Abstract
High-risk human papillomavirus (HR HPV) oncoproteins bind host cell proteins to dysregulate and uncouple apoptosis, senescence, differentiation, and growth. These pathways are important for both the viral life cycle and cancer development. HR HPV16 E6 (16E6) interacts with the cellular protein NFX1-123, and they collaboratively increase the growth and differentiation master regulator, Notch1. In 16E6 expressing keratinocytes (16E6 HFKs), the Notch canonical pathway genes Hes1 and Hes5 were increased with overexpression of NFX1-123, and their expression was directly linked to the activation or blockade of the Notch1 receptor. Keratinocyte differentiation genes Keratin 1 and Keratin 10 were also increased, but in contrast their upregulation was only indirectly associated with Notch1 receptor stimulation and was fully unlinked to growth arrest, increased p21Waf1/CIP1, or decreased proliferative factor Ki67. This leads to a model of 16E6, NFX1-123, and Notch1 differently regulating canonical and differentiation pathways and entirely uncoupling cellular arrest from increased differentiation.
Keywords: HPV E6, Keratinocytes, NFX1-123, Notch, Differentiation, Cell cycle
Introduction
Human Papillomaviruses (HPVs) are small double-stranded DNA viruses that infect mucosa and cutaneous epithelium, and the more than 100 genotypes of HPVs are divided into five genera. The alpha genus of HPV is further subdivided into HPV types termed low risk (LR HPV), which are associated with genital warts and benign lesions, and high risk (HR HPV), which can cause anogenital cancers. A long-term, persistent infection with a HR HPV type is the greatest risk factor for cervical cancer development (Moscicki, Schiffman et al., 2012; Rodriguez, Schiffman et al., 2010; Kjaer, van den Brule et al., 2002), and therefore the manner by which HR HPV persists and dysregulates its host cell, leading to cancer, is a focus of intensive research.
HPV depends upon both epithelial differentiation and continued cell cycling to complete its life cycle, and these processes are regulated by both the host cell and the virus (reviewed in (Doorbar, Quint et al., 2012)). HPV first infects the basal layer of stratified squamous epithelium, typically through micro-abrasions or anatomic epithelial transitions that expose these cells. Once the virus infects a basal cell, the episomal viral DNA migrates to the nucleus and is maintained at low copy numbers initially in proliferative epithelial cells while viral early proteins are expressed. As epithelial cells migrate to upper layers of stratified squamous epithelium, the expression of late viral proteins is induced, and the viral genome becomes amplified to thousands of copies per cell. Finally, virion assembly is coupled to late cellular differentiation, and infectious virus is released as host epithelial cells slough off. Despite our understanding of the normal HPV viral life cycle and normal epithelial differentiation, the molecular events that directly regulate the switch from viral genome maintenance to viral genome amplification and productive infection in an HPV-infected cell are not well understood, nor are the HPV-driven disruptions of epithelial differentiation, cell cycling, and cellular arrest pathways.
HR HPV type 16 (HPV 16) encodes two viral oncoproteins: E6 and E7. These oncoproteins perturb apoptosis and senescence by degradation of tumor suppressors p53 and Rb, dysregulation of angiogenesis, and modulation of keratinocyte growth and differentiation. HPV 16E6 specifically partners with cellular proteins to alter multiple epithelial and oncogenic pathways. One host cell protein bound by 16E6 is the cytoplasmic protein NFX1-123 (Katzenellenbogen, Egelkrout et al., 2007). NFX1-123 increases telomerase activity through post-transcriptional gene upregulation in HPV 16E6 expressing human foreskin keratinocytes (HFKs) (Katzenellenbogen, Vliet-Gregg et al., 2009; Katzenellenbogen, Egelkrout et al., 2007). Recently, our laboratory showed that NFX1-123 also synergizes with 16E6 to modulate Notch1 protein and mRNA expression (Vliet-Gregg, Hamilton et al., 2013). Both telomerase and Notch1 are important regulators of cellular senescence, growth, and differentiation.
The mammalian Notch gene family encodes evolutionary conserved type 1 transmembrane receptors that play a key role in cell fate determination and differentiation through cell-cell communication (Borggrefe & Oswald, 2009; Watt, Estrach et al., 2008). The Notch family of cell surface receptors is comprised of four transmembrane proteins (Notch1 to Notch4). In keratinocytes, Notch1 and Notch2 are primarily expressed. During canonical Notch1 signaling, ligands belonging to the Delta or Serrate/Jagged family bind the Notch receptor, and the receptor is cleaved by the gamma-secretase protease complex, releasing the Notch intracellular domain (NICD), to translocate to the nucleus (Watt, Estrach et al., 2008). In the nucleus, NICD associates with the DNA binding protein RBP-J (also known as CBF1) and its transcriptional coactivator Mastermind (MAML), activating downstream targets such as HES and HEY family members (Watt, Estrach et al., 2008). Notch1 signaling has been identified as both oncogenic and tumor suppressive in cervical cancer development and progression, dependent on the experimental design and cellular context (Yugawa, Handa et al., 2007; Veeraraghavalu, Pett et al., 2004; Bajaj, Maliekal et al., 2011; Henken, De-Castro et al., 2012; Lathion, Schaper et al., 2003; Maliekal, Bajaj et al., 2008; Rangarajan, Syal et al., 2001; Talora, Sgroi et al., 2002; Weijzen, Zlobin et al., 2003; Yugawa, Narisawa-Saito et al., 2010; Zagouras, Stifani et al., 1995).
In the present study, we investigate the roles Notch1 and NFX1-123 each play in Notch canonical pathway regulation, differentiation pathway regulation, and cellular growth and cycling in 16E6 HFKs. Endogenous NFX1-123 is expressed to a greater degree in HPV positive cervical cancer cell lines than in HFKs, and when 16E6 HFKs are transduced to overexpress NFX1-123, we found NFX1-123 had dual modes of action on the Notch canonical pathway and the differentiation pathway. In these 16E6 HFKs, increased NFX1-123 expression led to an augmented activation of canonical pathway genes Hes1 and Hes5, and this occurred directly through the Notch1 receptor. We also discovered that overexpressed NFX1-123 upregulated the gene expression of differentiation gene markers Keratin 1 and Keratin 10. However, this increase in differentiation gene expression in 16E6 HFKs with overexpressed NFX1-123 did not parallel the kinetics and magnitude of Notch canonical pathway gene activation, nor did it depend fully on the Notch1 receptor. Finally, the enhancement of the Notch canonical pathway and differentiation gene expression did not trigger the expected concomitant increase in cell cycle arrest or decrease in growth factor gene expression. The distinct activation of Notch canonical pathway genes and differentiation pathway genes by overexpressed NFX1-123 leads to a model of direct and indirect pathway regulation, respectively. It also highlights an uncoupling of keratinocyte differentiation from cell cycle exit in 16E6 HFKs through NFX1-123 overexpression, an unusual cellular phenotypic state that may support HPV persistence and cancer progression.
Material and Methods
Microarray
Microarray analysis has been previously described and is accessible through GEO (GSE43082) (Vliet-Gregg, Hamilton et al., 2013). Biological interpretation using Gene Ontology was performed using GeneSpring GX11.5.1 (Agilent Technologies, Santa Clara, CA).
Tissue culture
Primary human foreskin keratinocytes (HFKs) were cultured as described previously (Gewin, Myers et al., 2004). Briefly, HFKs were derived from neonatal foreskins and grown in EpiLife medium supplemented with calcium chloride (60 µM), human keratinocyte growth supplement (Life Technologies, Carlsbad, CA), and penicillin-streptomycin. HFKs cultured for cyclin detection were treated with 10µM of MG132 (Sigma, Saint Louis, MO), two hours prior to harvest of whole cell protein extracts. 293T cells were grown in Dulbecco’s modified Eagle’s medium (GIBCO-BRL, Carlsbad, CA) containing 10% fetal bovine serum and penicillin-streptomycin.
Retrovirus production and infection
Retrovirus was produced in 293T cells by a transient vesicular stomatitis virus G-pseudotyped virus (VSV-G) production protocol as previously described (Bartz & Vodicka, 1997). Lentivirus was also produced as previous described (Katzenellenbogen, Egelkrout et al., 2007). Retrovirus and lentivirus was serially collected, concentrated by ultracentrifuge, mixed with Polybrene (8 µg/mL) (EMD Millipore, Billerica, MA), and incubated with 50 to 60% confluent HFKs. Three hours later, the Epilife medium was replaced. For VSV-G pseudotyped virus infections (FNFX1-123WT, LXSN vector control, pBabe-puro 16E6) the cells were expanded 24 hours post-transduction, and after 48 hours the cells were placed under neomycin/G418 selection (50 µg/mL) or puromycin selection (0.5 µg/mL). All lentivirus infections (scramble) were confirmed by green fluorescent protein expression.
Quantitative real-time PCR and Taqman array
Total RNA was isolated with TRIzol reagent (Life Technologies, Carlsbad, CA). Total RNA (1µg) was DNase treated and used to generate cDNA with random hexamer primers and SuperScript II reverse transcriptase (Life Technoloiges, Carlsbad, CA). Quantitative real-time PCR was performed using an ABI StepOne Plus system (Applied Biosystems, Foster City, CA). Amplification was carried out using TaqMan master mix and the following pre-designed Taqman probes: GAPDH (4333764F), KRT1 (Hs00196158_m1), KRT10 (Hs00166289), LOR (HS01894962_s1), Notch1 (HS01062014_m1), HES1 (Hs00172878_m1), and HES5 (Hs01387463_g1) according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). Reactions were performed in triplicate. Data analysis was performed using the comparative threshold cycle method (Applied Biosystems, Foster City, CA) to determine relative expression levels, with GAPDH to normalize mRNA levels within each sample. Values graphed are the mean fold-change in each sample compared to control, and error bars graphed represent the standard deviation for each sample (n=3). Primer sequences for NFX1-123, p21Waf1/CIP1 and 36B4 were described previously (McDade, Patel et al., 2011; Katzenellenbogen, Egelkrout et al., 2007). Amplification was carried out as previously published (Katzenellenbogen, Egelkrout et al., 2007) using Power Sybr Green Master Mix (Life Technologies, Foster City, CA. Error bars using NFX1-123, p21Waf1/CIP1 and 36B4 primers represent 95% confidence intervals.
