Abstract
In this study, event-related potentials were used to investigate the effect of emotion on response inhibition. Participants performed an emotional go/no-go task that required responses to human faces associated with a “go” valence (i.e., emotional, neutral) and response inhibition to human faces associated with a “no-go” valence. Emotional content impaired response inhibition, as evidenced by decreased response accuracy and N2 amplitudes in no-go trials. More importantly, emotional expressions elicited larger N170 amplitudes than neutral expressions, and this effect was larger in no-go than in go trials, indicating that the perceptual processing of emotional expression had priority in inhibitory trials. In no-go trials, correlation analysis showed that increased N170 amplitudes were associated with decreased N2 amplitudes. Taken together, our findings suggest that that emotional content impairs response inhibition due to the prioritization of emotional content processing.
Descriptors: N170, N2, Emotion, Perceptual processing, Response inhibition, Event-related potentials(ERPs)
Response inhibition, which determines the ability to suppress actions that are inappropriate in a given context, is an important component of executive control (Mostofsky & Simmonds, 2008). Response inhibition tasks, such as the go/no-go task and the stop-signal task, have been used to measure inhibitory control in laboratory settings (Aron, 2007). For example, in the go/no-go task, a motor response is made to one stimulus class (go cues) and withheld to another (no-go cues); response inhibition is measured by the accuracy of no-go cues. Adapted response inhibition tasks are used to investigate the effects of emotional cues on response inhibition. In these adapted tasks, non-emotional response cues are replaced by emotional stimuli, such as emotional faces (Goldstein, et al., 2007; Hare, et al., 2008), pictures (Wang, et al., 2011), or words (Chiu, Holmes, & Pizzagalli, 2008). However, whether emotional perception biases response inhibition remains unclear.
Studies using the adapted tasks have suggested that the impacts of emotional content on behavior are weak and variable, with emotional stimuli impairing (Hare, et al., 2008; Schulz, et al., 2007), improving (Chiu, Holmes, & Pizzagalli, 2008; Pessoa, Padmala, Kenzer, & Bauer, 2012), or having no significant effect on the behavioral performance of response inhibition (Elliott, Rubinsztein, Sahakian, & Dolan, 2000; Murphy, et al., 1999; Shafritz, Collins, & Blumberg, 2006). These mixed results can be interpreted through a dual competition framework (Pessoa, 2009), whereby emotional content influences the competition between perceptual and executive control. When the threat of emotional content is low, the priority of emotion processing is “soft,” suggesting that emotional content facilitates behavioral performance. The perceptual processing of the emotional cue could attract further attention which in turn could be used for executive control, thereby enhancing behavioral performance, such as through increased no-go accuracy (Chiu, Holmes, & Pizzagalli, 2008) and short stop-signal response times (SSRTs) (Pessoa, Padmala, Kenzer, & Bauer, 2012). Conversely, when the threat of emotional content is high, the brain processes the emotional cue with “hard” prioritization, which may impair behavioral performance. The perceptual processing of the emotional cue may compete the attention resources needed for executive control, impairing behavioral performance, as reflected in decreased no-go accuracy (Hare, et al., 2008; Schulz, et al., 2007) and longer SSRTs (Pessoa, Padmala, Kenzer, & Bauer, 2012).
Based on this dual competition framework (Pessoa, 2009), increased visual cortex activation is expected when response inhibition signals load emotional content. Schulz et al. (2009) reported that blood oxygen level-dependent (BOLD) signals were increased in the inferior occipital gyrus and fusiform face area for no-go events in contrast to go events when facial expressions were used as stimuli. However, most functional magnetic resonance (MR) imaging studies have found that emotional response inhibition cues activate the inferior frontal gyrus and dorsolateral prefrontal, anterior cingulate, and insular cortices, but not the visual cortex (Hare, Tottenham, Davidson, Glover, & Casey, 2005; Hare, et al., 2008; Schulz, et al., 2009).
Event-related potentials (ERPs) reveal the time course of cortex activation for perceptual processing and response inhibition with a high degree of temporal resolution. The posterior N1 component is a negative peak occurring about 150 ms post-stimulus and is largest over the inferior occipital cortex when participants are asked to make discriminative responses (Hopf, Vogel, Woodman, Heinze, & Luck, 2002). The N1 component elicited by faces occurs about 170 ms post-stimulus in the posterior temporal scalp and is often referred to as N170 (Bentin, Allison, Puce, Perez, & McCarthy, 1996; Itier & Taylor, 2004). N170 amplitudes are higher for emotional faces than for neutral faces and indicate perception processing, during which the brain can distinguish emotional from neutral facial stimuli, but cannot distinguish among specific types of emotions (Luo, Feng, He, Wang, & Luo, 2010). On the other hand, the N2 and P3 components are associated with response inhibition. The N2 component, a negative-going with maximum amplitudes in frontal and central scalp locations at 200 and 400 ms post-stimulus, has been associated with response inhibition in go/no-go paradigms, with greater amplitudes for no-go than for go events (no-go effect) (Falkenstein, Hoormann, & Hohnsbein, 1999; Jodo & Kayama, 1992; Lewis, et al., 2008; Nieuwenhuis, Yeung, & Cohen, 2004; Nieuwenhuis, Yeung, Wery, & Ridderinkhof, 2003; Smith, Johnstone, & Barry, 2008). Decreased no-go N2 amplitudes have been observed in participants with low no-go accuracy (Beste, Willemssen, Saft, & Falkenstein, 2010; Falkenstein, Hoormann, & Hohnsbein, 1999). The P3 component is a large positive deflection occurring about 150 ms after N2. No-go P3 amplitudes have central distributions, whereas go P3 amplitudes tend to be maximal at parietal sites, termed no-go P3 “anteriorization” (Dimoska, Johnstone, & Barry, 2006; Falkenstein, Hoormann, & Hohnsbein, 1999; Kok, Ramautar, De Ruiter, Band, & Ridderinkhof, 2004; Nieuwenhuis, Yeung, & Cohen, 2004; Smith, Johnstone, & Barry, 2008). In Stop Signal tasks, central no-go P3 amplitudes were greater in successful than in failed inhibition trials (Liotti, Pliszka, Perez, Kothmann, & Woldorff, 2005), and for failed inhibition than for ignored stop signals (Schmajuk, Liotti, Busse, & Woldorff, 2006). Thus, the no-go P3 amplitude may reflect motor inhibition (Smith, Johnstone, & Barry, 2008) or monitoring/evaluation of the inhibition outcome (Schmajuk, Liotti, Busse, & Woldorff, 2006). In short, the N170, N2, and P3 ERP components are reliable indices of perceptual processing, inhibition, and monitoring, respectively.
