Abstract
Purpose of review
Remarkable progress has been achieved over the past 2 years in understanding the cellular actions of renalase, its pathophysiology and potential therapeutic utility.
Recent findings
There has been a paradigm shift in our thinking about the mechanisms underlying the cellular actions of renalase. We now understand that, independent of its enzymatic properties, renalase functions as a signaling molecule, a cytokine that interacts with a yet-to-be identified plasma membrane receptor(s) to activate protein kinase B and the mitogen-activated protein kinase pathway. These signaling properties are critical to its cytoprotective effects. New information regarding renalase’s enzymatic function as an α-nicotinamide adenine dinucleotide oxidase/anomerase will be reviewed. Lastly, we will discuss the association of certain single nucleotide polymorphisms in the renalase gene with type 1 diabetes and with ischemic stroke, and the clinical implications of these findings.
Summary
The consistent association of renalase single nucleotide polymorphisms and the development of type 1 diabetes is a great interest particularly because we now understand that renalase functions as a cytokine. Future work on renalase should focus on exploring the identity of its receptor(s), and its potential role as an immune modulator.
Keywords: cell signaling, cytokine, mitogen-activated protein kinase pathway, protein kinase B, type 1 diabetes
INTRODUCTION
Renalase is a secreted flavin adenine dinucleotide-containing protein (flavoprotein), hypothesized to participate in catecholamine metabolism [1,2]. Its crystal structure has been solved [3], and although most abundant in kidney, heart, skeletal muscle and liver, it is expressed in a wide variety of cells [4]. It circulates in plasma in which steady-state levels correlate with kidney mass and renal function, suggesting a renal origin for secreted renalase [4]. The renalase-deficient (knockout) mouse develops moderate hypertension and is more sensitive to cardiac and renal ischemic injury [5]. Administration of recombinant renalase rescues the phenotype. Single nucleotide polymorphisms (SNPs) in the renalase gene (rs2576178 GG genotype and rs2296545 CC) are associated with essential hypertension and cardiac dysfunction [6,7].
Significant progress has been achieved over the past 2 years in understanding the cellular actions of renalase, its pathophysiology and potential therapeutic utility. This review will summarize new findings, indicating that renalase functions as a signaling molecule, a cytokine that interacts with a yet-to-be identified plasma membrane receptor(s) to activate the mitogen-activated protein kinase (MAPK) pathway. These signaling properties are completely independent of renalase’s enzymatic function, and are yet critical to its cytoprotective effects. New information regarding renalase’s enzymatic function will also be reviewed. Lastly, we will discuss the association of certain polymorphisms in the renalase gene with type 1 diabetes and with ischemic stroke, and the clinical implications of these findings.
RENALASE FUNCTIONS AS A CYTOKINE, INDEPENDENT OF ITS ENZYMATIC ACTIVITY
Renalase is a flavoprotein, and the human genome contains only 90 genes encoding for flavin-dependent proteins [8]. The majority of flavoproteins are enzymes that catalyze oxidation–reduction processes in primary metabolic pathways, such as the citric acid cycle, β-oxidation and degradation of amino acids. They also play key roles in the biosynthesis of other essential cofactors and hormones, such as coenzyme A, coenzyme Q, heme, thyroxine and steroids. Renalase appears to be unique in that it functions as a cytokine in a manner that is completely independent of its enzymatic properties.
Renalase protects against ischemic and toxic acute kidney injury
Renalase knockout mice subjected to renal ischemia reperfusion developed significantly worse renal injury compared with renalase wild-type mice [9▪]. Administration of recombinant renalase ameliorated ischemic acute kidney injury (AKI) in renalase wild-type mice. Compared with wild-type, ischemic AKI in renalase knockout mice was associated with drastically worse renal tubular necrosis, inflammation and apoptosis. Mice subjected to renal ischemia reperfusion injury had significantly reduced kidney and plasma renalase levels and higher plasma catecholamine levels compared with the sham-operated mice.
Renalase was also protective in toxic AKI from cisplatin. Three days after treatment with cisplatin, plasma creatinine was significantly higher in renalase knockout mice compared with wild-type mice. Renalase-deficient mice developed worse renal histological injury compared with renalase wild-type mice. Renalase knockout mice showed more severe acute tubular necrosis compared with wild-type mice (injury score: knockout=56.62±7.38%, n=6, wild-type=17.07±4.03, n=5; P=0.0002) and a higher degree of apoptosis (two-fold increase in terminal deoxynucleotidyl transferase dUTP nick end labelling staining, P<0.005) and macrophage infiltration (35% increase in F4/80 staining, P<0.05) [10▪▪].