Immunoblotting
Western Blots were performed as described previously (Vliet-Gregg, Hamilton et al., 2013). Blots were probed with the following antibodies: actin (I-19) (1:1000; Scbt, Santa Cruz, CA), Cleaved Notch1 (Val1744) (1:1000; Cell Signaling, Danvers, MA), Cyclin A (H432) (1:500; Scbt, Santa Cruz, CA), Cyclin B1 (GNS1) (1:500; Scbt, Santa Cruz, CA), Cyclin E (C-19) (1:500; Scbt, Santa Cruz, CA), GAPDH (1:100,000, Abcam, Cambridge, MA) and Notch1 (5B5) (1:1000; Cell Signaling, Danvers, MA). The rabbit polyclonal anti-NFX1-123 antibody was generously provided by Dr. Ann Roman.
Immunofluorescence
16E6 HFK cell lines were cultured on glass coverslips and grown to 60% confluency as described previously (Katzenellenbogen, Vliet-Gregg et al., 2009). Cells were then fixed with 4% paraformaldehyde, permeabilized and stained with Ki67 antibody VP-K451 (1:1000 for 60 minutes, Vector Laboratories, Burlingame, CA) and TO-PRO3 nuclear stain (Life Technologies, Carlsbad, CA), as previously described (Katzenellenbogen, Vliet-Gregg et al., 2009). Confocal microscopy was conducted as previously described (Katzenellenbogen, Vliet-Gregg et al., 2009). For the Ki67 expression rates, more than 700 cells for both LXSN-expressing and FLAG-NFX1-123-expressing HFKs were counted. Ki67 expression was determined manually, and the percent of Ki67+ cells (Ki67+cells/total cells) and 95% confidence intervals were calculated using Excel (Redmond, WA).
Dual Immunohistochemistry
16E6 HFKs were grown in culture, rinsed with warm PBS, scraped off tissue culture plates, and centrifuged. The cell pellet was washed in PBS and resuspended in 10% neutral buffered formalin. The cell pellet was fixed for three to five days at 4°C and embedded in paraffin for staining. Antigen was retrieved by Trilogy steamer for 20 minutes with a 20 minute cool down. Cells were blocked with TCT Buffer and stained with Ki67 MIB1 (1:50 for 60 minutes; Dako #M7240, Carpinteria, CA), Keratin 1 (1:200 for 60 minutes; Covance #PRB-149P, Princeton, NJ), or an isogenic IgG control. Cells were counterstained with DAPI. Confocal microscopy was conducted as previously described (Katzenellenbogen, Vliet-Gregg et al., 2009). Mean gray scale value of Keratin 1 was quantified on Z-stack cell projections using ImageJ. Images were quantified after adjustment to isotype control threshold values.
Delta-1 Ligand and Gamma Secretase Inhibitor treatment
Chemical inhibition of Notch signaling was done by treating cells with 10µM gamma secretase inhibitor IX, DAPT (DAPT, #565784, Millipore, Billerica, MA). Delta-1 ligand (DL1) was produced as previously described (Varnum-Finney, Wu et al., 2000) and was a generous gift from Dr. Colleen Delaney for Notch1 activation. Briefly, 2.5 µg/mL of DL1 and 5 µg/mL of retronectin [a vehicle that is required for immobilizing Delta-1 ligand to the non-coated tissue culture plate] or retronectin alone as a control (Takara/Clontech, Mountain View, CA) were diluted in chilled PBS, coated onto non-tissue culture treated plates at 0.16 mL/cm2 and kept overnight at 4°C. Keratinocytes were then plated the following day after washing the plates with PBS. All experiments were done in keratinocytes at 50–60% confluency in culture because Notch signaling occurs between adjacent cells and cell-cell contact is an inducer of keratinocyte differentiation in culture. Keratinocytes used for these experiments were also of equivalent passages and were never cultured beyond passage fifteen.
Fluorescence-activated cell sorter (FACS) analysis
For the pulse chase experiments, cells were synchronized by growing to confluence and waiting an additional 24 hours before release. Cells were released and labeled with 200 µM BrdU for one hour, chased in BrdU-free media, and collected at indicated times. Cells were harvested by trypsinization and pelleted by centrifugation. All centrifugation steps were done for five minutes at 1000xg. Cell pellets were resuspended in cold phosphate-buffered saline (PBS); and while being vortexed, samples were fixed by the addition of cold 95% ethanol for overnight storage at 4°C. Nuclei were isolated from fixed cells by incubating pellets with 3mL of 0.08% pepsin, for 20 minutes at 37°C. Samples were then pelleted by centrifugation and the supernatant was aspirated. Nuclei were resuspended, while being vortexed, with 1.5mL 2M HCl and incubated for 15 minutes at 37°C. Twice the original sample volume of 0.1M Sodium Borate was then added while vortexing. Nuclei were pelleted by centrifugation and the supernatant was aspirated. The resulting pellet was then resuspended, with continuous vortexing, in 2mL IFA (150 mM NaCl, 4% fetal bovine serum, 0.1% sodium azide) plus 5% Tween20. Samples were once again pelleted by centrifugation and the supernatant was aspirated. Nuclei were stained with fluorescein isothiocyanate (FITC) conjugated anti-BrdU antibody (Becton Dickinson, Franklin Lakes, NJ), for 30 minutes on ice. Nuclei were pelleted, supernatant removed, and resulting pellet was resuspended in 250 µL of 5 µg/ml RNase A dissolved in IFA, transferred to 12 by 75-mm snap cap tubes, and incubated at 37°C for 15 minutes. Following incubation, 250 µL of 100 µg propidium iodide (PI) per ml was added. Nuclei were then analyzed using a BD FACS Scan instrument (Becton Dickinson) and FlowJo software (Ashland, OR).
Results
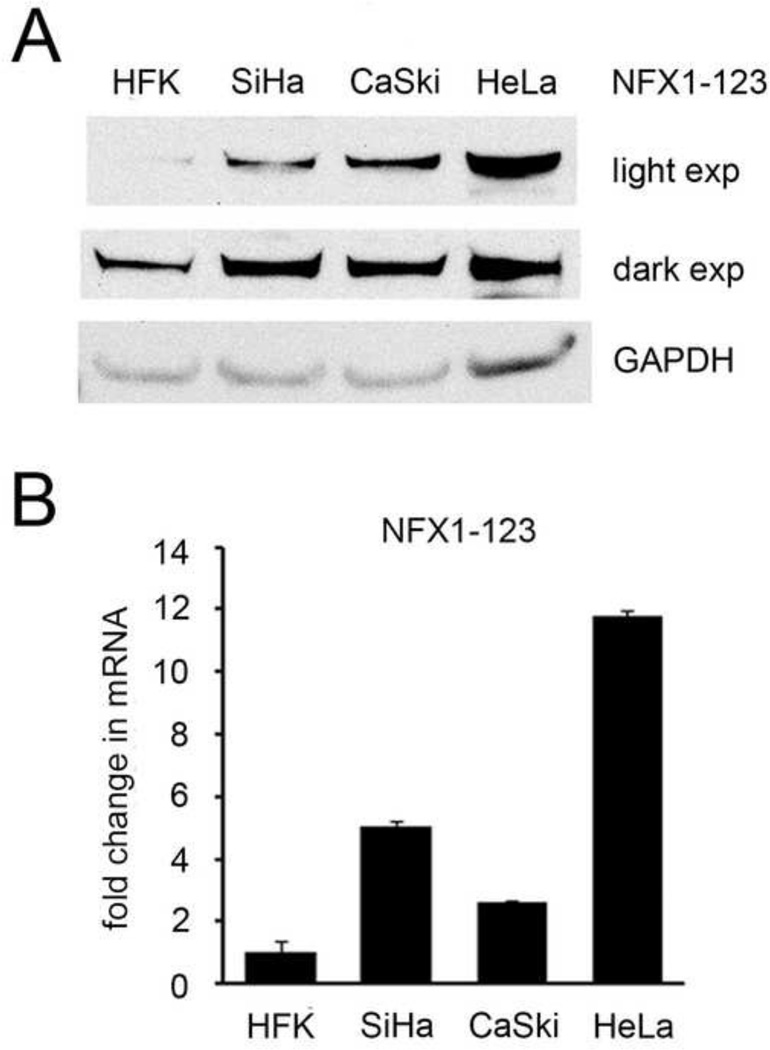
NFX1-123 expression increased in cervical cancer cell lines
NFX1-123 and 16E6 and collaboratively regulate hTERT and Notch1 expression (Katzenellenbogen, Egelkrout et al., 2007; Vliet-Gregg, Hamilton et al., 2013; Katzenellenbogen, Vliet-Gregg et al., 2009), and both hTERT and Notch1 drive cellular growth and malignant transformation (Katzenellenbogen, Vliet-Gregg et al., 2010; Henken, De-Castro et al., 2012). As hTERT and Notch1 can be increased in cervical cancer development and progression, we hypothesized that NFX1-123 may also be increased in HPV positive cervical cancer cell lines. Indeed, SiHa, CaSki, and HeLa cell lines all had increased protein and mRNA NFX1-123 expression (Figure 1A and B). Therefore, because NFX1-123 levels were increased, we chose to study further how a gain in NFX1-123 expression in 16E6 HFKs would affect downstream Notch canonical, growth, and differentiation pathways.
