Abstract
Background
Black patients with metastatic colorectal cancer have inferior survival compared to white patients. The purpose of this study was to examine disparity in specialist consultation and multimodality treatment and the impact that treatment inequality has on survival.
Methods
We identified 9935 non-Hispanic white and 1281 black patients with stage IV colorectal cancer aged 66 years and older from the Surveillance, Epidemiology, and End Results (SEER)–Medicare linked database. Logistic regression models identified race-based differences in consultation rates and subsequent treatment with surgery, chemotherapy, or radiation. Multivariable Cox regression models identified potential factors that explain race-based survival differences. All statistical tests were two-sided.
Results
Black patients had lower rates of consultation with surgery, medical oncology, and radiation oncology. Among patients seen in consultation, black patients received less surgery directed at the primary tumor, liver- or lung-directed surgery, chemotherapy, and radiotherapy. Unadjusted survival analysis found a 15% higher chance of dying for black patients compared with white patients (hazard ratio [HR] = 1.15; 95% confidence interval (CI) = 1.08 to 1.22; P < .001). Adjustment for patient, tumor, and demographic variables marginally reduced the risk of death (HR = 1.08; 95% CI = 1.01 to 1.15; P = .03). After adjustment for differences in treatment, the increased risk of death for black patients disappeared.
Conclusions
Our study shows racial disparity in specialist consultation as well as subsequent treatment with multimodality therapy for metastatic colorectal cancer, and it suggests that inferior survival for black patients may stem from this treatment disparity. Further research into the underlying causes of this inequality will improve access to treatment and survival in metastatic colorectal cancer.
Despite screening efforts and improvements in treatment, colorectal cancer remains the second leading cause of cancer death in the United States (1). Approximately 20% of patients have stage IV disease at diagnosis (1), and upwards of 50% of those initially with stage I to stage III disease will develop metastatic disease. The management of metastatic colorectal cancer is patient-specific, requires multidisciplinary input, and often involves multimodality treatment with chemotherapy, colon or rectal surgery, metastasectomy of liver or lung tumors, and radiotherapy (2,3).
In the United States, colorectal cancer disproportionally affects black patients, with higher incidence rates (1), more advanced stage at presentation (4,5), and decreased survival compared with other races (6). Numerous studies have addressed racial disparity in colorectal cancer (4–19), and unfortunately the survival gap between black and white patients may be widening, especially in patients with metastatic disease (15). Studies in populations of patients with locoregional colorectal cancer have found that black patients have lower consultation rates with specialists, as well as lower rates of subsequent treatment after consultation (11). Additionally, prospective clinical trial data often show equivalent survival among black and white patients receiving the same treatment, which suggests that referral and treatment may contribute to inferior overall survival in black patients compared with white patients (13,16). The question of race-based differences in referral patterns, subsequent treatment, and the potential impact on survival has not been addressed in patients with metastatic colorectal cancer.
The purpose of this study is to examine treatment disparity among black patients with metastatic colorectal cancer on a population-based level. Specifically we explored race-based differences in patient consultation with cancer specialists, as well as subsequent treatment. Finally, we analyzed the impact that treatment disparity has on survival.
Methods
Data
This study used the Surveillance, Epidemiology, and End Results (SEER)–Medicare linked database. The SEER program consists of cancer registries that account for 28% of the US population. The Medicare program provides federally funded health insurance for people aged 65 years and older. The SEER–Medicare linkage contains Medicare claims data for Medicare beneficiaries with cancer in the SEER database. This unique population-based dataset provides a valuable resource that allows researchers to track patterns of care over the continuum of the life of cancer patients.
Study Population
This study identified 22742 patients with stage IV colorectal cancer aged 66 years and older diagnosed between 2000 and 2007. We excluded patients with multiple primary tumors (n = 3169), as well as those diagnosed with cancer at death or autopsy (n = 1426). Patients with incomplete Medicare claims data (continuous part A and part B, without part C enrollment) for 12 months before diagnosis (to calculate comorbidity) through death or last follow-up were excluded (n = 6168). Finally, races other than non-Hispanic black or non-Hispanic white were excluded because of small numbers, which limited further analyses (n = 763). After the above exclusions, our final sample contained 11216 patients. A higher fraction of black patients had stage IV disease at presentation (20% black vs. 15% white). A higher fraction of black patients were excluded because of diagnosis at death or autopsy (8.5% black excluded vs. 7.3% white) and because of incomplete Medicare claim data (35% black excluded vs. 32% white). On the other hand, more white patients were excluded because of multiple primary tumors (22% white vs. 19% black). After all exclusion criteria were applied, a smaller proportion of black patients were excluded, and the overall fraction of black patients increased slightly from 9.2% to 11.4%.