Renalase’s protection against organ injury is independent of its enzymatic function
Independent of its enzymatic properties, renalase activates a receptor-mediated, prosurvival signaling cascade, with activation of protein kinase B (AKT), extracellular signal-regulated kinase (ERK), p38 mitogen-activated kinase, B-cell lymphoma 2 and inhibition of c-Jun N-terminal kinase (Fig. 1) [10▪▪]. We have identified the key region of the renalase molecule that mediates its cytoprotective action. Peptides (~20–24 amino acids, RP-220 and RPH220) (Fig. 2) derived from that region rapidly signal via MAPKs and fully mimic the protective effect of renalase in cells exposed to cisplatin. Additionally, they significantly ameliorated renal injury in mice either treated with cisplatin (15–20 mg/kg) or subjected to ischemic AKI (unilateral nephrectomy followed by 30 min of ischemia of remaining kidney). Administration of either RP-220 or RP-H220 was associated with lower serum creatinine, less tubular injury (light microscopy), decreased apoptosis, necrosis and inflammation.
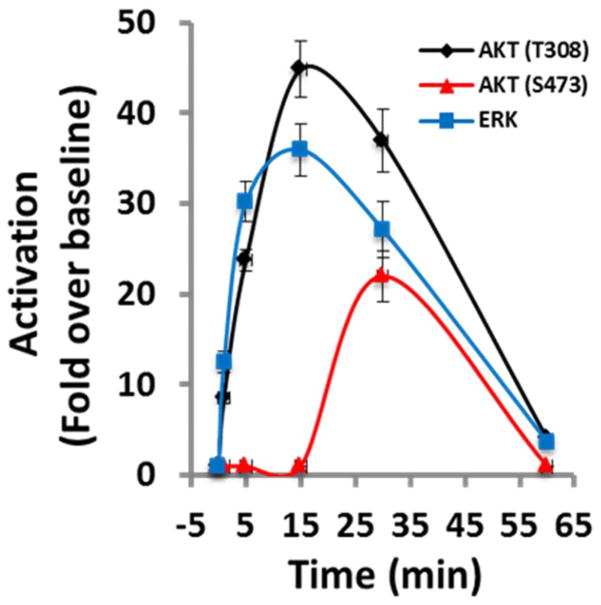
FIGURE 1.
Time course of renalase-dependent cell signaling. Human embryonic kidney cells (HK-2) incubated with renalase and activation of protein kinase B (AKT) and extracellular-signal regulated kinase (ERK) determined by Western blot analysis; representative blot is shown (n=3); signals normalized to glyceraldehyde 3-phosphate dehydrogenase loading control (n=3); changes over baseline statistically significant at from 1 to 60 min for ERK, and AKT (T308) and at 30 min only for AKT (S473).
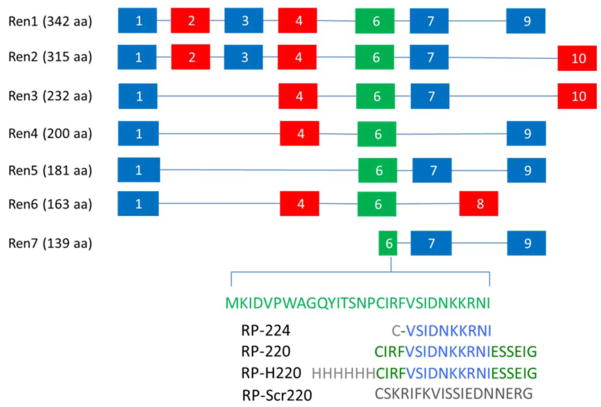
FIGURE 2.
Renalase isoforms Ren1–7: exons numbered from 1 to 10. RP-224, renalase peptide amino acid 224 233 of Ren1 or Ren2; RP-220, amino acids 220–239; RP-H220, histidine-tagged RP- 220; RP-Scr220, scrambled RP-220.
