Abstract
Context
Chemotherapy-induced peripheral neuropathy (CIPN) is a major dose-limiting and persistent consequence of numerous classes of antineoplastic agents, affecting up to 30%–40% of patients. To date, there is no effective prevention or therapy. An evolving hypothesis for reducing CIPN pain involves direct nerve stimulation to reduce the pain impulse.
Objectives
To evaluate the impact on CIPN associated with the MC5-A Calmare® therapy device.
Methods
The MC5-A Calmare® therapy device is designed to generate a patient-specific cutaneous electrostimulation to reduce the abnormal pain intensity. Sixteen patients from one center received one-hour interventions daily over 10 working days.
Results
Of 18 patients, 16 were evaluable. The mean age of the patients was 58.6 years—four men and 14 women—and the duration of CIPN was three months to eight years. The most common drugs were taxanes, platinums, and bortezomib (Velcade, Millenium Pharmaceuticals, Cambridge MA). At the end of the study (Day 10), a 20% reduction in numeric pain scores was achieved in 15 of 16 patients. The pain score fell 59% from 5.81 ± 1.11 before treatment to 2.38 ± 1.82 at the end of 10 days (P < 0.0001 by paired t-test). A daily treatment benefit was seen with a strong statistically significant difference between the preand post-daily pain scores (P < 0.001). Four patients had their CIPN reduced to zero. A repeated-measures analysis using the scores from all 10 days confirmed these results. No toxicity was seen. Some responses have been durable without maintenance.
Conclusion
Patient-specific cutaneous electrostimulation with the MC5-A Calmare® device appears to dramatically reduce pain in refractory CIPN patients with no toxicity. Further studies are underway to define the benefit, mechanisms of action, and optimal schedule.
Keywords: Pain, neuropathy, chemotherapy-induced peripheral neuropathy, neurocutaneous stimulation, taxanes, pain relief
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a major, often dose-limiting and persistent consequence of numerous classes of antineoplastic agents.1 The list includes taxanes; platinums; vinca alkaloids; and newer agents, including the proteosome inhibitor bortezomib and epithelones, such as ixabepilone.2 Although the incidence of CIPN varies with the drugs and schedules used, it commonly occurs in 30%–40% of patients. Classic stocking-and-glove pain is the predominant symptom and often persists for months to years after treatment.3
The mechanisms responsible for CIPN appear to be primarily a rapid direct damage to the nerve microtubules.4 To date, there are no effective neuroprotective agents to be given before or during chemotherapy. Although a long list of drugs and combinations have been assessed for treating CIPN, there is no standard effective therapy for CIPN.1 Tricyclic antidepressants,5,6 lamotrigine7 and gabapentin8 have been shown to have no or minimal benefit in reducing CIPN pain in randomized placebo-controlled trials. Magnesium and calcium infusions may have some protective effect in oxaliplatin-induced neuropathy9 but have no effect in other CIPNs. Topical baclofenamitriptyline-ketamine (BAK) gel reduced CIPN pain but not consistently or completely.10
An evolving hypothesis for reducing chronic pain involves direct nerve stimulation to reduce the pain impulse.11 Spinal cord stimulation has been successful in small nonrandomized series12,13 for chronic pain from reflex sympathetic dystrophy14 and postherpetic neuropathy15 but has not been assessed in CIPN. Acupuncture was effective for CIPN in one small nonrandomized series16 and is being actively tested. The hypothesized mechanisms by which nerve stimulation reduces pain include raising the “gate” threshold for pain at the spinal cord, reducing “wind up” (central sensitization of the spinal cord and brain that amplifies the abnormal feelings), reducing impulses from the damaged nerve, and allowing remodeling.
An electrocutaneous nerve stimulation device (MC5-A Calmare®, Competitive Technologies, Inc. Fairfield, CT) has shown some efficacy in relieving refractory chronic pain and was recently approved by the Food and Drug Administration (FDA). In the first trial, 11 cancer patients (three pancreas, four colon, and four gastric cancers) suffering from drug-resistant visceral pain were studied during 10 daily treatment sessions.17 Pain was quickly and markedly reduced from 8.6 out of 10 before the first treatment to 2.3 out of 10 after the first treatment and to less than 0.5 out of 10 at the end of 10 sessions (P < 0.0001 by paired t-test). Nine of 11 patients stopped pain drugs within the first five applications. No side effects were observed. These pain reductions continued until death; if the pain recurred later, it could be successfully retreated. In the second trial, 226 patients with neuropathic pain, including failed back surgery, brachial plexus neuropathy, and others, were treated.18 Eighty percent of patients responded with greater than 50% pain relief, 10% responded with pain relief from 25% to 49%, and 10% had no response (P < 0.0001 by paired t-test). No toxicities were noted. In the third trial (Marineo et al., unpublished data), 52 patients with chronic neuropathic pain were randomized to treatment with the MC5-A Calmare® device or treatment by the same expert group following the standard pharmacology guidelines.19 The patients had postsurgical, postherpetic, or narrow canal neuropathic syndromes. The mean pain intensity score at outset was 8.1 out of 10. At one month, the MC5-A Calmare® group had a decreased pain of 5.8 points (−91%), and the standard therapy group had a decreased pain of 0.7 points (−28%). After two and three months, the mean values were 1.4 and 2.0 in the MC5-A Calmare® group and 5.7 and 5.9 in the standard therapy group, respectively. Pain drug consumption decreased by 72% in the MC5-A Calmare® group, including opioids, antidepressants, and anticonvulsants, but not in the guideline group. Allodynia, or pain on touching of the skin, was reduced from 77% to 15% in the MC5-A Calmare® group at three months but not in the guideline group. All differences were both clinically and statistically significant. Again, no toxicities were observed.
