Abstract
Hair follicles have characteristic sizes corresponding to their cycle specific stage. However, how the anagen hair follicle specifies its size remains elusive. Here, we show that in response to prolonged ectopic Wnt10b-mediated β-catenin activation, regenerating anagen hair follicles grow larger in size. In particular, the hair bulb, dermal papilla and hair shaft become enlarged. While the formation of different hair types (Guard, Awl, Auchene, and Zigzag) is unaffected. Interestingly, we found the effect of exogenous WNT10b was mainly on Zigzag and less on the other kinds of hairs. We observed dramatically enhanced proliferation within the matrix, DP and hair shaft of the enlarged AdWnt10b-treated hair follicles compared with those of normal hair follicles at P98. Furthermore, expression of CD34, a specific hair stem cell marker, was increased in its number to the bulge region after AdWnt10b treatment. Ectopic expression of CD34 throughout the ORS region was also observed. Many CD34 positive hair stem cells were actively proliferating in AdWnt10b-induced hair follicles. Importantly, subsequent co-treatment with the Wnt inhibitor, DKK1, reduced hair follicle enlargement, decreased proliferation and maintained proper hair stem cell localization. Moreover, injection of DKK1 during early anagen significantly reduced the width of prospective hairs. Together, these findings strongly suggest that a balance of Wnt10b/DKK1 governs reciprocal signaling between cutaneous epithelium and mesenchyme to regulate proper hair follicle size.
Keywords: Wnt10b, DKK1, hair follicle size, hair regeneration, hair stem cells
Introduction
Hair follicles display different shapes, lengths and thickness during anagen, catagen and telogen phases of the hair cycle (1, 2). Furthermore, in mouse dorsal skin, there are four distinct hair follicle types with different shapes and sizes that successively emerge during hair development. These include Guard (primary hair), Auchene and Awl (secondary hair) and Zigzag (tertiary hair) hairs (3). In general, primary and secondary hair follicles are larger than tertiary hair follicles during anagen (4).
Hair size is strictly controlled by reciprocal epithelial–mesenchymal interactions. Epithelial keratinocytes of the hair matrix supply the hair shaft progenitor cells, while the dermal papilla (DP) is the critical mesenchymal signaling center that regulates epithelial behavior (5). In both human and murine hair follicles, hair size correlates with DP cell numbers (4, 6, 7). A recent study showed that hairs can switch progressively from smaller to larger types during the hair cycle due to increased DP cell numbers (4).
At the molecular level, hair size can be regulated by multiple factors that influence epithelium and/or mesenchyme. Ectopic epidermal expression of the BMP antagonist, Noggin, causes a marked increase in anagen hair follicle size and changes Zigzag hairs to become larger, Awl-like hair follicles (8). Epidermal Eda/Edar is required for primary hair placode formation (9-12), while Sox2+ cell depletion resulted in a loss of primary and secondary hair induction (3). Knocking out Sox2 in the dermal papilla reduced the overall rate of hair growth (13). Sox18 deletion reduced Zigzag hair numbers (14, 15). FGF20 signaling activity also plays a role in primary and secondary hair induction by influencing dermal condensation formation (16). The deletion or up-regulation of each of these molecules not only results in changes of hair size but also hair types. To date it still remains unknown how the anagen VI hair follicle size is specified. We also don’t know whether hair follicles can change their size without changing their hair type in subsequent hair cycles. Finally, the molecular mechanisms underlying the regulation of hair size remains unknown.
Wnts and their DKK antagonists regulate the morphology of ectodermal organs (17-20). Here we evaluate how alteration of the balance between Wnt and DKK can modulate the size of hair follicles. Wnt10b is a canonical Wnt member (21) that is expressed during hair induction and hair reconstitution (22, 23). Recent studies have shown that adenovirus mediated Wnt10b expression (AdWnt10b) leads to epithelial and melanocyte cell differentiation and elongated hair shafts (24-26). In our previous studies, we reported that WNT10b over-expression resulted in growth of vibrissae in vitro and early induction of hair follicles in vivo (27-29). In the present study, we enhanced Wnt activation by multiple injections of Wnt10b-expressing adenoviruses and provide evidence that WNT10b plays an important role in enlarging hair follicle size during regeneration. Furthermore, we found that the enlargements of hair follicle size could be partially rescued by administering DKK1, a Wnt pathway inhibitor. A single dose of DKK1 treatment led hairs to decrease in width. Collectively, our data suggest that a balance of Wnt10b/DKK1 signaling mediates epithelial–mesenchymal interactions during hair regeneration. These findings shed new light on how external macroenvironmental signaling communicates with the hair follicle to specify organ size at the cellular and molecular levels.
Materials and methods
Mice
Animal maintenance and utilization were approved by the Third Military Medical University in China. Female C57BL/6J mice at 8 weeks of age, corresponding to the second telogen phase of the hair cycle (28), were used for the adenovirus injection study. Female C57BL/6J mice at postnatal day 98 were used as controls.
