Abstract
The metabolism of polychlorinated biphenyls (PCBs) is complex and has an impact on toxicity and thereby assessment of PCB risks. A large number of reactive and stable metabolites are formed in the processes of biotransformation in biota in general and in humans in particular. The aim of this document is to provide an overview of PCB metabolism and to identify metabolites of concern and their occurrence. Emphasis is given to mammalian metabolism of PCBs and their hydroxyl, methylsulfonyl, and sulfated metabolites, especially those that persist in human blood. Potential intracellular targets and health risks are also discussed.
Keywords: Hydroxylated PCBs, polychlorobiphenylols, PCB methyl sulfones, PCB sulfates, human exposure, reactive intermediates
Introduction
Polychlorinated biphenyls (PCBs) are a class of industrial chemicals that were mass-produced globally from the late 1920s until their commercial production was banned, initially by the Toxic Substances Control Act (TSCA) in the United States in 1979, as a reaction to increasing numbers of reports of PCBs in humans and concern for adverse human health effects.
The commercial production of PCBs involved the batch chlorination of biphenyl with chlorine gas in the presence of a catalyst, resulting in the formation of complex mixtures containing a range of PCBs rather than just individual congeners (Erickson, 1997, Hansen, 1999, Erickson and Kaley, 2011). Typically, these PCB mixtures were sold under various trade names (such as Aroclor in the U.S., Kanechlor in Japan, or Clophen in Germany), often with numerical designations indicating percent chlorine by weight (e.g., Aroclor 1254 is 54% and Clophen A60 is 60%) (Silberhorn et al., 1990).
PCBs were used in a wide variety of applications that resulted in release to the environment. Open-ended applications included their uses as plasticizers in rubber and resins, in carbonless copy paper, in adhesives, in wax extenders, in dedusting agents, in paints, and in inks, while nominally closed system uses were in hydraulic fluids, heat-transfer fluids, and in lubricants. Finally, closed system uses of PCBs were primarily in capacitors and in transformers (Kimbrough et al., 1989). The estimated world production of PCBs has been variously estimated from about 1.2 million tons to 2 million tons, of which 0.2 to 0.4 million tons have become “environmentally available” (Tanabe, 1988, ATSDR, 2000). PCBs can now be detected all over the planet, from highly populated areas to the arctic region (Christensen et al., 2010, Gutleb et al., 2010, Macdonal et al., 2000, Ockenden et al., 2001, Wethington and Hornbuckle, 2005, Hu et al., 2010a). Although different for each congener, their resistance towards chemical and biological degradation explains their environmental persistence and omnipresence more than thirty years after their withdrawal from commercial mass production. From a public health perspective, this widespread distribution of PCBs indicates the need for a thorough understanding of the potential adverse effects associated with the parent congeners and their metabolites. This is of particular importance for populations living near, or eating fish from, PCB reservoirs such as the Great Lakes area, the Hudson River in New York or the area around Anniston, Alabama, a former Monsanto production site of PCBs (Custer et al., 2010, Fitzgerald et al., 2008, Goncharov et al., 2010, Goncharov et al., 2011, Silverstone et al., 2012).
PCBs that are the substrates for metabolic attack in exposed individuals arise from two sources. The major source of PCBs for the general population is the food supply (Feinberg et al., 2011, Schecter et al., 2010). A second and often overlooked source of exposure to PCBs is city air and the air of buildings that were constructed using PCBs in sealants, caulking, and other building materials (Ludewig et al., 2008, Persoon et al., 2010). Among these airborne PCBs are also most of the recently discovered nonlegacy PCBs, PCBs that are unintentionally formed as by-products during the manufacture of paints and dyes that are currently sold (Hu and Hornbuckle, 2010, Rodenburg et al., 2010a, Grossman, 2013).
PCBs in the food supply tend to be more highly chlorinated and are therefore poorer substrates for metabolic attack, while airborne PCBs tend to be more volatile, and possess fewer chlorine atoms (McFarland and Clarke, 1989, Robertson and Ludewig, 2011). The former group is longer lived/ more persistent, while the latter one, often referred to as transient or episodic, is composed of PCBs with comparatively short half-lives that are relatively quickly metabolized (Hansen, 2001, Zhao et al., 2010, Robertson and Ludewig, 2011). However, to date the metabolism of PCBs and the physiologic fate of the individual metabolites remain poorly understood.
Many biologic effects of PCBs are receptor-mediated (Luthe et al., 2008), including the well-described characteristics of PCBs as inducers of xenobiotic metabolism (Parkinson et al., 1983, Safe et al., 1985). On the other hand, PCB metabolism is generally regarded as a detoxication process due to the fact that a large proportion of all hydroxylated PCB metabolites (OH-PCBs) being formed are excreted from the body as such or after conjugation (Birnbaum, 1983, Ohta et al., 2015). However, it has become apparent that a variety of PCB toxication processes involves or depends on the metabolism of parent PCBs or their metabolic progeny. Electrophilic metabolic intermediates such as arene oxides may cause harm through their reactions with protein, DNA, or lipids (Pereg et al., 2001, Morck et al., 2002). In the event that OH-PCBs are further oxidized to (semi)quinones, these highly reactive species may also form covalent adducts with proteins, DNA and other endogenous compounds (Lin et al., 2000, Pereg et al., 2001, Robertson and Gupta, 2000, Song et al., 2009, Srinivasan et al., 2002, Amaro et al., 1996). Additionally, metabolites like OH-PCBs, PCB sulfates, and PCB methyl sulfones (MeSO2-PCBs) might be equally persistent as parent congeners and elicit their own toxicities.
In this review, we will attempt to emphasize the often overlooked issues of metabolism of PCBs and the role metabolism and metabolites play in toxication processes.
Environmental sources of PCBs
Traditional manufactured PCBs
Based on differences in their number of chlorine substituents, PCB congeners can be subdivided into semivolatile and relatively nonvolatile, with the higher chlorinated PCBs (HC-PCBs) typically being less volatile. These differences are also key determinants for their environmental availability and their routes of exposure. The majority of airborne PCBs found in major cities in the United States are lower-chlorinated ones (LC-PCBs) containing four or less chlorine substituents (Hu et al., 2010a, Persoon et al., 2010, Wethington and Hornbuckle, 2005). Both LC-PCBs and HC-PCBs were contained in traditionally manufactured commercial PCB products, however, HC-PCBs have a higher potential for bioaccumulation and biomagnification along the food chain (Barron et al., 1994, Troisi et al., 2001). As a result, human populations are typically exposed to less volatile, HC-PCBs via contaminated food, particularly fish (Domingo and Bocio, 2007, Weintraub and Birnbaum, 2008). Epidemiological studies have revealed a correlation between the consumption of contaminated fish and increased serum concentrations of such PCBs (Weintraub and Birnbaum, 2008).
Airborne PCBs
Although all PCBs are semivolatile, the LC-PCBs are most commonly reported. The highest concentrations of airborne PCBs are found in indoor air and in industrial and densely populated urban areas, for example in the cities of Chicago, Milwaukee, Toronto, Philadelphia and New York (Breivik et al., 2007, Hu et al., 2010a, Ockenden et al., 2001, Wethington and Hornbuckle, 2005, Sun et al., 2006, Du et al., 2009, Melymuk et al., 2012). The twenty most abundant of these airborne PCBs are summarized in Table 1. Volatilization of airborne PCBs is temperature dependent and can result in their release from environmental or industrial reservoirs, such as rivers, lakes, landfills or contaminated building materials (Persoon et al., 2010, Desborough and Harrad, 2011, Zhang et al., 2011, Simcik et al., 1999, Achman et al., 1993, Hsu et al., 2003, Rudel and Perovich, 2009).
Table 1.
The twenty most frequently detected PCB congeners in air and their potential sources
| Major Sources | Detection in | ||
|---|---|---|---|
| Paint pigments | Human serum | ||
| 52 | Aroclors 1016, 1242, 1248, 1254 | Y | Y |
| 20/28 | Aroclors 1016, 1242, 1248 | Y | Y |
| 11 | Pigment and dye production | Y | Y |
| 95 | Aroclor 1254 | Y | Y |
| 31 | Aroclors 1016, 1242, 1248 | Y | Y |
| 18/30 | Aroclors 1016, 1242 | Y | Y |
| 8 | Aroclors 1221, 1016, 1242 | Y | Y |
| 61/70/74/76 | Aroclors 1242, 1248, 1254 | Y | Y |
| 3 | Aroclor 1221 | Y | Y |
| 4 | Aroclor 1221, 1016, 1242 | Y | Y |
| 90/101/113 | Aroclor 1254 | Y | Y |
| 21/33 | Aroclors 1016, 1242 | Y | Y |
| 16 | Aroclors 1016, 1242 (>3%) | Y | Y |
| 15 | Aroclors 1016, 1242 | Y | Y |
| 110 | Aroclor 1254 | Y | Y |
| 17 | Aroclors 1016, 1242 (>3%) | ND | Y |
| 49/69 | Aroclors 1016, 1248 (>3) | ND | Y |
| 83/99 | Aroclor 1254 | ND | Y |
| 118 | Aroclor 1254 | Y | Y |
1) Congeners were ranked by frequency of occurrence in analyzed air samples.
2) For the co-eluting peaks, the bolded congeners are believed to be primary congeners.
3) Y: detected; ND: non-detectable
Human populations are more likely being exposed to lower-chlorinated, airborne PCBs by inhalation, rather than by ingestion (Harrad et al., 2009, Robertson and Ludewig, 2011). Indoor inhalation exposure to PCBs is of concern in schools and other buildings that were built in the 1950s and 1960s, as demonstrated by a number of studies investigating indoor exposure to PCBs in the United States and Europe (Herrick, 2010, Herrick et al., 2004, MacIntosh et al., 2012, Gabrio et al., 2000, Jamshidi et al., 2007, Harrad et al., 2010, Zhang et al., 2011). During this time, the caulking and other building materials used in construction contained high levels of PCBs and affected buildings still represent a major source for chronic inhalation exposures. A German study reporting measurements of several indicator PCBs in indoor air of contaminated schools found high concentrations of the lower-chlorinated PCB congeners 28 and 52 (Gabrio et al., 2000). Moreover, there was a significant correlation between PCB exposure and increased blood concentrations of PCBs in teachers who had worked in these contaminated school buildings. Another study reported a correlation between indoor PCB levels and serum PCB concentrations in residents in the Hudson River area (Fitzgerald et al., 2011). The public health relevance of indoor inhalation exposure is further highlighted by several studies indicating that indoor air concentrations of PCBs significantly exceeded those determined in their respective outdoor environments (Jamshidi et al., 2007, Menichini et al., 2007).
Nonlegacy PCBs
The common perception that PCBs are long-banned industrial contaminants that are no longer produced is unfortunately misleading. In recent years, it has been conclusively shown that non-Aroclor, or nonlegacy PCBs contaminate the environment of homes and cities and accumulate in the bodies of exposed populations. In 2008, the Iowa Superfund Research Program (ISRP) published the results of a large-scale air toxics monitoring program, demonstrating for the first time that PCB 11 (3,3’di-chlorobiphenyl), a non-Aroclor PCB that has been previously detected in New York/ New Jersey harbor surface and waste water (Litten et al., 2002), was ubiquitous in Chicago (Hu et al., 2008) and in Cleveland (Persoon et al., 2010) air. Since that initial discovery, researchers have reported the presence of nonlegacy PCBs in air samples around the world and it has been revealed that the most likely source of these contaminants is volatilization from common household paint (Basu et al., 2009, Choi et al., 2008). In 2010, a study of PCBs in pigments manufactured and sold as colorants in household paint revealed the presence of more than 50 nonlegacy PCBs (Hu and Hornbuckle, 2010). PCB 11 was the most commonly detected congener but many other PCBs, including several dioxin-like PCBs, were also highly abundant. Yellow, green, blue, and red pigments also contained PCB congeners, while white, black, and brown did not. The distinctive pattern of PCB congeners in the different pigments was an important clue to the chemical manufacturing processes that inadvertently produced these unwanted byproducts. Since then additional studies have shown the widespread presence of non-Aroclor PCBs in the environment and in consumer products. Their presence is most likely due to use of paint and pigments containing PCBs.
Even though they are only being discovered now, non-Aroclor PCBs have been present in the environment for at least 80 years, as evidenced by the presence of paint pigment derived PCBs in sediment core samples from the Great Lakes (Hu et al., 2011). As opposed to the Aroclor PCBs, however, PCB congeners associated with pigments are not exhibiting strong declines in the environment. And unlike Aroclors, these PCB congeners are still legally produced and distributed in the public. At this point, it has become clear that pigments are an important source of PCBs into the environment, especially into air but there are probably other sources. Theoretically, PCBs can be unintentionally produced from any chemical process that involves carbon, chlorine, and elevated temperatures or catalysts (Rudel and Perovich, 2009, Erickson, 2001). Consequently, many consumer products, including building materials that have been manufactured involving these chemical processes, might be tainted with PCBs which are eventually released into the environment (Rodenburg et al., 2010b, Shang et al., 2014).
Despite this knowledge, human uptake, metabolism and toxic response to PCB 11 and other nonlegacy PCBs remains poorly understood. Initial laboratory studies with animals and plants have demonstrated the potential of PCB 11 to become bioavailable to humans through inhalation and to be accessible for biotransformation (Hu et al., 2013, Zhu et al., 2013, Hu et al., 2012, Hu et al., 2010b, Hu et al., 2014). In 2013, PCB 11 was reportedly detected in human serum for the first time, thereby further emphasizing the need for a better understanding of the exposure, metabolism and toxicities of nonlegacy PCBs (Marek et al., 2013b).
PCB metabolism and relevant classes of PCB metabolites
Nomenclature of PCB metabolites
PCB metabolite nomenclature is based on PCB structure as originally numbered by Ballschmiter and coworkers (Ballschmiter et al., 1993). The Ballschmiter system assigns congeners in ascending numerical order according to their chlorination status beginning with monochlorinated PCBs and ending with the only decachlorinated congener, PCB 209. Accordingly, it is not possible to derive the nomenclature from the correct IUPAC name of the metabolite. A short stepwise guidance for abbreviations of hydroxyl, methylsulfonyl, sulfate, and glucuronic acid metabolites of PCBs can be derived from the nomenclature system originally proposed for OH-PCBs and MeSO2-PCBs (Maervoet et al., 2004). Step 1: Identify the Ballschmiter number of the PCB as if no substituents are attached to the molecule and note which one of the two phenyl rings is carrying the primed chlorine positions. Step 2: A functional group (e.g. hydroxyl (-OH), methylsulfonyl (-MeSO2) or sulfate (-OSO3−)) is given a non-primed or a primed number depending on the position where it is attached to the biphenyl moiety. Examples of full metabolite nomenclature are given in Tables 2 and 3. Eight-hundred-thirty-seven mono-hydroxylated metabolites are theoretically possible from the 209 PCB congeners, and this would also be true of the analogous PCB sulfates and PCB glucuronides derived from OH-PCBs. These OH-PCB congeners are listed in Table 2. In Table 3, those OH-PCBs identified in human plasma are listed together with their suggested or experimentally determined parent PCB congeners (Sjödin et al., 1998).
Table 2.
List of all possible mono-OH-PCB congeners, showing the calculated pKa values and octanol:water coefficients of the un-ionized tate
| ID1 | Compound1 | pKa1 | log P1 |
|---|---|---|---|
| 2-Chlorobiphenyl5 | |||
| 1 | 3-OH-PCB 1 | 8.36 | 4.07 |
| 2 | 4-OH-PCB 1 | 8.77 | 4.07 4.044 |
| 3 | 5-OH-PCB 1 | 9.29 | 4.04 |
| 4 | 6-OH-PCB 1 | 9.79 | 4.05 |
| 5 | 2'-OH-PCB 1 | 10.63 | 4.03 |
| 6 | 3'-OH-PCB 1 | 9.81 | 4.03 |
| 7 | 4'-OH-PCB 1 | 9.55 | 4.05 |
| 3-Chlorobiphenyl | |||
| 8 | 2-OH-PCB 2 | 9.1 | 4.04 |
| 9 | 4-OH-PCB2 | 7.89 | 4.07 |
| 10 | 5-OH-PCB 2 | 9.05 | 4.03 |
| 11 | 6-OH-PCB 2 | 9.99 | 4 |
| 12 | 2'-OH-PCB 2 | 10.49 | 3.99 |
| 13 | 3'-OH-PCB 2 | 9.85 | 4 |
| 14 | 4'-OH-PCB 2 | 9.41 | 4.01 |
| 4-Chlorobiphenyl | |||
| 15 | 2-OH-PCB 3 | 9.74 | 4 |
| 16 | 3-OH-PCB 3 | 8.33 | 4.04 |
| 17 | 2'-OH-PCB 3 | 10.5 9.482 |
3.98 3.512 |
| 18 | 3'-OH-PCB 3 | 9.87 9.622 |
3.98 3.802 |
| 19 | 4'-OH-PCB 3 | 9.42 9.712 9.613 |
3.97 3.772 3.773 4.044 |
| 2,2'-Dichlorobiphenyl | |||
| 20 | 3-OH-PCB 4 | 8.26 | 4.82 |
| 21 | 4-OH-PCB4 | 8.69 | 4.81 |
| 22 | 5-OH-PCB 4 | 9.19 | 4.79 |
| 23 | 6-OH-PCB4 | 9.86 | 4.78 |
| 2,3-Dichlorobiphenyl | |||
| 24 | 4-OH-PCB 5 | 7.2 | 4.83 |
| 25 | 5-OH-PCB 5 | 8.43 | 4.82 |
| 26 | 6-OH-PCB 5 | 9.17 | 4.78 |
| 27 | 2'-OH-PCB 5 | 10.52 | 4.78 |
| 28 | 3'-OH-PCB 5 | 9.75 | 4.8 |
| 29 | 4'-OH-PCB 5 | 9.5 | 4.79 |
| 2,3'-Dichlorobiphenyl | |||
| 30 | 3-OH-PCB 6 | 8.3 | 4.8 |
| 31 | 4-OH-PCB 6 | 8.71 | 4.77 |
| 32 | 5-OH-PCB 6 | 9.23 | 4.75 |
| 33 | 6-OH-PCB 6 | 9.67 | 4.76 |
| 34 | 2'-OH-PCB 6 | 9.14 | 4.77 |
| 35 | 4'-OH-PCB 6 | 7.98 8.173 |
4.81 4.213 |
| 36 | 5'-OH-PCB 6 | 8.95 | 4.77 |
| 37 | 6'-OH-PCB 6 | 10.01 | 4.76 |
| 2,4-Dichlorobiphenyl | |||
| 38 | 3-OH-PCB 7 | 6.81 | 4.78 |
| 39 | 5-OH-PCB 7 | 7.71 | 4.8 |
| 40 | 6-OH-PCB 7 | 8.92 | 4.77 |
| 41 | 2'-OH-PCB 7 | 10.53 | 4.76 |
| 42 | 3'-OH-PCB 7 | 9.76 | 4.75 |
| 43 | 4'-OH-PCB 7 | 9.51 | 4.77 |
| 2,4'-Dichlorobiphenyl | |||
| 44 | 3-OH-PCB 8 | 8.31 | 4.77 |
| 45 | 4-OH-PCB 8 | 8.73 8.703 |
4.77 4.573 |
| 46 | 5-OH-PCB 8 | 9.24 | 4.75 |
| 47 | 6-OH-PCB 8 | 9.68 | 4.75 |
| 48 | 2'-OH-PCB 8 | 9.77 | 4.76 |
| 49 | 3'-OH-PCB 8 | 8.23 | 4.79 |
| 2,5-Dichlorobiphenyl | |||
| 50 | 3-OH-PCB 9 | 7.49 | 4.8 |
| 51 | 4-OH-PCB 9 | 7.2 | 4.83 |