Mitogen-activated protein kinase activation critical for the protective effect of renalase peptides
The finding that renalase peptides were equally effective as recombinant renalase in protecting mice against AKI led us to search for mechanisms unrelated to renalase’s enzymatic activity. Chemical inhibition of ERK and AKT signaling completely abrogated the peptide’s protective action (Fig. 3), suggesting that the protective effect of renalase in ischemic AKI is mediated, at least in part, through ERK1/2 and Phosphatidylinositol-4,5-bisphosphate 3-kinase or AKT signaling [10▪▪].
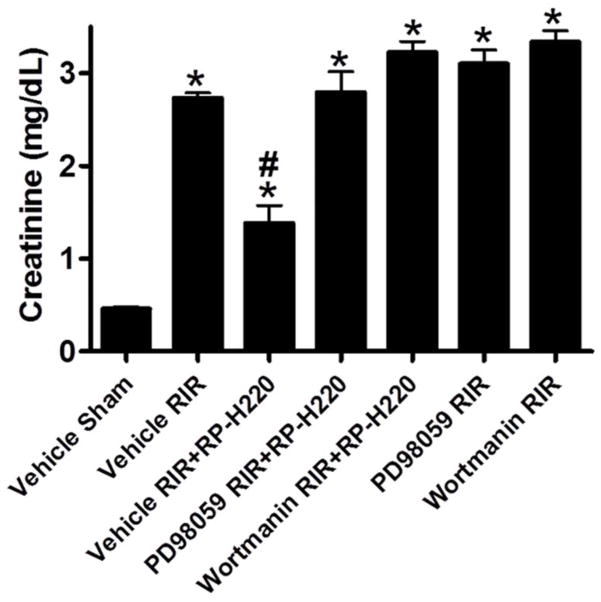
FIGURE 3.
Extracellular signal-regulated kinase or activation of protein kinase B inhibition abrogates protective effect of renalase peptide. Wild-type mice subjected to sham surgery or to 30 min of renal ischemia and reperfusion; RP-H220 or vehicle (saline) injected 10 min before renal ischemia. Extracellular signal-regulated kinase inhibitor PD98059 or the PI3K or activation of protein kinase B inhibitor wortmannin abrogated RP-H220’s protective effect.
These novel findings provide evidence that secreted renalase, which circulates as a dimer at a plasma concentration of ~4μg/ml (~54 nanomolar) [11], interacts with a membrane receptor to signal via the AKT and MAPK pathways. This signaling cascade is critical to the cytoprotective actions of renalase and renalase peptides.
ENZYMATIC PROPERTIES OF RENALASE
In the original description of renalase, we reported that the recombinant protein (Glutathione S-transferase fusion protein in Escherichia coli) could metabolize epinephrine, norepinephrine and dopamine in vitro [2]. We subsequently showed that recombinant renalase required nicotinamide adenine dinucleotide phosphate [NAD(P)H] for full activity [6,12]. An extensive search for putative renalase substrates identified only catecholamines and catecholamine-like compounds [12]. The renalase knockout mouse is a hypertensive mouse and has increased plasma catecholamines levels [5]. On the basis of the above, we concluded that the effects of recombinant renalase were mediated by decreased circulating catecholamines. The validity of our conclusion, however, has been questioned. The method used to assess renalase activity relied on the production of H2O2 as an indirect measure of oxidase activity. Because the measured rate of H2O2 synthesis was low, the putative oxidase activity of renalase was deemed unlikely to have physiologic significance [3,13]. Inaddition, recombinant renalase synthesized in E. coli as a histidine-tagged protein had no detectable oxidase activity and yet caused a marked decrease in BP when injected into rats [3].
A recent study indicates that recombinant renalase functions as an oxidase/anomerase, using molecular oxygen to convert α-NAD(P)H into β-NAD(P)+, with hydrogen peroxide as a reaction byproduct [14▪▪]. It is proposed that the α-anomer of NAD(P)H reduces the renalase flavin cofactor, which in turn reacts with dioxygen to form hydrogen peroxide and releases nicotinamide dinucleotide product in the β-form (Fig. 4). These processes are at least two orders of magnitude more rapid than any reported activity of renalase with catechol neurotransmitters. The kinetics and equilibria of renalase in turnover with α-NAD(P)H have been measured [15].
FIGURE 4.