Adenovirus and plasmid
Adenoviruses including Adwnt10b and AdGFP (control) used in this study were a gift from Dr. T.C. He, University of Chicago, USA. The adenoviruses were propagated in HEK293 cells to a final titer of 1×108 according to the published protocol (30). Full length DKK1 CDS sequence was cloned into a pEGFP-N1 vector at Kpn I and Hind III restriction enzyme sites, with the following primers Sense: 5'-CCCAAGCTTATGATGGTTGTGTGTGCAGCGG-3', Antisense:-5' GGGGTACCTTGTGTCTCTGGCAGGTGTGGAGC-3'. pEGFP-N1 plasmid information and expression in skin after injection were presented in our previous studies (31, 32).
Intradermal injection of Adenovirus in vivo
For adenovirus injection, 40ul 1×108 AdWnt10b or AdGFP adenoviruses were injected intradermally once a week for four weeks (Schematic drawing in Fig. 1a and Fig. S1a). The diffusion and expression of adenoviruses were observed by detecting LacZ and GFP in the adenovirus injected area as previously described (28). In the present study, we also observed WNT10b was increased in hair bulb, DP and hair shaft (Fig. S2a). Adenovirus injection experiments were repeated at least 40 times, with a hair regeneration rate of about 85%.
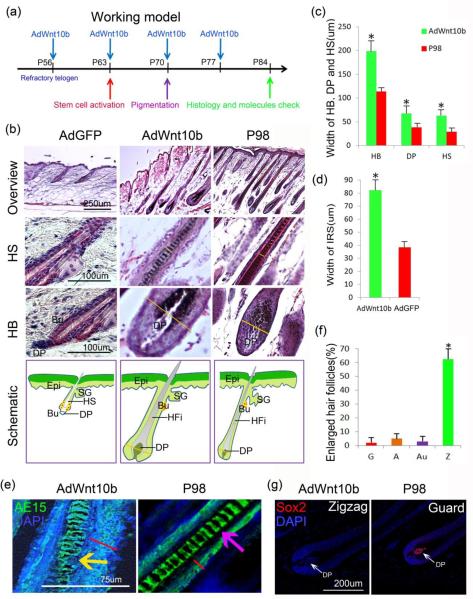
Figure 1.
AdWnt10b treatment enlarges hair follicle size without shifting its type. a. Schematic drawing showing the timing of multiple AdWnt10b injections, hair cycle events and check points. b-c. H&E staining and statistical chart showing that the width of HB, DP and HS was significantly increased after continuous AdWnt10b treatment. d-e. AE15 immunostaining and statistical chart revealed the IRS was broadened (Red line), and the hair fiber did not differentiate properly in the AdWnt10b-induced hair follicles (yellow arrow). f. About 65% of Zigzag hair follicles became enlarged, while only few Guard (G), Awl (A), Auchene (Au) hair follicles expanded in size. g. Wnt10b-induced larger Zigzag hair follicles were Sox2 negative while the normal Guard hairs were Sox2 positive. Epi, epidermis; SG, sebaceous gland; HFi, hair fiber; Bu, bulge; HB, hair bulb; HM, hair matrix; DP, dermal papilla; HS, hair shaft. *P < 0.05.
Transfection of naked plasmid in vivo
Naked plasmid was applied to the adenovirus treated area when hair follicles entered anagen (pigmentation appears; Schematic drawing in Fig. 3a and Fig. S4a) or later, during early anagen (P91, schematic drawing in Fig. 4a). 20ul DKK1 or pEGFP-N1 empty vector plasmid was injected at a concentration of 600 ug/ml (12ug total) to a 12.6 mm2 area in the center of the pigmented region (31, 32). Plasmid injection experiments were repeated five times. Authenticity of the naked plasmid intradermal injection was confirmed by PCR, immunostaining and direct fluorescence as described in our previous studies (31, 32). In the present study, most hair follicles (60.5±6.8%, n=100) in the plasmid injected skin were positive for the encoded GFP and DKK1 one week after DKK1 treatment (Fig. S4c). The AdWnt10b+DKK1 plasmid treated skin samples were harvested two weeks after plasmid injection (Fig. 3a). All hair follicles in the collected samples remained in anagen phase as evaluated by TUNEL staining (Fig. S1h and Fig. S4d). Skin samples were harvested one week after receiving a single DKK1 plasmid injection (Fig. 4a). The size of the central hair bulb and the middle hair shaft width were determined. Hair shaft length was measured from the epidermis to the tip of the hair bulb. BrdU diluted in PBS (100 mg/kg) was injected to the abdomen 4 hours before euthanasia.
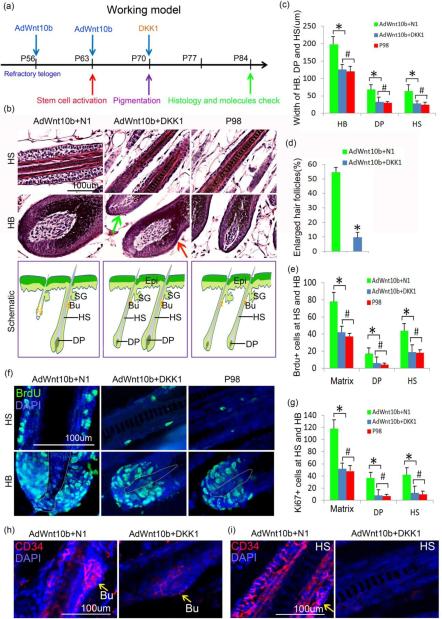
Figure 3.
