Abstract
Introduction
Maintenance of high tissue oxygenation (PtO2) is recommended during surgery because PtO2 is highly predictive of surgical site infection and colonic anastomotic leakage. However, surgical site perfusion is often sub-optimal, creating an obstructive hurdle for traditional, systemically applied therapies to maintain or increase surgical site PtO2. This research tested the hypothesis that insufflation of humidified-warm CO2 into the abdominal cavity would increase sub-peritoneal PtO2 during open abdominal surgery.
Materials and Methods
15 Wistar rats underwent laparotomy under general anesthesia. Three sets of randomized cross-over experiments were conducted in which the abdominal cavity was subjected to alternating exposure to 1) humidified-warm CO2 & ambient air; 2) humidified-warm CO2 & dry-cold CO2; and 3) dry-cold CO2 & ambient air. Sub-peritoneal PtO2 and tissue temperature were measured with a polarographic oxygen probe.
Results
Upon insufflation of humidified-warm CO2, PtO2 increased by 29.8 mmHg (SD 13.3; p<0.001), or 96.6% (SD 51.9), and tissue temperature by 3.0°C (SD 1.7 p<0.001), in comparison with exposure to ambient air. Smaller, but significant, increases in PtO2 were seen in experiments 2 and 3. Tissue temperature decreased upon exposure to dry-cold CO2 compared with ambient air (-1.4°C, SD 0.5, p = 0.001).
Conclusions
In a rat model, insufflation of humidified-warm CO2 into the abdominal cavity during open abdominal surgery causes an immediate and potentially clinically significant increase in PtO2. The effect is an additive result of the delivery of CO2 and avoidance of evaporative cooling via the delivery of the CO2 gas humidified at body temperature.
Introduction
Maintenance of adequate tissue perfusion and oxygenation is a fundamental principle taught to surgeons in training [1, 2]. Low tissue oxygenation is highly predictive of surgical site infection [3–5], as tissue oxygen partial pressure (PtO2) drives the production of bactericidal superoxide by phagocytes [6–8]. PtO2 predicts collagen deposition, and therefore wound strength [9–11] and integrity or leakage of colonic anastomoses [12]. Angiogenesis depends on adequate oxygenation and is enhanced by high PtO2 [13]. As such, increasing peri-operative PtO2 is a common recommendation for the prevention of surgical complications [1, 13–16].
Interventions to increase peri-operative PtO2 are traditionally applied systemically, usually under the control of the anesthetist rather than the surgeon. These include manipulating inspired gases to achieve increased arterial oxygen tension (PaO2) [4, 17–19] or hypercapnia [20–23]; reduction of sympathetic vasoconstriction by minimizing pain [24], cooling [25], hypovolemia [10, 19, 26] and nicotine [18, 27]; and by the use of regional anesthesia [28–30]. However, to achieve increased PtO2 specifically at the surgical site, where it is required to maximize surgical site healing and to minimize surgical site sepsis, these systemic treatments all critically depend on adequate surgical site tissue perfusion and, in spite of these techniques, surgical site perfusion is frequently suboptimal [3, 10, 18]. An intervention that can locally increase surgical site perfusion and PtO2 may be clinically important, as it may be able to overcome poor perfusion that is often observed in surgical patients.
Insufflation of humidified-warm CO2 into the abdominal cavity has been proposed as a therapy to increase surgical site tissue perfusion and PtO2 during open abdominal surgery [31]. The CO2 is heated to body temperature and humidified to avoid evaporative heat loss, as evaporative cooling contributes up to 50% of heat loss during open abdominal surgery [32]. Using an active humidification system and a specially designed gas diffuser, humidified-warm CO2 can be diffused into the open abdominal cavity at a low velocity, while at a flow rate high enough to create a local environment with a high concentration of CO2 [33–36]. Both delivery of CO2 and maintenance of a warmer tissue temperature, by reducing evaporative heat loss, are hypothesized to have a direct vasodilatory effect on local tissue [31]. Furthermore, CO2 may increase PtO2 via the Bohr Effect, a right shift in the oxygen hemoglobin dissociation curve resulting in release of more oxygen from hemoglobin [31]. The Bohr Effect may be a direct result of both increased local CO2 and the subsequent decrease in local pH, and may be further amplified by reducing evaporative cooling [31].
Previous research supports the hypothesis that both exposure to CO2 and maintenance of tissue temperature will contribute to an increase in PtO2. Topical CO2 has been shown to increase blood flow [37–39] and PtO2 [39] in skin. Intra-abdominal CO2 has been shown to increase sub-peritoneal PtO2 during laparoscopic surgery compared with open abdominal surgery without CO2 insufflation, both in humans [40] and in a murine model [41]. Furthermore, local warming significantly increases subcutaneous PtO2 [25, 42] and can increase capillary blood flow by 17-fold in the presence of vasoconstriction [25]. Insufflation of humidified-warm CO2 into the abdominal cavity increases wound temperature during open colorectal surgery [43, 44]. However, the magnitude of the influence of insufflation of humidified or dry CO2 into the abdominal cavity during open abdominal surgery has not yet been measured.
This research was designed to test the hypothesis that insufflation of humidified-warm CO2 into the open abdominal cavity during surgery would increase local PtO2 due to a combination of exposure to CO2, as well as by reduction in evaporative cooling in the abdominal cavity. We hypothesized that insufflation of either humidified-warm CO2, or dry-cold CO2, into the abdominal cavity during open abdominal surgery would increase sub-peritoneal PtO2, compared with laparotomy without gas insufflation. Furthermore, we hypothesized that insufflation of humidified-warm CO2 would increase sub-peritoneal PtO2 compared with insufflation of dry-cold CO2.
