Abstract
In the postgenomic era, systems biology has rapidly emerged as an exciting field predicted to enhance the molecular understanding of complex biological systems by the use of quantitative experimental and mathematical approaches. Systems biology studies how the components of a biological system (e.g. genes, transcripts, proteins, metabolites) interact to bring about defined biological function or dysfunction. Living systems may be divided into five dimensions of complexity: (i) molecular; (ii) structural; (iii) temporal; (iv) abstraction and emergence; and (v) algorithmic. Understanding the details of these dimensions in living systems is the challenge that systems biology aims to address. Here, we argue that the hair follicle (HF), one of the signature features of mammals, is a perfect and clinically relevant model for systems biology research. The HF represents a stem cell-rich, essentially autonomous mini-organ, whose cyclic transformations follow a hypothetical intrafollicular “hair cycle clock” (HCC). This prototypic neuroectodermal-mesodermal interaction system, at the cross-roads of systems and chronobiology, encompasses various levels of complexity as it is subject to both intrafollicular and extrafollicular inputs (e.g. intracutaneous timing mechanisms with neural and systemic stimuli). Exploring how the cycling HF addresses the five dimensions of living systems, we argue that a systems biology approach to the study of hair growth and cycling, in man and mice, has great translational medicine potential. Namely, the easily accessible human HF invites preclinical and clinical testing of novel hypotheses generated with this approach.
Keywords: anagen, BMP, chronobiology, clock genes, hair cycle, telogen, WNT
Introduction
“The problem of biology is not to stand aghast at the complexity but to conquer it” Sidney Brenner, Nobel Laureate (1)
Systems biology is a fast-evolving life sciences field that aims to establish how the components of a living system combine to cause function (2,3). Biological systems can be divided into five levels of complexity: (i) molecular; (ii) structural; (iii) temporal; (iv) abstraction and emergence; and (v) algorithmic (Table S1) (4).
In the past, cell cultures (particularly yeast) were often used in systems biology research to handle the complexity of living systems (2,5–8). These models are far removed from the reality of mammalian organisms. Identifying mammalian models that are sufficiently complex to encompass these five dimensions, and approach physiological relevance is an important challenge for systems biology (2,9).
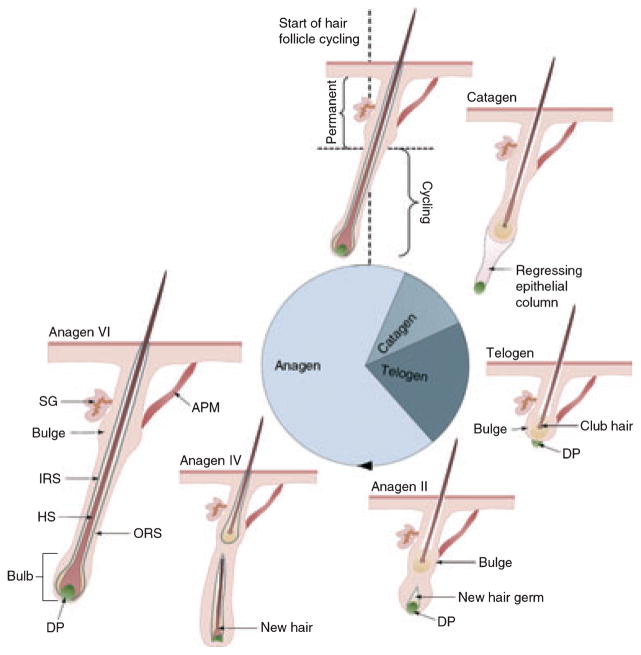
The hair follicle (HF) consists of multiple different cell populations of neural crest, ectodermal or mesodermal origin, which are distinct in their location, function and gene and protein expression characteristics (10–13). Additionally, the HF is a uniquely dynamic mini-organ that under-goes continuous cycling throughout adult life during which elements of its own morphogenesis are recapitulated (11,14) (Fig. 1). This transformation process arises under the dictates of an enigmatic oscillator system [the hair cycle clock (HCC)] (12,15,16). Hair growth disorders can be attributed, at large, to changes in the normal dynamic behaviour of the HF (12,13,15,17,18). Common hair diseases such as alopecia areata, telogen effluvium, hirsutism and hypertrichosis remain major, unsolved medical problems that call for new approaches in developing effective remedies. The HF is an attractive research model as hair growth, cycling and colour are of profound interest to biological and medical researchers, and a vast industry that caters to individuals who wish to manipulate these parameters. The study of HF cycling thus brings together systems biology, stem cell biology, regenerative medicine, chronobiology and translational medicine (16,19–21).
Figure 1.
The hair follicle enters a continuous cyclical process of organ regression (catagen), a relative ‘resting’ phase (telogen) and a growth phase (anagen) in the hair cycle. This process is altered in hair disorders such as hirsutism where hair follicles exhibit a prolonged anagen phase. The hair cycle demonstrates the dynamic nature of the HF with drastic molecular and structural changes associated with the passing of time (stage of hair cycle). DP, Dermal papilla; ORS, Outer root sheath; HS, Hair shaft; IRS, inner root sheath; SG, Sebaceous gland. Adapted after Schneider et al. 2009 Curr Biol (11).
Induction, spacing, orientation and morphogenesis of the HF (22) represent classical scenarios of developmental and stem cell systems biology (23–25). The hair patterning process is a prototypic neuroectodermal-mesodermal interaction system which is beautifully demonstrated by the pioneering work of Nagorcka and Mooney (26–28). Mathematical theory was utilized to explain the spatial patterns of morphogens and thus patterned HF formation. Systems biology research in HF development has been previously covered (25,29,30); therefore, here we focus on the cycling adult HF as a systems biology research model.
We argue that the HF, a signature organ of the mammalian species, is optimally suited to address challenges in medical and systems biology research by studying clinically relevant biological phenomena from a comprehensive systems biology perspective.