Sequential AdWnt10b+DKK1 hair follicle treatment decreased Wnt/β-catenin pathway activation, reduced proliferation in the hair matrix, DP and hair shaft, but maintained the proper localization of hair stem cells. a. Schematic drawing showing the timing of two injections of AdWnt10b followed by DKK1 treatment, hair cycle events and check points. b-c. H&E staining and statistical chart presenting the significantly decreased width of HB, DP and HS after AdWnt10b-DKK1 treatment. The results were similar to those of the P98 normal hair follicles. d. The enlarged hair follicles were significantly reduced from 54.3±4.3 (%) in the AdWnt10b+N1-treated group to 9.8±3.8 (%) in the AdWnt10b+DKK1-treated group. Note, not all hair follicles decreased in size (Red and green arrows in b). e-g. Immunostaining and statistical chart revealed BrdU+ and Ki67+ proliferating cells were decreased in AdWnt10b+DKK1-treated hair follicles compared within the AdWnt10b+N1-treated hair follicles, especially in the hair matrix, DP and hair shaft. The proliferative index was recovered to the P98 normal state. h. Immunostaining for CD34 indicated that after AdWnt10b+DKK1 treatment, hair stem cells were only located in the bulge region of the regenerated hair follicles. i. CD34+ cells were widespread in the hair shaft, especially the ORS region of AdWnt10b+N1 while not in those of AdWnt10b+DKK1 treated group. N1, control plasmid; Epi, epidermis; SG, sebaceous gland; HB, hair bulb; HM, hair matrix; DP, dermal papilla; HS, hair shaft. *P < 0.05; # no statistical difference.
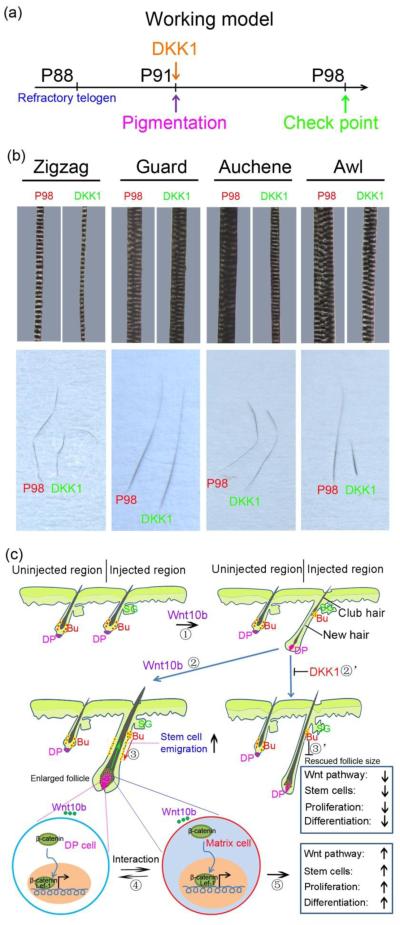
Figure 4.
DKK1 treatment decreased hair width. a. Schematic drawing showing the timing of DKK1 injection, hair follicle status and check points. b. DKK1 treatment narrowed the width of Zigzag, Auchene, and Awl hairs, and shortened the length of Awl hairs. c. Summary diagram showing that hair regeneration could be induced by ectopic WNT10b. Prolonged activation of Wnt signaling in the hair follicle would lead to greater interaction between the hair matrix epithelial cells and the DP mesenchymal cells, producing more proliferation and differentiation and broadening the HB, DP and HS. WNT10b could also promote migration of hair stem cells to sustain matrix proliferation. Interestingly, these processes can be rescued by giving DKK1 to inhibit regenerating hair follicles. Bu, bulge; DP, dermal papilla; HS, hair shaft.
Histology and immunofluorescence
Harvested samples were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin. Sections were cut at 5um and stained with hematoxylin and eosin (H&E) for 3min. The Adobe Photoshop CS3 ruler tool was used to analyze follicle width. For immunostaining, antigen retrieval was carried out by microwaving the tissues for 10 min in boiled citrate acid plus sodium citrate buffer. Then samples were incubated with primary antibodies against WNT10b (Goat, 1:100, Santa Cruz, CA, USA), β-catenin (Rabbit, Boster, Wuhan, China), Lef1 (Goat, 1:100, Santa Cruz, CA, USA), BrdU (mouse, 1:100, Sigma-Aldrich, St. Louis, MO, USA), Ki67 (mouse, 1:100, Sigma-Aldrich, St. Louis, MO, USA), AE15 (1:2, gift), CD34 (Rabbit, Boster, Wuhan, China), Sox2 (Goat, 1:100, R&D Systems, MN, USA), or β1-integrin (Rabbit, 1:100, Bioss, Beijing, China) overnight at 4°C and with Cy3-labeled fluorescent secondary antibodies (Beyotime, Nantong, China) for 2 hrs at 37°C. Sections were counterstained with DAPI (1:1000, Sigma-Aldrich, St. Louis, MO, USA). Fluorescence was checked by fluorescence microscopy (Nikon, Japan). The relative intensity ofβ1-integrin protein was measured using Image J.