Few studies have investigated the effects of emotion modulation effect on response inhibition using ERPs, and the results of such studies have been mixed (Chiu, Holmes, & Pizzagalli, 2008; Wang, et al., 2011). Using emotional words as go/no-go cues, Chiu et al. (2008) found that emotional stimuli elicited smaller go N2 and larger go P3 amplitudes than did neutral words, but had no effect on no-go components. The authors interpreted these emotional effects on go components as the allocation of enhanced cognitive resources to neutral stimuli during responses and speculated that the lack of an effect on the no-go component might be due to the relatively weak salience of emotional words. Wang et al. (2011) tested the effects of emotion on behavioral control using a two-choice oddball task with emotional content. Compared with neutral pictures, they observed larger N2 and smaller P3 amplitudes in response to unpleasant pictures, but larger P3 amplitudes in response to pleasant pictures. They suggested that increased cognitive control served to monitor conflict and inhibit responses evoked by irrelevant negative stimuli. However, the inhibitory control evoked by a “do Y” task may be an indirect mechanism, in contrast to the direct inhibitory control evoked by a “do not do X” task (Munakata, et al., 2011). Thus, the results of Wang et al. (2011) may indicate the emotional modulation of indirect inhibition control, rather than an effect on direct inhibition. Unfortunately, previous ERP studies have focused on emotional modulation of inhibition-related components, such as N2 and P3 (Albert, Lopez-Martin, & Carretie, 2010; Chiu, Holmes, & Pizzagalli, 2008; Wang, et al., 2011; Yu, Yuan, & Luo, 2009), but not on perceptual components, such as N1. Thus, whether the prioritization of perceptual processing of emotional cues affects response inhibition remains unclear.
The present study aimed to examine the hypothesis that emotion could modulate response inhibition via increased perceptual processing of emotional content. According to the dual competition framework (Pessoa, 2009), if emotional content enhances response inhibition, we would expect that emotional faces would evoke increased amplitudes or shorter latencies of inhibition-related components relative to neutral faces. Conversely, if emotional content impairs response inhibition, we would expect emotional faces to evoke attenuated amplitudes or delayed latencies of inhibition-related components relative to neutral faces. In either case, we expect emotional faces to elicit larger amplitudes of perception-related components within response inhibition. To evoke emotional salience, we used facial expressions as stimuli (Pessoa, Padmala, Kenzer, & Bauer, 2012).
Method
Participants
Twenty-five young Chinese adults (Asian ethnicity, mean age 23.16 ± 2.85 years, 12 females) participated in this study as paid volunteers. All participants were right handed and had normal or corrected-to-normal vision. None had a history of a neurological or psychiatric disorder. All participants provided written informed consent, and the study was approved by the Institutional Review Board of Beijing Normal University.
Stimuli and Procedure
Emotional stimuli consisted of 120 gray-scale pictures of faces selected from the Chinese Facial Affective Picture System (Wang & Luo, 2005). Forty facial expressions of the 120 individuals (60 females, 60 males) were happy, 40 were neutral, and 40 were unhappy (20 sad, 20 afraid). The size, luminance, background, spatial frequency, and contrast grade of the images were normalized.
We matched the degree of arousal associated with happy and unhappy expressions. In a pilot study, 100 college students (mean age 22.6 years, 50 females) rated the valence and arousal levels of the same 120 facial expressions using a previously described procedure (Britton, Taylor, Sudheimer, & Liberzon, 2006). The valence rating instructions were “Rate how unpleasantly or pleasantly the image makes you feel on a scale ranging from 1 to 9 (1 = very unpleasant, 5 = neutral, 9 = very pleasant).” The arousal rating instructions were “Rate how emotionally intense or aroused the image makes you feel on a scale ranging from 1 to 9 (1 = calm, 5 = somewhat aroused, 9 = excited).” Analysis of variance (ANOVA) revealed significant differences in valence [F (2, 117) = 1397.04, p < .001] and arousal [F (2, 117) = 435.07, p < .001] among happy, neutral, and unhappy expressions. Students gave higher valence ratings to happy expressions (mean 6.9 ± 0.5) than to neutral expressions (mean 4.6 ± 0.3), and higher ratings to neutral than to unhappy expressions (mean 2.7 ± 0.3; both p < .001). Arousal ratings were lower for neutral expressions (mean 6.1 ± 0.7) than for happy (mean 2.7 ± 0.3) and unhappy expressions (mean 6.1 ± 0.7; both p < .001), whereas they did not differ between happy and unhappy expressions (p > .99).
For the main study, participants sat in an electrically shielded, sound-attenuated, dimly lit room facing a computer monitor (cathode-ray tube, refresh rate 100 Hz). Facial expression stimuli were presented in the center of the screen and occupied 5.7 × 6.4° (width × height) of the visual angle. The stimuli were presented for 200 ms, followed by presentation of a blank screen for 800–1200 ms. Instructions regarding which kinds of expression served as a go or no-go cue were presented at the beginning of each block of stimulus presentation. Participants were instructed to press the “space” key as quickly and accurately as possible with an index finger when a go cue was presented and to withhold pressing the “space” key when a no-go cue appeared.