Systems biology in a nutshell
Systems biology is the study of how the components of a biological system (e.g. genes, transcripts, proteins, metabolites) interact to bring about function (and dysfunction) of that system (2,4,6,8,31). This discipline has risen in the postgenomic era as advances in molecular biology, and the production of high-throughput data such as deciphering the genome in the human genome project (4,32,33) still left a gap in our ability to translate the vast amounts of molecular knowledge to understanding biological function (7). Understanding biological systems by a transition from reductionist study on the molecular level to the systems level of life phenomena is systems biology (5,6,31,34). Appendix S1 provides further details about systems biology, emergence, mathematical modelling in systems biology and a summary of some important successes of systems biology in medical research (Table S2).
The hair follicle as a prototypic systems biology organ
HF cycling displays a number of properties that recommend it for systems biology research. The HF can be investigated at different levels that mirror the five dimensions of living systems Table S1 (4). We discuss some previous studies where this has already been explored in hair cycling research.
Molecular complexity of the hair follicle
Despite the divergent cell types and number of molecular players that interact within a HF, the total number of HF protagonists is finite compared to larger organs. Moreover, the murine HF is among the best-characterized mammalian organs at the gene and protein level (11,12,35).
Typically, profiling tools such as metabolomics, proteomics and genomics are used to acquire a systematic analysis of the molecular components in living systems (4). Time-course gene expression profiling of murine skin has led to the identification of novel candidates in hair cycle regulation. This method of eliciting gene expression during the hair cycle also addresses the temporal dimension of living systems (16). By clustering gene expression profiles by their pattern of expression during the murine hair cycle and subsequently grouping the clusters by gene function, novel genes and pathways have been identified as candidates in murine hair cycle control. Clock genes (those responsible for the circadian rhythm) have been demonstrated to be hair cycle dependent (16) and are prominently expressed in the secondary hair germ (SHG) (36) of telogen and early anagen HFs (16). Bmal1 knock-out mice displayed retarded anagen development and lack mitotic cells in the SHG, suggesting that clock genes regulate anagen progression via their effect on the cell cycle. These findings indicate that clock genes can regulate complex non-diurnal organ transformation such as HF cycling (16). Additionally, the systems approach used enabled novel candidates to be identified and allowed understanding of dynamic gene expression during the HF cycle.
The use of mouse mutants (11,12,22,37) represents an ideal system biology instrument in vivo (38). One particularly interesting example is the fuzzy phenotype, which results from a mutation in the Sgk3 gene (39) (Supplementary Figure S1) and exhibits strikingly accelerated HF cycling (40). Researchers often investigate one gene or protein of interest, however, functional overlap of hair cycle candidates (such as via common pathways) should not be overlooked, and a systems biology approach avoids this (Appendix S2 and Supplementary Figure S1 detail the link between Sgk3 and growth factor pathways). This approach can be complemented in the human system via organ culture of micro-dissected human anagen scalp HFs and perturbing their normal behaviour in vitro. Thus, the impact of defined test agents on hair shaft formation, follicular melanogenesis, catagen transformation, hair matrix keratinocyte proliferation and apoptosis and gene expression can be evaluated (41–44). From a systems biology point of view, the cycling HF connects molecular complexity (e.g. epithelial-mesenchymal signalling exchanges) with structural and temporal complexities (e.g. switch-on/switch-off hair shaft production, cyclic organ transformation, time course of HF cycling and disparate length of individual cycle stages).
Structural complexity of the hair follicle
The tissue compartments of the HF are stringently circumscribed and of well-defined composition. This makes the HF a microcosm and ideal target for systems biology research.
The structure of the HF can directly address the second dimension of living systems that Huang and Wikswo describe (4). HFs are easily accessible for experimentation, observation and perturbation because of their superficial location on the surface of mammalian bodies. This feature makes the HF ideal for systems biology research as access to molecular, structural and phenotypic information is readily available. In addition, the HF can be reduced into different structural levels; from cells (such as stem cells, dermal papilla (DP) cells, epithelial cells) to isolated mini-organs (e.g. HFs) to whole tissues (e.g. hair-bearing skin) and organisms (such as humans and mice) (45). Importantly, these different structural levels all exhibit systems properties within themselves (Appendix S1).
The structural unit of the HF can be considered an essentially autonomous organ as it is able to grow after dissection from its neurovascular supply and transplantation into another part of the integument (46). In addition, isolated human HFs can be maintained in organ culture (47,48). This is an exciting prospect for systems biologists as these mini-organs exhibit emergent properties of great biological relevance: controlled cell proliferation; differentiation; apoptosis and organ regeneration. In addition, the basic autonomous clock driving the HF cycle may reside in the HF itself (15,49). Nevertheless, the HF ‘system’ is also highly sensitive to extra-follicular signals, e.g. neural and vascular stimuli (22,49,50), thus increasing the level of system complexity.
The mature HF can be divided into the mesenchymal HF, consisting of the DP and connective tissue sheath, and the epithelial HF [the remaining portions; including transient amplifying cells of the hair matrix that envelope the DP, hair shaft, inner root sheath (IRS) and outer root sheath (ORS)]. The structural complexity of the HF is intertwined with the molecular complexity of this mini-organ and adequate epithelial-mesenchymal communication is vital for normal HF cycling (12,14,35,49,52). (Appendix S2 explores epithelial-mesenchymal interaction in the HF). The accessibility and exhibition of epithelial and mesenchymal communication in the HF make it an excellent tool for those interested in this type of communication; for example, in cancer research (53,54) and as general model for inter-tissue communication through time and space (2).