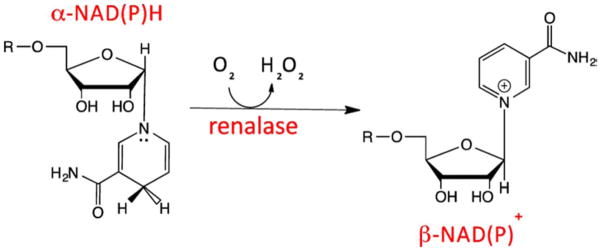
Renalase and α-NAD(P)H oxidase/anomerase: proposed scheme. Oxidation of α-dihydropyridyl ring of NAD(P), with transfer of two electrons to the flavin cofactor of renalase, and conversion of the ribose C1 from α to β configuration; reduced FAD cofactor is reoxidized by reacting with dioxygen-yielding hydrogen peroxide as a reaction byproduct.
The physiological relevance of renalase’s ability to convert α-NAD(P)H into β-NAD(P)+ has not been established. Because there appears to be a measurable rate of spontaneous conversion of β-NAD(P)H to α-NAD(P)H in biological solutions, and because α-NAD(P)H is not reported to participate in cellular reactions in humans, it is possible that renalase regulates an intracellular salvage pathway designed to maintain adequate cellular concentrations of β-NAD(P)H. Additional studies will be required to formally test that hypothesis. It should be noted, however, that renalase’s enzymatic activity does not appear to be relevant to its cellular signaling properties and cytoprotective actions [10▪▪].
RENALASE AND THE RENAL DOPAMINERGIC SYSTEM
There is extensive experimental support for the notion that the proximal tubule generatesdopamine, and that endogenous intrarenal dopamine contributes to the overall regulation of renal sodium and phosphate excretion. Changes in renal dopamine concentration modulate the signaling pathways that control renal tubular Pi reabsorption. Wild-type mice fed a high-phosphate diet acutely increased renal and urinary dopamine and phosphate excretion [16]. This was accompanied by increased dopamine synthesis by aromatic acid decarboxylase and decreased activity of renalase, monoamine oxidase A and monoamine oxidase B.A low-phosphate diet markedly decreased dopamine and phosphate excretion [16]. These data suggest that the rapid adaptation to changes in phosphate intake involves alterations in the key enzymes of dopamine metabolism.
Effect of renalase on renal phosphate transport
Renalase knockout mice maintained on a regular diet develop a moderate fall in plasma phosphate, which is accompanied by an increase in urinary phosphate (PO4−) excretion [17]. This could not be ascribed to an intrinsic renal defect in the knockout because both wild-type and knockout mice respond similarly to PO4− restriction by increasing renal catechol-O-methyltransferase-1 activity and markedly decreasing PO4− excretion. Instead, urinary dopamine increased by two folds, whereas urinary L-DOPA excretion decreased by two folds in the knockout mouse, indicating an upregulation of renal dopamine synthesis, which would in turn account for stimulated PO4− excretion, and moderately severe hypophosphatemia. The specific signal to increase renal dopamine synthesis, though unclear, is rather strong as it overcomes a compensatory increase in catechol-O-methyltransferase-1 activity in the knockout.
Interaction between the D5 dopamine receptors and renalase
Because the dopaminergic and sympathetic systems interact to regulate blood pressure, and because renalase could regulate circulating epinephrine levels and dopamine production in renal proximal tubules, it is possible that D1-like receptors regulate renalase expression in the kidney. Fenoldopan, a D1-like receptor agonist, increased renalase protein expression and function in renal proximal tubular cells from normotensive rats, but decreased expression and function in cells obtained from spontaneously hypertensive rats [18]. The regulation of renalase by the D1-like receptor appears to be mediated by D5 receptor signaling in a protein kinase C-dependent manner. These results suggest that aberrant regulation of renalase by the D5 receptor may contribute to the pathogenesis of hypertension.
DISEASE ASSOCIATION WITH RENALASE SINGLE NUCLEOTIDE POLYMORPHISM
Renalase is enclosed by a gene spanning ~310 kb and that contains many SNPs. Several renalase SNPs have been linked to common human diseases, such as hypertension and diabetes. The most consistent association of renalase SNPs has been with type 1 diabetes.
