Abstract
Currently, no animal models fully embody exfoliation syndrome (XFS) or exfoliation glaucoma (XFG). Both genetic and environmental factors appear critical for disease manifestation, and both must be considered when generating animal models. Because mice provide a powerful mammalian platform for modeling complex disease, this paper focuses on mouse models of XFS and XFG.
Exfoliation syndrome (XFS) is the most common identifiable cause of open-angle glaucoma. Despite its prevalence, little is understood about its molecular etiology or about the factors determining susceptibility and progression to exfoliation glaucoma (XFG). It is clear that XFS is a complex, age-related disease affected by both genetic and environmental factors. Nevertheless, both the genetic and environmental factors need better definition. 1-4 A strong association between single-nucleotide polymorphisms (SNPs) in the lysyl oxidase–like 1 (LOXL1) gene and XFS was identified in the Swedish and Icelandic populations using a genome-wide association study. 5. This association was replicated in other populations. 6-22 However, risk alleles of LOXL1 are very common in unaffected controls, and the mechanisms of action of these alleles are not clear. 7, 23 Risk alleles at contactin-associated protein-like 2 (CNTNAP2) loci also have significant associations between XFS and XFG in some but not all populations. 24-26. Mutations in CNTNAP2 cause neurologic disease, and it is not clear if allelic, genetic background or other differences modulate the phenotypic consequences of mutation in this gene. A host of environmental factors have been suggested to influence XFS but findings are often inconsistent across studies.3, 4
The challenge of modeling XFS/XFG
To understand the molecular mechanisms underlying XFS and progression to XFG tractable animal models are needed. Extremely few publications have studied animal models of XFS, and to our knowledge, only 2 animal models have been reported. 27, 28 There are no reports of models with all of the features of XFS with XFG. The lack of animal models has been suggested to reflect the typical occurrence of XFS at old ages, with the belief that model species do not live long enough to develop the condition. Although possible, we do not feel that the shorter lifespan of model species in absolute years prevents development of XFS. Disease susceptibility in model species increases with age relative to lifespan in a similar fashion to that in people. 29 For example, within a 2 years life span, mice often develop complex, age-related diseases that occur later in human life. On the other hand, differences in environment may be critical and profoundly impact whether or not model species develop XFS. The degree and nature of exposure to light (UV), low temperature, viruses and caffeine have all been suggested to impact development of XFS. 4, 30-33 Most laboratory animals are housed in cages with limited UV exposure, constant controlled temperature, limited or no exposure to pathogens and lack of caffeine and other lifestyle factors.
Genetic differences across species may also be important. In humans a high-risk genotype at the LOXL1 locus is typically necessary for manifestation of XFS, as high-risk alleles are present in almost all patients with XFS. 23
Thus, the challenge of modeling XFS/XFG in animals is to develop models reflecting these complex genetic and environmental risk factors.
Current Models
Porcine model
The first animal model was reported in pigs. 27 The authors fed pigs a high sucrose, high salt diet to induce cataracts. They reasoned that cataracts are common in XFS and that mature cataracts shed exfoliative material. After a few months on the diet the pigs developed cataracts and had an exfoliation-like material that contained crystallins. We are not aware of any follow up studies. The relevance of this model to the human disease is not yet clear, though it may caution against high salt and sugar intakes.