Statistic analysis
40 hair follicles for each group of AdWnt10b, AdGFP, P98, AdWnt10b+DKK1 and AdWnt10b+N1 were analyzed. All experiments were repeated at least 3 times and the determinations were performed in triplicate. Statistical significance was determined using the Student’s t-test (SPSS 13.0, SPSS Inc.; P<0.05). Results are shown as the mean ± SD.
Results
Prolonged Wnt10b over-expression increased the size of regenerated hair follicles
To determine whether WNT10b regulates hair follicle size during hair regeneration, we subcutaneously injected AdWnt10b into dorsal skin of the mouse once a week beginning in refractory telogen at P56. The regenerated hairs reached their largest size approximate 4 weeks after the initiation of AdWnt10b treatments (P84; Schematic drawing in Fig. 1a and Fig. S1a), and resembled anagen VI hairs found in P98 control mice. H&E staining (Fig. 1b) showed the width of AdWnt10b-induced hair bulbs and hair shafts were remarkably increased compared to untreated controls (Fig. 1c). In contrast, skin treated with AdGFP remained at telogen phase at P84 (Fig. 1b). The width of medulla cells in the hair fiber was also significantly broadened (Fig. 1e and Fig. S1c). The DP of AdWnt10b-treated hair follicles were significantly enlarged compared to those of the P98 normal mouse hair follicles (Fig. 1b-c). AE15 immunostaining showed that the IRS of AdWnt10b-infected hair follicles was significantly thicker than that of control hair follicles (Fig. 1d-e). Furthermore, compared to the control follicle, where the ORS is composed of one or two cell layers, the ORS of AdWnt10b-infected hair follicles increased to three to five layers (Fig. S1b red line).
Next, we examined whether the larger regenerating follicles observed originated from primary or secondary hairs. H&E staining showed that there was only one column of medulla cells indicating that these were Zigzag hairs (Fig. S1b). Additionally, among the four types of regenerated hair follicles, Zigzag hairs were most affected by Wnt10b overexpression (Fig. S1d). About 65% of Zigzag hair follicles had a larger size in mice treated 4-weeks with AdWnt10b compared to controls (Fig. 1f), while the size of other hair types were less affected (Fig. 1f). Unaffected Zigzag hair follicles were comparable in size to normal P98 hair follicles (Fig. S1e). Staining for Sox2, a marker of primary hairs, revealed that the enlarged AdWnt10b treated hairs were not converted to primary hairs (Fig. 1g). Based on these observations, we focused on changes in the Zigzag hairs after WNT10b treatments. The lengths of hair follicles were mostly unchanged compared with those of control mice at P98 (Fig. S1f). And the overall shape of all hair types remained normal after continuous AdWnt10b treatment (Fig. S1g).
Aberrant and over-activation of Wnt signaling in AdWnt10b-induced hair follicles
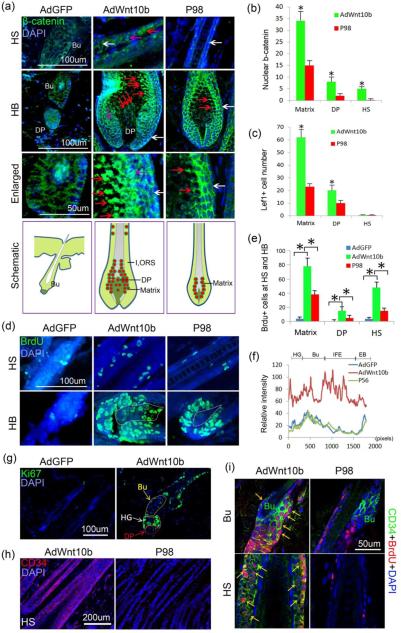
We next confirmed that 4 weeks of treatment with AdWnt10b induced ectopic Wnt10b signaling. Normally, WNT10b expression was observed in the hair matrix, with very weak expression in the ORS at P98. Prolonged treatment of the skin with ectopic AdWnt10b increased WNT10b expression levels significantly in the hair matrix, hair shaft, and DP (Fig. S2a). Nuclear β-catenin expression within the matrix was also elevated compared to control animals at P98 (Fig. 2a-b). Interestingly, β-catenin also accumulated in nuclei of the hair shaft and DP of WNT10b-induced hair follicles compared to controls (Fig. 2a-b). No nuclear β-catenin was detected in the AdGFP-treated hair follicles (Fig. 2a-b). Subsequently, we found that the number of Lef1 positive nuclei was dramatically increased in the matrix and DP of enlarged AdWnt10b-induced hair follicles, compared to those of P98 control animals (Fig. S2b and Fig. 2c). While in the hair follicle of the AdGFP-treated group, Lef1 was only expressed in the DP region (Fig. S2b).
Figure 2.