Given the dramatic pain relief obtained with no toxicity in a variety of pain syndromes and the dearth of effective therapies for CIPN, we sought to evaluate this electrocutaneous nerve stimulation device for CIPN. The objective of this pilot study was to determine if MC5-A Calmare® therapy decreased CIPN enough to justify larger randomized trials.
Methods
Recruitment, Inclusion, and Exclusion Criteria
Patients were recruited from our clinical oncology practice by physician referral and by advertisements posted in the waiting room. The inclusion and exclusion criteria are shown in Table 1.
Table 1.
Protocol Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age ≥ 18 years | Ongoing or <4 weeks since receiving potentially neurotoxic chemotherapy |
| Life expectancy ≥3 months | Other known causes of peripheral neuropathy before chemotherapy (e.g., diabetes, cervical or lumbar radiculopathy, HIV, amyloidosis, hypothyroidism) |
| ECOG Performance Status, 0–2 | Known brain or spinal metastases |
| Summary status of their cancer as stable or no evidence of disease for ≥1 month | Use of another investigational pain-directed therapy within 30 days |
| Peripheral pain for ≥1 month attributed to CIPN | Prior interventional actions for pain control, including celiac plexus blocks and an implanted drug delivery system (such as Medtronic Synchromed Medtronic, Inc., Fridley, MN) |
| Average daily pain score ≥5 of 10 | Any form of medical “metal” device (e.g., pacemakers, defibrillators, vascular clips or stents, cardiac valve, or joint replacements) |
| Prior treatment (>4 weeks) with paclitaxel or docetaxel, carboplatin, cis-platinum, oxaliplatin, vincristine, vinblastine, vinorelbine, or bortezomib | An adverse reaction to past use of a TENS unit |
| Women who were pregnant, nursing, or using active contraceptive | |
| Active coronary artery disease within last 6 months | |
| History of seizures | |
| Skin conditions preventing application of the electrodes | |
| Any other medical condition at the investigator discretion felt to compromise the study’s objectives |
ECOG = Eastern Cooperative Oncology Group; HIV = human immunodeficiency virus.
MC5-A Calmare® Therapy
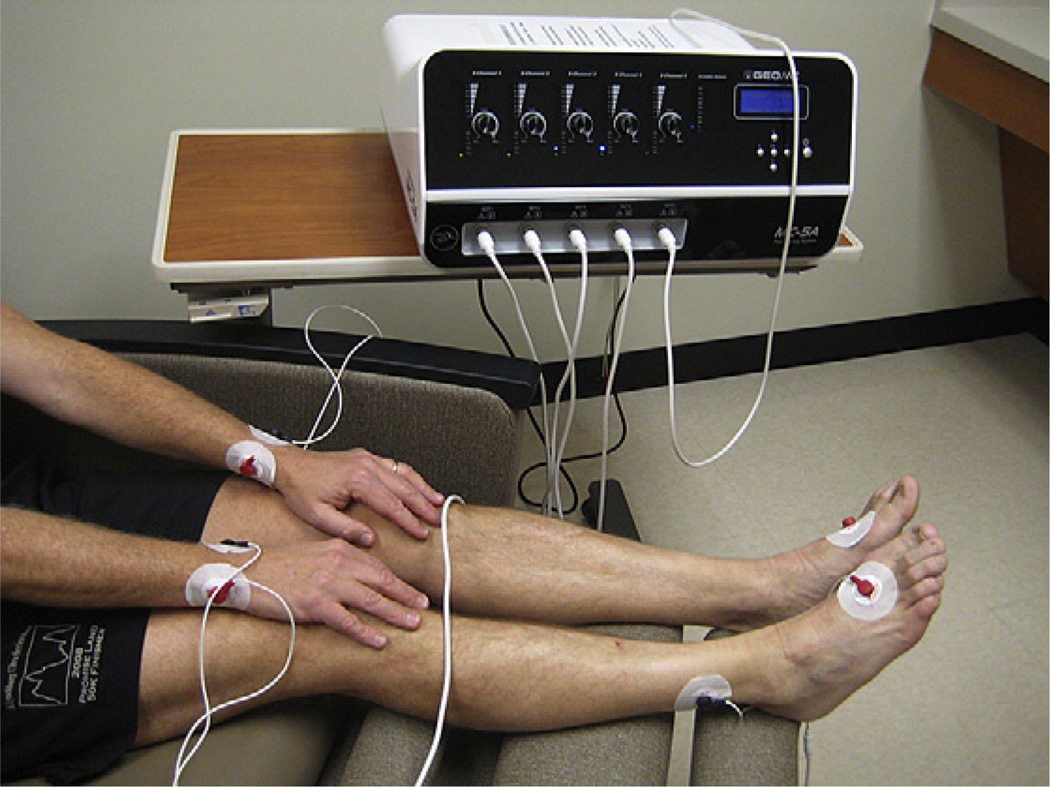
The goal of the MC5-A Calmare® device is to provide “nonpain” information to the cutaneous nerves to block the effect of pain information. The device consists of a multiprocessor apparatus able to simulate five artificial neurons by the application of surface electrodes on skin overlying the painful areas. It synthesizes 16 different types of nerve action potentials similar to the endogenous kinds and strings them into sequences. The device induces a transdermal modulation of pain responses by transmitting low-frequency stimulationto the patient’s nerves using surface electrodes in tandem at each patient’s specific pain areas (http://calmare.competitivetech.net/overview.html). Proprietary software uses algorithms to determine a patient-specific cutaneous electrostimulation to reduce the abnormal pain intensity. The device was approved by the U.S. FDA in February 2009. Figure 1 shows the device attached to a person to treat hand and foot CIPN.
