Abstract
Objective
To quantify cerebrovascular autoregulation as a function of gestational age (GA) and across the phases of the cardiac cycle.
Study design
The present study is a hypothesis-generating re-analysis of previously published data. Premature infants (n=179), with a GA range of 23 to 33 weeks were monitored with umbilical artery catheters and transcranial Doppler insonation of the middle cerebral artery for 1-hour sessions over the first week of life. Autoregulation was quantified by three methods, as a moving correlation coefficient between: 1) systolic arterial blood pressure (ABP) and systolic cerebral blood flow (CBF) velocity (Sx); 2) mean ABP and mean CBF velocity (Mx); and 3) diastolic ABP and diastolic CBF velocity (Dx). Comparisons of individual and cohort cerebrovascular pressure autoregulation were made across GA for each aspect of the cardiac cycle.
Results
Systolic, mean and diastolic ABP increased with GA (r=0.3, 0.4, and 0.4; p<0.0001). Systolic CBF velocity was pressure-passive in infants with the lowest GA, and Sx decreased with advancing GA (r=−0.3; p<0.001), indicating increased capacity for cerebral autoregulation during systole during development. By contrast, Dx was elevated, indicating dysautoregulation, in all subjects and showed minimal change with advancing GA (r=−0.06; p=0.05). Multivariate analysis confirmed that both GA (p<0.001) and "effective cerebral perfusion pressure" (ABP minus critical closing pressure; p<0.01) were associated with Sx.
Conclusion
Premature infants have low and usually pressure-passive diastolic CBF velocity. By contrast, the regulation of systolic CBF velocity by pressure autoregulation developed in this cohort between 23 and 33 weeks GA. Elevated effective cerebral perfusion pressure derived from the critical closing pressure was associated with dysautoregulation.
Keywords: Cerebrovascular pressure autoregulation, critical closing pressure, diastolic closing margin, prematurity
Introduction
The association between long-term neurodevelopmental impairment and prematurity are linked by hemodynamic disturbance and its treatment; hemorrhagic, hypoxic, and ischemic neonatal brain injury; disturbances of arterial blood gases; ventilator asynchrony; and sepsis.1–3 All of these variables are considered fundamental aspects of neonatal intensive care management. However, the optimization and standardization of care to promote improved neurodevelopmental outcomes is hindered by an inability to demonstrate clear linearity between a specific care strategy and neurodevelopmental outcome. For example, there is no consensus among neonatologists regarding the proper management of neonatal hypotension based on gestational age (GA) and postnatal age and the definition of hypotension.4, 5
From animal models, both ischemia and hyperperfusion have been implicated in the etiology of neonatal brain injury.6–11 This apparent contradiction has caused a divergence of mechanistic understanding of intraventricular hemorrhage (IVH), which is the most common and easily detectable of all neonatal neurologic injuries. Does the fragile vascular bed of the germinal matrix bleed from ischemia followed by reperfusion or from hyperemia driven by excessive arterial blood pressure (ABP)? The mature mammalian brain is protected from fluctuations in ABP by pressure autoregulation, which maintains constant cerebral blood flow (CBF) to the brain across a wide range of ABPs. Pressure autoregulation is mediated by dynamic cerebrovascular tone and resistance, a state termed pressure reactivity.12 Lack of reactivity of cerebrovascular tone to a change in ABP is termed pressure-passivity, a state in the mature brain that is associated with death and severe neurologic morbidity.13, 14 Although the etiology of IVH is multifactorial, disturbances of CBF and cerebral autoregulation play important roles.15–18
Pressure-passivity of the cerebral circulation has been observed in premature infants by a variety of methods, a finding inconsistently associated with outcome.18–22 Thus, the clinical relevance of pressure passivity in the premature infant has mostly been implied from adult data. The mammalian cerebral vasculature develops muscularity at approximately two-thirds gestation, which corresponds to 26–27 weeks’ gestation in humans, so extremely premature infants may lack the necessary arteriolar tone needed to impart pressure autoregulation.23, 24 The ontogeny of autoregulation is therefore germane to our interpretation of pressure-passivity in premature infants.