Lyst mutant mouse model
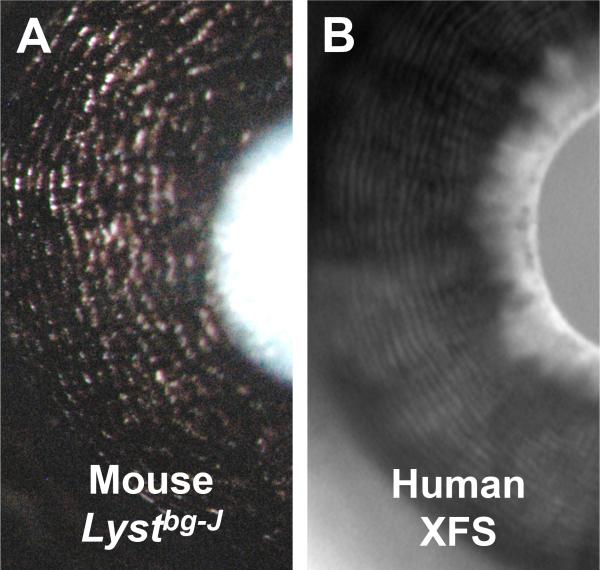
The other model is an inherited mouse model, which shares several features with human XFS. In addition to the accumulation of fibrillar material in the eye, patients with XFS have characteristic saw-tooth morphology of the iris pigment epithelium. 34, 35 This results in iris transillumination defects characterized by a specific concentric, circular transillumination pattern. 36 Similar to human patients, Lyst mutant mice have microscopically detectable deposits of fibrillin 1 positive material in the eye. 28 They also replicate the saw-tooth morphology of the iris pigment epithelium and have the same pattern of transillumination defects as human patients (Figure 1). In human XFS, increased susceptibility to oxidative stress has been suggested to contribute to the pathology 37, 38 and, as in other glaucomas, levels of transforming growth factor beta (TGF-β) superfamily members are elevated 39-42. The Lyst mutant iris disease involves oxidative damage 43 and TGF-β levels in Lyst mutant eyes remain to be tested. Thus, the mice have some XFS-like phenotypes, but lack clinically obvious XFS deposits or glaucoma. Although the degree of relevance of this mouse to human XFS is not yet clear, it is currently the most similar available model. Further evaluation of this model will be important. Similarly, evaluation of the LYST gene and functionally related genes in human patients is worthwhile.
Figure 1. Characteristic iris transillumination defects of XFS/XFG.
(A) Mouse homozygous for the Lystbg-J mutation imaged with standard slit-lamp illumination. Note concentric circles of transillumination defects (red). (B) Human XFS patient imaged with infrared videography showing concentric circles of transillumination defects (white). The two images have been scaled differently to promote comparison.
Future models
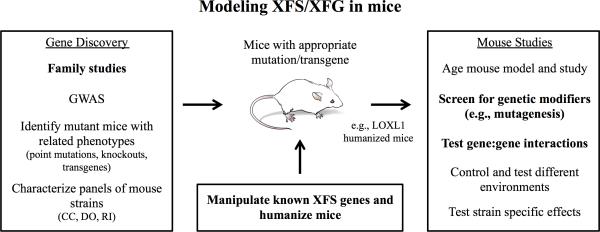
The housing environment may critically interact with genetics to determine if model species develop XFS. Thus, environment should be considered and manipulated when working to produce new models of XFS. Among other factors, the degree and nature of exposure to light (UV), low temperature, presence of specific viruses/microbes, dietary composition and caffeine intake may impact the development of XFS. 4, 30-33, 44 Using animal models, the importance of specific environmental factors could be clearly determined. It is now possible to abrogate or greatly decrease the amount of gene product produced by mutating genes in various species and a variety of species may contribute to improved understanding of XFS. Due to their small size, high fecundity, relatively low cost and the most powerful array of available genetic resources and tools for dissecting complex diseases and humanizing their genomes, mice will remain a very important model species. 45, 46 Mice are likely to add substantially to our mechanistic understanding of XFS (Figure 2).
Figure 2. Strategies to model XFS/XFG in mice.
