Abstract
Background
There is compelling evidence in humans that peripheral endocannabinoid signaling is disrupted in obesity. However, little is known about the corresponding central signaling. Here, we have investigated the relationship between gender, leptin, body mass index (BMI) and levels of the endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG) in the serum and cerebrospinal fluid (CSF) of primarily overweight to obese patients with osteoarthritis.
Methodology/Principal Findings
Patients (20 females, 15 males, age range 44-78 years, BMI range 24-42) undergoing total knee arthroplasty for end-stage osteoarthritis were recruited for the study. Endocannabinoids were quantified by liquid chromatography – mass spectrometry. AEA and 2-AG levels in the serum and CSF did not correlate with either age or BMI. However, 2-AG levels in the CSF, but not serum, correlated negatively with CSF leptin levels (Spearman’s ρ -0.48, P=0.0076, n=30). No such correlations were observed for AEA and leptin.
Conclusions/Significance
In the patient sample investigated, there is a negative association between 2-AG and leptin levels in the CSF. This is consistent with pre-clinical studies in animals, demonstrating that leptin controls the levels of hypothalamic endocannabinoids that regulate feeding behavior.
Introduction
The endocannabinoids anandamide (AEA) and 2-arachidonoyl glycerol (2-AG) are endogenous agonists of cannabinoid receptors and regulate a multitude of physiological processes including metabolism and food intake [1–3]. It is well-established that the endocannabinoid system plays a key role in the control of energy homeostasis and that these effects are brought about both centrally and peripherally (for reviews, see [4–7]). For example, activation of cannabinoid receptors by AEA and 2-AG promotes hyperphagia while cannabinoid receptor blockade reduces food intake and improves parameters such as fasting glucose and triglyceride levels in humans [8–13]. Leptin is a hormone produced primarily by adipose tissue and exerts its anorexigenic effects through activation of hypothalamic proopiomelanocortin neurons and downregulation of hypothalamic endocannabinoid levels [9, 14], suggesting an interplay between the central leptin and endocannabinoid systems, at least in rodents.
Previous studies have demonstrated that fasting is associated with reduced central and peripheral leptin levels in humans and elevated hypothalamic 2-AG levels in rodents [9, 15–17]. Notably, administration of leptin to rats reduces hypothalamic 2-AG levels [9], suggesting functional antagonism between the anorexigenic effects of leptin and orexigenic endocannabinoids [8]. Given this orexigenic role of the endocannabinoid system, it is perhaps not surprising that changes in the levels of the endocannabinoids and/or their synthetic or degradative enzymes have been reported in adipose tissue and plasma samples from obese, obese/diabetic patients, and patients with eating disorders [18–31]. Most studies examining central changes in endocannabinoid function have been undertaken using experimental animals following dietary intervention (see e.g. [32–34]).
Despite the documented opposing roles of endocannabinoids and leptin in the control of feeding and obesity, only one study has examined the interplay between central leptin and endocannabinoid levels in humans [28]. These authors found that 2-AG levels in the cerebrospinal fluid (CSF) were higher in an obesity-prone population (American Indians) than in Caucasians, but there was no correlation between CSF endocannabinoid and leptin levels [28]. In a recent study, we investigated associations between pain and endocannabinoid levels in the serum and CSF from elderly, overnight fasted overweight/obese individuals suffering from osteoarthritis [35]. In the present study, we have investigated the relationship between central and peripheral endocannabinoid levels, body-mass index (BMI), and leptin levels in these individuals.
Materials and Methods
Ethics Statement
The experiments conducted herein were approved by the Stony Brook University institutional review board and written consent was obtained from each patient.
Study participants
Total knee arthroplasty (TKA) patients (n = 35) scheduled for an elective unilateral TKA under spinal anesthesia and a femoral nerve block were enrolled for this study. Patients with documented rheumatoid arthritis, patients scheduled for bilateral TKA, and patients scheduled for a TKA revision were excluded from the current study. Details of the patient population used here are given in [35].
Experimental design & data collection
Immediately prior to the surgery, at the time of the spinal anesthesia, blood and CSF were collected from fasting (10–18 hours) patients.
Analysis of leptin levels
Blood was drawn from patients into tubes lacking anticoagulant (BD Vacutainer) and the resulting serum samples prepared, flash frozen, and stored at -80°C until use. CSF was collected and subjected to centrifugation at 1800 x g for 15 min at 4°C and the resulting supernatant was flash frozen in liquid nitrogen and stored at -80°C. Leptin levels were quantified using a leptin enzyme-linked immunosorbent assay (Quantikine immunoassay, R&D Systems). The samples were diluted by gender as suggested by the manufacturer. Each sample was assayed in triplicate using the protocol supplied by the manufacturer. The color intensities were read at 450 nm.
Quantification of Endocannabinoids
Serum and CSF (0.5 ml) were mixed with 3.5 ml of 2:1:1 CHCl3:MeOH:Tris (50 mM, pH 8) that was spiked with 4 ng d4-PEA, d2-OEA, d5-2-AG, and 400 pg d4-AEA. Following centrifugation, the organic layer was removed and the sample was extracted again with the same buffer. The organic layer was dried down under argon and resuspended with 120 μl of 2:1 CHCl3:MeOH and 10 μl was injected into a Thermo TSQ Quantum Access Triple Quadropole mass spectrometer in triplicate. Endocannabinoid quantification was performed exactly as described [35, 36].