Figure 1. NFX1-123 expression in epithelial cell lines.
(A) Representative immunoblot of NFX1-123 protein in whole cell extracts of HFKs and cervical cancer cell lines. GAPDH was used as a loading control. (B) Relative levels of NFX1-123 mRNA expression by qPCR. Error bars represent 95% confidence intervals from the technical triplicate replicates shown.
NFX1-123 and Notch regulation of canonical pathway genes in 16E6 HFKs
We previously published that Notch1 expression was modulated by the level of NFX1-123 in HFKs; 16E6 synergistically increased or decreased Notch1 dependent on the amount of NFX1-123 expressed (Vliet-Gregg, Hamilton et al., 2013). Multiple components of the Notch canonical pathway, like Hes1 and Hes5, were increased in 16E6 HFKs with greater NFX1-123, and were reduced when NFX1-123 was knocked down by short-hairpin RNA (Vliet-Gregg, Hamilton et al., 2013). Whether NFX1-123 drove expression of Hes1 and Hes5 directly itself or through Notch1 and its canonical signaling pathway was unclear, however.
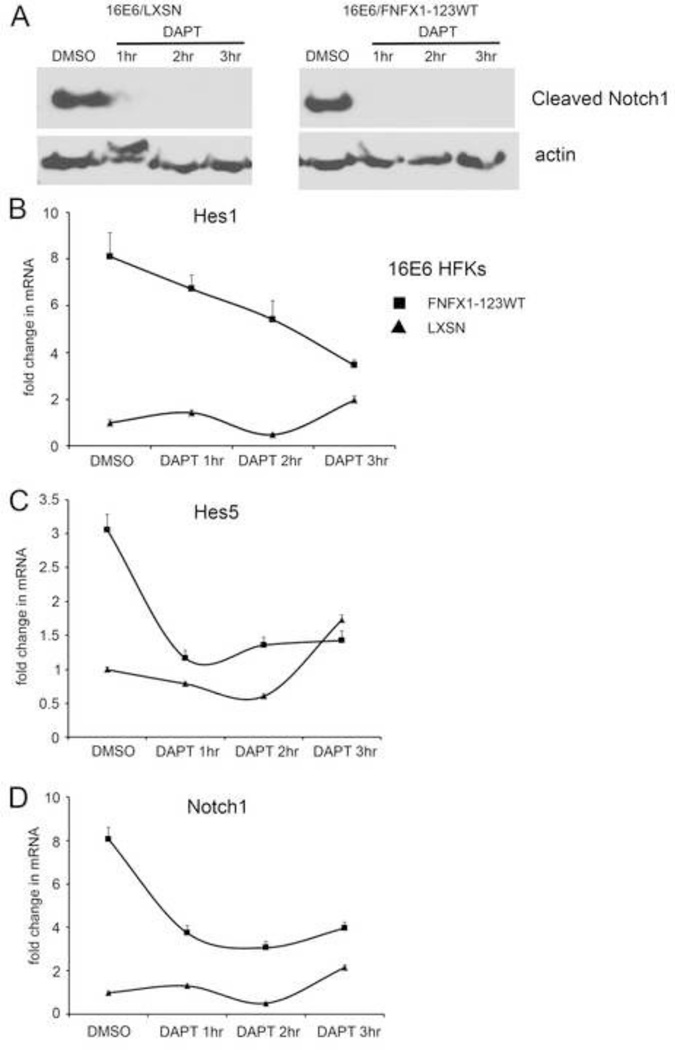
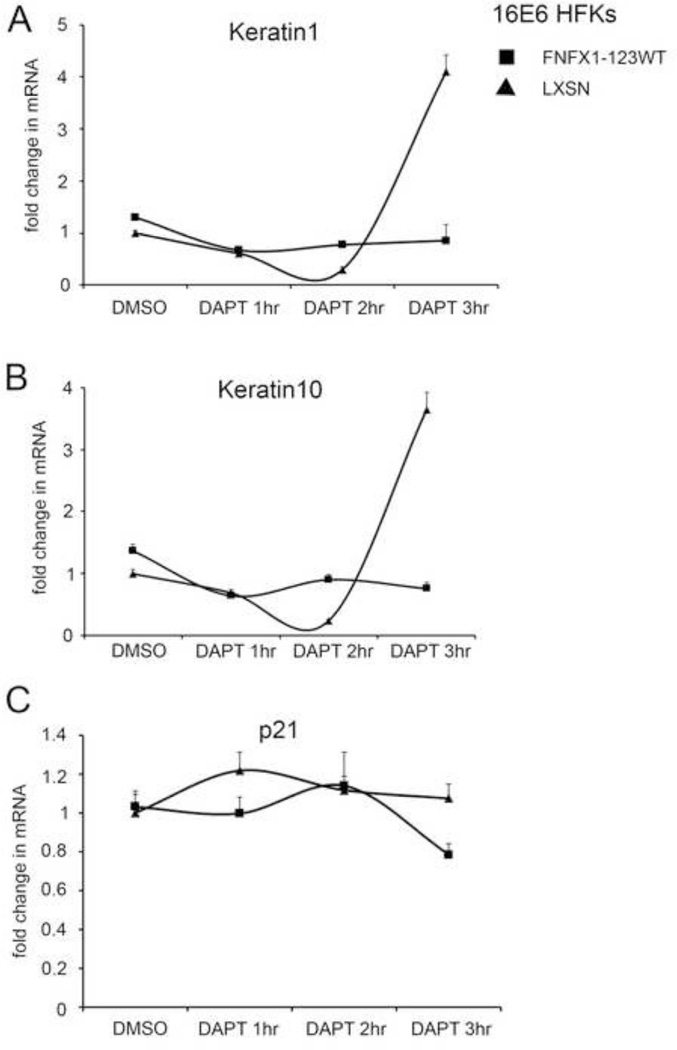
To determine this, the Notch1 receptor was either blocked or activated using the gamma secretase inhibitor DAPT or the ligand Delta-Ligand 1 (DL1), respectively. This was done in 16E6 HFKs transduced to overexpress NFX1-123 (16E6/FNFX1-123WT) or vector control (16E6/LXSN), noting that 16E6/FNFX1-123WT HFKs had increased Notch1 when compared to 16E6/LXSN HFKs (Vliet-Gregg, Hamilton et al., 2013). Cells were treated with DAPT to block cleavage of membrane-bound Notch to its active, intracellular form. Cleaved Notch1 (or NICD) was undetectable by whole cell extract immunoblot assay in both 16E6/FNFX1-123WT and 16E6/LXSN HFKs within one hour of treatment when compared to vehicle control (dimethyl sulfoxide (DMSO)) (Figure 2A). With DMSO treatment, 16E6/FNFX1-123WT HFKs had eightfold greater Hes1 mRNA and threefold greater Hes5 mRNA when compared to 16E6 HFKs with endogenous levels of NFX1-123 (16E6/LXSN) (Figure 2B and C). However, when Notch1 cleavage was blocked by DAPT, Hes1 and Hes5 mRNA in 16E6/FNFX1-123WT HFKs rapidly returned to amounts equivalent to 16E6/LXSN HFKs (Figure 2B and C). Notch1 mRNA itself was increased eightfold in DMSO-treated 16E6/FNFX1-123WT HFKs versus 16E6/LXSN HFKs; this too was reduced by more than half with DAPT treatment (Figure 2D). Without cleavage of Notch1 to its active intracellular form, the sustained increase of Hes1, Hes5 and Notch1 mRNA in 16E6/FNFX1-123WT cells fell to the level of 16E6/LXSN HFKs (Figure 2B–D, DMSO vs DAPT 3hr).
Figure 2. Chemical inhibition of Notch1 abrogated canonical pathway components.
(A) Representative immunoblot of cleaved Notch1 protein in 16E6 HFKs transduced with overexpressed FLAG-tagged NFX1-123 wild type (FNFX1-123WT) or LXSN vector control. Whole cell extracts from 16E6/LXSN and 16E6/FNFX1-123WT treated with either DMSO or 10µM DAPT were prepared at the indicated times. Actin was used as a loading control. Similar results were obtained in two additional experiments. (B) Relative levels of Hes1 mRNA (C) Hes5 mRNA, and (D) Notch1 mRNA were calculated using the ΔΔCT method, normalizing mRNA levels to GAPDH within each sample. Values shown were the mean fold change in each sample compared to the DMSO treated 16E6/LXSN vector control. Error bars represent the standard deviation for triplicate samples.