The present study is a hypothesis-generating re-analysis of previously published data.3, 15, 25–27 In the prior studies, cerebral autoregulation was found to be influenced by hypercapnia and associated with severe IVH. In addition, changes to cerebral hemodynamics occurred during surfactant administration and tracheal suctioning. In the current study, our purpose was different–to delineate the regulation of CBF across the cardiac cycle and determine the relationship of dysautoregulation to GA. While autoregulation is classically measured using low-frequency changes in mean ABP, we studied low-frequency changes in systolic, mean and diastolic ABP separately. We evaluated the capacity for autoregulation across the phases of the cardiac cycle because we observed low diastolic ABP and diastolic CBF velocity in the cohort during a preliminary quality check of the recordings (Figure 1). Low and nearly absent diastolic CBF velocity suggests that CBF in premature infants is likely dependent on systolic ABP, and not mean ABP. This observation may be the normal state of CBF for premature infants but has been described in mature subjects only in the presence of profound cerebrovascular pathology.28, 29
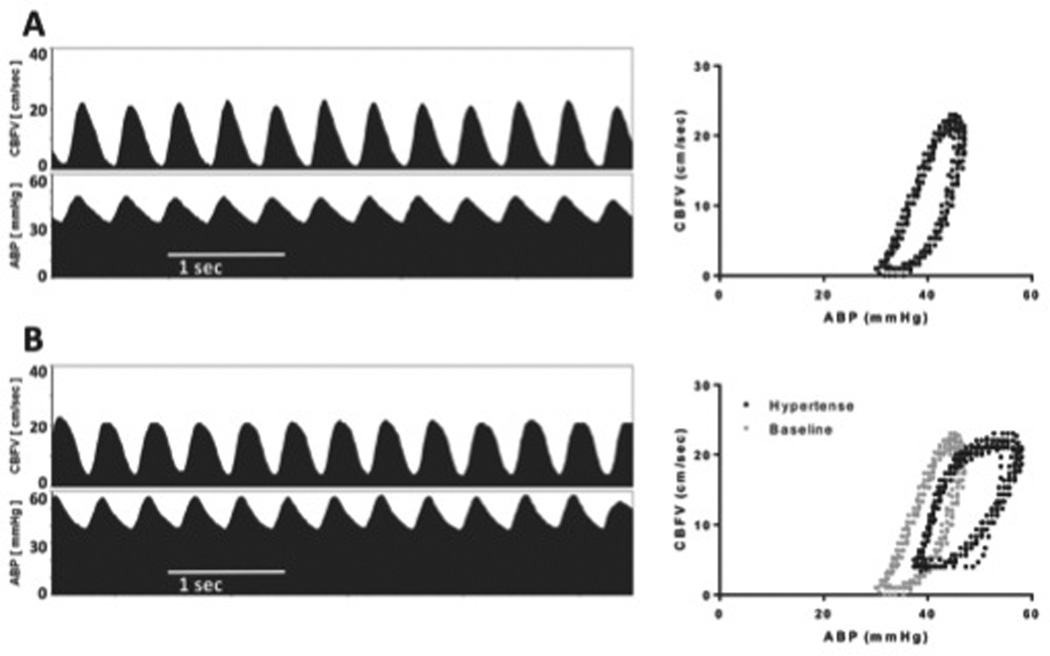
Figure 1.
Changes in middle cerebral artery CBF velocity in a premature infant (28 weeks’ gestation) after hypertension induced by tracheal suctioning. The panels on the left show CBF velocity and ABP in a 5-second window of time. The corresponding graphs to the right show the same CBF velocity recordings as a function of the ABP, illustrating changes during the cardiac cycle. A) At baseline, CBF velocity is noted to be low, and occasionally 0 during diastole for an ABP that is not uncommonly low for the product of 28 weeks’ gestation. B) One minute after a suctioning event, provoking sustained hypertension, CBF velocity during systole is similar to baseline, indicating intact autoregulation, but the diastolic CBF velocity is much higher than baseline, indicating impaired autoregulation.
We hypothesized an absence of diastolic CBF velocity autoregulation in premature infants, and a graded appearance of systolic CBF velocity autoregulation with advancing GA. We performed a multivariate analysis of factors related to the state of autoregulation in this cohort, including ABP normalized to critical closing pressure (CrCP), 5-minute Apgar score, GA, hour of life, vasopressor support and arterial carbon dioxide tension.
Methods
This report is a re-analysis of previously published, prospectively collected data from infants born from July 2002 to April 2008 to determine the ontogeny of cerebral autoregulation in premature infants. Approval was obtained by the Institutional Review Board at the University of Arkansas for Medical Sciences for the initial study period and informed consent was obtained prior to enrollment. The inclusion criteria for the initial studies were infants of very low birth weight (<1500 grams) who received mechanical ventilation and had an umbilical artery catheter in place. Infants were excluded if major congenital anomalies were present.
Physiologic data processing
All infants had measurement of ABP with an umbilical artery catheter placed for clinical monitoring. Middle cerebral artery CBF velocity was recorded with transcranial Doppler ultrasound (Nicolet Vascular/Natus Medical Incorporated, San Carlos, CA). Arterial carbon dioxide tension was continuously recorded with a Neotrend-L fiber-optic sensor (Diametrics Medical Ltd, St. Paul, MN). Analog data was simultaneously collected using a PowerLab 8 Channel data acquisition system (AD Instruments, Mountain View, CA).
Transcranial Doppler and ABP recordings at 200 Hz were analyzed using ICM+ software (ICM+, Cambridge Enterprise, UK). Signals were filtered for artifact using valid value ranges and by inspection for the presence of a physiologic waveform. Autoregulation was quantified by three methods, as a moving correlation coefficient between: 1) systolic ABP and systolic CBF velocity (Sx); 2) mean ABP and mean CBF velocity (Mx); and 3) diastolic ABP and diastolic CBF velocity (Dx). Of 1,037 subject sessions, 911 had sufficient data after artifact removal to render the Sx, and 688 subject sessions had sufficient data to render a CrCP. Data from 630 subject sessions, that had all the variables, were included in the final multivariate model. Trends of systolic and diastolic CBF velocity and ABP were made by sequential analysis of low-pass filtered, transformed 10-second segments. The algorithm used by ICM+ detects systolic peaks and diastolic valleys by the Pan-Tompkins method, rendering a single vector for systole and diastole covering the specified (10-second) buffer. Trends of mean CBF velocity and ABP were made from consecutive 10-second averages of the 200 Hz primary signals.
Autoregulation metrics
Sx was trended as the correlation between 30 consecutive samples of synchronous measures of systolic ABP and CBF velocity, updated at 60-second intervals from overlapping 300-second epochs. Both Mx and Dx were similarly trended as the correlation between 30 consecutive samples of synchronous measures of mean ABP and CBF velocity, and diastolic ABP and CBF velocity, respectively, and updated at 60-second intervals from overlapping 300-second epochs.