Statistical analysis
Zero-order correlation coefficients were determined using the statistical package built into the GraphPad Prism computer program for the Macintosh (GraphPad Software Inc., San Diego, CA, USA). First-order Spearman’s ρ values were calculated from the corresponding zero-order (bivariate) values using the formula of Lehmann [37]. Multiple linear regression analyses were conducted using using the IBS SPSS Statistics package, version 22. The rank-based two-way ANOVAs were calculated using the function raov in the Rfit package of the R computer program [38, 39]. We consider a p-value less than 0.05 as statistically significant.
Results
Endocannabinoid levels in the patient samples
The characteristics of the collective sample, stratified by gender and diagnosis of diabetes, are shown in Table 1. Given that the sample sizes are small, particularly for the diabetic subjects, the data are presented as medians and ranges, and a non-parametric rank-based two-way ANOVA [38] was used to assess significance. The body mass index (BMI) was higher in the diabetics vs. non-diabetics. As expected [40, 41], both serum and CSF leptin levels were significantly higher in the females than the males. The two-way ANOVAs confirmed these main effects and also found a significant interaction between gender and diabetes for the serum, but not CSF leptin (Table 1). Among the endocannabinoids, there were no significant main effects of either gender or diabetes, or interactions between gender and diabetes (Table 1).
Table 1. Characteristics of the study group stratified on the basis of gender (G) and incidence of diabetes (D).
| Diabetes | Females | Males | P value | |
|---|---|---|---|---|
| Age (years) | no | 68 (44–76, 14) | 70 (55–78, 11) | G: 0.44 |
| yes | 64 (51–78, 6) | 67 (59–70, 4) | D:0.30 | |
| GxD:0.91 | ||||
| BMI (kg/m2) | no | 34 (26–42, 14) | 28 (24–35, 11) | G: 0.066 |
| yes | 36 (29–40, 6) | 36 (33–39, 4) | D:0.002 | |
| GxD:0.064 | ||||
| Serum leptin | no | 45 (16–85, 14) | 9.3 (4.3–18, 9) | G: 0.0002 |
| yes | 36 (14–56, 6) | 28 (16–32, 3) | D:0.43 | |
| GxD:0.0095 | ||||
| CSF leptin | no | 290 (180–440, 14) | 140 (42–190, 9) | G: 0.0005 |
| yes | 240 (190–390, 6) | 210 (110–220, 3) | D:0.87 | |
| GxD:0.18 | ||||
| Serum AEA | no | 0.26 (0.11–0.54, 14) | 0.21 (0.11–0.33, 11) | G: 0.082 |
| yes | 0.28 (0.13–0.31, 5) | 0.15 (0.092–0.15, 4) | D:0.41 | |
| GxD:0.59 | ||||
| Serum 2-AG | no | 3.4 (0.91–6.1, 14) | 3.5 (0.96–5.0, 11) | G: 0.23 |
| yes | 3.3 (0.67–5.5, 6) | 1.4 (0.86–2.9, 4) | D:0.23 | |
| GxD:0.31 | ||||
| CSF AEA | no | 14 (6–22, 14) | 17 (4–30, 11) | G: 0.96 |
| yes | 11 (8–25, 5) | 10 (6–21, 4) | D:0.38 | |
| GxD:0.33 | ||||
| CSF 2-AG | no | 64 (30–220, 13) | 99 (14–170, 11) | G: 0.45 |
| yes | 99 (0–180, 5) | 100 (75–120, 4) | D:0.84 | |
| GxD:0.76 |
Data are given as medians with ranges, followed by the sample sizes in parentheses. Serum leptin levels are given as ng/ml while CSF leptin levels are given as pg/ml; serum AEA and 2-AG levels are given as ng/ml; and CSF AEA and 2-AG levels are given as pg/ml. P values are calculated using a non-parametric two-way robust Wilcoxon analysis [38]. Significant correlations are shown in boldface.
Correlation between leptin and endocannabinoid levels
Bivariate correlations were determined between the outcome parameters used in the present study. Before undertaking such correlations, it is important to determine whether the data are normally distributed because a parametric Pearson correlation is not appropriate if this is not the case. We used the D'Agostino & Pearson omnibus normality test and found that the serum 2-AG values were not normally distributed (all other variables reported passed this test). Consequently, we used non-parametric Spearman’s correlations for the whole dataset. The correlation coefficients for the endocannabinoids and for leptin are shown in Table 2, and the scatterplots for the endocannabinoids against the corresponding leptin concentrations are shown in Fig 1. As expected from the literature [15], CSF and serum leptin concentrations were highly correlated (Spearman’s ρ = 0.83, n = 31, P<0.0001) and thus it is not surprising that the significant association of BMI with leptin was seen for both serum and CSF leptin. In their original study, Schwartz et al. [41] reported that the best correlation for CSF leptin as dependent variable was seen in a model incorporating gender, plasma leptin and log plasma leptin as independents. However, this is likely to result in multicollinearity (a potential source of misleading results when one or more of the predictor variables are highly correlated, i.e. not independent), and this was indeed seen in our dataset. A simpler model with gender and either unlogged serum or logged serum leptin (but not both) gave similar r2 values (0.69 and 0.71) with both parameters contributing significantly, and the lowest level of collinearity (variance inflation factor value 1.8; as a rule of thumb, a value ≥5 indicates multicollinearity) was seen with the unlogged values (data not shown).