Fig. 1.
The MC5-A Calmare® device positioned to treat stocking-glove neuropathy.
Application of the MC5-A Calmare® Therapy Device
The MC5-A Calmare® device was applied according to manufacturer directions. Briefly, the area of CIPN was determined and assigned as closely as possible to dermatomes using a standard map. Next, electrodes similar to electrocardiogram gel pads were applied on the skin beyond the pain-affected area or on the most pain-free distal area. The opposing gel electrode was placed above the painful area, within the same dermatome. Five channels or sets of electrodes were used, as the device has five channels.
On Day 1, the treatment intensity was increased every 10 minutes to the maximum intensity individually bearable by the patient without pain or discomfort, similar to a mild bee sting. The treatment continued for a total of 60 minutes. Subsequent treatments began at the highest intensity tolerated at the previous treatment. Treatments were given daily for 10 days in a row, excluding weekends; no maintenance therapy was given. If, after three consecutive treatments, the patient did not experience any improvement, treatment was discontinued, and the treatment was considered a failure.
The electrical stimulation used in MC5-A Calmare® therapy is low, and the FDA has approved it as safe. The current is regulated, and there are “shutoffs” automatically with power overloads. At the highest setting, “70” on the dial from 10 to 70, the amperage (A) is 3.50–5.50 mA, with a voltage range of 6.5–12.5 V. The maximum current density is 0.0002009 W/cm2. Because so many other features of the MC5-A Calmare® device are different from transcutaneous electrical nerve stimulation (TENS) or other electrostimulation devices, the current value related to the charge phase is the only value that is comparable. The current value is the primary safety criterion for the FDA. An evaluation must consider all the possible combinations of different MC5-A Calmare® nonlinear waveforms, normalized to the root mean square value (the true voltage of a waveform that is variable and not steady), to compare these emissions with a typical square waveform such as that used in TENS. The average charge for “charge per phase” is 38.8 µC. This value is similar to conventional TENS devices approved by the FDA and in routine practice. The phase duration is 6.8–10.9 milliseconds, and the pulse rate is 43–52 Hz. Because the frequency of the device never exceeds 52 Hz, the mean energy delivered per second is generally less than most standard TENS devices that operate a square wave with the possibility of using frequencies greater than 52 Hz. Recent studies with TENS units have used a continuous pulse pattern, pulse width of 200 microseconds, a pulse frequency of 80 Hz, increased until the patient feels a strong sensation,20 but there is no direct comparison with MC5-A Calmare® therapy. The small amount of stimulation time (maximum of 50 minutes in a 24-hour period) and intermittent duty cycle fit with current suggestions for safety in implanted electrodes,21 but there are no similar standards for peripheral cutaneous stimulations.
Study Endpoints and Objectives
The primary objective was to determine if MC5-A Calmare® reduced CIPN pain in cancer patients by 20%. This reduction in pain score was based on the threshold used in the Cancer Pain Trial22 as important to focus groups of oncologists. Secondary objectives included 1) the use of different measurement scales for quantifying CIPN and their reproducibility in measuring neuropathic pain, because there is no “gold standard;”23 2) the impact on overall quality of life using the Uniscale;24 3) the change in use of pain drugs associated with therapy; 4) toxicity (measured by the Cancer Therapy Evaluation Program v. 4.0 standard toxicities scale [http://ctep.cancer.gov/reporting/ctc.html.]); and 5) confirm no worsening of the overall symptoms using the North Central Cancer Treatment Group Symptom Experience Diary, provided by Dr. Charles Loprinzi.
Patients were followed from the time of accrual to the end of three months, as stated in the protocol. Data were collected at entry (Day 1); the end of Weeks 1 and 2 of treatment; and Weeks 4, 8, and 12.