| 52 | 6-OH-PCB 9 | 8.28 | 4.75 |
| 53 | 2'-OH-PCB 9 | 10.52 | 4.77 |
| 54 | 3'-OH-PCB 9 | 9.75 | 4.75 |
| 55 | 4'-OH-PCB 9 | 9.5 9.253 |
4.78 4.173 4.634 |
| 2,6-Dichlorobiphenyl | |||
| 56 | 3-OH-PCB 10 | 7.73 | 4.82 |
| 57 | 4-OH-PCB 10 | 7.91 | 4.84 |
| 58 | 2'-OH-PCB 10 | 10.66 | 4.78 |
| 59 | 3'-OH-PCB 10 | 9.71 | 4.8 |
| 60 | 4'-OH-PCB 10 | 9.47 | 4.8 4.634 |
| 3,3'-Dichlorobiphenyl | |||
| 61 | 2-OH-PCB 11 | 8.99 | 4.75 |
| 62 | 4-OH-PCB 11 | 7.84 8.233 |
4.77 4.293 |
| 63 | 5-OH-PCB 11 | 8.99 | 4.73 |
| 64 | 6-OH-PCB 11 | 9.87 | 4.71 |
| 3,4-Dichlorobiphenyl | |||
| 65 | 2-OH-PCB 12 | 8.25 | 4.75 |
| 66 | 5-OH-PCB 12 | 7.47 | 4.79 |
| 67 | 6-OH-PCB 12 | 9.12 | 4.76 |
| 68 | 2'-OH-PCB 12 | 10.39 | 4.75 |
| 69 | 3'-OH-PCB 12 | 9.81 | 4.75 |
| 70 | 4'-OH-PCB 12 | 9.37 9.263 |
4.77 4.213 |
| 3,4'-Dichlorobiphenyl | |||
| 71 | 2-OH-PCB 13 | 9 | 4.73 |
| 72 | 4-OH-PCB 13 | 7.85 | 4.75 |
| 73 | 5-OH-PCB 13 | 9.01 | 4.72 |
| 74 | 6-OH-PCB 13 | 9.88 | 4.7 |
| 75 | 2'-OH-PCB 13 | 9.63 | 4.71 |
| 76 | 3'-OH-PCB 13 | 8.27 | 4.74 |
| 3,5-Dichlorobiphenyl | |||
| 77 | 2-OH-PCB 14 | 8.48 | 4.75 |
| 78 | 4-OH-PCB 14 | 6.35 6.943 |
4.8 4.273 |
| 79 | 2'-OH-PCB 14 | 10.38 | 4.74 |
| 80 | 3'-OH-PCB 14 | 9.79 | 4.75 |
| 81 | 4'-OH-PCB 14 | 9.35 6.942 |
4.76 4.272 |
| 4,4'-Dichlorobiphenyl | |||
| 82 | 2-OH-PCB 15 | 9.64 | 4.68 |
| 83 | 3-OH-PCB 15 | 8.29 | 4.73 |
| 2,2',3-Trichlorobiphenyl | |||
| 84 | 4-OH-PCB 16 | 7.13 | 5.24 |
| 85 | 5-OH-PCB 16 | 8.32 | 5.15 |
| 86 | 6-OH-PCB 16 | 9.24 | 5.22 |
| 87 | 3'-OH-PCB 16 | 8.2 | 5.19 |
| 88 | 4'-OH-PCB 16 | 8.64 | 5.2 |
| 89 | 5'-OH-PCB 16 | 9.13 | 5.16 |
| 90 | 6'-OH-PCB 16 | 9.74 | 5.2 |
| 2,2',4-Trichlorobiphenyl | |||
| 91 | 3-OH-PCB 17 | 6.71 | 5.25 |
| 92 | 5-OH-PCB 17 | 7.61 | 5.14 |
| 93 | 6-OH-PCB 17 | 8.99 | 5.17 |
| 94 | 3'-OH-PCB 17 | 8.21 | 5.15 |
| 95 | 4'-OH-PCB 17 | 8.65 | 5.26 |
| 96 | 5'-OH-PCB 17 | 9.14 | 5.22 |
| 97 | 6'-OH-PCB 17 | 9.75 | 5.1 |
| 2,2',5-Trichlorobiphenyl | |||
| 98 | 3-OH-PCB 18 | 7.4 | 5.19 |
| 99 | 4-OH-PCB 18 | 7.12 | 5.17 |
| 100 | 6-OH-PCB 18 | 8.36 | 5.23 |
| 101 | 3'-OH-PCB 18 | 8.2 | 5.15 |
| 102 | 4'-OH-PCB 18 | 8.64 | 5.26 |
| 103 | 5'-OH-PCB 18 | 9.13 | 5.21 |
| 104 | 6'-OH-PCB 18 | 9.74 | 5.11 |
| 2,2',6-Trichlorobiphenyl | |||
| 105 | 3-OH-PCB 19 | 7.64 | 5.23 |
| 106 | 4-OH-PCB 19 | 7.87 | 5.18 |
| 107 | 3'-OH-PCB 19 | 8.16 | 5.21 |
| 108 | 4'-OH-PCB 19 | 8.65 | 5.19 |
| 109 | 5'-OH-PCB 19 | 9.08 | 5.15 |
| 110 | 6'-OH-PCB 19 | 9.89 | 5.22 |
| 2,3,3'-Trichlorobiphenyl | |||
| 111 | 4-OH-PCB 20 | 7.15 | 5.19 |
| 112 | 5-OH-PCB 20 | 8.37 | 5.16 |
| 113 | 6-OH-PCB 20 | 9.05 | 5.12 |
| 114 | 2'-OH-PCB 20 | 9.02 | 5.2 |
| 115 | 4'-OH-PCB 20 | 7.93 | 5.18 |
| 116 | 5'-OH-PCB 20 | 8.89 | 5.16 |
| 117 | 6'-OH-PCB 20 | 9.9 | 5.1 |
| 2,3,4-Trichlorobiphenyl | |||
| 118 | 5-OH-PCB 21 | 6.85 | 5.3 |
| 119 | 6-OH-PCB 21 | 8.3 | 5.27 |
| 120 | 2'-OH-PCB 21 | 10.41 | 5.2 |
| 121 | 3'-OH-PCB 21 | 9.7 | 5.16 |
| 122 | 4'-OH-PCB 21 | 9.46 | 5.23 |
| 2,3,4'-Trichlorobiphenyl | |||
| 123 | 4-OH-PCB 22 | 7.16 | 5.18 |
| 124 | 5-OH-PCB 22 | 8.38 | 5.17 |
| 125 | 6-OH-PCB 22 | 9.06 | 5.11 |
| 126 | 2'-OH-PCB 22 | 9.65 | 5.1 |
| 127 | 3'-OH-PCB 22 | 8.17 | 5.15 |
| 2,3,5-Trichlorobiphenyl | |||
| 128 | 4-OH-PCB 23 | 5.66 | 5.32 |
| 129 | 6-OH-PCB 23 | 7.66 | 5.26 |
| 130 | 2'-OH-PCB 23 | 10.4 | 5.12 |
| 131 | 3'-OH-PCB 23 | 9.69 | 5.17 |
| 132 | 4'-OH-PCB 23 | 9.45 | 5.26 |
| 2,3,6-Trichlorobiphenyl | |||
| 133 | 4-OH-PCB 24 | 6.35 | 5.32 |
| 134 | 5-OH-PCB 24 | 6.87 | 5.3 |
| 135 | 2'-OH-PCB 24 | 10.54 | 5.21 |
| 136 | 3'-OH-PCB 24 | 9.65 | 5.16 |
| 137 | 4'-OH-PCB 24 | 9.42 | 5.22 |
| 2,3',4-Trichlorobiphenyl | |||
| 138 | 3-OH-PCB 25 | 6.75 | 5.18 |
| 139 | 5-OH-PCB 25 | 7.65 | 5.15 |
| 140 | 6-OH-PCB 25 | 8.81 | 5.1 |
| 141 | 2'-OH-PCB 25 | 9.03 | 5.11 |
| 142 | 4'-OH-PCB 25 | 7.94 7.773 |
5.23 4.793 |
| 143 | 5'-OH-PCB 25 | 8.9 | 5.23 |
| 144 | 6'-OH-PCB 25 | 9.91 | 5.12 |
| 2,3',5-Trichlorobiphenyl | |||
| 145 | 3-OH-PCB 26 | 7.43 | 5.14 |
| 146 | 4-OH-PCB 26 | 7.14 | 5.18 |
| 147 | 6-OH-PCB 26 | 8.17 | 5.17 |
| 148 | 2'-OH-PCB 26 | 9.02 | 5.12 |
| 149 | 4'-OH-PCB 26 | 7.93 | 5.22 |
| 150 | 5'-OH-PCB 26 | 8.89 | 5.21 |
| 151 | 6'-OH-PCB 26 | 9.9 | 5.12 |
| 2,3',6-Trichlorobiphenyl | |||
| 152 | 3-OH-PCB 27 | 7.67 | 5.15 |
| 153 | 4-OH-PCB 27 | 7.86 | 5.18 |
| 154 | 2'-OH-PCB 27 | 9.17 | 5.21 |
| 155 | 4'-OH-PCB 27 | 7.9 | 5.18 |
| 156 | 5'-OH-PCB 27 | 8.84 | 5.15 |
| 157 | 6'-OH-PCB 27 | 10.04 | 5.11 |
| 2,4,4'-Trichlorobiphenyl | |||
| 158 | 3-OH-PCB 28 | 6.76 | 5.14 |
| 159 | 5-OH-PCB 28 | 7.66 | 5.17 |
| 160 | 6-OH-PCB 28 | 8.82 | 5.1 |
| 161 | 2'-OH-PCB 28 | 9.66 | 5.12 |
| 162 | 3'-OH-PCB 28 | 8.18 | 5.17 |
| 2,4,5-Trichlorobiphenyl | |||
| 163 | 3-OH-PCB 29 | 5.95 | 5.29 |
| 164 | 6-OH-PCB 29 | 7.43 | 5.26 |
| 165 | 2'-OH-PCB 29 | 10.41 | 5.12 |
| 166 | 3'-OH-PCB 29 | 9.7 | 5.19 |
| 167 | 4'-OH-PCB 29 | 9.46 | 5.27 |
| 2,4,6-Trichlorobiphenyl | |||
| 168 | 3-OH-PCB 30 | 6.18 | 5.29 |
| 169 | 2'-OH-PCB 30 | 10.55 | 5.13 |
| 170 | 3'-OH-PCB 30 | 9.66 | 5.17 |
| 171 | 4'-OH-PCB 30 | 9.44 | 5.27 5.224 |
| 2,4',5-Trichlorobiphenyl | |||
| 172 | 3-OH-PCB 31 | 7.45 | 5.15 |
| 173 | 4-OH-PCB 31 | 7.16 | 5.2 |
| 174 | 6-OH-PCB 31 | 8.18 | 5.11 |
| 175 | 2'-OH-PCB 31 | 9.65 | 5.12 |
| 176 | 3'-OH-PCB 31 | 8.17 | 5.16 |
| 2,4',6-Trichlorobiphenyl | |||
| 177 | 3-OH-PCB 32 | 7.69 | 5.15 |
| 178 | 4-OH-PCB 32 | 7.87 | 5.19 |
| 179 | 2'-OH-PCB 32 | 9.79 | 5.1 |
| 180 | 3'-OH-PCB 32 | 8.13 | 5.15 |
| 2,3',4'-Trichlorobiphenyl | |||
| 181 | 3-OH-PCB 33 | 8.25 | 5.15 |
| 182 | 4-OH-PCB 33 | 8.68 8.333 |
5.27 5.013 |
| 183 | 5-OH-PCB 33 | 9.18 | 5.23 |
| 184 | 6-OH-PCB 33 | 9.57 | 5.1 |
| 185 | 2'-OH-PCB 33 | 8.28 | 5.21 |
| 186 | 5'-OH-PCB 33 | 7.37 | 5.18 |
| 187 | 6'-OH-PCB 33 | 9.15 | 5.12 |
| 2,3',5'-Trichlorobiphenyl | |||
| 188 | 3-OH-PCB 34 | 8.24 | 5.15 |
| 189 | 4-OH-PCB 34 | 8.66 | 5.27 |
| 190 | 5-OH-PCB 34 | 9.17 | 5.24 |
| 191 | 6-OH-PCB 34 | 9.55 | 5.1 |
| 192 | 2'-OH-PCB 34 | 8.52 | 5.19 |
| 193 | 4'-OH-PCB 34 | 6.43 6.572 6.713 |
5.2 4.742 4.743 |
| 3,3',4-Trichlorobiphenyl | |||
| 194 | 2-OH-PCB 35 | 8.13 | 5.17 |
| 195 | 5-OH-PCB 35 | 7.41 | 5.15 |
| 196 | 6-OH-PCB 35 | 9.01 | 5.11 |
| 197 | 2'-OH-PCB 35 | 8.88 | 5.11 |
| 198 | 4'-OH-PCB 35 | 7.8 7.822 7.823 |
5.23 4.742 4.743 |
| 199 | 5'-OH-PCB 35 | 8.95 | 5.23 |
| 200 | 6'-OH-PCB 35 | 9.77 8.563 |
5.14 4.863 |
| 3,3',5-Trichlorobiphenyl | |||
| 201 | 2-OH-PCB 36 | 8.36 | 5.11 |
| 202 | 4-OH-PCB 36 | 6.29 6.722 6.783 |
5.18 4.832 4.833 |
| 203 | 2'-OH-PCB 36 | 8.87 | 5.1 |
| 204 | 4'-OH-PCB 36 | 7.78 7.862 7.863 |
5.24 4.872 4.873 |
| 205 | 5'-OH-PCB 36 | 8.93 | 5.23 |
| 206 | 6'-OH-PCB 36 | 9.76 | 5.15 |
| 3,4,4'-Trichlorobiphenyl | |||
| 207 | 2-OH-PCB 37 | 8.14 | 5.12 |
| 208 | 5-OH-PCB 37 | 7.43 | 5.15 |
| 209 | 6-OH-PCB 37 | 9.02 | 5.13 |
| 210 | 2'-OH-PCB 37 | 9.52 | 5.13 |
| 211 | 3'-OH-PCB 37 | 8.23 | 5.19 |
| 3,4,5-Trichlorobiphenyl | |||
| 212 | 2-OH-PCB 38 | 7.62 | 5.26 |
| 213 | 2'-OH-PCB 38 | 10.27 | 5.13 |
| 214 | 3'-OH-PCB 38 | 9.75 | 5.2 |
| 215 | 4'-OH-PCB 38 | 9.32 | 5.26 |
| 3,4',5-Trichlorobiphenyl | |||
| 216 | 2-OH-PCB 39 | 8.38 | 5.11 |
| 217 | 4-OH-PCB 39 | 6.31 6.812 |
5.18 4.842 5.224 |
| 218 | 2'-OH-PCB 39 | 9.51 | 5.15 |
| 219 | 3'-OH-PCB 39 | 8.21 | 5.2 |
| 2,2',3,3'-Tetrachlorobiphenyl | |||
| 220 | 4-OH-PCB 40 | 7.08 | 5.89 |
| 221 | 5-OH-PCB 40 | 8.26 | 5.89 |
| 222 | 6-OH-PCB 40 | 9.12 | 5.86 |
| 2,2',3,4-Tetrachlorobiphenyl | |||
| 223 | 5-OH-PCB 41 | 6.75 | 5.87 |
| 224 | 6-OH-PCB 41 | 8.37 | 5.85 |
| 225 | 3'-OH-PCB 41 | 8.15 | 5.88 |
| 226 | 4'-OH-PCB 41 | 8.6 | 5.93 |
| 227 | 5'-OH-PCB 41 | 9.08 | 5.92 |
| 228 | 6'-OH-PCB 41 | 9.64 | 5.85 |
| 2,2',3,4'-Tetrachlorobiphenyl | |||
| 229 | 4-OH-PCB 42 | 7.09 | 5.9 |
| 230 | 5-OH-PCB 42 | 8.28 | 5.91 |
| 231 | 6-OH-PCB 42 | 9.13 | 5.86 |
| 232 | 3'-OH-PCB 42 | 6.65 | 5.86 |
| 233 | 5'-OH-PCB 42 | 7.55 | 5.88 |
| 234 | 6'-OH-PCB 42 | 8.88 | 5.84 |
| 2,2',3,5-Tetrachlorobiphenyl | |||
| 235 | 4-OH-PCB 43 | 5.58 | 5.91 |
| 236 | 6-OH-PCB 43 | 7.74 | 5.85 |
| 237 | 3'-OH-PCB 43 | 8.14 | 5.89 |
| 238 | 4'-OH-PCB 43 | 8.59 | 5.94 |
| 239 | 5'-OH-PCB 43 | 9.07 | 5.91 |
| 240 | 6'-OH-PCB 43 | 9.63 | 5.86 |
| 2,2',3,5'-Tetrachlorobiphenyl | |||
| 241 | 4-OH-PCB 44 | 7.08 | 5.9 |
| 242 | 5-OH-PCB 44 | 8.26 | 5.9 |
| 243 | 6-OH-PCB 44 | 9.12 | 5.86 |
| 244 | 3'-OH-PCB 44 | 7.34 | 5.87 |
| 245 | 4'-OH-PCB 44 | 7.07 | 5.9 |
| 246 | 6'-OH-PCB 44 | 8.25 | 5.85 |
| 2,2',3,6-Tetrachlorobiphenyl | |||
| 247 | 4-OH-PCB 45 | 6.31 | 5.9 |
| 248 | 5-OH-PCB 45 | 6.77 | 5.88 |
| 249 | 3'-OH-PCB 45 | 8.1 | 5.88 |
| 250 | 4'-OH-PCB 45 | 8.6 | 5.93 |
| 251 | 5'-OH-PCB 45 | 9.02 | 5.92 |
| 252 | 6'-OH-PCB 45 | 9.78 | 5.87 |
| 2,2',3,6'-Tetrachlorobiphenyl | |||
| 253 | 4-OH-PCB 46 | 7.09 | 5.89 |
| 254 | 5-OH-PCB 46 | 8.22 | 5.89 |
| 255 | 6-OH-PCB 46 | 9.27 | 5.87 |
| 256 | 3'-OH-PCB 46 | 7.58 | 5.88 |
| 257 | 4'-OH-PCB 46 | 7.82 | 5.92 |
| 2,2',4,4'-Tetrachlorobiphenyl | |||
| 258 | 3-OH-PCB 47 | 6.66 | 5.87 |
| 259 | 5-OH-PCB 47 | 7.56 | 5.9 |
| 260 | 6-OH-PCB 47 | 8.89 | 5.87 |
| 2,2',4,5-Tetrachlorobiphenyl | |||
| 261 | 3-OH-PCB 48 | 5.85 | 5.88 |
| 262 | 6-OH-PCB 48 | 7.51 | 5.85 |
| 263 | 3'-OH-PCB 48 | 8.15 | 5.89 |
| 264 | 4'-OH-PCB 48 | 8.6 | 5.93 |
| 265 | 5'-OH-PCB 48 | 9.08 | 5.91 |
| 266 | 6'-OH-PCB 48 | 9.64 | 5.86 |
| 2,2',4,5'-Tetrachlorobiphenyl | |||
| 267 | 3-OH-PCB 49 | 6.65 | 5.87 |
| 268 | 5-OH-PCB 49 | 7.55 | 5.9 |
| 269 | 6-OH-PCB 49 | 8.88 | 5.87 |
| 270 | 3'-OH-PCB 49 | 7.35 | 5.89 |
| 271 | 4'-OH-PCB 49 | 7.08 | 5.91 |
| 272 | 6'-OH-PCB 49 | 8.26 | 5.84 |
| 2,2',4,6-Tetrachlorobiphenyl | |||
| 273 | 3-OH-PCB 50 | 6.09 | 5.87 |
| 274 | 3'-OH-PCB 50 | 8.11 | 5.88 |
| 275 | 4'-OH-PCB 50 | 8.61 | 5.93 |
| 276 | 5'-OH-PCB 50 | 9.03 | 5.91 |
| 277 | 6'-OH-PCB 50 | 9.79 | 5.86 |
| 2,2',4,6'-Tetrachlorobiphenyl | |||
| 278 | 3-OH-PCB 51 | 6.61 | 5.87 |
| 279 | 5-OH-PCB 51 | 7.5 | 5.87 |
| 280 | 6-OH-PCB 51 | 9.03 | 5.85 |
| 281 | 3'-OH-PCB 51 | 7.59 | 5.89 |
| 282 | 4'-OH-PCB 51 | 7.83 | 5.93 |
| 2,2',5,5'-Tetrachlorobiphenyl | |||
| 283 | 3-OH-PCB 52 | 7.34 | 5.89 |
| 284 | 4-OH-PCB 52 | 7.07 | 5.91 |
| 285 | 6-OH-PCB 52 | 8.25 | 5.84 |
| 2,2',5,6'-Tetrachlorobiphenyl | |||
| 286 | 3-OH-PCB 53 | 7.3 | 5.87 |
| 287 | 4-OH-PCB 53 | 7.08 | 5.9 |
| 288 | 6-OH-PCB 53 | 8.41 | 5.85 |
| 289 | 3'-OH-PCB 53 | 7.58 | 5.88 |
| 290 | 4'-OH-PCB 53 | 7.82 | 5.93 |
| 2,2',6,6'-Tetrachlorobiphenyl | |||
| 291 | 3-OH-PCB 54 | 7.54 | 5.89 |
| 292 | 4-OH-PCB 54 | 7.83 | 5.92 |
| 2,3,3',4-Tetrachlorobiphenyl | |||
| 293 | 5-OH-PCB 55 | 6.79 | 5.87 |
| 294 | 6-OH-PCB 55 | 8.19 | 5.85 |
| 295 | 2'-OH-PCB 55 | 8.92 | 5.85 |
| 296 | 4'-OH-PCB 55 | 7.89 | 5.92 |
| 297 | 5'-OH-PCB 55 | 8.84 | 5.9 |
| 298 | 6'-OH-PCB 55 | 9.79 | 5.87 |
| 2,3,3',4'-Tetrachlorobiphenyl | |||
| 299 | 4-OH-PCB 56 | 7.11 | 5.91 |
| 300 | 5-OH-PCB 56 | 8.32 | 5.9 |
| 301 | 6-OH-PCB 56 | 8.95 | 5.87 |
| 302 | 2'-OH-PCB 56 | 8.17 | 5.84 |
| 303 | 5'-OH-PCB 56 | 7.31 | 5.88 |
| 304 | 6'-OH-PCB 56 | 9.03 | 5.84 |
| 2,3,3',5-Tetrachlorobiphenyl | |||
| 305 | 4-OH-PCB 57 | 5.61 | 5.88 |
| 306 | 6-OH-PCB 57 | 7.55 | 5.85 |
| 307 | 2'-OH-PCB 57 | 8.91 | 5.83 |
| 308 | 4'-OH-PCB 57 | 7.87 | 5.92 |
| 309 | 5'-OH-PCB 57 | 8.83 | 5.9 |
| 310 | 6'-OH-PCB 57 | 9.78 | 5.88 |
| 2,3,3',5'-Tetrachlorobiphenyl | |||
| 311 | 4-OH-PCB 58 | 7.1 | 5.91 |
| 312 | 5-OH-PCB 58 | 8.31 | 5.9 |
| 313 | 6-OH-PCB 58 | 8.93 | 5.88 |
| 314 | 2'-OH-PCB 58 | 8.4 | 5.83 |
| 315 | 4'-OH-PCB 58 | 6.38 | 5.9 |
| 2,3,3',6-Tetrachlorobiphenyl | |||
| 316 | 4-OH-PCB 59 | 6.3 | 5.88 |
| 317 | 5-OH-PCB 59 | 6.81 | 5.86 |
| 318 | 2'-OH-PCB 59 | 9.06 | 5.86 |
| 319 | 4'-OH-PCB 59 | 7.85 | 5.92 |
| 320 | 5'-OH-PCB 59 | 8.78 | 5.91 |
| 321 | 6'-OH-PCB 59 | 9.92 | 5.87 |
| 2,3,4,4'-Tetrachlorobiphenyl | |||
| 322 | 5-OH-PCB 60 | 6.81 | 5.86 |
| 323 | 6-OH-PCB 60 | 8.2 | 5.84 |
| 324 | 2'-OH-PCB 60 | 9.55 | 5.85 |
| 325 | 3'-OH-PCB 60 | 8.12 | 5.89 |
| 2,3,4,5-Tetrachlorobiphenyl | |||
| 326 | 6-OH-PCB 61 | 6.81 | 5.92 |
| 327 | 2'-OH-PCB 61 | 10.3 | 5.87 |
| 328 | 3'-OH-PCB 61 | 9.64 | 5.9 |
| 329 | 4'-OH-PCB 61 | 9.41 | 5.93 5.814 |
| 2,3,4,6-Tetrachlorobiphenyl | |||
| 330 | 5-OH-PCB 62 | 5.33 | 5.94 |
| 331 | 2'-OH-PCB 62 | 10.44 | 5.87 |
| 332 | 3'-OH-PCB 62 | 9.6 | 5.9 |
| 333 | 4'-OH-PCB 62 | 9.39 | 5.94 |
| 2,3,4',5-Tetrachlorobiphenyl | |||
| 334 | 4-OH-PCB 63 | 5.62 | 5.88 |
| 335 | 6-OH-PCB 63 | 7.56 | 5.83 |
| 336 | 2'-OH-PCB 63 | 9.54 | 5.87 |
| 337 | 3'-OH-PCB 63 | 8.11 | 5.9 |
| 2,3,4',6-Tetrachlorobiphenyl | |||
| 338 | 4-OH-PCB 64 | 6.31 | 5.89 |
| 339 | 5-OH-PCB 64 | 6.82 | 5.87 |
| 340 | 2'-OH-PCB 64 | 9.68 | 5.86 |
| 341 | 3'-OH-PCB 64 | 8.07 | 5.9 |
| 2,3,5,6-Tetrachlorobiphenyl | |||
| 342 | 4-OH-PCB 65 | 4.81 | 5.95 |
| 343 | 2'-OH-PCB 65 | 10.43 | 5.87 |
| 344 | 3'-OH-PCB 65 | 9.59 | 5.89 |
| 345 | 4'-OH-PCB 65 | 9.37 | 5.94 |
| 2,3',4,4'-Tetrachlorobiphenyl | |||
| 346 | 3-OH-PCB 66 | 6.7 | 5.88 |
| 347 | 5-OH-PCB 66 | 7.6 | 5.9 |
| 348 | 6-OH-PCB 66 | 8.7 | 5.87 |
| 349 | 2'-OH-PCB 66 | 8.18 | 5.83 |
| 350 | 5'-OH-PCB 66 | 7.33 | 5.89 |
| 351 | 6'-OH-PCB 66 | 9.04 | 5.87 |
| 2,3',4,5-Tetrachlorobiphenyl | |||
| 352 | 3-OH-PCB 67 | 5.89 | 5.86 |
| 353 | 6-OH-PCB 67 | 7.31 | 5.84 |
| 354 | 2'-OH-PCB 67 | 8.92 | 5.84 |
| 355 | 4'-OH-PCB 67 | 7.89 | 5.91 |
| 356 | 5'-OH-PCB 67 | 8.84 | 5.89 |
| 357 | 6'-OH-PCB 67 | 9.79 | 5.89 |
| 2,3',4,5'-Tetrachlorobiphenyl | |||
| 358 | 3-OH-PCB 68 | 6.69 | 5.88 |
| 359 | 5-OH-PCB 68 | 7.59 | 5.9 |
| 360 | 6-OH-PCB 68 | 8.69 | 5.87 |
| 361 | 2'-OH-PCB 68 | 8.41 | 5.85 |
| 362 | 4'-OH-PCB 68 | 6.39 6.302 6.303 |
5.91 5.332 5.333 |
| 2,3',4,6-Tetrachlorobiphenyl | |||
| 363 | 3-OH-PCB 69 | 6.12 | 5.86 |
| 364 | 2'-OH-PCB 69 | 9.07 | 5.84 |
| 365 | 4'-OH-PCB 69 | 7.87 | 5.92 |
| 366 | 5'-OH-PCB 69 | 8.8 | 5.9 |
| 367 | 6'-OH-PCB 69 | 9.93 | 5.88 |
| 2,3',4',5-Tetrachlorobiphenyl | |||
| 368 | 3-OH-PCB 70 | 7.39 | 5.89 |
| 369 | 4-OH-PCB 70 | 7.11 | 5.92 |
| 370 | 6-OH-PCB 70 | 8.06 | 5.84 |
| 371 | 2'-OH-PCB 70 | 8.17 | 5.83 |
| 372 | 5'-OH-PCB 70 | 7.31 | 5.89 |
| 373 | 6'-OH-PCB 70 | 9.03 | 5.87 |
| 2,3',4',6-Tetrachlorobiphenyl | |||
| 374 | 3-OH-PCB 71 | 7.63 | 5.9 |
| 375 | 4-OH-PCB 71 | 7.82 | 5.92 |
| 376 | 2'-OH-PCB 71 | 8.32 | 5.84 |
| 377 | 5'-OH-PCB 71 | 7.27 | 5.88 |
| 378 | 6'-OH-PCB 71 | 9.17 | 5.84 |
| 2,3',5,5'-Tetrachlorobiphenyl | |||
| 379 | 3-OH-PCB 72 | 7.37 | 5.9 |
| 380 | 4-OH-PCB 72 | 7.09 | 5.91 |
| 381 | 6-OH-PCB 72 | 8.05 | 5.84 |
| 382 | 2'-OH-PCB 72 | 8.4 | 5.85 |
| 383 | 4'-OH-PCB 72 | 6.38 | 5.91 |
| 2,3',5',6-Tetrachlorobiphenyl | |||
| 384 | 3-OH-PCB 73 | 7.62 | 5.9 |
| 385 | 4-OH-PCB 73 | 7.81 | 5.93 |
| 386 | 2'-OH-PCB 73 | 8.55 | 5.84 |
| 387 | 4'-OH-PCB 73 | 6.36 | 5.89 |
| 2,4,4',5-Tetrachlorobiphenyl | |||
| 388 | 3-OH-PCB 74 | 5.9 | 5.85 |
| 389 | 6-OH-PCB 74 | 7.32 | 5.83 |
| 390 | 2'-OH-PCB 74 | 9.55 | 5.88 |
| 391 | 3'-OH-PCB 74 | 8.12 | 5.9 |
| 2,4,4',6-Tetrachlorobiphenyl | |||
| 392 | 3-OH-PCB 75 | 6.14 | 5.86 |
| 393 | 2'-OH-PCB 75 | 9.69 | 5.87 |
| 394 | 3'-OH-PCB 75 | 8.08 | 5.9 |
| 2,3',4',5'-Tetrachlorobiphenyl | |||
| 395 | 3-OH-PCB 76 | 8.19 | 5.89 |
| 396 | 4-OH-PCB 76 | 8.62 | 5.93 |
| 397 | 5-OH-PCB 76 | 9.12 | 5.91 |
| 398 | 6-OH-PCB 76 | 9.45 | 5.85 |
| 399 | 2'-OH-PCB 76 | 7.66 | 5.85 |
| 3,3',4,4'-Tetrachlorobiphenyl | |||
| 400 | 2-OH-PCB 77 | 8.03 | 5.84 |
| 401 | 5-OH-PCB 77 | 7.37 | 5.9 |
| 402 | 6-OH-PCB 77 | 8.9 | 5.87 |
| 3,3',4,5-Tetrachlorobiphenyl | |||
| 403 | 2-OH-PCB 78 | 7.51 | 5.83 |
| 404 | 2'-OH-PCB 78 | 8.77 | 5.85 |
| 405 | 4'-OH-PCB 78 | 7.75 6.362 |
5.92 5.282 |
| 406 | 5'-OH-PCB 78 | 8.89 | 5.9 |
| 407 | 6'-OH-PCB 78 | 9.65 | 5.88 |
| 3,3',4,5'-Tetrachlorobiphenyl | |||
| 408 | 2-OH-PCB 79 | 8.01 | 5.85 |
| 409 | 5-OH-PCB 79 | 7.35 | 5.89 |
| 410 | 6-OH-PCB 79 | 8.89 | 5.87 |
| 411 | 2'-OH-PCB 79 | 8.26 | 5.86 |
| 412 | 4'-OH-PCB 79 | 6.26 6.363 |
5.91 5.283 |
| 3,3',5,5'-Tetrachlorobiphenyl | |||
| 413 | 2-OH-PCB 80 | 8.25 | 5.86 |
| 414 | 4-OH-PCB 80 | 6.24 | 5.91 |
| 3,4,4',5-Tetrachlorobiphenyl | |||
| 415 | 2-OH-PCB 81 | 7.52 | 5.84 |
| 416 | 2'-OH-PCB 81 | 9.41 | 5.87 |
| 417 | 3'-OH-PCB 81 | 8.17 | 5.89 |
| 2,2',3,3',4-Pentachlorobiphenyl | |||
| 418 | 5-OH-PCB 82 | 6.69 | 6.55 |
| 419 | 6-OH-PCB 82 | 8.26 | 6.51 |
| 420 | 4'-OH-PCB 82 | 7.04 | 6.57 |
| 421 | 5'-OH-PCB 82 | 8.22 | 6.57 |
| 422 | 6'-OH-PCB 82 | 9.02 | 6.53 |
| 2,2',3,3',5-Pentachlorobiphenyl | |||
| 423 | 4-OH-PCB 83 | 5.53 | 6.56 |
| 424 | 6-OH-PCB 83 | 7.63 | 6.51 |
| 425 | 4'-OH-PCB 83 | 7.03 | 6.57 |
| 426 | 5'-OH-PCB 83 | 8.2 | 6.58 |
| 427 | 6'-OH-PCB 83 | 9.01 | 6.53 |
| 2,2',3,3',6-Pentachlorobiphenyl | |||
| 428 | 4-OH-PCB 84 | 6.26 | 6.59 |
| 429 | 5-OH-PCB 84 | 6.72 | 6.55 |
| 430 | 4'-OH-PCB 84 | 7.04 | 6.59 |
| 431 | 5'-OH-PCB 84 | 8.16 | 6.57 |
| 432 | 6'-OH-PCB 84 | 9.16 | 6.54 |
| 2,2',3,4,4'-Pentachlorobiphenyl | |||
| 433 | 5-OH-PCB 85 | 6.7 | 6.54 |
| 434 | 6-OH-PCB 85 | 8.27 | 6.52 |
| 435 | 3'-OH-PCB 85 | 6.6 | 6.52 |
| 436 | 5'-OH-PCB 85 | 7.5 | 6.54 |
| 437 | 6'-OH-PCB 85 | 8.77 | 6.52 |
| 2,2',3,4,5-Pentachlorobiphenyl | |||
| 438 | 6-OH-PCB 86 | 6.89 | 6.5 |
| 439 | 3'-OH-PCB 86 | 8.09 | 6.56 |
| 440 | 4'-OH-PCB 86 | 8.55 | 6.61 |
| 441 | 5'-OH-PCB 86 | 9.02 | 6.58 |
| 442 | 6'-OH-PCB 86 | 9.52 | 6.53 |
| 2,2',3,4,5'-Pentachlorobiphenyl | |||
| 443 | 5-OH-PCB 87 | 6.69 | 6.54 |
| 444 | 6-OH-PCB 87 | 8.26 | 6.52 |
| 445 | 3'-OH-PCB 87 | 7.29 | 6.55 |
| 446 | 4'-OH-PCB 87 | 7.03 | 6.56 |
| 447 | 6'-OH-PCB 87 | 8.15 | 6.51 |
| 2,2',3,4,6-Pentachlorobiphenyl | |||
| 448 | 5-OH-PCB 88 | 5.23 | 6.53 |
| 449 | 3'-OH-PCB 88 | 8.05 | 6.57 |
| 450 | 4'-OH-PCB 88 | 8.56 | 6.62 |
| 451 | 5'-OH-PCB 88 | 8.97 | 6.58 |
| 452 | 6'-OH-PCB 88 | 9.67 | 6.52 |
| 2,2',3,4,6'-Pentachlorobiphenyl | |||
| 453 | 5-OH-PCB 89 | 6.65 | 6.55 |
| 454 | 6-OH-PCB 89 | 8.41 | 6.51 |
| 455 | 3'-OH-PCB 89 | 7.53 | 6.57 |
| 456 | 4'-OH-PCB 89 | 7.78 | 6.6 |
| 2,2',3,4',5-Pentachlorobiphenyl | |||
| 457 | 4-OH-PCB 90 | 5.55 | 6.54 |
| 458 | 6-OH-PCB 90 | 7.64 | 6.52 |
| 459 | 3'-OH-PCB 90 | 6.59 | 6.54 |
| 460 | 5'-OH-PCB 90 | 7.49 | 6.55 |
| 461 | 6'-OH-PCB 90 | 8.76 | 6.53 |
| 2,2',3,4',6-Pentachlorobiphenyl | |||
| 462 | 4-OH-PCB 91 | 6.27 | 6.57 |
| 463 | 5-OH-PCB 91 | 6.73 | 6.57 |
| 464 | 3'-OH-PCB 91 | 6.55 | 6.54 |
| 465 | 5'-OH-PCB 91 | 7.45 | 6.56 |
| 466 | 6'-OH-PCB 91 | 8.91 | 6.53 |
| 2,2',3,5,5'-Pentachlorobiphenyl | |||
| 467 | 4-OH-PCB 92 | 5.53 | 6.54 |
| 468 | 6-OH-PCB 92 | 7.63 | 6.52 |
| 469 | 3'-OH-PCB 92 | 7.28 | 6.55 |
| 470 | 4'-OH-PCB 92 | 7.02 | 6.58 |
| 471 | 6'-OH-PCB 92 | 8.13 | 6.52 |
| 2,2',3,5,6-Pentachlorobiphenyl | |||
| 472 | 4-OH-PCB 93 | 4.77 | 6.55 |
| 473 | 3'-OH-PCB 93 | 8.04 | 6.58 |