Wnt10b-induced hair follicles were accompanied by excessive activation of the Wnt/β-catenin pathway, increased proliferation, and hair stem cell activation and migration. a-b. Immunostaining and statistical chart showed nuclear β-catenin, a key mediator of the Wnt signaling pathway, was dramatically increased in the Matrix, HS and DP in the AdWnt10b-treated group compared within AdGFP and normal P98 hair follicle controls (Red arrow, nuclear β-catenin; pink arrow, cytoplasm β-catenin; white arrow, membrane β-catenin). c. Statistical chart of Lef1, a downstream Wnt signaling target, was also markedly augmented in the Matrix and DP region, but not in the HS. d-e. Immunostaining and statistical chart reveals BrdU-labeled proliferating cells were increased in Wnt10b-induced enlarged hair follicles. f. Statistical graph showing β1-integrin expression was significantly increased in the HG, Bu and IFE regions of AdWnt10b-treated hair follicles. g. Ki67+ proliferating cells were located in the bulge, second hair germ and DP region 6d after the first AdWnt10 treatment. h. CD34 was unexpectedly localized to the hair shaft, especially in the ORS region. i. Double staining displayed colocalization of CD34 and BrdU both in the bulge and hair shaft of AdWnt10b-induced hair follicles but not in those of normal control hair follicles. Bu, bulge; HB, hair bulb; DP, dermal papilla; HS, hair shaft; HG, second hair germ; IFE, interfollicular epidermis; EB, epidermal basal layer. *P < 0.05.
AdWnt10b induced hair follicle components show excessive proliferation
To investigate the mechanisms underlying enlargement of AdWnt10b-induced hair follicles, we examined proliferative activities of hair follicles using BrdU labeling. We observed that more BrdU labeled cells were located in the hair shaft (especially in the ORS), matrix and DP of AdWnt10b-treated mice (Fig. 2d-e). Ki67 staining confirmed that proliferation was increased. A statistically significant increase in nuclear Ki67 in the hair matrix, DP and hair shaft was observed in AdWnt10b-treated mouse hair follicles compared to those found in P98 control hair follicles (Fig. S3a-b). In contrast, AdGFP-treated hair follicles remained in telogen and contained very few BrdU+ or Ki67+ cells (Fig. S3a-b).
Increased and aberrant distribution cells with hair stem markers in AdWnt10b induced hair follicles
To examine progenitor cells that replenish the hyperproliferative hair follicles, we examined the expression of hair stem cell markers. Six days after the first AdWnt10b injection, expression of β1-integrin, a hair stem cell marker (33), was significantly enhanced in the bulge and the interfollicular epidermis compared to similar regions in the AdGFP treated group and normal telogen hair follicle (P56) (Fig. 2f and Fig. S3c). Moreover, CD34+ cells were also increased in the bulge region of the AdWnt10b-treated hair follicles compared with AdGFP-treated hair follicles (Fig. S3e-f). Interestingly, Ki67 immunostaining showed that the bulge, the DP and especially the second hair germ (HG) all displayed increased cell proliferation (Fig. 3g and Fig. S3d).
Surprisingly, CD34 expression was detected in the hair shaft of regenerated AdWnt10b-treated hair follicles from the bulge to the upper hair bulb, with particularly strong expression observed in the ORS (Fig. 2h). Double staining showed that CD34+/BrdU+ cells were colocalized both in the bulge and ORS of the regenerated AdWnt10b-treated hair follicles, compared to the P98 hair follicles which had very few cells that expressed both markers (Fig. 2i and Fig. S3g-h).
DKK1 decreased hair follicle sizes by blocking AdWnt10b induced activation of Wnt signaling
H&E staining showed that the size of most hair bulbs and hair shafts after DKK1 treatment were similar to those of control animals at P98 (Fig. 3b). Quantification of hair follicle widths showed a significant decrease in AdWnt10b+DKK1-treated animals compared to AdWnt10b+N1-treated animals (Fig. 3c). Moreover, the DP size of the AdWnt10b+DKK1-treated group decreased compared to the AdWnt10b+N1-treated group and was similar to that of P98 controls (Fig. 3c). However, AdWnt10b+DKK1-treated hair follicles were similar in length to those of P98 control animals (Fig. S4b). Additionally, the proportion of enlarged hair follicles was reduced in the AdWnt10b+DKK1-treated group compared to the AdWnt10b+N1-treated group (Fig. 3d).
We next explored whether DKK1 suppression of Wnt induced β-catenin expression blocked hair follicle enlargement after AdWnt10b treatment. Immunostaining revealed that numbers of cells with β-catenin positive nuclei in the AdWnt10b+N1-treated hair matrix and DP were greater than those found in either the AdWnt10b+DKK1-treated mice or P98 control mice. In addition, β-catenin expression was only associated with the membrane of the hair shaft cells of the AdWnt10b+DKK1-treated hair follicles (Fig. S5a, c). Moreover, Lef1 expression increased in animals treated with AdWnt10b+N1 compared to AdWnt10b+DKK1 treated or control P98 mice (Fig. S5b, d).
DKK1 treatment after AdWnt10b induction partially rescued the phenotypes
It is likely that the emaciated hair follicle phenotype observed following treatment with AdWnt10b+DKK1 was due to perturbed proliferation or differentiation. The AdWnt10b+N1 treated group displayed hyperproliferative hair matrix, DP and hair shaft cells (Fig. 3e-f). However, subsequent treatment with AdWnt10b+DKK1 restored proliferation to near control levels (Fig. 3e-f).
The levels of cell proliferation was also examined using Ki67 immunostaining and, consistent with the BrdU proliferation data, revealed that nuclear Ki67+ was dramatically decreased in the hair matrix, DP and hair shaft of AdWnt10b+DKK1 hair follicles to levels comparable to those of the P98 hair follicles (Fig. 3g and Fig. S6). Moreover, we found that proper hair differentiation events were also partially rescued by DKK1 overexpression (Fig. S7a-b).