Covariables Studied
Race was the primary covariable of interest, and it was obtained from SEER using the definition from the 2000 US Census and Bureau of Vital Statistics (20). In addition to race, other patient- and tumor-related variables obtained from SEER included age, marital status, disease site (colon or rectum), primary tumor size, tumor grade, geographic location, and socioeconomic status. Year of diagnosis was included as a categorical covariable (years 2000–2007) to account for trends over time. Individual SEER cancer registries were reclassified into East (Connecticut, New Jersey), Midwest (Detroit, Iowa), South (Atlanta, Rural Georgia, Kentucky, Louisiana), and West (San Francisco, Hawaii, New Mexico, Seattle, Utah, San Jose, Los Angeles, Greater California). Socioeconomic status was estimated by median regional household income divided into quintiles. Median regional household income was determined from the 2000 Census using Census tract data preferably over zip code data and secondarily using race and age adjusted data preferably over unadjusted data. Patients without household income data (1%) were grouped into the bottom quintile (20). Comorbidity was assessed during the year before diagnosis using inpatient and outpatient Medicare claims (21) with the Deyo adaptation (22) of the Charlson comorbidity index (23). Patient care in a teaching hospital was defined as any indirect medical education payment during a hospitalization after the patient’s diagnosis.
Identifying Consultation with a Specialist
Patient consultation with a surgeon, medical oncologist, or radiation oncologist was defined as the presence of a Medicare claim from a specialist within the timeframe extending from 1 month before diagnosis through death. The specialty of a physician was determined from 2 independent sources. First, we used the Medicare carrier file provider specialty data (HCFA codes) to identify consultation with a surgeon, medical oncologist, or radiation oncologist. Second, we linked unique physician identifier numbers (UPIN) and national provider identifier (NPI) numbers from the Medicare dataset with the American Medical Association (AMA) Masterfile. The AMA Masterfile collects specialty data on physicians and provided a secondary source to identify consultation with a surgeon, medical oncologist, or radiation oncologist. Finally, all patients who underwent surgery, received chemotherapy, or received radiotherapy were assumed to have been seen in consultation by a surgeon, medical oncologist, or radiation oncologist, respectively.
Treatment Characteristics
The treatment modalities evaluated in this study included chemotherapy, surgery, and radiation therapy; this information was obtained from Medicare claims data. Because of the heterogeneous and dynamic course of metastatic colorectal cancer, treatment can occur anytime from diagnosis through death. Therefore, this study captured treatment in the timeframe extending from diagnosis through death. Surgery in patients with metastatic colorectal cancer typically serves one of three general purposes: 1) resect the primary tumor, 2) resect metastatic disease in the liver or lung, and 3) attempt to bypass bowel obstruction from the tumor. Therefore, the delivery of surgery was classified into three groups: 1) surgery directed at the primary tumor in the colon or rectum, 2) surgery directed at the liver or lung, and 3) bowel diversion or placement of a stoma (without surgery directed at the primary). The delivery of chemotherapy and radiotherapy were captured from Medicare as described elsewhere (24,25). Radioactive implants (brachytherapy) and radioisotopes, coded separately in SEER and Medicare, were not counted as radiotherapy (n = 12). Table 1 has the specific codes from SEER and Medicare used to capture these treatments.
Table 1.