Materials and Methods
Ethics and animal care
Approval for this study was granted by the University of Wollongong Animal Ethics Committee (AE 10–24). Female Wistar rats were used in strict accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes [45]. All surgery was performed under isoflurane anesthesia, and all efforts were made to minimize suffering. Prior to surgery, the rats were housed two rats to a cage with ad-libitum access to food and water, and with PVC tubes as environment enrichment. The rats were maintained in a temperature controlled environment with diurnal variation of light and monitoring for signs of health every 1–2 days. Immediately following the experiments, the rats were euthanized by carbon dioxide asphyxiation, while still anesthetized.
Induction and monitoring of general anesthesia
All rats were weighed and then pre-warmed in the animal handler’s hands for 10 minutes immediately prior to induction of general anesthesia, using a blanket and heating lamp. General anesthesia was induced with inhalant isoflurane in air via a nose cone. Throughout anesthesia the rats rested on a Small Animal Far Infra-Red Warming Pad (Kent Scientific Corporation, Connecticut, USA) to maintain body temperature. Following induction, the rats underwent endotracheal intubation with a 16 G blunted intravenous catheter under direct vision of the entrance to trachea, illuminated by a red LED shone through the skin of the throat. The endotracheal catheter was connected to a ventilator (Rodent Ventilator 7025, Ugo Basile, Varese, Italy) with ventilator gas flow of 350 ml/kg/min, with a tidal volume of 1 ml, as pilot measurements showed that this minute ventilation maintained PaCO2 in a normal range for all treatment groups. The endotracheal catheter was secured around the snout of the rat and a small piece of gauze was placed in the mouth to collect oral secretions. Depth of anesthesia was monitored via continuous measurement of heart rate and pulse pressure using a pressure transducer placed over the skin of the neck in the area of the carotid artery, and also by arterial oxygen hemoglobin saturation monitoring using a pulse oximeter (Animal Oximeter Pod ML325/AC, AD Instruments, Dunedin, New Zealand) placed on a paw. Core body temperature was measured every 5–10 minutes with a rectal thermometer (Surgipak Flexible Digital Thermometer, Vega Technologies Inc., Taipei, Taiwan). An insulating “sock” was placed over the rat’s tail to reduce radiated heat loss. A heat lamp was positioned approximately 25cm above the rat, and was only used if the rat’s temperature was falling to below 36°C. Any use of the heat lamp was recorded. Ambient temperature and humidity of the operating room was recorded with a hygrometer (HygroPalm22 Portable Humidity & Temperature Meter, Rotronic, Switzerland). Insensible fluid loss was replaced with warmed 0.9% sodium chloride delivered sub-cutaneously at 10 ml/kg/hr, once an hour, in accordance to Australian guidelines for the promotion of wellbeing of animals used for scientific purposes [46].
Surgical procedure
Following intubation, the abdomen was clipped with an electric hair clipper (Oster Golden A5 two speed, Model 5-50A, Sunbeam Products, Florida, USA). An inverted “L” shaped laparotomy incision was then made to create adequate exposure of the parietal peritoneum for insertion of the tissue oxygen partial pressure (PtO2) probe. This consisted of a 60 mm long midline laparotomy incision, starting approximately 10 mm caudal to the xiphoid process. A further 40 mm long perpendicular extension of that incision was then made across the left side of the abdominal wall, from the rostral end of the first incision. A small clamp (5mm wide) was attached to the skin of the abdominal wall flap at the intersection point of the two incisions. The abdominal wall was then gently reflected towards the lower left quadrant so as to expose the parietal peritoneum. The skin was clamped so as to minimize tension on the peritoneum. To further expose the parietal peritoneum, by way of relaxation of the left abdominal wall, the left hind leg was flexed and secured using tape across the foot.
Measurement of Tissue Oxygen Partial Pressure
PtO2 was measured using a combined temperature and polarographic oxygen tension probe (Licox CC1P1, Gesellschaft fu¨r Medizinische Sondensysteme, GmBH, Kiel, Germany). Each probe is calibrated by the manufacturer and is supplied with an individual calibration card that is inserted into the monitor prior to use. The accuracy of the probe is ±10% for PtO2 and ± 0.2 C for temperature. To position the PtO2 probe a 16G intra venous catheter was tunneled beneath the peritoneal membrane from the lower left quadrant to the upper left quadrant under direct vision. The needle was then removed and the PtO2 probe was inserted into the catheter. Finally, the catheter was gently retracted so that a minimum of 30 mm length of probe remained within the tissue, ensuring that the measurement portion of the probe was fully embedded. Dissection conducted during pilot investigations showed that the probe was embedded in the musculature of the abdominal wall. The PtO2 probe was then connected to a Licox CMP Oxygen and Temperature Monitor (Gesellschaft fu¨r Medizinische Sondensysteme, GmBH, Kiel, Germany) to allow continuous recording of PtO2 and tissue temperature throughout the experiment.
Experimental design
Three sets of experiments were conducted in a randomized cross-over design. The first treatment was randomized and then treatment was alternated so that each rat received both treatments at least twice. Treatments were administered for a minimum of 15 minutes, ensuring that any initial steep change in PtO2 was captured in its entirety.
Experiment 1: 5 rats alternatively exposed to insufflation of humidified-warm CO2 or ambient air
Experiment 2: 7 rats alternatively exposed to insufflation of humidified-warm CO2 or dry-cold CO2 insufflation
Experiment 3: 3 rats alternatively exposed to insufflation of dry-cold CO2 or ambient air
Sample size calculation for experiment one was based on a minimal clinically significant difference between conditions of 15 mmHg, between subject standard deviation of 35 mmHg, correlation between observation on the same subject as 0.7, α = 0.0, β = 0.20, and assuming two crossovers per animal. Sample size calculations for the subsequent experiments were carried out at the completion of each experiment with the aim of minimizing the number of animals used. This resulted in a different number of animals per experiment.