Temporal complexity of the hair follicle
Living systems exhibit different temporal properties and scales. For example, in the heart, depolarization of myocytes takes 1 ms; the cardiac cycle, 1 s; and longevity of the organism, gigaseconds (4). The temporal dimension of living systems (Table S1) is well reflected in HF biology because this organ exhibits its own unique temporal cycle; the hair cycle. These cycling events are typical examples of patterns, namely breaks of homogeneity leading to the emergence of new structure (55). Temporal complexity of HF cycling also relates directly to structural and molecular complexities as these themselves exhibit dramatic hair cycle-dependent changes. During the hair cycle, the HF shows complex, patterned phenomena that are temporospatially restricted (55,56). Despite the large number of molecular candidates implicated in HF cycling control, such as IGF-1 (57), hepatocyte growth factor (HGF) (58– 60) (anagen promoters), fibroblast growth factor-5 (FGF-5) (61–63) and neurotrophins (catagen inducers) (64,65), the mechanisms regulating its timing remain elusive (11,12,15,16,18,49). An integrated systems approach to this problem has been lacking with researchers often looking only at one gene or protein of interest (66).
Organ-cultured human HFs are able to synthesize a hair fibre (at rates (41) and with a keratinization process (67) similar to that in vivo) and subsequently enter a catagen-like stage i.e. in the absence of extrafollicular tissue, neural, vascular or endocrine signals. A full hair cycle is not exhibited in this in vitro model, however, this model has advantages for researching the human anagen–catagen transition as the occurrence rate of catagen HFs in vivo is low (68) compared to the majority of HFs that spontaneously enter catagen with this model (45).
Because oscillatory behaviour is a classical emergent phenomenon (69), the cycling HF is an ideal mammalian system for systems biology studies. The full temporal complexity of HF cycling is evident by the recent discovery that clock genes may play an important role in this “intrinsic” HCC (16), thus joining circadian oscillator systems (daily) with the rhythmic organ remodelling process that spans weeks (mice) or even years (man). The HF and skin exhibit circadian rhythms in gene transcription and protein expression (16,70–75). Therefore, this tissue operates on various time-scales simultaneously (74). To fully understand how these distinct chronobiological systems interact constitutes a major systems biology research challenge. Routine hair research approaches cannot hope to master this challenge if hair biologists do not cooperate closely with chronobiologists and systems biologists (11,16,74,76).
Abstraction and Emergence
The Follicular Automaton
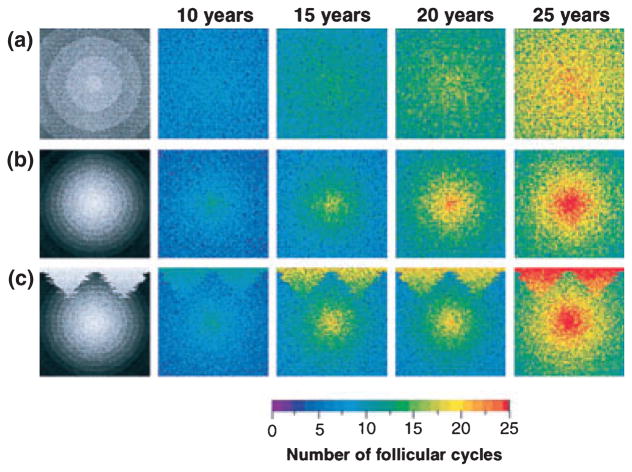
In order to cycle, HFs exhibit oscillations in structural and molecular properties. However, on a high level of abstraction, HF cycling can be studied without explicit reference to the exact molecular mechanisms that produce it. As a starting point to understand HF cycling, a rather abstract mathematical model was proposed; the follicular automaton model (FAM) (77,78). The FAM aimed to establish a model of the dynamics of human scalp hair to predict long-term changes of scalp hair growth and thus understand (and, ideally, predict) the manner by which different balding patterns occur (Fig. 2).
Figure 2.
Spatio-temporal simulations of the follicular automaton model (FAM) showing different patterns of hair growth. Experimental (phototrichogram) data collected over 14 years was used to approximate the distribution of durations of hair cycle stages in each hair follicle for each patient by using a log normal distribution. The FAM was defined using the assumptions that (i) each follicle is independent; (ii) each follicle traverses the cycle in the order of anagen-telogen- latency phases; and (iii) after latency the follicle may either enter a new cycle or undergo death or miniaturization. The figure shows the simulation of human hair growth over ‘25 years’ using the FAM and demonstrates how different patterns of alopecia could be achieved by simulating a population of HFs on a ‘scalp’. (a) ‘Hair follicles’ were arranged on a grid (‘scalp’) with hair follicles programmed with different mean durations of the anagen phase across a gradient as shown in left column (decreasing mean from periphery of the ‘scalp’ to the centre). The hair follicles were programmed to ‘die’ after a set number of cycles. The final hair pattern at 25 years corresponds to a diffuse alopecia commonly seen in women. (b) As in a, but steeper gradient set across the scalp to produce more dramatic balding pattern in the centre. (c) Grid programmed with temporal conditions as well as central gradient to achieve the final hair pattern consistent with androgenetic alopecia (male pattern baldness) as shown in Figures b and c. This model has limitations, for example, the phototrichogram method provides only approximate temporal information regarding the durations of hair cycle stages. The data represents observations from the skin’s surface and provides no more specific information on the molecular and temporal changes at the HF level. The assumption that the results obtained with this method can relate quantitatively to the molecular timings of the human hair cycle is tentative. This is demonstrated by the fact that catagen (which lasts a few weeks in human scalp hair) is not captured using this method. Adapted after Halloy et al. 2000 Proc Natl Acad Sci USA (77). Copyright 2000.