It is possible that the lower proliferative activities of the hair bulb and hair shafts in response to AdWnt10b+DKK1 treatment, may be due to reduced migration of hair stem cells from the bulge to the matrix which act drive hair follicle regeneration. To explore this hypothesis, we examined the stem cell distribution and found CD34 was still strongly expressed in the bulge region of both AdWnt10b+N1 and AdWnt10b+DKK1 treated groups (Fig. 3h). However, there were few CD34+ cells in the hair shaft region of the AdWnt10b+DKK1-induced hair follicles, compared to the abundant CD34+ staining in the AdWnt10b+N1-induced hair follicles (especially in the ORS region) (Fig. 3i).
Decrease of hair widths after DKK1 treatment
To further test the influence of DKK1 on hair size regulation, we subcutaneously injected DKK1 at early anagen when the skin became pigmented and examined hair size one week later (Fig. 4a). Most types of hairs displayed decreased widths after DKK1 treatment. This was especially true for secondary hairs (Auchene and Awl) and tertiary hairs (Zigzag), whose hair shafts had fewer columns of medulla cells (Fig. 4b). Unexpectedly, we observed that Awl hairs were significantly shorter after DKK1 treatment; whereas, other hair types retained normal lengths that were comparable to hairs of control animals at P98 (Fig. 4b lower panels).
Discussion
Precise formation of organ size is regulated by complex biological processes requiring interactions that coordinate a response to autonomous factors and the extrinsic environment. The roles of multiple signals including Eda/Edar, BMP, FGF, Sox2/Sox18 in specifying hair type and size have been well characterized (3, 8, 12, 14-16). In the present study, we demonstrate that Wnt10b/DKK1 co-operation is involved in regulating anagen hair follicle sizes, including the hair bulb width, DP sizes as well as the overall thickness of hair shafts. Notably, these events occur without altering hair types.
Injecting up to four weekly doses of highly concentrated AdWnt10b into dorsal skin to continuously activate Wnt signaling significantly increased the size of regenerated hair follicles. It was recently reported that epidermal over-expression of Noggin inhibited BMP signaling and led to increased hair bulb size. This also resulted in the conversion of kinked Zigzag and Auchene hairs into straight Awl-like hairs (8). Another study reported that some Zigzag and Auchene hairs can transform to larger Awl hair types in the following hair cycle (4). Here, we found that after WNT10b treatment, the enlarged regenerated Zigzag hair follicles still maintained their hair types. Our data suggest that WNT10b solely regulates hair follicle size and does not alter the type of hairs that regenerate.
We also determined how WNT10b exerted its effect on hair follicle enlargement. Previous studies revealed that WNT10b was strongly expressed in the hair matrix region of anagen VI hair follicles, during the most robust growth period of the hair cycle (26, 28). WNT10b was also shown to promote growth of vibrissae in vitro (29). These studies indicate that WNT10b might facilitate hair follicle proliferation. Indeed, here we demonstrated that the enlarged hair follicles contained greater numbers of nuclear β-catenin, Lef1+, BrdU+ and Ki67+ cells. Taken together, these data suggest that WNT10b may have a proliferative effect on hair matrix and DP cells and that interactive signaling between extra epithelial and mesenchymal cells may later lead to the enlarged hair follicle phenotype. Furthermore, since the hair fiber and IRS are derived from hair keratinocyte precursors (34), differentiation of the increased matrix cell progenitor pool or their progeny may lead to a thickened hair shaft.
Hair matrix progenitor cells differentiate from hair stem cells during hair regeneration (35, 36). In the present study, we found aberrant localization of hyperproliferative hair stem cells during WNT10b induction. Therefore, WNT10b might stimulate hair stem cells to proliferate and migrate from the bulge region to replenish hair matrix cells.
DKK1 is a specific endogenous Wnt antagonist (37). When hair ORS or DP cells were cultured in nevus sebaceus sebocyte-conditioned media, DKK1 was increased but WNT10b was decreased (38). When we administered DKK1 after AdWnt10b, the number of enlarged anagen VI hair follicles was dramatically decreased, suggesting that a balance between WNT10b and DKK1 can regulate hair follicle size. Although DKK1 was reported to promote hair follicle regression (39), it is possible that an alternative mechanism may be used here. Furthermore, the absence of CD34+ bulge stem cells in the ORS region after DKK1 treatment, further confirmed that WNT10b not only affected cell proliferation and differentiation, but also promoted hair stem cell migration.
Notably, in our study, DP size correlated with hair follicle size. It was reported that epidermal activation of β-catenin results in ectopic hair formation associated with increased fibroblast proliferation (40). It was also shown that inactivation of β-catenin within the developing hair follicle DP leads to reduced proliferation (5). Therefore, it is possible that the WNT10b-induced canonical Wnt/β-catenin pathway could also directly promote proliferation and enlargement of the DP. In addition, WNT10b was reported to promote differentiation of mesenchymal cells toward myofibroblasts (41). However, WNT10b is not expressed in the skin dermal-lineage under normal conditions (26, 28). It may function by mediating epithelial – mesenchymal interactions but not by reprogramming DP cell properties that determines hair types. Furthermore, several lines of evidence suggest that the hair type could be identified by DP markers. Sox2 marks the DPs of the larger primary and secondary hair follicles, while Sox18 marks the DPs of the smaller tertiary Zigzag hair follicles. So we might speculate that different types of DP have different sensitivities to ectopic WNT10b, which led to the larger Zigzag hairs but not those of primary and secondary hair follicles. Exactly how the balance of the Wnt10b and DKK1 pair regulates the maintenance of signals in the DP remains unknown.