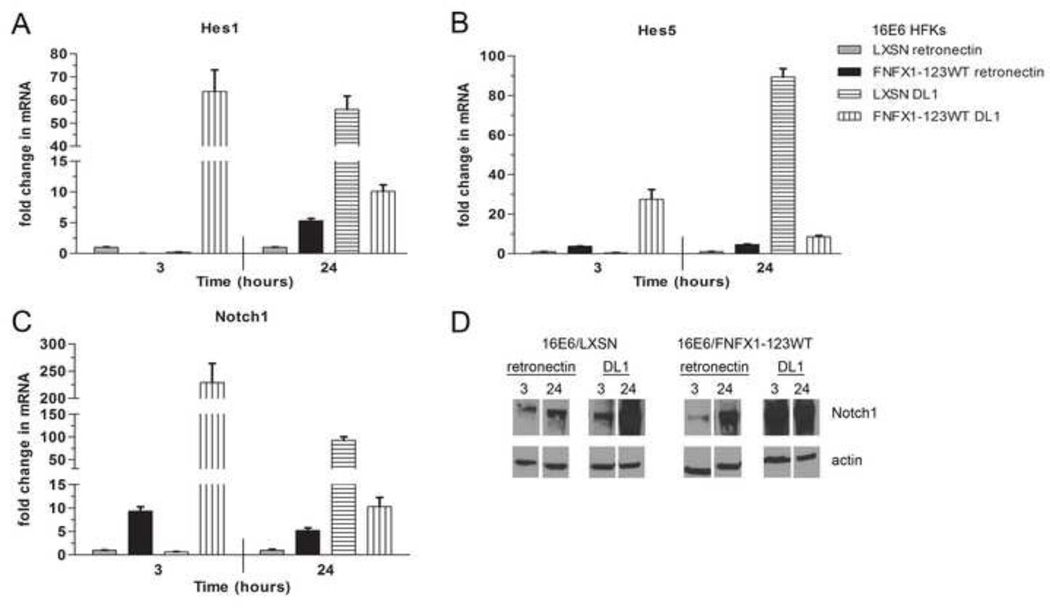
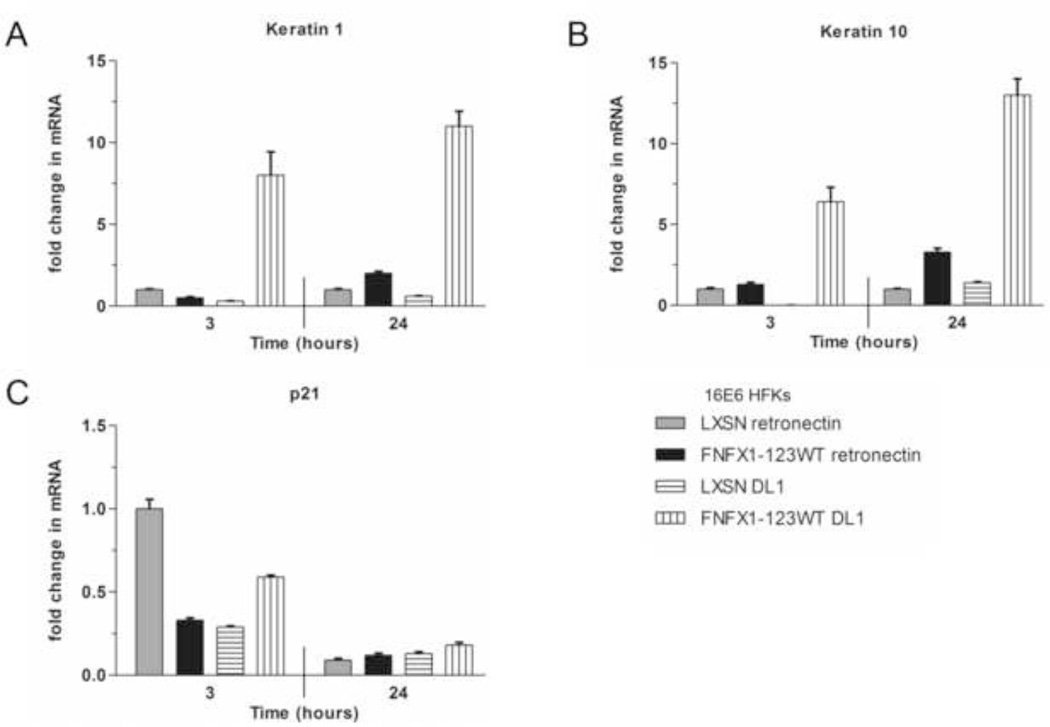
The Notch1 receptor was then activated by culturing 16E6/FNFX1-123WT or 16E6/LXSN HFKs in the presence of Delta-1 ligand (DL1) immobilized with retronectin or retronectin alone. Ligand immobilization with retronectin is required for Notch signal induction by DL1 (Varnum-Finney, Wu et al., 2000), with DL1 triggering Notch1 receptor activation and subsequent cleavage to its intracellular, active form (NICD). Hes1 and Hes5 expression had a robust and rapid induction in response to Notch1 receptor activation by DL1, increasing within three hours. In 16E6/FNFX1-123WT cells this mRNA increase was more than sixty times greater for Hes1 and thirty times higher for Hes5 than in 16E6/LXSN HFKs (Figure 3A and B). Notch1 mRNA also increased more than two hundredfold within three hours of culture with DL1 (Figure 3C and D). Notch1, Hes1 and Hes5 mRNA did increase in 16E6/LXSN HFKs cultured with DL1, but this did not occur until 24 hours (Figure 3A–C). Therefore, when triggered by DL1, increased NFX1-123 and Notch1 expression in 16E6/FNFX1-123WT HFKs led to greater and more rapid induction of Hes1, Hes5, and Notch1 when compared to 16E6/LXSN cells. Using retronectin without DL1, there was no change in Hes1, Hes5, or Notch1 transcript levels in 16E6/LXSN HFKs, but in 16E6/FNFX1-123WT HFKs where Notch1 is persistently increased, there were modest increases in Hes1, Hes5, and Notch1 mRNA levels (Figure 3A–D). These Notch canonical pathway studies, where Notch1 receptor was blocked or activated collectively, support the conclusion that NFX1-123 requires Notch1 itself to drive expression of Hes1 and Hes5, and when NFX1-123 itself is overexpressed it leads to a sustained greater Notch1 expression and therefore more dramatic and rapid activation or reduction in Notch canonical pathway targets.
Figure 3. DL1 activated Notch1 canonical pathway signaling.
16E6/LXSN and 16E6/FNFX1-123WT HFKs were plated on dishes coated with purified Delta-1 ligand (DL1) immobilized on retronectin or retronectin alone. Whole cell extracts were harvested for RNA and protein at the indicated times. Similar results were found in at least three independent experiments. (A) Relative levels of Hes1 mRNA (B) Hes5 mRNA and (C) Notch1 mRNA were calculated using the ΔΔCT method, normalizing mRNA levels to GAPDH within each sample. Values shown were the mean fold change in each sample compared to the 16E6/LXSN vector control plated on retronectin. Error bars represent the standard deviation for triplicate samples. (D) Representative immunoblot of Notch1 protein in whole cell extracts of 16E6/LXSN and 16E6/FNFX1-123WT HFKs at the indicated times. Actin shown as a loading control.
Differentiation gene expression but not p21WAF1/Cip1 increased by NFX1-123 in 16E6 HFKs
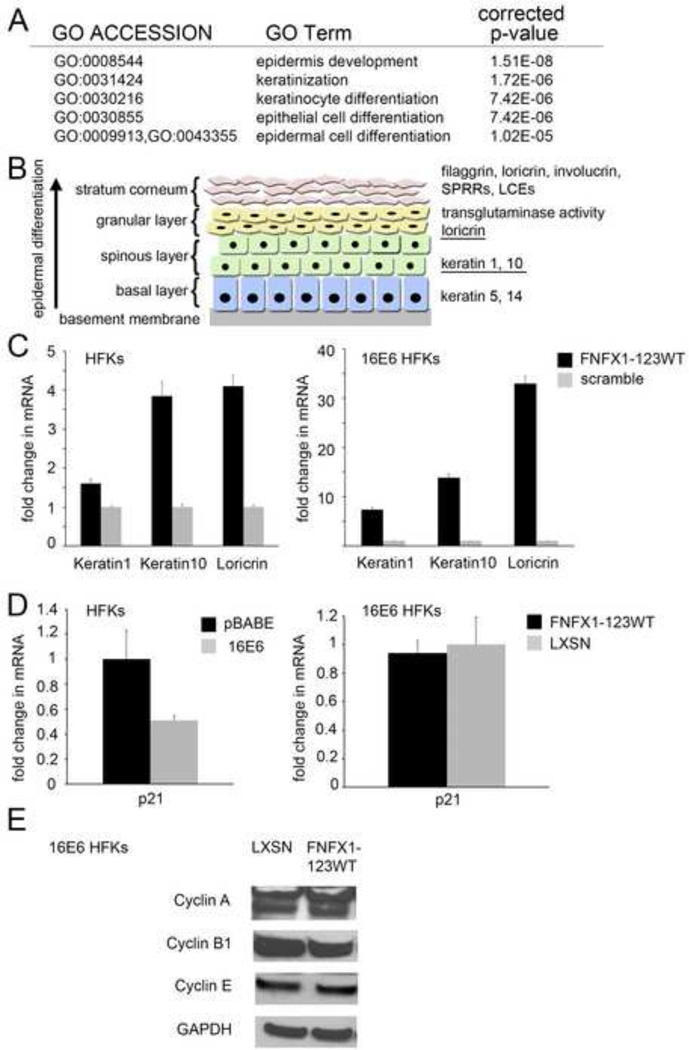
We previously conducted a microarray study to determine genes modulated by NFX1-123 in 16E6 HFKs (Vliet-Gregg, Hamilton et al., 2013). Data from this microarray study, analyzed using Gene Ontology terms for Biological Process classification, revealed that epidermal and keratinocyte differentiation were pathways significantly perturbed through NFX1-123 overexpression in 16E6 HFKs when compared to an isogenic scramble shRNA control (Figure 4A). Genes found to be increased twofold or more by microarray analysis that supported these identified pathway changes included the keratinocyte differentiation markers late cornified envelope proteins (LCEs), loricrin, and small proline rich proteins (SPRRs) (noted in model, Figure 4B) (Vliet-Gregg, Hamilton et al., 2013). Because the epidermal and keratinocyte differentiation pathways were significantly upregulated in the microarray study, we chose to quantify gene expression changes of several markers that should be increased during keratinocyte differentiation: Keratin 1 and Keratin 10 (early markers of differentiation in stratified squamous epithelium), and Loricrin (increased in our microarray) (underlined in Figure 4B) (Vliet-Gregg, Hamilton et al., 2013). FNFX1-123WT HFKs with no 16E6 co-expression had only a subtle increase in Keratin 1 mRNA, one-and-a-halffold, when compared to isogenic scramble shRNA control cells (scramble). Keratin 10 and Loricrin mRNA were moderately increased, up to fourfold in these cells (Figure 4C, left panel). These genes in 16E6/FNFX1-123WT HFKs revealed enhanced expression, seven- to-thirtyfold, of Keratin 1, Keratin 10 and Loricrin mRNA compared to 16E6/scramble cells (Figure 4C, right panel). Therefore, 16E6 co-expression led to greater levels of Keratin 1, Keratin 10, and Loricrin in HFKs with overexpressed NFX1-123.