| 474 | 4'-OH-PCB 93 | 8.55 | 6.62 |
| 475 | 5'-OH-PCB 93 | 8.96 | 6.58 |
| 476 | 6'-OH-PCB 93 | 9.66 | 6.53 |
| 2,2',3,5,6'-Pentachlorobiphenyl | |||
| 477 | 4-OH-PCB 94 | 5.55 | 6.57 |
| 478 | 6-OH-PCB 94 | 7.79 | 6.5 |
| 479 | 3'-OH-PCB 94 | 7.52 | 6.57 |
| 480 | 4'-OH-PCB 94 | 7.77 | 6.61 |
| 2,2',3,5',6-Pentachlorobiphenyl | |||
| 481 | 4-OH-PCB 95 | 6.26 | 6.58 |
| 482 | 5-OH-PCB 95 | 6.72 | 6.56 |
| 483 | 3'-OH-PCB 95 | 7.24 | 6.58 |
| 484 | 4'-OH-PCB 95 | 7.03 | 6.59 |
| 485 | 6'-OH-PCB 95 | 8.3 | 6.51 |
| 2,2',3,6,6'-Pentachlorobiphenyl | |||
| 486 | 4-OH-PCB 96 | 6.27 | 6.57 |
| 487 | 5-OH-PCB 96 | 6.68 | 6.56 |
| 488 | 3'-OH-PCB 96 | 7.48 | 6.58 |
| 489 | 4'-OH-PCB 96 | 7.78 | 6.6 |
| 2,2',3,4',5'-Pentachlorobiphenyl | |||
| 490 | 4-OH-PCB 97 | 7.04 | 6.56 |
| 491 | 5-OH-PCB 97 | 8.22 | 6.57 |
| 492 | 6-OH-PCB 97 | 9.02 | 6.53 |
| 493 | 3'-OH-PCB 97 | 5.79 | 6.53 |
| 494 | 6'-OH-PCB 97 | 7.4 | 6.51 |
| 2,2',3,4',6'-Pentachlorobiphenyl | |||
| 495 | 4-OH-PCB 98 | 7.05 | 6.59 |
| 496 | 5-OH-PCB 98 | 8.17 | 6.57 |
| 497 | 6-OH-PCB 98 | 9.17 | 6.53 |
| 498 | 3'-OH-PCB 98 | 6.03 | 6.54 |
| 2,2',4,4',5-Pentachlorobiphenyl | |||
| 499 | 3-OH-PCB 99 | 5.81 | 6.51 |
| 500 | 6-OH-PCB 99 | 7.41 | 6.5 |
| 501 | 3'-OH-PCB 99 | 6.6 | 6.54 |
| 502 | 5'-OH-PCB 99 | 7.5 | 6.55 |
| 503 | 6'-OH-PCB 99 | 8.77 | 6.52 |
| 2,2',4,4',6-Pentachlorobiphenyl | |||
| 504 | 3-OH-PCB 100 | 6.04 | 6.54 |
| 505 | 3'-OH-PCB 100 | 6.57 | 6.55 |
| 506 | 5'-OH-PCB 100 | 7.46 | 6.56 |
| 507 | 6'-OH-PCB 100 | 8.92 | 6.53 |
| 2,2',4,5,5'-Pentachlorobiphenyl | |||
| 508 | 3-OH-PCB 101 | 5.79 | 6.52 |
| 509 | 6-OH-PCB 101 | 7.39 | 6.51 |
| 510 | 3'-OH-PCB 101 | 7.29 | 6.54 |
| 511 | 4'-OH-PCB 101 | 7.03 | 6.59 |
| 512 | 6'-OH-PCB 101 | 8.14 | 6.52 |
| 2,2',4,5,6'-Pentachlorobiphenyl | |||
| 513 | 3-OH-PCB 102 | 5.76 | 6.54 |
| 514 | 6-OH-PCB 102 | 7.56 | 6.49 |
| 515 | 3'-OH-PCB 102 | 7.53 | 6.56 |
| 516 | 4'-OH-PCB 102 | 7.78 | 6.61 |
| 2,2',4,5',6-Pentachlorobiphenyl | |||
| 517 | 3-OH-PCB 103 | 6.03 | 6.54 |
| 518 | 3'-OH-PCB 103 | 7.25 | 6.56 |
| 519 | 4'-OH-PCB 103 | 7.04 | 6.58 |
| 520 | 6'-OH-PCB 103 | 8.31 | 6.52 |
| 2,2',4,6,6'-Pentachlorobiphenyl | |||
| 521 | 3-OH-PCB 104 | 5.99 | 6.55 |
| 522 | 3'-OH-PCB 104 | 7.49 | 6.56 |
| 523 | 4'-OH-PCB 104 | 7.79 | 6.62 |
| 2,3,3',4,4'-Pentachlorobiphenyl | |||
| 524 | 5-OH-PCB 105 | 6.75 | 6.53 |
| 525 | 6-OH-PCB 105 | 8.08 | 6.51 |
| 526 | 2'-OH-PCB 105 | 8.06 | 6.49 |
| 527 | 5'-OH-PCB 105 | 7.27 | 6.54 |
| 528 | 6'-OH-PCB 105 | 8.93 | 6.51 |
| 2,3,3',4,5-Pentachlorobiphenyl | |||
| 529 | 6-OH-PCB 106 | 6.69 | 6.46 |
| 530 | 2'-OH-PCB 106 | 8.8 | 6.51 |
| 531 | 4'-OH-PCB 106 | 7.84 | 6.57 |
| 532 | 5'-OH-PCB 106 | 8.78 | 6.55 |
| 533 | 6'-OH-PCB 106 | 9.68 | 6.53 |
| 2,3,3',4',5-Pentachlorobiphenyl | |||
| 534 | 4-OH-PCB 107 | 5.57 | 6.54 |
| 535 | 6-OH-PCB 107 | 7.44 | 6.51 |
| 536 | 2'-OH-PCB 107 | 8.05 | 6.5 |
| 537 | 5'-OH-PCB 107 | 7.25 | 6.52 |
| 538 | 6'-OH-PCB 107 | 8.92 | 6.51 |
| 2,3,3',4,5'-Pentachlorobiphenyl | |||
| 539 | 5-OH-PCB 108 | 6.73 | 6.54 |
| 540 | 6-OH-PCB 108 | 8.07 | 6.51 |
| 541 | 2'-OH-PCB 108 | 8.3 | 6.51 |
| 542 | 4'-OH-PCB 108 | 6.34 | 6.55 |
| 2,3,3',4,6-Pentachlorobiphenyl | |||
| 543 | 5-OH-PCB 109 | 5.27 | 6.5 |
| 544 | 2'-OH-PCB 109 | 8.96 | 6.51 |
| 545 | 4'-OH-PCB 109 | 7.81 | 6.58 |
| 546 | 5'-OH-PCB 109 | 8.74 | 6.57 |
| 547 | 6'-OH-PCB 109 | 9.82 | 6.53 |
| 2,3,3',4',6-Pentachlorobiphenyl | |||
| 548 | 4-OH-PCB 110 | 6.26 | 6.57 |
| 549 | 5-OH-PCB 110 | 6.76 | 6.55 |
| 550 | 2'-OH-PCB 110 | 8.21 | 6.51 |
| 551 | 5'-OH-PCB 110 | 7.21 | 6.54 |
| 552 | 6'-OH-PCB 110 | 9.06 | 6.54 |
| 2,3,3',5,5'-Pentachlorobiphenyl | |||
| 553 | 4-OH-PCB 111 | 5.55 | 6.54 |
| 554 | 6-OH-PCB 111 | 7.43 | 6.51 |
| 555 | 2'-OH-PCB 111 | 8.29 | 6.52 |
| 556 | 4'-OH-PCB 111 | 6.33 | 6.55 |
| 2,3,3',5,6-Pentachlorobiphenyl | |||
| 557 | 4-OH-PCB 112 | 4.76 | 6.53 |
| 558 | 2'-OH-PCB 112 | 8.95 | 6.51 |
| 559 | 4'-OH-PCB 112 | 7.8 | 6.59 |
| 560 | 5'-OH-PCB 112 | 8.72 | 6.58 |
| 561 | 6'-OH-PCB 112 | 9.81 | 6.54 |
| 2,3,3',5',6-Pentachlorobiphenyl | |||
| 562 | 4-OH-PCB 113 | 6.24 | 6.57 |
| 563 | 5-OH-PCB 113 | 6.75 | 6.56 |
| 564 | 2'-OH-PCB 113 | 8.44 | 6.52 |
| 565 | 4'-OH-PCB 113 | 6.31 | 6.57 |
| 2,3,4,4',5-Pentachlorobiphenyl | |||
| 566 | 6-OH-PCB 114 | 6.7 | 6.48 |
| 567 | 2'-OH-PCB 114 | 9.43 | 6.52 |
| 568 | 3'-OH-PCB 114 | 8.06 | 6.54 |
| 2,3,4,4',6-Pentachlorobiphenyl | |||
| 569 | 5-OH-PCB 115 | 5.28 | 6.52 |
| 570 | 2'-OH-PCB 115 | 9.57 | 6.53 |
| 571 | 3'-OH-PCB 115 | 8.02 | 6.55 |
| 2,3,4,5,6-Pentachlorobiphenyl | |||
| 572 | 2'-OH-PCB 116 | 10.32 | 6.5 |
| 573 | 3'-OH-PCB 116 | 9.54 | 6.59 |
| 574 | 4'-OH-PCB 116 | 9.33 | 6.61 |
| 2,3,4',5,6-Pentachlorobiphenyl | |||
| 575 | 4-OH-PCB 117 | 4.77 | 6.54 |
| 576 | 2'-OH-PCB 117 | 9.56 | 6.53 |
| 577 | 3'-OH-PCB 117 | 8.01 | 6.55 |
| 2,3',4,4',5-Pentachlorobiphenyl | |||
| 578 | 3-OH-PCB 118 | 5.84 | 6.51 |
| 579 | 6-OH-PCB 118 | 7.21 | 6.5 |
| 580 | 2'-OH-PCB 118 | 8.06 | 6.5 |
| 581 | 5'-OH-PCB 118 | 7.27 | 6.53 |
| 582 | 6'-OH-PCB 118 | 8.93 | 6.52 |
| 2,3',4,4',6-Pentachlorobiphenyl | |||
| 583 | 3-OH-PCB 119 | 6.08 | 6.54 |
| 584 | 2'-OH-PCB 119 | 8.22 | 6.51 |
| 585 | 5'-OH-PCB 119 | 7.22 | 6.53 |
| 586 | 6'-OH-PCB 119 | 9.07 | 6.51 |
| 2,3',4,5,5'-Pentachlorobiphenyl | |||
| 587 | 3-OH-PCB 120 | 5.83 | 6.51 |
| 588 | 6-OH-PCB 120 | 7.2 | 6.49 |
| 589 | 2'-OH-PCB 120 | 8.3 | 6.5 |
| 590 | 4'-OH-PCB 120 | 6.34 | 6.56 |
| 2,3',4,5',6-Pentachlorobiphenyl | |||
| 591 | 3-OH-PCB 121 | 6.06 | 6.54 |
| 592 | 2'-OH-PCB 121 | 8.45 | 6.52 |
| 593 | 4'-OH-PCB 121 | 6.32 | 6.55 |
| 2,3,3',4',5'-Pentachlorobiphenyl | |||
| 594 | 4-OH-PCB 122 | 7.06 | 6.56 |
| 595 | 5-OH-PCB 122 | 8.26 | 6.56 |
| 596 | 6-OH-PCB 122 | 8.83 | 6.53 |
| 597 | 2'-OH-PCB 122 | 7.55 | 6.5 |
| 2,3',4,4',5'-Pentachlorobiphenyl | |||
| 598 | 3-OH-PCB 123 | 6.64 | 6.52 |
| 599 | 5-OH-PCB 123 | 7.54 | 6.55 |
| 600 | 6-OH-PCB 123 | 8.59 | 6.51 |
| 601 | 2'-OH-PCB 123 | 7.56 | 6.5 |
| 2,3',4',5,5'-Pentachlorobiphenyl | |||
| 602 | 3-OH-PCB 124 | 7.33 | 6.53 |
| 603 | 4-OH-PCB 124 | 7.05 | 6.57 |
| 604 | 6-OH-PCB 124 | 7.95 | 6.51 |
| 605 | 2'-OH-PCB 124 | 7.55 | 6.49 |
| 2,3',4',5',6-Pentachlorobiphenyl | |||
| 606 | 3-OH-PCB 125 | 7.57 | 6.56 |
| 607 | 4-OH-PCB 125 | 7.77 | 6.6 |
| 608 | 2'-OH-PCB 125 | 7.7 | 6.51 |
| 3,3',4,4',5-Pentachlorobiphenyl | |||
| 609 | 2-OH-PCB 126 | 7.41 | 6.49 |
| 610 | 2'-OH-PCB 126 | 7.91 | 6.5 |
| 611 | 5'-OH-PCB 126 | 7.31 | 6.53 |
| 612 | 6'-OH-PCB 126 | 8.79 | 6.51 |
| 3,3',4,5,5'-Pentachlorobiphenyl | |||
| 613 | 2-OH-PCB 127 | 7.39 | 6.5 |
| 614 | 2'-OH-PCB 127 | 8.15 | 6.5 |
| 615 | 4'-OH-PCB 127 | 6.21 | 6.53 |
| 2,2',3,3',4,4'-Hexachlorobiphenyl | |||
| 616 | 5-OH-PCB 128 | 6.64 | 7.13 |
| 617 | 6-OH-PCB 128 | 8.15 | 7.11 |
| 2,2',3,3',4,5-Hexachlorobiphenyl | |||
| 618 | 6-OH-PCB 129 | 6.78 | 7.04 |
| 619 | 4'-OH-PCB 129 | 6.99 | 7.18 |
| 620 | 5'-OH-PCB 129 | 8.16 | 7.2 |
| 621 | 6'-OH-PCB 129 | 8.9 | 7.12 |
| 2,2',3,3',4,5'-Hexachlorobiphenyl | |||
| 622 | 5-OH-PCB 130 | 6.63 | 7.15 |
| 623 | 6-OH-PCB 130 | 8.14 | 7.12 |
| 624 | 4'-OH-PCB 130 | 5.49 5.042 |
7.13 6.172 |
| 625 | 6'-OH-PCB 130 | 7.53 | 7.11 |
| 2,2',3,3',4,6-Hexachlorobiphenyl | |||
| 626 | 5-OH-PCB 131 | 5.17 | 7.11 |
| 627 | 4'-OH-PCB 131 | 7 | 7.17 |
| 628 | 5'-OH-PCB 131 | 8.11 | 7.2 |
| 629 | 6'-OH-PCB 131 | 9.05 | 7.11 |
| 2,2',3,3',4,6'-Hexachlorobiphenyl | |||
| 630 | 5-OH-PCB 132 | 6.59 | 7.14 |
| 631 | 6-OH-PCB 132 | 8.29 | 7.1 |
| 632 | 4'-OH-PCB 132 | 6.22 | 7.16 |
| 633 | 5'-OH-PCB 132 | 6.67 | 7.14 |
| 2,2',3,3',5,5'-Hexachlorobiphenyl | |||
| 634 | 4-OH-PCB 133 | 5.48 | 7.13 |
| 635 | 6-OH-PCB 133 | 7.52 | 7.1 |
| 2,2',3,3',5,6-Hexachlorobiphenyl | |||
| 636 | 4-OH-PCB 134 | 4.7 | 7.17 |
| 637 | 4'-OH-PCB 134 | 6.99 | 7.17 |
| 638 | 5'-OH-PCB 134 | 8.1 | 7.2 |
| 639 | 6'-OH-PCB 134 | 9.05 | 7.12 |
| 2,2',3,3',5,6'-Hexachlorobiphenyl | |||
| 640 | 4-OH-PCB 135 | 5.49 | 7.14 |
| 641 | 6-OH-PCB 135 | 7.68 | 7.1 |
| 642 | 4'-OH-PCB 135 | 6.21 | 7.17 |
| 643 | 5'-OH-PCB 135 | 6.66 | 7.15 |
| 2,2',3,3',6,6'-Hexachlorobiphenyl | |||
| 644 | 4-OH-PCB 136 | 6.22 | 7.16 |
| 645 | 5-OH-PCB 136 | 6.62 | 7.15 |
| 2,2',3,4,4',5-Hexachlorobiphenyl | |||
| 646 | 6-OH-PCB 137 | 6.79 | 7.07 |
| 647 | 3'-OH-PCB 137 | 6.54 | 7.12 |
| 648 | 5'-OH-PCB 137 | 7.44 | 7.2 |
| 649 | 6'-OH-PCB 137 | 8.66 | 7.12 |
| 2,2',3,4,4',5'-Hexachlorobiphenyl | |||
| 650 | 5-OH-PCB 138 | 6.64 | 7.18 |
| 651 | 6-OH-PCB 138 | 8.15 | 7.13 |
| 652 | 3'-OH-PCB 138 | 5.75 | 7.11 |
| 653 | 6'-OH-PCB 138 | 7.29 | 7.08 |
| 2,2',3,4,4',6-Hexachlorobiphenyl | |||
| 654 | 5-OH-PCB 139 | 5.18 | 7.11 |
| 655 | 3'-OH-PCB 139 | 6.51 | 7.13 |
| 656 | 5'-OH-PCB 139 | 7.4 | 7.2 |
| 657 | 6'-OH-PCB 139 | 8.81 | 7.12 |
| 2,2',3,4,4',6'-Hexachlorobiphenyl | |||
| 658 | 5-OH-PCB 140 | 6.6 | 7.15 |
| 659 | 6-OH-PCB 140 | 8.3 | 7.11 |
| 660 | 3'-OH-PCB 140 | 5.98 | 7.12 |
| 2,2',3,4,5,5'-Hexachlorobiphenyl | |||
| 661 | 6-OH-PCB 141 | 6.78 | 7.05 |
| 662 | 3'-OH-PCB 141 | 7.23 | 7.17 |
| 663 | 4'-OH-PCB 141 | 6.98 | 7.22 |
| 664 | 6'-OH-PCB 141 | 8.03 | 7.1 |
| 2,2',3,4,5,6-Hexachlorobiphenyl | |||
| 665 | 3'-OH-PCB 142 | 8 | 7.25 |
| 666 | 4'-OH-PCB 142 | 8.51 | 7.21 |
| 667 | 5'-OH-PCB 142 | 8.92 | 7.17 |
| 668 | 6'-OH-PCB 142 | 9.56 | 7.12 |
| 2,2',3,4,5,6'-Hexachlorobiphenyl | |||
| 669 | 6-OH-PCB 143 | 6.94 | 7.05 |
| 670 | 3'-OH-PCB 143 | 7.47 | 7.15 |
| 671 | 4'-OH-PCB 143 | 7.73 | 7.23 |
| 2,2',3,4,5',6-Hexachlorobiphenyl | |||
| 672 | 5-OH-PCB 144 | 5.17 | 7.11 |
| 673 | 3'-OH-PCB 144 | 7.19 | 7.16 |
| 674 | 4'-OH-PCB 144 | 6.99 | 7.22 |
| 675 | 6'-OH-PCB 144 | 8.19 | 7.1 |
| 2,2',3,4,6,6'-Hexachlorobiphenyl | |||
| 676 | 5-OH-PCB 145 | 5.14 | 7.08 |
| 677 | 3'-OH-PCB 145 | 7.43 | 7.16 |
| 678 | 4'-OH-PCB 145 | 7.74 | 7.23 |
| 2,2',3,4',5,5'-Hexachlorobiphenyl | |||
| 679 | 4-OH-PCB 146 | 5.49 5.042 |
7.19 6.232 |
| 680 | 6-OH-PCB 146 | 7.53 | 7.1 |
| 681 | 3'-OH-PCB 146 | 5.73 | 7.11 |
| 682 | 6'-OH-PCB 146 | 7.28 | 7.08 |
| 2,2',3,4',5,6-Hexachlorobiphenyl | |||
| 683 | 4-OH-PCB 147 | 4.73 | 7.12 |
| 684 | 3'-OH-PCB 147 | 6.49 | 7.14 |
| 685 | 5'-OH-PCB 147 | 7.39 | 7.19 |
| 686 | 6'-OH-PCB 147 | 8.8 | 7.11 |
| 2,2',3,4',5,6'-Hexachlorobiphenyl | |||
| 687 | 4-OH-PCB 148 | 5.51 | 7.14 |
| 688 | 6-OH-PCB 148 | 7.69 | 7.1 |
| 689 | 3'-OH-PCB 148 | 5.97 | 7.14 |
| 2,2',3,4',5',6-Hexachlorobiphenyl | |||
| 690 | 4-OH-PCB 149 | 6.22 | 7.2 |
| 691 | 5-OH-PCB 149 | 6.67 | 7.17 |
| 692 | 3'-OH-PCB 149 | 5.7 | 7.13 |
| 693 | 6'-OH-PCB 149 | 7.45 | 7.1 |
| 2,2',3,4',6,6'-Hexachlorobiphenyl | |||
| 694 | 4-OH-PCB 150 | 6.23 | 7.18 |
| 695 | 5-OH-PCB 150 | 6.63 | 7.15 |
| 696 | 3'-OH-PCB 150 | 5.93 | 7.12 |
| 2,2',3,5,5',6-Hexachlorobiphenyl | |||
| 697 | 4-OH-PCB 151 | 4.72 | 7.23 |
| 698 | 3'-OH-PCB 151 | 7.18 | 7.15 |
| 699 | 4'-OH-PCB 151 | 6.98 | 7.21 |
| 700 | 6'-OH-PCB 151 | 8.19 | 7.11 |
| 2,2',3,5,6,6'-Hexachlorobiphenyl | |||
| 701 | 4-OH-PCB 152 | 4.73 | 7.11 |
| 702 | 3'-OH-PCB 152 | 7.42 | 7.16 |
| 703 | 4'-OH-PCB 152 | 7.73 | 7.23 |
| 2,2',4,4',5,5'-Hexachlorobiphenyl | |||
| 704 | 3-OH-PCB 153 | 5.75 | 7.15 |
| 705 | 6-OH-PCB 153 | 7.29 | 7.09 |
| 2,2',4,4',5,6'-Hexachlorobiphenyl | |||
| 706 | 3-OH-PCB 154 | 5.71 | 7.12 |
| 707 | 6-OH-PCB 154 | 7.46 | 7.1 |
| 708 | 3'-OH-PCB 154 | 5.98 | 7.15 |
| 2,2',4,4',6,6'-Hexachlorobiphenyl | |||
| 709 | 3-OH-PCB 155 | 5.94 | 7.15 |
| 2,3,3',4,4',5-Hexachlorobiphenyl | |||
| 710 | 6-OH-PCB 156 | 6.59 | 7.1 |
| 711 | 2'-OH-PCB 156 | 7.95 | 7.09 |
| 712 | 5'-OH-PCB 156 | 7.21 | 7.18 |
| 713 | 6'-OH-PCB 156 | 8.81 | 7.16 |
| 2,3,3',4,4',5'-Hexachlorobiphenyl | |||
| 714 | 5-OH-PCB 157 | 6.69 | 7.18 |
| 715 | 6-OH-PCB 157 | 7.97 | 7.13 |
| 716 | 2'-OH-PCB 157 | 7.44 | 7.09 |
| 2,3,3',4,4',6-Hexachlorobiphenyl | |||
| 717 | 5-OH-PCB 158 | 5.22 | 7.12 |
| 718 | 2'-OH-PCB 158 | 8.1 | 7.1 |
| 719 | 5'-OH-PCB 158 | 7.16 | 7.19 |
| 720 | 6'-OH-PCB 158 | 8.95 | 7.14 |
| 2,3,3',4,5,5'-Hexachlorobiphenyl | |||
| 721 | 6-OH-PCB 159 | 6.58 | 7.09 |
| 722 | 2'-OH-PCB 159 | 8.18 | 7.11 |
| 723 | 4'-OH-PCB 159 | 6.29 | 7.2 |
| 2,3,3',4,5,6-Hexachlorobiphenyl | |||
| 724 | 2'-OH-PCB 160 | 8.84 | 7.11 |
| 725 | 4'-OH-PCB 160 | 7.76 | 7.17 |
| 726 | 5'-OH-PCB 160 | 8.68 | 7.17 |
| 727 | 6'-OH-PCB 160 | 9.7 | 7.11 |
| 2,3,3',4,5',6-Hexachlorobiphenyl | |||
| 728 | 5-OH-PCB 161 | 5.21 | 7.11 |
| 729 | 2'-OH-PCB 161 | 8.33 | 7.1 |
| 730 | 4'-OH-PCB 161 | 6.27 | 7.2 |
| 2,3,3',4',5,5'-Hexachlorobiphenyl | |||
| 731 | 4-OH-PCB 162 | 5.52 | 7.18 |
| 732 | 6-OH-PCB 162 | 7.33 | 7.1 |
| 733 | 2'-OH-PCB 162 | 7.43 | 7.09 |
| 2,3,3',4',5,6-Hexachlorobiphenyl | |||
| 734 | 4-OH-PCB 163 | 4.72 | 7.12 |
| 735 | 2'-OH-PCB 163 | 8.09 | 7.11 |
| 736 | 5'-OH-PCB 163 | 7.15 | 7.17 |
| 737 | 6'-OH-PCB 163 | 8.94 | 7.14 |
| 2,3,3',4',5',6-Hexachlorobiphenyl | |||
| 738 | 4-OH-PCB 164 | 6.21 | 7.21 |
| 739 | 5-OH-PCB 164 | 6.7 | 7.16 |
| 740 | 2'-OH-PCB 164 | 7.59 | 7.1 |
| 2,3,3',5,5',6-Hexachlorobiphenyl | |||
| 741 | 4-OH-PCB 165 | 4.71 | 7.13 |
| 742 | 2'-OH-PCB 165 | 8.32 | 7.11 |
| 743 | 4'-OH-PCB 165 | 6.26 | 7.19 |
| 2,3,4,4',5,6-Hexachlorobiphenyl | |||
| 744 | 2'-OH-PCB 166 | 8.16 | 6.94 |
| 745 | 3'-OH-PCB 166 | 7.57 | 6.95 |
| 2,3',4,4',5,5'-Hexachlorobiphenyl | |||
| 746 | 3-OH-PCB 167 | 5.78 | 7.13 |
| 747 | 6-OH-PCB 167 | 7.09 | 7.08 |
| 748 | 2'-OH-PCB 167 | 7.44 | 7.12 |
| 2,3',4,4',5',6-Hexachlorobiphenyl | |||
| 749 | 3-OH-PCB 168 | 6.02 | 7.16 |
| 750 | 2'-OH-PCB 168 | 7.6 | 7.09 |
| 3,3',4,4',5,5'-Hexachlorobiphenyl | |||
| 751 | 2-OH-PCB 169 | 7.29 | 7.11 |
| 2,2',3,3',4,4',5-Heptachlorobiphenyl | |||
| 752 | 6-OH-PCB 170 | 6.67 | 7.55 |
| 753 | 5'-OH-PCB 170 | 6.58 | 7.65 |
| 754 | 6'-OH-PCB 170 | 8.04 | 7.62 |
| 2,2',3,3',4,4',6-Heptachlorobiphenyl | |||
| 755 | 5-OH-PCB 171 | 5.12 | 7.6 |
| 756 | 5'-OH-PCB 171 | 6.54 | 7.65 |
| 757 | 6'-OH-PCB 171 | 8.19 | 7.63 |
| 2,2',3,3',4,5,5'-Heptachlorobiphenyl | |||
| 758 | 6-OH-PCB 172 | 6.66 | 7.57 |
| 759 | 4'-OH-PCB 172 | 5.44 4.732 |
7.65 6.552 |
| 760 | 6'-OH-PCB 172 | 7.41 | 7.62 |
| 2,2',3,3',4,5,6-Heptachlorobiphenyl | |||
| 761 | 4'-OH-PCB 173 | 6.95 | 7.68 |
| 762 | 5'-OH-PCB 173 | 8.05 | 7.67 |
| 763 | 6'-OH-PCB 173 | 8.94 | 7.6 |
| 2,2',3,3',4,5,6'-Heptachlorobiphenyl | |||
| 764 | 6-OH-PCB 174 | 6.83 | 7.49 |
| 765 | 4'-OH-PCB 174 | 6.17 | 7.68 |
| 766 | 5'-OH-PCB 174 | 6.61 | 7.65 |
| 2,2',3,3',4,5',6-Heptachlorobiphenyl | |||
| 767 | 5-OH-PCB 175 | 5.11 | 7.6 |
| 768 | 4'-OH-PCB 175 | 5.45 | 7.66 |
| 769 | 6'-OH-PCB 175 | 7.58 | 7.62 |
| 2,2',3,3',4,6,6'-Heptachlorobiphenyl | |||
| 770 | 5-OH-PCB 176 | 5.08 | 7.56 |
| 771 | 4'-OH-PCB 176 | 6.18 | 7.67 |
| 772 | 5'-OH-PCB 176 | 6.57 | 7.66 |
| 2,2',3,3',4,5',6'-Heptachlorobiphenyl | |||
| 773 | 5-OH-PCB 177 | 6.53 | 7.65 |
| 774 | 6-OH-PCB 177 | 8.18 | 7.63 |
| 775 | 4'-OH-PCB 177 | 4.68 | 7.62 |
| 2,2',3,3',5,5',6-Heptachlorobiphenyl | |||
| 776 | 4-OH-PCB 178 | 4.67 | 7.64 |
| 777 | 4'-OH-PCB 178 | 5.44 | 7.66 |
| 778 | 6'-OH-PCB 178 | 7.57 | 7.61 |
| 2,2',3,3',5,6,6'-Heptachlorobiphenyl | |||
| 779 | 4-OH-PCB 179 | 4.68 | 7.6 |
| 780 | 4'-OH-PCB 179 | 6.17 | 7.67 |
| 781 | 5'-OH-PCB 179 | 6.56 | 7.65 |
| 2,2',3,4,4',5,5'-Heptachlorobiphenyl | |||
| 782 | 6-OH-PCB 180 | 6.67 | 7.61 |
| 783 | 3'-OH-PCB 180 | 5.69 | 7.64 |
| 784 | 6'-OH-PCB 180 | 7.18 | 7.61 |
| 2,2',3,4,4',5,6-Heptachlorobiphenyl | |||
| 785 | 3'-OH-PCB 181 | 6.45 | 7.64 |
| 786 | 5'-OH-PCB 181 | 7.34 | 7.65 |
| 787 | 6'-OH-PCB 181 | 8.7 | 7.63 |
| 2,2',3,4,4',5,6'-Heptachlorobiphenyl | |||
| 788 | 6-OH-PCB 182 | 6.84 | 7.56 |
| 789 | 3'-OH-PCB 182 | 5.92 | 7.65 |
| 2,2',3,4,4',5',6-Heptachlorobiphenyl | |||
| 790 | 5-OH-PCB 183 | 5.12 | 7.64 |
| 791 | 3'-OH-PCB 183 | 5.65 | 7.64 |
| 792 | 6'-OH-PCB 183 | 7.34 | 7.61 |
| 2,2',3,4,4',6,6'-Heptachlorobiphenyl | |||
| 793 | 5-OH-PCB 184 | 5.09 | 7.6 |
| 794 | 3'-OH-PCB 184 | 5.88 | 7.65 |
| 2,2',3,4,5,5',6-Heptachlorobiphenyl | |||
| 795 | 3'-OH-PCB 185 | 7.13 | 7.66 |
| 796 | 4'-OH-PCB 185 | 6.94 | 7.69 |
| 797 | 6'-OH-PCB 185 | 8.08 | 7.49 |
| 2,2',3,4,5,6,6'-Heptachlorobiphenyl | |||
| 798 | 3'-OH-PCB 186 | 7.37 | 7.66 |
| 799 | 4'-OH-PCB 186 | 7.69 | 7.69 |
| 2,2',3,4',5,5',6-Heptachlorobiphenyl | |||
| 800 | 4-OH-PCB 187 | 4.68 4.082 |
7.65 6.772 |
| 801 | 3'-OH-PCB 187 | 5.64 | 7.64 |
| 802 | 6'-OH-PCB 187 | 7.33 | 7.61 |
| 2,2',3,4',5,6,6'-Heptachlorobiphenyl | |||
| 803 | 4-OH-PCB 188 | 4.69 | 7.64 |
| 804 | 3'-OH-PCB 188 | 5.87 | 7.65 |
| 2,3,3',4,4',5,5'-Heptachlorobiphenyl | |||
| 805 | 6-OH-PCB 189 | 6.47 | 7.6 |
| 806 | 2'-OH-PCB 189 | 7.33 | 7.6 |
| 2,3,3',4,4',5,6-Heptachlorobiphenyl | |||
| 807 | 2'-OH-PCB 190 | 7.99 | 7.59 |
| 808 | 5'-OH-PCB 190 | 7.1 | 7.65 |
| 809 | 6'-OH-PCB 190 | 8.84 | 7.63 |
| 2,3,3',4,4',5',6-Heptachlorobiphenyl | |||
| 810 | 5-OH-PCB 191 | 5.16 | 7.62 |
| 811 | 2'-OH-PCB 191 | 7.48 | 7.61 |
| 2,3,3',4,5,5',6-Heptachlorobiphenyl | |||
| 812 | 2'-OH-PCB 192 | 8.22 | 7.61 |
| 813 | 4'-OH-PCB 192 | 6.22 | 7.66 |
| 2,3,3',4',5,5',6-Heptachlorobiphenyl | |||
| 814 | 4-OH-PCB 193 | 4.67 | 7.64 |
| 815 | 2'-OH-PCB 193 | 7.47 | 7.61 |
| 2,2',3,3',4,4',5,5'-Octachlorobiphenyl | |||
| 816 | 6-OH-PCB 194 | 6.56 | 8.04 |
| 2,2',3,3',4,4',5,6-Octachlorobiphenyl | |||
| 817 | 5'-0H CB 195 | 6.48 | 8.14 |
| 818 | 6'-0H CB 195 | 8.08 | 8.09 |
| 2,2',3,3',4,4',5,6'-Octachlorobiphenyl | |||
| 819 | 6-OH-PCB 196 | 6.72 | 7.97 |
| 820 | 5'-OH-PCB 196 | 5.06 | 8.14 |
| 2,2',3,3',4,4',6,6'-Octachlorobiphenyl | |||
| 821 | 5-OH-PCB 197 | 5.03 | 8.1 |
| 2,2',3,3',4,5,5',6-Octachlorobiphenyl | |||
| 822 | 4'-OH-PCB 198 | 5.4 | 8.14 |
| 823 | 6'-OH-PCB 198 | 7.46 | 7.98 |
| 2,2',3,3',4,5,5',6'-Octachlorobiphenyl | |||
| 824 | 6-OH-PCB 199 | 6.71 | 7.97 |
| 825 | 4'-OH-PCB 199 | 4.63 | 8.15 |
| 2,2',3,3',4,5,6,6'-Octachlorobiphenyl | |||
| 826 | 4'-OH-PCB 200 | 6.13 | 8.14 |
| 827 | 5'-OH-PCB 200 | 6.51 | 8.16 |
| 2,2',3,3',4,5',6,6'-Octachlorobiphenyl | |||
| 828 | 5-OH-PCB 201 | 5.02 | 8.02 |
| 829 | 4'-OH-PCB 201 | 4.64 | 8.15 |
| 2,2',3,3',5,5',6,6'-Octachlorobiphenyl | |||
| 830 | 4-OH-PCB 202 | 4.63 | 8.12 |
| 2,2',3,4,4',5,5',6-Octachlorobiphenyl | |||
| 831 | 3'-OH-PCB 203 | 5.59 | 8.14 |
| 832 | 6'-OH-PCB 203 | 7.23 | 7.98 |
| 2,2',3,4,4',5,6,6'-Octachlorobiphenyl | |||
| 833 | 3'-OH-PCB 204 | 5.82 | 8.14 |
| 2,3,3',4,4',5,5',6-Octachlorobiphenyl | |||
| 834 | 2'-OH-PCB 205 | 7.37 | 8.06 |
| 2,2',3,3',4,4',5,5',6-Nonachlorobiphenyl | |||
| 835 | 6'-OH-PCB 206 | 6.61 | 8.19 |
| 2,2',3,3',4,4',5,6,6'-Nonachlorobiphenyl | |||
| 836 | 5'-OH-PCB 207 | 4.97 | 8.23 |
| 2,2',3,3',4,5,5',6,6'-Nonachlorobiphenyl | |||
| 837 | 4'-OH-PCB 208 | 4.59 | 8.25 |
Taken from Rayne and Forest (2010) unless otherwise denoted.