Gene Discovery: A main priority is to identify genes associated with XFS. Large families provide a rare but important resource for identifying causal genes - they may provide the most effective mode of gene discovery. Genome-wide association studies (GWAS) remain important, but gene discovery can be difficult due to complexity and very large sample sizes are needed for further progress. The characterization of mice with mutations or transgenes affecting specific genes may also identify new XFS genes. Another avenue for gene discovery is characterization of a series of mouse strains that are specifically tailored for identifying genes that underlie complex diseases like XFS, such as Collaborative Cross (CC), Diversity Outcross (DO) and Recombinant Inbred (RI) mice. Mice can be screened for XFS based on the presence of exfoliation, concentric circular iris defects, or high IOP and glaucoma. Mouse models: Once human genes are identified, mouse models can be made by targeted mutagenesis or by making transgenic mice. A top priority is to create a humanized mouse model containing a high-risk allele of LOXL1. BAC transgenic mice with a human LOXL1 allele and its regulatory elements will likely best recapitulate LOXL1-associated disease risk. Mouse studies: Humanized LOXL1 mice and other mouse models need to be studied in the context of aging, genetic modifiers, environmental conditions, and gene-environment interactions. Using chemical mutagenesis to induce random point mutations in humanized LOXL1 mice is an unbiased way to uncover genetic modifiers of LOXL1. Generating combined mutants with LOXL1 variants and Lyst, humanized CNTNAP2, or newly discovered XFS genes is also an effective strategy to understand genetic interactions. Priority areas that may provide the quickest route to useful mouse models are indicated in bold.
Mice with human alleles of LOXL1 and CNTNAP2
As the LOXL1 genotype is critically important in the development of XFS, an important step in producing a new mouse model of XFS is to make mice with variant forms of the Loxl1 gene. Mice with a completely nonfunctional allele of Loxl1 have an elastic tissue disease 47 but do not develop XFS. 48, 49 Although it is worth aging and assessing these mice in different environments, it remains possible that a null allele will not cause XFS. Since the specific DNA change(s) that render susceptibility to XFS are not clearly defined and may be intronic, making mice that are transgenic for a human bacterial artificial chromosome (BAC) containing a high-risk human allele of LOXL1 is a high priority. If multiple high-risk alleles are defined from different populations, mice can be made with alleles conferring different degrees of risk with initial bias towards those with the strongest effect. Also, it will be important to evaluate multiple transgenic mouse lines with different expression levels.
LOXL1 genotype alone is unlikely to be sufficient to induce XFS, as many individuals with a high-risk allele do not have the disease. Thus, it will be important both to combine the human LOXL1 transgene with mutations in other genes (such as Lyst) and to assess the effects of different environments. Decreased antioxidant capacity and increased propensity to inflammation may be important in XFS and XFG. 38, 50, 51 Mutations that alter these systems can be introduced into the transgenic mice by breeding. Altered TGF-β signaling has been implicated in XFS, but transgenic or knockout mice affecting this pathway have not been demonstrated to develop XFS. Combining human LOXL1 transgenes with mutations affecting TGF-β pathways may prove valuable, especially for the development of high IOP and glaucoma. 52-55 Although less clearly important, mutations in CNTNAP2 are also implicated in XFS and it is worth assessing mice with CNTNAP2 mutations and humanizing mice with human high-risk alleles.