Table 2. Correlations between endocannabinoid and leptin concentrations and the corresponding parameters for age, BMI, AEA and 2-AG.
| Parameter 1 | Parameter 2 | Serum | CSF |
|---|---|---|---|
| Age | Leptin | -0.20 (P = 0.26, n = 33) | -0.015 (P = 0.94, n = 32) |
| AEA | -0.012 (P = 0.95, n = 34) | -0.058 (P = 0.75, n = 34) | |
| 2-AG | 0.042 (P = 0.81, n = 35) | -0.27 (P = 0.13, n = 33) | |
| BMI | Leptin | 0.65 (P<0.0001, n = 33) | 0.49 (P = 0.0048, n = 32) |
| AEA | 0.18 (P = 0.30, n = 34) | -0.031 (P = 0.86, n = 34) | |
| 2-AG | -0.13 (P = 0.47, n = 35) | -0.019 (P = 0.91, n = 33) | |
| Leptin | AEA | 0.29 (P = 0.10, n = 33) | -0.17 (P = 0.35, n = 31) |
| 2-AG | -0.09 (P = 0.62, n = 33) | -0.48 (P = 0.0076, n = 30) | |
| AEA | 2-AG | 0.18 (P = 0.31, n = 34) | 0.13 (P = 0.47, n = 32) |
Values are Spearman’s ρ, in deference to the non-normal distribution of serum 2-AG in the sample. The other parameters passed the D'Agostino & Pearson omnibus normality test. Significant correlations are shown in boldface.
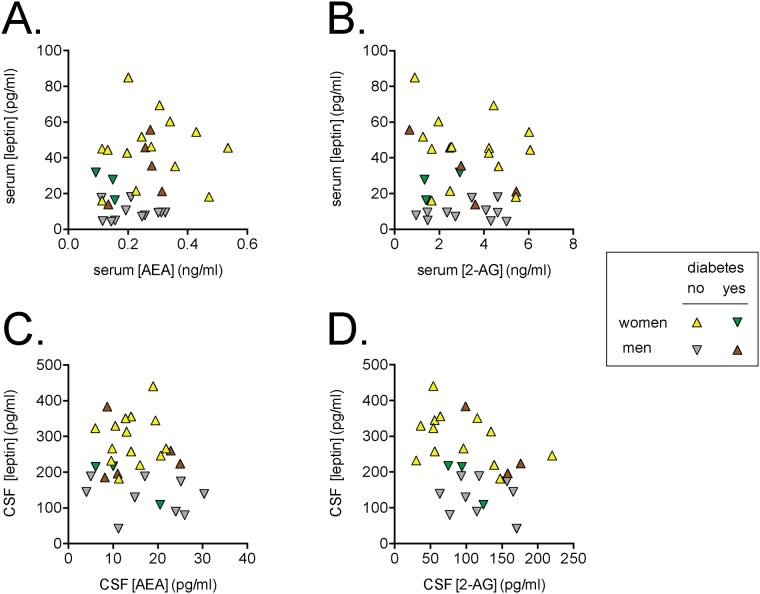
Fig 1. Relationship between endocannabinoid and leptin levels in the serum (A, B) and CSF (C, D).
Shown are the data for AEA (Panels A, C) and 2-AG (Panels B, D). The Spearman ρ values are provided in Table 2.
There was no significant association between AEA and the corresponding leptin levels. As reported previously for this patient population [35], serum AEA and CSF AEA levels were significantly (p < 0.05) correlated. No such correlation between serum and CSF 2-AG levels was seen or between serum 2-AG and serum leptin levels (Table 2). However, a significant negative correlation (ρ = -0.48, n = 30, P = 0.0076) was observed between CSF 2-AG and leptin levels (Table 2, Fig 1).
To exclude the possibility that the negative correlation between leptin and 2-AG was a result of an underlying distribution of another significantly associated parameter, higher-order correlations controlling for additional parameters were applied. The first order Spearman’s ρ value for CSF leptin vs. CSF 2-AG controlling for BMI, remained significant (ρ = -0.50, n = 30, P = 0.0059). Given that both the CSF leptin and the CSF 2-AG values passed the D'Agostino & Pearson omnibus normality test, it is possible to explore the relationship between these two variables further in multiple regression analyses. The statistical program we used allows different approaches for how the independent variables are presented. We used backward elimination (where all the candidate variables are initially included, and variables are removed when such deletion improves the model), with CSF 2-AG as the dependent variable and with age, gender, incidence of diabetes, BMI and CSF leptin as the independent variables. Using this approach, the only significant variable remaining in the final model was CSF leptin, and the same was true when forward selection (when variables are added to the model) was tested. Given that the data indicate an association rather than causality, the reverse analysis, i.e. where CSF leptin was the dependent variable, was also investigated. Both methods selected CSF 2-AG and diabetes as significant predictors. The backward elimination analyses are summarized in S1 and S2 Tables. Thus, the association between CSF leptin and 2-AG levels found here is not induced by the other parameters investigated.
Discussion
The endocannabinoid system regulates appetite and metabolism [8]. Previous work in rodents suggests an antagonistic relationship between the central endocannabinoid system and leptin [9]. Indeed, administration of leptin to normal Sprague-Dawley rats reduced hypothalamic levels of both AEA and 2-AG [9]. Obese Zucker (fa/fa) rats with defective leptin signaling possess higher hypothalamic 2-AG levels than lean controls, whereas AEA levels are not changed. Elevated hypothalamic 2-AG levels are observed in ob/ob mice (where there is no leptin production) compared to controls. Furthermore, fasting elevates while feeding reduces hypothalamic 2-AG levels in rats [16] and fasting results in reduced leptin levels in the CSF of humans [15, 17]. Based upon these studies, fasting in humans would be expected to produce an inverse gradient between CSF 2-AG and leptin levels. Indeed, such a negative correlation between 2-AG and leptin was observed in the current study, suggesting that the control of 2-AG production by leptin seen in the rodent hypothalamus [9] may also occur in humans.