Treatment conditions
Preliminary testing showed that the abdominal cavity of a rat is too shallow to create a local environment of high CO2 gas concentration. This is due to the relatively thin abdominal wall of the rat compared with humans, and the minimal use of retraction in this model in order to protect the peritoneum from physical trauma. To ensure the abdomen was exposed to a stable high concentration of CO2, the rat was placed in a plastic container with a 9 x 12 cm hole in the top through which the CO2 was insufflated. CO2 being heavier than air, air was then displaced from the container by the CO2 and the abdomen was in a stable, high CO2 concentration environment. The CO2 was continuously insufflated into the container at 9 L/min via a gas diffuser (VitaDiffuser, Cardia Innovation, Sweden) that ensures the gas enters the container at a low velocity, thereby reducing turbulence whilst allowing high CO2 gas concentrations within the container. The CO2 entered the container at the diffuser and continually overflowed out of the hole at the top of the container, creating a continuous flow of CO2 over the abdominal wound. Pilot measurements of CO2 concentration using a CheckMate II gas analyzer (PBI Dansensor, Denmark) showed that the environment within the box is maintained at > 90% CO2. The ability to create an environment of high CO2 concentration within a surgical cavity has been well documented, both with and without humidification [33, 35, 36]. In the humidified-warm CO2 conditions, the CO2 was humidified and warmed using a humidifier controller and delivered to the gas diffuser by a heated delivery tube (HumiGard, Fisher and Paykel Healthcare, New Zealand). Independent testing has shown that the humidifier delivers >98.0% relative humidity at 37°C [47].
Data analysis
PtO2 and tissue temperature measurements were averaged over the last minute for each treatment condition. Each treatment was paired with the alternate treatment (the first treatment with the second, third with the fourth etc.) to give a change in PtO2 and tissue temperature for each cross-over trial. The Shapiro-Wilk test was used to check the normality of the data set for each experiment. When the assumption of normality was satisfied, a paired Student’s t-test was conducted to test whether the mean change between paired treatment conditions differed from zero. When the assumption of normality was not satisfied, a Hodges-Lehman median difference and Wilcoxon Signed Rank test was used. P-value <0.05 was considered statistically significant for all tests.
Results
Animals and arterial oxygen saturation
The average weight of the rats was 294 g (SD 27), and did not differ between experiments (p = 0.34). There was no difference in arterial oxygen saturation (SpO2) during PtO2 data collection between conditions in any of the experiments (p = 0.48, 0.51, 0.46 for experiments 1–3)
Sub-peritoneal tissue oxygenation partial pressure (PtO2) and tissue temperature
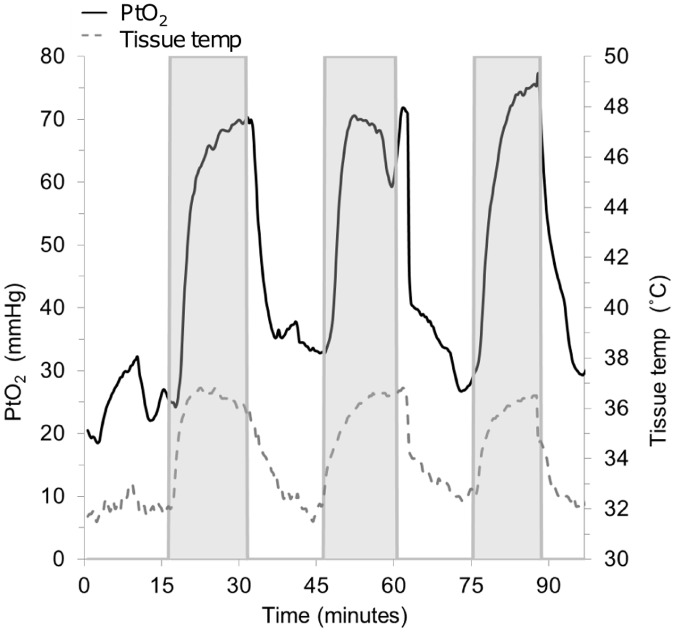
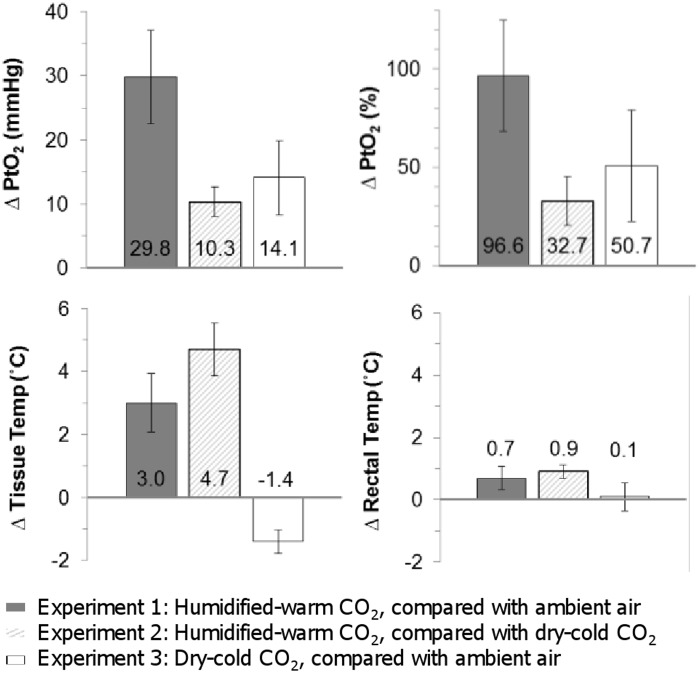
Upon insufflation of humidified-warm CO2, both PtO2 and tissue temperature increased almost immediately following the start of each period of gas insufflation, and was reversed each time gas insufflation was stopped (Fig. 1). Mean sub-peritoneal PtO2 increased by 29.8 mmHg (SD 13.3, p<0.001), or 96.6% (SD 51.9), and tissue temperature by 3.0°C (SD 1.7 p<0.001) compared with exposure to ambient air (experiment 1) (Table 1 and Fig. 2).