Analysis Plan
The primary endpoint was a minimum 20% reduction in the numeric pain score on a 0–10 scale. Sample size was determined by an anticipated effect size of 20%, for example, a 20% reduction in pain score from Day 1 to Day 10; 15 patients would be required (giving 5% alpha and 80% power) to detect a difference of 1.6 starting at 8 out of 10, assuming a standard deviation (SD) of 2. Participants were asked to score their pain before and after the treatment on each day. We chose pain “right now” (identical to Question 5 on the Brief Pain Inventory) to give the best index of the pain relief attributable to the device and least likely to be influenced by other factors, such as opioid timing, drug peaks and valleys, and so on.
In the statistical analysis, three null hypotheses were tested: first, there would be no difference between the mean pre- and post-treatment pain scores; second, the pain scores would remain constant over time; and third, the rate of decrease, if any, would be similar in the pre- and post-treatment groups. A repeated-measures, random-effects analysis of variance was performed to test these hypotheses. This analysis accounted for the correlations among the subjects over the 10 days using an autoregressive structure; this assumes that the correlations of the scores between days that are closer together are higher and become smaller as the number of days in between increases. The study was approved by the Massey Cancer Center Protocol Review and Monitoring System, Virginia Commonwealth University Investigation Review Board, and registered at clinicaltrials.gov (MCV-MCC-12110, NCT00952 848).
Results
A total of 18 patients were enrolled between July 2009 and October 2009. Table 2 lists their respective age, gender, CIPN chemotherapies, duration of CIPN symptoms, and preceding pain-directed therapies. The average age was 58.6 ± 10.4 years; four were men and 14 were women; 10 were Caucasians, six African Americans, one Native American, and one other. The unadjusted mean “pain now” score before treatment was 5.81 (SD 1.11). The predominant past CIPN-associated treatment was taxane or bortezomib administration. All had stable CIPN for at least three months before the trial. Two patients were excluded from the final analysis, as specified in the protocol: one for progressive disease requiring chemotherapy before she started MC5-A Calmare® therapy and another who had transportation problems and could not receive three consecutive treatments. Of the 18, 16 were evaluable as prespecified in the protocol and had full data sets for the 10 days and subsequent follow-up. One patient (number 16 in Table 2) had a seizure and was diagnosed with brain metastases after Day 6 of treatment; hence, she stopped treatment as specified in the protocol, although she wanted to continue because her pain was reduced by half and her sensation and walking were markedly improved. Her last value for pain (3 out of 10) was carried forward on Days 7, 8, 9, and 10 as specified in the protocol.
Table 2.
Patient Characteristics
| UPN | Age, Sex | Disease | CIPN Drugs | Symptom Duration (Months) |
Prior Treatments for CIPN |
|---|---|---|---|---|---|
| 1 | 59, F | Lung | Carboplatin, paclitaxel | 3 | Opioids, duloxetine |
| 2a | 42, F | Breast | Docetaxel | 12 | ADs, others |
| 3a | 76, F | Lymphoma | Cyclophosphamide, procarbazine | 24 | Gabapentin |
| 4 | 54, F | Breast | Paclitaxel, docetaxel | 78 | Gabapentin, opioids, pregabalin, others |
| 5 | 46, M | Myeloma | Thalidomide | 48 | Opioids, ADs |
| 6 | 56, M | Colon | Oxaliplatin, fluorouracil, leucovorin | 9 | Magnesium, opioids, ADs |
| 7 | 60, F | Breast | Adriamycin, paclitaxel | 30 | Opioids, ADs, pregabalin |
| 8 | 76, F | Lymphoma | Flavopiradol, bortezomib | 6 | Opioids, ADs |
| 9 | 67, F | Colon | Oxaliplatin | 24 | Opioids, ADs, pregabalin, gabapentin |
| 10 | 56, F | Breast | Taxanes | 48 | Opioids, ADs, gabapentin |
| 11 | 60, F | Breast | Taxanes | 96 | Opioids, carbamazepine |
| 12 | 53, M | Colon | FOLFOX + bevacizumab | 30 | Opioids, gabapentin, carbamazepine, duloxetine |
| 13 | 52, F | Hodgkins | MOPP | 5 | Opioids, duloxetine, carbamazepine |
| 14 | 62, F | Myeloma | Bortezomib + flavopiradol | 48 | Opioids, gabapentin, carbamazepine, duloxetine |
| 15 | 63, F | Breast | Docetaxel | 5 | Gabapentin |
| 16 | 75, F | Breast | Paclitaxel | 4 | Gabapentin |
| 17 | 40, M | Colon | FOLFOX | 3 | ADs, tramadol, venlafaxine |
| 18 | 59, F | Breast | Paclitaxel | 24 | Gabapentin |
UPN = unique patient number; FOLFOX = fluorouracil, oxaliplatin, leucovorin; MOPP = mechlorethamine, vincristine, prednisone, procarbazine; ADs = antidepressants.
Not evaluable patients. Patient no. 2 was never treated because of rapidly progressive disease immediately after enrollment. Patient no. 3 only completed two treatments because of transportation problems.
Technically, all patients were able to define the peripheral nerve distribution of maximal pain. Nearly all patients achieved settings of 50–70 on the MC5-A Calmare® device, near the maximum that is potentially delivered. This does not correlate with voltage or amperage but with the intensity of the signal, as described in the Methods section earlier.