Other XFS gene and models
Various efforts to identify XFS genes are underway, including genome wide association studies and genetic studies in human families. Due to the complexity of the disease these studies are not easy, but once more genes are identified they can be assessed in mice using the approaches discussed above. Genetic studies in families with many affected individuals may have the highest likelihood of success and are very important. Mutagenesis screens in mice are another powerful approach for providing animal models and discovering disease mechanism. 46, 56-59 With adequate aging and attention to environment, such screens may provide key new models of XFS and XFG. One important strategy for discovering XFS genes and pathways would be to perform a mutagenesis screen using mice that are transgenic for human high-risk alleles of LOXL1. Modifier screens to identify genetic differences that alter phenotypes caused by the LOXL1 transgene (or other human alleles) between mouse strains may prove valuable. 60-63
Footnotes
Disclosure: The authors declare no conflict of interest
References
- 1.Schlötzer-Schrehardt U. Genetics and genomics of pseudoexfoliation syndrome/glaucoma. Middle East Afr J Ophthalmol. 2011;18:30–36. doi: 10.4103/0974-9233.75882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sein J, Galor A, Sheth A, et al. Exfoliation syndrome: new genetic and pathophysiologic insights. Curr Opin Ophthalmol. 2013;24:167–174. doi: 10.1097/ICU.0b013e32835d5d11. [DOI] [PubMed] [Google Scholar]
- 3.Damji KF, Bains HS, Stefansson E, et al. Is pseudoexfoliation syndrome inherited? A review of genetic and nongenetic factors and a new observation. Ophthalmic Genet. 1998;19:175–185. doi: 10.1076/opge.19.4.175.2310. [DOI] [PubMed] [Google Scholar]
- 4.Stein JD, Pasquale LR, Talwar N, et al. Geographic and climatic factors associated with exfoliation syndrome. Arch Ophthalmol. 2011;129:1053–1060. doi: 10.1001/archophthalmol.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorleifsson G, Magnusson KP, Sulem P, et al. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science. 2007;317:1397–1400. doi: 10.1126/science.1146554. [DOI] [PubMed] [Google Scholar]
- 6.Aragon-Martin JA, Ritch R, Liebmann J, et al. Evaluation of LOXL1 gene polymorphisms in exfoliation syndrome and exfoliation glaucoma. Mol Vis. 2008;14:533–541. [PMC free article] [PubMed] [Google Scholar]
- 7.Challa P, Schmidt S, Liu Y, et al. Analysis of LOXL1 polymorphisms in a United States population with pseudoexfoliation glaucoma. Mol Vis. 2008;14:146–149. [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Jia L, Wang N, et al. Evaluation of LOXL1 polymorphisms in exfoliation syndrome in a Chinese population. Mol Vis. 2009;15:2349–2357. [PMC free article] [PubMed] [Google Scholar]
- 9.Fan BJ, Pasquale L, Grosskreutz CL, et al. DNA sequence variants in the LOXL1 gene are associated with pseudoexfoliation glaucoma in a U.S. clinic-based population with broad ethnic diversity. BMC Med Genet. 2008;9:5. doi: 10.1186/1471-2350-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fingert JH, Alward WL, Kwon YH, et al. LOXL1 mutations are associated with exfoliation syndrome in patients from the midwestern United States. Am J Ophthalmol. 2007;144:974–975. doi: 10.1016/j.ajo.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 11.Fuse N, Miyazawa A, Nakazawa T, et al. Evaluation of LOXL1 polymorphisms in eyes with exfoliation glaucoma in Japanese. Mol Vis. 2008;14:1338–1343. [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi H, Gotoh N, Ueda Y, et al. Lysyl oxidase-like 1 polymorphisms and exfoliation syndrome in the Japanese population. Am J Ophthalmol. 2008;145:582–585. doi: 10.1016/j.ajo.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Hewitt AW, Sharma S, Burdon KP, et al. Ancestral LOXL1 variants are associated with pseudoexfoliation in Caucasian Australians but with markedly lower penetrance than in Nordic people. Hum Mol Genet. 2008;17:710–716. doi: 10.1093/hmg/ddm342. [DOI] [PubMed] [Google Scholar]