The FAM has generated useful formation on the dynamics of human hair cycling, with a level of abstraction that may be advantageous (4). On a population level, the distribution of HFs in the skin provides a striking example of a self-organized spatio-temporal pattern and molecular detail is not required to demonstrate this. In addition, the model simulation of the evolution of hair patterns is hypothesis stimulating i.e. the pattern of hair growth on the human scalp can be manipulated to elicit male pattern baldness, diffuse alopecia and ‘normal’ hair growth (Fig. 2). This provokes hypotheses about HF parameters such as “heterogeneous distribution of follicular parameters produces a diffuse pattern” whereas “gradients in mean duration of anagen phase (e.g. from centre to periphery of the scalp) cause a central balding pattern”. The authors also determine that the independence of HFs and variability in the length of the anagen phase are major determinants of collective dynamics of human hair cycling. This model could be advanced, predictions tested, implemented and improved by including molecular signals and further refined by experimental data and hair cycle staging.
Despite its limitations (see legend, Fig. 2); this model is an elegant example of how combined experimental and theoretical methods can be optimally utilized to explore specific scientific problems (here: to provide a basis for long-term prognoses of human scalp hair growth in response to manipulations), make predictions and create new hypotheses. The dynamic behaviour of single HFs and HF populations can be approximated by a simple model (abstraction). Thus, the FAM perfectly illustrates a classical systems biology approach to hair research.
Intrafollicular and extrafollicular communication in hair follicle dynamics
The HF exhibits emergent properties, i.e. properties that exist in complex systems that are not demonstrated by the components alone and cannot be predicted through understanding the separated parts. For example, understanding the properties of hydrogen and oxygen does not equate to understanding the properties of water (32). The HF is an emergent organ; the group of cells comprising the HF do not function in the same way when isolated than when operating together within the mini-organ. For example, isolated DP cells will not produce a hair shaft (35,52).
Recently, Plikus et al. (50) have shown that adult pelage HFs in mice exhibit hair cycle domains (i.e. spatially distinct HF populations that cycle synchronously within a defined skin territory). These hair cycle domains show the propagation of regenerating HFs as wave patterns (79,80). (Fig. 3). Through directed experimental techniques, spatial– temporal patterns in HF activity were directly linked to expression levels of the BMPs (Bmp2 and Bmp4) within hair cycle domains (Fig. 3). Cyclic changes were found in Bmp2 and Bmp4 that were asynchronous to hair cyclic changes, WNT/β-catenin signalling and noggin (a BMP antagonist) expression.
Figure 3.
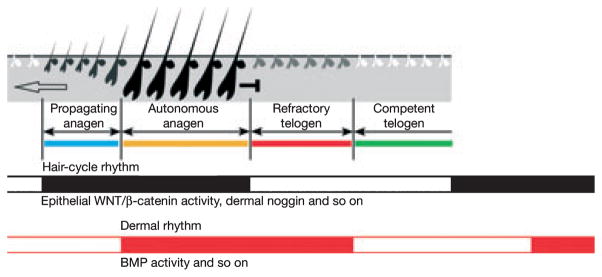
Demonstration of the emergent property of murine hair follicles exhibiting this behaviour in hair cycle domains. In situ hybridization was carried out on longitudinal sections of murine dorsal skin that were arranged spatially and temporally. These experiments revealed how the propagation of hair cycle waves on the dorsum may arise. Spatio-temporal patterns in hair follicle activity were directly linked to expression levels of the BMPs within hair cycle domains. Cyclic changes in Bmp2 and Bmp4 expression were asynchronous to hair cycle changes and that of WNT/β-catenin signalling. Noggin, a BMP antagonist, was expressed in a similar pattern to WNT. The figure shows the schematic summary of the hair cycle rhythm (in black) and the dermal rhythm (red). Together they define four new functional stages that correspond to the ability to propagate and respond to hair cycle propagation, these are; propagating anagen, autonomous anagen, refractory telogen and competent telogen. Refractory telogen is characterized by low noggin, high BMPs and low WNT signalling. Competent telogen is defined by low noggin, low BMPs whereas, during propagating anagen high noggin levels and low BMPs are found. Autonomous anagen is distinguished by the expression of high noggin and high BMPs. Catagen is omitted for simplification. Reprinted by permission from Macmillan Publishers Ltd: Plikus et al. 2008 Nature (50). Copyright 2008.
On the basis of these studies, the hair cycle was redefined [in the context of a population of HFs and the intrafollicular status of the skin (50)], from the traditional anagen, catagen and telogen stages, into functional phases of propagating anagen, autonomous anagen, refractory telogen and competent telogen (Fig. 3). This study addresses the population dynamics of cycling murine HFs, i.e. the population behaviour of HFs to either propagate and cycle in waves or not. Human HFs, however, behave differently to murine HFs in that they do not exhibit hair cycle domains and behave independently and stochastically or at the very most, in follicular units (81). This murine study cannot be extrapolated to explain the behaviour of human HFs as yet, however, it could be that a similar mechanism may exist between follicular units rather than cycle domains, but there is as yet no evidence to support this idea.
The functional phases of the hair cycle proposed in this study, in terms of ability to propagate a wave, can be used to define spatial–temporal relationships and controls within murine skin. Plikus et al. (50) demonstrate that stem cell regeneration is subject to control of biological rhythm. This work shows how the intrafollicular clock communicates with intracutaneous (but extrafollicular) timing mechanisms and lend themselves to the quantification and mathematical modelling of BMPs, noggin, WNT and β-catenin levels as a function of time to thereby predict and elucidate their function in the hair cycle.
Plikus et al. (50) have managed to demonstrate that the emergent properties of HFs within its macro-environment are essential for explaining function. Moreover, this study offers a great example of how emergent properties and molecular, structural and temporal complexity are all involved in the process of cyclic tissue transformation events. This provides yet further evidence that the HF is a classic systems biology tool.