The moving correlation method is patterned after the methods first used in the neurosurgical unit at Cambridge University.30 The 10-second averages of ABP and CBF velocity measurements remove pulse and respiratory frequency variation from the correlation analysis. ABP oscillations at the respiratory frequency exceed the maximum frequency limit that will provoke a consistent reaction from the cerebral vasculature.31 Limiting the epoch of correlation analysis to 300 seconds amplifies the contribution of spontaneous slow wave activity (Lundberg’s B waves) known to be relevant to autoregulation and diminishes the contribution of slower confounding drifts of CBF caused by changes in arterial carbon dioxide tension, transfusions and fluid boluses, temperature changes, etc.32, 33 Thus, although the analysis is performed in the time domain, the filters and sampling epochs render a correlation analysis that is relatively specific to frequencies of ABP changes between 0.003 and 0.05 Hz.
The moving correlation method detects dysautoregulation as a positive correlation coefficient. Intact autoregulation is detected as a negative, or near-zero correlation. The Mx has been extensively studied in adult populations, for whom values greater than 0.3 have been associated with poor outcomes.34
Critical Closing Pressure
In a developmentally mature population, ABP is a reasonable surrogate for cerebral perfusion pressure. In premature infants, however, this assumption is potentially confounded by a lower ABP relative to CrCP of the cerebral vasculature. CrCP is the threshold ABP at which CBF ceases as ABP is lowered.35, 36 Conversely, CrCP can be conceptualized as the ABP at which CBF begins as ABP is raised from zero. Thus, the effective perfusion pressure of the mammalian brain is ABP minus CrCP.28 CrCP is greater than 0 mm Hg.37–39 In the mature brain, CrCP has been reported to be nearly 30 mmHg, which is above the mean ABP of many infants in this cohort.36 Thus, inter-subject variability of CrCP could have a profound confounding effect on the assumption that mean ABP is a surrogate for cerebral perfusion pressure in premature infants. We normalized ABP to CrCP to render a “mean closing margin” (effective mean cerebral perfusion pressure = mean ABP – CrCP) and a “diastolic closing margin” (effective diastolic cerebral perfusion pressure = diastolic ABP – CrCP).28 CrCP was determined by analyzing transcranial Doppler and ABP recordings. The CrCP equations are derived from a model of cerebrovascular impedance to an alternating CBF at the frequency of the cardiac cycle. The full derivation has been previously published.40 Values of CrCP are rendered from 20-second epochs and updated in non-overlapping 20-second intervals over each recording session.
Statistics
Subject characteristics including GA, Apgar scores, gender, mode of delivery, exposure to antenatal steroids and multiple gestation pregnancies as well as the use of vasopressor support during each recording session were included in the database. Single, mean values of the study variables and independent variables were recorded for each study session. These included Sx, Mx, Dx; systolic, mean and diastolic ABP; systolic, mean and diastolic CBF velocity; CrCP; mean closing margin; diastolic closing margin; mean arterial carbon dioxide tension, maximum arterial carbon dioxide tension and carbon dioxide fluctuation (maximum – minimum arterial carbon dioxide tension) during the recording session.
Univariate analyses of CBF velocity and autoregulation were performed against GA, reported as regression and Pearson correlation coefficients. This was done with respect to the cardiac cycle, analyzing systole, diastole and mean values separately. From this preliminary analysis, Sx was determined to have the most robust relationship with GA and was used as the primary marker of autoregulation for subsequent multivariate analyses.
Univariate analyses of factors potentially contributing to autoregulation (Sx) were performed with an a priori threshold of p<0.1 for inclusion in the multivariate regression model. These variables included GA at the time of study session, hour of life, 5-minute Apgar score, use of vasopressor support (as a binary variable), ABPs, closing margins and metrics of arterial carbon dioxide tension. Tests of normality (Shapiro-Wilk test) and constant variance were performed (p=0.565 and p=0.07, respectively). For inconsistently repeated measures, multiple linear regression with generalized estimation of equations was performed based on robust covariance matrix using the method proposed by Liang and Zeger and the MATLAB toolbox kit published by Ratcliffe and Shults.41, 42
Results
Infant characteristics
Premature infants (n=179) with GA 26.2 ± 2 weeks (mean ± SD, range 23–33 weeks) and birth weight 824 ± 237 grams had 1-hour recordings of ABP and middle cerebral artery CBF velocity twice daily for 3 days and then daily for the next 4 days during the first week of life (median 4 recording sessions per subject). Of this cohort of patients, 96 (54%) were female, 127 (70%) were born by Cesarean section, 163 (91%) were exposed to antenatal steroids, and 47 (26%) were from multiple gestation pregnancies. The Apgar scores (median, interquartile range) at 1 and 5 minutes were 4 (2 to 6) and 6 (5 to 7), respectively. 172 (92%) of the infants survived to hospital discharge with 3 deaths occurring outside of the initial monitoring period.