Leptin is an adipokine that regulates appetite, metabolism, and inflammation and one of its primary roles is to reduce food intake [42–44]. Obesity is associated with chronically elevated leptin levels that may give rise to leptin resistance [45]. Recent work has shown that peripheral endocannabinoid signaling contributes to leptin resistance [11]. In contrast, lack of hypothalamic endocannabinoid signaling leads to leptin resistance in animals fed a high fat diet, whereas its actions are potentiated in chow-fed animals [46]. In our study, the negative correlation between CSF leptin and 2-AG suggests that the reciprocal regulation of central leptin and endocannabinoid signaling remains functionally coupled in overweight fasting humans. Because our serum and CSF samples were obtained during the morning hours, it is tempting to speculate that the inverse relationship between leptin and 2-AG may reflect an increase in the morning orexigenic drive. This is supported by a very recent clinical study demonstrating that 2-AG levels increase while leptin levels decrease during sleep and throughout the morning [47].
Our results are in contrast to the study of Jumpertz et al [28], who did not observe a correlation between leptin and 2-AG levels in the CSF of healthy, middle aged overweight individuals, which the authors attributed to possible leptin resistance in this cohort. Although our sample comprised older patients with osteoarthritis, it is unlikely that the endocannabinoid and leptin levels were affected by the underlying osteoarthritis (see below). However, it is prudent to consider the present findings in context of this co-morbidity and again to suggest that further validation studies are needed. Lastly, the negative correlation between CSF 2-AG and leptin in the fasting subjects would infer that sufficient 2-AG levels derived from the hypothalamus are reflected in the CSF for the effect to be specific to the hypothalamic endocannabinoid signaling process.
Importantly, the CSF endocannabinoid levels reported herein are in line with the literature (Table 3). Most of the previously reported data are for AEA, with mean values for controls ranging from below the limit of detection to 11.65±7.53 pmol/ml (Table 3). Similarly, 2-AG levels ranging from below the limit of detection to 160±110 pmol/mg (61±42 ng/ml) have been reported; and our values are within this range. Lastly, the CSF leptin levels reported herein are similar to those previously observed in subjects without underlying osteoarthritis [48, 49], arguing against osteoarthritis-induced alterations in leptin levels.
Table 3. Literature values of AEA and 2-AG (+1-AG) levels in the CSF.
| Study | Control/Patients | n | AEA | 2-AG |
|---|---|---|---|---|
| Giuffrida et al. [50] | Controls | 81 | 0.007±0.002 b | |
| Schizophrenic patients: Antipsychotic-naïve | 24 | 0.057±0.011 b | ||
| 1 °G antipsychotic treated | 36 | 0.031±0.012 b | ||
| 2 °G antipsychotic treated | 31 | 0.062±0.016 b | ||
| Pisani et al [51] | Controls | 14 | 5.23±1.81 a | |
| Untreated PD patients | 16 | 10.7±3.2 a | ||
| Sarchielli et al. [52] | Controls | 20 | 0.39±0.09 b | Below detection limit (0.2 pmol/sample) for all groups |
| Chronic migraine | 15 | 0.21±0.06 b | ||
| Probable migraine + PAOH | 15 | 0.22±0.05 b | ||
| Centonze et al. [53] | Controls | 11 | 2.4±1.3 | ~75–100 c |
| Relapsing-remitting MS | 11 | 20.3±15.7 | ||
| Leweke et al. [54] | Controls | 81 | range 0 - ~0.07 c | |
| Schizophrenics | 44 | range 0 - ~0.33 c | ||
| Di Filippo et al. [55] | Controls | 20 | 0.085±0.0019 a | 1.068±0.123 a |
| Stable remitting-relapsing MS | 20 | 0.0048±0.0009 a | 0.824±0.104 a | |
| MS during relapse | 15 | 0.0068±0.0009 a | 0.997±0.127 a | |
| Secondary phase MS | 15 | 0.0036±0.0006 a | 0.762±0.098 a | |
| Koethe et al. [56] | Controls | 81 | Median <0.001, IQR <0.001–0.005 | |
| Prodromal psychotic patients | 27 | Median 0.006, IQR <0.001–0.073 | ||
| Koppel et al. [57] | Elderly controls and Late onset AD patients | 35 | Below detection limit | For all cases mean 0.205, range 0.027–0.704 |
| Koethe et al. [58] | Controls before and after sleep deprivation | 20 | Very low levels shown in Fig 1 of paper | |
| Romigi et al. [59] | Controls | 9,6 d | 11.65±7.53 a | 160±110 a |
| Untreated epilepsy | 9,6 d | 2.55±1.78 a | 210±147 a | |
| Pisani et al. [60] | Controls | 37 | 4.59±1.65 a | |
| De novo PD | 38 | 9.76±3.26 a | ||
| PD, treatment withdrawal | 8 | 11.19±3.23 a | ||
| PD, under treatment | 10 | 6.76±3.41 a | ||
| Jumpertz et al. [28] | Controls (BMI 34±8 a kg/m2) | 27 | 0.06±0.08 a | 14.2±6.85 a |
| Morgan et al. [61] | Controls | 13 | ~0.13 c | ~10 c |
| Light cannabis users | 10 | ~0.16 c | ~18 c | |
| Heavy cannabis users | 10 | ~0.10 c | ~23 c |
aMeans ± SD;
bmeans ±SE;
cvalues not given explicitly in the text but estimated from the figures;
drefers to samples analysed for AEA and 2-AG, respectively.
Abbreviations: 1 °G and 2 °G, first- and second-generation, respectively; AD, Alzheimer’s disease; IQR, interquartile range; MS, multiple sclerosis; PAOH, probable analgesic overuse headache; PD, Parkinson’s disease.
