dry year.
In summary, our results suggest that the impact of P. downsi on finch reproductive success is not mediated by a stress response in breeding females. Nestling finches may experience stress responses due to P. downsi parasitism; however, we were unable to test this hypothesis because most nestlings died before we could obtain adequate blood samples. Several studies have demonstrated increased levels of corticosterone in nestlings in response to ectoparasites in the nest (Eggert et al., 2010; Kitaysky et al., 2001; Raouf et al., 2006). Breeding adults can leave the nest, temporarily escaping parasites. Nestlings, by contrast, are essentially captive in the nest until they fledge. Thus, nest parasites may be a more intense and persistent stressor to nestlings, exacerbating negative fitness consequences.
Acknowledgments
We thank Galápagos National Park, the Charles Darwin Foundation, Priscilla Espin Vargas, Miriam Clayton, Lori Neuman-Lee, Leilani Lucus, and Michael Bingham for various forms of assistance. We also thank Fred Adler, Sarah Bush and three anonymous referees for comments that greatly improved the manuscript. The work was supported by NSF grant DEB-0816877 to DHC, the Society of Integrative and Comparative Biology (Grant-in-Aid of Research to SAK), the American Ornithologists’ Union (Research Award to SAK), and University of Utah Biology Department (Student Research Award to SAK). All procedures were approved by the University of Utah Institutional Animal Care and Use Committee (protocol #07-08004).
References
- Boughton RK, Atwell JW, Schoech SJ. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens) J. Parasitol. 2006;92:941–948. doi: 10.1645/GE-769R.1. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Hahn TP. Integrating stress physiology, environmental change, and behavior in free-living sparrows. Horm. Behav. 2003;43:115–123. doi: 10.1016/s0018-506x(02)00020-x. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Patterson SH, Hahn TP. In search of relationships between acute adrenocortical response and fitness. Gen. Comp. Endocrinol. 2008;157:288–295. doi: 10.1016/j.ygcen.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Brown CR, Brown MB, Raouf SA, Smith LC, Wingfield JC. Effects of endogenous steroid hormone levels on annual survival in Cliff Swallows. Ecology. 2005;86:1034–1046. [Google Scholar]
- Causton CE, Peck SB, Sinclair BJ, Roque-Albelo L, Hodgson CJ, Landry B. Alien insects: threats and implications for conservation of Galápagos Islands. Ann. Entomol. Soc. Am. 2006;99:121–143. [Google Scholar]
- Clayton DH, Tompkins DM. Ectoparasite virulence is linked to mode of transmission. Proc. R. Soc. 1994;256:211–217. doi: 10.1098/rspb.1994.0072. [DOI] [PubMed] [Google Scholar]
- Eggert LMF, Jodice PGR, O’Reilly KM. Stress response of brown pelican nestlings to ectoparasite infection. Gen. Comp. Endocrinol. 2010;166:33–38. doi: 10.1016/j.ygcen.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Fessl B, Couri MS, Tebbich S. Philornis downsi Dodge & Aitken, new to the Galapagos Islands, (Diptera, Muscidae) Studia Dipterologica. 2001;8:317–322. [Google Scholar]
- Fessl B, Sinclair BJ, Kleindorfer S. The life-cycle of Philornis downsi (Diptera: Muscidae) parasitizing Darwin’s finches and its impacts on nestling survival. Parasitology. 2006;133:739–747. doi: 10.1017/S0031182006001089. [DOI] [PubMed] [Google Scholar]
- Fessl B, Tebbich S. Philornis downsi – a recently discovered parasite on the Galápagos archipelago – a threat for Darwin’s finches? Ibis. 2002;144:445–451. [Google Scholar]
- French SS, DeNardo DF, Greives TJ, Strand CR, Demas GE. Human disturbance alters endocrine and immune responses in the Galapagos marine iguana (Amblyrhynchus cristatus) Horm. Behav. 2010;58:792–799. doi: 10.1016/j.yhbeh.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant PR. Ecology and Evolution of Darwin’s Finches. Princeton, NJ: Princeton University Press; 1999. [Google Scholar]
- Huber SK. Effects of the introduced parasite Philornis downsi on nestling growth and mortality in the medium ground finch (Geospiza fortis) Biol. Conserv. 2008;141:601–609. [Google Scholar]