Taken from (Tampal et al., 2002).
Calculated from Advanced Chemistry Development (ACD) I-Lab Web Service using ACD/pKa 8.03 or ACD/LogP 8.02 as appropriate.
Calculated LogP values from Bradbury et al. (1996).
Parent congeneric PCB nomenclature consistent with Ballschmiter (1980) and EPA on-line list. See Mills et al. (2007) for a comparison of congener nomenclatures.
Table 3.
Polychlorinated biphenylols (OH-PCBs) identified in human plasma and their suggested parent compound. Parent compounds in bold as determined by Sjödin and coworkers (Soechitram et al., 2004).
| Abbreviation | Structure | Parentcompound | |
|---|---|---|---|
| via epoxide or direct insertion |
via 1,2-shift |
||
| 5'-OH-PCB 66 | 3-OH-2',4,4',5-tetraCB | PCB 66 | PCB 68 |
| 4'-OH-PCB 79 | 4-OH-3,3',4',5-tetraCB | PCB 79 | PCB 77 |
| 3'-OH-PCB 85 | 3-OH-2,2',3',4,4'-pentaCB | PCB 85 | PCB 105 PCB 82 |
| 4'-OH-PCB 120 | 4-OH-2',3,4',5,5'-pentaCB | PCB 120 | PCB 118 |
| 4'-OH-PCB 101 | 4-OH-2,2',4',5,5'-pentaCB | PCB 101 | PCB 99 |
| 3-OH-PCB 118 | 3-OH-2,3',4,4',5-pentaCB | PCB 118 | PCB 107 PCB 126 |
| 4-OH-PCB 107 | 4-OH-2,3,3',4',5-pentaCB | PCB 107 | PCB 105, PCB 118 |
| 4'-OH-PCB 108 | 4-OH-2',3,3',4',5-pentaCB | PCB 108 | PCB 105 |
| 4-OH-PCB 134 | 4-OH-2,2',3,3',5,6-hexaCB | PCB 134 | PCB 131 |
| 4'-OH-PCB 97 | 4-OH-2,2',3,4',5'-pentaCB | PCB 97 | PCB 99 |
| 3'-OH-PCB 184 | 3-OH-2,2',3',4,4',6,6'-heptaCB | PCB 184 | PCB 176 |
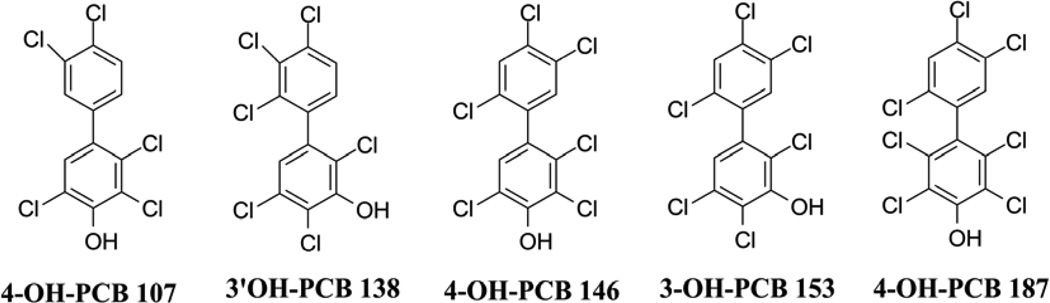
| 3-OH-PCB 153 | 3-OH-2,2',4,4',5,5'-hexaCB | PCB 153 | PCB 146 PCB 167 |
| 4-OH-PCB 146 | 4-OH-2,2',3,4',5,5'-hexaCB | PCB 146 | PCB 138, PCB 153 |
| 3',4-diOHCB 90 | 3',4 –diOH-2,2',3,4',5-pentaCB | ||
| 2',4 –diOH-PCB 107 | 2',4 –diOH-2,3,3',4',5-pentaCB | PCB 105 PCB 118 | |
| 4'-OH-PCB 127 | 4-OH-3,3',4',5,5'-pentaCB | PCB 127 | PCB 126 |
| 3'-OH-PCB 138 | 3-OH-2,2',3',4,4',5-hexaCB | PCB 138 | PCB 130 PCB 157 |
| 4'-OH-PCB 130 | 4-OH-2,2',3,3',4',5-hexaCB | PCB 130 | PCB 138 PCB 128 |
| 4-OH-PCB 163 | 4-OH-2,3,3',4',5,6-hexaCB | PCB 163 | PCB 158 |
| 4-OH-PCB 178 | 4-OH-2,2',3,3',5,5',6-heptaCB | PCB 178 | PCB 175 |
| 3'-OH-PCB 182 | 3-OH-2,2',3',4,4',5',6-heptaCB | PCB 182 | PCB 174 PCB 180 |
| 3'-OH-PCB 183 | 3-OH-2,2',3',4,4',5,6'-heptaCB | PCB 183 | PCB175 PCB 191 |
| 4'-OH-PCB 175 | 4-OH-2,2',3,3',4',5,6'-heptaCB | PCB 175 | PCB 183 PCB 171 |
| 4-OH-PCB 187 | 4-OH-2,2',3,4',5,5',6-heptaCB | PCB 187 | PCB 183 |
| 4'-OH-PCB 159 | 4-OH-2',3,3',4',5,5'-hexaCB | PCB 159 | PCB 156 |
| 4-OH-PCB 162 | 4-OH-2,3,3',4',5,5'-hexaCB | PCB 162 | PCB 157 |
| 4-OH-PCB 177 | 4-OH-2,2',3,3',4',5,6-heptaCB | PCB 177 | PCB 171 |
| 4-OH-PCB 202 | 4-OH-2,2',3,3',5,5',6,6'-octaCB | PCB 202 | PCB 201 |
| 3'-OH-PCB 180 | 3-OH-2,2',3',4,4',5,5'-heptaCB | PCB 180 | PCB 172 PCB 189 |
| 4'-OH-PCB 172 | 4-OH-2,2',3,3',4',5,5'-heptaCB | PCB 172 | PCB 170 PCB 180 |
| 4-OH-PCB 193 | 4-OH-2,3,3',4',5,5',6-heptaCB | PCB 193 | PCB 191 |
| 4,3'-diOH-PCB 187 | 4,3'-diOH-2,2',3,4',5,5',6-heptaCB | PCB 187 | PCB 183 |
| 4,4'-diOH-PCB 178 | 4,4'-diOH-2,2',3,3',5,5',6-heptaCB | PCB 183 | |
| 3'-OH-PCB 203 | 3-OH-2,2',3',4,4',5,5',6'-octaCB | PCB 203 | PCB 198 PCB 205 |
| 4'-OH-PCB 198 | 4-OH-2,2',3,3',4',5,5',6'-octaCB | PCB 198 | PCB 195 PCB 203 |
| 4'-OH-PCB 199 | 4-OH-2,2',3,3',4',5,5',6-octaCB | PCB 199 | PCB 196 |
| 4,4'-diOH-PCB 202 | 4,4'-diOH-2,2',3,3',5,5',6,6'-octaCB | ||
| 4'-OH-PCB 208 | 4-OH-2,2',3,3',4',5,5',6,6'-nonaCB | PCB 208 | PCB 207 |
PCB metabolism
The rate and extent of PCB metabolism depends on the number and positions of chlorines in the molecule (Kato et al., 1980, Matthews and Anderson, 1975, Mills et al., 1985, Schnellmann et al., 1985). Overall, the fewer number of chlorine atoms on biphenyl, the faster the metabolism. Also, availability of vicinal non-chlorine substituted positions, especially in meta- and para-positions of the biphenyl core, increases the chances of cytochrome P450 (CYP) mediated transformation (Mills et al., 1985). Consequently, the fate of individual PCBs within the human body depends on their structural properties. Conventional HC-PCBs are fairly resistant towards biotransformation reactions and due to their high lipophilicity, they tend to be retained in adipose tissues or in plasma where they can be frequently detected at high concentrations of more than 10 µg/g lipid weight (Fangstrom et al., 2002, Kutz et al., 1991). LC-PCBs, by contrast, are often transiently detected in serum and their rapid disappearance is assumed to be related to their higher susceptibility for metabolic conversion (Robertson and Ludewig, 2011, Hansen, 1999)
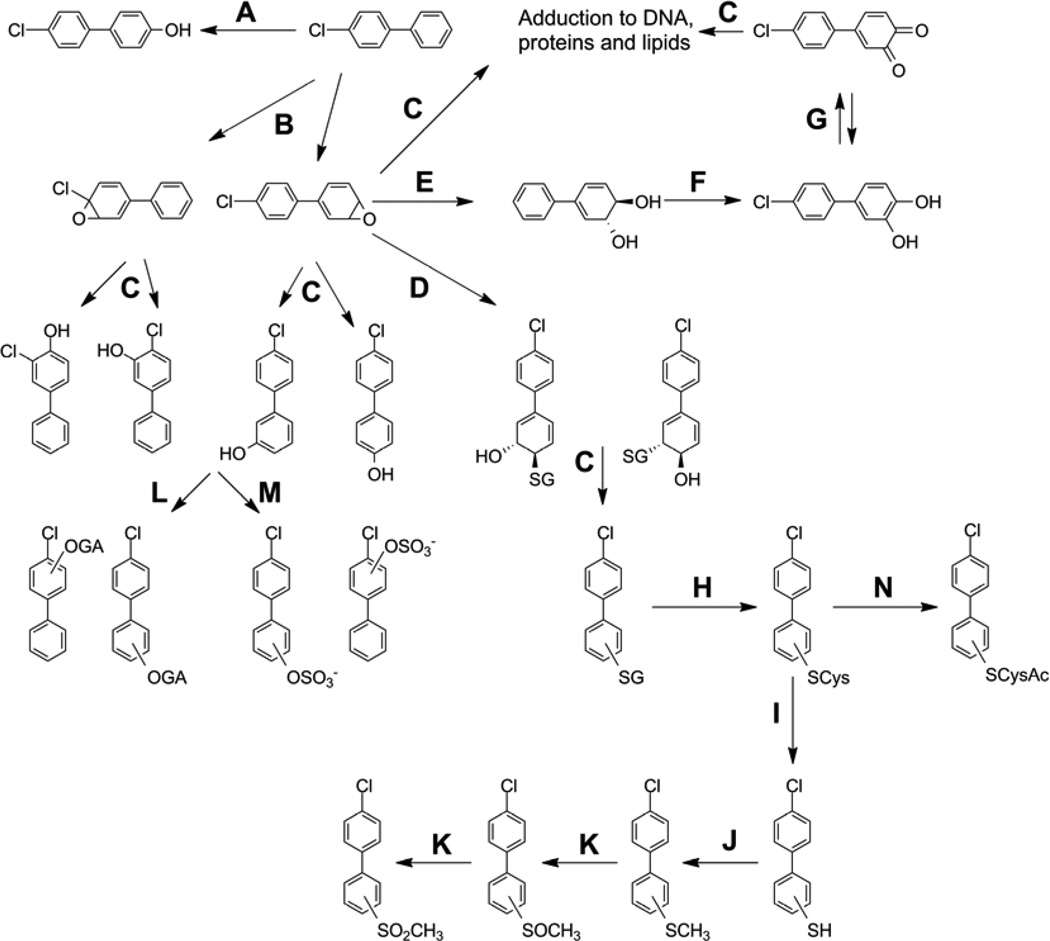
The main pathways for PCB metabolism are shown in Figure 1 (Safe, 2001, Letcher et al., 2000), with enzymes involved suggested. The initial step includes the oxidation of PCBs to OH-PCBs by hepatic cytochromes P-450 (CYP) enzymes (Bergman et al., 1994b, Matthews and Kato, 1979). Structurally different PCB congeners may be metabolized by different enzymes of the CYP superfamily. Non-ortho substituted (so called co-planar or dioxin-like) PCB congeners are metabolized predominately by CYP1A enzymes, while multiple ortho-substituted PCBs are substrates for CYP2B enzymes (Kaminsky et al., 1981, Lu et al., 2013, Lu and Wong, 2011, McGraw and Waller, 2006, Waller et al., 1999, Warner et al., 2009). These observations are also confirmed by more efficient binding of structurally related PCBs to appropriate cytochrome P-450 isoforms (Hrycay and Bandiera, 2003, Kania-Korwel et al., 2008a).
Figure 1.
General metabolic scheme for a representative lower-chlorinated PCB congener, PCB 3. Enzymes involved in the metabolism are indicated by the letters A, B and D-N with the letter C indicating non-enzymatic transformations. Enzymes suggested for these transformations are listed as follows: A, Cytochrome P-450 (CYP) enzyme system, Direct insertion in meta position; CYP2B (rodents); B, Cytochrome P-450 enzyme system; CYB2B1 (rodents); CYP3A4 (humans); Non-coplanar PCBs: CYB2B, 2C, 3A ; C, Non-enzymatic reaction; D, Glutathione S-transferase; E, Epoxide hydrolase; F, Dihydrodiol dehydrogenase (AKR1C); G, Autooxidation and/or Peroxidases; H, γ–Glutamyl transpeptidase, then cysteinylglycine dipeptidase; I, Cysteine S-conjugate β-lyase; J, Thiol S-methyltransferase; K, CYP and/or FAD-containing monooxygenases (FMO); L, UDP-glucuronosyl transferase (UGT); M, Sulfotransferase (SULT); N, Cysteine S-conjugate N-acetyltransferase
Depending on the PCB congener, the initial CYP-dependent monooxygenation can result from direct electrophilic addition of oxygen or may involve the formation of a transient reactive arene oxide (Guengerich, 2001, Jerina and Daly, 1974, Preston et al., 1983). In fact, OH-PCBs are most easily formed from PCBs with 2,3-, 2,5- and 2,6-dichlorination or 2,3,6-trichlorination patterns or PCB epoxides where the phenyl ring has none or a lower number of chlorine atom substituents in any position except the 4-position. PCB congeners with 4-, 3,4-, 3,5-, 2,4,5-, 2,3,4,6- or 2,3,5,6- chlorine substitutions are less efficiently metabolized and they tend to form epoxides between a chlorine substituted carbon and an unsubstituted carbon. Each of the epoxides will yield two isomeric OH-PCBs. While OH-PCBs with at least one unsubstituted carbon next to the phenol group appear to be rapidly eliminated from organisms, including humans, the chlorine may undergo a 1,2-shift (Guroff et al., 1967) yielding rearranged polychlorobiphenylols. OH-PCBs with neighboring chlorine substituted carbons to the phenol group are retained in the blood (Letcher et al., 2000).
Transient PCB epoxides may also form adducts with biomacromolecules, isomerize to mono-hydroxy PCBs or hydrolyze to form PCB dihydrodiols (McLean et al., 1996b, McLean et al., 1996a, Kaminsky et al., 1981). Alternatively, PCB epoxides may react with glutathione (GSH) to form a dihydro-glutathione-hydroxyl-substituted PCB metabolite that can form a fully aromatic PCB glutathione conjugate through the loss of water (Figure 1) (Letcher et al., 2000, Bakke et al., 1983, Bakke et al., 1982). Each PCB undergoing this type of metabolism will yield two PCB glutathione conjugates. These GS-PCBs are degraded in a stepwise fashion to the corresponding cysteine conjugates that may be N-acetylated to form mercapturic acids, a route of degradation known as the mercapturic acid pathway (Bakke et al., 1982, Letcher et al., 2000). The corresponding PCB thiols are formed via cysteine S-conjugate β-lyase catalyzed cleavage of the C-S bond in the cysteine conjugate. The PCB thiols are methylated and oxidized in two steps to sulfoxides and finally to the corresponding MeSO2-PCBs, highly hydrophobic Lewis bases (Kallenborn and Huhnerfuss, 2001).
OH-PCBs have also been shown to undergo multiple oxidation reactions leading to more than a single hydroxyl substituent on the biphenyl structure (Bergman et al., 1994b, James, 2001). Alternatively, the formation of dihydroxylated PCB metabolites can be directly catalyzed from parent PCBs by CYP2B enzymes (Lu et al., 2013, McLean et al., 1996a, Waller et al., 1999). Dihydroxylation can result in the formation of catechols and other hydroquinones (McLean et al., 1996a), that possess toxicological relevance in that they promote oxidative stress (Song et al., 2008b). OH-PCBs also represent substrates for conjugation reactions catalyzed by sulfotransferases (SULTs) or UDP-glucuronosyl transferases (UGTs) to yield their respective sulfate or glucuronic acid conjugates (Daidoji et al., 2005, Dhakal et al., 2012, Matthews and Kato, 1979).
Chirality of PCB metabolites
Nineteen PCBs and their metabolites are optically active (or chiral) because they exist as stable rotational isomers, called atropisomers, that are non-superimposable mirror images of each other (Lehmler et al., 2010, Mannschreck et al., 1985, Püttmann et al., 1989, Püttmann et al., 1986). Only non-coplanar PCBs with three or four ortho-chlorine substituents possess sufficient torsional strain to facilitate the formation of stable atropisomer pairs that can be separated chromatographically (Kaiser, 1974, Norstrom et al., 2006, Haglund, 1996). PCBs 84, 91, 95, 132, 136, 149, 174 and/ or their metabolites have been shown to accumulate enantioselectively in mammalian tissues (Lehmler et al., 2010), in birds (Jörundsdottir et al., 2006), in plants (Zhai et al., 2011) and in humans (Ellerichmann et al., 1998, Hovander et al., 2004).
Theoretically, 456 of the 837 possible mono-hydroxylated PCB metabolites are chiral (Nezel et al., 1997). These atropisomers may or may not be generated in a 1:1 ratio, but the metabolites, like PCB atropisomers, seem to have different half-lives in animal tissues and in biota (Püttmann et al., 1989, Larsson et al., 2004, Larsson et al., 2002, Chu et al., 2003a). The atropselective formation of chiral OH-PCBs has been shown both in vivo (Kania-Korwel et al., 2008b, Kania-Korwel et al., 2012) and in vitro (Kania-Korwel et al., 2011, Wu et al., 2011, Lu et al., 2013, Wu et al., 2013a, Wu et al., 2014, Wu et al., 2013b, Zhai et al., 2013b). The recently identified OH-PCB metabolites of five chiral PCBs, PCB 91, 95, 132, 136, and 149, are chiral themselves, but were not previously identified in environmental samples, including human blood, due to the lack of authentic standards. The atropselective formation of these OH-PCBs results in changes of enantiomeric fractions of the parent compound (Warner et al., 2009). It was demonstrated using pure atropisomers, that biotransformation of (−)-PCB 136 leads to the formation of single enantiomer of 5-OH-PCB 136, while the biotransformation of (+)-PCB 136 results in the formation of the other enantiomer of that major metabolite (Wu et al., 2011). Considering that pure PCB atropisomers can elicit different toxicological responses (Pessah et al., 2009, Lehmler et al., 2005, Yang et al., 2014), these findings may have implications for risk assessment associated with those metabolites. Optically active MeSO2-PCBs identified in humans and laboratory animals to date are atropisomers of 5’-MeSO2-PCB 132 and 3-MeSO2-PCB 149 (Ellerichmann et al., 1998, Norstrom et al., 2006).
Reactive (epoxide and (semi)quinone) PCB intermediates
Hepatic microsomes are capable of metabolizing lower chlorinated biphenyls, mono-, di-, and trichlorobiphenyls to catechols and hydroquinones (Robertson and Gupta, 2000, McLean et al., 1996a, Oakley et al., 1996). Likewise, the potential for microsomal formation of PCB catechols derived from penta- and hexachlorinated PCBs (e.g. PCB 136) was demonstrated (Lu et al., 2013, Wu et al., 2013a, Wu et al., 2014). One-electron oxidation of a PCB hydroquinone or catechol, or single-electron reduction of a PCB quinone, results in a semi-quinone radical with subsequent formation of reactive oxygen species (e.g. superoxide anion radical, hydrogen peroxide, and hydroxyl radical) and the PCB quinone (Song et al., 2008a, Song et al., 2008b). In addition to the potential for generation of toxic oxygen species, the metabolic pathways of PCBs may include the formation of electrophilic PCB arene oxides and quinones that may bind to nucleophilic sites on cellular macromolecules (Robertson and Gupta, 2000, Lin et al., 2000, Qin et al., 2013, Wangpradit et al., 2009, James, 2001). In fact, a large number of in vitro studies have demonstrated adduct formation of PCBs and their metabolites, in particular PCB quinones, to proteins, RNA, DNA or lipids (Robertson and Gupta, 2000, Morck et al., 2002, Ludewig, 2001, Klasson Wehler et al., 1989, Klasson Wehler et al., 1993, Zhao et al., 2004).
Even though most evidence of PCB adduct formation points towards a primary involvement of LC-PCBs, there is limited evidence available supporting the potential of HC-PCBs for adduct formation with DNA/RNA and/or protein. An in vivo study in mice demonstrated covalent binding of 2,2’,3,3’,6,6’-hexachlorobiphenyl (PCB 136) to RNA, proteins, and DNA in liver, muscle, and kidneys and of 2,2’,4,4’,5,5’- hexachlorobiphenyl (PCB 153) to RNA and proteins in liver (Morales and Matthews, 1979). Another study showed binding of PCB 153 to nuclear proteins and DNA in livers of treated rats (Daubeze and Narbonne, 1984). Further evidence of the presence of reactive intermediates of PCBs forming reaction products with biomolecules is the observation that non-extractable residues are present after exposure to radiolabeled PCBs (Pereg et al., 2001, Klasson Wehler et al., 1989, Klasson Wehler et al., 1993, Morck et al., 2002, Tampal et al., 2003). However, the identity of these adducts have so far only been poorly characterized, although two classes of PCB electrophiles, arene oxides and (semi)quinones, appear to be involved. The binding to lipids appears to involve phospholipids (Morck et al., 2002).
The ability of eight mono- to hexachlorinated biphenyls to form DNA adducts following bioactivation with hepatic microsomes from different species (rat, mouse, and human) was investigated (Pereg et al., 2002). Interestingly, only the lower chlorinated congeners with up to three chlorine atoms were capable of DNA adduction. Based on structural identification of PCB adducts to DNA, the suggested formation of DNA adducts involves PCB quinone metabolites (Zhao et al., 2004).
Binding indices have shown 15- to 30-fold greater binding of PCBs to peptides than to DNA (Pereg et al., 2001). Protein adduction is initiated through a reaction of the PCB metabolites with nitrogen and sulfur nucleophiles (Amaro et al., 1996). Sulfur nucleophiles are much more reactive with PCB-derived electrophiles than nitrogen nucleophiles. Therefore one would predict that protein sulfhydryls would be a preferred target (both on the basis of reactivity and abundance). In addition, using glutathione (GSH) as a target peptide, two distinctive, structure-dependent mechanisms by which PCB quinones are capable of forming protein adducts were revealed (Song et al., 2009). While PCB quinones without chlorine substituents in the quinone ring typically undergo a Michael addition to form a GSH adduct, GSH adduction of PCB quinones with chlorine substituents in the quinone ring tends to involve nonenzymatic displacement of such chlorines. Lin et al. reported an implied involvement of reactive quinones in the liver and brain of rats exposed to 2,2’,5,5’-tetrachlorobiphenyl (PCB 52) (Lin et al., 2000). The adducts seemed to be unstable in vivo since the estimated half-life of the adducts was 2.5-fold shorter than the turnover rate of liver cytosolic protein.
Altogether, it is evident that PCBs are precursors of biomacromolecule adducts. This has most clearly been shown for the lower chlorinated biphenyls, but a number of studies on higher chlorinated biphenyls confirm the possibility of adduct formation. This is mediated by the metabolic formation of reactive intermediates, arene oxides, and (semi)quinones.
Hydroxylated PCB metabolites
Some OH-PCBs are stable (and extractable) metabolites that are retained in the body. Approximately 40 different OH-PCBs have been identified in human blood as first reported by Bergman (Bergman et al., 1994a) and thereafter in a series of other studies (Marek et al., 2013b, Quinete et al., 2014, Park et al., 2008, Park et al., 2007, Park et al., 2009), as well as in blood from wildlife (Fängström et al., 2005a, Letcher et al., 2000, Gutleb et al., 2010). There are 837 possible OH-PCB congeners (Table 2); the chemical structures of several that have been commonly detected in human blood (Table 3) are shown in Figure 2. All of these have 5 or more chlorines. Human plasma or serum concentrations of these most abundant OH-PCB congeners, as determined in human populations from Canada, the Faroe Islands, Latvia, the Netherlands, Nicaragua, Russia, Slovakia, and Sweden, are given in Table 4. All studies, except for the study of humans from the Russian Arctic, provided comparisons with PCB 153, the most abundant PCB congener. The OH-PCBs are present in concentrations similar in range as many PCB congeners, except for those PCBs that are the most prevalent/persistent. Since OH-PCBs in the blood are reversibly bound to proteins, their accumulation is not lipid dependent (Gutleb et al., 2010, Purkey et al., 2004). Therefore it is more correct to report concentrations in serum or plasma on the basis of fresh weights. However, for comparative reasons the concentrations are often provided based on lipid weight. It was shown that the OH-PCB congener concentrations may reach median concentrations of 2–300 ng/g fat, with individual levels up to 940 ng/g lipid weight (Table 4). Comparisons with plasma levels of PCB 153 indicate the high prevalence of various OH-PCBs. In fact, plasma concentrations of the most abundant OH-PCB congeners reach about 30% of those determined for PCB 153, with a variation among studies of 11–82%. The practical implication is that OH-PCBs present at the highest concentrations always exceed the concentrations of a large number of individual PCB congeners.
Figure 2.
Chemical structures of the most common OH-PCB congeners present in human blood (c.f. Table 2). The full names of the five OH-PCBs are: 2,3,3’,4’,5-pentachloro-4-biphenylol (4-OH-PCB 107); 2,2’,3’,4,4’,5- hexachloro-3-biphenylol (3’-OH-PCB 138); 2,2’,3,4’,5,5’- hexachloro-4-biphenylol (4-OH-PCB 146); 2,2’,4,4’,5,5’- hexachloro-3-biphenylol (3-OH-PCB 153) and 2,2’,3,4’,5,5’,6 - heptachloro-4-biphenylol (4-OH-PCB 187).
Table 4.
Median and range (min-max) concentrations (ng/g lipid weight) of OH-PCBs and CB-153 in plasma from humans at different geographical areas.
| Country | Yeara | N | 4-OH-PCB 107 |
3'-OH-PCB 138 |
4-OH-PCB 146 |
3-OH-PCB 153 |
4-OH-PCB 187 |
PCB-153 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Slovakia R1 | ||||||||||
| Stropkov/Svidnik | 2001 | female/male | 175 | 26 (0.95–84) | 26 (6.5–92) | 58 (15–260) | 240 (51–1200) | |||
| Micahlovce | female/male | 122 | 62 (3.7–710) | 73 (6.7–940) | 130 (11–1500) | 570 (100–7200) | ||||
| The Netherlands R2 | 1998/00 | maternal | 51 | 10 (0.8–38) | 7 (1.3–26) | 10 (3–27) | 5 (1.4–13) | 20 (7–49) | 100 (43–290) | |
| cord plasma | 51 | 14 (4–350) | 18 (6–50) | 23 (8–58) | 14 (3–38) | 38 (17–69) | 115 (25–250) | |||
| Faroe Islands R3 | 1994/95 | maternal | 57 | 54 (5.7–300) | 33 (7.5–260) | 88 (22–360) | 31 (5.8–230) | 150 (39–600) | 430 (99–1500) | |
| 2000/01 | children (7 yr) | 42 | 130 (8.5–580) | 31 (2.0–130) | 68 (4.9–480) | 23 (1.9–100) | 99 (12–470) | 310 (25–1300) | ||
| Sweden R4 | 1995 | female | lowb | 16 | 36 (0–59) | 41 (4–72) | 50 (13–73) | |||
| highb | 16 | 81 (28–480) | 93 (43–270) | 290 (150–460) | ||||||
| Sweden R5 | 1991 | male | lowc | 20 | 36 (15–110) | 20 (7.5–45) | 39 (12–140) | 15 (5.1–31) | 74 (54–110) | 220 (120–390) |
| highc | 12 | 58 (27–290) | 28 (14–68) | 66 (43–290) | 20 (11–51) | 68 (57–280) | 450 (280–1000) | |||
| Latvia R5 | 1993 | male | lowc | 19 | 82 (31–150) | 18 (9.1–33) | 31 (17–62) | 12 (7.3–28) | 34 (23–61) | 160 (100–230) |
| highc | 26 | 290 (87–770) | 74 (29–230) | 160 (57–540) | 57 (16–280) | 120 (66–430) | 920 (320–1700) | |||
| The Netherlands R6 | 2001/02 | maternal | 90 | 3.5 (n.d.–20) | 14 (5.7–110) | 11 (4.7–74) | 63 (19–230) | |||
| cord plasma | 9 | 7.7 (n.d. –23) | 41 (13–180) | 30 (13–87) | 62 (26–110) | |||||
| Sweden R7 | maternal | earlyd | 6 | 52 (27–130) | 26 (9.9–59) | 40 (30–84) | 95 (41–160) | |||
| lated | 8 | 14 (9.3–31) | 14 (5.3–22) | 26 (14–39) | 64 (32–75) | |||||
| Nicaragua R8 | 2002 | female | lowb | 4 | 2 (1–5) | 14 (6–61) | ||||
| highb | 4 | 16 (9–27) | 100 (49–220) | |||||||
| Canada e, f, R9 | ||||||||||
| Nunavik | 1993/96 | Cord plasma | 10 | 6 (1.5–22) | 5 (1.5–18) | 18 (2–67) | 9.5 (2–32) | 24 6.5–78) | 131 (24–670) | |
| Lower N. Shore | 10 | 24 (3–84) | 11 (4.5–46) | 40 (8–250) | 12 (5–37) | 48 (27–130) | 215 (54–680) | |||
| Southern Quebeck | 10 | 5.5 (1.5–22) | 2.5 (1.5–8) | 6 (2–29) | 3 (1.5–7) | 14 (5–48) | 52 (15–100) | |||
| Swedene, R10 | 2000/01 | maternal | 15 | 5 (2–15) | 4.5 (1–27) | 14 (6–61) | 3.5 (0.5–18) | 24 (12–49) | 56 (27–200) | |
| cord plasma | 15 | 2.5 (<0.05–5.5) | 4.5 (1–28) | 10 (4–27) | 2.5 (0.5–16) | 12 (6.5–22) | 44 (20–110) | |||
| Russian Arcticg, R11 | 2003 | Female/male | high | 15 | 280 (200–920) | 170 (92–340) | 190 (87–350) | 190 (100–490) | 140 (74–460) |
n.d. = not detected;
Sampling year
The groups are divided according to PCB153 concentrations, low and high, respectively.