Taken together, our data provide compelling evidence that the Wnt10b-DKK1 interaction can orchestrate hair follicle sizes by regulating hair matrix, DP and hair stem cell behaviors, including cell proliferation, differentiation and migration (Fig. 4c). The reciprocal interactions between epithelial and mesenchymal cells influenced by Wnt10b and DKK1 may also specify hair follicle types. Furthermore, these data identify potential mechanisms controlling hair follicle miniaturization which may be utilized during aging or androgenetic alopecia and provide future directions to study the hair follicle response to external insults such as environmental pollution and radiation that target hair stem cells, hair matrix and DP cells.
Supplementary Material
Acknowledgements
This study was supported by grants 30972645, 11172338 and 11032012 from the National Nature Science Foundation of China and CSTC, Program for New Century Excellent Talents in University (NCET-10-0879), Innovation and Attracting Talents Program for College and University (‘111’ Project) (B06023), China. RW and CMC are supported by US NIH grant AR 42177 and AR 60306. We thank Dr. T.C. He (The University of Chicago) for the generous gifts of Wnt10b and control Adenoviruses. We thank Dr. Eve Kandyba (University of Southern California) and Dr. Lishi Li (The Rockefeller University) for carefully revising the manuscript.
Footnotes
Conflict of interests
The authors have declared no conflict of interest.
Reference
- 1.Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 2.Plikus MV, Mayer JA, de la Cruz D, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–2823. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi W, Wu E, Morgan BA. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development. 2013;140:1676–1683. doi: 10.1242/dev.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott K, Stephenson TJ, Messenger AG. Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: implications for the control of hair follicle size and androgen responses. J Invest Dermatol. 1999;113:873–877. doi: 10.1046/j.1523-1747.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 7.Van Scott EJ, Ekel TM. Geometric relationships between the matrix of the hair bulb and its dermal papilla in normal and alopecic scalp. The Journal of investigative dermatology. 1958;31:281–287. doi: 10.1038/jid.1958.121. [DOI] [PubMed] [Google Scholar]
- 8.Sharov AA, Sharova TY, Mardaryev AN, et al. Bone morphogenetic protein signaling regulates the size of hair follicles and modulates the expression of cell cycle-associated genes. Proc Natl Acad Sci U S A. 2006;103:18166–18171. doi: 10.1073/pnas.0608899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui CY, Kunisada M, Piao Y, Childress V, Ko MS, Schlessinger D. Dkk4 and Eda regulate distinctive developmental mechanisms for subtypes of mouse hair. PLoS One. 2010;5:e10009. doi: 10.1371/journal.pone.0010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fliniaux I, Mikkola ML, Lefebvre S, Thesleff I. Identification of dkk4 as a target of Eda-A1/Edar pathway reveals an unexpected role of ectodysplasin as inhibitor of Wnt signalling in ectodermal placodes. Dev Biol. 2008;320:60–71. doi: 10.1016/j.ydbio.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Tomann P, Andl T, et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Dev Cell. 2009;17:49–61. doi: 10.1016/j.devcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mou C, Jackson B, Schneider P, Overbeek PA, Headon DJ. Generation of the primary hair follicle pattern. Proc Natl Acad Sci U S A. 2006;103:9075–9080. doi: 10.1073/pnas.0600825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clavel C, Grisanti L, Zemla R, et al. Sox2 in the dermal papilla niche controls hair growth by fine-tuning BMP signaling in differentiating hair shaft progenitors. Dev Cell. 2012;23:981–994. doi: 10.1016/j.devcel.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James K, Hosking B, Gardner J, Muscat GE, Koopman P. Sox18 mutations in the ragged mouse alleles ragged-like and opossum. Genesis. 2003;36:1–6. doi: 10.1002/gene.10190. [DOI] [PubMed] [Google Scholar]
- 15.Pennisi D, Gardner J, Chambers D, et al. Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nat Genet. 2000;24:434–437. doi: 10.1038/74301. [DOI] [PubMed] [Google Scholar]
- 16.Huh SH, Narhi K, Lindfors PH, et al. Fgf20 governs formation of primary and secondary dermal condensations in developing hair follicles. Genes Dev. 2013;27:450–458. doi: 10.1101/gad.198945.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chodankar R, Chang CH, Yue Z, et al. Shift of localized growth zones contributes to skin appendage morphogenesis: role of the Wnt/beta-catenin pathway. J Invest Dermatol. 2003;120:20–26. doi: 10.1046/j.1523-1747.2003.12008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haara O, Fujimori S, Schmidt-Ullrich R, Hartmann C, Thesleff I, Mikkola ML. Ectodysplasin and Wnt pathways are required for salivary gland branching morphogenesis. Development. 2011;138:2681–2691. doi: 10.1242/dev.057711. [DOI] [PubMed] [Google Scholar]