Algorithmic complexity of the cycling hair follicle
Living organisms must be able to ‘compute’ inputs and convert these to outputs. As an example, gene regulatory networks within cells process a signal e.g. stimulation by a hormone (input) into changes in gene expression, protein production, enyzme activity and complex cellular phenomena (outputs) (Table S1). The ‘computational centre’ (HCC) that drives the hair cycle in the HF has not been identified (15). The existing theoretical models that simulate hair cycling (77,78,82) do not model the ‘computations’ of the HF during this process. A systems biology approach to hair cycling research should carefully identify the drivers of the hair cycle (internal computer or HCC) whereby inputs to the cycling HF are converted to the outputs; such as a hair shaft formation, regression of the HF, stem cell activation and HF regeneration. In addition, we need to ensure that we have the computational means to create models that can handle the complexity of such simulations (4).
Perspectives – Tackling the human hair cycle
We have delineated why the HF is an ideal tool for systems biology research; it is a unique mammalian feature, easily accessible, defined in form and function and addresses all the dimensions of living systems as an essentially autonomous mini-organ. It is evident that the dimensions of living systems overlap and are intertwined and the HF demonstrates this flawlessly; for example, molecular changes in BMPs are coordinated with spatio-temporal changes in the propagation of hair cycle waves and associated structural alterations of HFs as they traverse the hair cycle (50,51).
Human hair cycle research highlights the intertwining pathways that are relevant in the regulation and function of a multicellular mini-organ. Interesting recent findings show that circadian genes are differentially expressed in anagen and catagen human HFs (83). The cycling HF is thus relevant to regenerative medicine researchers, chronobiologists and stem cell biologists to name but a few (20). More comprehensive and dynamic analyses of the human hair cycle, e.g. via perturbation with siRNA as well as microarray, metabolomic and proteomic analyses, combined with the use of mathematical modelling, is a most promising course of research for both systems biologists and investigators of human HF biology and pathology. Also, the systems biology approach to hair research advocated here, at long last, may help to unravel the enigmatic HCC. This, in turn, could facilitate the development of novel therapeutic agents for the more effective management of common hair growth disorders.
Conclusions
In summary:
HF cycling in mice and man offers an excellent, clinically relevant, research model for systems biology and also stem cell, chronobiology, regenerative medicine and neuroendocrinology research;
The HF model deserves to be fully discovered by dermatological and systems biology research communities;
We propose that a systems biology approach to human HF cycling via the use of mathematical modelling coupled to experimental work would greatly further hair cycle research.
Supplementary Material
Acknowledgments
Writing of this viewpoint was supported in part by funding from the EPSRC and BBSRC to YA and a grant by Manchester NIHR Biomedical Research Centre to RP. GB is supported by EPSRC and CMC is supported by NIAMS, NIH. YA wishes to thank Mr. Marc Goodfellow and Professor CEM Griffiths for their comments and continued support.
Footnotes
Additional Supporting Information may be found in the online version of this article:
Figure S1. Sgk3 mutation and the hair cycle: molecular causes of Fuzzy phenotype and links between SGK3 and growth factor pathways.
Table S1. The five dimensions of living systems and hair cycle relevance.
Table S2. Summary table of successes of systems biology research in medical studies.
Appendix S1. Supporting text providing more detail about systems biology, the definition of emergence, the use of mathematical modelling in systems biology and a summary of important successes of systems biology in medical research.
Appendix S2. Supporting text on the links between SGK3 and growth factor function in human hair follicle anagen–catagen transition.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Duncan DE. Discover Dialogue: Sydney Brenner. In: Powell CS, Adcroft PG, Keating R, Wooden T, Weintraub P, editors. Discover Science, Technology and the Future. New York: Discover Media LLC; 2004. [Last accessed 6th May 2010]. http://discovermagazine.com/2004/apr/discover-dialogue. [Google Scholar]
- 2.Klipp E, Liebermeister W, Wierling C, Kowald A, Lehrach H, Herwig R. Systems Biology: A textbook. Weinheim: Wiley-VCH; 2009. [Google Scholar]
- 3.Academy of Medical Sciences and the Royal Academy of Engineering. Systems Biology: a vision for engineering and medicine. A report from the Academy of Medical Sciences and The Royal Academy of Engineering. London: Academy of Medical Sciences and the Royal Academy of Engineering; 2007. pp. 1–65. [Google Scholar]
- 4.Huang S, Wikswo J. Dimensions of systems biology. In: Amara SG, Bamberg E, Gudermann T, Lill E, Hebert SC, Jahn R, Lederer WJ, Miyajima A, Offermans S, editors. Reviews of Physiology Biochemistry and Pharmacology. Berlin: Springer Berlin Heidelberg; 2006. pp. 81–104. [DOI] [PubMed] [Google Scholar]
- 5.Westerhoff HV, Palsson BO. The evolution of molecular biology into systems biology. Nature. 2004;22:1249–1252. doi: 10.1038/nbt1020. [DOI] [PubMed] [Google Scholar]