- Huber SK, Owen JP, Koop JAH, King MO, Grant PR, Grant BR, Clayton DH. Ecoimmunity in Darwin’s finches: invasive parasites trigger acquired immunity in the medium ground finch (Geospiza fortis) PLoS One. 2010;5:e8605. doi: 10.1371/journal.pone.0008605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone CP, Reina RD, Lill A. Interpreting indices of physiological stress in free-living vertebrates. J. Comp. Physiol. B Biochem. System. Environ. Physiol. 2012;182:861–879. doi: 10.1007/s00360-012-0656-9. [DOI] [PubMed] [Google Scholar]
- Kitaysky AS, Wingfield JC, Piatt JF. Dynamics of food availability, body condition and physiological stress response in breeding black-legged kittiwakes. Funct. Ecol. 1999;13:577–584. [Google Scholar]
- Kitaysky AS, Wingfield JC, Piatt JF. Corticosterone facilitates begging and affects resource allocation in the black-legged kittiwake. Behav. Ecol. 2001;12:619–625. [Google Scholar]
- Koop JAH, Le Bohec C, Clayton DH. Dry year does not reduce invasive parasitic fly prevalence or abundance in Darwin’s finch nests. Ecol. Evol. in press. [Google Scholar]
- Koop JAH, Huber SK, Laverty SM, Clayton DH. Experimental demonstration of the fitness consequences of an introduced parasite of Darwin’s finches. PLoS One. 2011;6:e19706. doi: 10.1371/journal.pone.0019706. http://dx.doi.org/10.1371/journal.pone.0019706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop JAH, Owen JP, Knutie SA, Aguilar MA, Clayton DH. Experimental demonstration of a parasite-induced immune response in wild birds: Darwin’s finches and introduced nest flies. Ecol. Evol. 2013 doi: 10.1002/ece3.651. http://dx.doi.org/10.1002/ece3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CA, Wingfield JC. Hormonal correlates of breeding status, nest construction, and parental care in multiple-brooded northern mockingbirds, Mimus polyglottos. Horm. Behav. 1995;29:12–30. doi: 10.1006/hbeh.1995.1002. [DOI] [PubMed] [Google Scholar]
- Love OP, Breuner CW, Vezina F, Williams TD. Mediation of a corticosterone-induced reproductive conflict. Horm. Behav. 2004;46:59–65. doi: 10.1016/j.yhbeh.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Marshall AG. The Ecology of Ectoparasitic Insects. London: Academic Press Inc; 1981. [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Olaymeni FO. Haematological changes in Guinea fowls (Numida meleagris galeata, Pallas) following haemorrhage. Int. J. Poultry Sci. 2009;8:1093–1095. [Google Scholar]
- Palmer J, Klugman KP, Hattingh J. Haematological changes associated with haemorrhage in the pigeon. Comp. Biochem. Phys. A. 1979;63:587–589. [Google Scholar]
- Peig J, Green AJ. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos. 2009;118:1883–1891. [Google Scholar]
- Raouf SA, Smith LC, Brown MB, Wingfield JC, Brown CR. Glucocorticoid hormone levels increase with group size and parasite load in cliff swallows. Anim. Behav. 2006;71:39–48. [Google Scholar]
- Sapolsky RM, Romero M, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Mumme RL, Wingfield JC. Corticosterone, reproductive status, and body mass in a cooperative breeder, the Florida scrub-jay (Aphelocoma coerulescens) Physiol. Zool. 1997;70:68–73. doi: 10.1086/639545. [DOI] [PubMed] [Google Scholar]
- Siegel HS. Physiological stress in birds. Bioscience. 1980;30:529–534. [Google Scholar]
- Silverin B. Corticosterone-binding proteins and behavioural effects of high plasma levels of corticosterone during the breeding period in the pied flycatcher. Gen. Comp. Endocrinol. 1986;64:67–74. doi: 10.1016/0016-6480(86)90029-8. [DOI] [PubMed] [Google Scholar]
- Sockman KW, Schwabl H. Plasma corticosterone in nestling American kestrels: effects of age, handling stress, yolk androgens, and body condition. Gen. Comp. Endocrinol. 2001;122:205–212. doi: 10.1006/gcen.2001.7626. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J. Psychosomat. Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Kitaysky AS. Endocrine responses to unpredictable environmental events: stress or anti-stress hormones? Integr. Comp. Biol. 2002;42:600–609. doi: 10.1093/icb/42.3.600. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. Ecological bases of hormone–behavior interactions: the “emergency life history stage”. Am. Zool. 1998;38:191–206. [Google Scholar]
- Wingfield JC, Silverin B. Effects of corticosterone on territorial behavior of free-living male song sparrows Melospiza melodia. Horm. Behav. 1986;20:405–417. doi: 10.1016/0018-506x(86)90003-6. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Smith JP, Farner DS. Endocrine responses of white-crowned sparrows to environmental-stress. Condor. 1982;84:399–409. [Google Scholar]