- 14.Lee KY, Ho SL, Thalamuthu A, et al. Association of LOXL1 polymorphisms with pseudoexfoliation in the Chinese. Mol Vis. 2009;15:1120–1126. [PMC free article] [PubMed] [Google Scholar]
- 15.Mabuchi F, Sakurada Y, Kashiwagi K, et al. Lysyl oxidase-like 1 gene polymorphisms in Japanese patients with primary open angle glaucoma and exfoliation syndrome. Mol Vis. 2008;14:1303–1308. [PMC free article] [PubMed] [Google Scholar]
- 16.Mori K, Imai K, Matsuda A, et al. LOXL1 genetic polymorphisms are associated with exfoliation glaucoma in the Japanese population. Mol Vis. 2008;14:1037–1040. [PMC free article] [PubMed] [Google Scholar]
- 17.Mossbock G, Renner W, Faschinger C, et al. Lysyl oxidase-like protein 1 (LOXL1) gene polymorphisms and exfoliation glaucoma in a Central European population. Mol Vis. 2008;14:857–861. [PMC free article] [PubMed] [Google Scholar]
- 18.Ozaki M, Lee KY, Vithana EN, et al. Association of LOXL1 gene polymorphisms with pseudoexfoliation in the Japanese. Invest Ophthalmol Vis Sci. 2008;49:3976–3980. doi: 10.1167/iovs.08-1805. [DOI] [PubMed] [Google Scholar]
- 19.Pasutto F, Krumbiegel M, Mardin CY, et al. Association of LOXL1 common sequence variants in German and Italian patients with pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1459–1463. doi: 10.1167/iovs.07-1449. [DOI] [PubMed] [Google Scholar]
- 20.Ramprasad VL, George R, Soumittra N, et al. Association of non-synonymous single nucleotide polymorphisms in the LOXL1 gene with pseudoexfoliation syndrome in India. Mol Vis. 2008;14:318–322. [PMC free article] [PubMed] [Google Scholar]
- 21.Tanito M, Minami M, Akahori M, et al. LOXL1 variants in elderly Japanese patients with exfoliation syndrome/glaucoma, primary open-angle glaucoma, normal tension glaucoma, and cataract. Mol Vis. 2008;14:1898–1905. [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X, Zabriskie NA, Hau VS, et al. Genetic association of LOXL1 gene variants and exfoliation glaucoma in a Utah cohort. Cell Cycle. 2008;7:521–524. doi: 10.4161/cc.7.4.5388. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Chen LJ, Zhang M, et al. Ethnicity-based subgroup meta-analysis of the association of LOXL1 polymorphisms with glaucoma. Mol Vis. 2010;16:167–177. [PMC free article] [PubMed] [Google Scholar]
- 24.Krumbiegel M, Pasutto F, Schlötzer-Schrehardt U, et al. Genome-wide association study with DNA pooling identifies variants at CNTNAP2 associated with pseudoexfoliation syndrome. Eur J Hum Genet. 2011;19:186–193. doi: 10.1038/ejhg.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malukiewicz G, Lesiewska-Junk H, Linkowska K, et al. Analysis of CNTNAP2 polymorphisms in Polish population with pseudoexfoliation syndrome. Acta Ophthalmol. 2012;90:e660–661. doi: 10.1111/j.1755-3768.2012.02395.x. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu A, Takano Y, Shi D, et al. Evaluation of CNTNAP2 gene polymorphisms for exfoliation syndrome in Japanese. Mol Vis. 2012;18:1395–1401. [PMC free article] [PubMed] [Google Scholar]
- 27.Veromann S, Sunter A, Juronen E, et al. Eye lens crystallins: a component of intraocular pseudoexfoliative material. Ophthalmic Res. 2004;36:51–54. doi: 10.1159/000076110. [DOI] [PubMed] [Google Scholar]