- 6.Bruggeman FJ, Westerhoff HV. The Nature of Systems Biology. Trends Microbiol. 2006;15:45–50. doi: 10.1016/j.tim.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Sauer U, Heinemann M, Zamboni N. Getting Closer to the Whole Picture. Science. 2007;316:550–551. doi: 10.1126/science.1142502. [DOI] [PubMed] [Google Scholar]
- 8.Di Ventura B, Lemerle C, Michalodimitrakis K, Serrano L. From in vivo to in silico biology and back. Nature. 2006;443:527–533. doi: 10.1038/nature05127. [DOI] [PubMed] [Google Scholar]
- 9.Makarow M, Hojgaard L, Ceulemans R European Science Foundation. Setting Science Agendas for Europe: Advancing Systems Biology for Medical Applications ESF Science Policy Briefing. 2008;35:1–12. [Google Scholar]
- 10.Fuchs E. Beauty is skin deep: the fascinating biology of the epidermis and its appendages. Harvey Lect. 1998;94:47–77. [PubMed] [Google Scholar]
- 11.Schneider MR, Schmidt-Ullrich R, Paus R. The Hair Follicle as a Dynamic Miniorgan. Curr Biol. 2009;19:R132–R142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Stenn KS, Paus R. Controls of Hair Follicle Cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 13.Paus R, Cotsarelis G. The Biology of Hair Follicles. N Eng J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs E, Merrill BJ, Jamora C, DasGupta R. At the roots of a never-ending cycle. Dev Cell. 2001;1:13–25. doi: 10.1016/s1534-5807(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 15.Paus R, Foitzik K. In search of the “hair cycle clock”: a guided tour. Differentiation. 2004;72:489–511. doi: 10.1111/j.1432-0436.2004.07209004.x. [DOI] [PubMed] [Google Scholar]
- 16.Lin KK, Kumar V, Geyfman M, et al. Circadian Clock Genes Contribute to the Regulation of Hair Follicle Cycling. PLoS Genet. 2009;5:e1000573. doi: 10.1371/journal.pgen.1000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadshiew IM, Foitzik K, Arck PC, Paus R. Burden of Hair Loss: Stress and the Underestimated Psychosocial Impact of Telogen Effluvium and Androgenetic Alopecia. J Invest Dermatol. 2004;123:455–457. doi: 10.1111/j.0022-202X.2004.23237.x. [DOI] [PubMed] [Google Scholar]
- 18.Cotsarelis G, Millar SE. Towards a molecular understanding of hair loss and its treatment. Trends Mol Med. 2001;7:293–301. doi: 10.1016/s1471-4914(01)02027-5. [DOI] [PubMed] [Google Scholar]
- 19.Ito M, Kizawa K, Hamada K, Cotsarelis G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation. 2004;72:548–557. doi: 10.1111/j.1432-0436.2004.07209008.x. [DOI] [PubMed] [Google Scholar]
- 20.Yu BD, Mukhopadhyay A, Wong C. Skin and hair: models for exploring organ regeneration. Hum Mol Genet. 2008;17:R54–R59. doi: 10.1093/hmg/ddn086. [DOI] [PubMed] [Google Scholar]
- 21.Batista RTB, Ramirez DB, Santos RD, del Rosario MCI, Mendoza ER. EUCLIS– An information system for circadian systems biology. IET Syst Biol. 2007;1:266–273. doi: 10.1049/iet-syb:20060078. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–261. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- 23.MacArthur BD, Ma’ayan A, Lemischka IR. Systems biology of stem cell fate and cellular reprogramming. Nat Rev Mol Cell Biol. 2009;10:672–681. doi: 10.1038/nrm2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanag VK, Epstein IR. Pattern formation mechanisms in reaction-diffusion systems. Int J Dev Biol. 2009;53:673–681. doi: 10.1387/ijdb.072484vv. [DOI] [PubMed] [Google Scholar]
- 25.Baker RE, Schnell S, Maini PK. Waves and patterning in developmental biology: vertebrate segmentation and feather bud formation as case studies. Int J Dev Biol. 2009;53:783–794. doi: 10.1387/ijdb.072493rb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagorcka BN, Mooney JR. The role of a reaction-diffusion system in the formation of hair fibres. J Theor Biol. 1982;98:575–607. doi: 10.1016/0022-5193(82)90139-4. [DOI] [PubMed] [Google Scholar]
- 27.Nagorcka BN, Mooney JR. The role of a reaction-diffusion system in the initiation of primary hair follicles. J Theor Biol. 1985;114:243–272. doi: 10.1016/s0022-5193(85)80106-5. [DOI] [PubMed] [Google Scholar]
- 28.Mooney JR, Nagorcka BN. Spatial patterns produced by a reaction-diffusion system in primary hair follicles. J Theor Biol. 1985;115:299–317. doi: 10.1016/s0022-5193(85)80102-8. [DOI] [PubMed] [Google Scholar]
- 29.Sick S, Reinker S, Timmer J, Schlake T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism [see comment] Science. 2006;314:1447–1450. doi: 10.1126/science.1130088. [DOI] [PubMed] [Google Scholar]
- 30.Stark J, Andl T, Millar SE. Hairy Math: Insights into Hair-Follicle Spacing and Orientation. Cell. 2007;128:17–20. doi: 10.1016/j.cell.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 31.Kitano H. Systems Biology: A brief overview. Science. 2002;295:1662–1664. doi: 10.1126/science.1069492. [DOI] [PubMed] [Google Scholar]
- 32.Aderem A. Systems Biology: Its Practice and Challenges. Cell. 2005;121:511– 513. doi: 10.1016/j.cell.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 33.O’Malley MA, Dupré J. Fundamental issues in systems biology. Bioessays. 2005;27:1270–1276. doi: 10.1002/bies.20323. [DOI] [PubMed] [Google Scholar]
- 34.Noble D. Prologue: Mind Over Molecule: Activating Biological Demons. Ann N Y Acad Sci. 2008;1123:xi–xix. doi: 10.1196/annals.1420.000. [DOI] [PubMed] [Google Scholar]