Arterial blood pressure and cerebral blood flow velocity
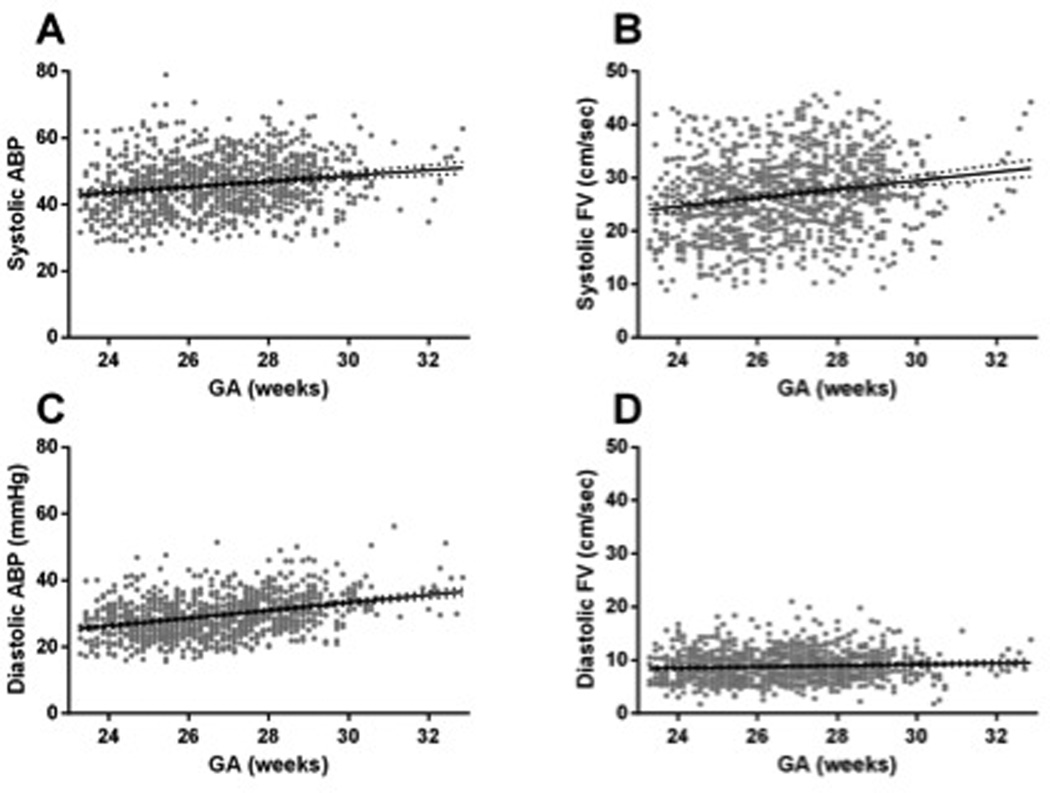
As previously described, we found significant relationships for increased ABP as GA increased.43–45 Diastolic and systolic ABP increased with GA by 1.2 ± 0.1 mm Hg and 0.8 ± 0.1 mm Hg per week of gestation, respectively (r=0.43 and 0.26; p<0.001 for both). Although diastolic ABP increased more than systolic ABP, there was little increase in diastolic FV compared with systolic CBF velocity. Diastolic CBF velocity increased by 0.1 ± 0.05 cm/sec/week of gestation whereas systolic CBF velocity increased by 0.8 ± 0.1 cm/sec/week gestation (r=0.09 and 0.24; p=0.003 and p<0.001 respectively; Figure 2).
Figure 2.
ABP and CBF velocity are shown as a function of GA. A and B) Systolic ABP and CBF velocity both trend upward between 23 and 33 weeks gestation (r = 0.26 and 0.24 respectively, p<0.001). C and D) While diastolic ABP trends upward by more than 1 mm Hg/week gestation in this same developmental period, diastolic CBF velocity shows very little increase, clustering near the functional 0 reading of the transcranial Doppler used (r = 0.43 and 0.009; p < 0.001 and p = 0.003 respectively).
Pressure autoregulation and gestation
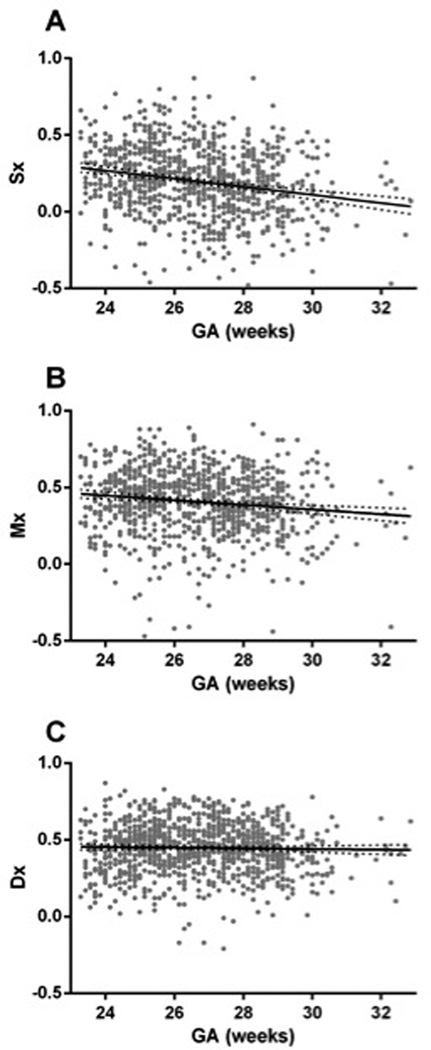
For the whole cohort across all recording sessions, the mean Sx was 0.20 ± 0.22 compared with a mean Dx of 0.45 ± 0.16 (p<0.001 by paired t-test). With advancing GA, we observed a significant and obvious decrease in Sx, indicating improved regulation of systolic CBF velocity across changes in ABP (r=−0.3, p<0.001). By contrast, Dx changed only slightly, albeit significantly as GA increased (r=−0.06, p=0.05; Figure 3).