All values are in pmol/ml.
One observation at odds with the literature, however, is the finding of a significant correlation between serum and CSF AEA (but not 2-AG) levels. In four separate studies [28, 50, 56, 61] no such correlation was found, although a positive correlation was seen for schizophrenic patients treated with first-generation antipsychotics [50]. Very recently completed data (i.e. after this manuscript was submitted) by one of us suggests that there is a large inter-sample variation in plasma AEA and 2-AG levels for any given individual when the samples are either taken at a one hour interval or at the same time of day on two different occasions (L. Lindgren, M. Gouveia-Figueira, M.L. Nodding and Christopher J. Fowler, manuscript in preparation). This may account for the differences in plasma:CSF correlation coefficients between the studies. Additionally, it may be possible that in the patient population examined here, the underlying osteoarthritis has produced an effect upon AEA turnover centrally and/or peripherally, thereby inducing a correlation. There is evidence that both inflammatory and anti-inflammatory cytokines can affect components of the endocannabinoid system, such as cannabinoid receptors and fatty acid amide hydrolase [62–64] so in theory this is possible although unlikely because the serum endocannabinoid levels obtained here are similar to those reported for healthy patients without underlying osteoarthritis [65–67]. Furthermore, brain and plasma AEA levels were found not to be altered in an animal model of osteoarthritis [68].
A second potential factor to consider may be the body weight range of the patient sample. In our dataset, only 2 individuals were classified as normal weight (BMI<25) whilst 9 were overweight (25<BMI<30), 22 were obese (30<BMI<40) and 2 were morbidly obese BMI>40). Thus, our findings relate to an overweight/obese population. This may be of importance for the observed and interpreted results, although the literature is somewhat divergent with respect to the association between circulating endocannabinoid levels and body weight / BMI. Indeed, some studies have reported elevated levels of both AEA and 2-AG in overweight and obese individuals [18, 22], some have reported elevations in only one of the endocannabinoids [19, 21, 28, 31], while others have reported no change in either metabolite [24]. The only study investigating the CSF was that of Jumpertz et al. [28], who reported a negative correlation between BMI and AEA, but not 2-AG, levels; but this association was lost in a multivariate analysis adjusting for plasma AEA concentrations. However, our data reveal that central and circulating endocannabinoid levels do not correlate with BMI at least in an osteoarthritis patient population.
In conclusion, the present study investigated the relationship between obesity factors and peripheral and central endocannabinoids in a population of overweight to obese individuals with severe osteoarthritis. Because endocannabinoid levels are dynamically regulated by feeding and fasting in rodents and humans [16, 20, 69], it is clear that the relationship between circulating and central endocannabinoids and obesity-related parameters is complex and most likely dependent upon the patient populations selected. Therefore, it should be noted that the conclusions of our study are restricted to this patient category and some of the differences between the present study and other published data for other patient categories may reflect this fact. Nonetheless, the present study provides evidence for the tight coupling between central endocannabinoid and leptin levels and suggests that the regulation of 2-AG levels by leptin observed in the rodent hypothalamus may likewise exist in humans.
Supporting Information
B in the table refers to the unstandardized coefficients, and these are only shown for analyses with significant ANOVA values.
(DOCX)
B in the table refers to the unstandardized coefficients, and these are only shown for CSF 2-AG, gender and the constant; the other variables did not reach significance in any case (P > 0.3).
(DOCX)
Acknowledgments
We would like to thank Robert Rieger at the Stony Brook Proteomics Center for help with mass spectrometry.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Department of Anesthesiology and National Institutes of Health grants DA035923 and DA035949. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14(6):667–75. Epub 2008/06/03. doi: nm1775 [pii] 10.1038/nm1775 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jo YH, Chen YJ, Chua SC Jr, Talmage DA, Role LW. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron. 2005;48(6):1055–66. Epub 2005/12/21. doi: S0896-6273(05)00886-X [pii] 10.1016/j.neuron.2005.10.021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Vry J, Schreiber R, Eckel G, Jentzsch KR. Behavioral mechanisms underlying inhibition of food-maintained responding by the cannabinoid receptor antagonist/inverse agonist SR141716A. Eur J Pharmacol. 2004;483(1):55–63. Epub 2004/01/08. doi: S0014299903025305 [pii]. . [DOI] [PubMed] [Google Scholar]
- 4. Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005;8(5):585–9. Epub 2005/04/28. doi: nn1457 [pii] 10.1038/nn1457 . [DOI] [PubMed] [Google Scholar]