Fig 1. Representative data from one rat in experiment 1 (Insufflation of humidified-warm CO2 vs exposure to ambient air).
The shaded areas show each period of insufflation of humidified-warm CO2. A rapid increase in both PtO2 and tissue temperature is seen each time gas insufflation is started, and is reversed when insufflation is stopped.
Table 1. Tissue oxygen tension (PtO2) and tissue temperature results for each experiment.
| Experiment | Intervention | Control | Number of rats | Number of trials | PtO2 | Tissue Temperature | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Control (mmHg) | Mean change with intervention (mmHg) | p | Mean Control (°C) | Mean change with intervention (°C) | p | |||||||
| Compared to control | Compared to control | |||||||||||
| 1 | Humidified—warm CO2 | Ambient air | 5 | 13 | 33.2 (8.2) | 29.8 (13.3) | <0.001 | 34.0 (1.6) | 3.0 (1.7) | <0.001 | ||
| 2 | Humidified—warm CO2 | Dry—cold CO2 | 7 | 20 | 33.3 (7.2) | 10.3 (5.1) | <0.001* | 31.4 (1.6) | 4.7 (1.9) | <0.001 | ||
| 3 | Dry—cold CO2 | Ambient air | 3 | 7 | 28.0 (7.2) | 14.1 (7.2) | 0.005 | 32.1 (1.4) | -1.4 (0.5) | 0.001 | ||
All results show ‘mean (standard deviation)’ unless stated
* Related-samples Hodges-Lehman median difference shown, and Wilcoxon matched pair signed rank test used as assumption of normality was not satisfied.
Fig 2. Change in PtO2 (shown as both an absolute change (mmHg) and relative change (%)), tissue temperature and rectal temperature affected by the intervention condition of each experiment.
Error bars show 95% confidence intervals. Where error bars do not cross zero, the intervention had a statistically significant effect compared with the control condition.
Smaller, but significant, increases in PtO2 were seen when the two components of the therapy, exposure to humidity/warmth and exposure to CO2, were observed separately. Experiment 2 showed an increase in PtO2 of 10.3 mmHg (SD 5.1 p<0.001), or 32.7% (SD 16.6) upon the delivery of humidity and warmth in the constant presence of CO2 insufflation. Experiment 3 showed an increase in PtO2 of 14.1 (SD 7.2, p = 0.005), or 50.7% (SD 37.9), upon insufflation of dry-cold CO2 compared with exposure to ambient air.
With a similar pattern to PtO2, tissue temperature increased upon the addition of humidity and warmth by 4.7°C (SD 1.9, p < 0.001), in the constant presence of CO2 insufflation (experiment 2). However, in contrast to PtO2, tissue temperature decreased by 1.4°C (SD 0.5, p = 0.001) during insufflation of dry-cold CO2 compared with exposure to ambient air (experiment 3).
Body temperature maintenance
There was an increase in body temperature following insufflation of humidified-warm CO2 of 0.7°C (SD 0.7, p = 0.001) when compared to exposure to ambient air (experiment 1), and 0.9°C (SD 0.5, p < 0.001) when compared to exposure to dry-cold CO2 (experiment 2) (Fig. 2). There was no significant change in body temperature during insufflation of dry-cold CO2 compared to exposure to ambient air (experiment 3) (Fig. 2). There was no significant difference in the average heat pad setting between conditions in any of the experiments. The heat lamp was normally not required to maintain normothermia, but was used half as often in the conditions in which humidity and warmth were delivered.
Discussion
This research was designed to test the hypothesis that insufflation of humidified-warm CO2 into the open abdominal cavity during surgery would increase local PtO2 by a combination of the delivery of both CO2 and by reducing evaporative cooling in the peritoneal cavity. The first set of randomized cross-over trials showed that insufflation of humidified-warm CO2 causes an immediate and significant increase in PtO2 compared with exposure to ambient air, an average increase of 29.8 mmHg or 96.6%. Two subsequent sets of randomized cross-over trials showed that exposure to CO2 and reduction of evaporative cooling by exposure to humidity/warmth separately have an additive effect on PtO2. Furthermore, the increase in PtO2 upon the delivery of humidity/warmth may be explained by a concomitant increase in tissue temperature, in contrast to the drop in tissue temperature observed when dry-cold CO2 was insufflated in comparison with ambient air. To our knowledge this is the first report of the effect of CO2 insufflation during open abdominal surgery on PtO2.
The observed increase in PtO2 upon exposure to CO2 is consistent with previous reports of increased PtO2 upon topical CO2 application to the skin [39], and intra-abdominal exposure of CO2 during laparoscopy [40, 41]. The enhanced maintenance of tissue and core body temperature observed upon exposure to humidified-warm CO2 is also consistent with previous reports in human trials in colo-rectal surgery [43, 44]. Furthermore, the decrease in local tissue temperature observed with exposure to dry-cold CO2 is also consistent with human results that showed decreased local tissue temperature with the use of dry-cold CO2 in open cardiac surgery [48].