Figure 4. FNFX1-123WT induced differentiation markers in 16E6 HFKs.
(A) Top statistically significant Gene Ontology (GO) terms for Biological Process classification of the differentially increased gene expression comparing 16E6 HFKs transduced with overexpressed FLAG-tagged NFX1-123 wild type (FNFX1-123WT) and isogenic scramble short hairpin RNA control (scramble). (B) Markers of differentiation in stratified squamous epithelium. The expression of keratins and other structural proteins shift with differentiation. (Illustration adapted from (Hoffjan & Stemmler, 2007)). (C) HFKs and 16E6 HFKs transduced with FNFX1-123WT or a scramble shRNA control were analyzed by qPCR. Relative levels of Keratin1, Keratin10 and Loricrin mRNA were calculated using the ΔΔCT method, normalizing mRNA levels to GAPDH within each sample. Values shown are the mean fold change in each sample compared to the scramble vector control. Error bars represent the standard deviation for triplicate samples. (D) HFKs transduced with 16E6 or a vector control (pBABE), and then FNFX1-123WT or a second vector control (LXSN), were analyzed for p21Waf1/CIP1 mRNA levels using the housekeeping gene 36B4 as control. Values shown were the mean fold change in each sample compared to the scramble vector control. Error bars represent 95% confidence intervals from the technical triplicate replicates shown. (E) Representative immunoblot of Cyclin A, B1, and E protein in whole cell extracts of 16E6/LXSN and 16E6/FNFX1-123WT HFKs. GAPDH shown as a loading control.
Surprisingly, while mRNA expression of differentiation marker genes were increased and differentiation pathways were significantly altered when NFX1-123 was overexpressed in 16E6 HFKs, genes involved in cell cycle regulation were unperturbed. We found no evidence for increases in cell cycle arrest genes by microarray (Vliet-Gregg, Hamilton et al., 2013) or subsequent Gene Ontology analyses. To confirm this, we quantified the expression of p21WAF1/Cip1, a cell cycle arrest gene. The p21WAF1/Cip1 gene is directly activated by p53 and by Notch1 (Rangarajan, Talora et al., 2001; Warfel & El-Deiry, 2013) to trigger cell cycle arrest. It is known that p21WAF1/Cip1 expression is reduced in 16E6 expressing cells through p53 degradation and inactivation of the p150 transcription factor (Huibregtse, Scheffner et al., 1991; Parroche, Touka et al., 2011; Scheffner, Huibregtse et al., 1993). We wanted to determine if 16E6 affected p21WAF1/Cip1 levels in cells overexpressing NFX1-123, and if p21WAF1/Cip1 levels were modulated by the augmented Notch1 expression in 16E6/FNFX1-123WT HFKs. As expected, p21WAF1/Cip1 mRNA was detected in vector control (pBABE) HFKs, and it was reduced by half when 16E6 was expressed (Figure 4D). In 16E6/FNFX1-123WT HFKs there was no change in p21WAF1/Cip1 mRNA expression when compared to 16E6/LXSN HFKs (Figure 4D), despite greater Notch1 and differentiation gene expression (Vliet-Gregg, Hamilton et al., 2013) and Figures 2–4. We also found no change in Cyclin A, B1, or E protein expression in 16E6/FNFX1-123WT HFKs when compared to cells with endogenous levels of NFX1-123 (Figure 4E). Higher levels of NFX1-123 (FNFX1-123WT) increased keratinocyte differentiation gene expression and was further increased by 16E6 co-expression in HFKs, but surprisingly this was not accompanied by concomitant cell cycle arrest or senescence as marked by p21WAF1/Cip1 expression.
Regulation of differentiation and cell cycle arrest pathway genes by NFX1-123 and Notch in 16E6 HFKs
Notch1 functions as a master regulator of growth and differentiation in keratinocytes and during cervical cancer development (Maliekal, Bajaj et al., 2008; Henken, De-Castro et al., 2012; Lathion, Schaper et al., 2003; Rangarajan, Syal et al., 2001; Rangarajan, Talora et al., 2001; Zagouras, Stifani et al., 1995). Because NFX1-123 increases Notch1 expression in 16E6 HFKs, and greater levels of Notch1 may drive increased differentiation marker expression and pathway activation, it was important to determine if increased differentiation gene expression and pathway changes in 16E6/FNFX1-123WT HFKs occurred directly via Notch1 receptor activation, similar to the Notch canonical pathway genes Hes1 and Hes5. As a corollary, we wanted to elucidate whether activation or blockade of the Notch1 receptor in 16E6/FNFX1-123WT and 16E6/LXSN HFKs led to interlinked regulation of differentiation and cell cycle arrest or whether they were fully uncoupled.
To explore this, we expanded on our previous studies and treated 16E6/FNFX1-123WT or 16E6/LXSN HFKs with DAPT to block Notch1 receptor cleavage and activation, and serially collected lysates for quantitation of both differentiation and cell cycle arrest genes. The profile of Keratin 1 and Keratin 10 mRNA changes did not parallel those of the Notch1 canonical pathway genes Hes1 and Hes5 (compare Figure 2B and C to Figure 5A and B). In both 16E6/FNFX1-123WT and 16E6/LXSN HFKs, there was no decrease in differentiation marker gene expression with inhibition of Notch1 activation. In fact, at three hours of DAPT treatment in 16E6/LXSN HFKs, the transcript levels of Keratin 1 and Keratin 10 increased (Figure 5A and B). Furthermore, the cell cycle arrest gene p21WAF1/Cip1 mRNA was unchanged when Notch1 cleavage was blocked (Figure 5C). When later time points were evaluated, no decrease or increase in Keratin 1, Keratin 10 or p21WAF1/Cip1 mRNA levels was identified (data not shown). Therefore, differentiation marker and cell cycle arrest gene expression did not depend on activated, cleaved Notch1 in either 16E6 cell type.
Figure 5. Differentiation markers unperturbed by chemical inhibition of Notch1 in 16E6 HFKs with overexpressed NFX1-123.
16E6 HFKs transduced with overexpressed FLAG-tagged NFX1-123 wild type (FNFX1-123WT) or LXSN vector control treated with either DMSO or 10µM DAPT were prepared at the indicated times. Similar results were obtained in three independent experiments. (A) Relative levels of Keratin 1 mRNA and (B) Keratin 10 mRNA were calculated using the ΔΔCT method, normalizing mRNA levels to GAPDH within each sample. Values shown were the mean fold change in each sample compared to the DMSO treated 16E6/LXSN vector control. Error bars represent the standard deviation for triplicate samples. (C) Transcript levels for p21Waf1/CIP1 were assessed by quantitative real-time PCR using primers and normalized to the housekeeping gene 36B4 as control. Values shown were the mean fold change in each sample compared to the DMSO treated 16E6/LXSN vector control. Error bars represent 95% confidence intervals from the technical triplicate replicates shown.
When Notch1 receptor was activated by immobilized DL1, Keratin1 and Keratin 10 increased, but the kinetics of their increased expression were dramatically different than the Notch canonical pathway target genes Hes1 and Hes5 (compare Figure 6 to Figure 3). At three hours, Keratin 1 and Keratin 10 had a less robust increase in expression that continued to rise at 24 hours, unlike Hes1 and Hes5 (Figure 6A and B). Also, only 16E6/FNFX1-123WT HFKs had an increase in Keratin 1 and Keratin 10 levels when activated by DL1, as those cells had greater baseline Notch1 expression. Finally, we found p21 WAF1/Cip1 mRNA fell over the course of DL1 activation (Figure 6C). So, Notch1 activation by DL1 did slowly lead to moderate increases in differentiation gene expression, but only in cells with greater Notch1 expression (16E6/FNFX1-123WT HFKs, Figure 6 vertical striped bars), and activation of Notch1 did not trigger increased p21WAF1/Cip1 expression.
Figure 6. Notch1 stimulation by DL1 activated differentiation markers in 16E6 HFKs with overexpressed NFX1-123.
16E6/LXSN and 16E6/FNFX1-123WT HFKs were plated on dishes coated with purified Delta-1 ligand (DL1) immobilized on retronectin or retronectin alone. Whole cell extracts were harvested for RNA and protein at the indicated times. Similar results were found in at least three independent experiments. (A) Relative levels of Keratin 1 mRNA and (B) Keratin 10 mRNA were calculated using the ΔΔCT method, normalizing mRNA levels to GAPDH within each sample. Values shown were the mean fold change in each sample compared to the 16E6/LXSN vector control plated on retronectin. Error bars represent the standard deviation for each triplicate. (C) Transcript levels for p21Waf1/CIP1 were assessed by quantitative real-time PCR using primers and normalized to the housekeeping gene 36B4 as control. Values shown were the mean fold change in each sample compared to the DMSO treated 16E6/LXSN vector control. Error bars represent 95% confidence intervals from the technical triplicate replicates shown.
No growth suppression with increased differentiation gene expression by NFX1-123 in 16E6 HFKs
Higher NFX1-123 and Notch1 protein and mRNA expression did not increase the cell cycle arrest gene p21WAF1/Cip1 in 16E6 HFKs (Figure 4D and 6C) However, we wanted to assess if other genes involved in growth or proliferation were repressed, leading to slowed growth or cell cycle blockade in 16E6/FNFX1-123WT HFKs compared to 16E6/LXSN HFKs. Additionally, there may be subsets of 16E6/FNFX1-123WT HFKs that express greater amounts of differentiation markers and concomitantly do not express proliferation markers, but this difference was undetectable when averaged across a population of cells. To address these issues, we quantified differentiation gene and growth marker expression on a cell-by-cell basis in 16E6/FNFX1-123WT and 16E6/LXSN HFKs.