- 5. Quarta C, Mazza R, Obici S, Pasquali R, Pagotto U. Energy balance regulation by endocannabinoids at central and peripheral levels. Trends in molecular medicine. 2011;17(9):518–26. 10.1016/j.molmed.2011.05.002 . [DOI] [PubMed] [Google Scholar]
- 6. DiPatrizio NV, Piomelli D. The thrifty lipids: endocannabinoids and the neural control of energy conservation. Trends Neurosci. 2012;35(7):403–11. 10.1016/j.tins.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martins CJ, Genelhu V, Di Marzo V, Francischetti EA. The endocannabinoid system—back to the scene of cardiometabolic risk factors control? Horm Metab Res. 2014;46(8):529–36. 10.1055/s-0034-1375653 . [DOI] [PubMed] [Google Scholar]
- 8. Kunos G, Osei-Hyiaman D, Liu J, Godlewski G, Batkai S. Endocannabinoids and the control of energy homeostasis. J Biol Chem. 2008;283(48):33021–5. 10.1074/jbc.R800012200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410(6830):822–5. Epub 2001/04/12. 10.1038/35071088 . [DOI] [PubMed] [Google Scholar]
- 10. Van Gaal LF, Scheen AJ, Rissanen AM, Rossner S, Hanotin C, Ziegler O. Long-term effect of CB1 blockade with rimonabant on cardiometabolic risk factors: two year results from the RIO-Europe Study. Eur Heart J. 2008. Epub 2008/04/18. doi: ehn076 [pii] 10.1093/eurheartj/ehn076 . [DOI] [PubMed] [Google Scholar]
- 11. Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T, et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012;16(2):167–79. 10.1016/j.cmet.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watanabe T, Kubota N, Ohsugi M, Kubota T, Takamoto I, Iwabu M, et al. Rimonabant ameliorates insulin resistance via both adiponectin-dependent and adiponectin-independent pathways. J Biol Chem. 2009;284(3):1803–12. 10.1074/jbc.M807120200 . [DOI] [PubMed] [Google Scholar]
- 13. Liu J, Zhou L, Xiong K, Godlewski G, Mukhopadhyay B, Tam J, et al. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology. 2012;142(5):1218–28 e1 10.1053/j.gastro.2012.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–4. 10.1038/35078085 . [DOI] [PubMed] [Google Scholar]
- 15. Hagan MM, Havel PJ, Seeley RJ, Woods SC, Ekhator NN, Baker DG, et al. Cerebrospinal fluid and plasma leptin measurements: covariability with dopamine and cortisol in fasting humans. J Clin Endocrinol Metab. 1999;84(10):3579–85. . [DOI] [PubMed] [Google Scholar]
- 16. Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136(4):550–7. 10.1038/sj.bjp.0704767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab. 1996;81(9):3419–23. 10.1210/jcem.81.9.8784108 . [DOI] [PubMed] [Google Scholar]
- 18. Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Janke J, Batkai S, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54(10):2838–43. Epub 2005/09/28. doi: 54/10/2838 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Monteleone P, Matias I, Martiadis V, De Petrocellis L, Maj M, Di Marzo V. Blood levels of the endocannabinoid anandamide are increased in anorexia nervosa and in binge-eating disorder, but not in bulimia nervosa. Neuropsychopharmacology. 2005;30(6):1216–21. Epub 2005/04/21. doi: 1300695 [pii] 10.1038/sj.npp.1300695 . [DOI] [PubMed] [Google Scholar]
- 20. Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91(8):3171–80. Epub 2006/05/11. doi: jc.2005-2679 [pii] 10.1210/jc.2005-2679 . [DOI] [PubMed] [Google Scholar]
- 21. Cote M, Matias I, Lemieux I, Petrosino S, Almeras N, Despres JP, et al. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes (Lond). 2007;31(4):692–9. Epub 2007/01/17. doi: 0803539 [pii] 10.1038/sj.ijo.0803539 . [DOI] [PubMed] [Google Scholar]
- 22. Di Marzo V, Cote M, Matias I, Lemieux I, Arsenault BJ, Cartier A, et al. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia. 2009;52(2):213–7. Epub 2008/10/31. 10.1007/s00125-008-1178-6 . [DOI] [PubMed] [Google Scholar]
- 23. Annuzzi G, Piscitelli F, Di Marino L, Patti L, Giacco R, Costabile G, et al. Differential alterations of the concentrations of endocannabinoids and related lipids in the subcutaneous adipose tissue of obese diabetic patients. Lipids Health Dis. 2010;9:43. Epub 2010/04/30. doi: 1476-511X-9-43 [pii] 10.1186/1476-511X-9-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sipe JC, Scott TM, Murray S, Harismendy O, Simon GM, Cravatt BF, et al. Biomarkers of endocannabinoid system activation in severe obesity. PLoS One. 2010;5(1):e8792 10.1371/journal.pone.0008792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Banni S, Carta G, Murru E, Cordeddu L, Giordano E, Sirigu AR, et al. Krill oil significantly decreases 2-arachidonoylglycerol plasma levels in obese subjects. Nutr Metab (Lond). 2011;8(1):7. Epub 2011/02/01. doi: 1743-7075-8-7 [pii] 10.1186/1743-7075-8-7 ; PubMed Central PMCID: PMC3048484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bennetzen MF, Wellner N, Ahmed SS, Ahmed SM, Diep TA, Hansen HS, et al. Investigations of the human endocannabinoid system in two subcutaneous adipose tissue depots in lean subjects and in obese subjects before and after weight loss. Int J Obes (Lond). 2011;35(11):1377–84. 10.1038/ijo.2011.8 . [DOI] [PubMed] [Google Scholar]