- 28.Trantow CM, Mao M, Petersen GE, et al. Lyst mutation in mice recapitulates iris defects of human exfoliation syndrome. Invest Ophthalmol Vis Sci. 2009;50:1205–1214. doi: 10.1167/iovs.08-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Pasquale LR, Wiggs JL, Willett WC, et al. The Relationship between caffeine and coffee consumption and exfoliation glaucoma or glaucoma suspect: a prospective study in two cohorts. Invest Ophthalmol Vis Sci. 2012;53:6427–6433. doi: 10.1167/iovs.12-10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ringvold A. Exfoliation syndrome immunological aspects. Acta Ophthalmol Suppl. 1988;184:35–43. doi: 10.1111/j.1755-3768.1988.tb02626.x. [DOI] [PubMed] [Google Scholar]
- 32.Detorakis ET, Kozobolis VP, Pallikaris IG, et al. Detection of herpes simplex virus in pseudoexfoliation syndrome and exfoliation glaucoma. Acta Ophthalmol Scand. 2002;80:612–616. doi: 10.1034/j.1600-0420.2002.800610.x. [DOI] [PubMed] [Google Scholar]
- 33.Resnikoff S, Filliard G, Dell'Aquila B. Climatic droplet keratopathy, exfoliation syndrome, and cataract. Br J Ophthalmol. 1991;75:734–736. doi: 10.1136/bjo.75.12.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asano N, Schlötzer-Schrehardt U, Naumann GO. A histopathologic study of iris changes in pseudoexfoliation syndrome. Ophthalmology. 1995;102:1279–1290. [PubMed] [Google Scholar]
- 35.Eagle RC, Jr., Font RL, Fine BS. The basement membrane exfoliation syndrome. Arch Ophthalmol. 1979;97:510–515. doi: 10.1001/archopht.1979.01020010254014. [DOI] [PubMed] [Google Scholar]
- 36.Fingert JH, Burden JH, Wang K, et al. Circumferential iris transillumination defects in exfoliation syndrome. J Glaucoma. 2013;22:555–558. doi: 10.1097/IJG.0b013e318255da16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlötzer-Schrehardt U, Naumann GO. Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol. 2006;141:921–937. doi: 10.1016/j.ajo.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 38.Erdurmus M, Yagci R, Atis O, et al. Antioxidant status and oxidative stress in primary open angle glaucoma and pseudoexfoliative glaucoma. Curr Eye Res. 2011;36:713–718. doi: 10.3109/02713683.2011.584370. [DOI] [PubMed] [Google Scholar]
- 39.Schlötzer-Schrehardt U, Zenkel M, Küchle M, et al. Role of transforming growth factor-beta1 and its latent form binding protein in pseudoexfoliation syndrome. Exp Eye Res. 2001;73:765–780. doi: 10.1006/exer.2001.1084. [DOI] [PubMed] [Google Scholar]
- 40.Picht G, Welge-Luessen U, Grehn F, et al. Transforming growth factor beta 2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefes Arch Clin Exp Ophthalmol. 2001;239:199–207. doi: 10.1007/s004170000252. [DOI] [PubMed] [Google Scholar]
- 41.Kottler UB, Jünemann AG, Aigner T, et al. Comparative effects of TGF-beta 1 and TGF-beta 2 on extracellular matrix production, proliferation, migration, and collagen contraction of human Tenon's capsule fibroblasts in pseudoexfoliation and primary open-angle glaucoma. Exp Eye Res. 2005;80:121–134. doi: 10.1016/j.exer.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 42.Inatani M, Tanihara H, Katsuta H, et al. Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 2001;239:109–113. doi: 10.1007/s004170000241. [DOI] [PubMed] [Google Scholar]
- 43.Trantow CM, Hedberg-Buenz A, Iwashita S, et al. Elevated oxidative membrane damage associated with genetic modifiers of Lyst-mutant phenotypes. PLoS Genet. 2010;6:e1001008. doi: 10.1371/journal.pgen.1001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yilmaz A, Ayaz L, Tamer L. Selenium and pseudoexfoliation syndrome. Am J Ophthalmol. 2011;151:272–276. e271. doi: 10.1016/j.ajo.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen D, Xu T. The expanding role of mouse genetics for understanding human biology and disease. Dis Model Mech. 2008;1:56–66. doi: 10.1242/dmm.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.John SW, Anderson MG, Smith RS. Mouse genetics: a tool to help unlock the mechanisms of glaucoma. J Glaucoma. 1999;8:400–412. [PubMed] [Google Scholar]