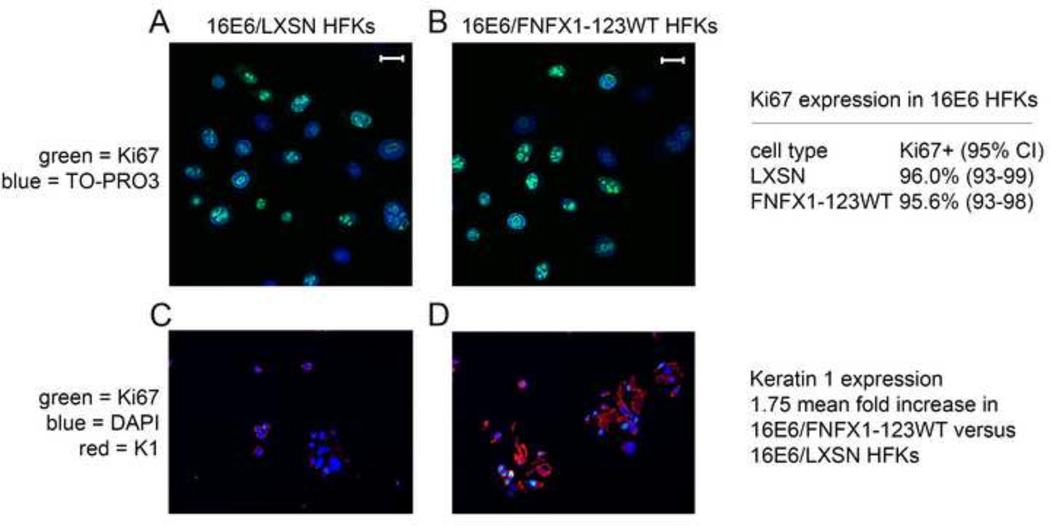
To evaluate whether there were differences in cell growth and proliferation factor expression in cells with greater NFX1-123, 16E6/FNFX1-123WT and 16E6/LXSN HFKs were stained for Ki67. We found 96% of 16E6/LXSN HFKs to be Ki67 positive, and this remained unchanged when NFX1-123 was overexpressed (16E6/FNFX1-123WT) (Figure 7A and B). Therefore equal numbers of 16E6 HFKs expressed Ki67 irrespective of NFX1-123 levels. These cells were then co-stained for Keratin 1 and Ki67 to assess whether there were cells with greater differentiation marker gene expression that segregated into separate populations from cells with growth factor expression. Keratin 1 protein expression was nearly twofold greater in 16E6/FNFX1-123WT HFKs than in 16E6/LXSN HFKs, and its staining was homogeneous across the cell population (Figure 7C and D). Again, as in Figure 7A and B, more than 95% of 16E6 HFKs expressed Ki67 regardless of the expression level of NFX1-123 or Keratin 1 (Figure 7C and D). Therefore, nearly all 16E6 HFKs with increased NFX1-123 still expressed a proliferation factor, Ki67, even as NFX1-123, Notch1, and differentiation markers, such as Keratin 1, were increased.
Figure 7. Ki67 expression unchanged with overexpression of FNFX1-123WT and increased Keratin 1.
Immunofluorescent staining was done in 16E6 HFKs transduced with overexpressed FLAG-tagged NFX1-123 wild type (FNFX1-123WT) or LXSN vector control. (A) Ki67 staining of 16E6/LXSN HFKs and (B) 16E6/FNFX1-123WT HFKs. Bar = 10 micron; Green = Ki67; Blue = TO-PRO3, nuclear stain. Quantification of percent positive Ki67 expression by immunofluorescent staining was done in three independent 16E6 HFK cell lines with increased NFX1-123 (FNFX1-123WT) versus three vector control (LXSN) HFKs. Expression pattern of the indicated protein was assessed by dual immunohistochemical (IHC) analysis of pelleted, fixed, paraffin embedded cells. (C) 16E6/LXSN HFKs and (D) 16E6/FNFX1-123WT HFKs stained for Ki67 and Keratin1 (K1). Green = Ki67; Blue = DAPI; Red = Keratin 1. Similar results were observed in three independent experiments using three distinct 16E6 HFK cell lines.
Normal cell cycle progression in 16E6 HFKs with increased NFX1-123
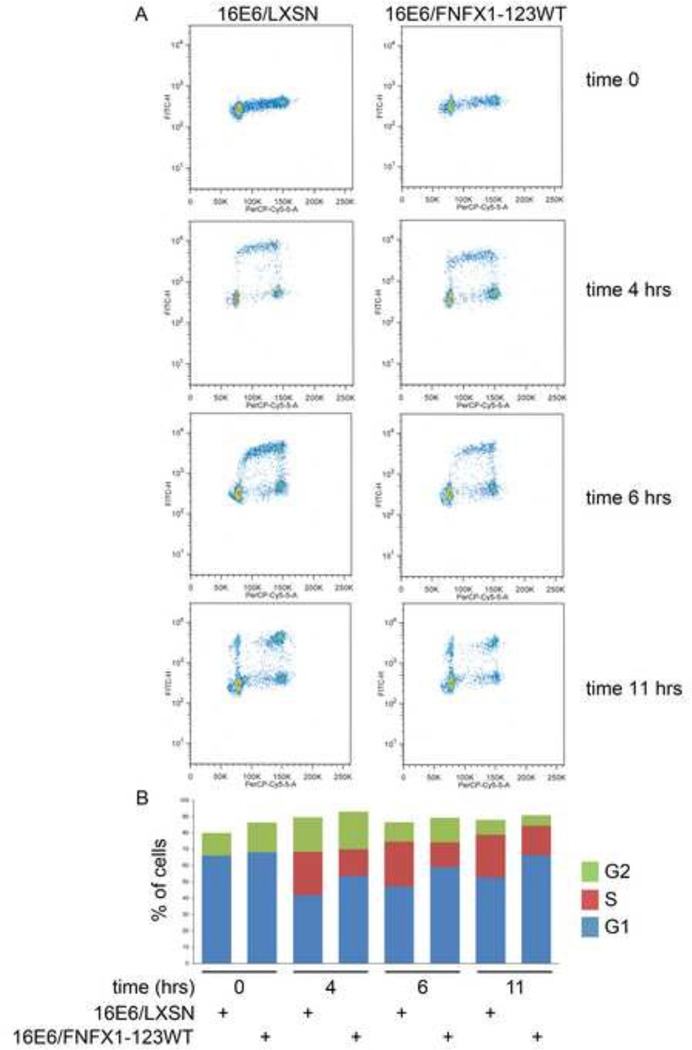
Even with similar p21WAF1/Cip1 and Ki67 expression in 16E6/FNFX1-123WT and 16E6/LXSN HFKs, the rate of cell cycle progression in 16E6/FNFX1-123WT HFKs might be slowed with both increased Notch1 and differentiation pathway gene expression. We performed cell cycle analysis using fluorescence-activated cell sorting to compare the cell cycle profiles of density arrested and released 16E6/FNFX1-123WT HFKs to 16E6/LXSN HFKs. Interestingly both of these cell types were cycling, and there was no slowing in 16E6/FNFX1-123WT HFKs (Figure 8A). In fact, a greater fraction of 16E6/FNFX1-123WT HFKs were already in G2 at four hours after release when compared to 16E6/LXSN, and by six hours, a greater number of 16E6/FNFX1-123WT HFKs had returned to G1 when compared to 16E6/LXSN HFKs (Figure 8B). 16E6 HFKs with overexpressed NFX1-123 had increased Notch1, canonical pathway, and differentiation gene expression but had uncoupled the typical reflexive activation of cell cycle arrest shown by unchanged p21, Ki67, and cell cycle profiles.
Figure 8. Cell cycle unchanged in 16E6 HFKs expressing increased NFX1-123.
BrdU pulse-chase experiment in 16E6 HFKs transduced with overexpressed FLAG-tagged NFX1-123 wild type (FNFX1-123WT) or LXSN vector control. Cells were synchronized by density arrest, released in BrdU for one hour, then chased in BrdU-free media for the indicated times. Cells were stained with Propidium Iodide (PI) and cell cycle progression was monitored by FACS analysis. Similar results were found in least three independent HFK cell line experiments. (B) Graphical representation of flow cytometry analysis.