- 27. Cable JC, Tan GD, Alexander SP, O'Sullivan SE. The activity of the endocannabinoid metabolising enzyme fatty acid amide hydrolase in subcutaneous adipocytes correlates with BMI in metabolically healthy humans. Lipids Health Dis. 2011;10:129 10.1186/1476-511X-10-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jumpertz R, Guijarro A, Pratley RE, Piomelli D, Krakoff J. Central and peripheral endocannabinoids and cognate acylethanolamides in humans: association with race, adiposity, and energy expenditure. J Clin Endocrinol Metab. 2011;96(3):787–91. Epub 2010/12/24. 10.1210/jc.2010-2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quercioli A, Montecucco F, Pataky Z, Thomas A, Ambrosio G, Staub C, et al. Improvement in coronary circulatory function in morbidly obese individuals after gastric bypass-induced weight loss: relation to alterations in endocannabinoids and adipocytokines. Eur Heart J. 2013;34(27):2063–73. 10.1093/eurheartj/eht085 . [DOI] [PubMed] [Google Scholar]
- 30. Abdulnour J, Yasari S, Rabasa-Lhoret R, Faraj M, Petrosino S, Piscitelli F, et al. Circulating endocannabinoids in insulin sensitive vs. Insulin resistant obese postmenopausal women. A MONET group study. Obesity (Silver Spring). 2014;22(1):211–6. 10.1002/oby.20498 . [DOI] [PubMed] [Google Scholar]
- 31. Fernandez-Aranda F, Sauchelli S, Pastor A, Gonzalez ML, de la Torre R, Granero R, et al. Moderate-vigorous physical activity across body mass index in females: moderating effect of endocannabinoids and temperament. PLoS One. 2014;9(8):e104534 10.1371/journal.pone.0104534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Artmann A, Petersen G, Hellgren LI, Boberg J, Skonberg C, Nellemann C, et al. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim Biophys Acta. 2008;1781(4):200–12. Epub 2008/03/05. doi: S1388-1981(08)00035-8 [pii] 10.1016/j.bbalip.2008.01.006 . [DOI] [PubMed] [Google Scholar]
- 33. Massa F, Mancini G, Schmidt H, Steindel F, Mackie K, Angioni C, et al. Alterations in the hippocampal endocannabinoid system in diet-induced obese mice. J Neurosci. 2010;30(18):6273–81. Epub 2010/05/07. doi: 30/18/6273 [pii] 10.1523/JNEUROSCI.2648-09.2010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rojo ML, Soderstrom I, Olsson T, Fowler CJ. Changes in cannabinoid CB(1) receptor functionality in the female rat prefrontal cortex following a high fat diet. Life Sci. 2013;92(13):757–62. 10.1016/j.lfs.2013.02.002 . [DOI] [PubMed] [Google Scholar]
- 35. Azim S, Nicholson J, Rebecchi MJ, Galbavy W, Feng T, Reinsel R, et al. Endocannabinoids and acute pain after total knee arthroplasty. Pain. 2015;156(2):341–7. 10.1097/01.j.pain.0000460315.80981.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaczocha M, Rebecchi MJ, Ralph BP, Teng YH, Berger WT, Galbavy W, et al. Inhibition of Fatty Acid binding proteins elevates brain anandamide levels and produces analgesia. PLoS One. 2014;9(4):e94200 10.1371/journal.pone.0094200 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lehmann R. General derivation of partial and multiple rank correlation coefficients Biometrical journal Biometrische Zeitschrift. 1977;19(4):229–36. [Google Scholar]
- 38. Kloke JD, McKean JW. Rfit: Rank-based Estimation for Linear Models. R J. 2012;4(2):57–64. WOS:000313198000008. [Google Scholar]
- 39.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, Available: http://www.R-project.org/. Accessed 2014 September 23.
- 40. Hickey MS, Israel RG, Gardiner SN, Considine RV, McCammon MR, Tyndall GL, et al. Gender differences in serum leptin levels in humans. Biochemical and molecular medicine. 1996;59(1):1–6. . [DOI] [PubMed] [Google Scholar]
- 41. Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D Jr. Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2(5):589–93. . [DOI] [PubMed] [Google Scholar]
- 42. Otero M, Lago R, Gomez R, Dieguez C, Lago F, Gomez-Reino J, et al. Towards a pro-inflammatory and immunomodulatory emerging role of leptin. Rheumatology (Oxford). 2006;45(8):944–50. 10.1093/rheumatology/kel157 . [DOI] [PubMed] [Google Scholar]
- 43. Dube JJ, Bhatt BA, Dedousis N, Bonen A, O'Doherty RM. Leptin, skeletal muscle lipids, and lipid-induced insulin resistance. Am J Physiol Regul Integr Comp Physiol. 2007;293(2):R642–50. Epub 2007/05/11. doi: 00133.2007 [pii] 10.1152/ajpregu.00133.2007 . [DOI] [PubMed] [Google Scholar]
- 44. Otero M, Lago R, Lago F, Casanueva FF, Dieguez C, Gomez-Reino JJ, et al. Leptin, from fat to inflammation: old questions and new insights. FEBS Lett. 2005;579(2):295–301. 10.1016/j.febslet.2004.11.024 . [DOI] [PubMed] [Google Scholar]
- 45. Sainz N, Barrenetxe J, Moreno-Aliaga MJ, Martinez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64(1):35–46. 10.1016/j.metabol.2014.10.015 . [DOI] [PubMed] [Google Scholar]
- 46. Cardinal P, Andre C, Quarta C, Bellocchio L, Clark S, Elie M, et al. CB1 cannabinoid receptor in SF1-expressing neurons of the ventromedial hypothalamus determines metabolic responses to diet and leptin. Molecular metabolism. 2014;3(7):705–16. 10.1016/j.molmet.2014.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hanlon EC, Tasali E, Leproult R, Stuhr KL, Doncheck E, de Wit H, et al. Circadian rhythm of circulating levels of the endocannabinoid 2-arachidonoylglycerol. J Clin Endocrinol Metab. 2015;100(1):220–6. 10.1210/jc.2014-3455 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fujioka K, Patane J, Lubina J, Lau D. CSF leptin levels after exogenous administration of recombinant methionyl human leptin. JAMA. 1999;282(16):1517–8. . [DOI] [PubMed] [Google Scholar]