Medicare and Surveillance, Epidemiology, and End Results codes used in study
| Variable | Codes used to define variable |
|---|---|
| Surgery | |
| Surgery consult | AMA Masterfile specialty codes: AS, ASO, CRS, GS, SO |
| Medicare carrier file specialty codes: 02, 28, 91 | |
| Surgery directed at primary tumor | CPT codes: 44140–44160, 45110–45119 |
| Procedure codes: 457.1–457.6, 457.9, 458.1–458.3, 484.0, 484.1–484.3, 484.9, 485.0–485.2, 485.9, 486.1–486.5, 486.9 | |
| SEER primary site surgery code: 30–80 | |
| Liver surgery | CPT codes: 47120, 47122, 47125, 47130 |
| Procedure codes: 502.2, 503 | |
| Lung surgery | CPT codes: 32440, 32442, 32445, 32480, 32482, 32484, 32486, 32488, 32500, 32503, 32504, 32520, 32522, 32525, 32657, 32663 |
| Procedure codes: 321, 322.0, 322.9, 323, 323.0, 323.9, 324, 324.1, 324.9, 325, 325.0, 325.9, 326 | |
| Diversion surgery | CPT code: 44130 |
| Procedure code: 459.0–459.5 | |
| Ostomy placement | CPT codes: 44300, 44310, 44320, 44322 |
| Procedure codes: 461.0–461.4 | |
| Chemotherapy | |
| Medical oncology consult | AMA Masterfile specialty codes: ON, HO, HEM Medicare carrier file specialty codes: 82, 83, 90 |
| Chemotherapy | CPT codes: J8999–J9999, J8520–J8521, Q0083–Q0085, 96400–96599 |
| Diagnosis codes: V581, V662, V672 | |
| Revenue center codes: 0331, 0332, 0335 | |
| Procedure code: 99.25 | |
| NDC codes: 00004110013, 00004110020, 00004110051, 00004110113, 00004110116, 00004110150, 00004110151 | |
| Radiation therapy | |
| Radiation oncology consult | AMA Masterfile specialty codes: RO |
| Medicare carrier file specialty codes: 92 | |
| Radiation delivery | CPT codes: 61793, 61796, 61797–9, 61800, 63620–1, 77371–3, 77401–16, 77418, 77421–3, 77520, 77522–3, 77525, 0082T, 0197T, G0173-4, G0243, G0251, G0339-40 |
| SEER radiation code: 1 | |
Statistical Analysis
Differences in patient and tumor characteristics between black and white patients were assessed with χ2 tests. Continuous covariables were divided into categorical covariables to assess for nonlinear trends. We evaluated the impact of race on treatment with surgery, chemotherapy, or radiotherapy across the study population with univariate and multivariable logistic regression models. Black–white treatment disparity with treatment was discovered, so we next sought to determine whether the source of this disparity was from lack of consultation with a cancer specialist or lack of treatment after consultation. Black–white disparity in consultation rates with a cancer specialist and black–white disparity in subsequent treatment with surgery, chemotherapy, or radiation were assessed with univariate and multivariable logistic regression models. Potential interactions between race and all other covariables listed above were examined. Overall survival was defined from the date of diagnosis through death or last follow-up (December 31, 2009). Unadjusted survival differences between black and white patients were demonstrated graphically with Kaplan–Meier plots. Sequentially constructed multivariable Cox regression models were used to determine potential groups of factors that explain race-based survival differences. The multivariable survival analysis included treatment variables that have an accepted impact on survival, such as the delivery of chemotherapy and surgery on liver or lung metastases. Other treatment variables without clear evidence of a survival benefit, such as primary tumor surgery, ostomy/diversion placement, and radiotherapy, were excluded from the multivariable survival analysis. All analyses were conducted with SAS version 9.3 (SAS Institute Inc, Cary, NC). This study was deemed exempt from institutional review board approval by the University of California San Diego. All statistical tests were two-sided.
Results
Baseline differences between the 9935 white patients (89%) and 1281 black patients (11.4%) with stage IV colorectal cancer are demonstrated in Table 2. In general, black patients were more likely to be younger at diagnosis, be unmarried, live in the South or Midwest, have more comorbid disease, and have colon primary tumors. Also, black patients were more likely to be treated at a teaching hospital, likely secondary to the higher proportion residing in metropolitan areas. Of the entire study population, 72% had surgery directed at their primary colon or rectal tumor, 5.0% had a liver or lung resection, and 6.6% had a surgical diversion or ostomy placement. Fifty percent of the population received chemotherapy, and 13% received radiotherapy.
Table 2.