To exclude the oddball effect, we used an equal-probability go/no-go task (Chiu, Holmes, & Pizzagalli, 2008). Given the inclusion of three facial categories, equal numbers of go/no-go trials could not be presented for the three levels of valence and two levels of arousal. Thus, two levels of valence (e.g., happy and neutral or unhappy and neutral) were used in each block. The experiments consisted of four trial blocks: (1) happy go/neutral no-go, (2) happy no-go/neutral go, (3) unhappy go/neutral no-go, and (4) unhappy no-go/neutral go. Within each block, valence and arousal levels and response types had equal probabilities. A total of 40 neutral and 40 emotional (happy or unhappy) expressions were used as go and no-go cues within each block. Each of the four blocks was repeated four times in random order, separated by short breaks (2 minutes), and the response hand was counterbalanced among subjects. Blocks utilizing happy expressions were considered positive and those utilizing unhappy expressions were referred to as negative. Participants completed four short practice blocks (12 trials each) to familiarize themselves with the procedure before the formal ERP experiment. The E-prime software (Psychology Software Tools, Inc., Pittsburgh, PA, USA) was used to present stimuli.
Recording
Electroencephalographic (EEG) data were collected with a 64-channel BrainAmp MR amplifier (Brain Products, Munich, Germany) and Brain Vision Recorder software (version 2.01; Brain Products). Data were sampled at 500 Hz (16-bit precision, 0.01–100-Hz bandwidth) with reference to the right mastoid. Electro-oculographic (EOG) data were recorded above and below the left eye (vertical EOG) and lateral to the outer canthi of the eyes (horizontal EOG). Interelectrode impedance was maintained below 5 kΩ. Trials in which reaction times (RTs) exceeded three standard deviations (SDs) of the individual’s typical performance were considered to represent extreme data and were eliminated.
Data Analysis
RTs were analyzed using two-way repeated-measures ANOVA, with block (positive, negative) and expression (emotional, neutral) characteristics serving as within-subjects factors. Response accuracy was examined using three-way repeated-measures ANOVA, with block (positive, negative), expression (emotional, neutral), and response (go, no-go) types serving as within-subjects factors. To measure the N2 and P3 components, EEG signals were re-referenced offline to the algebraic average of the left and right mastoids (Vogel & Luck, 2000).
To measure the N170 component, EEG signals were re-referenced offline to the average of all scalp sites (Joyce & Rossion, 2005; Muhlberger, et al., 2009). The analyzer performed digital low-pass filtering of individual EEG data with zero phase shift (16 Hz, 24 dB/octave) (Beste, Willemssen, Saft, & Falkenstein, 2010; Sehlmeyer, et al., 2010) and corrected for horizontal and vertical ocular artifacts using a method implemented with BrainVision Analyzer 2 software (Brain Product, Munich, Germany). Stimulus-locked ERPs were segmented into epochs from 200 ms before to 800 ms after stimulus presentation, and baseline-corrected to the pre-stimulus activation. Epochs in which deflection (peak-to-peak) exceeded ± 80 μV were excluded.
The EEG data were averaged separately according to conditions (emotional/neutral × go/no-go × positive/negative) and electrodes. The N170, N2, and P3 components were identified via inspection of the grand-averaged waveforms under each condition. Based on the topographical distribution of grand-averaged ERP activity and data from previous studies (Luo, Feng, He, Wang, & Luo, 2010; Vogel & Luck, 2000), different electrode sets and time windows were selected for these components. Although scoring choices were based on the inspection of data collected in the present study, they were in agreement with extensive published reports on these components in relevant experimental contexts.
The N170 component (130–200 ms) was analyzed at the P7, P8, PO7, and PO8 sites. Since the N2 component is followed by the P3 component, the N2 component (280–400 ms) was measured between 280 to 400 ms after stimuli onset and analyzed at the F1–4, Fz, FC1–4, FCz, C1–4, and Cz electrode sites. The P3 component (300–700 ms) was analyzed at the C1–4, Cz, CP1–4, CPz, P1–4, and Pz electrode sites. The peak amplitudes (baseline to peak) and peak latencies (stimulus onset to peak) of these ERPs were scored at each electrode site. The components were defined by the averaged peak amplitudes and latencies across electrode sites. Component amplitudes and latencies were examined using three-way repeated-measures ANOVA, with block (positive, negative), expression (emotional, neutral), and response (go, no-go) types serving as within-subjects factors. Post-hoc comparisons were made using the Sidak procedure (α < .05). To test the expression effect in go and no-go trials, planned contrasts were performed.
To test the relationship between the perception and inhibition processes, we used Spearman’s rho correlation to examine associations among N170, N2, and P3 amplitudes in no-go trials. Values are reported as means ± SDs throughout the text.
Results
Behavioral Data
Table 1 shows the results of participants’ behavioral performance. RTs to emotional expressions (492 ± 63 ms) were faster than those to neutral expressions [544 ± 68 ms; F (1, 24) = 78.80, p < .001, ]. RTs were faster in positive blocks (508 ± 65 ms) than in negative blocks [527 ± 63 ms; F (1, 24) = 48.91, p < .001, ].
Table 1.
Behavioral Performance in Different Emotional Expression Conditions
| Positive Blocks | Negative Blocks | |||
|---|---|---|---|---|
|
| ||||
| Neutral | Happiness | Neutral | Unhappiness | |
| Go Acc (%) | 96 ± 4 | 97 ± 4 | 96 ± 4 | 97 ± 3 |
| No-go Acc (%) | 98 ± 2 | 94 ± 4 | 97 ± 3 | 94 ± 4 |
| Go RT (ms) | 534 ± 69 | 482 ± 66 | 553 ± 69 | 501 ± 61 |
Note: Go Acc= Go accuracy, No-go Acc=No-go accuracy, Go RT=Go response time, Values presented as Mean(Standard Deviation), ms = millisecond.
The accuracy of responses showed a response type × expression interaction effect [F (1, 24) = 49.23, p < .001, ; Figure 1A]. No-go responses to emotional expressions (94% ± 4%) were less accurate than those to neutral expressions (97% ± 2%, p < .001), but the accuracy of go responses did not differ between emotional and neutral expressions (p = .15). These findings suggest that emotional content selectively impaired behavior inhibition.