The 30 mmHg increase in PtO2 observed upon insufflation of humidified-warm CO2 is higher than the threshold of 15–25 mmHg that is widely considered as clinically significant [3, 49, 50]. A 25 mmHg increase in PtO2 has been shown to predict a 30% drop in surgical site infection rate [3]. Furthermore, a 40–50% fall in PtO2 is highly predictive of anastomotic leakage [12], suggesting that the 97% increase in PtO2 measured upon insufflation of humidified-warm CO2 may have a clinically important positive impact of anastomotic healing. Importantly, the results suggest that this local (surgical field) therapy may be at least as effective at increasing PtO2 during the operative period as systemically applied intraoperative therapies. An increase in intra-operative PaO2 from 150 to 300 mmHg increases PtO2 by approximately 19–20 mmHg in non-obese patients [17]. The increase in PtO2 drops to 11 mmHg in obese patients, likely due to reduced perfusion related to adiposity [17]. Manipulation of end tidal CO2 from 30 to 45 mmHg increases PtO2 by 12–16 mmHg [20, 23]. The observed effect of humidified-warm CO2 on PtO2 in the current study is larger than that achieved by aggressive fluid management [26], and by the use of thoracic epidural anesthesia [28–30].
While intra-abdominal insufflation of humidified-warm CO2 during open abdominal surgery may cause an increase in PtO2 of a similar magnitude to systemic therapies, the innovation of intra-abdominal insufflation of humidified-warm CO2 is that it achieves a local increase in PtO2 that may be able to overcome limitations of systemic therapies. The results of the current study suggest that a local effect was achieved, as the change in tissue temperature in the surgical site (sub-peritoneal in the abdominal wall) was at least 4-fold greater than the observed change in rectal temperature. It is likely that the insufflation of humidified-warm CO2 locally increases tissue oxygenation by mimicking normal metabolic regulation of oxygen delivery. This is most likely a combination of both a local increase in micro-perfusion through vasodilation, and a decrease in oxygen hemoglobin affinity. Increase in perfusion has previously been measured in response to topical CO2 [37–39] and local heating [25], and has been reported with concomitant increase in PtO2 [25, 39]. Delivery of oxygen to wounds is critically dependent on micro-perfusion to the surgical site. Oxygen dissipates radially from the vasculature to the tissue and PtO2 can drop rapidly just 20 μm from the vessel wall [51]. Micro-perfusion is dramatically altered by vasoconstriction and by arteriovenous shunts, which can allow oxygenated blood to bypass capillary beds resulting in higher venous PO2 than capillary PO2 [51]. The potential for inadequate perfusion to the surgical site is an obstructive hurdle for the effectiveness of systemic interventions to increase PtO2 [52]. We propose that maintenance of a warm, humid, high CO2 surgical site environment during open surgery in the abdomen is a solution that appears to increase perfusion directly at the target site and thereby increase local tissue oxygenation.
The implementation of intra-abdominal humidified-warm CO2 in open abdominal surgery into clinical practice is simplified by the fact that intra-abdominal CO2 is already established as the recommended gas for the creation of pneumoperitoneum for laparoscopic surgery [53]. Due to early concerns, the effect of intra-abdominal CO2 exposure has been broadly investigated, especially in respect to post-operative adhesion formation and tumor implantation. Recent mechanistic animal research concludes that exposure to dry CO2 pneumoperitoneum does not increase post-operative adhesion formation compared to exposure to dry air, when pneumoperitoneum is established at an appropriately low pressure and with adequate ventilatory support so as to avoid tissue hypoxia [41, 54, 55]. Furthermore, human trials have found that the rates of adhesive bowel obstruction [56, 57] and cancer survival [58] for laparoscopic surgery with CO2 pneumoperitoneum are at least on-par with open abdominal surgery. In light of this evidence, a recent study that suggested a CO2 operating environment increases adhesion formation [59] has been criticized as not using an adequately standardized adhesion model [60]. Another point of clinical interest is the fate of the excess CO2 that overflows from an open abdominal cavity. The Coanda effect causes the CO2 to flow out of the wound attached to the adjacent surface, similar to gas flow in avionics. A combination of the negative buoyancy of CO2 and the Coanda effect causes the CO2 to drop to the floor of the operating room and therefore operating staff are not exposed to high inspired CO2 [61, 62].
The rat model used for this research included endotracheal intubation, replacement of insensible fluid loss, and continuous monitoring of depth of anesthesia by a dedicated anesthetist. Although applicability to the human clinical setting is inferred, there is some evidence that the PtO2 response is similar between species, as similar increases in PtO2 have been reported in rodent [41] and human [4]surgical models in response to increased inspired oxygen. The use of a rat model had several advantages over measurement in humans. Most notably, the model allowed for the expensive PtO2 probe to be re-used for several rats, avoiding the expense of single use probes that would be required in the human clinical setting. This enabled several different experiments to be conducted, which would not have been economically feasible in a human model. The model also allowed complete assurance that the PtO2 probe remained in place and that the overlaying tissue remained free of surgical fluids, blood or bowel.
Continuous monitoring of arterial CO2 partial pressure (PaCO2) was not conducted in this model, as we determined that the extra complication of femoral artery cannulation in an already long procedure was not justified. It is possible that a systemic increase in PaCO2 contributed to the measured increase in PtO2. During the development of the current rat model, blood gas measurements were taken at different minute ventilation rates in 6 rats with and without humidified-warm CO2 insufflation. The results, supplied in supplemental files, suggest that insufflation of humidified-warm CO2 increases PaCO2 by 7 mmHg at the minute ventilation used in the current study (note that the results were not statistically significant and therefore did not justify increasing minute ventilation during CO2 insufflation in this protocol). Human data suggests that a 7 mmHg increase in PaCO2 would increase PtO2 by just 5–7 mmHg [20, 23]. It is, therefore, unlikely that an increase in PaCO2 alone is sufficient to explain the large increase in PtO2 observed in the current study.