Description of patient characteristics according to race
| Variable | White | Black |
|---|---|---|
| Total | 9935 | 1281 |
| Age at diagnosis, y | ||
| 66–69 | 1622 (16) | 285 (22) |
| 70–74 | 2209 (22) | 318 (25) |
| 75–79 | 2362 (24) | 258 (20) |
| 80–84 | 2029 (20) | 222 (17) |
| ≥85 | 1713 (17) | 198 (15) |
| Urban/Rural | ||
| Metro area ≥250000 | 5292 (53) | 916 (72) |
| Metro area <1000000 | 2870 (29) | 254 (20) |
| Urban area ≥20,000 | 646 (7) | 46 (4) |
| Urban area 2500–19999 | 908 (9) | 54 (4) |
| Rural area <2500 | 219 (2) | 11 (1) |
| Teaching hospital | ||
| Yes | 5080 (51) | 861 (67) |
| Region | ||
| East | 2635 (27) | 283 (22) |
| Midwest | 1669 (17) | 276 (22) |
| South | 1784 (18) | 435 (34) |
| West | 3847 (39) | 287 (22) |
| Income | ||
| Bottom quintile | 1454 (15) | 730 (57) |
| 2nd quintile | 2093 (21) | 184 (14) |
| 3rd quintile | 2107 (21) | 163 (13) |
| 4th quintile | 2156 (22) | 126 (10) |
| Top quintile | 2125 (21) | 78 (6) |
| Sex | ||
| Male | 4649 (47) | 559 (44) |
| Female | 5286 (53) | 722 (56) |
| Marital status | ||
| Married | 4834 (49) | 414 (32) |
| Other | 5101 (51) | 867 (68) |
| Charlson score | ||
| 0 | 5880 (59) | 675 (53) |
| 1 | 2360 (24) | 307 (24) |
| 2 | 982 (10) | 165 (13) |
| ≥3 | 713 (7) | 134 (10) |
| Year of diagnosis | ||
| 2000 | 1232 (12) | 176 (14) |
| 2001 | 1260 (13) | 169 (13) |
| 2002 | 1325 (13) | 136 (11) |
| 2003 | 1313 (13) | 169 (13) |
| 2004 | 1305 (13) | 178 (14) |
| 2005 | 1248 (13) | 159 (12) |
| 2006 | 1167 (12) | 154 (12) |
| 2007 | 1085 (11) | 140 (11) |
| Tumor site | ||
| Colon | 7692 (77) | 1066 (83) |
| Rectal | 2243 (23) | 215 (17) |
| Primary tumor size | ||
| ≤5 cm | 3640 (37) | 414 (32) |
| >5 cm | 2836 (29) | 349 (27) |
| Unknown | 3459 (35) | 518 (40) |
| Primary tumor grade | ||
| Well to moderately differentiated | 5197 (52) | 717 (56) |
| Poorly to undifferentiated | 2838 (29) | 260 (20) |
| Unknown | 1900 (19) | 304 (24) |
First we looked for treatment differences between black and white patients across the whole study population. Black patients were statistically significantly less likely to receive surgery directed at their primary tumor, liver- or lung-directed surgery, chemotherapy, and radiation therapy (Table 3). Black patients were 10% less likely to have primary tumor surgery, 40% less likely to have liver- or lung-directed surgery, 17% less likely to receive chemotherapy, and 30% less likely to receive radiotherapy. Among patients who received chemotherapy, white patients were more likely to receive more than one chemotherapy agent (69% vs. 63%; P = 0.01). The multivariable analysis presented in Table 3 shows that the statistically significant treatment differences between black and white patients held after controlling for potential confounding covariables.
Table 3.
Racial differences in treatment for stage IV colorectal cancer (n = 11216)
| Treatment | Racial group, No. (%) | Unadjusted odds ratio (95% CI)* | P | Adjusted odds ratio (95% CI)† | P | |
|---|---|---|---|---|---|---|
| White | Black | |||||
| Surgery | ||||||
| Primary tumor | 6531 (66) | 757 (59) | 0.75 (0.67 to 0.85) | <.001 | 0.81 (0.67 to 0.97) | .02 |
| Liver or lung | 471 (4.7) | 37 (2.9) | 0.60 (0.43 to 0.84) | .002 | 0.62 (0.42 to 0.90) | .01 |
| Diversion/ostomy | 586 (5.9) | 83 (6.5) | 1.11 (0.87 to 1.40) | .45 | 0.84 (0.64 to 1.12) | .23 |
| Chemotherapy | 4784 (48) | 515 (40) | 0.72 (0.64 to 0.81) | <.001 | 0.82 (0.71 to 0.94) | .005 |
| Radiotherapy | 1294 (13) | 117 (9) | 0.67 (0.55 to 0.82) | <.001 | 0.75 (0.60 to 0.95) | .02 |
* Odds ratios and P values comparing treatment for black vs white patients from a univariate logistic regression analysis. All statistical tests were two-sided. CI = confidence interval.
† Odds ratios and P values comparing treatment for black vs white patients from a multivariable analysis adjusted for age at diagnosis, sex, Charlson comorbidity score, tumor site, primary tumor size, tumor grade, geographic region, income level, urban/rural, and year of diagnosis. All statistical tests were two-sided.