Figure 1.
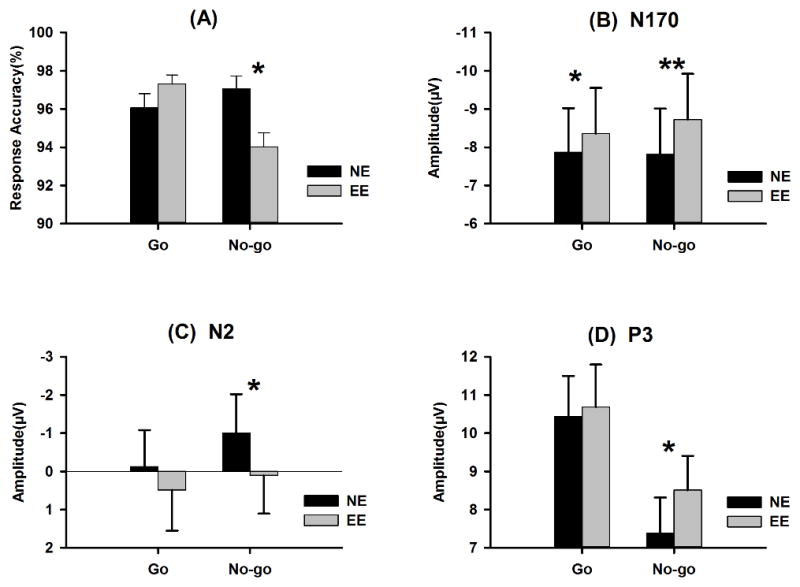
Mean response accuracies (A), N170 (B), N2 (C) and P3 (D) amplitudes in no-go and go trials, specified for emotional and neutral expressions. NE: neutral expression, EE: emotional expression
ERP Data
Grand mean ERPs for go and no-go stimuli, emotional and neutral expressions, and positive and negative blocks are shown in Figures 2–4, respectively. Response accuracy and the effects of the interaction between expression and response type on N170, N2, and P3 amplitudes are shown in Figure 1. Effect summaries for the N170, N2, and P3 components are shown in Table 2.
Figure 2.
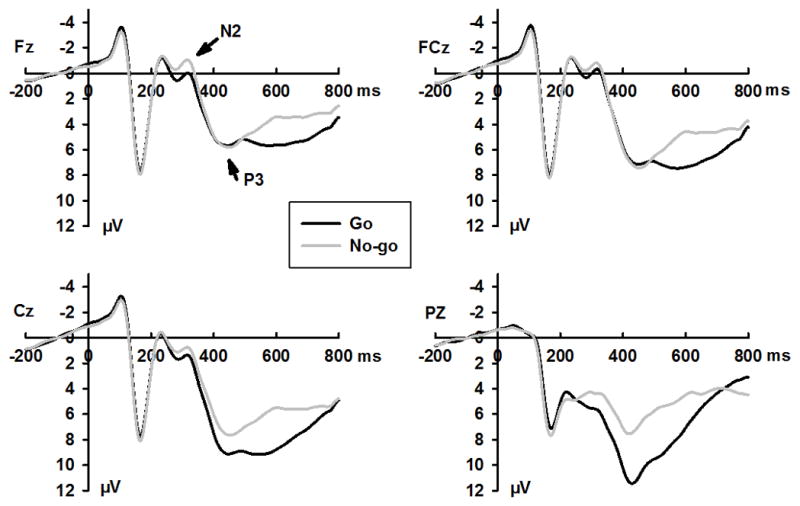
Grand averaged ERPs waveform for no-go and go trials at selected sites
Figure 4.
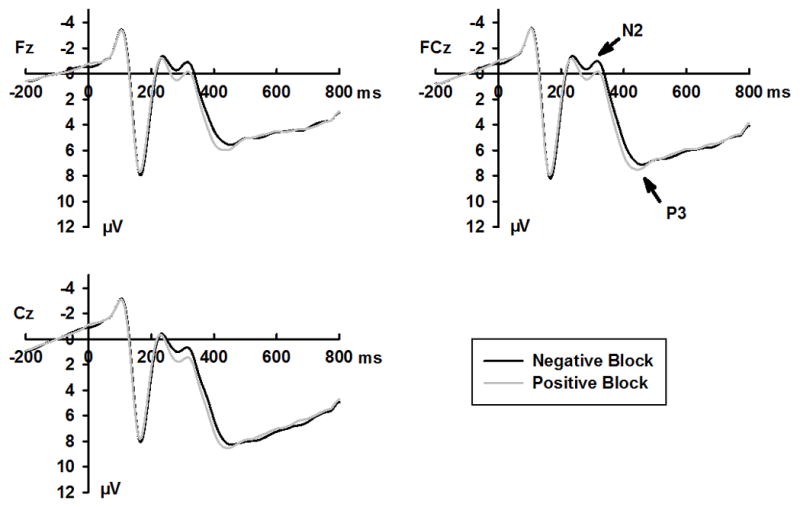
Grand averaged ERPs waveform for positive and negative blocks at selected sites
Table 2.
Signi cant Results for the ERP Components (df=1, 24)
| Component | Effect | F | P |
|
||
|---|---|---|---|---|---|---|
| N170 | amplitude | E | 29.6 | .001 | 0.57 | |
| E × R | 6.2 | .020 | 0.21 | |||
| latency | E × B | 7.5 | .011 | 0.24 | ||
| N2 | amplitude | R | 5.3 | .030 | 0.18 | |
| E | 11.9 | .002 | 0.35 | |||
| B | 7.0 | .014 | 0.23 | |||
| P3 | amplitude | R | 13.9 | .001 | 0.37 | |
| E | 5.8 | .024 | 0.19 | |||
| latency | B | 24.6 | .000 | 0.51 | ||
| R | 5.1 | .034 | 0.17 | |||
| E × R | 15.1 | .001 | 0.39 | |||
Note for this table: R, Response Type (go vs. no-go); E, Expression (emotional vs. neutral); B, Block (positive vs. negative).