Divided in groups according to consumption of fatty fish: low = 0–1 meals/month and high > 12 fish meals/month, 10th–90th percentile range
Pregnancy time, early: 9–13 weeks and late: 31–36 weeks
Data has been normalized for lipid content at 0.2% according to (Soechitram et al., 2004) and (Guvenius et al., 2003)
Geometric mean
Samples chosen with high PCB value.
References :
: (Hagmar et al., 2001);
: (Sjodin et al., 2000);
: (Meijer et al., 2004);
: (Cuadra et al., 2006);
: (Sandau et al., 2002);
: (Hofvander, 2006).
OH-PCBs are efficiently transferred from the maternal to the fetal blood via the placenta (Guvenius et al., 2003, Meerts et al., 2002, Morse et al., 1996, Soechitram et al., 2004). In fact, the transplacental transfer seems to be more efficient for the OH-PCBs (Lucier et al., 1978) than for the PCBs (Gallenberg et al., 1990, Ring et al., 1988) themselves. This is most likely due to a lower relative lipid content in the blood of the fetus. It is also necessary to consider this difference in maternal-fetal transfer from a risk perspective. On the other hand, basically no, or a very limited, transfer of OH-PCBs occurs from mothers to their nursing children via the milk, which was shown both in laboratory animals (Meerts et al., 2002, Morse et al., 1996) and in humans (Malmberg et al., 2004, Fangstrom et al., 2005, Guvenius et al., 2002). The concentrations of PCB 153 in mother’s milk have been shown to be almost three orders of magnitude higher than those determined for 4-OH-PCB 187, the most common OH-PCB. This partitioning behavior is presumably due to the more polar and less lipophilic characteristics of the OH-PCBs.
To date, the in vivo formation of OH-PCBs from LC-PCBs has not been extensively characterized and deserves further scientific attention. Initial studies using rats exposed to PCB 11 by inhalation exposure, however, clearly indicate their rapid hydroxylation and subsequent elimination (Hu et al., 2014, Hu et al., 2013). While many OH-PCB metabolites are good substrates for conjugation reactions (James, 2001), the ease of formation of glucuronic acid and sulfate conjugates is highly structure-dependent, an observation that is supported by the persistence of certain OH-PCBs in serum (Tampal et al., 2002, Liu et al., 2009, Bergman et al., 1994b, Gutleb et al., 2010). In addition, both conjugation reactions, glucuronidation and sulfation, may be inhibited by OH-PCBs (Ekuase et al., 2011, James, 2001, Kester et al., 2000, Liu et al., 2006, Liu et al., 2011, Liu et al., 2009, Schuur et al., 1998b, van den Hurk et al., 2002, Wang et al., 2006).
PCB methyl sulfones
About 60 MeSO2-PCBs have been detected in environmental samples (Letcher et al., 2000). The precursor PCB congeners are well known for all MeSO2-PCBs that so far have been identified in humans and in wildlife. All the 22 MeSO2-PCB metabolites that have been structurally identified, and their parent PCB congeners, are listed in Table 5. In addition, recent data show the occurrence of up to fifty or more MeSO2-PCB congeners in human serum, but so far the majority of these are still not structurally identified (Hovander et al., 2006). Studies on the formation and retention of MeSO2-PCBs in humans and wildlife published through 1999 were reviewed in detail by Letcher and co-workers (Letcher et al., 2000).
Table 5.
PCB methyl sulfone (MeSO2-PCBs) identified in humans and their parent compounds are indicated. Chiral MeSO2-PCBs are marked with an x in the table.
| Structure | Abbreviation | Chiral MeSO2-CBs |
Parent compound |
|---|---|---|---|
| 3-MeSO2-2,2´,4´,5-tetraCB | 3´-MeSO2-PCB 49 | PCB 49 | |
| 4-MeSO2-2,2’,4´,5-tetraCB | 4´-MeSO2-PCB 49 | PCB 49 | |
| 3-MeSO2-2,2´,5,5´-tetraCB | 3-MeSO2-PCB 52 | PCB 52 | |
| 4-MeSO2-2,2´,5,5´-tetraCB | 4-MeSO2-PCB 52 | PCB 52 | |
| 3-MeSO2-2,4´,5,6-tetraCB | 5-MeSO2-PCB 64 | PCB 64 | |
| 4-MeSO2-2,3,4´,6-tetraCB | 4-MeSO2-PCB 64 | PCB 64 | |
| 3-MeSO2-2,3´,4´,5-tetraCB | 3-MeSO2-PCB 70 | PCB 70 | |
| 4-MeSO2-2,3´,4´,5-tetraCB | 4-MeSO2-PCB 70 | PCB 70 | |
| 3-MeSO2-2,2´,3´,4´,5-pentaCB | 3´-MeSO2-PCB 87 | PCB 87 | |
| 4-MeSO2-2,2´,3´,4´,5-pentaCB | 4´-MeSO2-PCB 87 | PCB 87 | |
| 3-MeSO2-2,2´,4´,5,6-pentaCB | 5-MeSO2- PCB 91 | x | PCB 91 |
| 4-MeSO2-2,2´,3,4´,6-pentaCB | 4-MeSO2- PCB 91 | x | PCB 91 |
| 3-MeSO2-2,2´,4´,5,5´-pentaCB | 3´-MeSO2-PCB 101 | PCB 101 | |
| 4-MeSO2-2,2´,4´,5,5´-pentaCB | 4´-MeSO2-PCB 101 | PCB 101 | |
| 3-MeSO2-2,2´,3´,4´,5,6-hexaCB | 5´-MeSO2-PCB 132 | x | PCB 132 |
| 4-MeSO2-2,2´,3,3´,4´,6-hexaCB | 4´-MeSO2-PCB 132 | x | PCB 132 |
| 3-MeSO2-2,2´,3´,4´,5,5´-hexaCB | 3´-MeSO2-PCB 141 | PCB 141 | |
| 4-MeSO2-2,2´,3´,4´,5,5´-hexaCB | 4´-MeSO2-PCB 141 | PCB 141 | |
| 3-MeSO2-2,2´,4´,5,5´,6-hexaCB | 3-MeSO2-PCB 149 | x | PCB 149 |
| 4-MeSO2-2,2´,3,4´,5´,6-hexaCB | 4-MeSO2-PCB 149 | x | PCB 149 |
| 3-MeSO2-2,2´,3´,4´,5,5´,6-heptaCB | 5´-MeSO2-PCB 174 | x | PCB 174 |
| 4-MeSO2-2,2´,3,3´,4´,5´,6-heptaCB | 4´-MeSO2-PCB 174 | x | PCB 174 |
Given their neutral lipophilic character, MeSO2-PCBs are present primarily in body lipids. In humans, MeSO2-PCBs that have been identified are generally present only at low concentrations, 1% or less compared to the PCB concentrations. They accumulate with high selectivity in certain tissues, such as liver and lung (Letcher et al., 2000, Bergman et al., 1979, Larsson and Bergman, 1998). The MeSO2-PCB concentrations are notably high in the liver of mammals (Larsson and Bergman, 1998, Bergman et al., 1994b) and likewise, human livers have been shown to accumulate MeSO2-PCBs (Weistrand and Noren, 1997, Ellerichmann et al., 1998). The majority of these metabolites have the MeSO2 substituent in the meta-position of their respective biphenyl ring. The binding characteristics of MeSO2-PCBs in liver tissue have not been elucidated, but 4-MeSO2-PCBs that accumulate in lung bronchial epithelium of rodents were specifically located in non-ciliated cells, the Clara cells, which contain uteroglobin, alternatively known as PCB binding protein (Lund et al., 1985, Lund et al., 1987). This accumulation in the lung may be seen as an excretion pathway for the MeSO2-PCBs. However, if transported up the airways they may be swallowed and possibly reabsorbed from the gut. Data on human concentrations of MeSO2-PCBs are summarized in Table 6.
Table 6.
Median and range (min-max) concentrations (ng/g lipid weight) of methylsulfonyl-PCBs in humans from four European countries and from Canada. References to the scientific reports are given in the table.
| Country | yeara | n | 4’-MeSO2-PCB 87 |
4’-MeSO2-PCB 101 |
4-MeSO2-PCB 149 |
ΣMeSO2-PCB | PCB-153 | No. cong.b |
|---|---|---|---|---|---|---|---|---|
| Plasma | ||||||||
| SlovakiaR1 | 2001 | |||||||
| Stropkov/Svidnik | 175 | 0.24 (LOQ-1.4) | 0.22 (LOQ-1.4) | 1.0c (0.19–9.2) | 1.5 (0.34–11) | 240 (51–1200) | 3 | |
| Micahlovce | 122 | 0.85 (LOQ-22) | 0.75 (LOQ-9.9) | 2.7c (0.25–72) | 4.2 (0.41–100) | 570 (100–7200) | 3 | |
| Faroe IslandsR12 | ||||||||
| maternal | 1994/95 | 10 | 5.1 (1.9–15) | 4.5 (1.6–11) | 7.5c (2.4–25) | 73 (24–189) | 730 (490–1500) | 11 |
| children | 2000/01 | 10 | 4.0 (1.7–6.5) | 3.6 (1.3–6.6) | 8.6c (3.4–17) | 70 (23–103) | 820 (620–1300) | 11 |
| SwedenR12 | 2002 | 9 | 0.88 (0.14–2.4) | 0.50 (0.14–1.5) | 1.2c (0.28–4.6) | 5.6 (0.90–17) | 290 (140–800) | 11 |
| SwedenR13 | 1997d | 11 | 2.0 (0.81–5.6) | 210 (110–380) | 17 | |||
| Milk | ||||||||
| Sweden R14 | 1972 | pe/75 | 2.1 | 0.78 | 2.0 | 9.2 | 210 | 23 |
| Sweden R14 | 1984/85 | pe/102 | 0.70 | 0.32 | 0.64 | 3.1 | 100 | 23 |
| Sweden R14 | 1992 | pe/20 | 0.33 | 0.13 | 0.35 | 1.6 | 96 | 23 |
| CanadaR15 | 1992 | 50 | 0.33f | 0.087f | 0.81f | 12 | ||
| Adipose tissue | ||||||||
| SwedenR16 | 1997d | 7 | 0.82 (0.48–3.6) | 0.40 (0.21–2.6) | 0.71 (0.41–5.1) | 2.9 (2.0–9.0) | 280 (140–590) | 24 |
| SwedenR17 | 1994 | 5 | 1 (0.2–2) | 0.3 (0.2–1) | 1 (0.4–2) | 6 (2) | 240 (140–740) | 24 |
| BelgiumR18 | 2002 | 11 | 0.33g (0.13–0.98) | 0.27g (0.12–0.93) | 0.08g (n.d.–0.17) | 1.57g (0.33–4.3) | 110 (40–250) | 26 |
| Liver | ||||||||
| SwedenR16 | 1997d | 7 | 1.2 (0.61–2.61) | 0.54 (0.18–1.5) | 1.7 (0.69–11) | 28 (12–358) | 230 (110–610) | 24 |
| Sweden R17 | 1994 | 5 | 1 (0.4–2) | 0.3 (0.2–2) | 1 (0.1–11) | 34 (12–358) | 200 (120–620) | 24 |
| Belgium R18 | 2002 | 11 | 0.35g (n.d.–0.87) | 0.23g (n.d.–1.0) | 0.10g (n.d.–0.42) | 9.3g (1.7–27) | 78 (11–240) | 26 |
| Lung | ||||||||
| Belgium R18 | 2002 | 11 | 0.55g (n.d.–1.1) | 0.46g (n.d.–1.2) | 0.08g (n.d.–0.89) | 2.7g (n.d.–12) | 120g (19–800) | 26 |
n.d. = not detected; LOQ = limit of quantification;
Sampling year;
Number of MeSO2-PCB congeners in the ΣMeSO2-PCB;
include concentration of the MeSO2-hexaCB (unknown);
The publication year;
pooled sample/number of subjects in the pool;
Data has been recalculated for a lipid content of 4%;
Mean values.
References:
: (Noren et al., 1996);
: (Chu et al., 2003b);
: (Chu et al., 2003b).
PCB sulfates
A number of studies have been published that identified OH-PCBs as substrates and inhibitors for recombinant human sulfotransferases SULT1A1, SULT1E1, and SULT2A1 (Ekuase et al., 2011, Kester et al., 2000, Liu et al., 2006, Wang et al., 2006). Moreover, the susceptibility of several OH-PCBs to serve as substrates or inhibitors for rat SULT1A1 and rat SULT2A3, the presumptive rat homolog for human SULT2A1, was assessed (Liu et al., 2009). The majority of the OH-PCBs examined were found to be excellent substrates for certain SULT isoforms while being inhibitors for others. A general conclusion of these studies on structure-activity relationships was that 4’-hydroxylated PCBs without chlorine substituents in the adjacent 3’ or 5’ positions were typically substrates for SULT1A1 and inhibitors for SULT2A1, whereas OH-PCBs possessing the 3’,5’-dichloro-4’hydroxy substitution pattern were usually identified as substrates for SULT2A1 and inhibitors of SULT1A1. The latter group also constitutes the most potent inhibitors of SULT1E1 (Kester et al., 2000). Based on the small number of OH-PCBs with only a single chlorine atom in a position ortho to the phenol that have been examined to date, they appear to have more subtle and complex interactions with family 1 and 2 SULTs that affect substrate/inhibitor specificities of the enzymes.
However, evidence for the formation of PCB sulfates in vivo was lacking until sulfate ester metabolites derived from PCB 3 were recently detected in serum and urine samples collected from male Sprague-Dawley rats exposed to PCB 3 by intraperitoneal injection or inhalation (Dhakal et al., 2013, Dhakal et al., 2012, Dhakal et al., 2014). Interestingly, PCB sulfates appeared to be major metabolites in these studies, far outweighing glucuronidation. Moreover, the most abundant PCB sulfate in serum was identified as 4’-OSO3−-PCB 3 and its serum concentrations greatly exceeded those determined for 3’-OSO3−-PCB 3 and 2’-OSO3−-PCB 3, thereby indicating the 4’ position on the unsubstituted aryl ring as a primary target for oxidation and subsequent sulfation reactions. In addition, serum concentrations of 4’-OSO3−-PCB 3 were approximately 60-fold higher than those of its hydroxylated precursor, 4’-OH-PCB 3. Thus, this indicated that sulfation is a major metabolic pathway for this lower-chlorinated OH-PCB in the rat. The presence of sulfate metabolites derived from PCB 3 has also been confirmed in poplar plants (Zhai et al., 2013a).
PCB metabolite associated toxicities
Despite the fact that xenobiotic metabolism is primarily regarded as a detoxication process, there is increasing evidence that various classes of PCB metabolites exhibit their own toxicities, including carcinogenic, neurologic and endocrine effects (Brouwer et al., 1999, Knerr and Schrenk, 2006, Silberhorn et al., 1990, Tilson and Kodavanti, 1998). These findings raise important questions such as whether many of the toxic effects previously attributed to parent PCBs are in fact caused by their metabolites. In this section, we attempt to summarize the scientific evidence for the involvement of PCB metabolites in PCB-associated toxicities with an emphasis on carcinogenesis and thyroid disruption.
Reactive (epoxide and (semi)quinone) PCB intermediates
PCBs have recently been classified as “carcinogenic to humans” by the International Agency for Research on Cancer (IARC) (Lauby-Secretan et al., 2013). While this classification was based on positive correlation between PCB exposure and incidences of melanoma and limited evidence of an involvement in the development of breast cancer and non-Hodgkin lymphoma, exposure to PCBs has also been correlated with an increased incidence of other malignancies including hepatocellular carcinoma and lung cancer (Onozuka et al., 2009, Todaka et al., 2009). In addition, exposure to several Aroclor PCB mixtures resulted in the formation of thyroid neoplasms in male Sprague-Dawley rats, a finding that is in agreement with similar in vivo studies (Mayes et al., 1998, Vansell et al., 2004). Although for a long time PCBs were thought to be strictly promoting carcinogens, a lower chlorinated congener (PCB3) was shown to induced point mutations in rat livers in vivo (Lehmann et al., 2007), and, in agreement with this, recent evidence suggests that particularly the lower-chlorinated congeners can be oxidized to genotoxic metabolites, such as arene oxides and quinone species (Espandiari et al., 2003, Espandiari et al., 2004, Robertson and Ludewig, 2011). In fact, the quinone metabolites of PCB3 increase gene mutations in vitro at low micromolar concentrations (Zettner et al., 2007), induce strand breaks (Xie et al., 2010), bind to and inhibit the nuclear protein topoisomerase II (Bender et al., 2006, Bender and Osheroff, 2007, Srinivasan et al., 2001, Srinivasan et al., 2002) and reduce telomerase activity resulting in shortened chromosomal telomeres in cells in culture (Jacobus et al., 2008). Interestingly, only the para-dihydroxy metabolite of PCB3 induced polyploidization and only the ortho-dihydroxy metabolite caused sister chromatid exchanges (Flor and Ludewig, 2010) indicating a binding to other, so far unidentified cellular proteins, as mediators of these genotoxic effects. While adduction of PCB metabolites to DNA and DNA-related proteins may not be the primary target of PCB adduction, it may have a profound impact on the carcinogenicity observed with PCB exposure. The metabolic activation pathway of PCB 3 to its ultimate carcinogenic species was demonstrated in a rat model (Espandiari et al., 2004). In this study, which examined several PCB3 metabolites, a probable proximate carcinogen, the para-hydroxylated 4’-OHPCB 3 and an ultimate carcinogen, the 3,4-benzoquinone PCB 3, were identified as cancer initiating compounds. PCB associated DNA-adduction is described in more detail in the respective section of this review. In contrast to LC-PCBs, the tumor promoting activity of certain PCB mixtures has been partially attributed to their enzyme-inducing properties (Alvares et al., 1977, Knerr and Schrenk, 2006). The fact that the higher-chlorinated congeners are generally better inducers than LC-PCBs might explain the synergistic carcinogenicity of PCB mixtures as opposed to individual congeners that was observed in previous in vivo studies (Hansen, 1998, Sleight, 1985). Another mechanism by which hydroxylated, and potentially quinone metabolites of PCBs exert tumor promoting activity is through inhibition of gap junctional intercellular communication (Machala et al., 2004). For a more detailed discussion of the genotoxicity of PCBs, please see (Lehmann et al., 2007, Ludewig et al., 2008, Senthilkumar et al., 2011).
Besides their involvement in carcinogenesis, reactive PCB metabolites have been shown to induce oxidative stress, resulting in profound cytotoxic effects observed in vitro. For example 4-OH-PCB 11 was capable of inducing cytotoxic effects in immortalized human prostate epithelial cells (Zhu et al., 2013). The mechanism of cytotoxicity resulting in decreased cell viability was demonstrated to involve an OH-PCB dependent increase in the formation of reactive oxygen species, in particular intracellular superoxide and hydroperoxides.
Hydroxylated PCB metabolites
While PCBs in general are known endocrine disruptors that can target various endocrine systems, including the estrogen and thyroid systems, hydroxylated PCBs also appear to be toxicologically relevant key players in PCB mediated endocrine disruption. For example, certain OH-PCBs have been shown to interact with the estrogen receptor, acting either as receptor agonists or antagonists (Connor et al., 1997, DeCastro et al., 2006, Krishnan and Safe, 1993, Ma and Sassoon, 2006, Machala et al., 2004). Interestingly, several hydroxyl LC-PCBs had up to 100% higher efficacy than estradiol (Machala et al., 2004). Although they were much less potent than estradiol, they acted in an additive mode. A study investigating the estrogenic activity in human serum of men exposed to PCBs indicated a distinct and congener-dependent pattern, with higher-chlorinated congeners being strictly antiestrogenic and lower-chlorinated ones exerting estrogenic effects (Pliskova et al., 2005). This finding was in agreement with previous observations in vivo indicating that LC-PCBs were more estrogenic than higher-chlorinated congeners, which could be a result of their increased potential for metabolic conversion (Bitman and Cecil, 1970, Hansen, 1998). Also, exposure of ovarian cells to ng/ml amounts of PCB3 or its hydroxylated metabolites increased secretion of estradiol, an effect that was caused by an increase in aromatase activity and which was most pronounced with the dihydroxy PCB3 (Ptak et al., 2005, Ptak et al., 2006). In addition to exerting direct estrogenic and antiestrogenic effects through receptor binding or increased estrogen production, OH-PCBs have also been shown to induce estrogenicity indirectly through inhibition of the estrogen sulfotransferase (i.e., SULT1E1) (Kester et al., 2000, Kester et al., 2002). Inhibition of this SULT isoform slows down the rate of inactivation of estrogens by sulfation and thus results in increased levels of the active hormones.
OH-PCBs have also been identified as one class of PCB metabolites that act as disruptors of thyroid homeostasis (Meerts et al., 2002, Morse et al., 1996, Meerts et al., 2004) and a clear relationship between elevated OH-PCB levels and decreased thyroid hormone levels has been observed in animal (Dallaire et al., 2009, Otake et al., 2007, van den Berg et al., 1988, Morse et al., 1993) and human (Kato et al., 2004) studies. Serum hypothyroxinemia is among the most frequently reported adverse health effects in human populations exposed to PCBs (Kodavanti and Curras-Collazo, 2010, Patrick, 2009). Alongside observed direct thyroid effects in wildlife, these observations led to the classification of PCBs as thyroid-disrupting chemicals (TDCs) (Knerr and Schrenk, 2006, Mayes et al., 1998, Pearce and Braverman, 2009). Due to the role of thyroid hormones as stimulants and regulators for cellular proliferation and differentiation, thyroid disruption is particularly critical during human fetal development (Patrick, 2009, Giera et al., 2011). Serum concentrations of both the pro-hormone L-thyroxine (T4) and the active hormone triiodothyronine (T3) are extremely low (3.5–6.5 pM free T3, 0.9–2.8 nM total T3, 10–23 pM free T4, 58–161 nM total T4) and even small changes in these concentrations can result in developmental toxicities (Patrick, 2009). Neurodevelopmental effects of alterations in thyroid hormones have been observed in infants and laboratory animals as a result of subclinical maternal hypothyroidism during the first trimester of pregnancy, and include decreased cognitive and motor function, mental retardation, and low IQ scores (Vermiglio et al., 2004, Zoeller and Rovet, 2004). Epidemiological studies report visual recognition deficits, impaired executive functioning and speech problems as adverse neurodevelopmental effects in PCB-exposed populations (Jugan et al., 2010, Pearce and Braverman, 2009). In adults, subclinical hypothyroidism primarily affects lipid metabolism and is associated with increased serum lipid concentrations and a higher incidence of obesity (Asvold et al., 2007, Patrick, 2009). These effects may also contribute to the increased risk of cardiovascular effects such as atherosclerosis and myocardial infarction that was observed in PCB exposed populations (Razvi et al., 2008).
Interestingly, while most studies report negative correlations between PCB and thyroid hormone levels, fewer studies indicate positive correlations, particularly between serum PCB levels and free thyroid hormone concentrations (Bloom et al., 2009, Langer et al., 2008). In addition, a few studies report no correlation between elevated plasma PCB and thyroid hormone levels (Jugan et al., 2010). These findings indicate that thyroid effects may not just be related to the extent of the exposure but are in fact congener- or metabolite-dependent. In vivo animal models clearly indicate a negative correlation between PCB concentrations and T4 levels (Jugan et al., 2010, Meerts et al., 2002, Meerts et al., 2004). PCBs have been suggested to affect the thyroid homeostatic system at various stages in a congener-dependent manner and there is increasing evidence for distinct roles of various classes of PCB metabolites.
A suggested major contributing mechanism in environmental contaminant induced hypothyroxinemia is the displacement of T4 from its binding sites on the thyroid hormone transport protein transthyretin (TTR) (Kodavanti and Curras-Collazo, 2010). As opposed to parent PCBs, all OH-PCBs retained in human and wildlife blood seem to be competitors for T4 binding sites on TTR (Brouwer et al., 1998, Malmberg et al., 2004, Darnerud et al., 1996, Gutleb et al., 2010). In addition, the binding potencies of OH-PCBs have been shown to be up to an order of magnitude greater than the binding of T4 (Rickenbacher et al., 1986, Chauhan et al., 2000). Moreover, TTR has been suggested to facilitate the transport of bound ligands across the blood-brain barrier and the placenta and thus, binding to TTR may also play a role in the distribution of OH-PCBs to the placenta and the brain (Brouwer et al., 1998, Lans et al., 1993, Mortimer et al., 2012). In fact, exposure of pregnant mice to PCBs resulted in elevated serum and brain levels of OH-PCBs in the developing fetus (Meerts et al., 2002, Meerts et al., 2004, Morse et al., 1995, Morse et al., 1996). After birth, the OH-PCBs in brain were no longer detected, suggesting an increased susceptibility exists in this highly sensitive moment of brain development (Jacobson et al., 1990, Boucher et al., 2009, Darras, 2008).
Another potential mechanism by which OH-PCBs interfere with thyroid homeostasis is represented by the inhibition of SULT-catalyzed sulfation of thyroid hormones (Schuur et al., 1998a), since OH-PCBs are a well-known class of substrates and inhibitors of a variety of sulfotransferase enzymes (Liu et al., 2009, Ekuase et al., 2011, Kester et al., 2000, Kester et al., 2002, Wang et al., 2006).
OH-PCBs also possess neurotoxic potential (Pessah et al., 2006, Londono et al., 2010, Sharma and Kodavanti, 2002) in that they have been shown to interact more potently with the RyR receptors than parent compounds (Pessah et al., 2006) and they have been shown to affect the Ca2+ homeostasis in neuronal cells (Londono et al., 2010). More recently it was revealed that OH-PCB induced muscle dysfunction actually depends on their interactions with RyR receptors (Niknam et al., 2013).
PCB methyl sulfones
The selective tissue retention of MeSO2-PCBs in lung tissue has been hypothesized to be at least partially accountable for respiratory problems in Yusho patients (Kato et al., 1995). In liver, several MeSO2-PCBs have been found to induce microsomal drug metabolizing enzymes, and in some cases metabolites were even more potent than the respective parent compounds (Kato et al., 1995, Kato et al., 1999, Kato et al., 1997). In particular, certain MeSO2-PCBs were capable of inducing CYP2B1, CYP2B2, CYP3A2, and CYP2C6 (Kato et al., 1995). In addition, they were strong inhibitors of CYP11B1, an enzyme that is required in corticosterone biosynthesis and antagonists for the glucocorticoid receptor, which further indicated their potential as endocrine disruptors (Johansson et al., 1998). The MeSO2-PCBs may also induce UDP-glucuronosyltransferase (Kato et al., 2000), which in turn was hypothesized to affect the thyroid hormone homeostasis (Kato et al., 2000). Finally, MeSO2-PCBs are also capable of reducing thyroid hormone levels in laboratory animals (Kato et al., 1998, Kato et al., 1999, Kato et al., 2000). These effects, were observed both in mink dams and also in their offspring, where metabolites were transferred from mothers (Lund et al., 1999).
PCB sulfates
While OH-PCBs have long been known as a class of competitive ligands for TTR, we have recently demonstrated that PCB sulfates represent another group of high-affinity ligands for the T4-binding sites on the protein (Grimm et al., 2013). Interestingly, the sulfates bound with similar or higher affinity than their corresponding OH-PCBs, which for the first time indicated a potential toxicological significance of PCB sulfates in thyroid hormone disruption. This increased affinity as compared to OH-PCBs may be the result of the presence of the anionic sulfate group, which resembles the alanyl moiety on T4 and is assumed to facilitate hydrogen bonding interactions with lysine residues in the T4 binding site. Authentic PCB sulfate standards were not available until relatively recently (Li et al., 2010), and this may explain the lack of previous mechanistic studies on their biological and/or toxicological potential.
Toxicological relevance of PCB metabolites and research needs
So why should we care about the formation and physiologic fate of PCB metabolites, particularly those derived from the more readily metabolized LC-PCBs?
First, due to their environmental persistence and widespread occurrence, traditionally manufactured PCBs remain a public health hazard. Even though human exposure levels appear to be overall declining (Jugan et al., 2010), the measured PCB concentrations can vary greatly between exposed individuals, from almost undetectable to peak concentrations of more than 100 µg/g lipid weight (Langer et al., 2008). Assuming an average plasma lipid concentration of 7.35 g/l (El Majidi et al., 2014) and an average molecular weight of 300 g/mol for PCBs, these 31 measurements translate into plasma PCB concentrations from the picomolar to low micromolar range (2.45 µM). OHPCB and MeSO2-metabolite concentrations are frequently correlated to parent PCB levels and are typically one to two orders of magnitude below PCB concentrations, a finding that is reflective of the relative resistance toward bioconversion exhibited by mostly HC-PCBs (Letcher et al., 2000, Marek et al., 2013b).
Second, recent evidence for the omnipresence of lower-chlorinated and/or nonlegacy PCBs in our environment and the increasing evidence for their associated toxicities, clearly indicate their role as chemicals of interest from a public health perspective. A target group of particular concern are school children daily exposed to lower-chlorinated, airborne PCBs in older buildings built primarily in the 1950s and 1960s (Herrick, 2010, Herrick et al., 2004, MacIntosh et al., 2012). As opposed to HC-PCBs, these LC-PCBs are readily metabolized which may be the primary reason, why the parent congeners are typically present only at very low to undetectable concentrations (Hansen, 2001, Robertson and Ludewig, 2011). Low blood levels are often misinterpreted as an indication for low exposure and therefore low relevance when in fact they should be taken as an indication of continuous, steady-state, and therefore potentially significant overall exposure to compounds that have a high propensity to be bioactivated to potentially harmful metabolites. To date research has revealed four groups of potentially toxic metabolites of primarily these LC-PCBs: i: reactive metabolic intermediates from PCBs, particularly the quinones; ii: hydroxylated PCB metabolites, iii: PCB methyl sulfones and iv: PCB sulfates.
While there is substantial evidence for the formation and toxic potential of these metabolites derived from both in vitro and in vivo studies, the extent of their formation and exposure levels in human populations have not yet been explored. However, considering air PCB concentrations of up to of 5.5 ng/m3, for example in Chicago (Hu et al., 2010a, Persoon et al., 32 2010), and several hundred to thousand ng/m3 in indoor air of schools (Herrick et al., 2004, Liebl et al., 2004) and evidence for the presence of certain lower-chlorinated, airborne PCBs, such as PCB 11, in plasma of exposed populations (Marek et al., 2013b), quantitative assessment of LC-PCB and metabolite exposure levels is highly desirable.
It is not yet possible to assess human exposure to reactive metabolic intermediates of PCBs (arene oxides, semiquinones, quinones or their bound residues) even though the neutral, lipophilic MeSO2-PCB congeners may be taken as a measure of arene oxide formation. Moreover, it is relevant to assess human levels of OH-PCBs and their conjugated PCB metabolites, such as MeSO2-PCBs, PCB sulfates, and PCB glucuronides, since the parent PCB congeners are in many cases present only at trace concentrations or remain undetectable. The major OH-PCBs are present at concentrations in the same range as many of the PCB congeners and hence relevant for exposure assessments to PCBs. MeSO2- PCBs have strong tissue and cell specific retention leading to higher local (cellular) concentrations than general tissue levels. The physiological fates and biological activities of PCB sulfates are still relatively poorly understood and additional studies will be required to assess their significance in PCB metabolism and toxicities.