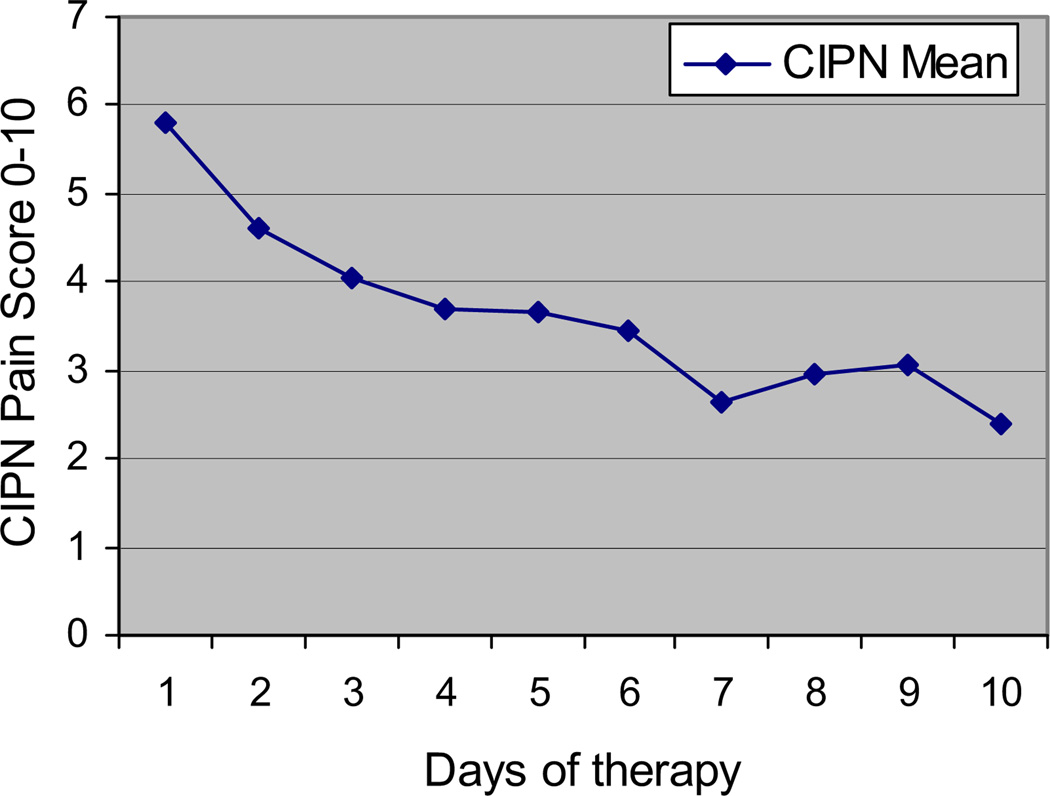
The primary endpoint, a reduction in numeric rating scale (NRS) pain of 20% by the end of the study, was met by 15 of the 16 (94%) evaluable patients: P < 0.0001 by Fischer’s exact test. The data showed a reduction in the pain score on each day and a decreasing trend in the pain over the 10 days after the start of the treatment. The observed score fell 59% from 5.81 ± 1.11 before starting treatment to 2.38 ± 1.82 at the end of 10 days (P < 0.0001 by paired t-test), as shown in Fig. 2.
Fig. 2.
Effect of MC5-A Calmare® therapy on pain scores during treatment. P < 0.0001 by paired t-test, two-tailed.
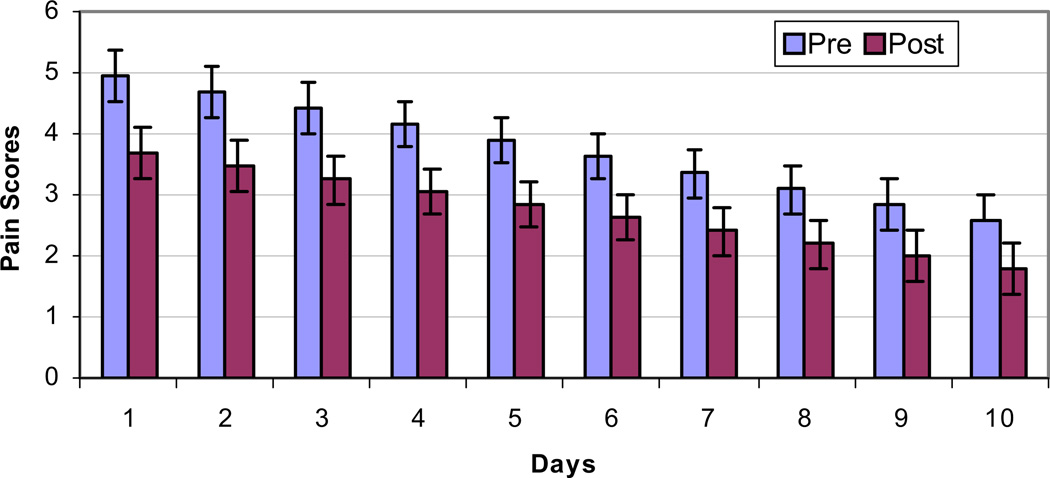
Using a repeated-measures analysis that adjusted the pain scores based on the correlation and the variability in measurements (both between patients and from day to day), the predicted means in the pre- and post-treatment groups were 4.93 (±0.42) and 1.78 (±0.42), respectively. Therefore, the overall benefit of the treatment was a 64% reduction in CIPN pain scores from start to end (P < 0.001). The scores did not stay constant over time (Hypothesis 2, mentioned earlier); the estimated pre- and post-pain scores were 3.74 (±0.38) and 2.72 (±0.38), respectively, a daily reduction in pain score of 1.02, or 27% (P < 0.001). Also, there was a statistically significant decreasing linear trend over the 10 days after the start of the treatment (P < 0.0001). This trend was similar, irrespective of whether the pre- or the post-pain scores were considered (P < 0.4). These results are shown in Table 3 and graphically in Fig. 3.