- 35.Rendl M, Lewis L, Fuchs E. Molecular Dissection of Mesenchymal–Epithelial Interactions in the Hair Follicle. PLoS Biol. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panteleyev AA, Jahoda CAB, Christiano AM. Hair follicle predetermination. J Cell Sci. 2001;114:3419–3431. doi: 10.1242/jcs.114.19.3419. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura M, Sundberg JP, Paus P. Mutant laboratory mice with abnormalities in hair follicle morphogenesis, cycling, and/or structure: annotated tables. Exp Dermatol. 2001;10:369–390. doi: 10.1034/j.1600-0625.2001.100601.x. [DOI] [PubMed] [Google Scholar]
- 38.Alonso L, Okada H, Pasolli HA, et al. Sgk3 links growth factor signaling to maintenance of progenitor cells in the hair follicle. J Cell Biol. 2005;170:559– 570. doi: 10.1083/jcb.200504131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sundberg JP, Peters EMJ, Paus R. Analysis of Hair Follicles in Mutant Laboratory Mice. J Investig Dermatol Symp Proc. 2005;10:264–270. doi: 10.1111/j.1087-0024.2005.10126.x. [DOI] [PubMed] [Google Scholar]
- 40.Mecklenburg L, Tobin DJ, Cirlan MV, Craciun C, Paus R. Premature termination of hair follicle morphogenesis and accelerated hair follicle cycling in Iasi congenital atrichia (fzica) mice points to fuzzy as a key element of hair cycle control. Exp Dermatol. 2005;14:561–570. doi: 10.1111/j.0906-6705.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 41.Philpott MP, Sanders D, Westgate GE, Kealey T. Human hair growth in vitro: a model for the study of hair follicle biology. J Dermatol Sci. 1994;7 (Suppl):S55–S72. doi: 10.1016/0923-1811(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 42.Kloepper JE, Sugawara K, Al-Nuaimi Y, Gáspár E, van Beek N, Paus R. Methods in hair research: how to objectively distinguish between anagen and catagen in human hair follicle organ culture. Exp Dermatol. 2010;19:305– 312. doi: 10.1111/j.1600-0625.2009.00939.x. [DOI] [PubMed] [Google Scholar]
- 43.Bodo E, Biro T, Telek A, et al. A hot new twist to hair biology: involvement of vanilloid receptor-1 (VR1/TRPV1) signaling in human hair growth control. Am J Pathol. 2005;166:985–998. doi: 10.1016/S0002-9440(10)62320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bodo E, Tobin DJ, Kamenisch Y, et al. Dissecting the impact of chemotherapy on the human hair follicle: a pragmatic in vitro assay for studying the pathogenesis and potential management of hair follicle dystrophy. Am J Pathol. 2007;171:1153–1167. doi: 10.2353/ajpath.2007.061164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogers GE, Hynd PI. Animal Models and Culture Methods in the Study of Hair Growth. Clin Dermatol. 2001;19:105–119. doi: 10.1016/s0738-081x(00)00121-8. [DOI] [PubMed] [Google Scholar]
- 46.Maurer M, Peters EMJ, Botchkarev VA, Paus R. Intact hair follicle innervation is not essential for anagen induction and development. Arch Dermatol Res. 1998;290:574–578. doi: 10.1007/s004030050354. [DOI] [PubMed] [Google Scholar]
- 47.Philpott MP, Green MR, Kealey T. Human hair growth in vitro. J Cell Sci. 1990;97:463–471. doi: 10.1242/jcs.97.3.463. [DOI] [PubMed] [Google Scholar]
- 48.Philpott MP, Sanders DA, Kealey T. Whole hair follicle culture. Dermatol Clin. 1996;14:595–607. doi: 10.1016/s0733-8635(05)70387-9. [DOI] [PubMed] [Google Scholar]
- 49.Paus R, Muller-Rover S, Botchkarev VA. Chronobiology of the hair follicle: hunting the “hair cycle clock”. J Investig Dermatol Symp Proc. 1999;4:338– 345. doi: 10.1038/sj.jidsp.5640241. [DOI] [PubMed] [Google Scholar]
- 50.Plikus MV, Mayer JA, de la Cruz D, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plikus MV, Widelitz RB, Maxson R, Chuong CM. Analyses of regenerative wave patterns in adult hair follicle populations reveal macro-environmental regulation of stem cell activity. Int J Dev Biol. 2009;53:857–868. doi: 10.1387/ijdb.072564mp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tobin DJ, Gunin A, Magerl M, Handijski B, Paus R. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme: implications for hair growth control. J Invest Dermatol. 2003;120:895–904. doi: 10.1046/j.1523-1747.2003.12237.x. [DOI] [PubMed] [Google Scholar]
- 53.Hu M, Polyak K. Molecular characterisation of the tumour microenvironment in breast cancer. Eur J Cancer. 2008;44:2760–2765. doi: 10.1016/j.ejca.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faratian D, Clyde RG, Crawford JW, Harrison DJ. Systems pathology—taking molecular pathology into a new dimension. Nat Rev Clin Oncol. 2009;6:455– 464. doi: 10.1038/nrclinonc.2009.102. [DOI] [PubMed] [Google Scholar]
- 55.Widelitz RB, Baker RE, Plikus M, et al. Distinct Mechanisms Underlie Pattern Formation in the Skin and Skin Appendages. Birth Defects Res. 2006;78:280– 291. doi: 10.1002/bdrc.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chuong CM, Dhouailly D, Gilmore S, et al. What is the biological basis of pattern formation of skin lesions? Exp Dermatol. 2006;15:547–564. doi: 10.1111/j.1600-0625.2006.00448_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rudman SM, Philpott MP, Thomas GA, Kealey T. The role of IGF-I in human skin and its appendages: morphogen as well as mitogen? J Invest Dermatol. 1997;109:770–777. doi: 10.1111/1523-1747.ep12340934. [DOI] [PubMed] [Google Scholar]
- 58.Jindo T, Tsuboi R, Takamori K, Ogawa H. Local injection of hepatocyte growth factor/scatter factor (HGF/SF) alters cyclic growth of murine hair follicles. J Invest Dermatol. 1998;110:338–342. doi: 10.1046/j.1523-1747.1998.00144.x. [DOI] [PubMed] [Google Scholar]
- 59.Jindo T, Tsuboi R, Imai R, Takamori K, Rubin JS, Ogawa H. The effect of hepatocyte growth factor/scatter factor on human hair follicle growth. J Dermatol Sci. 1995;10:229–232. doi: 10.1016/0923-1811(95)00429-v. [DOI] [PubMed] [Google Scholar]