Discussion
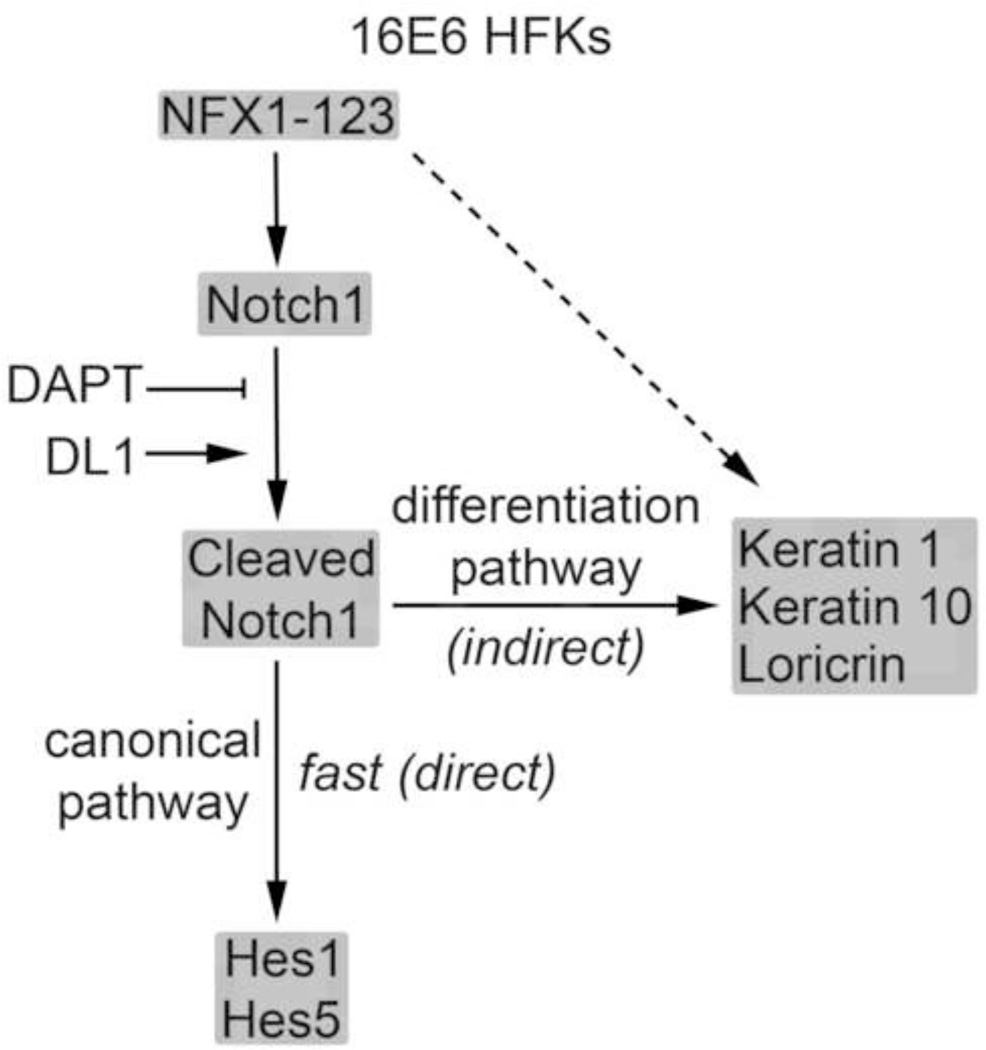
These studies identified a separation in gene and pathway regulation for 16E6 HFKs with increased expression of NFX1-123. In 16E6 HFKs, Notch1, Notch canonical pathway genes Hes1 and Hes5, and differentiation pathway genes Keratin 1 and Keratin 10 were all altered by greater NFX1-123 expression. However, NFX1-123 in these cells did not increase p21WAF1/Cip1 mRNA, change Ki67 expression, or cause cell cycle arrest. These findings lead to a dual-faceted model of pathway regulation by NFX1-123 and Notch1 in 16E6 HFKs (Figure 9). NFX1-123 regulates Notch1 expression in an augmented manner in 16E6 cells. When the Notch1 receptor is activated by DL1, Hes1, Hes5, and Notch1 mRNA expression quickly increase (Figure 3). This first mode of action on the canonical pathway is rapid, suggesting that the effect is directly due to Notch1 and NFX1-123. Consistent with this, when the Notch1 receptor cleavage and activation was blocked by gamma secretase inhibitor, the increased expression of Hes1 and Hes5 mRNA fell rapidly to the level of 16E6 HFKs with endogenous expression levels of NFX1-123 (Figure 2). These data point again to NFX1-123 driving Hes1 and Hes5 expression through activated, cleaved Notch1. They also point to Notch1 regulating its own expression when it is cleaved and activated or chemically blocked (Figure 2D and Figure 3C and D). We propose that the greater activation of Hes1, Hes5, and Notch1 expression in 16E6/FNFX1-123WT HFKs compared to 16E6/LXSN HFKs is due to the greater level of full length Notch1 found in these cells. With more Notch1 receptor available for activation by DL1, more cleaved Notch can be produced, leading to the direct, rapid, and robust downstream gene target response, and when Notch1 receptor is blocked, the fall in its downstream genes is also fast.
Figure 9. Model of 16E6 and NFX1-123WT regulation of Notch1, Notch canonical pathway genes and keratinocyte differentiation pathway genes.
Conversely, the differentiation pathway is regulated in 16E6 HFKs by NFX1-123 and Notch1 in a mode distinct from the canonical pathway. Keratin 1 and Keratin 10 mRNA expression are unperturbed by blocking cleavage of Notch1 in 16E6/FNFX1-123WT HFKs (Figure 5). However, when Notch1 was activated by DL1, Keratin 1 and Keratin 10 expression were increased in 16E6/FNFX1-123WT HFKs (Figure 6). The activation profiles of these differentiation markers were more gradual and less robust than the canonical pathway genes, which depended on the overexpression of NFX1-123 and Notch1. Keratin 1 and Keratin 10 did not increase in 16E6 HFKs with endogenous levels of NFX1-123 and Notch1. In our model of 16E6 HFKs, Notch1 has an indirect effect on the differentiation pathway and its genes only when NFX1-123 is overexpressed.
Finally, an uncoupling of Notch1 and differentiation pathway upregulation from growth arrest in 16E6 HFKs was also identified in our studies. There was no evidence of an upregulation in growth arrest genes in our microarray screen when 16E6 HFKs with overexpressed NFX1-123 were compared to those with endogenous levels of NFX1-123 (Vliet-Gregg, Hamilton et al., 2013). Additionally no difference was found in expression of p21WAF1/Cip1 (Figure 4), despite it being a Notch1 regulated gene, and there was no decrease in percent of Ki67 expression in cells or cell cycle rates in 16E6/FNFX1-123WT HFKs (Figures 7 and 8). There was no heterogeneity in growth factor or differentiation gene expression among 16E6/FNFX1-123WT and 16E6/LXSN HFKs at a population level or on a cell-by-cell basis, and 16E6 HFKs with greater expression of NFX1-123 progressed normally through the cell cycle, if not faster than 16E6/LXSN HFKs. This suggests that 16E6 cells with greater NFX1-123 expression increase Notch pathway canonical and differentiation genes while concurrently avoiding cell cycle exit and growth arrest.
It is well-known that HPV requires epithelial cells to both divide and differentiate during its viral life cycle, but it is less clear if cellular division and differentiation happen simultaneously within the same cell or whether these changes occur separately in distinct layers of stratified squamous epithelium. Recent studies support our findings and have found increased Notch1 expression co-occurring with both an increased growth potential and differentiation marker expression in cervical cancer cell lines as well as in HFKs expressing HPV 31 (Pattabiraman, Hong et al., 2014). Additionally, the parsing of differentiation and growth arrest pathways has been seen in the context of 16E7 expression (Lefort, Mandinova et al., 2007; Muller, Alunni-Fabbroni et al., 1999; Nguyen, Lefort et al., 2006), so there is precedence for an HPV oncoprotein uncoupling differentiation from cellular growth.
The increases we found in differentiation pathway regulation by Gene Ontology (Figure 4A), and differentiation gene expression (Figure 4C) required greater NFX1-123 expression and were specifically augmented by 16E6 co-expression. In a microarray study we conducted of non-16E6 expressing HFKs, two differentiation genes had twofold or greater mRNA levels with overexpression of NFX1-123 alone (GEO (GSE43082)); these genes plus several more were increased when NFX1-123 was overexpressed and 16E6 was co-expressed (Vliet-Gregg, Hamilton et al., 2013). These findings support a hypothesis that NFX1-123 regulates the keratinocyte differentiation pathway, and this is synergistically increased by 16E6 while avoiding changes to cell growth.
Greater NFX1-123 (typically overexpressed threefold in 16E6/FNFX1-123WT HFKs) drives augmented hTERT expression and telomerase activity (Katzenellenbogen, Egelkrout et al., 2007; Katzenellenbogen, Vliet-Gregg et al., 2009), and clinically greater telomerase activity parallels worsening cervical disease (Ault, Allen et al., 2005; Branca, Giorgi et al., 2006). Increased NFX1-123 expression may be an important driver of both telomerase activation and cellular dysregulation with high risk E6, and the combination of increased NFX1-123 with 16E6 affecting host cells’ differentiation and growth pathways is important to study. Additional studies are ongoing to examine the mechanism by which NFX1-123 and 16E6 increase differentiation gene expression collaboratively, the factors that link Notch1 and differentiation directly or indirectly through NFX1-123 and 16E6, and how these changes influence the HPV viral life cycle.
Conclusions
Our findings point to a model of keratinocytes where HR HPV oncogenes -specifically HPV 16E6 - and increased NFX1-123 lead to greater Notch1 and canonical pathway gene expression as well as differentiation pathway gene expression, while entirely untethering them from concomitant growth arrest. This cellular phenotype, driven by NFX1-123 expression, may lead to both growth and differentiation within the same cell and an ideal host cell environment for a long-lived and productive HPV infection.
Highlights.
16E6 and NFX1-123 increased the Notch canonical pathway through Notch1
16E6 and NFX1-123 increased the differentiation pathway indirectly through Notch1
16E6 and NFX1-123 increased differentiation gene expression without growth arrest
Increased NFX1-123 with 16E6 may create an ideal cellular phenotype for HPV
Acknowledgements
We are grateful to Dr. Galloway for helpful discussions, Dr. Delaney for advice and Delta Ligand 1 reagent, and FACS analysis support from Kristin Robinson. We appreciate review of this manuscript by Dr. Lagunoff, Dr. Geballe, and Dr. Roman. This work was supported by NIH R01 CA 172742A1 to R.A.K. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest to disclose.