In conclusion, the current study has shown that insufflation of humidified-warm CO2 into the abdominal cavity during open abdominal surgery in a rat model causes an immediate and clinically significant increase in PtO2. Furthermore, the effect is an additive result of the delivery of CO2 and avoidance of evaporative cooling via the delivery of the gas humidified at body temperature. This finding may have important clinically implications, as the local application of the therapy may be able to overcome inadequate local micro-perfusion, which limits delivery of supplemental oxygen to the surgical site in many surgical patients.
Supporting Information
(XLSX)
(XLSX)
(XLS)
(XLS)
Acknowledgments
The authors wish to thank Vanessa Sluyter for her dedicated approach to assisting in the data collection for this research. Without this assistance the project would not have been possible.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was funded by Fisher & Paykel Healthcare Ltd (Auckland, New Zealand). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Davis B, Rivadeneira DE. Complications of Colorectal Anastomoses: Leaks, Strictures, and Bleeding. Surg Clin North Am. 2013;93(1):61–87. 10.1016/j.suc.2012.09.014 [DOI] [PubMed] [Google Scholar]
- 2. Taflampas P, Christodoulakis M, Tsiftsis DD. Anastomotic leakage after low anterior resection for rectal cancer: Facts, obscurity, and fiction. Surg Today. 2009;39(3):183–8. 10.1007/s00595-008-3835-2 [DOI] [PubMed] [Google Scholar]
- 3. Hopf HW, Hunt TK, West JM, Blomquist P, Goodson Iii WH, Jensen JA, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132(9):997–1005. [DOI] [PubMed] [Google Scholar]
- 4. Greif R, Akça O, Horn EP, Kurz A, Sessler DI. Supplemental perioperative oxygen to reduce the incidence of surgical wound infection. N Engl J Med. 2000;342(3):161–7. [DOI] [PubMed] [Google Scholar]
- 5. Govinda R, Kasuya Y, Bala E, Mahboobi R, Devarajan J, Sessler DI, et al. Early postoperative subcutaneous tissue oxygen predicts surgical site infection. Anesth Analg. 2010;111(4):946–52. 10.1213/ANE.0b013e3181e80a94 [DOI] [PubMed] [Google Scholar]
- 6. Allen DB, Maguire JJ, Mahdavian M, Wicke C, Marcocci L, Scheuenstuhl H, et al. Wound hypoxia and acidosis limit neutrophil bacterial killing mechanisms. Arch Surg. 1997;132(9):991–6. [DOI] [PubMed] [Google Scholar]
- 7. Babior BM. Oxygen dependent microbial killing by phagocytes. (Second of two parts). N Engl J Med. 1978;298(13):721–5. [DOI] [PubMed] [Google Scholar]
- 8. Babior BM. Oxygen-dependent microbial killing by phagocytes. (First of two parts). N Engl J Med. 1978;298(12):659–68. [DOI] [PubMed] [Google Scholar]
- 9. Jonsson K, Jensen JA, Goodson Iii WH, Scheuenstuhl H, West J, Hopf HW, et al. Tissue oxygenation, anemia, and perfusion in relation to wound healing in surgical patients. Ann Surg. 1991;214(5):605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hartmann M, Jonsson K, Zederfeldt B. Effect of tissue perfusion and oxygenation on accumulation of collagen in healing wounds: Randomized study in patients after major abdominal operations. Eur J Surg. 1992;158(10):521–6. [PubMed] [Google Scholar]
- 11. Jonsson K, Jensen A, Goodson WHI, Hunt TK. Wound healing in subcutaneous tissue of surgical patients in relation to oxygen availability. Surg Forum. 1986;37:86–8. [Google Scholar]
- 12. Sheridan WG, Lowndes RH, Young HL. Tissue oxygen tension as a predictor of colonic anastomotic healing. Dis Colon Rectum. 1987;30(11):867–71. [DOI] [PubMed] [Google Scholar]
- 13. Hunt TK, Gimbel M, Sen CK. Revascularization of wounds: The oxygen-hypoxia paradox Angiogenesis: An Integrative Approach From Science to Medicine. Boston: Springer; 2008. p. 541–59. [Google Scholar]
- 14. Hopf HW, Rollins MD. Wounds: An overview of the role of oxygen. Antioxidants and Redox Signaling. 2007;9(8):1183–92. [DOI] [PubMed] [Google Scholar]
- 15. Sessler DI. Non-pharmacologic prevention of surgical wound infection. Anesthesiol Clin. 2006. June;24(2):279–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoshida M, Nabeshima T, Gomi H, Lefor AT. Technology and the Prevention of Surgical Site Infections. Journal of Surgical Education. 2007. October;64(5):302–10. [DOI] [PubMed] [Google Scholar]
- 17. Kabon B, Nagele A, Reddy D, Eagon C, Fleshman JW, Sessler DI, et al. Obesity decreases perioperative tissue oxygenation. Anesthesiology. 2004;100(2):274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jonsson K, Jensen JA, Goodson Iii WH. Assessment of perfusion in postoperative patients using tissue oxygen measurements. Br J Surg. 1987;74(4):263–7. [DOI] [PubMed] [Google Scholar]
- 19. Chang N, Goodson Iii WH, Gottrup F, Hunt TK. Direct measurement of wound and tissue oxygen tension in postoperative patients. Ann Surg. 1983;197(4):470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akca O, Liem E, Suleman MI, Doufas AG, Galandiuk S, Sessler DI. Effect of intra-operative end-tidal carbon dioxide partial pressure on tissue oxygenation. Anaesthesia. 2003;58(6):536–42. [DOI] [PubMed] [Google Scholar]