N170
The N170 amplitudes for emotional expressions (−8.54 ± 5.96 μV) were larger than those for neutral expressions (−7.84 ± 5.87 μV; Figure 2, Table 2). A response type × expression interaction effect was observed (Table 2). Although N170 amplitudes were larger for emotional than for neutral expressions in go (p = .004) and no-go (p < .001) trials, the effect size of expression (emotional – neutral) was larger in no-go (0.91 μV) than in go (0.49 μV) trials (Figure 1B). This enhanced emotional processing in no-go trials might be the result of larger N170 amplitudes elicited by emotional expressions in no-go (−8.73 ± 5.97 μV) than in go (−8.36 ± 5.96 μV) trials (p < .001), whereas neutral expressions did not elicit such an effect (go, −7.87 ± 5.77 μV; no-go, −7.82 ± 5.98 μV; p > .73).
N2
N2 amplitudes were larger in no-go (−0.45 ± 4.95 μV) than in go (0.18 ± 4.99 μV) trials (Figure 2, Table 2). Neutral expressions elicited larger N2 amplitudes (−0.57 ± 4.81 μV) than did emotional expressions (0.30 ± 5.11 μV). Larger N2 amplitudes were also observed in negative blocks (−0.45 ± 4.93 μV) than in positive blocks (0.18 ± 4.99 μV; Figure 4). No response type × expression interaction effect was observed [F (1, 24) = 1.03, p = .32, ]. The planned contrasts revealed that neutral expressions elicited larger N2 amplitudes than did emotional expressions in no-go trials (−1.00 ± 5.07 μV, Vs. 0.11 ± 5.00 μV, p = .004), but not in go trials (−0.13 ± 4.77 μV, Vs. 0.49 ± 5.35 μV, p = .10; Figure 1C).
P3
The P3 amplitudes showed the main effect with response type, as amplitudes were larger for go trials (10.57 ± 5.34 μV) than for no-go trials (7.95 ± 4.45 μV; Figure 2, Table 2). Emotional expressions elicited larger P3 amplitudes (9.60 ± 4.66 μV) than did neutral expressions (8.92 ± 4.63 μV; Table 2). The response type × expression interaction had a marginal effect [F (1, 24) = 3.29, p = .082, ]. Planned contrasts revealed that emotional expressions elicited larger P3 amplitudes than neutral expressions in no-go trials (8.51 ± 4.47 μV vs. 7.389 ± 4.64 μV, p = .009), but not in go trials (10.69 ± 5.52 μV vs. 10.44 ± 5.29 μV, p = .484; Figure 1D). P3 latencies showed a significant expression × response type interaction (Table 2). No-go P3 latencies were longer for emotional expressions (451.61 ± 39.27 ms) than for neutral expressions (431.76 ± 41.08 ms, p = .001), but go P3 latency did not differ between emotional (456.82 ± 42.04 ms) and neutral (463.67 ± 52.40 ms) expressions (p = .17).
Correlation analyses revealed that no-go N170 amplitudes were negatively correlated with no-go N2 (r = −.61, p < .001) and P3 (r = −0.58, p < .01) amplitudes. Because no-go N170 and N2 amplitudes were negative-going, the negative correlation indicated that larger (more negative) N170 amplitudes were associated with smaller (more positive) N2 amplitudes.
Discussion
The behavioral and ERP data revealed that emotional expressions impaired response inhibition. Inhibitory accuracy and no-go N2 amplitudes were decreased for emotional expressions in comparison with neutral expressions. More importantly, emotional expressions elicited larger N170 amplitudes than did neutral expressions, and this effect was larger in no-go than in go trials. Moreover, increased no-go N170 amplitudes were correlated with decreased no-go N2 amplitudes. Taking all of these data into account, impaired response inhibition might reasonably be considered to be the result of increased perceptual processing of emotional content in no-go trials.
Our finding of lower inhibitory accuracy in response to emotional than to neutral expressions is in line with previous findings (Hare, et al., 2008; Schulz, et al., 2007). For example, Schulz et al. (2007) found that subjects’ performance was worse in emotional than in non-emotional go/no-go tasks. Impaired response inhibition was also reflected by attenuated no-go N2 amplitudes elicited by emotional faces; such attenuation has been observed in subjects with low no-go accuracy (Beste, Willemssen, Saft, & Falkenstein, 2010; Falkenstein, Hoormann, & Hohnsbein, 1999). For example, Falkenstein et al. (1999) observed lower no-go N2 amplitudes in subjects with high false-alarm rates than in those with low false-alarm rates in a go/no-go task. Increased no-go N2 amplitudes have also been observed under conditions of further inhibition, such as response inhibition under pronounced time pressure (Jodo & Kayama, 1992) and primed by response cues (Kopp, Mattler, Goertz, & Rist, 1996). Thus, our data showed that emotional expressions impaired response inhibition at the behavioral and electrophysiological levels.
Impaired response inhibition may result from competition for processing resources due to increased perceptual processing of emotional content in no-go trials. The present study revealed that no-go N170 and N2 amplitudes were negatively correlated, indicating that stronger perceptual processing was associated with weaker inhibition processing. Emotional expressions elicited larger N170 amplitudes in no-go than in go trials, indicating that more resources were recruited for the perceptual processing of inhibition cues than for the processing of response cues in the presence of emotional content. In contrast with the enhanced no-go N170 component, the inhibition-related no-go N2 component showed attenuated amplitudes and decreased accuracy of response inhibition in trials with emotional content. Hence, it is reasonable to associate impaired response inhibition with stronger emotional processing.
It has been reported that the perception processing of inhibition cues may be associated with response inhibition. Boehler et al. (2009) found that go stimuli elicited larger N1 amplitudes in unsuccessful stop trials than in successful stop trials, but the N1 Stop Signal component was larger for successful than for unsuccessful stop trials. They interpreted these results as indicating that enhanced processing of go stimuli facilitated response, whereas enhanced processing of Stop Signals facilitated inhibition (Boehler, et al., 2009). In studies employing go/no-go tasks, no-go trials are often compared with go trials. The N1 component does not differ between no-go and go trials in adult participants (Eimer, 1993), which may indicate that perceptual processing leads to successful responses as well as successful inhibition. Thus, the competition hypothesis may be suitable for interpreting impaired response inhibition in emotional trials. An alternative interpretation is that emotional stimuli inhibit the recruitment of cognitive resources for inhibition processing. Unfortunately, we could not exclude this possibility in the present study.