Type 1 diabetes
There is a strong association between type 1 diabetes and polymorphisms within the human leukocyte antigen (HLA) region, indicating an immune basis for the disease. A recent genome-wide association study found that approximately 42 loci affect the risk of diabetes [19]. Most importantly, it identified 18 novel loci, and the strongest evidence of association among these novel regions was achieved at rs10509540 (combined P=1.3×10−28), located on chromosome 10q23.31 in the renalase (RNLS) gene [19]. In another recent study, a statistical test of colocalization was applied to type 1 diabetes-associated regions and confirmed a potential role for renalase in the development of autoimmune pancreatic β-cell destruction [20]. Outside the HLA region, RNLS, rs10509540, and the IL2 SNP rs2069763 have been shown to have an age-at-diagnosis effect on pediatric-onset type 1 diabetes, and for both loci, subjects with the susceptible homozygous phenotype were diagnosed ~7 months earlier than those with the protective genotypes [21▪]. Because an effect of rs10509540, RNLS (10q23.31), was not detected in adult-onset autoimmune diabetes, it, along with the HLA class II alleles, distinguishes pediatric-onset type 1 diabetes from adult-onset autoimmune diabetes [22]. Renalase is expressed in T lymphocytes, and the mechanisms that underlie its potential role in the development of type 1 diabetes should be investigated.
Ischemic stroke
An association between renalase and stroke was first noted in a study designed to investigate renalase SNPs in patients with type 2 diabetes [23]. It was reported that 66% of hypertensive patients with stroke were GG homozygotes at rs10887800 SNP. This association was confirmed in an independent cohort of 130 stroke patients without diabetes, suggesting that the G allele rs10887800 might be useful in identifying patients at increased risk of stroke. In another study, serum renalase was significantly lower in prevalent patients on hemodialysis with a history of stroke than in those without stroke [24].
Correlation between renalase SNPs and severity of intracranial cerebral atherosclerotic vascular stenosis was examined in 212 ischemic stroke patients and 244 healthy controls from the northern Chinese Han population [25]. SNP rs10887800 in the renalase gene was associated with severe intracranial cerebral atherosclerotic vascular stenosis.
Similar results were obtained with a different cohort, a northern Chinese Han population [26]. In 507 ischemic stroke patients and 503 sexmatched controls, rs10887800 and rs2576178 SNPs were significantly associated with ischemic stroke and hypertension. Taken together, the above studies suggest that renalase may be involved in the pathogenesis of ischemic stroke. The specific mechanism is unclear, but may relate to the role of renalase in blood pressure regulation and endothelial dysfunction [27].
Organ transplantation
The time course of plasma and urine renalase levels and activity were studied for up to 90 days in 26 end-stage renal disease patients receiving a cadaver kidney transplant [28]. Although plasma creatinine, epinephrine and renalase decreased significantly at 2 weeks and 3 months after transplant, renalase levels remained approximately three fold above control levels 3 months after transplant, indicating a disconnect between renalase levels, and renal function and plasma epinephrine. Given that renalase functions as a cytokine, it will be important to identify the other signals that are likely to participate in the regulation of renalase secretion in plasma.
CONCLUSION
Our understanding of the physiology and pathophysiology of renalase has expanded dramatically over the past 2 years. The discovery that, independent of its intrinsic enzymatic properties, renalase functions as a signaling molecule that interacts with a yet-to-be identified plasma membrane receptor to activate the MAPK pathway and increase cell survival, is a major step forward. This should serve as the basis for further work aimed at exploring the mechanistic details of the renalase signaling cascade (Fig. 5), including the identification of the renalase receptor(s). The potential therapeutic utility of renalase administration in AKI should be explored. Lastly, we are not unaware of the possibility that abnormal regulation of renalase expression and function may impact the survival of cancer cells, and that, in some circumstances, inhibition of renalase expression or function could be beneficial.
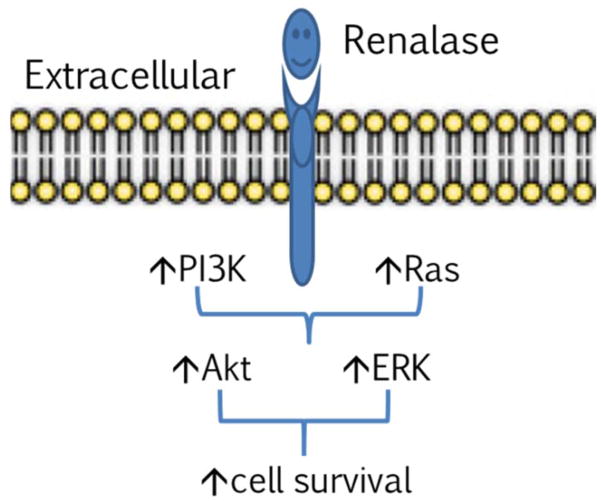
FIGURE 5.