Research needs for reactive (epoxide and (semi)quinone) PCB intermediates
PCB adducts with DNA and proteins in humans may be impossible to identify. Risks of adduct formation to DNA and other biomacromolecules can therefore only fully be assessed indirectly if we know the complete PCB congener profile and concentration pattern of the individual exposure to PCBs. This requirement is primarily based on the need for concentration data for lower chlorinated biphenyls and other congeners that are rapidly metabolized and therefore disappearing in humans despite often continuous, ongoing exposure from the 33 environment (Grossman, 2013). Exposures to lower chlorinated biphenyls would be primarily through inhalation of contaminated air in cities, buildings or locations near PCB-contaminated waste sites (Herrick, 2010, Hu and Hornbuckle, 2010, Hu et al., 2010a, MacIntosh et al., 2012, Marek et al., 2013b). The majority of exposures to PCBs in the general population, however, is thought to arise through dietary sources (Domingo and Bocio, 2007). Food as source for LC-PCBs is often overlooked, but may be high for certain food items such as plant oils, possibly due to airborne exposure onto crops or from contaminated sewage sludge used as fertilizer (Ludewig et al., 2007). However, since most HC-PCBs biomagnify in the food chain and they are therefore the major contributors to dietary PCB exposure. HC-PCBs also bioaccumulate in humans resulting in the observed age-dependent increase in body burden over a lifetime. For these congeners it would seem possible to model the reactive intermediate concentrations based on internal concentrations of PCB congeners. The exposure to LC-PCBs with short half-lives may have a small impact on the total PCB body burden at any given time, but may well be the major source of PCBs that form reactive intermediates (Robertson and Gupta, 2000, Robertson and Ludewig, 2011). The formation of reactive intermediates, arene oxides, semi-quinones, and quinones, is therefore related to PCB concentration patterns in individuals. Other important questions relate to organand species-specificity of activation pathways and therefore likelihood of adduct formation. As described above, the chlorination pattern of a PCB congener determines the substrate specificity for CYP forms which in turn my influence organ and cell-specific formation of oxidized metabolites. In addition, cells with specific oxidizing enzymes, like myeloperoxidase in bone marrow cells, may be most likely to produce reactive quinone metabolites and therefore more likely to form DNA and protein adduct (Xie et al., 2010). Understanding of these specific enzyme requirements may elucidate the mechanism of the observed organ-specific carcinogenicity of 34 PCBs. Finally, an understanding of the activation pathway and mechanisms of toxicity will enable the development of chemoprotective regiments. Potential toxicities of the reactive intermediates are thus 1. directly related to the internal PCB congener concentrations including those from short-lived lower chlorinated congeners that can only be estimated by assessing the external exposures, 2. affected by species, organ and cell-specific bioactivation capacities, and 3. determined by the cellular consequences of the adduction to a specific macromolecule. Data for all three parameters are currently incomplete, thereby preventing true data-driven risk assessment.
Research needs for OH-PCBs
Human populations can be exposed to OH-PCBs by two different sources. While metabolic conversion of PCBs was for a long time considered the only source of OH-PCBs, it has also been demonstrated that OH-PCBs were originally present in Aroclors and they are still present in detectable amounts in the environment (Bergman et al., 1994b, Marek et al., 2013a, Matthews and Kato, 1979). Moreover, little or no data exist about OH-PCBs in our foodstuffs. OH-PCBs are either excreted as such or as their conjugates, or they may be further metabolized to dihydroxylated PCBs. However, some of the OH-PCBs are strongly retained in blood as protein bound phenolic metabolites with reasonably long half-lives (Bergman et al., 1994b, Malmberg et al., 2004, Oberg et al., 2002). Considering that OH-PCBs formed from internal PCB congeners lead to a continuous exposure to these metabolites, it should be possible to model some OH-PCB concentrations based on known PCB levels in a healthy normal human. However, since OH-PCB congeners can be present in high concentrations (10% – 30% of the PCB level) which are often higher than many of the individual PCB congeners (Marek et al., 2013b, Hisada et al., 2013, Nomiyama et al., 2010), it is relevant to determine the concentrations of these metabolites and 35 potential sources separately. The goal is primarily to promote dose-response linkages between effects of the OH-PCBs and their concentrations, a research field that needs further development. Assessing PCB induced adverse health effects for many toxic endpoints can be improved by including data on human concentrations and effects of OH-PCBs.
Research needs for PCB methyl sulfones
In contrast to the retained OH-PCB metabolites, MeSO2-PCBs are neutral metabolites that are present at much lower concentrations in humans than OH-PCBs (<4% of the dominant OH-PCB congener) (Bergman et al., 1979, Letcher et al., 2000). MeSO2-PCBs have specific and strong tissue or even cell specificity, particularly in the liver and lung (Bergman et al., 1979, Larsson and Bergman, 1998, Letcher et al., 2000). Whether or not this translates into organ and cell specific toxic effects has not been fully investigated. Another difference between methylsulfone and OH PCBs is that the MeSO2-PCBs are the final product of the most rapidly metabolized PCB congeners which consequently are present in humans only at trace or even non-detectable concentrations. Since these metabolites derive from exposure to some of the most rapidly metabolized PCB congeners, they may serve as markers for exposure to these congeners and of the non-quantifyable arene oxide and quinone metabolites of these PCBs.
Research needs for PCB sulfates
Although the toxicological relevance of PCB sulfation is not yet known, a variety of in vitro and in vivo studies suggest that the formation of PCB sulfates is a potentially significant metabolic pathway for LC-PCBs in humans (Dhakal et al., 2012, Dhakal et al., 2014, Ekuase et al., 2011, Grimm et al., 2013, Liu et al., 2006, Liu et al., 2011, Liu et al., 2009, Zhai et al., 2013a). The 36 fact that, in the past, human serum has been almost exclusively analyzed for parent PCBs and only recently for their hydroxylated metabolites indicates that overall PCB exposure levels in exposed populations may have been underestimated, at least with respect to the LC-PCBs (Hovander et al., 2000, Marek et al., 2013b, Dirtu and Covaci, 2010). Thus development of sulfate metabolites in urine or blood as biomarker of exposure to LC-PCBs is a promising avenue to obtain realistic exposure data for humans in their natural environments.
Summary of PCB metabolism and associated toxicities.
Although many biologic effects of parent PCBs are receptor-mediated, including the well-described characteristics of PCBs as inducers of xenobiotic metabolism, other PCB toxication processes involve the metabolism of PCBs themselves or their metabolic progeny.
PCBs are in general metabolized via initial oxidation to arene oxides, but may also undergo direct insertion of hydroxyl groups. These reactions, catalyzed by CYPs, are regio- and stereo-selective. Retention of PCB atropisomers in tissues, for example, may be highly enantioselective.
Reactive electrophilic PCB metabolites, arene oxides, semi-quinones and quinones, may form adducts to biomacromolecules, i.e. proteins, DNA, RNA and lipids.
The major stable PCB metabolites are polychlorobiphenylols (OH-PCBs) that are, depending on their structure, either rapidly metabolized, excreted or retained in certain compartments in the body, primarily the blood.
Major OH-PCB congeners derived from HC-PCBs that are found in blood are present in concentrations similar to the most persistent individual PCB congeners. They are also more 37 easily than the parent compounds transferred via the placenta to the fetus.
PCB congeners with non-chlorinated meta-/para-positions and chlorinated neighboring ortho-/meta-positions and a slowly reacting second phenyl ring are rapidly metabolized; these PCB congeners form OH-PCBs and MeSO2-PCBs.
Several MeSO2-PCBs are accumulated in a highly tissue-specific manner, especially in liver and lung.
Recent evidence supports sulfation as a major metabolic pathway for LC-PCBs in vitro and in vivo, and provides initial evidence for biological activity of the resulting sulfate ester metabolites.
Conjugated PCB metabolites such as PCB sulfates and glucuronides may have been overlooked classes of PCB metabolites in the past and total PCB exposure levels, particularly to LC-PCBs in exposed populations, may have been underestimated. Novel procedures to reliably quantify PCB sulfates and potentially other conjugated metabolites, such as glucuronic acid derivatives, in human serum could fill this significant gap in the literature and could help to more accurately estimate human exposure levels.
Acknowledgements
This review was in part the work product of the European Food Safety Authority (EFSA) working group on non-dioxin like PCBs, 2005. Where the authors own work was mentioned, those studies were supported by grants from the NIH (ES07380 and ES013661), DOD, EPA and the EU R&D projects PCBRISK, COMPARE, ANEMONE and RENCO. LWR would also like to recognize the Alexander von Humboldt Foundation for financial support.
Contributor Information
FA Grimm, Interdisciplinary Graduate Program in Human Toxicology, Department of Pharmaceutical Sciences & Experimental Therapeutics, University of Iowa.
D Hu, Department of Civil and Environmental Engineering, University of Iowa.
I Kania-Korwel, Department of Occupational & Environmental Health, University of Iowa.
HJ Lehmler, Interdisciplinary Graduate Program in Human Toxicology, Department of Occupational & Environmental Health, University of Iowa.
G Ludewig, Interdisciplinary Graduate Program in Human Toxicology, Department of Occupational & Environmental Health, University of Iowa.
KC Hornbuckle, Interdisciplinary Graduate Program in Human Toxicology, Department of Civil and Environmental Engineering, University of Iowa.
MW Duffel, Interdisciplinary Graduate Program in Human Toxicology, Department of Pharmaceutical Sciences & Experimental Therapeutics, University of Iowa.
A Bergman, Swedish Toxicology Sciences Research Center (SWETOX), Forskargatan 20, SE-151 36 Södertälje, SWEDEN.
LW Robertson, Interdisciplinary Graduate Program in Human Toxicology, Department of Occupational & Environmental Health, University of Iowa.
References
- Alvares AP, Fischbein A, Anderson KE, Kappas A. Alterations in drug metabolism in workers exposed to polychlorinated biphenyls. Clin Pharmacol Ther. 1977;22:140–146. doi: 10.1002/cpt1977222140. [DOI] [PubMed] [Google Scholar]
- Amaro AR, Oakley GG, Bauer U, Spielmann HP, Robertson LW. Metabolic activation of PCBs to quinones: reactivity toward nitrogen and sulfur nucleophiles and influence of superoxide dismutase. Chem Res Toxicol. 1996;9:623–629. doi: 10.1021/tx950117e. [DOI] [PubMed] [Google Scholar]
- Asvold BO, Vatten LJ, Nilsen TI, Bjoro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. The HUNT Study. Eur J Endocrinol. 2007;156:181–186. doi: 10.1530/eje.1.02333. [DOI] [PubMed] [Google Scholar]
- ATSDR . Toxicological profile for polychlorinated biphenyls (PCBs) U.S. Dept. Health Services, Public Health Service; 2000. [PubMed] [Google Scholar]
- Bakke JE, Bergman AL, Larsen GL. Metabolism of 2,4',5-trichlorobiphenyl by the mercapturic acid pathway. Science. 1982;217:645–647. doi: 10.1126/science.6806905. [DOI] [PubMed] [Google Scholar]
- Bakke JE, Feil VJ, Bergman A. Metabolites of 2,4',5-trichlorobiphenyl in rats. Xenobiotica. 1983;13:555–564. doi: 10.3109/00498258309052295. [DOI] [PubMed] [Google Scholar]
- Ballschmiter K, Mennel A, Buyten J. Long chain alkyl-polysiloxanes as non-polar stationary phases in capillary gas chromatography. Fresenius' Journal of Analytical Chemistry. 1993;346:396–402. [Google Scholar]
- Ballschmiter K, Zell M. Analysis of polychlorinated-biphenyls (PCB) by glass-capillary gas-chromatography - composition of technical Aroclor-PCB and Clophen-PCB mixtures. Fresenius J Anal Chem. 1980;302:20–31. [Google Scholar]
- Barron MG, Yurk JJ, Crothers DB. Assessment of Potential Cancer Risk from Consumption of PCBs Bioaccumulated in Fish and Shellfish. Environ Health Perspect. 1994;102:562–567. doi: 10.1289/ehp.94102562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu I, Arnold KA, Venier M, Hites RA. Partial pressures of PCB-11 in air from several Great Lakes sites. Environmental Science & Technology. 2009;43:6488–6492. doi: 10.1021/es900919d. [DOI] [PubMed] [Google Scholar]
- Bender RP, Lehmler HJ, Robertson LW, Ludewig G, Osheroff N. Polychlorinated biphenyl quinone metabolites poison human topoisomerase IIalpha: altering enzyme function by blocking the N-terminal protein gate. Biochemistry. 2006;45:10140–10152. doi: 10.1021/bi0524666. [DOI] [PubMed] [Google Scholar]
- Bender RP, Osheroff N. Mutation of cysteine residue 455 to alanine in human topoisomerase IIalpha confers hypersensitivity to quinones: enhancing DNA scission by closing the N-terminal protein gate. Chem Res Toxicol. 2007;20:975–981. doi: 10.1021/tx700062t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman A, Brandt I, Jansson B. Accumulation of methylsulfonyl derivatives of some bronchial-seeking polychlorinated biphenyls in the respiratory tract of mice. Toxicol Appl Pharmacol. 1979;48:213–220. doi: 10.1016/0041-008x(79)90026-7. [DOI] [PubMed] [Google Scholar]
- Bergman A, Klasson-Wehler E, Kuroki H. Selective retention of hydroxylated PCB metabolites in blood. Environ Health Perspect. 1994a;102:464–469. doi: 10.1289/ehp.94102464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman A, Norstrom RJ, Haraguchi K, Kuroki H, Beland P. PCB and DDE methyl sulfones in mammals from Canada and Sweden. Environmental Toxicology and Chemistry. 1994b;13:121–128. [Google Scholar]
- Birnbaum LS. Distribution and excretion of 2,3,6,2',3',6'- and 2,4,5,2',4',5'-hexachlorobiphenyl in senescent rats. Toxicol Appl Pharmacol. 1983;70:262–272. doi: 10.1016/0041-008x(83)90102-3. [DOI] [PubMed] [Google Scholar]
- Bitman J, Cecil HC. Estrogenic activity of DDT analogs and polychlorinated biphenyls. J Agric Food Chem. 1970;18:1108–1112. doi: 10.1021/jf60172a019. [DOI] [PubMed] [Google Scholar]
- Bloom MS, Vena JE, Olson JR, Kostyniak PJ. Assessment of polychlorinated biphenyl congeners, thyroid stimulating hormone, and free thyroxine among New York state anglers. Int J Hyg Environ Health. 2009;212:599–611. doi: 10.1016/j.ijheh.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Boucher O, Muckle G, Bastien CH. Prenatal exposure to polychlorinated biphenyls: a neuropsychologic analysis. Environ Health Perspect. 2009;117:7–16. doi: 10.1289/ehp.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury S, Mekenyan O, Ankley G. Quantitative structure-activity relationships for polychlorinated hydroxybiphenyl estrogen receptor binding affinity—an assessment of conformer flexibility. Environ Toxicol Chem. 1996;15:1945–1954. [Google Scholar]
- Breivik K, Sweetman A, Pacyna JM, Jones KC. Towards a global historical emission inventory for selected PCB congeners--a mass balance approach 3. An update. Sci Total Environ. 2007;377:296–307. doi: 10.1016/j.scitotenv.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Brouwer A, Longnecker MP, Birnbaum LS, Cogliano J, Kostyniak P, Moore J, Schantz S, Winneke G. Characterization of potential endocrine-related health effects at low-dose levels of exposure to PCBs. Environ Health Perspect. 1999;107(Suppl 4):639–649. doi: 10.1289/ehp.99107s4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer A, Morse DC, Lans MC, Schuur AG, Murk AJ, Klasson-Wehler E, Bergman A, Visser TJ. Interactions of persistent environmental organohalogens with the thyroid hormone system: mechanisms and possible consequences for animal and human health. Toxicol Ind Health. 1998;14:59–84. doi: 10.1177/074823379801400107. [DOI] [PubMed] [Google Scholar]
- Chauhan KR, Kodavanti PR, Mckinney JD. Assessing the role of ortho-substitution on polychlorinated biphenyl binding to transthyretin, a thyroxine transport protein. Toxicol Appl Pharmacol. 2000;162:10–21. doi: 10.1006/taap.1999.8826. [DOI] [PubMed] [Google Scholar]
- Choi SD, Baek SY, Chang YS, Wania F, Ikonomou MG, Yoon YJ, Park BK, Hong S. Passive air sampling of polychlorinated biphenyls and organochlorine pesticides at the Korean Arctic and Antarctic research stations: implications for long-range transport and local pollution. Environmental Science & Technology. 2008;42:7125–7131. doi: 10.1021/es801004p. [DOI] [PubMed] [Google Scholar]
- Christensen H, Heggberget TM, Gutleb AC. Polychlorinated biphenyls and reproductive performance in otters from the Norwegian coast. Arch Environ Contam Toxicol. 2010;59:652–660. doi: 10.1007/s00244-010-9510-9. [DOI] [PubMed] [Google Scholar]
- Chu S, Covaci A, Haraguchi K, Voorspoels S, van de Vijver K, Das K, Bouquegneau JM, De Coen W, Blust R, Schepens P. Levels and enantiomeric signatures of methyl sulfonyl PCB and DDE metabolites in livers of harbor porpoises (Phocoena phocoena) from the Southern North Sea. Environ Sci Technol. 2003a;37:4573–4578. doi: 10.1021/es034241t. [DOI] [PubMed] [Google Scholar]
- Chu S, Covaci A, Jacobs W, Haraguchi K, Schepens P. Distribution of methyl sulfone metabolites of polychlorinated biphenyls and p,p'-DDE in human tissues. Environ Health Perspect. 2003b;111:1222–1227. doi: 10.1289/ehp.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor K, Ramamoorthy K, Moore M, Mustain M, Chen I, Safe S, Zacharewski T, Gillesby B, Joyeux A, Balaguer P. Hydroxylated polychlorinated biphenyls (PCBs) as estrogens and antiestrogens: structure-activity relationships. Toxicol Appl Pharmacol. 1997;145:111–123. doi: 10.1006/taap.1997.8169. [DOI] [PubMed] [Google Scholar]
- Cuadra SN, Linderholm L, Athanasiadou M, Jakobsson K. Persistent organic pollutants in children working in a waste disposal site and in female high fish consumers in Managua, Nicaragua. Ambio. 2006;35:109–116. doi: 10.1579/0044-7447(2006)35[109:popicw]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Custer TW, Custer CM, Gray BR. Polychlorinated biphenyls, dioxins, furans, and organochlorine pesticides in belted kingfisher eggs from the upper Hudson River basin, New York, USA. Environ Toxicol Chem. 2010;29:99–110. doi: 10.1002/etc.26. [DOI] [PubMed] [Google Scholar]
- Daidoji T, Gozu K, Iwano H, Inoue H, Yokota H. UDP-glucuronosyltransferase isoforms catalyzing glucuronidation of hydroxy-polychlorinated biphenyls in rat. Drug Metab Dispos. 2005;33:1466–1476. doi: 10.1124/dmd.105.004416. [DOI] [PubMed] [Google Scholar]
- Dallaire R, Muckle G, Dewailly E, Jacobson SW, Jacobson JL, Sandanger TM, Sandau CD, Ayotte P. Thyroid hormone levels of pregnant inuit women and their infants exposed to environmental contaminants. Environ Health Perspect. 2009;117:1014–1020. doi: 10.1289/ehp.0800219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnerud PO, Morse D, Klasson-Wehler E, Brouwer A. Binding of a 3,3',4,4'-tetrachlorobiphenyl (CB-77) metabolite to fetal transthyretin and effects on fetal thyroid hormone levels in mice. Toxicology. 1996;106:105–114. doi: 10.1016/0300-483x(95)03169-g. [DOI] [PubMed] [Google Scholar]
- Darras VM. Endocrine disrupting polyhalogenated organic pollutants interfere with thyroid hormone signalling in the developing brain. Cerebellum. 2008;7:26–37. doi: 10.1007/s12311-008-0004-5. [DOI] [PubMed] [Google Scholar]
- Daubeze M, Narbonne JF. Incorporation of labeled 2,4,5,2',4',5' - hexachlorobiphenyl into the nuclear fraction of rat hepatocytes in vivo. Toxicology. 1984;31:315–318. doi: 10.1016/0300-483x(84)90112-4. [DOI] [PubMed] [Google Scholar]
- Decastro BR, Korrick SA, Spengler JD, Soto AM. Estrogenic activity of polychlorinated biphenyls present in human tissue and the environment. Environ Sci Technol. 2006;40:2819–2825. doi: 10.1021/es051667u. [DOI] [PubMed] [Google Scholar]
- Dhakal K, Adamcakova-Dodd A, Lehmler HJ, Thorne PS, Robertson LW. Sulfate conjugates are urinary markers of inhalation exposure to 4-chlorobiphenyl (PCB3) Chem Res Toxicol. 2013;26:853–855. doi: 10.1021/tx4001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakal K, He X, Lehmler HJ, Teesch LM, Duffel MW, Robertson LW. Identification of sulfated metabolites of 4-chlorobiphenyl (PCB3) in the serum and urine of male rats. Chem Res Toxicol. 2012;25:2796–2804. doi: 10.1021/tx300416v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakal K, Uwimana E, Adamcakova-Dodd A, Thorne PS, Lehmler HJ, Robertson LW. Disposition of Phenolic and Sulfated Metabolites after Inhalation Exposure to 4-Chlorobiphenyl (PCB3) in Female Rats. Chem Res Toxicol. 2014 doi: 10.1021/tx500150h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirtu AC, Covaci A. Estimation of daily intake of organohalogenated contaminants from food consumption and indoor dust ingestion in Romania. Environ Sci Technol. 2010;44:6297–6304. doi: 10.1021/es101233z. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Bocio A. Levels of PCDD/PCDFs and PCBs in edible marine species and human intake: a literature review. Environ Int. 2007;33:397–405. doi: 10.1016/j.envint.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Du S, Wall SI, Cacia D, Rodenburg LA. Passive air sampling for polychlorinated biphenyls in the Philadelphia metropolitan area. Environ Sci Technol. 2009;43:1287–1292. doi: 10.1021/es802957y. [DOI] [PubMed] [Google Scholar]
- Ekuase EJ, Liu Y, Lehmler HJ, Robertson LW, Duffel MW. Structure-activity relationships for hydroxylated polychlorinated biphenyls as inhibitors of the sulfation of dehydroepiandrosterone catalyzed by human hydroxysteroid sulfotransferase SULT2A1. Chem Res Toxicol. 2011;24:1720–1728. doi: 10.1021/tx200260h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Majidi N, Bouchard M, Carrier G. Systematic analysis of the relationship between standardized biological levels of polychlorinated biphenyls and thyroid function in pregnant women and newborns. Chemosphere. 2014;98:1–17. doi: 10.1016/j.chemosphere.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Ellerichmann T, Bergmann A, Franke S, Huehnerfuss H, Jakobsson E, Koenig WA, Larsson C. Gas chromatographic enantiomer separations of chiral PCB methyl sulfons and identification of selectively retained enantiomers in human liver. Fresenius Environmental Bulletin. 1998;7:244–257. [Google Scholar]
- Erickson MD. Analytical Chemistry of PCBs. Boca Raton: CRC Lewis Publishers; 1997. [Google Scholar]
- Erickson MD. Introduction: PCB properties, uses, occurrence, and regulatory history. In: Robertson LW, Hansen LG, editors. PCBs: recent advances in environmental toxicology and health effects. Lexington, Kentucky: The University Press of Kentucky; 2001. [Google Scholar]
- Erickson MD, Kaley RG., 2nd Applications of polychlorinated biphenyls. Environ Sci Pollut Res Int. 2011;18:135–151. doi: 10.1007/s11356-010-0392-1. [DOI] [PubMed] [Google Scholar]
- Espandiari P, Glauert HP, Lehmler HJ, Lee EY, Srinivasan C, Robertson LW. Polychlorinated biphenyls as initiators in liver carcinogenesis: resistant hepatocyte model. Toxicol Appl Pharmacol. 2003;186:55–62. doi: 10.1016/s0041-008x(02)00018-2. [DOI] [PubMed] [Google Scholar]
- Espandiari P, Glauert HP, Lehmler HJ, Lee EY, Srinivasan C, Robertson LW. Initiating activity of 4-chlorobiphenyl metabolites in the resistant hepatocyte model. Toxicol Sci. 2004;79:41–46. doi: 10.1093/toxsci/kfh097. [DOI] [PubMed] [Google Scholar]
- Fängström B, Athanasiadou M, Athanassiadis I, Weihe P, Bergman A. Hydroxylated PCB metabolites in nonhatched fulmar eggs from the Faroe Islands. Ambio. 2005a;34:184–187. [PubMed] [Google Scholar]
- Fangstrom B, Athanasiadou M, Grandjean P, Weihe P, Bergman A. Hydroxylated PCB metabolites and PCBs in serum from pregnant Faroese women. Environ Health Perspect. 2002;110:895–899. doi: 10.1289/ehp.110-1240989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fängström B, Hovander L, Bignert A, Athanassiadis I, Linderholm L, Grandjean P, Weihe P, Bergman A. Concentrations of PBDE, PCB and OH-PCBs in serum from pregnant Faroese women and their children seven years later. Environmental Science & Technology. 2005b;39:9457–9463. doi: 10.1021/es0513032. [DOI] [PubMed] [Google Scholar]
- Fangstrom B, Strid A, Grandjean P, Weihe P, Bergman A. A retrospective study of PBDEs and PCBs in human milk from the Faroe Islands. Environ Health. 2005;4:12. doi: 10.1186/1476-069X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg M, Soler L, Contenot S, Verger P. Assessment of seasonality in exposure to dioxins, furans and dioxin-like PCBs by using long-term food-consumption data. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28:502–512. doi: 10.1080/19440049.2011.553844. [DOI] [PubMed] [Google Scholar]
- Fitzgerald EF, Belanger EE, Gomez MI, Cayo M, Mccaffrey RJ, Seegal RF, Jansing RL, Hwang SA, Hicks HE. Polychlorinated biphenyl exposure and neuropsychological status among older residents of upper Hudson River communities. Environ Health Perspect. 2008;116:209–215. doi: 10.1289/ehp.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EF, Shrestha S, Palmer PM, Wilson LR, Belanger EE, Gomez MI, Cayo MR, Hwang SA. Polychlorinated biphenyls (PCBs) in indoor air and in serum among older residents of upper Hudson River communities. Chemosphere. 2011;85:225–231. doi: 10.1016/j.chemosphere.2011.06.027. [DOI] [PubMed] [Google Scholar]
- Flor S, Ludewig G. Polyploidy-induction by dihydroxylated monochlorobiphenyls: structure-activity-relationships. Environ Int. 2010;36:962–969. doi: 10.1016/j.envint.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrio T, Piechotowski I, Wallenhorst T, Klett M, Cott L, Friebel P, Link B, Schwenk M. PCB-blood levels in teachers, working in PCB-contaminated schools. Chemosphere. 2000;40:1055–1062. doi: 10.1016/s0045-6535(99)00353-7. [DOI] [PubMed] [Google Scholar]
- Gallenberg LA, Ring BJ, Vodicnik MJ. The influence of time of maternal exposure to 2,4,5,2',4',5'-hexachlorobiphenyl on its accumulation in their nursing offspring. Toxicol Appl Pharmacol. 1990;104:1–8. doi: 10.1016/0041-008x(90)90276-z. [DOI] [PubMed] [Google Scholar]
- Giera S, Bansal R, Ortiz-Toro TM, Taub DG, Zoeller RT. Individual polychlorinated biphenyl (PCB) congeners produce tissue- and gene-specific effects on thyroid hormone signaling during development. Endocrinology. 2011;152:2909–2919. doi: 10.1210/en.2010-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharov A, Bloom M, Pavuk M, Birman I, Carpenter DO. Blood pressure and hypertension in relation to levels of serum polychlorinated biphenyls in residents of Anniston, Alabama. J Hypertens. 2010;28:2053–2060. doi: 10.1097/HJH.0b013e32833c5f3e. [DOI] [PubMed] [Google Scholar]
- Goncharov A, Pavuk M, Foushee HR, Carpenter DO. Blood pressure in relation to concentrations of PCB congeners and chlorinated pesticides. Environ Health Perspect. 2011;119:319–325. doi: 10.1289/ehp.1002830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm FA, Lehmler HJ, He X, Robertson LW, Duffel MW. Sulfated metabolites of polychlorinated biphenyls are high-affinity ligands for the thyroid hormone transport protein transthyretin. Environ Health Perspect. 2013;121:657–662. doi: 10.1289/ehp.1206198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman E. Nonlegacy PCBs: pigment manufacturing by-products get a second look. Environ Health Perspect. 2013;121:A86–A93. doi: 10.1289/ehp.121-a86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem Res Toxicol. 2001;14:611–650. doi: 10.1021/tx0002583. [DOI] [PubMed] [Google Scholar]