Table 3.
Effect of Electrocutaneous Stimulation with MC5-A Calmare® Therapy on Pain Scores
| Measure | Before | After | P-value, Statistical Test |
|---|---|---|---|
| Reduction in pain by 20% | 0 | 15/16 (94%) | <0.0001, Fischer’s exact test |
| CIPN pain score | 5.81 ± 1.11 | 2.38 ± 1.82 (−59%) | <0.0001, paired t-test |
| Adjusted pain scores | 4.9 ± 0.4 | 1.8 ± 0.4 (−64%) | <0.0001, repeated-measures analysis |
| Daily reduction in pain scores | 3.74 ± 0.38 | 2.72 ± 0.38 (1.02, −27%) | <0.001, repeated-measures analysis |
Fig. 3.
Effect of MC5-A Calmare® therapy on pain scores. Overall reduction in pain score is 64%; P < 0.001 by repeated-measures analysis of variance.
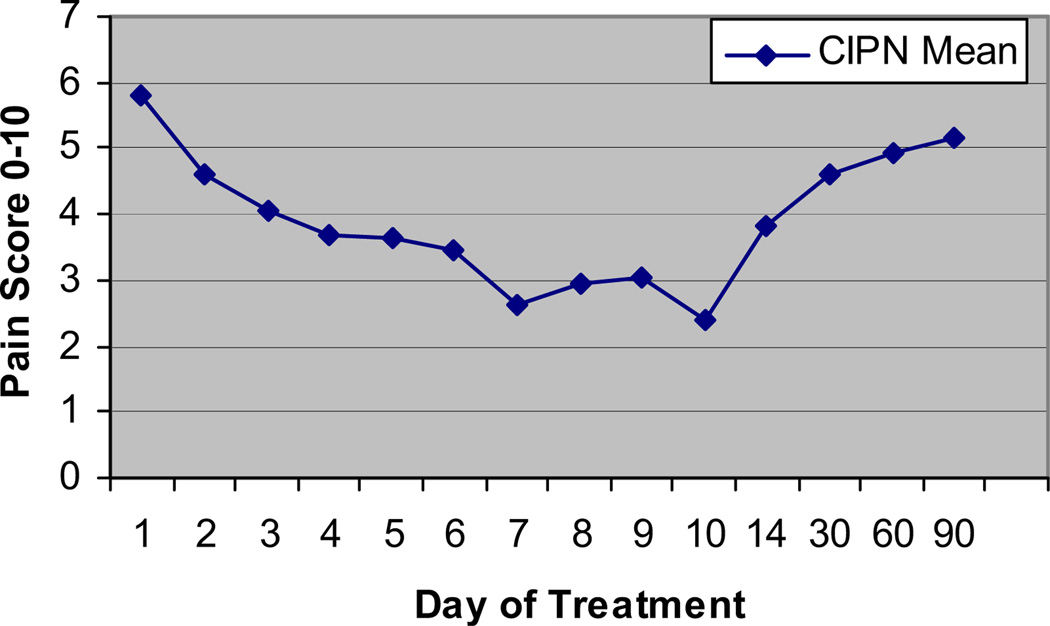
The effect of the MC5-A Calmare® was variable after the study. Two patients had sustained absence of pain without any maintenance. Most patients had their pain gradually return to pretreatment levels one or two months after treatment ended, as shown in Fig. 4. The protocol has been amended to allow retreatment with a brief course for acceptable pain control, followed by maintenance therapy.
Fig. 4.
Duration of effect of MC5-A Calmare® therapy on pain scores: pain relief over time.
The absence of toxicity was noticeable. No subjects complained of any side effects. The experienced clinical research nurse noted no toxicities.
Several participants noted a dramatic and unexpected benefit from a substantial recovery of “normal” sensation, leading to marked improvement in overall function. The first patient’s pain score went to 0, but more importantly to her, she had normal sensation and could walk, pick up her grandchildren, and feel their hair. Several other patients reported at least partial relief of numbness, but this was not explicitly measured. Two patients developed symptomatic brain metastases, which were not attributed to therapy. One patient noted complete resolution of motor abnormalities in her legs once her pain and numbness disappeared; she developed a brain metastasis and seizure after Day 6 and, hence, did not continue treatment but still had benefit weeks later.