The results of the present study support the hypothesis that the “hard” prioritization of emotional processing impaired behavioral performance (Pessoa, 2009). According to the dual competition framework (Pessoa, 2009), emotional content may improve or degrade performance depending on its threat level. The threat level of facial expressions, particularly those associated with positive valence, was inferred a priori to be relatively low, leading to the expectation of improved performance; however, performance impairment was observed in this study. Emotional expressions have been found to impair behavior performance in other studies, as reflected by factors such as decreased no-go accuracy (Hare, et al., 2008; Schulz, et al., 2007) and slower SSRTs (Pessoa, Padmala, Kenzer, & Bauer, 2012). We thus speculated that the “hard” prioritization of emotional processing might occur only when either the threat level or the significance of the emotional content is high. First, information used to guide individual behavior, such as no-go signaling, is important for survival and social adaption. Second, emotional facial expressions provide information about the expresser’s feelings and intentions. Thus, the processing of emotional stimuli containing significant clues about ongoing behavior is important for survival in a given context. Consequently, integrated information about response inhibition and emotion may be more significant than information about either factor alone. Previous studies have suggested that the N170 component reflects the encoding of important relational features, such as emotional expressions (Itier & Taylor, 2004; Luo, Feng, He, Wang, & Luo, 2010), the self (Keyes, Brady, Reilly, & Foxe, 2010), and personally important faces (Caharel, et al., 2002; Herzmann, Schweinberger, Sommer, & Jentzsch, 2004). For example, the image of a participant’s own face increased the N170 amplitude compared with images of a friend or stranger (Keyes, Brady, Reilly, & Foxe, 2010). In line with these findings, the present data showed that emotional cues evoked larger no-go N170 amplitudes than did neutral go cues, suggesting that emotional no-go cues are more significant than emotional go cues in humans. In addition, rewards can impair response inhibition: Padmal et al. (2010) found that SSRTs were longer under reward (for correct go responses) conditions than under neutral control conditions, with attenuated activation of a subset of regions related to inhibition, such as the right inferior frontal gyrus, left precentral gyrus, and bilateral putamen. The impaired response inhibition were not attributable to reward-facilitated go responses because go RTs were not shortened under the reward condition compared with the neutral condition. In that study (Padmala & Pessoa, 2010), the reward condition was thus a significant, but not high-threat, condition relative to the neutral condition. Future studies should compare the effects of significant and high-threat affective stimuli on executive function.
The P3 component also reflected impaired response inhibition. In line with the results of previous studies (Bokura, Yamaguchi, Matsubara, & Kobayashi, 2002; Falkenstein, Hoormann, & Hohnsbein, 2002), the no-go P3 distribution showed more anterior topography than the go P3. The no-go P3 component may indicate the evaluation of inhibition processing outcomes (Beste, Willemssen, Saft, & Falkenstein, 2010; Schmajuk, Liotti, Busse, & Woldorff, 2006). Several studies, including the present study, have found that emotional expressions elicited larger P3 amplitudes than neutral expressions in expression-recognizing tasks, indicating that greater attention resources are devoted to emotional expressions (Muhlberger, et al., 2009; Williams, Palmer, Liddell, Song, & Gordon, 2006). Williams et al. (2006) found an enhanced P3 amplitude (280–450 ms) in response to expressions of fear relative to neutral expressions, suggesting that signals of potential danger serve to enhance ongoing stimulus elaboration and context evaluation. ERP data obtained in the present study also showed longer latencies in response to emotional than to neutral expressions in the no-go P3, but not the go P3, component. Furthermore, Maguire et al. (2009) found that the no-go P3 latency elicited by a difficult task was significantly longer than that evoked by relatively easy tasks. Similarly, we found that participants had more difficulty inhibiting responses to emotional expressions than to neutral expressions, as indicated by reduced no-go accuracy for emotional expressions. Thus, the P3 effect may reflect the impairment of inhibition by emotional content. These results may reflect the selective occurrence of enhanced elaboration and evaluation in response inhibition trials involving go/no-go tasks.
Emotion modulates the N2 and P3 components during response inhibition tasks, but the effect may vary depending on the type of emotional content and the frequency of emotional stimuli. Chiu et al. (2008) found that emotional words elicited smaller N2 and larger P3 amplitudes than did neutral words in go, but not in no-go, trials. The difference between these results and ours may reflect our use of emotional expressions, which have more direct biological and social relevance than emotional words (Schacht & Sommer, 2009). Facial expressions provide the perceiver not only with emotional information about an individual’s affective state, but also with potential action cues. Wang et al. (2011) observed larger N2 and smaller P3 amplitudes (deviant–standard difference) in response to unpleasant than to neutral pictures and larger P3 amplitudes in response to pleasant than to neutral pictures. However, deviant stimuli (emotional pictures) were presented less frequently (15%) than standard stimuli (85%) (Wang, et al., 2011). Because the N2 amplitude is enhanced for infrequent stimuli (Nieuwenhuis, Yeung, Wery, & Ridderinkhof, 2003), the findings of Wang et al. (2011) may be confounded by the rare probability of deviant stimuli.
Conclusion
In this study, we measured the behavioral and ERP effects of the modulation of emotional expression on response inhibition. Subjects’ performance was less accurate when emotional expressions directed response inhibition. Consistent with this impaired behavioral performance, emotional expressions elicited attenuated no-go N2 amplitudes and increased no-go P3 amplitudes and latency relative to neutral expressions. More importantly, the perceptual processing of emotional content was overactivated in response inhibition trials, as indicated by selectively increased no-go N170 amplitudes in response to emotional expressions. These results support the hypothesis that emotional content impairs executive function due to the prioritization of emotional content processing (Pessoa, 2009).