To understand the source of the race-based differences in treatment, we next explored whether black patients were seen in consultation by cancer specialists and, of those seen, what fraction were subsequently treated (Table 4). On multivariable analysis, black patients were less likely to be seen in consultation by a surgeon, and black patients had a trend toward lower consultation rates with a medical oncologist. Among the patients seen in consultation, black patients were less likely to receive liver- or lung-directed surgery and chemotherapy. There was a trend toward decreased radiation oncology consultation rates and subsequent treatment with radiotherapy for black patients (Table 4); however, the radiation treatment rate among the whole population was lower for this population (Table 3), suggesting that both decreased consultation rates and decreased consultation after treatment factor into the population-wide reduction in treatment with radiation. We found no evidence of statistical interaction between race and other covariables.
Table 4.
Racial differences in referral to specialist and subsequent treatment for stage IV colorectal cancer
| Endpoint | Racial group, No. (%) | Unadjusted odds ratio (95% CI)* | P | Adjusted odds ratio (95% CI)† | P | |
|---|---|---|---|---|---|---|
| White | Black | |||||
| Consult with surgeon | 8995 (91) | 1125 (88) | 0.75 (0.63 to 0.90) | .002 | 0.73 (0.58 to 0.91) | .005 |
| If consult, probability of primary tumor surgery | 6531 (73) | 757 (67) | 0.78 (0.68 to 0.89) | <.001 | 0.87 (0.72 to 1.07) | .19 |
| If consult, probability of liver or lung surgery | 471 (5.2) | 37 (3.3) | 0.62 (0.44 to 0.87) | .005 | 0.64 (0.44 to 0.93) | .02 |
| If consult, probability of diversion/ostomy | 586 (6.5) | 83 (7.4) | 1.14 (0.90 to 1.45) | .27 | 0.87 (0.66 to 1.16) | .34 |
| Consult with medical oncologist | 8146 (82) | 1036 (81) | 0.93 (0.80 to 1.08) | .33 | 0.86 (0.72 to 1.02) | .09 |
| If consult, probability of chemotherapy | 4784 (59) | 515 (50) | 0.70 (0.61 to 0.79) | <.001 | 0.85 (0.73 to 0.99) | .04 |
| Consult with radiation oncologist | 2222 (22) | 213 (17) | 0.69 (0.59 to 0.81) | <.001 | 0.84 (0.70 to 1.01) | .06 |
| If consult, probability of radiation therapy | 1294 (58) | 117 (55) | 0.87 (0.66 to 1.16) | .35 | 0.72 (0.52 to 1.01) | .06 |
* Odds ratios and P values comparing consultation and subsequent treatment for black vs white patients from a univariable logistic regression. All statistical tests were two-sided. CI = confidence interval.
† Odds ratios and P values comparing consultation and subsequent treatment for black vs white patients from a multivariable logistic regression adjusted for age at diagnosis, sex, Charlson comorbidity score, tumor site, primary tumor size, tumor grade, geographic region, income level, urban/rural, and year of diagnosis. All statistical tests were two-sided.
Next we focused on racial differences in the timing of specialist consultation and treatment. The median time from diagnosis to medical oncology consult was 16 days, from diagnosis to surgery consult was 14 days, and from diagnosis to radiation oncology consult was 18 days. No statistically significant differences existed in time from diagnosis to consult with any specialist for black patients compared with white patients. The time from consult through receipt of chemotherapy was 4 days longer for black patients compared with white patients (P = .005), whereas no statistically significant differences existed with respect to time from consult to surgery or consult to radiation. With radiotherapy, the median time from diagnosis through consult was 4 days earlier for those who subsequently received radiation compared with those who did not receive radiation (P < .001). With chemotherapy and surgery, the time from diagnosis through consult did not substantially vary by whether the patient was subsequently treated or not.