- Guroff G, Daly JW, Jerina DM, Renson J, Witkop B, Udenfriend S. Hydroxylation-induced migration: the NIH shift. Recent experiments reveal an unexpected and general result of enzymatic hydroxylation of aromatic compounds. Science. 1967;157:1524–1530. doi: 10.1126/science.157.3796.1524. [DOI] [PubMed] [Google Scholar]
- Gutleb AC, Cenijn P, Velzen M, Lie E, Ropstad E, Skaare JU, Malmberg T, Bergman A, Gabrielsen GW, Legler J. In vitro assay shows that PCB metabolites completely saturate thyroid hormone transport capacity in blood of wild polar bears (Ursus maritimus) Environ Sci Technol. 2010;44:3149–3154. doi: 10.1021/es903029j. [DOI] [PubMed] [Google Scholar]
- Guvenius DM, Aronsson A, Ekman-Ordeberg G, Bergman A, Noren K. Human prenatal and postnatal exposure to polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenylols, and pentachlorophenol. Environ Health Perspect. 2003;111:1235–1241. doi: 10.1289/ehp.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guvenius DM, Hassanzadeh P, Bergman A, Noren K. Metabolites of polychlorinated biphenyls in human liver and adipose tissue. Environ Toxicol Chem. 2002;21:2264–2269. [PubMed] [Google Scholar]
- Haglund P. Isolation and characterization of polychlorinated biphenyl (PCB) atropisomers. Chemosphere. 1996;32:8. [Google Scholar]
- Hagmar L, Rylander L, Dyremark E, Klasson-Wehler E, Erfurth EM. Plasma concentrations of persistent organochlorines in relation to thyrotropin and thyroid hormone levels in women. Int Arch Occup Environ Health. 2001;74:184–188. doi: 10.1007/s004200000213. [DOI] [PubMed] [Google Scholar]
- Hansen LG. Stepping backward to improve assessment of PCB congener toxicities. Environ Health Perspect. 1998;106(Suppl 1):171–189. doi: 10.1289/ehp.98106s1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LG. The ortho side of PCBs: Occurrence and disposition. Boston: Kluwer Academic Publishers Boston; 1999. [Google Scholar]
- Hansen LG. Identification of steady state and episodic PCB congeners from mulitple pathway exposures. In: Robertson LW, Hansen LG, editors. PCBs: Recent Advances in Environmental Toxicology and Health Effects. Lexington, Kentucky, US: The University Press of Kentucky; 2001. [Google Scholar]
- Harrad S, Goosey E, Desborough J, Abdallah MA, Roosens L, Covaci A. Dust from U.K. primary school classrooms and daycare centers: the significance of dust as a pathway of exposure of young U.K. children to brominated flame retardants and polychlorinated biphenyls. Environ Sci Technol. 2010;44:4198–4202. doi: 10.1021/es100750s. [DOI] [PubMed] [Google Scholar]
- Harrad S, Ibarra C, Robson M, Melymuk L, Zhang X, Diamond M, Douwes J. Polychlorinated biphenyls in domestic dust from Canada, New Zealand, United Kingdom and United States: implications for human exposure. Chemosphere. 2009;76:232–238. doi: 10.1016/j.chemosphere.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Herrick RF. PCBs in school-persistent chemicals, persistent problems. New Solut. 2010;20:115–126. doi: 10.2190/NS.20.1.h. [DOI] [PubMed] [Google Scholar]
- Herrick RF, Mcclean MD, Meeker JD, Baxter LK, Weymouth GA. An unrecognized source of PCB contamination in schools and other buildings. Environ Health Perspect. 2004;112:1051–1053. doi: 10.1289/ehp.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisada A, Shimodaira K, Okai T, Watanabe K, Takemori H, Takasuga T, Noda Y, Shirakawa M, Kato N, Yoshinaga J. Serum levels of hydroxylated PCBs, PCBs and thyroid hormone measures of Japanese pregnant women. Environ Health Prev Med. 2013;18:205–214. doi: 10.1007/s12199-012-0306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofvander L. Ph.D. Thesis. Department of Environmental Chemistry, University of Stockholm; 2006. Polychlorinated biphenyls and their metabolites in human blood: Method development, identification and quantification. [Google Scholar]
- Hovander L, Athanasiadou M, Asplund L, Jensen S, Wehler EK. Extraction and cleanup methods for analysis of phenolic and neutral organohalogens in plasma. J Anal Toxicol. 2000;24:696–703. doi: 10.1093/jat/24.8.696. [DOI] [PubMed] [Google Scholar]
- Hovander L, Linderholm L, Athanasiadou M, Athanassiadis I, Bignert A, Fängström B, Kocan A, Petrik J, Trnovec T, Bergman A. Levels of PCBs and their metabolites in the serum of residents of a highly contaminated area in eastern Slovakia. Environmental Science & Technology. 2006;40:3696–3703. doi: 10.1021/es0525657. [DOI] [PubMed] [Google Scholar]
- Hovander L, Linderholm L, Athanasiadou M, Athanassiadis I, Trnovec T, Kocan A, Petrik J, Bergman A. Analysis of PCB and PCB metabolites in humans from eastern Slovakia. Organohalogen Compounds. 2004;66:3525–3531. [Google Scholar]
- Hrycay EG, Bandiera SM. Spectral interactions of tetrachlorobiphenyls with hepatic microsomal cytochrome P450 enzymes. Chem. Biol. Interact. 2003;146:285–296. doi: 10.1016/j.cbi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hu D, Hornbuckle KC. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ Sci Technol. 2010;44:2822–2827. doi: 10.1021/es902413k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Lehmler HJ, Martinez A, Wang K, Hornbuckle KC. Atmospheric PCB congeners across Chicago. Atmos Environ (1994) 2010a;44:1550–1557. doi: 10.1016/j.atmosenv.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Martinez A, Hornbuckle KC. Discovery of non-aroclor PCB (3,3'-dichlorobiphenyl) in Chicago air. Environ Sci Technol. 2008;42:7873–7877. doi: 10.1021/es801823r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Martinez A, Hornbuckle KC. Sedimentary Records of Non-Aroclor and Aroclor PCB mixtures in the Great Lakes. Journal of Great Lakes Research. 2011;37:359–364. doi: 10.1016/j.jglr.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Adamcakova-Dodd A, Lehmler HJ, Hu D, Hornbuckle K, Thorne PS. Subchronic inhalation exposure study of an airborne polychlorinated biphenyl mixture resembling the Chicago ambient air congener profile. Environ Sci Technol. 2012;46:9653–9662. doi: 10.1021/es301129h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Adamcakova-Dodd A, Lehmler HJ, Hu D, Kania-Korwel I, Hornbuckle KC, Thorne PS. Time course of congener uptake and elimination in rats after short-term inhalation exposure to an airborne polychlorinated biphenyl (PCB) mixture. Environ Sci Technol. 2010b;44:6893–6900. doi: 10.1021/es101274b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Adamcakova-Dodd A, Thorne PS. The fate of inhaled (14)C-labeled PCB11 and its metabolites in vivo. Environ Int. 2014;63:92–100. doi: 10.1016/j.envint.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Lehmler HJ, Adamcakova-Dodd A, Thorne PS. Elimination of inhaled 3,3'-dichlorobiphenyl and the formation of the 4-hydroxylated metabolite. Environ Sci Technol. 2013;47:4743–4751. doi: 10.1021/es3049114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Humphrey HE. Effects of in utero exposure to polychlorinated biphenyls and related contaminants on cognitive functioning in young children. J Pediatr. 1990;116:38–45. doi: 10.1016/s0022-3476(05)81642-7. [DOI] [PubMed] [Google Scholar]
- Jacobus JA, Flor S, Klingelhutz A, Robertson LW, Ludewig G. 2-(4'-Chlorophenyl)-1,4-Benzoquinone Increases the Frequency of Micronuclei and Shortens Telomeres. Environ Toxicol Pharmacol. 2008;25:267–272. doi: 10.1016/j.etap.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MO. Polychlorinated Biphenyls: Metabolism and Metabolites. In: Robertson L, Hansen LG, editors. PCBs, Recent Advances in Environmental Toxicology and Health Effects. Lexington, KY: The University Press of Kentucky; 2001. [Google Scholar]
- Jamshidi A, Hunter S, Hazrati S, Harrad S. Concentrations and chiral signatures of polychlorinated biphenyls in outdoor and indoor air and soil in a major U.K. conurbation. Environ Sci Technol. 2007;41:2153–2158. doi: 10.1021/es062218c. [DOI] [PubMed] [Google Scholar]
- Jerina DM, Daly JW. Arene oxides: a new aspect of drug metabolism. Science. 1974;185:573–582. doi: 10.1126/science.185.4151.573. [DOI] [PubMed] [Google Scholar]
- Johansson M, Larsson C, Bergman A, Lund BO. Structure-activity relationship for inhibition of CYP11B1-dependent glucocorticoid synthesis in Y1 cells by aryl methyl sulfones. Pharmacol Toxicol. 1998;83:225–230. doi: 10.1111/j.1600-0773.1998.tb01473.x. [DOI] [PubMed] [Google Scholar]
- Jörundsdottir H, Norstrom K, Olsson M, Pham-tuan H, Hühnerfuss H, Bignert A, Bergman A. Temporal trend of bis(4-chlorophenyl) sulfone, methylsulfonyl-DDE and -PCBs in Baltic guillemot (Uria aalge) egg 1971–2001 - A comparison to 4,4'-DDE and PCB trends. Environ. Pollut. 2006;141:226–237. doi: 10.1016/j.envpol.2005.08.054. [DOI] [PubMed] [Google Scholar]
- Jugan ML, Levi Y, Blondeau JP. Endocrine disruptors and thyroid hormone physiology. Biochem Pharmacol. 2010;79:939–947. doi: 10.1016/j.bcp.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Kaiser K. On the optical activity of polychlorinated biphenyls. Environ. Poll. 1974;7:93–101. [Google Scholar]
- Kallenborn R, Huhnerfuss H, editors. Chiral Environmental Pollutants - Trace Analysis and Ecotoxicology. Berlin, Heidelberg: Springer Verlag; 2001. [Google Scholar]
- Kaminsky LS, Kennedy MW, Adams SM, Guengerich FP. Metabolism of dichlorobiphenyls by highly purified isozymes of rat liver cytochrome P-450. Biochemistry. 1981;20:7379–7384. doi: 10.1021/bi00529a009. [DOI] [PubMed] [Google Scholar]
- Kania-korwel I, Barnhart CD, Stamou M, Truong KM, El-komy MH, Lein PJ, Veng-pedersen P, Lehmler HJ. 2,2',3,5',6-Pentachlorobiphenyl (PCB 95) and its hydroxylated metabolites are enantiomerically enriched in female mice. Environ Sci Technol. 2012;46:11393–11401. doi: 10.1021/es302810t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-korwel I, Duffel MW, Lehmler HJ. Gas chromatographic analysis with chiral cyclodextrin phases reveals the enantioselective formation of hydroxylated polychlorinated biphenyls by rat liver microsomes. Environ. Sci. Technol. 2011;45:9590–9596. doi: 10.1021/es2014727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-korwel I, Hrycay EG, Bandiera S, Lehmler H-J. 2,2',3,3',6,6'-hexachlorobiphenyl (PCB 136) atropisomers interact enantioselectively with hepatic microsomal cytochrome P450 enzymes. Chem. Res. Toxicol. 2008a;21:1295–1303. doi: 10.1021/tx800059j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-korwel I, Vyas S, Song Y, Lehmler HJ. Gas chromatographic separation of methoxylated polychlorinated biphenyl atropisomer. J. Chromatogr. A. 2008b;1207:146–154. doi: 10.1016/j.chroma.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Mckinney JD, Matthews HB. Metabolism of symmetrical hexachlorobiphenyl isomers in the rat. Toxicol. Appl. Pharma. 1980;53:389–398. doi: 10.1016/0041-008x(80)90352-x. [DOI] [PubMed] [Google Scholar]
- Kato Y, Haraguchi K, Kawashima M, Yamada S, Masuda Y, Kimura R. Induction of hepatic microsomal drug-metabolizing enzymes by methylsulphonyl metabolites of polychlorinated biphenyl congeners in rats. Chem Biol Interact. 1995;95:257–268. doi: 10.1016/0009-2797(94)03564-o. [DOI] [PubMed] [Google Scholar]
- Kato Y, Haraguchi K, Shibahara T, Masuda Y, Kimura R. Reduction of thyroid hormone levels by methylsulfonyl metabolites of polychlorinated biphenyl congeners in rats. Arch Toxicol. 1998;72:541–544. doi: 10.1007/s002040050540. [DOI] [PubMed] [Google Scholar]
- Kato Y, Haraguchi K, Shibahara T, Shinmura Y, Masuda Y, Kimura R. The induction of hepatic microsomal UDP-glucuronosyltransferase by the methylsulfonyl metabolites of polychlorinated biphenyl congeners in rats. Chem Biol Interact. 2000;125:107–115. doi: 10.1016/s0009-2797(99)00168-4. [DOI] [PubMed] [Google Scholar]
- Kato Y, Haraguchi K, Shibahara T, Yumoto S, Masuda Y, Kimura R. Reduction of thyroid hormone levels by methylsulfonyl metabolites of tetra- and pentachlorinated biphenyls in male Sprague-Dawley rats. Toxicol Sci. 1999;48:51–54. doi: 10.1093/toxsci/48.1.51. [DOI] [PubMed] [Google Scholar]
- Kato Y, Haraguchi K, Tomiyasu K, Hiroyuki S, Isogai M, Masuda Y, Kimura R. Structure-dependent induction of CYP2B1/2 by 3-methylsulfonyl metabolites of polychlorinated biphenyl congeners in rats. Environ Toxicol Pharmacol. 1997;3:137–144. doi: 10.1016/s1382-6689(97)00150-6. [DOI] [PubMed] [Google Scholar]
- Kato Y, Ikushiro S, Haraguchi K, Yamazaki T, Ito Y, Suzuki H, Kimura R, Yamada S, Inoue T, Degawa M. A possible mechanism for decrease in serum thyroxine level by polychlorinated biphenyls in Wistar and Gunn rats. Toxicol Sci. 2004;81:309–315. doi: 10.1093/toxsci/kfh225. [DOI] [PubMed] [Google Scholar]
- Kester MH, Bulduk S, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Bergman A, Safe SH, Kuiper GG, Schuur AG, Brouwer A, Visser TJ. Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs. Endocrinology. 2000;141:1897–1900. doi: 10.1210/endo.141.5.7530. [DOI] [PubMed] [Google Scholar]
- Kester MH, Bulduk S, Van toor H, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Schuur AG, Brouwer A, Visser TJ. Potent inhibition of estrogen sulfotransferase by hydroxylated metabolites of polyhalogenated aromatic hydrocarbons reveals alternative mechanism for estrogenic activity of endocrine disrupters. J Clin Endocrinol Metab. 2002;87:1142–1150. doi: 10.1210/jcem.87.3.8311. [DOI] [PubMed] [Google Scholar]
- Kimbrough RD, Jensen AA, editors. Halogenated Biphenyls, Terphenyls, Naphthalenes, Dibenzodioxins and Related Products. 2nd Ed. 1989. [Google Scholar]
- Klasson wehler E, Jonsson J, Bergman A, Brandt I, Darnerud PO. 3,3',4,4'-Tetrachlorobiphenyl and 3,3',4,4',5-pentachlorobiphenyl: tissue localization and metabolic fate in the mourse. Chemosphere. 1989;19:809–812. [Google Scholar]
- Klasson Wehler E, Lindberg L, Joensson CJ, Bergman A. Tissue retention and metabolism of 2,3,4,3',4'-pentachlorobiphenyl in mink and mouse. Chemosphere. 1993;27:2397–2412. [Google Scholar]
- Knerr S, Schrenk D. Carcinogenicity of "non-dioxinlike" polychlorinated biphenyls. Crit Rev Toxicol. 2006;36:663–694. doi: 10.1080/10408440600845304. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Curras-collazo MC. Neuroendocrine actions of organohalogens: thyroid hormones, arginine vasopressin, and neuroplasticity. Front Neuroendocrinol. 2010;31:479–496. doi: 10.1016/j.yfrne.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Safe S. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), and dibenzofurans (PCDFs) as antiestrogens in MCF-7 human breast cancer cells: quantitative structure-activity relationships. Toxicol Appl Pharmacol. 1993;120:55–61. doi: 10.1006/taap.1993.1086. [DOI] [PubMed] [Google Scholar]
- Kutz FW, Wood PH, Bottimore DP. Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue. Rev Environ Contam Toxicol. 1991;120:1–82. doi: 10.1007/978-1-4612-3080-9_1. [DOI] [PubMed] [Google Scholar]
- Langer P, Kocan A, Tajtakova M, Koska J, Radikova Z, Ksinantova L, Imrich R, Huckova M, Drobna B, Gasperikova D, Sebokova E, Klimes I. Increased thyroid volume, prevalence of thyroid antibodies and impaired fasting glucose in young adults from organochlorine cocktail polluted area: outcome of transgenerational transmission? Chemosphere. 2008;73:1145–1150. doi: 10.1016/j.chemosphere.2008.06.067. [DOI] [PubMed] [Google Scholar]
- Lans MC, Klasson-Wehler E, Willemsen M, Meussen E, Safe S, Brouwer A. Structure-dependent, competitive interaction of hydroxy-polychlorobiphenyls, -dibenzo-p-dioxins and -dibenzofurans with human transthyretin. Chem Biol Interact. 1993;88:7–21. doi: 10.1016/0009-2797(93)90081-9. [DOI] [PubMed] [Google Scholar]
- Larsdotter M, Darnerud P, Aune M, Glynn A, Bjerselius R. Serum concentrations of pentachlorophenol (PCP), polychlorinated biphenyls (PCBs), and hydroxylated metabolites of PCB during pregnancy and lactation (in Swedish) [Available Jan. 16, 2006];Swedish EPA Report on Contract 2190104, 2005. 2005 / http://www.naturvardsverket.se/dokument/mo/modok/export/klorfenoler.pdf/ [Google Scholar]
- Larsson C, Bergman A. Synthesis of radiolabelled methylsulphonyl CBs with specific retention in the rat liver. Organohalogen Compounds. 1998;35:127–130. [Google Scholar]
- Larsson C, Ellerichmann T, Huhnerfuss H, Bergman A. Chiral PCB methyl sulfones in rat tissues after exposure to technical PCBs. Environ Sci Technol. 2002;36:2833–2838. doi: 10.1021/es025512n. [DOI] [PubMed] [Google Scholar]
- Larsson C, Norstrom K, Athanansiadis I, Bignert A, Konig WA, Bergman A. Enantiomeric specificity of methylsulfonyl-PCBs and distribution of bis(4-chlorophenyl) sulfone, PCB, and DDE methyl sulfones in grey seal tissues. Environ Sci Technol. 2004;38:4950–4955. doi: 10.1021/es049361v. [DOI] [PubMed] [Google Scholar]
- Lauby-secretan B, Loomis D, Grosse Y, El ghissassi F, Bouvard V, Benbrahim-tallaa L, Guha N, Baan R, Mattock H, Straif K. Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 2013;14:287–288. doi: 10.1016/S1470-2045(13)70104-9. [DOI] [PubMed] [Google Scholar]
- Lehmann L, H LE, P AK, L WR, Ludewig G. 4-monochlorobiphenyl (PCB3) induces mutations in the livers of transgenic Fisher 344 rats. Carcinogenesis. 2007;28:471–478. doi: 10.1093/carcin/bgl157. [DOI] [PubMed] [Google Scholar]
- Lehmler HJ, Harrad SJ, Huhnerfuss H, Kania-korwel I, Lee CM, Lu Z, Wong CS. Chiral polychlorinated biphenyl transport, metabolism, and distribution: a review. Environ Sci Technol. 2010;44:2757–2766. doi: 10.1021/es902208u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmler HJ, Robertson LW, Garrison AW, Kodavanti PR. Effects of PCB 84 enantiomers on [3H]-phorbol ester binding in rat cerebellar granule cells and 45Ca2+-uptake in rat cerebellum. Toxicol Lett. 2005;156:391–400. doi: 10.1016/j.toxlet.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Letcher RJ, Klasson-wehler E, Bergman A. Methyl sulfone and hydroxylated metabolites of polychlorinated biphenyls. In: Paasivirta J, editor. New Types of Persistent Halogenated Compounds. Berlin: Springer-Verlag; 2000. [Google Scholar]
- Li X, Parkin S, Duffel MW, Robertson LW, Lehmler HJ. An efficient approach to sulfate metabolites of polychlorinated biphenyls. Environ Int. 2010;36:843–848. doi: 10.1016/j.envint.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl B, Schettgen T, Kerscher G, Broding HC, Otto A, Angerer J, Drexler H. Evidence for increased internal exposure to lower chlorinated polychlorinated biphenyls (PCB) in pupils attending a contaminated school. Int J Hyg Environ Health. 2004;207:315–324. doi: 10.1078/1438-4639-00296. [DOI] [PubMed] [Google Scholar]
- Lin PH, Sangaiah R, Ranasinghe A, Upton PB, La DK, Gold A, Swenberg JA. Formation of quinonoid-derived protein adducts in the liver and brain of Sprague-Dawley rats treated with 2,2',5,5'-tetrachlorobiphenyl. Chem Res Toxicol. 2000;13:710–718. doi: 10.1021/tx000030f. [DOI] [PubMed] [Google Scholar]
- Litten S, Fowler B, Luszniak D. Identification of a novel PCB source through analysis of 209 PCB congeners by US EPA modified method 1668. Chemosphere. 2002;46:1457–1459. doi: 10.1016/s0045-6535(01)00253-3. [DOI] [PubMed] [Google Scholar]
- Liu Y, Apak TI, Lehmler HJ, Robertson LW, Duffel MW. Hydroxylated polychlorinated biphenyls are substrates and inhibitors of human hydroxysteroid sulfotransferase SULT2A1. Chem Res Toxicol. 2006;19:1420–1425. doi: 10.1021/tx060160+. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lehmler HJ, Robertson LW, Duffel MW. Physicochemical properties of hydroxylated polychlorinated biphenyls aid in predicting their interactions with rat sulfotransferase 1A1 (rSULT1A1) Chem Biol Interact. 2011;189:153–160. doi: 10.1016/j.cbi.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Smart JT, Song Y, Lehmler HJ, Robertson LW, Duffel MW. Structure-activity relationships for hydroxylated polychlorinated biphenyls as substrates and inhibitors of rat sulfotransferases and modification of these relationships by changes in thiol status. Drug Metab Dispos. 2009;37:1065–1072. doi: 10.1124/dmd.108.026021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londono M, Shimokawa N, Miyazaki W, Iwasaki T, Koibuchi N. Hydroxylated PCB induces Ca2+ oscillations and alterations of membrane potential in cultured cortical cells. J Appl Toxicol. 2010;30:334–342. doi: 10.1002/jat.1501. [DOI] [PubMed] [Google Scholar]
- Lu Z, Kania-korwel I, Lehmler HJ, Wong CS. Stereoselective formation of mono- and dihydroxylated polychlorinated biphenyls by rat cytochrome P450 2B1. Environ Sci Technol. 2013;47:12184–12192. doi: 10.1021/es402838f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Wong CS. Factors affecting phase I stereoselective biotransformation of chiral polychlorinated biphenyls by rat cytochrome P-450 2B1 isozyme. Environ Sci Technol. 2011;45:8298–8305. doi: 10.1021/es200673q. [DOI] [PubMed] [Google Scholar]
- Lucier GW, Mcdaniel OS, Schiller CM, Matthews HB. Structural requirements for the accumulation of chlorinated biphenyl metabolites in the fetal rat intestine. Drug Metab Dispos. 1978;6:584–590. [PubMed] [Google Scholar]
- Ludewig G. Cancer initiation by PCBs. In: Robertson LW, Hansen LG, editors. PCBs, Recent Advances in Environmental Toxicology and Health Effects. Lexington: The University Press of Kentucky; 2001. [Google Scholar]
- Ludewig G, Esch H, Robertson L. Polyhalogenierte Bi- und Terphenyle. In: Dunkelberg H, Gebel T, Hartwig A, editors. Handbuch der Lebensmitteltoxikologie: Belastungen, Wirkungen, Lebensmittelsicherheit, Hygiene. Weinheim: Wiley-VCH; 2007. [Google Scholar]
- Ludewig G, Lehmann L, Esch H, Robertson LW. Metabolic Activation of PCBs to Carcinogens in Vivo - A Review. Environ Toxicol Pharmacol. 2008;25:241–246. doi: 10.1016/j.etap.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B-O, Örberg J, Bergman Å, Larsson C, Bergman A, Bäcklin B-M, Håkansson H, Madej A, Brouwer A, Brunström B. Chronic and reproductive toxicity of a mixture of 15 methylsulfonyl-polychlorinated biphenyls and 3-methylsulfonyl-2,2-bis-(4-chlorophenyl)-1,1-dichloroethene in mink (Mustela vison) Environmental Toxicology and Chemistry. 1999;18:292–298. [Google Scholar]
- Lund J, Brandt I, Poellinger L, Bergman A, Klasson-wehler E, Gustafsson JA. Target cells for the polychlorinated biphenyl metabolite 4,4'-bis(methylsulfonyl)-2,2',5,5'-tetrachlorobiphenyl. Characterization of high affinity binding in rat and mouse lung cytosol. Mol Pharmacol. 1985;27:314–323. [PubMed] [Google Scholar]
- Lund J, Nordlund L, Devereux T, Glaumann H, Gustafsson JA. Physicochemical and immunological characterization of binding protein for PCB methyl sulfones. Chemosphere. 1987;16:1677–1680. [Google Scholar]
- Luthe G, Jacobus JA, Robertson LW. Receptor interactions by polybrominated diphenyl ethers versus polychlorinated biphenyls: a theoretical Structure-activity assessment. Environ Toxicol Pharmacol. 2008;25:202–210. doi: 10.1016/j.etap.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Sassoon DA. PCBs exert an estrogenic effect through repression of the Wnt7a signaling pathway in the female reproductive tract. Environ Health Perspect. 2006;114:898–904. doi: 10.1289/ehp.8748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonal RW, Barrie LA, Bidleman TF, Diamond ML, Gregor DJ, Semkin RG, Strachan WM, Li YF, Wania F, Alaee M, Alexeeva LB, Backus SM, Bailey R, Bewers JM, Gobeil C, Halsall CJ, Harner T, Hoff JT, Jantunen LM, Lockhart WL, Mackay D, Muir DC, Pudykiewicz J, Reimer KJ, Smith JN, Stern GA. Contaminants in the Canadian Arctic: 5 years of progress in understanding sources, occurrence and pathways. Sci Total Environ. 2000;254:93–234. doi: 10.1016/s0048-9697(00)00434-4. [DOI] [PubMed] [Google Scholar]
- Machala M, Blaha L, Lehmler HJ, Pliskova M, Majkova Z, Kapplova P, Sovadinova I, Vondracek J, Malmberg T, Robertson LW. Toxicity of hydroxylated and quinoid PCB metabolites: inhibition of gap junctional intercellular communication and activation of aryl hydrocarbon and estrogen receptors in hepatic and mammary cells. Chem Res Toxicol. 2004;17:340–347. doi: 10.1021/tx030034v. [DOI] [PubMed] [Google Scholar]
- Macintosh DL, Minegishi T, Fragala MA, Allen JG, Coghlan KM, Stewart JH, Mccarthy JF. Mitigation of building-related polychlorinated biphenyls in indoor air of a school. Environ Health. 2012;11:24. doi: 10.1186/1476-069X-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maervoet J, Covaci A, Schepens P, Sandau CD, Letcher RJ. A reassessment of the nomenclature of polychlorinated biphenyl (PCB) metabolites. Environ Health Perspect. 2004;112:291–294. doi: 10.1289/ehp.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg T, Hoogstraate J, Bergman A, Klasson wehler E. Pharmacokinetics of two major hydroxylated polychlorinated biphenyl metabolites with specific retention in rat blood. Xenobiotica. 2004;34:581–589. doi: 10.1080/00498250410001713078. [DOI] [PubMed] [Google Scholar]
- Mannschreck A, Pustet N, Robertson L, Oesch F, Püttmann M. Enantiomers of polychlorinated biphenyls: Semi-preparative enrichment by liquid chromatography. Liebigs Ann. Chem. 1985:2101–2103. [Google Scholar]
- Marek RF, Martinez A, Hornbuckle KC. Discovery of hydroxylated polychlorinated biphenyls (OH-PCBs) in sediment from a lake Michigan waterway and original commercial aroclors. Environ Sci Technol. 2013a;47:8204–8210. doi: 10.1021/es402323c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek RF, Thorne PS, Wang K, Dewall J, Hornbuckle KC. PCBs and OH-PCBs in serum from children and mothers in urban and rural U.S. communities. Environ Sci Technol. 2013b;47:3353–3361. doi: 10.1021/es304455k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HB, Anderson MW. Effect of chlorination on the distribution and excretion of polychlorinated biphenyls. Drug Metab. Disp. 1975;3:371–380. [PubMed] [Google Scholar]
- Matthews HB, Kato S. The metabolism and disposition of halogenated aromatics. Ann N Y Acad Sci. 1979;320:131–137. doi: 10.1111/j.1749-6632.1979.tb56596.x. [DOI] [PubMed] [Google Scholar]
- Mayes BA, Mcconnell EE, Neal BH, Brunner MJ, Hamilton SB, Sullivan TM, Peters AC, Ryan MJ, Toft JD, Singer AW, Brown JF, Jr, Menton RG, Moore JA. Comparative carcinogenicity in Sprague-Dawley rats of the polychlorinated biphenyl mixtures Aroclors 1016, 1242, 1254, and 1260. Toxicol Sci. 1998;41:62–76. doi: 10.1093/toxsci/41.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcfarland VA, Clarke JU. Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: considerations for a congener-specific analysis. Environ Health Perspect. 1989;81:225–239. doi: 10.1289/ehp.8981225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgraw JESR, Waller DP. Specific human CYP 450 isoform metabolism of a pentachlorobiphenyl (PCB-IUPAC# 101) Biochem Biophys Res Commun. 2006;344:129–133. doi: 10.1016/j.bbrc.2006.03.122. [DOI] [PubMed] [Google Scholar]
- Mclean MR, Bauer U, Amaro AR, Robertson LW. Identification of catechol and hydroquinone metabolites of 4-monochlorobiphenyl. Chem Res Toxicol. 1996a;9:158–164. doi: 10.1021/tx950083a. [DOI] [PubMed] [Google Scholar]
- Mclean MR, Robertson LW, Gupta RC. Detection of PCB adducts by the 32P-postlabeling technique. Chem Res Toxicol. 1996b;9:165–171. doi: 10.1021/tx9500843. [DOI] [PubMed] [Google Scholar]
- Meerts IA, Assink Y, Cenijn PH, Van den berg JH, Weijers BM, Bergman A, Koeman JH, Brouwer A. Placental transfer of a hydroxylated polychlorinated biphenyl and effects on fetal and maternal thyroid hormone homeostasis in the rat. Toxicol Sci. 2002;68:361–371. doi: 10.1093/toxsci/68.2.361. [DOI] [PubMed] [Google Scholar]
- Meerts IA, Hoving S, Van den berg JH, Weijers BM, Swarts HJ, Van der beek EM, Bergman A, Koeman JH, Brouwer A. Effects of in utero exposure to 4-hydroxy-2,3,3',4',5-pentachlorobiphenyl (4-OH-CB107) on developmental landmarks, steroid hormone levels, and female estrous cyclicity in rats. Toxicol Sci. 2004;82:259–267. doi: 10.1093/toxsci/kfh251. [DOI] [PubMed] [Google Scholar]
- Meijer L, Weiss J, Van velzen M, Brouwer A, Bergman A, Sauer P. Flame retardants, polychlorinated biphenyls and insecticides in pregnant women in the northern part of The Netherlands. Organohalogen Compounds. 2004;66:3552–3556. [Google Scholar]