Figure 3.
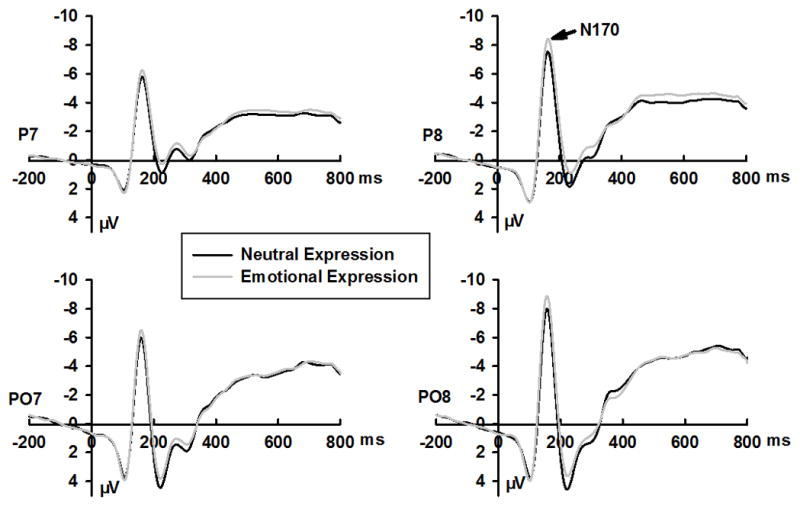
Grand averaged ERPs waveform for neutral and emotional expressions at selected sites
Acknowledgments
This work was supported by the First-class Disciplines of Shanghai Colleges and Universities (Psychology) and the Engagement Fund of Outstanding Doctoral Dissertation of Beijing Normal University (08047) grants to Suyong Yang, the NSFC (30930031), Ministry of Science & Technology 973 Program (2014CB744600, 2011CB711000), the foundation of the National Key laboratory of Human Factors Engineering (HF2012-K-03), the Fundamental Research Funds for the Central Universities grants to Yuejia Luo, and the National Institutes of Health (UL1RR033173; UL1TR000117; TL1TR000115) grants to L. S. Broster.
References
- Albert J, Lopez-Martin S, Carretie L. Emotional context modulates response inhibition: neural and behavioral data. Neuro Image. 2010;49:914–921. doi: 10.1016/j.neuroimage.2009.08.045. [DOI] [PubMed] [Google Scholar]
- Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological Studies of Face Perception in Humans. J Cogn Neurosci. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C, Willemssen R, Saft C, Falkenstein M. Response inhibition subprocesses and dopaminergic pathways: Basal ganglia disease effects. Neuropsychologia. 2010;48:366–373. doi: 10.1016/j.neuropsychologia.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Boehler CN, Munte TF, Krebs RM, Heinze HJ, Schoenfeld MA, Hopf JM. Sensory MEG responses predict successful and failed inhibition in a stop-signal task. Cereb Cortex. 2009;19:134–145. doi: 10.1093/cercor/bhn063. [DOI] [PubMed] [Google Scholar]
- Bokura H, Yamaguchi S, Matsubara M, Kobayashi S. Frontal lobe contribution to response inhibition process--an ERP study and aging effect. International Congress Series. 2002;1232:17–20. [Google Scholar]
- Britton JC, Taylor SF, Sudheimer KD, Liberzon I. Facial expressions and complex IAPS pictures: Common and differential networks. Neuro Image. 2006;31:906–919. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Caharel S, Poiroux S, Bernard C, Thibaut F, Lalonde R, Rebai M. ERPs associated with familiarity and degree of familiarity during face recognition. International Journal of Neuroscience. 2002;112:1499–1512. doi: 10.1080/00207450290158368. [DOI] [PubMed] [Google Scholar]
- Chiu PH, Holmes AJ, Pizzagalli DA. Dissociable recruitment of rostral anterior cingulate and inferior frontal cortex in emotional response inhibition. Neuro Image. 2008;42:988–997. doi: 10.1016/j.neuroimage.2008.04.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimoska A, Johnstone SJ, Barry RJ. The auditory-evoked N2 and P3 components in the stop-signal task: Indices of inhibition, response-conflict or error-detection? Brain and Cognition. 2006;62:98–112. doi: 10.1016/j.bandc.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Eimer M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biological Psychology. 1993;35:123–138. doi: 10.1016/0301-0511(93)90009-w. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI. Neuro Report. 2000;11:1739–1744. doi: 10.1097/00001756-200006050-00028. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychologica. 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. Inhibition-related ERP components: Variation with modality, age, and time-on-task. Journal of Psychophysiology. 2002;16:167–175. [Google Scholar]
- Goldstein M, Brendel G, Tuescher O, Pan H, Epstein J, Beutel M, Yang Y, Thomas K, Levy K, Silverman M, Clarkin J, Posner M, Kernberg O, Stern E, Silbersweig D. Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: An emotional linguistic go/no-go fMRI study. Neuro Image. 2007;36:1026–1040. doi: 10.1016/j.neuroimage.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biological Psychiatry. 2005;57:624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological Substrates of Emotional Reactivity and Regulation in Adolescence During an Emotional Go-Nogo Task. Biological Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzmann G, Schweinberger SR, Sommer W, Jentzsch I. What’s special about personally familiar faces? A multimodal approach. Psychophysiology. 2004;41:688–701. doi: 10.1111/j.1469-8986.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- Hopf JM, Vogel E, Woodman G, Heinze HJ, Luck SJ. Localizing visual discrimination processes in time and space. J Neurophysiol. 2002;88:2088–2095. doi: 10.1152/jn.2002.88.4.2088. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Taylor MJ. N170 or N1? Spatiotemporal differences between object and face processing using ERPs. Cerebral Cortex. 2004;14:132–142. doi: 10.1093/cercor/bhg111. [DOI] [PubMed] [Google Scholar]
- Jodo E, Kayama Y. Relation of a negative ERP component to response inhibition in a Go/No-go task. Electroencephalography and Clinical Neurophysiology. 1992;82:477–482. doi: 10.1016/0013-4694(92)90054-l. [DOI] [PubMed] [Google Scholar]
- Joyce C, Rossion B. The face-sensitive N170 and VPP components manifest the same brain processes: The effect of reference electrode site. Clinical Neurophysiology. 2005;116:2613–2631. doi: 10.1016/j.clinph.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Keyes H, Brady N, Reilly RB, Foxe JJ. My face or yours? Event-related potential correlates of self-face processing. Brain and Cognition. 2010;72:244–254. doi: 10.1016/j.bandc.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Kok A, Ramautar JR, De Ruiter MB, Band GPH, Ridderinkhof KR. ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology. 2004;41:9–20. doi: 10.1046/j.1469-8986.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- Kopp B, Mattler U, Goertz R, Rist F. N2, P3 and the lateralized readiness potential in a nogo task involving selective response priming. Electroencephalography and Clinical Neurophysiology. 1996;99:19–27. doi: 10.1016/0921-884x(96)95617-9. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Granic I, Lamm C, Zelazo PD, Stieben J, Todd RM, Moadab I, Pepler D. Changes in the neural bases of emotion regulation associated with clinical improvement in children with behavior problems. Development and Psychopathology. 2008;20:913–939. doi: 10.1017/S0954579408000448. [DOI] [PubMed] [Google Scholar]