- 21. Akça O, Sessler DI, Delong D, Keijner R, Ganzel B, Doufas AG. Tissue oxygenation response to mild hypercapnia during cardiopulmonary bypass with constant pump output. Br J Anaesth. 2006;96(6):708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hager H, Reddy D, Mandadi G, Pulley D, Eagon JC, Sessler DI, et al. Hypercapnia improves tissue oxygenation in morbidly obese surgical patients. Anesth Analg. 2006;103(3):677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Akça O, Doufas AG, Morioka N, Iscoe S, Fisher J, Sessler DI. Hypercapnia improves tissue oxygenation. Anesthesiology. 2002;97(4):801–6. [DOI] [PubMed] [Google Scholar]
- 24. Akça O, Melischek M, Scheck T, Hellwagner K, Arkiliç CF, Kurz A, et al. Postoperative pain and subcutaneous oxygen tension. Lancet. 1999;354(9172):41–2. [DOI] [PubMed] [Google Scholar]
- 25. Sheffield CW, Sessler DI, Hopf HW, Schroeder M, Moayeri A, Hunt TK, et al. Centrally and locally mediated thermoregulatory responses alter subcutaneous oxygen tension. Wound Repair Regen. 1996;4(3):339–45. [DOI] [PubMed] [Google Scholar]
- 26. Arkiliç CF, Taguchi A, Sharma N, Ratnaraj J, Sessler DI, Read TE, et al. Supplemental perioperative fluid administration increases tissue oxygen pressure. Surgery. 2003;133(1):49–55. [DOI] [PubMed] [Google Scholar]
- 27. Jensen JA, Goodson WH, Hopf HW, Hunt TK. Cigarette smoking decreases tissue oxygen. Arch Surg. 1991;126(9):1131–4. [DOI] [PubMed] [Google Scholar]
- 28. Kabon B, Fleischmann E, Treschan T, Taguchi A, Kapral S, Kurz A. Thoracic Epidural Anesthesia Increases Tissue Oxygenation during Major Abdominal Surgery. Anesth Analg. 2003;97(6):1812–7. [DOI] [PubMed] [Google Scholar]
- 29. Treschan TA, Taguchi A, Ali SZ, Sharma N, Kabon B, Sessler DI, et al. The effects of epidural and general anesthesia on tissue oxygenation. Anesth Analg. 2003;96(6):1553–7. [DOI] [PubMed] [Google Scholar]
- 30. Buggy DJ, Doherty WL, Hart EM, Pallett EJ. Postoperative wound oxygen tension with epidural or intravenous analgesia: A prospective, randomized, single-blind clinical trial. Anesthesiology. 2002;97(4):952–8. [DOI] [PubMed] [Google Scholar]
- 31. Persson M, van der Linden J. Intraoperative CO2 insufflation can decrease the risk of surgical site infection. Med Hypotheses. 2008;71(1):8–13. 10.1016/j.mehy.2007.12.016 [DOI] [PubMed] [Google Scholar]
- 32. Roe CF. Effect of bowel exposure on body temperature during surgical operations. The American Journal of Surgery. 1971;122(1):13–5. [DOI] [PubMed] [Google Scholar]
- 33. Persson M, Svenarud P, Van Der Linden J. What is the optimal device for carbon dioxide deairing of the cardiothoracic wound and how should It be positioned? J Cardiothorac Vasc Anesth. 2004;18(2):180–4. [DOI] [PubMed] [Google Scholar]
- 34. Persson M, Van der Linden J. De-airing of a cardiothoracic wound cavity model with carbon dioxide: Theory and comparison of a gas diffuser with conventional tubes. J Cardiothorac Vasc Anesth. 2003;17(3):329–35. [DOI] [PubMed] [Google Scholar]
- 35. Svenarud P, Persson M, Van der Linden J. Efficiency of a gas diffuser and influence of suction in carbon dioxide deairing of a cardiothoracic wound cavity model. J Thorac Cardiovasc Surg. 2003;125(5):1043–9. [DOI] [PubMed] [Google Scholar]
- 36. Svenarud P, Persson M, Van der Linden J. Intermittent or continuous carbon dioxide insufflation for de-airing of the cardiothoracic wound cavity? An experimental study with a new gas-diffuser. Anesth Analg. 2003;96(2):321–7. [DOI] [PubMed] [Google Scholar]
- 37. Diji A. Local vasodilator action of carbon dioxide on blood vessels of the hand. J Appl Physiol. 1959;14(3):414–6. [DOI] [PubMed] [Google Scholar]
- 38. Minamiyama M, Yamamoto A. Direct evidence of the vasodilator action of carbon dioxide on subcutaneous microvasculature in rats by use of intra-vital video-microscopy. Journal of Biorheology. 2010;24(1):42–6. [Google Scholar]
- 39. Hartmann BR, Bassenge E, Pittler M. Effect of carbon dioxide-enriched water and fresh water on the cutaneous microcirculation and oxygen tension in the skin of the foot. Angiology. 1997;48(4):337–43. [DOI] [PubMed] [Google Scholar]
- 40. Gianotti L, Nespoli L, Rocchetti S, Vignali A, Nespoli A, Braga M. Gut oxygenation and oxidative damage during and after laparoscopic and open left-sided colon resection: A prospective, randomized, controlled clinical trial. Surg Endosc. 2011;25(6):1835–43. 10.1007/s00464-010-1475-2 [DOI] [PubMed] [Google Scholar]
- 41. Bourdel N, Matsuzaki S, Bazin JE, Pouly JL, Mage G, Canis M. Peritoneal tissue-oxygen tension during a carbon dioxide pneumoperitoneum in a mouse laparoscopic model with controlled respiratory support. Hum Reprod. 2007;22(4):1149–55. [DOI] [PubMed] [Google Scholar]
- 42. Ikeda T, Tayefeh F, Sessler DI, Kurz A, Plattner O, Petschnigg B, et al. Local radiant heating increases subcutaneous oxygen tension. Am J Surg. 1998;175(1):33–7. [DOI] [PubMed] [Google Scholar]