The secondary endpoints showed minimal change with MC5-A Calmare® therapy. There was no consistent effect on the other pain scales (data not shown). There was no difference in morphine oral equivalent dose from Day 1 to Day 10 or afterward; three patients decreased their dose but the average stayed at 110–150 mg morphine oral equivalents per day (data not shown.) There was no change in formal quality of life or symptoms other than pain, as assessed by the Symptom Assessment Diary (data not shown.)
Discussion
CIPN is a common distressing symptom that rarely responds to conventional drug therapies.1 In this study, the MC5-A Calmare® cutaneous stimulation device reduced pain scores by a total of 59% in 10 days of treatment. No side effects were observed or reported despite careful monitoring. Several patients reported return of more normal sensation, although that was not a specifically measured outcome. For most patients, the pain returned to original intensity over the two-month period after MC5-A Calmare® treatment ended; preliminary experience has been that patients can be retreated successfully and maintained (Marineo et al., unpublished data).
There are strengths and weaknesses to this pilot study. One of the strengths of the study is that it was performed at an expert center in a relatively uniform patient cohort meeting a strict definition for established CIPN. The intervention used a standard reproducible technique for electrode application. The weaknesses include the fact that it was a single site, an unblinded study, and the short-term follow-up. In some but not all patients, the pain returned to its initial levels. Larger studies potentially can explore possible factors associated with this phenomenon and the potential for response to retreatment; in other types of cancer pain, retreatment followed by maintenance leads to recovery of the original benefit (Marineo et al., unpublished data).
There could be a possible placebo effect, but most reported placebo effects are much smaller than the reduction observed here. In the recent North Central Cancer Treatment Group placebo-controlled trial of BAK gel in similar patients, the BAK gel showed only a small marginally significant effect (P = 0.053, in a trial of 208 patients).10 In the lamotrigine trial,7 the pain score in the placebo group decreased over 10 weeks by 0.4 units from an average of 4.2, a less than 10% decrease. In the trial of gabapentin vs. placebo,8 there was no effect noted from placebo. In a randomized trial of sham vs. real percutaneous electrostimulation for back pain with inserted needles with or without stimulation, the sham group had a 9% reduction in back pain.25 Placebo was no different from TENS in the two randomized trials for cancer pain treatment.26 The 59% reduction in pain scores observed here with the MC5-A Calmare® is much larger than the improvement reported in the aforementioned placebo-controlled trials and is similar to the other trials of MC5-A Calmare® therapy17,18 (Marineo et al., unpublished data,) that observed with direct nerve stimulation,11–15 and that observed with the infusion of a local anesthetic plus opioid into the spinal fluid.22 There are no data from nonspecific TENS in CIPN;27 hence, we cannot make any comparisons.
In summary, MC5-A Calmare® therapy in a small cohort of patients caused a dramatic 59% reduction in CIPN pain beginning over the first several days of treatment, which is durable in some patients, with no side effects. Future studies will seek to determine the efficacy compared with sham or placebo treatment using newly available CIPN scales28 that also will assess function, optimal scheduling for treatment, and the need for maintenance therapy.
Acknowledgments
Research support was received from Massey Cancer Center 5 P30 CA16059 (T.J.S., W.P., V.R., P.D.), GO8 LM0095259 from the National Library of Medicine (T.J.S.) and R01CA116227-01 (T.J.S.) from the National Cancer Institute. The funders were not involved in the conduct of the study or development of the submission.
Footnotes
Disclosures
The authors declare no conflicts of interest.
References
- 1.Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44:1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Yardley DA. Proactive management of adverse events maintains the clinical benefit of ixabepilone. Oncologist. 2009;14:448–455. doi: 10.1634/theoncologist.2008-0284. [DOI] [PubMed] [Google Scholar]
- 3.Kannarkat G, Lasher EE, Schiff D. Neurologic complications of chemotherapy agents. Curr Opin Neurol. 2007;20:719–725. doi: 10.1097/WCO.0b013e3282f1a06e. [DOI] [PubMed] [Google Scholar]
- 4.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13:27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 5.Kautio A-L, Haanpaa M, Saarto T, et al. Amitriptyline in the treatment of chemotherapy-induced neuropathic symptoms. J Pain Symptom Manage. 2008;35:31–39. doi: 10.1016/j.jpainsymman.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 6.Hammack JE, Michalak JC, Loprinzi CL, et al. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain. 2002;98:195–203. doi: 10.1016/s0304-3959(02)00047-7. [DOI] [PubMed] [Google Scholar]