- 47.Liu X, Zhao Y, Gao J, et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 48.Wiggs JL, Pasquale LR, Pawlyk B, Xu X, Connolly E, Kim I, Miller JW, Rhee D, Haddadin R, Grosskreutz C, Chen T, Fan BJ, Haines JL, Li T. LOXL1 null mouse phenotype suggests that loss of lysyl oxidase like 1 function contributes to exfoliation glaucoma.. 59th Annual Meeting of The American Society of Human Genetics; Honolulu, HI. 2009. [Google Scholar]
- 49.Wiggs JL, Pawlyk B, Connolly E, et al. Disruption of the blood-aqueous barrier and lens abnormalities in mice lacking Lysyl Oxidase-like 1 (LOXL1). Invest Ophthalmol Vis Sci. 2014;55:856–64. doi: 10.1167/iovs.13-13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zenkel M, Kruse FE, Naumann GO, et al. Impaired cytoprotective mechanisms in eyes with pseudoexfoliation syndrome/glaucoma. Invest Ophthalmol Vis Sci. 2007;48:5558–5566. doi: 10.1167/iovs.07-0750. [DOI] [PubMed] [Google Scholar]
- 51.Schlötzer-Schrehardt U. [Oxidative stress and pseudoexfoliation glaucoma]. Klin Monbl Augenheilkd. 2010;227:108–113. doi: 10.1055/s-0028-1109977. [DOI] [PubMed] [Google Scholar]
- 52.Fleenor DL, Shepard AR, Hellberg PE, et al. TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006;47:226–234. doi: 10.1167/iovs.05-1060. [DOI] [PubMed] [Google Scholar]
- 53.Bachmann B, Birke M, Kook D, et al. Ultrastructural and biochemical evaluation of the porcine anterior chamber perfusion model. Invest Ophthalmol Vis Sci. 2006;47:2011–2020. doi: 10.1167/iovs.05-1393. [DOI] [PubMed] [Google Scholar]
- 54.Shepard AR, Millar JC, Pang IH, et al. Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci. 2010;51:2067–2076. doi: 10.1167/iovs.09-4567. [DOI] [PubMed] [Google Scholar]
- 55.Robertson JV, Golesic E, Gauldie J, et al. Ocular gene transfer of active TGF-beta induces changes in anterior segment morphology and elevated IOP in rats. Invest Ophthalmol Vis Sci. 2010;51:308–318. doi: 10.1167/iovs.09-3380. [DOI] [PubMed] [Google Scholar]
- 56.Vreugde S, Erven A, Kros CJ, et al. Beethoven, a mouse model for dominant, progressive hearing loss DFNA36. Nat Genet. 2002;30:257–258. doi: 10.1038/ng848. [DOI] [PubMed] [Google Scholar]
- 57.Nair KS, Hmani-Aifa M, Ali Z, et al. Alteration of the serine protease PRSS56 causes angle-closure glaucoma in mice and posterior microphthalmia in humans and mice. Nat Genet. 2011;43:579–584. doi: 10.1038/ng.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lachke SA, Alkuraya FS, Kneeland SC, et al. Mutations in the RNA granule component TDRD7 cause cataract and glaucoma. Science. 2011;331:1571–1576. doi: 10.1126/science.1195970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beckers J, Wurst W, de Angelis MH. Towards better mouse models: enhanced genotypes, systemic phenotyping and envirotype modelling. Nat Rev Genet. 2009;10:371–380. doi: 10.1038/nrg2578. [DOI] [PubMed] [Google Scholar]
- 60.Rubio-Aliaga I, Soewarto D, Wagner S, et al. A genetic screen for modifiers of the delta1-dependent notch signaling function in the mouse. Genetics. 2007;175:1451–1463. doi: 10.1534/genetics.106.067298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mohan S, Baylink DJ, Srivastava AK. A chemical mutagenesis screen to identify modifier genes that interact with growth hormone and TGF-beta signaling pathways. Bone. 2008;42:388–395. doi: 10.1016/j.bone.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 62.Matera I, Watkins-Chow DE, Loftus SK, et al. A sensitized mutagenesis screen identifies Gli3 as a modifier of Sox10 neurocristopathy. Hum Mol Genet. 2008;17:2118–2131. doi: 10.1093/hmg/ddn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu X, Dobbie M, Tunningley R, et al. ENU mutagenesis screen to establish motor phenotypes in wild-type mice and modifiers of a pre-existing motor phenotype in tau mutant mice. J Biomed Biotechnol. 2011;2011:130947. doi: 10.1155/2011/130947. [DOI] [PMC free article] [PubMed] [Google Scholar]