Figure 3.
Pressure autoregulation of systolic, mean and diastolic CBF velocity as a function of GA. A) The moving correlation between systolic ABP and systolic CBF velocity (Sx) decreased by 0.03 ± 0.003 correlation units/week of gestation (r = −0.3; p < 0.001). B) The moving correlation between mean ABP and CBF velocity (Mx) decreased by 0.015 ± 0.003 correlation units/week of gestation (r = −0.2; p < 0.001). C) The moving correlation between diastolic ABP and CBF velocity (Dx) decreased by only 0.002 ± 0.002 correlation units/week of gestation (r = −0.06; p = 0.05).
Factors influencing pressure autoregulation in the premature infant
We confirmed our observation that Sx decreased with advancing GA in a multivariate analysis of factors thought to influence pressure autoregulation. Univariate analysis was done using a threshold of p-value of 0.1; see Table 1. Hour of life showed a trend toward significance and 5-minute Apgar score was significantly associated with Sx and both were included in the multivariate model. Use of vasopressor support showed a trend for worsening autoregulation that was not significant, but was entered into the final model as a marker of provider perception of circulatory insufficiency.
Table 1.
Univariate regression analysis of factors associated with impaired pressure autoregulation. Autoregulation was quantified as a moving correlation coefficient between systolic ABP and systolic CBF velocity, denoted by Sx. Sx was the dependent variable for the equation.
| R | P Value | n | |
|---|---|---|---|
| GA* | −0.303 | 8.12E-21 | 911 |
| HOL* | −0.0547 | 0.0988 | 911 |
| Ap5* | −0.08 | 0.0157 | 911 |
| Pressors* | 0.0506 | 0.127 | 911 |
| SABP | 0.0291 | 0.38 | 911 |
| MABP | −0.0358 | 0.28 | 911 |
| CM | 0.151 | 0.0000736 | 688 |
| DCM* | 0.209 | 3.14E-08 | 688 |
| CO2 | −0.0168 | 0.614 | 907 |
| CO2Max | 0.00156 | 0.964 | 831 |
| CO2Fluc* | 0.0654 | 0.0596 | 831 |
Variables included in the multivariate regression analysis are indicated with an asterisk.
HOL: hour of life; AP5: 5-minute Apgar score; GA: gestational age; Pressors: use of any vasopressors; CM: closing margin (MABP - CrCP); DCM: diastolic closing margin = diastolic ABP - CrCP; CO2 mean arterial carbon dioxide tension; CO2Max: maximum recorded arterial carbon dioxide tension; CO2Fluc: maximum -minimum arterial carbon dioxide tensions.
Neither systolic nor mean ABPs were associated with changes in Sx, but when ABP was normalized to CrCP, an increased closing margin (ABP – CrCP) was associated with higher Sx (r=0.151, p<0.001). This means that a higher effective cerebral perfusion pressure was associated with worse autoregulation. Interestingly, comparing systole, mean, and diastolic values, the diastolic closing margin (diastolic ABP – CrCP) was more prominently related to Sx (r=0.2, p<0.001). The direction was similar to that seen with the mean closing margin, as higher effective diastolic cerebral perfusion pressure was associated with worse autoregulation. Since diastolic closing margin was most significantly related to Sx in univariate analyses of ABP measurements, it was the ABP measure that was included in the multivariate model.
Continuous intra-arterial carbon dioxide monitoring allowed us to examine the relationship between mean, maximum and fluctuations in arterial carbon dioxide tension on pressure autoregulation. Of these, only the fluctuations of arterial carbon dioxide tension were associated with Sx (r=0.07; p=0.06) and included in the multiple regression model.
Multivariate regression including all of these variables confirmed that both lower GA and higher diastolic closing margin were associated with dysautoregulation as defined by Sx; Table 2. These findings demonstrate that the more premature the infant, the greater the likelihood for CBF to be dysautoregulated in systole. In addition, higher diastolic closing margin, indicating higher cerebral perfusion pressure, was associated with dysautoregulation.
Table 2.
Multivariate regression of factors associated with impaired pressure autoregulation.
| Variable | P value |
|---|---|
| HOL | 0.731 |
| Ap5 | 0.867 |
| Pressors | 0.659 |
| GA | <0.001* |
| DCM | <0.01* |
| CO2Fluc | 0.199 |
HOL: hour of life; AP5: 5-minute Apgar score; Pressors: use of any vasopressors; GA: gestational age; DCM: diastolic closing margin = diastolic ABP – CrCP; CO2fluc: maximum – minimum arterial carbon dioxide tension.
Discussion
These data demonstrate evidence for the development of cerebrovascular pressure autoregulation after the second trimester, between 23 and 33 weeks in human premature infants. In this cohort, we observed an association between GA and intact cerebral autoregulation during systole. However, during diastole, these same infants had low diastolic ABPs, and low or near absent diastolic CBF velocity and demonstrated dysautoregulation during diastole.
We observed that ABP is not predictive of the state of autoregulation in this cohort, and show evidence that inter-subject differences in the CrCP confound the assumption that ABP is a proxy for cerebral perfusion pressure. Prior studies of autoregulation in premature infants have used uncorrected ABP as a surrogate for cerebral perfusion pressure. When ABP was normalized to CrCP, rendering a “closing margin,” or “effective cerebral perfusion pressure,” we found a strong relationship between cerebral perfusion pressure and the state of autoregulation quantified by Sx. Interestingly, this relationship was in the opposite direction of our original hypothesis, which was that dysautoregulation in premature infants is associated with shock and hypotension. Rather, in this cohort, high cerebral perfusion pressure was associated with dysautoregulation.