- Melymuk L, Robson M, Helm PA, Diamond ML. PCBs, PBDEs, and PAHs in Toronto air: spatial and seasonal trends and implications for contaminant transport. Sci Total Environ. 2012;429:272–280. doi: 10.1016/j.scitotenv.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Menichini E, Iacovella N, Monfredini F, Turrio-baldassarri L. Atmospheric pollution by PAHs, PCDD/Fs and PCBs simultaneously collected at a regional background site in central Italy and at an urban site in Rome. Chemosphere. 2007;69:422–434. doi: 10.1016/j.chemosphere.2007.04.078. [DOI] [PubMed] [Google Scholar]
- Mills RA, Millis CD, Dannan GA, Guengerich FP, Aust SD. Studies on the structure-activity relationships for the metabolism of polybrominated biphenyls by rat liver microsomes. Toxicol Appl Pharmacol. 1985;78:96–104. doi: 10.1016/0041-008x(85)90309-6. [DOI] [PubMed] [Google Scholar]
- Mills SA, 3rd, Thal DI, Barney J. A summary of the 209 PCB congener nomenclature. Chemosphere. 2007;68:1603–1612. doi: 10.1016/j.chemosphere.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Morales NM, Matthews HB. In vivo binding of 2,3,6,2',3',6' - hexachlorobiphenyl and 2,4,5,2',4',5' - hexachlorobiphenyl to mouse liver macromolecules. Chem Biol Interact. 1979;27:99–110. doi: 10.1016/0009-2797(79)90153-4. [DOI] [PubMed] [Google Scholar]
- Morck A, Larsen G, Wehler EK. Covalent binding of PCB metabolites to lipids: route of formation and characterization. Xenobiotica. 2002;32:625–640. doi: 10.1080/00498250210130573. [DOI] [PubMed] [Google Scholar]
- Morse DC, Groen D, Veerman M, Van amerongen CJ, Koeter HB, Smits van prooije AE, Visser TJ, Koeman JH, Brouwer A. Interference of polychlorinated biphenyls in hepatic and brain thyroid hormone metabolism in fetal and neonatal rats. Toxicol Appl Pharmacol. 1993;122:27–33. doi: 10.1006/taap.1993.1168. [DOI] [PubMed] [Google Scholar]
- Morse DC, Wehler EK, Van DE, Pas M, De bie AT, Van bladeren PJ, Brouwer A. Metabolism and biochemical effects of 3,3',4,4'-tetrachlorobiphenyl in pregnant and fetal rats. Chem Biol Interact. 1995;95:41–56. doi: 10.1016/0009-2797(94)03347-1. [DOI] [PubMed] [Google Scholar]
- Morse DC, Wehler EK, Wesseling W, Koeman JH, Brouwer A. Alterations in rat brain thyroid hormone status following pre- and postnatal exposure to polychlorinated biphenyls (Aroclor 1254) Toxicol Appl Pharmacol. 1996;136:269–279. doi: 10.1006/taap.1996.0034. [DOI] [PubMed] [Google Scholar]
- Mortimer RH, Landers KA, Balakrishnan B, Li H, Mitchell MD, Patel J, Richard K. Secretion and transfer of the thyroid hormone binding protein transthyretin by human placenta. Placenta. 2012;33:252–256. doi: 10.1016/j.placenta.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Newsome WH, Davies D. Determination of PCB metabolites in Canadian human milk. Chemosphere. 1996;33:559–565. doi: 10.1016/0045-6535(96)00199-3. [DOI] [PubMed] [Google Scholar]
- Nezel T, Müller-plathe F, Müller MD, Buser H-R. Theoretical considerations about chiral PCBs and their methylthio and methylsulfonyl metabolites being possibly present as stable enantiomers. Chemosphere. 1997;35:1895–1906. [Google Scholar]
- Niknam Y, Feng W, Cherednichenko G, Dong Y, Joshi SN, Vyas SM, Lehmler HJ, Pessah IN. Structure-activity relationship of selected meta- and para-hydroxylated non-dioxin like polychlorinated biphenyls: from single RyR1 channels to muscle dysfunction. Toxicol Sci. 2013;136:500–513. doi: 10.1093/toxsci/kft202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomiyama K, Murata S, Kunisue T, Yamada TK, Mizukawa H, Takahashi S, Tanabe S. Polychlorinated biphenyls and their hydroxylated metabolites (OH-PCBs) in the blood of toothed and baleen whales stranded along Japanese coastal waters. Environ Sci Technol. 2010;44:3732–3738. doi: 10.1021/es1003928. [DOI] [PubMed] [Google Scholar]
- Noren K, Lunden A, Pettersson E, Bergman A. Methylsulfonyl metabolites of PCBs and DDE in human milk in Sweden, 1972–1992. Environ Health Perspect. 1996;104:766–772. doi: 10.1289/ehp.104-1469405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norstrom K, Eriksson J, Haglund J, Silvari V, Bergman A. Enantioselective formation of methyl sulfone metabolites of 2,2',3,3',4,6'-hexachlorobiphenyl in rat. Environ Sci Technol. 2006;40:7649–7655. doi: 10.1021/es061584t. [DOI] [PubMed] [Google Scholar]
- Oakley GG, Robertson LW, Gupta RC. Analysis of polychlorinated biphenyl-DNA adducts by 32P-postlabeling. Carcinogenesis. 1996;17:109–114. doi: 10.1093/carcin/17.1.109. [DOI] [PubMed] [Google Scholar]
- Oberg M, Sjodin A, Casabona H, Nordgren I, Klasson-wehler E, Hakansson H. Tissue distribution and half-lives of individual polychlorinated biphenyls and serum levels of 4-hydroxy-2,3,3',4',5-pentachlorobiphenyl in the rat. Toxicol Sci. 2002;70:171–182. doi: 10.1093/toxsci/70.2.171. [DOI] [PubMed] [Google Scholar]
- Ockenden WA, Lohmann R, Shears JR, Jones KC. The significance of PCBs in the atmosphere of the southern hemisphere. Environ Sci Pollut Res Int. 2001;8:189–194. doi: 10.1007/BF02987384. [DOI] [PubMed] [Google Scholar]
- Ohta S, Haraguchi K, Kato Y, Endo T, Kimura O, Koga N. Distribution and excretion of 2,2′,3,4′,5,5′,6-heptachlorobiphenyl (CB187) and its metabolites in rats and guinea pigs. Chemosphere. 2015;118:5–11. doi: 10.1016/j.chemosphere.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Onozuka D, Yoshimura T, Kaneko S, Furue M. Mortality after exposure to polychlorinated biphenyls and polychlorinated dibenzofurans: a 40-year follow-up study of Yusho patients. Am J Epidemiol. 2009;169:86–95. doi: 10.1093/aje/kwn295. [DOI] [PubMed] [Google Scholar]
- Otake T, Yoshinaga J, Enomoto T, Matsuda M, Wakimoto T, Ikegami M, Suzuki E, Naruse H, Yamanaka T, Shibuya N, Yasumizu T, Kato N. Thyroid hormone status of newborns in relation to in utero exposure to PCBs and hydroxylated PCB metabolites. Environ Res. 2007;105:240–246. doi: 10.1016/j.envres.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Park JS, Bergman A, Linderholm L, Athanasiadou M, Kocan A, Petrik J, Drobna B, Trnovec T, Charles MJ, Hertz-picciotto I. Placental transfer of polychlorinated biphenyls, their hydroxylated metabolites and pentachlorophenol in pregnant women from eastern Slovakia. Chemosphere. 2008;70:1676–1684. doi: 10.1016/j.chemosphere.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Linderholm L, Charles MJ, Athanasiadou M, Petrik J, Kocan A, Drobna B, Trnovec T, Bergman A, Hertz-picciotto I. Polychlorinated biphenyls and their hydroxylated metabolites (OH-PCBS) in pregnant women from eastern Slovakia. Environ Health Perspect. 2007;115:20–27. doi: 10.1289/ehp.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Petreas M, Cohn BA, Cirillo PM, Factor-litvak P. Hydroxylated PCB metabolites (OH-PCBs) in archived serum from 1950–60s California mothers: a pilot study. Environ Int. 2009;35:937–942. doi: 10.1016/j.envint.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson A, Safe SH, Robertson LW, Thomas PE, Ryan DE, Reik LM, Levin W. Immunochemical quantitation of cytochrome P-450 isozymes and epoxide hydrolase in liver microsomes from polychlorinated or polybrominated biphenyl-treated rats. A study of structure-activity relationships. J Biol Chem. 1983;258:5967–5976. [PubMed] [Google Scholar]
- Patrick L. Thyroid disruption: mechanism and clinical implications in human health. Altern Med Rev. 2009;14:326–346. [PubMed] [Google Scholar]
- Pearce EN, Braverman LE. Environmental pollutants and the thyroid. Best Pract Res Clin Endocrinol Metab. 2009;23:801–813. doi: 10.1016/j.beem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Pereg D, Robertson LW, Gupta RC. DNA adduction by polychlorinated biphenyls: adducts derived from hepatic microsomal activation and from synthetic metabolites. Chem Biol Interact. 2002;139:129–144. doi: 10.1016/s0009-2797(01)00292-7. [DOI] [PubMed] [Google Scholar]
- Pereg D, Tampal N, Espandiari P, Robertson LW. Distribution and macromolecular binding of benzo[a]pyrene and two polychlorinated biphenyl congeners in female mice. Chem Biol Interact. 2001;137:243–258. doi: 10.1016/s0009-2797(01)00256-3. [DOI] [PubMed] [Google Scholar]
- Persoon C, Peters TM, Kumar N, Hornbuckle KC. Spatial distribution of airborne polychlorinated biphenyls in Cleveland, Ohio and Chicago, Illinois. Environ Sci Technol. 2010;44:2797–2802. doi: 10.1021/es901691s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Hansen LG, Albertson TE, Garner CE, Ta TA, Do Z, Kim KH, Wong PW. Structure-activity relationship for noncoplanar polychlorinated biphenyl congeners toward the ryanodine receptor-Ca2+ channel complex type 1 (RyR1) Chem Res Toxicol. 2006;19:92–101. doi: 10.1021/tx050196m. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Lehmler HJ, Robertson LW, Perez CF, Cabrales E, Bose DD, Feng W. Enantiomeric specificity of (−)-2,2',3,3',6,6'-hexachlorobiphenyl toward ryanodine receptor types 1 and 2. Chem Res Toxicol. 2009;22:201–207. doi: 10.1021/tx800328u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliskova M, Vondracek J, Canton RF, Nera J, Kocan A, Petrik J, Trnovec T, Sanderson T, Van den berg M, Machala M. Impact of polychlorinated biphenyls contamination on estrogenic activity in human male serum. Environ Health Perspect. 2005;113:1277–1284. doi: 10.1289/ehp.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston BD, Miller JA, Miller EC. Non-arene oxide aromatic ring hydroxylation of 2,2',5,5'-tetrachlorobiphenyl as the major metabolic pathway catalyzed by phenobarbital-induced rat liver microsomes. J Biol Chem. 1983;258:8304–8311. [PubMed] [Google Scholar]
- Ptak A, Ludewig G, Lehmler HJ, Wojtowicz AK, Robertson LW, Gregoraszczuk EL. Comparison of the actions of 4-chlorobiphenyl and its hydroxylated metabolites on estradiol secretion by ovarian follicles in primary cells in culture. Reprod Toxicol. 2005;20:57–64. doi: 10.1016/j.reprotox.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Ptak A, Ludewig G, Robertson L, Lehmler HJ, Gregoraszczuk EL. In vitro exposure of porcine prepubertal follicles to 4-chlorobiphenyl (PCB3) and its hydroxylated metabolites: effects on sex hormone levels and aromatase activity. Toxicol Lett. 2006;164:113–122. doi: 10.1016/j.toxlet.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Purkey HE, Palaninathan SK, Kent KC, Smith C, Safe SH, Sacchettini JC, Kelly JW. Hydroxylated polychlorinated biphenyls selectively bind transthyretin in blood and inhibit amyloidogenesis: rationalizing rodent PCB toxicity. Chem Biol. 2004;11:1719–1728. doi: 10.1016/j.chembiol.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Püttmann M, Mannschreck A, Oesch F, Robertson L. Chiral effects in the induction of drug-metabolizing enzymes using synthetic atropisomers of polychlorinated biphenyls (PCBs) Biochem Pharmacol. 1989;38:1345–1352. doi: 10.1016/0006-2952(89)90342-0. [DOI] [PubMed] [Google Scholar]
- Püttmann M, Oesch F, Robertson L, Mannschreck A. Characteristics of polychlorinated biphenyl (PCB) atropisomers. Chemosphere. 1986;15:2061–2064. [Google Scholar]
- Qin X, Lehmler HJ, Teesch LM, Robertson LW, Duffel MW. Chlorinated Biphenyl Quinones and Phenyl-2,5-benzoquinone Differentially Modify the Catalytic Activity of Human Hydroxysteroid Sulfotransferase hSULT2A1. Chem Res Toxicol. 2013 doi: 10.1021/tx400207q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinete N, Schettgen T, Bertram J, Kraus T. Occurrence and distribution of PCB metabolites in blood and their potential health effects in humans: a review. Environ Sci Pollut Res Int. 2014 doi: 10.1007/s11356-014-3136-9. [DOI] [PubMed] [Google Scholar]
- Rayne S, Forest K. pK(a) values of the monohydroxylated polychlorinated biphenyls (OH-PCBs), polybrominated biphenyls (OH-PBBs), polychlorinated diphenyl ethers (OH-PCDEs), and polybrominated diphenyl ethers (OH-PBDEs) J Environ Sci Health A Tox Hazard Subst Environ Eng. 2010;45:1322–1346. doi: 10.1080/10934529.2010.500885. [DOI] [PubMed] [Google Scholar]
- Razvi S, Shakoor A, Vanderpump M, Weaver JU, Pearce SH. The influence of age on the relationship between subclinical hypothyroidism and ischemic heart disease: a metaanalysis. J Clin Endocrinol Metab. 2008;93:2998–3007. doi: 10.1210/jc.2008-0167. [DOI] [PubMed] [Google Scholar]
- Rickenbacher U, Mckinney JD, Oatley SJ, Blake CC. Structurally specific binding of halogenated biphenyls to thyroxine transport protein. J Med Chem. 1986;29:641–648. doi: 10.1021/jm00155a010. [DOI] [PubMed] [Google Scholar]
- Ring BJ, Seitz KR, Vodicnik MJ. Transfer of 2,4,5,2',4',5'-hexachlorobiphenyl across the in situ perfused guinea pig placenta. Toxicol Appl Pharmacol. 1988;96:7–13. doi: 10.1016/0041-008x(88)90241-4. [DOI] [PubMed] [Google Scholar]
- Robertson LW, Gupta R. Metabolism of polychlorinated biphenyls (PCBs) generates eletrophiles and reactive oxygen species that damage DNA. In: Williams GM, Aruoma OI, editors. Molecular Drug Metabolism and Toxicology. OICA International; 2000. [Google Scholar]
- Robertson LW, Ludewig G. Polychlorinated Biphenyl (PCB) carcinogenicity with special emphasis on airborne PCBs. Gefahrst Reinhalt Luft. 2011;71:25–32. [PMC free article] [PubMed] [Google Scholar]
- Rodenburg LA, Du S, Fennell DE, Cavallo GJ. Evidence for widespread dechlorination of polychlorinated biphenyls in groundwater, landfills, and wastewater collection systems. Environ Sci Technol. 2010a;44:7534–7540. doi: 10.1021/es1019564. [DOI] [PubMed] [Google Scholar]
- Rodenburg LA, Guo J, Du S, Cavallo GJ. Evidence for unique and ubiquitous environmental sources of 3,3'-dichlorobiphenyl (PCB 11) Environmental Science & Technology. 2010b;44:2816–2821. doi: 10.1021/es901155h. [DOI] [PubMed] [Google Scholar]
- Rudel RA, Perovich LJ. Endocrine disrupting chemicals in indoor and outdoor air. Atmos Environ (1994) 2009;43:170–181. doi: 10.1016/j.atmosenv.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S. Hydroxylated polychlorinated biphenyls (PCBs) and organochlorine pesticides as potential endocrine disruptors. Handbook of Environmental Chemistry. 2001;3:155–167. [Google Scholar]
- Safe S, Bandiera S, Sawyer T, Robertson L, Safe L, Parkinson A, Thomas PE, Ryan DE, Reik LM, Levin W, et al. PCBs: structure-function relationships and mechanism of action. Environ Health Perspect. 1985;60:47–56. doi: 10.1289/ehp.856047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandanger TM, Dumas P, Berger U, Burkow IC. Analysis of HO-PCBs and PCP in blood plasma from individuals with high PCB exposure living on the Chukotka Peninsula in the Russian Arctic. J Environ Monit. 2004;6:758–765. doi: 10.1039/b401999g. [DOI] [PubMed] [Google Scholar]
- Sandau CD, Ayotte P, Dewailly E, Duffe J, Norstrom RJ. Pentachlorophenol and hydroxylated polychlorinated biphenyl metabolites in umbilical cord plasma of neonates from coastal populations in Quebec. Environ Health Perspect. 2002;110:411–417. doi: 10.1289/ehp.02110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Colacino J, Haffner D, Patel K, Opel M, Papke O, Birnbaum L. Perfluorinated Compounds, Polychlorinated Biphenyl, and Organochlorine Pesticide Contamination in Composite Food Samples from Dallas, Texas. Environ. Health Perspect. 2010;118:796–802. doi: 10.1289/ehp.0901347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnellmann RG, Vickers AEM, Sipes IG. Metabolism and disposition of polychlorinated biphenyls. In: Hodgson EBJR, Philpot RM, editors. Reviews in Biochemical Toxicology. New York, Asterdam, Oxford: Elsevier; 1985. [Google Scholar]
- Schuur AG, Brouwer A, Bergman A, Coughtrie MW, Visser TJ. Inhibition of thyroid hormone sulfation by hydroxylated metabolites of polychlorinated biphenyls. Chem Biol Interact. 1998a;109:293–297. doi: 10.1016/s0009-2797(97)00140-3. [DOI] [PubMed] [Google Scholar]
- Schuur AG, Legger FF, Van meeteren ME, Moonen MJ, Van leeuwen-bol I, Bergman A, Visser TJ, Brouwer A. In vitro inhibition of thyroid hormone sulfation by hydroxylated metabolites of halogenated aromatic hydrocarbons. Chem Res Toxicol. 1998b;11:1075–1081. doi: 10.1021/tx9800046. [DOI] [PubMed] [Google Scholar]
- Senthilkumar PK, Klingelhutz AJ, Jacobus JA, Lehmler H, Robertson LW, Ludewig G. Airborne polychlorinated biphenyls (PCBs) reduce telomerase activity and shorten telomere length in immortal human skin keratinocytes (HaCat) Toxicol Lett. 2011;204:64–70. doi: 10.1016/j.toxlet.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang H, Li Y, Wang T, Wang P, Zhang H, Zhang Q, Jiang G. The presence of polychlorinated biphenyls in yellow pigment products in China with emphasis on 3,3'-dichlorobiphenyl (PCB 11) Chemosphere. 2014;98:44–50. doi: 10.1016/j.chemosphere.2013.09.075. [DOI] [PubMed] [Google Scholar]
- Sharma R, Kodavanti PR. In vitro effects of polychlorinated biphenyls and hydroxy metabolites on nitric oxide synthases in rat brain. Toxicol Appl Pharmacol. 2002;178:127–136. doi: 10.1006/taap.2001.9328. [DOI] [PubMed] [Google Scholar]
- Silberhorn EM, Glauert HP, Robertson LW. Carcinogenicity of polyhalogenated biphenyls: PCBs and PBBs. Crit Rev Toxicol. 1990;20:440–496. doi: 10.3109/10408449009029331. [DOI] [PubMed] [Google Scholar]
- Silverstone AE, Rosenbaum PF, Weinstock RS, Bartell SM, Foushee HR, Shelton C, Pavuk M. Polychlorinated biphenyl (PCB) exposure and diabetes: results from the Anniston Community Health Survey. Environ Health Perspect. 2012;120:727–732. doi: 10.1289/ehp.1104247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin A, Hagmar L, Klasson-wehler E, Bjork J, Bergman A. Influence of the consumption of fatty Baltic Sea fish on plasma levels of halogenated environmental contaminants in Latvian and Swedish men. Environ Health Perspect. 2000;108:1035–1041. doi: 10.1289/ehp.108-1240159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A, Tullsten AK, Klasson-wehler E. Identification of the parent compounds to selectively retained hydroxylated PCB metabolites in rat blood plasma. Organohalogen Compounds. 1998;37:365–368. [Google Scholar]
- Sleight S. Effects of PCBs and related compounds on hepatocarcinogenesis in rats and mice. Environ Health Perspect. 1985;60:35–39. doi: 10.1289/ehp.856035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soechitram SD, Athanasiadou M, Hovander L, Bergman A, Sauer PJ. Fetal exposure to PCBs and their hydroxylated metabolites in a Dutch cohort. Environ Health Perspect. 2004;112:1208–1212. doi: 10.1289/ehp.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Buettner GR, Parkin S, Wagner BA, Robertson LW, Lehmler HJ. Chlorination increases the persistence of semiquinone free radicals derived from polychlorinated biphenyl hydroquinones and quinones. J Org Chem. 2008a;73:8296–8304. doi: 10.1021/jo801397g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Wagner BA, Lehmler HJ, Buettner GR. Semiquinone radicals from oxygenated polychlorinated biphenyls: electron paramagnetic resonance studies. Chem Res Toxicol. 2008b;21:1359–1367. doi: 10.1021/tx8000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Wagner BA, Witmer JR, Lehmler HJ, Buettner GR. Nonenzymatic displacement of chlorine and formation of free radicals upon the reaction of glutathione with PCB quinones. Proc Natl Acad Sci U S A. 2009;106:9725–9730. doi: 10.1073/pnas.0810352106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A, Lehmler HJ, Robertson LW, Ludewig G. Production of DNA strand breaks in vitro and reactive oxygen species in vitro and in HL-60 cells by PCB metabolites. Toxicol Sci. 2001;60:92–102. doi: 10.1093/toxsci/60.1.92. [DOI] [PubMed] [Google Scholar]
- Srinivasan A, Robertson LW, Ludewig G. Sulfhydryl binding and topoisomerase inhibition by PCB metabolites. Chem Res Toxicol. 2002;15:497–505. doi: 10.1021/tx010128+. [DOI] [PubMed] [Google Scholar]
- Sun P, Basu I, Hites RA. Temporal trends of polychlorinated biphenyls in precipitation and air at chicago. Environ Sci Technol. 2006;40:1178–1183. doi: 10.1021/es051725b. [DOI] [PubMed] [Google Scholar]
- Tampal N, Lehmler HJ, Espandiari P, Malmberg T, Robertson LW. Glucuronidation of hydroxylated polychlorinated biphenyls (PCBs) Chem Res Toxicol. 2002;15:1259–1266. doi: 10.1021/tx0200212. [DOI] [PubMed] [Google Scholar]
- Tampal N, Myers S, Robertson LW. Binding of polychlorinated biphenyls/metabolites to hemoglobin. Toxicol Lett. 2003;142:53–60. doi: 10.1016/s0378-4274(02)00484-8. [DOI] [PubMed] [Google Scholar]
- Tanabe S. PCB problems in the future: foresight from current knowledge. Environ Pollut. 1988;50:5–28. doi: 10.1016/0269-7491(88)90183-2. [DOI] [PubMed] [Google Scholar]
- Tilson HA, Kodavanti PR. The neurotoxicity of polychlorinated biphenyls. Neurotoxicology. 1998;19:517–525. [PubMed] [Google Scholar]
- Todaka T, Hori T, Hirakawa H, Kajiwara J, Yasutake D, Onozuka D, Iida T, Furue M. Concentrations of polychlorinated biphenyls in blood of Yusho patients over 35 years after the incident. Chemosphere. 2009;74:902–909. doi: 10.1016/j.chemosphere.2008.10.042. [DOI] [PubMed] [Google Scholar]
- Troisi GM, Haraguchi K, Kaydoo DS, Nyman M, Aguilar A, Borrell A, Siebert U, Mason CF. Bioaccumulation of polychlorinated biphenyls (PCBs) and dichlorodiphenylethane (DDE) methyl sulfones in tissues of seal and dolphin morbillivirus epizootic victims. J Toxicol Environ Health A. 2001;62:1–8. doi: 10.1080/00984100050201622. [DOI] [PubMed] [Google Scholar]
- Van den berg KJ, Zurcher C, Brouwer A. Effects of 3,4,3',4'-tetrachlorobiphenyl on thyroid function and histology in marmoset monkeys. Toxicol Lett. 1988;41:77–86. doi: 10.1016/0378-4274(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Van den hurk P, Kubiczak GA, Lehmler HJ, James MO. Hydroxylated polychlorinated biphenyls as inhibitors of the sulfation and glucuronidation of 3-hydroxy-benzo[a]pyrene. Environ Health Perspect. 2002;110:343–348. doi: 10.1289/ehp.02110343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansell NR, Muppidi JR, Habeebu SM, Klaassen CD. Promotion of thyroid tumors in rats by pregnenolone-16alpha-carbonitrile (PCN) and polychlorinated biphenyl (PCB) Toxicol Sci. 2004;81:50–59. doi: 10.1093/toxsci/kfh197. [DOI] [PubMed] [Google Scholar]
- Vermiglio F, Lo presti VP, Moleti M, Sidoti M, Tortorella G, Scaffidi G, Castagna MG, Mattina F, Violi MA, Crisa A, Artemisia A, Trimarchi F. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab. 2004;89:6054–6060. doi: 10.1210/jc.2004-0571. [DOI] [PubMed] [Google Scholar]
- Waller SC, He YA, Harlow GR, He YQ, Mash EA, Halpert JR. 2,2',3,3',6,6'-hexachlorobiphenyl hydroxylation by active site mutants of cytochrome P450 2B1 and 2B11. Chem Res Toxicol. 1999;12:690–699. doi: 10.1021/tx990030j. [DOI] [PubMed] [Google Scholar]
- Wang LQ, Lehmler HJ, Robertson LW, James MO. Polychlorobiphenylols are selective inhibitors of human phenol sulfotransferase 1A1 with 4-nitrophenol as a substrate. Chem Biol Interact. 2006;159:235–246. doi: 10.1016/j.cbi.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Wangpradit O, Mariappan SV, Teesch LM, Duffel MW, Norstrom K, Robertson LW, Luthe G. Oxidation of 4-chlorobiphenyl metabolites to electrophilic species by prostaglandin H synthase. Chem Res Toxicol. 2009;22:64–71. doi: 10.1021/tx800300t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner NA, Martin JW, Wong CS. Chiral polychlorinated biphenyls are biotransformed enantioselectively by mammalian cytochrome P-450 isozymes to form hydroxylated metabolites. Environ Sci Technol. 2009;43:114–121. doi: 10.1021/es802237u. [DOI] [PubMed] [Google Scholar]
- Weintraub M, Birnbaum LS. Catfish consumption as a contributor to elevated PCB levels in a non-Hispanic black subpopulation. Environ Res. 2008;107:412–417. doi: 10.1016/j.envres.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Weistrand C, Noren K. Methylsulfonyl metabolites of PCBs and DDE in human tissues. Environ Health Perspect. 1997;105:644–649. doi: 10.1289/ehp.97105644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weistrand C, Noren K, Nilsson A. Occupational exposure. Organochlorine compounds in blood plasma from potentially exposed workers. PCB, PCN, PCDD/F, HCB and methylsulfonyl metabolites of PCB. Environmental Science and Pollution Research International. 1997;4:2–9. doi: 10.1007/BF02986256. [DOI] [PubMed] [Google Scholar]
- Wethington DM, 3RD, Hornbuckle KC. Milwaukee, WI, as a source of atmospheric PCBs to Lake Michigan. Environ Sci Technol. 2005;39:57–63. doi: 10.1021/es048902d. [DOI] [PubMed] [Google Scholar]
- Wu X, Duffel M, Lehmler HJ. Oxidation of polychlorinated biphenyls by liver tissue slices from phenobarbital-pretreated mice is congener-specific and atropselective. Chem Res Toxicol. 2013a;26:1642–1651. doi: 10.1021/tx400229e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Kammerer A, Lehmler HJ. Microsomal oxidation of 2,2',3,3',6,6'-hexachlorobiphenyl (PCB 136) results in species-dependent chiral signatures of the hydroxylated metabolites. Environ Sci Technol. 2014;48:2436–2444. doi: 10.1021/es405433t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Kania-korwel I, Chen H, Stamou M, Dammanahalli KJ, Duffel M, Lein PJ, Lehmler HJ. Metabolism of 2,2',3,3',6,6'-hexachlorobiphenyl (PCB 136) atropisomers in tissue slices from phenobarbital or dexamethasone-induced rats is sex-dependent. Xenobiotica. 2013b;43:933–947. doi: 10.3109/00498254.2013.785626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Pramanik A, Duffel MW, Hrycay EG, Bandiera SM, Lehmler HJ, Kania-korwel I. 2,2',3,3',6,6'-Hexachlorobiphenyl (PCB 136) is enantioselectively oxidized to hydroxylated metabolites by rat liver microsomes. Chem. Res. Toxicol. 2011;24:2249–2257. doi: 10.1021/tx200360m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Wang K, Robertson LW, Ludewig G. Investigation of mechanism(s) of DNA damage induced by 4-monochlorobiphenyl (PCB3) metabolites. Environ Int. 2010;36:950–961. doi: 10.1016/j.envint.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Kania-korwel I, Ghogha A, Chen H, Stamou M, Bose DD, Pessah IN, Lehmler HJ, Lein PJ. PCB 136 atropselectively alters morphometric and functional parameters of neuronal connectivity in cultured rat hippocampal neurons via ryanodine receptor-dependent mechanisms. Toxicol Sci. 2014;138:379–392. doi: 10.1093/toxsci/kft334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettner MA, Flor S, Ludewig G, Wagner J, Robertson LW, Lehmann L. Quinoid metabolites of 4-monochlorobiphenyl induce gene mutations in cultured Chinese hamster v79 cells. Toxicol Sci. 2007;100:88–98. doi: 10.1093/toxsci/kfm204. [DOI] [PubMed] [Google Scholar]
- Zhai G, Hu D, Lehmler HJ, Schnoor JL. Enantioselective biotransformation of chiral PCBs in whole poplar plants. Environ Sci Technol. 2011;45:2308–2316. doi: 10.1021/es1033662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai G, Lehmler HJ, Schnoor JL. Sulfate metabolites of 4-monochlorobiphenyl in whole poplar plants. Environ Sci Technol. 2013a;47:557–562. doi: 10.1021/es303807f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai G, Wu X, Lehmler HJ, Schnoor JL. Atropisomeric determination of chiral hydroxylated metabolites of polychlorinated biphenyls using HPLC-MS. Chem Cent J. 2013b;7:183. doi: 10.1186/1752-153X-7-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Diamond ML, Robson M, Harrad S. Sources, emissions, and fate of polybrominated diphenyl ethers and polychlorinated biphenyls indoors in Toronto, Canada. Environ Sci Technol. 2011;45:3268–3274. doi: 10.1021/es102767g. [DOI] [PubMed] [Google Scholar]
- Zhao HX, Adamcakova-dodd A, Hu D, Hornbuckle KC, Just CL, Robertson LW, Thorne PS, Lehmler HJ. Development of a synthetic PCB mixture resembling the average polychlorinated biphenyl profile in Chicago air. Environ Int. 2010;36:819–827. doi: 10.1016/j.envint.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Narang A, Ding X, Eadon G. Characterization and quantitative analysis of DNA adducts formed from lower chlorinated PCB-derived quinones. Chem Res Toxicol. 2004;17:502–511. doi: 10.1021/tx034245b. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Mapuskar KA, Marek RF, Xu W, Lehmler HJ, Robertson LW, Hornbuckle KC, Spitz DR, Aykin-burns N. A new player in environmentally induced oxidative stress: polychlorinated biphenyl congener, 3,3'-dichlorobiphenyl (PCB11) Toxicol Sci. 2013;136:39–50. doi: 10.1093/toxsci/kft186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol. 2004;16:809–818. doi: 10.1111/j.1365-2826.2004.01243.x. [DOI] [PubMed] [Google Scholar]