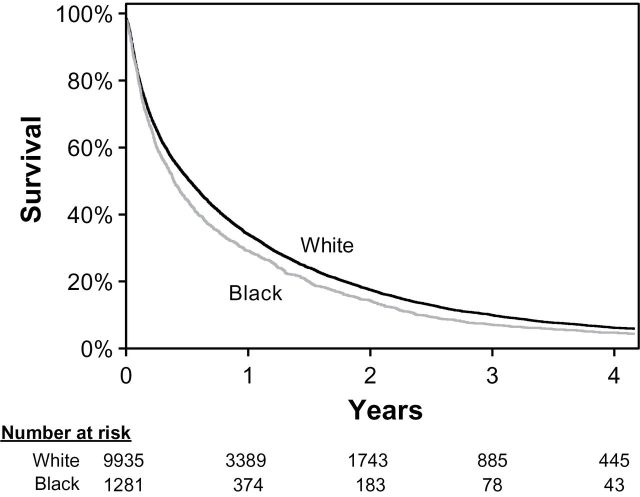
Next, we examined survival in colorectal cancer. Among the entire study cohort of 11216 patients, 10648 (95%) died within our study follow-up period. The median survival for the whole study cohort was 6.0 months. The unadjusted median survival was 4.7 months for black patients, compared with 6.3 months for white patients (P < .001) (Figure 1). We attempted to determine the underlying factors driving this black–white survival difference with a multivariable analysis (Table 5). In the unadjusted analysis, black patients had a 15% higher risk of dying compared with white patients (hazard ratio [HR] = 1.15; 95% confidence interval [CI] = 1.08 to 1.22; P < .001). Adjustment for age, sex, comorbidity, tumor site, primary tumor size, and tumor grade had no material effect on the risk of death for black patients. After further adjustment for income, location, and year of diagnosis, the risk of death for black patients attenuated to 8% (HR = 1.08; 95% CI = 1.01 to 1.15; P = .03). No statistically significant racial difference in risk of dying was observed after adjustment for treatment, including chemotherapy and surgery directed at liver or lung metastases (HR = 1.01; 95% CI = 0.95 to 1.08; P = .70). This analysis suggests that the survival differences between black and white patients are explained by a combination of demographic factors (decrease of adjusted HR from 1.15 to 1.08), and treatment differences (decrease of adjusted HR from 1.08 to 1.01).
Figure 1.
Overall survival among patients with stage IV colorectal cancer stratified by race.
This figure shows an unadjusted Kaplan–Meier analysis of overall survival for white and black patients.
Table 5.
Race and survival among black and white patients with stage IV colorectal cancer
| Model | Overall survival for black vs white patients HR (95% CI)* | P |
|---|---|---|
| Unadjusted | 1.15 (1.08 to 1.22) | <.001 |
| Prior model with adjustment for patient covariables† | 1.17 (1.10 to 1.24) | <.001 |
| Prior model with adjustment for tumor characteristics‡ | 1.15 (1.08 to 1.22) | <.001 |
| Prior model with adjustment for demographic covariables§ | 1.08 (1.01 to 1.15) | .03 |
| Prior model with adjustment for treatmentǁ | 1.01 (0.95 to 1.08) | .70 |
* Hazard ratio (HR) and 95% confidence interval (CI) estimated from a multivariable Cox regression. All statistical tests were two-sided.
† Clinical covariables include age at diagnosis, sex, and Charlson comorbidity score.‡ Tumor characteristics include tumor site, tumor size, and histologic grade.§ Demographics include geographic region, income level, urban/rural, and year of diagnosis.ǁ Treatment includes chemotherapy and surgery of the liver or lung.
Finally, we examined the impact that treatment has on survival with a multivariable analysis controlling for age, sex, comorbidity, tumor site, primary tumor size, tumor grade, geographic region, income level, urban/rural, year of diagnosis, and race. After controlling for these confounders, treatment with chemotherapy or surgery directed at liver or lung metastases were both independently associated with increased survival. The receipt of chemotherapy was associated with a 66% decreased risk of death (HR = 0.34; 95% CI = 0.33 to 0.36; P < .001), and surgery directed at liver or lung lesions was associated with a 53% decreased risk of death (HR = 0.47; 95% CI = 0.42 to 0.52; P < .001).
Discussion
This study identified several findings related to racial disparity for black patients with metastatic colorectal cancer. Our results demonstrate disparity in specialist consultation, as well as subsequent treatment with multimodality therapy. Additionally, our analyses suggest that inferior survival in metastatic colorectal cancer for black patients compared with white patients may result from such treatment disparity.
The racial inequality with treatment of metastatic colorectal cancer observed in this study agrees with comparable analyses in patients with localized colorectal cancer (8,11). With nonmetastatic stage II and III rectal cancer, Morris et al. found that black patients had decreased rates of consultation with medical oncology and surgical oncology, as well as decreased treatment with chemotherapy and radiotherapy after consultation (11). Similarly, Baldwin et al. studied stage III colon cancer and found decreased rates of consultation with a medical oncologist and decreased rates of subsequent treatment with chemotherapy among blacks (8). Obeida et al. found that black patients received newer chemotherapy agents less frequently than white patients (12). Additionally, our findings are consistent with research in other cancers, including nonmetastatic esophageal (26), pancreatic (27), and lung (28) cancers, that show black patients have lower rates of consultation and lower rates of subsequent treatment after consultation with a specialist. Altogether, these findings imply that racial inequality in cancer treatment does not stop after consultation. The administrative data used in this study, like other epidemiology studies, lack the granularity to isolate the underlying causes of inequality. Although the true cause of these black–white differences remains unknown, potential explanations may include the following: 1) conscious or unconscious provider biases, 2) patient mistrust, 3) health literacy, 4) patient–physician communication breakdown, 5) health-care access barriers, or 6) race-based differences in disease biology.