With the present analysis, we have observed for the first time that when Doppler ultrasound is used to study autoregulation in premature infants, evaluation of the systolic phase of the cardiac cycle is more likely to demarcate optimal care strategies than evaluation of mean values across the cardiac cycle. Infants in our cohort demonstrated ability to autoregulate the surge of CBF velocity during systole before any apparent autoregulation of diastolic CBF velocity. We also confirm previous beliefs that ABP is a poor surrogate of cerebral perfusion pressure in premature infants, and that diastolic closing margin may be a better marker of cerebral perfusion pressure in this population.
Although not the major focus of the study, we did examine the relationship between autoregulation and effective cerebral perfusion pressure. The importance of this finding may be questioned by the fact that the measurement used for determining CrCP is still somewhat investigative. There is no gold standard to measure CrCP and measurements of CrCP are still subject to debate in various animal and human models. Clinically, the limitation of ABP to describe cerebral perfusion pressure becomes a conundrum, as there is no convenient or non-research method to derive CrCP. The association between elevated cerebral perfusion pressure and dysautoregulation should not prompt new management recommendations at this time because dysautoregulation, by itself, is not a meaningful clinical outcome. Thus, if our novel findings can be replicated, then perhaps ABP minus CrCP can be used as a more accurate marker of cerebral perfusion pressure.
The main limitation of the study is that previously published data were re-analyzed for the new variables of autoregulation and CrCP. On the other hand, the breadth and temporal resolution of the original dataset from 179 premature infants during the first week of life is one of the largest ever collected with such high fidelity of physiological variables. Thus, re-analysis of this robust dataset using software that can capitalize on the quality of the data was warranted. Changes in practice may have occurred since the original dataset was obtained, therefore the authors do not support new blood pressure management based on the data presented here. Finally, the need for high-quality resolution of systole and diastole resulted in some data dropout. Although the study was still adequately powered, unexpected bias may have been introduced by the data loss.
In conclusion, this study demonstrates for the first time development of autoregulation in human premature infants between 23 and 33 weeks’ gestation. Stark differences between systolic and diastolic CBF velocity autoregulation were seen with no evidence for autoregulation of diastolic CBF velocity in the premature infants of this cohort. Dysautoregulation of systolic CBF velocity was associated with both lower GA and higher effective cerebral perfusion pressure. These results are informative for future studies of autoregulation, effective cerebral perfusion pressure and neurocognitive outcomes in premature infants.
Acknowledgements
Dr. Kaiser was supported by the National Institutes of Health (1K23NS43185, RR20146, and 1R01NS060674) and the University of Arkansas for Medical Sciences Translational Research Institute (1UL1RR029884).
The technical assistance of Natalie C. Sikes and Melanie J. Mason, and the support of the University of Arkansas for Medical Sciences neonatologists, NICU nurses, respiratory therapists, and ultrasound technicians, are gratefully appreciated.
Each author listed on this manuscript has seen and approved the submission of this version of the manuscripts and takes full responsibility for the manuscript. Dr. Rhee conceptualized the reanalysis of the primary data, performed the data analysis, drafted the initial manuscript and reviewed and revised the manuscript. Ms. Kibler, Drs. Easley, Andropoulos, Czosnyka, Smielewski and Rusin reviewed and revised the manuscript. Dr. Varsos performed data analysis and reviewed and revised the manuscript. Dr. Brady performed data analysis, reviewed and revised the manuscript. Dr. Kaiser conceptualized and designed the initial study, collected the primary data, performed the data analysis and reviewed and revised the manuscript.