Working model of renalase as a cytokine. Extracellular renalase interacts with a plasma membrane receptor to activate protein kinase B and extracellular signal-regulated kinase and increase cell survival.
KEY POINTS.
Renalase is a cytokine that interacts with a plasma membrane receptor to activate protein kinase B (AKT) and the MAPK pathway.
The cytoprotective effects of renalase depend on its signaling properties, and not on its enzymatic function.
There is a strong and consistent association between renalase SNPs and the development of type 1 diabetes, suggesting an immune regulatory function of renalase.
Acknowledgments
Funding Sources: This work was supported in part by VA Connecticut (H.V., R.S. and G.V.D.), National Basic Research Program of China 973 Program 2012CB517600, 2012CB517602, Shanghai Health Bureau ZXSNXD-CC-ZDYJ002 (W.L.)
Footnotes
Conflicts of interest
G.V.D. is a named inventor on several issued patents for the discovery and therapeutic utility of renalase.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Li G, Xu J, Wang P, et al. Catecholamines regulate the activity, secretion, and synthesis of renalase. Circulation. 2008;117:1277–1282. doi: 10.1161/CIRCULATIONAHA.107.732032. [DOI] [PubMed] [Google Scholar]
- 2.Xu J, Li G, Wang P, et al. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest. 2005;115:1275–1280. doi: 10.1172/JCI24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milani M, Ciriello F, Baroni S, et al. FAD-binding site and NADP reactivity in human renalase: a new enzyme involved in blood pressure regulation. J Mol Biol. 2011;411:463–473. doi: 10.1016/j.jmb.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Hennebry SC, Eikelis N, Socratous F, et al. Renalase, a novel soluble FAD-dependent protein, is synthesized in the brain and peripheral nerves. Mol Psychiatry. 2010;15:234–236. doi: 10.1038/mp.2009.74. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Xu J, Velazquez H, et al. Renalase deficiency aggravates ischemic myocardial damage. Kidney Int. 2011;79:853–860. doi: 10.1038/ki.2010.488. [DOI] [PubMed] [Google Scholar]
- 6.Farzaneh-Far R, Desir GV, Na B, et al. A functional polymorphism in renalase (Glu37Asp) is associated with cardiac hypertrophy, dysfunction, and ischemia: data from the heart and soul study. PLoS One. 2010;5:e13496. doi: 10.1371/journal.pone.0013496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Q, Fan Z, He J, et al. Renalase gene is a novel susceptibility gene for essential hypertension: a two-stage association study in northern Han Chinese population. J Mol Med (Berl) 2007;85:877–885. doi: 10.1007/s00109-006-0151-4. [DOI] [PubMed] [Google Scholar]
- 8.Lienhart W-D, Gudipati V, Macheroux P. The human flavoproteome. Arch Biochem Biophys. 2013;535:150–162. doi: 10.1016/j.abb.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9▪.Lee HT, Kim JY, Kim M, et al. Renalase protects against ischemic AKI. J Am Soc Nephrol. 2013;24:445–455. doi: 10.1681/ASN.2012090943. This study demonstrates that renalase knockout mice have more severe ischemic renal injury, and that recombinant renalase administration ameliorates ischemic injury by decreasing apoptosis and inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10▪▪.Wang L, Velazquez H, Moeckel G, et al. Renalase prevents AKI independent of amine oxidase activity. J Am Soc Nephrol. 2014;25:1226–1235. doi: 10.1681/ASN.2013060665. This study establishes the signaling properties of renalase, and that it functions as a cytokine and activates protein kinase B and the MAPK pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Przybylowski P, Malyszko J, Kozlowska S, et al. Serum renalase depends on kidney function but not on blood pressure in heart transplant recipients. Transplant Proc. 2011;43:3888–3891. doi: 10.1016/j.transproceed.2011.08.075. [DOI] [PubMed] [Google Scholar]