In addition to treatment disparity, a critical finding of our study relates to the association between treatment, race, and survival. We found that black patients had a 15% increased risk of death compared with white patients; however, underlying patient and demographic factors only partly explained this disparity. Of note, our analysis found that 47% of the relative survival difference between black and white patients was attributable to treatment differences, and after accounting for these treatment differences, the race-based survival difference completely disappeared. Prospective cohort studies evaluating black–white differences, and analyses of clinical trial data have yielded mixed results, with some studies finding no survival difference (13,16), and others finding inferior survival for black patients (19); however, these studies lack analyses that control for important confounding factors such as comorbidity, socioeconomic status, and geography, which make direct comparisons with the results of our study difficult. Interestingly, in loco-regional nonmetastatic colorectal cancer, an analysis with SEER–Medicare data that controlled for socioeconomic status, comorbidity, and other confounders found that treatment differences failed to explain the survival differences between black and white patients (18). The difference between the importance of treatment in nonmetastatic and metastatic colorectal cancer is difficult to explain, although further understanding of these differences could shed light on the underlying causes of disparity. As with all epidemiology studies, this analysis does not show causality, and the link between treatment and survival could reflect unmeasured confounding variables. Regardless, the link between race, treatment, and survival strongly supports further research in this area.
There are potential limitations to this study worth mentioning. The SEER–Medicare data used in this study does not include patients aged less than 65 years. Although we found no variation in the race-based differences among the different age groups in this study, caution should be used when generalizing these results to patients aged less than 65 years. The median survival of 6 months for our entire study cohort was similar to other SEER registry studies involving older patients with metastatic colorectal cancer (10); however, 6 months is lower than the survival typically reported in contemporary chemotherapy trials (29–32). The survival difference between this study and clinical trials likely arises from the fact that only half of the patients in SEER received chemotherapy, which raises the question of age discrimination. Additionally, clinical trials typically include a select group of patients with higher performance status, lower disease burdens, and fewer comorbidities, which would tend to result in superior survival. A third limitation relates to the lack of clinical information on disease burden aside from the size of the primary colorectal tumor. Although all patients in this study had stage IV disease, black patients could present with more advanced or diffuse disease, which could render them less amenable to receiving treatment and could explain the inferior survival observed in our study. Additionally, the administrative data used in this study lack detail with regard to treatment specifics such as the number of chemotherapy cycles delivered, schedules, and doses, as well as details of surgery and radiotherapy. A final limitation inherent with research using registry and billing data relates to the lack the level of detail needed to conduct the in-depth analysis required to identify potential reasons for discrepancies in treatment.
In conclusion, this study demonstrates racial inequality for black patients compared with non-Hispanic white patients in specialist consultation and receipt of multidisciplinary treatment of metastatic colorectal cancer, as well as a detriment in survival associated with such disparities. These findings emphasize the need for further research into patient–physician communication, patient and provider biases, and racial hurdles both inside and outside the health-care system. Identifying and eliminating these racial barriers will improve survival in black patients with metastatic colorectal cancer.
Funding
This work was supported by a Young Investigator Award from the American Society of Clinical Oncology (to JDM) and a research collaboration grant from Varian Medical Systems (to JDM and QTL).