- 43. Frey JM, Janson M, Svanfeldt M, Svenarud PK, van der Linden JA. Local insufflation of warm humidified CO2 increases open wound and core temperature during open colon surgery: A randomized clinical trial. Anesth Analg. 2012;115(5):1204–511. 10.1213/ANE.0b013e31826ac49f [DOI] [PubMed] [Google Scholar]
- 44. Frey JMK, Janson M, Svanfeldt M, Svenarud PK, van der Linden JA. Intraoperative local insufflation of warmed humidified CO2 increases open wound and core temperatures: A randomized clinical trial. World J Surg. 2012;36(11):2567–75. 10.1007/s00268-012-1735-5 [DOI] [PubMed] [Google Scholar]
- 45. National Health and Medical Research Council. Australian code for the care and use of animals for scientific purposes. 8th ed Canberra: National Health and Medical Research Council; 2013. [Google Scholar]
- 46. National Health and Medical Research Council. Guidelines to promote the wellbeing of animals used for scientific purposes: The assessment and alleviation of pain and distress in research animals. Canberra: National Health and Medical Research Council; 2008. [Google Scholar]
- 47. Sammour T, Kahokehr A, Hill AG. Independent testing of the Fisher & Paykel Healthcare MR860 Laparoscopic Humidification System. Minim Invasive Ther Allied Technol. 2010;19(4):219–23. 10.3109/13645701003644475 [DOI] [PubMed] [Google Scholar]
- 48. Frey JM, Svegby HK, Svenarud PK, van der Linden JA. CO2 insufflation influences the temperature of the open surgical wound. Wound Repair Regen. 2010. July;18(4):378–82. 10.1111/j.1524-475X.2010.00602.x [DOI] [PubMed] [Google Scholar]
- 49. Akça O, Kurz A, Fleischmann E, Buggy D, Herbst F, Stocchi L, et al. Hypercapnia and surgical site infection: A randomized trial. Br J Anaesth. 2013;111(5):759–67. 10.1093/bja/aet233 [DOI] [PubMed] [Google Scholar]
- 50. Bakri MH, Nagem H, Sessler DI, Mahboobi R, Dalton J, Akça O, et al. Transdermal oxygen does not improve sternal wound oxygenation in patients recovering from cardiac surgery. Anesth Analg. 2008. June 2008;106(6):1619–26. 10.1213/ane.0b013e3181732e82 [DOI] [PubMed] [Google Scholar]
- 51. Tsai AG, Johnson PC, Intaglietta M. Oxygen gradients in the microcirculation. Physiol Rev. 2003. July;83(3):933–63. [DOI] [PubMed] [Google Scholar]
- 52. Qadan M, Cheadle WG. Controversies in host defense against surgical site infection. Expert Review of Anti-infective Therapy. 2009;7(9):1043–7. 10.1586/eri.09.88 [DOI] [PubMed] [Google Scholar]
- 53. Neudecker J, Sauerland S, Neugebauer E, Bergamaschi R, Bonjer HJ, Cuschieri A, et al. The European Association for Endoscopic Surgery clinical practice guideline on the pneumoperitoneum for laparoscopic surgery. Surg Endosc. 2002;16(7):1121–43. [DOI] [PubMed] [Google Scholar]
- 54. Matsuzaki S, Canis M, Bazin JE, Darcha C, Pouly JL, Mage G. Effects of supplemental perioperative oxygen on post-operative abdominal wound adhesions in a mouse laparotomy model with controlled respiratory support. Hum Reprod. 2007;22(10):2702–6. [DOI] [PubMed] [Google Scholar]
- 55. Matsuzaki S, Jardon K, Maleysson E, D'Arpiany F, Canis M, Bazin JE, et al. Carbon dioxide pneumoperitoneum, intraperitoneal pressure, and peritoneal tissue hypoxia: a mouse study with controlled respiratory support. Surg Endosc. 2010;24(11):2871–80. 10.1007/s00464-010-1069-z [DOI] [PubMed] [Google Scholar]
- 56. Reshef A, Hull TL, Kiran RP. Risk of adhesive obstruction after colorectal surgery: The benefits of the minimally invasive approach may extend well beyond the perioperative period. Surgical Endoscopy and Other Interventional Techniques. 2013;27(5):1717–20. 10.1007/s00464-012-2663-z [DOI] [PubMed] [Google Scholar]
- 57. Saklani AP, Naguib N, Shah PR, Mekhail P, Winstanley S, Masoud AG. Adhesive intestinal obstruction in laparoscopic vs open colorectal resection. Colorectal Disease. 2013;15(1):80–4. 10.1111/j.1463-1318.2012.03098.x [DOI] [PubMed] [Google Scholar]
- 58. Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW Jr, et al. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007;246(4):655–62. [DOI] [PubMed] [Google Scholar]
- 59. De Vries A, Mårvik R, Kuhry E. To perform operative procedures in an optimized local atmosphere: Can it reduce post-operative adhesion formation? International Journal of Surgery. 2013;11(10):1118–22. 10.1016/j.ijsu.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 60. Mynbaev OA, Eliseeva M, Tinelli A, Malvasi A, Kosmas IP, Medvediev MV, et al. An inexact study design produced misleading conclusions: To perform operative procedures in an optimized local atmosphere: Can it reduce post-operative adhesion formation? De Vries A, Mårvik R, Kuhry E. [Int J Surg 11 (2013) 1118–1122]. International Journal of Surgery. 2014;12(2):190–1. 10.1016/j.ijsu.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 61. Persson M, van der Linden J. A simple system for intraoperative antiseptic wound ventilation. J Hosp Infect. 2003;55(2):152–3. [DOI] [PubMed] [Google Scholar]
- 62. Cater JE, van der Linden J. Simulation of carbon dioxide insufflation via a diffuser in an open surgical wound model. Med Eng Phys. 2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLS)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.