- 60.Lindner G, Menrad A, Gherardi E, et al. Involvement of hepatocyte growth factor/scatter factor and met receptor signaling in hair follicle morphogenesis and cycling. FASEB J. 2000;14:319–332. doi: 10.1096/fasebj.14.2.319. [DOI] [PubMed] [Google Scholar]
- 61.Kawano M, Komi-Kuramochi A, Asada M, et al. Comprehensive analysis of FGF and FGFR expression in skin: FGF18 is highly expressed in hair follicles and capable of inducing anagen from telogen stage hair follicles. J Invest Dermatol. 2005;124:877–885. doi: 10.1111/j.0022-202X.2005.23693.x. [DOI] [PubMed] [Google Scholar]
- 62.Hebert JM, Rosenquist T, Gotz J, Martin GR. FGF5 as a regulator of the hair growth cycle: evidence from targeted and spontaneous mutations. Cell. 1994;78:1017–1025. doi: 10.1016/0092-8674(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 63.Rosenquist TA, Martin GR. Fibroblast growth factor signalling in the hair growth cycle: expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev Dyn. 1996;205:379–386. doi: 10.1002/(SICI)1097-0177(199604)205:4<379::AID-AJA2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 64.Botchkarev VA, Botchkareva NV, Welker P, et al. A new role for neurotrophins: involvement of brain-derived neurotrophic factor and neurotrophin-4 in hair cycle control. FASEB J. 1999;13:395–410. doi: 10.1096/fasebj.13.2.395. [DOI] [PubMed] [Google Scholar]
- 65.Botchkarev VA, Welker P, Albers KM, et al. A New Role for Neurotrophin-3. Involvement in the Regulation of Hair Follicle Regression (Catagen) Am J Pathol. 1998;153:785–799. doi: 10.1016/S0002-9440(10)65621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stenn KS, Prouty SM, Seiberg M. Molecules of the cycling hair follicle - a tabulated review. J Dermatol Sci. 1994;7 (Suppl):S109–S124. doi: 10.1016/0923-1811(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 67.Thibaut S, Collin C, Langbein L, Schweizer J, Gautier B, Bernard BA. Hair keratin pattern in human hair follicles grown in vitro. Exp Dermatol. 2003;12:160–164. doi: 10.1034/j.1600-0625.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 68.Whiting DA. Light Microscopy of Vertical and Horizontal Sections of Scalp Biopsies. Fairfield: Canfield Publishing; 2004. The Structure of the Human Hair Follicle. [Google Scholar]
- 69.Whittington MA, Traubb RD, Kopellc N, Ermentroutd B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- 70.Zanello SB, Jackson DM, Holick MF. Expression of the Circadian Clock Genes clock and period1 in Human Skin. J Invest Dermatol. 2000;115:757–760. doi: 10.1046/j.1523-1747.2000.00121.x. [DOI] [PubMed] [Google Scholar]
- 71.Bjarnson GA, Jordan RCK, Wood PA, et al. Circadian Expression of Clock Genes in Human Oral Mucosa and Skin Association with Specific Cell-Cycle Phases. Am J Pathol. 2001;158:1793–1801. doi: 10.1016/S0002-9440(10)64135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bjarnson GA, Jordan R. Rhythms in Human Gastrointestinal Mucosa and Skin. Chronobiol Int. 2002;19:129–140. doi: 10.1081/cbi-120002595. [DOI] [PubMed] [Google Scholar]
- 73.Kawara S, Mydlarski R, Mamelak AJ, et al. Low-dose Ultraviolet B Rays Alter the mRNA Expression of the Circadian Clock Genes in Cultured Human Keratinocytes. J Invest Dermatol. 2002;119:1220–1223. doi: 10.1046/j.1523-1747.2002.19619.x. [DOI] [PubMed] [Google Scholar]
- 74.Mehling A, Fluhr JW. Chronobiology: Biological Clocks and Rhythms of the Skin. Skin Pharmacol Physiol. 2006;19:182–189. doi: 10.1159/000093113. [DOI] [PubMed] [Google Scholar]
- 75.Tanioka M, Yamada H, Doi M, et al. Molecular Clocks in Mouse Skin. J Invest Dermatol. 2009;129:1225–1232. doi: 10.1038/jid.2008.345. [DOI] [PubMed] [Google Scholar]
- 76.Lin KK, Chudova D, Hatfield GW, Smyth P, Andersen B. Identification of hair cycle-associated genes from time-course gene expression profile data by using replicate variance. Proc Natl Acad Sci USA. 2004;101:15955–15960. doi: 10.1073/pnas.0407114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Halloy J, Bernard BA, Loussouarn G, Goldbeter A. Modeling the dynamics of human hair cycles by a follicular automaton. Proc Natl Acad Sci USA. 2000;97:8328–8333. doi: 10.1073/pnas.97.15.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Halloy J, Bernard BA, Loussouarn G, Goldbeter A. The follicular automaton model: effect of stochasticity and of synchronization of hair cycles. J Theor Biol. 2002;214:469–479. doi: 10.1006/jtbi.2001.2474. [DOI] [PubMed] [Google Scholar]
- 79.Ma L, Liu J, Wu T, et al. ‘Cyclic alopecia’ in Msx2 mutants: defects in hair cycling and hair shaft differentiation. Development. 2003;130:379–389. doi: 10.1242/dev.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suzuki N, Hirata M, Kondo S. Traveling stripes on the skin of a mutant mouse. PNAS. 2003;100:9680–9685. doi: 10.1073/pnas.1731184100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jimenez F, Ruifernández JM. Distribution of Human Hair in Follicular Units. Dermatol Surg. 1999;25:294–298. doi: 10.1046/j.1524-4725.1999.08114.x. [DOI] [PubMed] [Google Scholar]
- 82.Kolinko V, Littler CM. Mathematical modeling for the prediction and optimization of laser hair removal. Lasers Surg Med. 2000;26:164–176. doi: 10.1002/(sici)1096-9101(2000)26:2<164::aid-lsm7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 83.Al-Nuaimi Y, Philpott M, Kloepper J, et al. A role for clock-related genes in human hair growth control? J Invest Dermatol. 2009;129:S67. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.