- 7.Rao RD, Flynn PJ, Sloan JA, et al. Efficacy of lamotrigine in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled trial, N01C3. Cancer. 2008;112:2802–2808. doi: 10.1002/cncr.23482. [DOI] [PubMed] [Google Scholar]
- 8.Rao RD, Michalak JC, Sloan JA, et al. North Central Cancer Treatment Group. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3) Cancer. 2007;110:2110–2118. doi: 10.1002/cncr.23008. [DOI] [PubMed] [Google Scholar]
- 9.Nikcevich DA, Grothey A, Sloan JA, et al. A phase III randomized, placebo-controlled, double-blind study of intravenous calcium/magnesium to prevent oxaliplatin-induced sensory neurotoxicity, N04C7. J Clin Oncol. 2008 May 20;26(Suppl) doi: 10.1200/JCO.2013.52.0536. abstract 4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barton DL, Wos E, Qin R, et al. A randomized controlled trial evaluating a topical treatment for chemotherapy-induced neuropathy: NCCTG trial N06CA. J Clin Oncol. 2009;27(15 Suppl) abstract 9531. [Google Scholar]
- 11.Folletti A, Durrer A, Buchser E. Neurostimulation technology for the treatment of chronic pain: a focus on spinal cord stimulation. Expert Rev Med Devices. 2007;4:201–214. doi: 10.1586/17434440.4.2.201. [DOI] [PubMed] [Google Scholar]
- 12.de Leon-Casasola OA. Spinal cord and peripheral nerve stimulation techniques for neuropathic pain. J Pain Symptom Manage. 2009;38(2 Suppl):S28–S38. doi: 10.1016/j.jpainsymman.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Mailis-Gagnon A, Furlan AD, Sandoval JA, Taylor R. Spinal cord stimulation for chronic pain. Cochrane Database Syst Rev. 2004;3 doi: 10.1002/14651858.CD003783.pub2. CD003783. [DOI] [PubMed] [Google Scholar]
- 14.Harke H, Gretenkort P, Ladleif HU, Rahman S. Spinal cord stimulation in sympathetically maintained complex regional pain syndrome type I with severe disability. A prospective clinical study. Eur J Pain. 2005;9:363–373. doi: 10.1016/j.ejpain.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Harke H, Gretenkort P, Ladleif HU, Koester P, Rahman S. Spinal cord stimulation in postherpetic neuralgia and in acute herpes zoster pain. Anesth Analg. 2002;94:694–700. doi: 10.1097/00000539-200203000-00040. [DOI] [PubMed] [Google Scholar]
- 16.Wong R, Sagar S. Acupuncture treatment for chemotherapy-induced peripheral neuropathy—a case series. Acupunct Med. 2006;24:87–91. doi: 10.1136/aim.24.2.87. [DOI] [PubMed] [Google Scholar]
- 17.Marineo G. Untreatable pain resulting from abdominal cancer: new hope from biophysics? JOP. 2003;4:1–10. [PubMed] [Google Scholar]
- 18.Sabato AF, Marineo G, Gatti A. Calmare therapy. Minerva Anestesiol. 2005;71:479–482. [PubMed] [Google Scholar]
- 19.Attal N, Cruccu G, Haanpää M, et al. EFNS Task Force. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13:1153–1169. doi: 10.1111/j.1468-1331.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- 20.Bennett MI, Johnson MI, Brown SR, et al. Feasibility study of transcutaneous electrical nerve stimulation (TENS) for cancer bone pain. J Pain. 2010;11:351–359. doi: 10.1016/j.jpain.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Agnew WF, McCreery DB. Considerations for safety with chronically implanted nerve electrodes. Epilepsia. 1990;31(Suppl 2):S27–S32. doi: 10.1111/j.1528-1157.1990.tb05845.x. [DOI] [PubMed] [Google Scholar]
- 22.Smith TJ, Staats PJ, Pool G, et al. Randomized clinical trial of comprehensive medical management of refractory cancer pain vs. comprehensive medical management plus intrathecal implantable drug delivery systems. J Clin Oncol. 2002;20:4040–4049. doi: 10.1200/JCO.2002.02.118. [DOI] [PubMed] [Google Scholar]
- 23.Loprinzi CL, Paice J. Neuropathic pain from chemotherapy in cancer: diagnosis and treatment dilemmas. WHO Cancer Pain Release. 2008;21:6–7. [Google Scholar]
- 24.Sloan JA, Loprinzi CL, Kuross SA, et al. Randomized comparison of four tools measuring overall quality of life in patients with advanced cancer. J Clin Oncol. 1998;15:3662–3673. doi: 10.1200/JCO.1998.16.11.3662. [DOI] [PubMed] [Google Scholar]
- 25.Ghoname EA, Craig WF, White PF, et al. Percutaneous electrical nerve stimulation for low back pain: a randomized crossover study. JAMA. 1999;281:818–823. doi: 10.1001/jama.281.9.818. [DOI] [PubMed] [Google Scholar]
- 26.Robb KA, Bennett MI, Johnson MI, Simpson KJ, Oxberry SG. Transcutaneous electrical nerve stimulation (TENS) for cancer pain in adults. Cochrane Database Syst Rev. 2008;3 doi: 10.1002/14651858.CD006276.pub2. CD006276. [DOI] [PubMed] [Google Scholar]
- 27.Visovsky C, Collins M, Abbott L, Aschenbrenner J, Hart C. Putting evidence into practice: evidence-based interventions for chemotherapy-induced peripheral neuropathy. Clin J Oncol Nurs. 2007;11:901–913. doi: 10.1188/07.CJON.901-913. [DOI] [PubMed] [Google Scholar]
- 28.Postma TJ, Aaronson NK, Heimans JJ, et al. EORTC Quality of Life Group. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer. 2005;41:1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]