References
- 1.Seri I, editor. Circulatory support of the sick newborn infant. London: WB Saunders Co; 2001. [DOI] [PubMed] [Google Scholar]
- 2.Sarkar S, Bhagat I, Dechert R, Schumacher RE, Donn SM. Severe intraventricular hemorrhage in preterm infants: comparison of risk factors and short-term neonatal morbidities between grade 3 and grade 4 intraventricular hemorrhage. Amer J Perinatol. 2009;26(06):419–424. doi: 10.1055/s-0029-1214237. 18.05.2009. [DOI] [PubMed] [Google Scholar]
- 3.Kaiser JR, Gauss CH, Williams DK. The effects of hypercapnia on cerebral autoregulation in ventilated very low birth weight infants. Pediatr Res. 2005;58(5):931–935. doi: 10.1203/01.pdr.0000182180.80645.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Aweel I, Pursley D, Rubin L, Sharh B, Weisberger S, Richardson D. Variations in prevalence of hypotension, hypertension, and vasopressor use in NICUs. J Perinatol. 2001;21(5):272–278. doi: 10.1038/sj.jp.7210563. [DOI] [PubMed] [Google Scholar]
- 5.Laughon M, Bose C, Allred E, O'Shea TM, Marter LJV, Bednarek F, et al. Factors associated with treatment for hypotension in extremely low gestational age newborns during the first postnatal week. Pediatrics. 2007;119(2):273–280. doi: 10.1542/peds.2006-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perlman J, Volpe JJ. Intraventricular hemorrhage in extremely small premature infants. Am J Dis Child. 1986;140(11):1122–1124. doi: 10.1001/archpedi.1986.02140250048034. [DOI] [PubMed] [Google Scholar]
- 7.Volpe JJ. Brain injury in the premature infant. Neuropathology, clinical aspects, pathogenesis, and prevention. Clin Perinatol. 1997;24(3):567–587. [PubMed] [Google Scholar]
- 8.Del Toro J, Louis PT, Goddard-Finegold J. Cerebrovascular regulation and neonatal brain injury. Pediatr Neurol. 1991;7(1):3–12. doi: 10.1016/0887-8994(91)90098-6. [DOI] [PubMed] [Google Scholar]
- 9.Ment LR, Stewart WB, Duncan CC, Pitt BR, Rescigno A, Cole J. Beagle puppy model of perinatal cerebral infarction. J Neurosurg. 1985;63(3):441–447. doi: 10.3171/jns.1985.63.3.0441. [DOI] [PubMed] [Google Scholar]
- 10.Ment LR, Stewart WB, Duncan CC, Pitt BR. Beagle puppy model of perinatal cerebral insults. J Neurosurg. 1986;65(6):847–850. doi: 10.3171/jns.1986.65.6.0847. [DOI] [PubMed] [Google Scholar]
- 11.Lynch JK, Nelson KB. Epidemiology of perinatal stroke. Curr Opin Pediatr. 2001;13(6):499–505. doi: 10.1097/00008480-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Lee JK, Kibler KK, Benni PB, Easley RB, Czosnyka M, Smielewski P, et al. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke. 2009;40(5):1820–1826. doi: 10.1161/STROKEAHA.108.536094. [DOI] [PubMed] [Google Scholar]
- 13.Aries MJH, Czosnyka M, Budohoski KP, Steiner LA, Lavinio A, Kolias AG, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40(8):2456–2463. doi: 10.1097/CCM.0b013e3182514eb6. [DOI] [PubMed] [Google Scholar]
- 14.Steiner L, Czosnyka M, Piechnik S, Smielewski P, Chatfield D, Menon DK, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30(4):733–738. doi: 10.1097/00003246-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Lightburn M, Gauss C, Williams D, Kaiser JR. Cerebral blood flow velocities in extremely low birth weight infants with hypotension and infants with normal blood pressure. J Pediatr. 2009;154(6):824–828. doi: 10.1016/j.jpeds.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perlman J, Goodman S, Kreusser K, Volpe JJ. Reduction in intraventricular hemorrhage by elimination of fluctuating cerebral blood-flow velocity in preterm infants with respiratory distress syndrome. N Engl J Med. 1985;312(21):1353–1357. doi: 10.1056/NEJM198505233122104. [DOI] [PubMed] [Google Scholar]
- 17.Pryds O, Greisen G, Lou H, Friis-Hansen B. Heterogeneity of cerebral vasoreactivity in preterm infants supported by mechanical ventilation. Pediatrics. 1989;115(4):638–645. doi: 10.1016/s0022-3476(89)80301-4. [DOI] [PubMed] [Google Scholar]
- 18.Tsuji M, Saul JP, du Plessis A, Eichenwald E, Sobh J, Crocker R, et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. 2000;106(4):625–632. doi: 10.1542/peds.106.4.625. [DOI] [PubMed] [Google Scholar]
- 19.Bassan H, Gauvreau K, Newburger J, Tsuji M, Limperopoulos C, Soul JS, et al. Identification of pressure passive cerebral perfusion and its mediators after infant cardiac surgery. Pediatr Res. 2005;57(1):35–41. doi: 10.1203/01.PDR.0000147576.84092.F9. [DOI] [PubMed] [Google Scholar]
- 20.Gilmore MM, Stone BS, Shepard JA, Czosnyka M, Easley RB, Brady KM. Relationship between cerebrovascular dysautoregulation and arterial blood pressure in the premature infant. J Perinatol. 2011;31(11):722–729. doi: 10.1038/jp.2011.17. [DOI] [PubMed] [Google Scholar]
- 21.Soul J, Hammer P, Tsuji M, Saul J, Bassan H, Limperopoulos C, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007;61(4):467–473. doi: 10.1203/pdr.0b013e31803237f6. [DOI] [PubMed] [Google Scholar]
- 22.Wong F, Leung T, Austin T, Wilkinson M, Meek J, Wyatt J, et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics. 2008;121(3):e604–e611. doi: 10.1542/peds.2007-1487. [DOI] [PubMed] [Google Scholar]