This study used the linked SEER–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc; and the SEER Program tumor registries in the creation of the SEER–Medicare database.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. [DOI] [PubMed] [Google Scholar]
- 2. National Comprehensive Cancer Network. Colon Cancer, Version 3.2013. http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf Accessed on January 29, 2013. [Google Scholar]
- 3. National Comprehensive Cancer Network. Rectal Cancer, Version 4.2013. http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf Accessed on January 29, 2013. [Google Scholar]
- 4. Marcella S, Miller JE. Racial differences in colorectal cancer mortality. The importance of stage and socioeconomic status. J Clin Epidemiol. 2001;54(4):359–66. [DOI] [PubMed] [Google Scholar]
- 5. Mayberry RM, Coates RJ, Hill HA, et al. Determinants of black/white differences in colon cancer survival. J Natl Cancer Inst. 1995;87(22):1686–1693. [DOI] [PubMed] [Google Scholar]
- 6. Clegg LX, Li FP, Hankey BF, et al. Cancer survival among US whites and minorities: a SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch Intern Med. 2002;162(17):1985–1993. [DOI] [PubMed] [Google Scholar]
- 7. Ayanian JZ, Zaslavsky AM, Fuchs CS, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol. 2003;21(7):1293–1300. [DOI] [PubMed] [Google Scholar]
- 8. Baldwin LM, Dobie SA, Billingsley K, et al. Explaining black-white differences in receipt of recommended colon cancer treatment. J Natl Cancer Inst. 2005;97(16):1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Demissie K, Oluwole OO, Balasubramanian BA, et al. Racial differences in the treatment of colorectal cancer: a comparison of surgical and radiation therapy between whites and blacks. Ann Epidemiol. 2004;14(3):215–221. [DOI] [PubMed] [Google Scholar]
- 10. Golan T, Urban D, Berger R, et al. Changing prognosis of metastatic colorectal adenocarcinoma: differential improvement by age and tumor location. Cancer. 2013;119(16):3084–3091. [DOI] [PubMed] [Google Scholar]
- 11. Morris AM, Billingsley KG, Hayanga AJ, et al. Residual treatment disparities after oncology referral for rectal cancer. J Natl Cancer Inst. 2008;100(10):738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Obeidat NA, Pradel FG, Zuckerman IH, et al. Racial/ethnic and age disparities in chemotherapy selection for colorectal cancer. Am J Manag Care. 2010;16(7):515–522. [PubMed] [Google Scholar]
- 13. Polite BN, Sing A, Sargent DJ, et al. Exploring racial differences in outcome and treatment for metastatic colorectal cancer: results from a large prospective observational cohort study (BRiTE). Cancer. 2012;118(4):1083–1090. [DOI] [PubMed] [Google Scholar]
- 14. Potosky AL, Harlan LC, Kaplan RS, et al. Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. J Clin Oncol. 2002;20(5):1192–1202. [DOI] [PubMed] [Google Scholar]
- 15. Robbins AS, Siegel RL, Jemal A. Racial disparities in stage-specific colorectal cancer mortality rates from 1985 to 2008. J Clin Oncol. 2012;30(4):401–405. [DOI] [PubMed] [Google Scholar]
- 16. Sanoff HK, Sargent DJ, Green EM, et al. Racial differences in advanced colorectal cancer outcomes and pharmacogenetics: a subgroup analysis of a large randomized clinical trial. J Clin Oncol. 2009;27(25):4109–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schrag D, Rifas-Shiman S, Saltz L, et al. Adjuvant chemotherapy use for Medicare beneficiaries with stage II colon cancer. J Clin Oncol. 2002;20(19):3999–4005. [DOI] [PubMed] [Google Scholar]
- 18. White A, Vernon SW, Franzini L, et al. Racial disparities in colorectal cancer survival: to what extent are racial disparities explained by differences in treatment, tumor characteristics, or hospital characteristics? Cancer. 2010;116(19):4622–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yothers G, Sargent DJ, Wolmark N, et al. Outcomes among black patients with stage II and III colon cancer receiving chemotherapy: an analysis of ACCENT adjuvant trials. J Natl Cancer Inst. 2011;103(20):1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. [DOI] [PubMed] [Google Scholar]
- 22. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 23. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 24. Virnig BA, Warren JL, Cooper GS, et al. Studying radiation therapy using SEER-Medicare-linked data. Med Care. 2002;40(8 Suppl):49–54. [DOI] [PubMed] [Google Scholar]
- 25. Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(8 Suppl):55–61. [DOI] [PubMed] [Google Scholar]
- 26. Steyerberg EW, Earle CC, Neville BA, et al. Racial differences in surgical evaluation, treatment, and outcome of locoregional esophageal cancer: a population-based analysis of elderly patients. J Clin Oncol. 2005;23(3):510–517. [DOI] [PubMed] [Google Scholar]
- 27. Murphy MM, Simons JP, Ng SC, et al. Racial differences in cancer specialist consultation, treatment, and outcomes for locoregional pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16(11):2968–2977. [DOI] [PubMed] [Google Scholar]
- 28. Earle CC, Neumann PJ, Gelber RD, et al. Impact of referral patterns on the use of chemotherapy for lung cancer. J Clin Oncol. 2002;20(7):1786–1792. [DOI] [PubMed] [Google Scholar]
- 29. de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938–2947. [DOI] [PubMed] [Google Scholar]
- 30. Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25(13):1670–1676. [DOI] [PubMed] [Google Scholar]
- 31. Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22(1):23–30. [DOI] [PubMed] [Google Scholar]
- 32. Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343(13):905–914. [DOI] [PubMed] [Google Scholar]