- 12.Desir GV, Tang L, Wang P, et al. Renalase lowers ambulatory blood pressure by metabolizing circulating adrenaline. J Am Heart Assoc. 2012;1:e002634. doi: 10.1161/JAHA.112.002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boomsma F, Tipton KF. Renalase, a catecholamine-metabolising enzyme? J Neural Transm. 2007;114:775–776. doi: 10.1007/s00702-007-0672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪▪.Beaupre BA, Carmichael BR, Hoag MR, et al. Renalase is an alpha-NAD(P)H oxidase/anomerase. J A Chem Soc. 2013;135:13980–13987. doi: 10.1021/ja407384h. The authors provided evidence that renalase specifically oxidizes α-NADH and converts it into β-NAD+ and suggested that renalase could represent the key player in an in-vivo salvage pathway that prevents the accumulation of the metabolically inert α-NADH. [DOI] [PubMed] [Google Scholar]
- 15.Beaupre BA, Hoag MR, Carmichael BR, Moran GR. Kinetics and equilibria of the reductive and oxidative half-reactions of human renalase with α-NADPH. Biochemistry. 2013;52:8929–8937. doi: 10.1021/bi401185m. [DOI] [PubMed] [Google Scholar]
- 16.Weinman EJ, Biswas R, Steplock D, et al. Increased renal dopamine and acute renal adaptation to a high-phosphate diet. Am J Physiol Renal Physiol. 2011;300:F1123–F1129. doi: 10.1152/ajprenal.00744.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sizova D, Velazquez H, Sampaio-Maia B, et al. Renalase regulates renal dopamine and phosphate metabolism. Am J Physiol Renal Physiol. 2013;305:F839–F844. doi: 10.1152/ajprenal.00616.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Lu X, Yang J, et al. Regulation of renalase expression by D5 dopamine receptors in rat renal proximal tubule cells. Am J Physiol Renal Physiol. 2014;306:F588–F596. doi: 10.1152/ajprenal.00196.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett JC, Clayton DG, Concannon P, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace C, Rotival M, Cooper JD, et al. Statistical colocalization of monocyte gene expression and genetic risk variants for type 1 diabetes. Hum Mol Genet. 2012;21:2815–2824. doi: 10.1093/hmg/dds098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪.Howson JMM, Cooper JD, Smyth DJ, et al. Evidence of gene-gene interaction and age-at-diagnosis effects in type 1 diabetes. Diabetes. 2012;61:3012–3017. doi: 10.2337/db11-1694. Outside the HLA region, only RNLS, rs10509540, and the IL2 SNP rs2069763 have been shown to have an age-at-diagnosis effect on pediatric-onset type 1 diabetes, and subjects with the susceptible homozygous phenotype were diagnosed ~7 months earlier than those with the protective genotypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howson JMM, Rosinger S, Smyth DJ, et al. Genetic analysis of adult-onset autoimmune diabetes. Diabetes. 2011;60:2645–2653. doi: 10.2337/db11-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buraczynska M, Zukowski P, Buraczynska K, et al. Renalase gene polymorphisms in patients with type 2 diabetes, hypertension and stroke. Neuromolecular Med. 2011;13:321–327. doi: 10.1007/s12017-011-8158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malyszko J, Koc-Zorawska E, Malyszko JS, et al. Renalase, stroke, and hypertension in hemodialyzed patients. Ren Fail. 2012;34:727–731. doi: 10.3109/0886022X.2012.681534. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Wang Z, Liu Y, et al. Association of imaging classification of intracranial cerebral atherosclerotic vascular stenosis in ischemic stroke and renalase gene polymorphisms. J Mol Neurosci. 2014;52:461–466. doi: 10.1007/s12031-013-0110-9. [DOI] [PubMed] [Google Scholar]
- 26.Zhang R, Li X, Liu N, et al. An association study on renalase polymorphisms and ischemic stroke in a Chinese population. Neuromolecular Med. 2013;15:396–404. doi: 10.1007/s12017-013-8227-0. [DOI] [PubMed] [Google Scholar]
- 27.Zbroch E, Malyszko J, Malyszko J, et al. Renalase, kidney function, and markers of endothelial dysfunction in renal transplant recipients. Pol Arch Med Wewn. 2012;122:40–44. [PubMed] [Google Scholar]
- 28.Quelhas-Santos J, Soares-Silva I, Fernandes-Cerqueira C, et al. Plasma and urine renalase levels and activity during the recovery of renal function in kidney transplant recipients. Exp Biol Med. 2014;239:502–508. doi: 10.1177/1535370214522182. [DOI] [PubMed] [Google Scholar]