- Liotti M, Pliszka SR, Perez R, Kothmann D, Woldorff MG. Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex. 2005;41:377–388. doi: 10.1016/s0010-9452(08)70274-0. [DOI] [PubMed] [Google Scholar]
- Luo WB, Feng WF, He WQ, Wang NY, Luo YJ. Three stages of facial expression processing: ERP study with rapid serial visual presentation. Neuro Image. 2010;49:1857–1867. doi: 10.1016/j.neuroimage.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire MJ, Brier MR, Moore PS, Ferree TC, Ray D, Mostofsky S, Hart J, Jr, Kraut MA. The influence of perceptual and semantic categorization on inhibitory processing as measured by the N2-P3 response. Brain and Cognition. 2009;71:196–203. doi: 10.1016/j.bandc.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Simmonds DJ. Response inhibition and response selection: Two sides of the same coin. Journal of Cognitive Neuroscience. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Muhlberger A, Wieser MJ, Herrmann MJ, Weyers P, Troger C, Pauli P. Early cortical processing of natural and artificial emotional faces differs between lower and higher socially anxious persons. Journal of Neural Transmission. 2009;116:735–746. doi: 10.1007/s00702-008-0108-6. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O Reilly RC. A unified framework for inhibitory control. Trends in Cognitive Sciences. 2011;15:453–459. doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES. Emotional bias and inhibitory control processes in mania and depression. Psychological Medicine. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Cohen JD. Stimulus modality, perceptual overlap, and the go/no-go N2. Psychophysiology. 2004;41:157–160. doi: 10.1046/j.1469-8986.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Wery VdW, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Interactions between cognition and motivation during response inhibition. Neuropsychologia. 2010;48:558–565. doi: 10.1016/j.neuropsychologia.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends in Cognitive Sciences. 2009;13:160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, Kenzer A, Bauer A. Interactions Between Cognition and Emotion During Response Inhibition. Emotion. 2012;12:192–197. doi: 10.1037/a0024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht A, Sommer W. Emotions in word and face processing: Early and late cortical responses. Brain and Cognition. 2009;69:538–550. doi: 10.1016/j.bandc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Schmajuk M, Liotti M, Busse L, Woldorff MG. Electrophysiological activity underlying inhibitory control processes in normal adults. Neuropsychologia. 2006;44:384–395. doi: 10.1016/j.neuropsychologia.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Clerkin SM, Halperin JM, Newcorn JH, Tang CY, Fan J. Dissociable neural effects of stimulus valence and preceding context during the inhibition of responses to emotional faces. Human Brain Mapping. 2009;30:2821–2833. doi: 10.1002/hbm.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KP, Fan J, Magidina O, Marks DJ, Hahn B, Halperin JM. Does the emotional go/no-go task really measure behavioral inhibition?: Convergence with measures on a non-emotional analog. Archives of Clinical Neuropsychology. 2007;22:151–160. doi: 10.1016/j.acn.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C, Konrad C, Zwitserlood P, Arolt V, Falkenstein M, Beste C. ERP indices for response inhibition are related to anxiety-related personality traits. Neuropsychologia. 2010;48:2488–2495. doi: 10.1016/j.neuropsychologia.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Collins SH, Blumberg HP. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. NeuroImage. 2006;31:468–475. doi: 10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Smith JL, Johnstone SJ, Barry RJ. Movement-related potentials in the Go/NoGo task: The P3 reflects both cognitive and motor inhibition. Clinical Neurophysiology. 2008;119:704–714. doi: 10.1016/j.clinph.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;37:190–203. [PubMed] [Google Scholar]
- Wang Y, Luo Y-j. Standardization and Assessment of College Students’ Facial Expression of Emotion. Chinese Journal of Clinical Psychology. 2005;13:396–398. [Google Scholar]
- Wang Y, Yang J, Yuan J, Fu A, Meng X, Li H. The impact of emotion valence on brain processing of behavioral inhibitory control: Spatiotemporal dynamics. Neuroscience Letters. 2011;502:112–116. doi: 10.1016/j.neulet.2011.07.039. [DOI] [PubMed] [Google Scholar]
- Williams LM, Palmer D, Liddell BJ, Song L, Gordon E. The ‘when’ and ‘where’ of perceiving signals of threat versus non-threat. Neuro Image. 2006;31:458–467. doi: 10.1016/j.neuroimage.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Yu F, Yuan J, Luo YJ. Auditory-induced emotion modulates processes of response inhibition: an event-related potential study. Neuro Report. 2009;20:25–30. doi: 10.1097/WNR.0b013e32831ac9b1. [DOI] [PubMed] [Google Scholar]