Reference List
- Ault KA, Allen HK, Phillips SL, Zimmerman MB, Klingelhutz AJ. Telomerase activity as a potential diagnostic marker for triage of abnormal Pap smears. J Low.Genit.Tract.Dis. 2005;9:93–99. doi: 10.1097/00128360-200504000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj J, Maliekal TT, Vivien E, Pattabiraman C, Srivastava S, Krishnamurthy H, Giri V, Subramanyam D, Krishna S. Notch signaling in CD66+ cells drives the progression of human cervical cancers. Cancer Res. 2011;71:4888–4897. doi: 10.1158/0008-5472.CAN-11-0543. [DOI] [PubMed] [Google Scholar]
- Bartz SR, Vodicka MA. Production of high-titer human immunodeficiency virus type 1 pseudotyped with vesicular stomatitis virus glycoprotein. Methods. 1997;12:337–342. doi: 10.1006/meth.1997.0487. [DOI] [PubMed] [Google Scholar]
- Borggrefe T, Oswald F. The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol.Life Sci. 2009;66:1631–1646. doi: 10.1007/s00018-009-8668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branca M, Giorgi C, Ciotti M, Santini D, Di BL, Costa S, Benedetto A, Bonifacio D, Di BP, Paba P, Accardi L, Mariani L, Ruutu M, Syrjanen S, Favalli C, Syrjanen K. Upregulation of telomerase (hTERT) is related to the grade of cervical intraepithelial neoplasia, but is not an independent predictor of high-risk human papillomavirus, virus persistence, or disease outcome in cervical cancer. Diagn.Cytopathol. 2006;34:739–748. doi: 10.1002/dc.20554. [DOI] [PubMed] [Google Scholar]
- Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, Stanley MA. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30:F55–F70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- Gewin L, Myers H, Kiyono T, Galloway DA. Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex. Genes and Development. 2004;18:2269–2282. doi: 10.1101/gad.1214704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henken FE, De-Castro AJ, Rosl F, Bosch L, Meijer CJ, Snijders PJ, Steenbergen RD. The functional role of Notch signaling in HPV-mediated transformation is dose-dependent and linked to AP-1 alterations. Cell Oncol.(Dordr.) 2012;35:77–84. doi: 10.1007/s13402-011-0062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffjan S, Stemmler S. On the role of the epidermal differentiation complex in ichthyosis vulgaris, atopic dermatitis and psoriasis. Br.J.Dermatol. 2007;157:441–449. doi: 10.1111/j.1365-2133.2007.07999.x. [DOI] [PubMed] [Google Scholar]
- Huibregtse JM, Scheffner M, Howley PM. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenellenbogen RA, Egelkrout EM, Vliet-Gregg P, Gewin LC, Gafken PR, Galloway DA. NFX1-123 and Poly(A) Binding Proteins Synergistically Augment Activation of Telomerase in Human Papillomavirus Type 16E6 Expressing Cells. J.Virol. 2007;81:3786–3796. doi: 10.1128/JVI.02007-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenellenbogen RA, Vliet-Gregg P, Xu M, Galloway DA. NFX1-123 increases hTERT expression and telomerase activity posttranscriptionally in human papillomavirus type 16 E6 keratinocytes. J.Virol. 2009;83:6446–6456. doi: 10.1128/JVI.02556-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenellenbogen RA, Vliet-Gregg P, Xu M, Galloway DA. Cytoplasmic poly(A) binding proteins regulate telomerase activity and cell growth in human papillomavirus type 16 E6-expressing keratinocytes. J.Virol. 2010;84:12934–12944. doi: 10.1128/JVI.01377-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer SK, van den Brule AJ, Paull G, Svare EI, Sherman ME, Thomsen BL, Suntum M, Bock JE, Poll PA, Meijer CJ. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ. 2002;325:572. doi: 10.1136/bmj.325.7364.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathion S, Schaper J, Beard P, Raj K. Notch1 can contribute to viral-induced transformation of primary human keratinocytes. Cancer Res. 2003;63:8687–8694. [PubMed] [Google Scholar]
- Lefort K, Mandinova A, Ostano P, Kolev V, Calpini V, Kolfschoten I, Devgan V, Lieb J, Raffoul W, Hohl D, Neel V, Garlick J, Chiorino G, Dotto GP. Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCKalpha kinases. Genes and Development. 2007;21:562–577. doi: 10.1101/gad.1484707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliekal TT, Bajaj J, Giri V, Subramanyam D, Krishna S. The role of Notch signaling in human cervical cancer: implications for solid tumors. Oncogene. 2008;27:5110–5114. doi: 10.1038/onc.2008.224. [DOI] [PubMed] [Google Scholar]
- McDade SS, Patel D, McCance DJ. p63 maintains keratinocyte proliferative capacity through regulation of Skp2-p130 levels. J.Cell Sci. 2011;124:1635–1643. doi: 10.1242/jcs.084723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscicki AB, Schiffman M, Burchell A, Albero G, Giuliano AR, Goodman MT, Kjaer SK, Palefsky J. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine. 2012;30:F24–F33. doi: 10.1016/j.vaccine.2012.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C, Alunni-Fabbroni M, Kowenz-Leutz E, Mo X, Tommasino M, Leutz A. Separation of C/EBPalpha-mediated proliferation arrest and differentiation pathways. Proc.Natl.Acad.Sci.U.S.A. 1999;96:7276–7281. doi: 10.1073/pnas.96.13.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen BC, Lefort K, Mandinova A, Antonini D, Devgan V, Della GG, Koster MI, Zhang Z, Wang J, Tommasi dV, Kitajewski J, Chiorino G, Roop DR, Missero C, Dotto GP. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes and Development. 2006;20:1028–1042. doi: 10.1101/gad.1406006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parroche P, Touka M, Mansour M, Bouvard V, Thepot A, Accardi R, Carreira C, Roblot GG, Sylla BS, Hasan U, Tommasino M. Human papillomavirus type 16 E6 inhibits p21(WAF1) transcription independently of p53 by inactivating p150(Sal2) Virology. 2011;417:443–448. doi: 10.1016/j.virol.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Pattabiraman C, Hong S, Gunasekharan VK, Pranatharthi A, Bajaj J, Srivastava S, Krishnamurthy H, Ammothumkandy A, Giri VG, Laimins LA, Krishna S. CD66+ cells in cervical pre-cancers are partially differentiated progenitors with neoplastic traits. Cancer Res. 2014 doi: 10.1158/0008-5472.CAN-14-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan A, Syal R, Selvarajah S, Chakrabarti O, Sarin A, Krishna S. Activated Notch1 signaling cooperates with papillomavirus oncogenes in transformation and generates resistance to apoptosis on matrix withdrawal through PKB/Akt. Virology. 2001;286:23–30. doi: 10.1006/viro.2001.0867. [DOI] [PubMed] [Google Scholar]
- Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, Aster JC, Krishna S, Metzger D, Chambon P, Miele L, Aguet M, Radtke F, Dotto GP. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman ME, Solomon D, Guillen D, Alfaro M, Morales J, Hutchinson M, Katki H, Cheung L, Wacholder S, Burk RD. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J.Natl.Cancer Inst. 2010;102:315–324. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- Talora C, Sgroi DC, Crum CP, Dotto GP. Specific down-modulation of Notch1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation. Genes and Development. 2002;16:2252–2263. doi: 10.1101/gad.988902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum-Finney B, Wu L, Yu M, Brashem-Stein C, Staats S, Flowers D, Griffin JD, Bernstein ID. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J.Cell Sci. 2000;23 Pt 113:4313–4318. doi: 10.1242/jcs.113.23.4313. [DOI] [PubMed] [Google Scholar]
- Veeraraghavalu K, Pett M, Kumar RV, Nair P, Rangarajan A, Stanley MA, Krishna S. Papillomavirus-mediated neoplastic progression is associated with reciprocal changes in JAGGED1 and manic fringe expression linked to notch activation. J.Virol. 2004;78:8687–8700. doi: 10.1128/JVI.78.16.8687-8700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliet-Gregg PA, Hamilton JR, Katzenellenbogen RA. NFX1-123 and Human Papillomavirus 16E6 Increase Notch Expression in Keratinocytes. J.Virol. 2013;87:13741–13750. doi: 10.1128/JVI.02582-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfel NA, El-Deiry WS. p21WAF1 and tumourigenesis: 20 years after. Curr.Opin.Oncol. 2013;25:52–58. doi: 10.1097/CCO.0b013e32835b639e. [DOI] [PubMed] [Google Scholar]
- Watt FM, Estrach S, Ambler CA. Epidermal Notch signalling: differentiation, cancer and adhesion. Curr.Opin.Cell Biol. 2008;20:171–179. doi: 10.1016/j.ceb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijzen S, Zlobin A, Braid M, Miele L, Kast WM. HPV16 E6 and E7 oncoproteins regulate Notch-1 expression and cooperate to induce transformation. J.Cell Physiol. 2003;194:356–362. doi: 10.1002/jcp.10217. [DOI] [PubMed] [Google Scholar]
- Yugawa T, Handa K, Narisawa-Saito M, Ohno S, Fujita M, Kiyono T. Regulation of Notch1 gene expression by p53 in epithelial cells. Mol.Cell Biol. 2007;27:3732–3742. doi: 10.1128/MCB.02119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yugawa T, Narisawa-Saito M, Yoshimatsu Y, Haga K, Ohno S, Egawa N, Fujita M, Kiyono T. DeltaNp63alpha repression of the Notch1 gene supports the proliferative capacity of normal human keratinocytes and cervical cancer cells. Cancer Res. 2010;70:4034–4044. doi: 10.1158/0008-5472.CAN-09-4063. [DOI] [PubMed] [Google Scholar]
- Zagouras P, Stifani S, Blaumueller CM, Carcangiu ML, Artavanis-Tsakonas S. Alterations in Notch signaling in neoplastic lesions of the human cervix. Proc.Natl.Acad.Sci.U.S.A. 1995;92:6414–6418. doi: 10.1073/pnas.92.14.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]