- 19.Li A, Chen M, Jiang TX, et al. Shaping organs by a wingless-int/Notch/nonmuscle myosin module which orients feather bud elongation. Proc Natl Acad Sci U S A. 2013;110:E1452–1461. doi: 10.1073/pnas.1219813110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukhopadhyay M, Gorivodsky M, Shtrom S, et al. Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development. 2006;133:2149–2154. doi: 10.1242/dev.02381. [DOI] [PubMed] [Google Scholar]
- 21.Siar CH, Nagatsuka H, Han PP, et al. Differential expression of canonical and non-canonical Wnt ligands in ameloblastoma. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2012;41:332–339. doi: 10.1111/j.1600-0714.2011.01104.x. [DOI] [PubMed] [Google Scholar]
- 22.Reddy S, Andl T, Bagasra A, et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 23.Sriwiriyanont P, Lynch KA, Maier EA, Hahn JM, Supp DM, Boyce ST. Morphogenesis of chimeric hair follicles in engineered skin substitutes with human keratinocytes and murine dermal papilla cells. Exp Dermatol. 2012;21:783–785. doi: 10.1111/exd.12003. [DOI] [PubMed] [Google Scholar]
- 24.Ouji Y, Yoshikawa M, Moriya K, Ishizaka S. Effects of Wnt-10b on hair shaft growth in hair follicle cultures. Biochem Biophys Res Commun. 2007;359:516–522. doi: 10.1016/j.bbrc.2007.05.135. [DOI] [PubMed] [Google Scholar]
- 25.Ouji Y, Yoshikawa M, Moriya K, Nishiofuku M, Matsuda R, Ishizaka S. Wnt-10b, uniquely among Wnts, promotes epithelial differentiation and shaft growth. Biochem Biophys Res Commun. 2008;367:299–304. doi: 10.1016/j.bbrc.2007.12.091. [DOI] [PubMed] [Google Scholar]
- 26.Ye J, Yang T, Guo H, et al. Wnt10b promotes differentiation of mouse hair follicle melanocytes. Int J Med Sci. 2013;10:691–698. doi: 10.7150/ijms.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei MX, Chuong CM, Widelitz RB. Tuning Wnt signals for more or fewer hairs. J Invest Dermatol. 2013;133:7–9. doi: 10.1038/jid.2012.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li YH, Zhang K, Yang K, et al. Adenovirus-mediated Wnt10b overexpression induces hair follicle regeneration. J Invest Dermatol. 2013;133:42–48. doi: 10.1038/jid.2012.235. [DOI] [PubMed] [Google Scholar]
- 29.Li YH, Zhang K, Ye JX, Lian XH, Yang T. Wnt10b promotes growth of hair follicles via a canonical Wnt signalling pathway. Clinical and experimental dermatology. 2011;36:534–540. doi: 10.1111/j.1365-2230.2011.04019.x. [DOI] [PubMed] [Google Scholar]
- 30.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lei M, Bai X, Yang T, et al. Gsdma3 is a new factor needed for TNF-alpha-mediated apoptosis signal pathway in mouse skin keratinocytes. Histochemistry and cell biology. 2012;138:385–396. doi: 10.1007/s00418-012-0960-1. [DOI] [PubMed] [Google Scholar]
- 32.Lei M, Gao X, Yang L, Yang T, Lian X. Gsdma3 gene is needed for the induction of apoptosis-driven catagen during mouse hair follicle cycle. Histochemistry and cell biology. 2011;136:335–343. doi: 10.1007/s00418-011-0845-8. [DOI] [PubMed] [Google Scholar]
- 33.Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai J, Lee J, Kopan R, Ma L. Genetic interplays between Msx2 and Foxn1 are required for Notch1 expression and hair shaft differentiation. Developmental biology. 2009;326:420–430. doi: 10.1016/j.ydbio.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Jiang TX, Hughes MW, et al. Progressive alopecia reveals decreasing stem cell activation probability during aging of mice with epidermal deletion of DNA methyltransferase 1. J Invest Dermatol. 2012;132:2681–2690. doi: 10.1038/jid.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong Y, Li W, Shang C, et al. Brg1 governs a positive feedback circuit in the hair follicle for tissue regeneration and repair. Dev Cell. 2013;25:169–181. doi: 10.1016/j.devcel.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- 38.Lee WJ, Cha HW, Lim HJ, Lee SJ, Kim do W. The effect of sebocytes cultured from nevus sebaceus on hair growth. Exp Dermatol. 2012;21:796–798. doi: 10.1111/j.1600-0625.2012.01572.x. [DOI] [PubMed] [Google Scholar]
- 39.Kwack MH, Kim MK, Kim JC, Sung YK. Dickkopf 1 promotes regression of hair follicles. The Journal of investigative dermatology. 2012;132:1554–1560. doi: 10.1038/jid.2012.24. [DOI] [PubMed] [Google Scholar]
- 40.Collins CA, Kretzschmar K, Watt FM. Reprogramming adult dermis to a neonatal state through epidermal activation of beta-catenin. Development. 2011;138:5189–5199. doi: 10.1242/dev.064592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei J, Melichian D, Komura K, et al. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis and rheumatism. 2011;63:1707–1717. doi: 10.1002/art.30312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.