- 23.Szymonowicz W, Walker A, Yu V, Stewart M, Cannata J, Cussen L. Regional cerebral blood flow after hemorrhagic hypotension in the preterm, near-term, and newborn lamb. Pediatr Res. 1990;28(4):361–366. doi: 10.1203/00006450-199010000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Helou S, Koehler R, Gleason C, Jones M, Jr, Traystman R. Cerebrovascular autoregulation during fetal development in sheep. Am J Physiol. 1994;266:H1069–H1074. doi: 10.1152/ajpheart.1994.266.3.H1069. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser JR, Gauss CH, Williams DK. Surfactant administration acutely affects cerebral and systemic hemodynamics and gas exchange in very-low-birth-weight infants. J Pediatr. 2004;144(6):809–814. doi: 10.1016/j.jpeds.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 26.Kaiser J, Gauss C, Williams D. Tracheal suctioning is associated with prolonged disturbances of cerebral hemodynamics in very low birth weight infants. J Perinatol. 2007;28(1):34–41. doi: 10.1038/sj.jp.7211848. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser J, Gauss C, Williams D. The effects of closed tracheal suctioning plus volume guarantee on cerebral hemodynamics. J Perinatol. 2011;31(10):671–676. doi: 10.1038/jp.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varsos GV, de Riva N, Smielewski P, Pickard J, Brady K, Reinhard M, et al. Critical closing pressure during intracranial pressure plateau waves. Neurocrit Care. 2013;18(3):341–348. doi: 10.1007/s12028-013-9830-5. [DOI] [PubMed] [Google Scholar]
- 29.Nelson RJ, Czosnyka M, Pickard JD, Maksymowicz W, Perry S, Lovick AHJ. Experimental aspects of cerebrospinal hemodynamics: the relationship between blood flow velocity waveform and cerebral autoregulation. Neurosurgery. 1992;31(4):705–710. doi: 10.1227/00006123-199210000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Czosnyka M, Smielewski P, Kirkpatrick P, Menon DK, Pickard JD. Monitoring of cerebral autoregulation in head-injured patients. Stroke. 1996;27(10):1829–1834. doi: 10.1161/01.str.27.10.1829. [DOI] [PubMed] [Google Scholar]
- 31.Fraser CD, III, Brady K, Rhee CJ, Easley RB, Kibler KK, Smielewski P, et al. The frequency response of cerebral autoregulation. J Appl Physiol. 2013;115(1):52–56. doi: 10.1152/japplphysiol.00068.2013. [DOI] [PubMed] [Google Scholar]
- 32.Lundberg N, Troupp H, Lorin H. Continuous recording of the ventricular-fluid pressure in patients with severe acute traumatic brain injury. A preliminary report. J Neurosurg. 1965;22(6):581–590. doi: 10.3171/jns.1965.22.6.0581. [DOI] [PubMed] [Google Scholar]
- 33.Steinmeier R, Bauhuf C, Hubner U, Bauer RD, Fahlbusch R, Laumer R, et al. Slow rhythmic oscillations of blood pressure, intracranial pressure, microcirculation, and cerebral oxygenation: dynamic interrelation and time course in humans. Stroke. 1996;27(12):2236–2243. doi: 10.1161/01.str.27.12.2236. [DOI] [PubMed] [Google Scholar]
- 34.Czosnyka M, Smielewski P, Lavinio A, Pickard JD, Panerai R. An assessment of dynamic autoregulation from spontaneous fluctuations of cerebral blood flow velocity: a comparison of two models, index of autoregulation and mean flow index. Anesth Analg. 2008;106(1):234–239. doi: 10.1213/01.ane.0000295802.89962.13. [DOI] [PubMed] [Google Scholar]
- 35.Nichol J, Girling F, Jerrard W, Claxton E, Burton A. Fundamental instability of the small blood vessels and critical closing pressures in vascular beds. Am J Physiol. 1951;164(2):330–344. doi: 10.1152/ajplegacy.1951.164.2.330. [DOI] [PubMed] [Google Scholar]
- 36.Panerai R. The critical closing pressure of the cerebral circulation. Med Engineer Phys. 2003;25(8):621–632. doi: 10.1016/s1350-4533(03)00027-4. [DOI] [PubMed] [Google Scholar]
- 37.Dewey R, Pieper H, Hunt W. Experimental cerebral hemodynamics. Vasomotor tone, critical closing pressure, and vascular bed resistance. J Neurosurg. 1974;41(5):597–606. doi: 10.3171/jns.1974.41.5.0597. [DOI] [PubMed] [Google Scholar]
- 38.Michel E, Zernikow B, von Twickel J, Hillebrand S, Jorch G. Critical closing pressure in preterm neonates: towards comprehensive model of cerebral autoregulation. Neurol Res. 1995;17(2):149–155. doi: 10.1080/01616412.1995.11740304. [DOI] [PubMed] [Google Scholar]
- 39.Panerai R, Kelsall A, Rennie J, Evans D. Estimation of critical closing pressure in the cerebral circulation of newborns. Neuropediatrics. 1995;26(3):168–173. doi: 10.1055/s-2007-979748. [DOI] [PubMed] [Google Scholar]
- 40.Varsos GV, Richards H, Kasprowicz M, Budohoski KP, Brady KM, Reinhard M, et al. Critical closing pressure determined with a model of cerebrovascular impedance. J Cereb Blood Flow Metab. 2013;33(2):235–243. doi: 10.1038/jcbfm.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 42.Ratcliffe S, Shults J. GEEQBOX: A Matlab toolbox for generalized estimating equations and quasi-least squares. J Stat Softw. 2008;25(14):1–14. [Google Scholar]
- 43.Spinazzola R, Harper R, de Soler M, Lesser M. Blood pressure values in 500- to 750-gram birthweight infants in the first week of life. J Perinatol. 1991;11(2):147–151. [PubMed] [Google Scholar]
- 44.Lee J, Rajadurai VS, Tan KW. Blood pressure standards for very low birthweight infants during the first day of life. Arch Dis Child Fetal Neonatal. 1999;81(3):F168–F170. doi: 10.1136/fn.81.3.f168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cunningham S, Symon A, Elton R, Zhu C, McIntosh N. Intra-arterial blood pressure reference ranges, death and morbidity in very low birthweight infants during the first seven days of life. Early Hum Dev. 1999;56(2–3):151–165. doi: 10.1016/s0378-3782(99)00038-9. [DOI] [PubMed] [Google Scholar]