- 49. Nam SY, Kratzsch J, Kim KW, Kim KR, Lim SK, Marcus C. Cerebrospinal fluid and plasma concentrations of leptin, NPY, and alpha-MSH in obese women and their relationship to negative energy balance. J Clin Endocrinol Metab. 2001;86(10):4849–53. 10.1210/jcem.86.10.7939 . [DOI] [PubMed] [Google Scholar]
- 50. Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, et al. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology. 2004;29(11):2108–14. 10.1038/sj.npp.1300558 . [DOI] [PubMed] [Google Scholar]
- 51. Pisani A, Fezza F, Galati S, Battista N, Napolitano S, Finazzi-Agro A, et al. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson's disease patients. Ann Neurol. 2005;57(5):777–9. Epub 2005/04/27. 10.1002/ana.20462 . [DOI] [PubMed] [Google Scholar]
- 52. Sarchielli P, Pini LA, Coppola F, Rossi C, Baldi A, Mancini ML, et al. Endocannabinoids in chronic migraine: CSF findings suggest a system failure. Neuropsychopharmacology. 2007;32(6):1384–90. Epub 2006/11/23. doi: 1301246 [pii] 10.1038/sj.npp.1301246 . [DOI] [PubMed] [Google Scholar]
- 53. Centonze D, Bari M, Rossi S, Prosperetti C, Furlan R, Fezza F, et al. The endocannabinoid system is dysregulated in multiple sclerosis and in experimental autoimmune encephalomyelitis. Brain. 2007;130(Pt 10):2543–53. Epub 2007/07/13. doi: awm160 [pii] 10.1093/brain/awm160 . [DOI] [PubMed] [Google Scholar]
- 54. Leweke FM, Giuffrida A, Koethe D, Schreiber D, Nolden BM, Kranaster L, et al. Anandamide levels in cerebrospinal fluid of first-episode schizophrenic patients: impact of cannabis use. Schizophr Res. 2007;94(1–3):29–36. 10.1016/j.schres.2007.04.025 [DOI] [PubMed] [Google Scholar]
- 55. Di Filippo M, Pini LA, Pelliccioli GP, Calabresi P, Sarchielli P. Abnormalities in the cerebrospinal fluid levels of endocannabinoids in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2008;79(11):1224–9. Epub 2008/06/07. doi: jnnp.2007.139071 [pii] 10.1136/jnnp.2007.139071 . [DOI] [PubMed] [Google Scholar]
- 56. Koethe D, Giuffrida A, Schreiber D, Hellmich M, Schultze-Lutter F, Ruhrmann S, et al. Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. Br J Psychiatry. 2009;194(4):371–2. 10.1192/bjp.bp.108.053843 . [DOI] [PubMed] [Google Scholar]
- 57. Koppel J, Bradshaw H, Goldberg TE, Khalili H, Marambaud P, Walker MJ, et al. Endocannabinoids in Alzheimer's disease and their impact on normative cognitive performance: a case-control and cohort study. Lipids Health Dis. 2009;8:2. Epub 2009/01/16. doi: 1476-511X-8-2 [pii] 10.1186/1476-511X-8-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Koethe D, Schreiber D, Giuffrida A, Mauss C, Faulhaber J, Heydenreich B, et al. Sleep deprivation increases oleoylethanolamide in human cerebrospinal fluid. J Neural Transm. 2009;116(3):301–5. 10.1007/s00702-008-0169-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Romigi A, Bari M, Placidi F, Marciani MG, Malaponti M, Torelli F, et al. Cerebrospinal fluid levels of the endocannabinoid anandamide are reduced in patients with untreated newly diagnosed temporal lobe epilepsy. Epilepsia. 2010;51(5):768–72. 10.1111/j.1528-1167.2009.02334.x . [DOI] [PubMed] [Google Scholar]
- 60. Pisani V, Moschella V, Bari M, Fezza F, Galati S, Bernardi G, et al. Dynamic changes of anandamide in the cerebrospinal fluid of Parkinson's disease patients. Mov Disord. 2010;25(7):920–4. Epub 2010/05/13. 10.1002/mds.23014 . [DOI] [PubMed] [Google Scholar]
- 61. Morgan CJ, Page E, Schaefer C, Chatten K, Manocha A, Gulati S, et al. Cerebrospinal fluid anandamide levels, cannabis use and psychotic-like symptoms. Br J Psychiatry. 2013;202(5):381–2. 10.1192/bjp.bp.112.121178 . [DOI] [PubMed] [Google Scholar]
- 62. Borner C, Hollt V, Kraus J. Activation of human T cells induces upregulation of cannabinoid receptor type 1 transcription. Neuroimmunomodulation. 2007;14(6):281–6. Epub 2008/02/22. doi: 000117809 [pii] 10.1159/000117809 . [DOI] [PubMed] [Google Scholar]
- 63. Maccarrone M, Valensise H, Bari M, Lazzarin N, Romanini C, Finazzi-Agro A. Progesterone up-regulates anandamide hydrolase in human lymphocytes: role of cytokines and implications for fertility. J Immunol. 2001;166(12):7183–9. Epub 2001/06/08. . [DOI] [PubMed] [Google Scholar]
- 64. De Chiara V, Motta C, Rossi S, Studer V, Barbieri F, Lauro D, et al. Interleukin-1beta alters the sensitivity of cannabinoid CB1 receptors controlling glutamate transmission in the striatum. Neuroscience. 2013;250:232–9. 10.1016/j.neuroscience.2013.06.069 . [DOI] [PubMed] [Google Scholar]
- 65. Hill MN, Bierer LM, Makotkine I, Golier JA, Galea S, McEwen BS, et al. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology. 2013;38(12):2952–61. 10.1016/j.psyneuen.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ho WS, Hill MN, Miller GE, Gorzalka BB, Hillard CJ. Serum contents of endocannabinoids are correlated with blood pressure in depressed women. Lipids Health Dis. 2012;11:32 10.1186/1476-511X-11-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hauer D, Schelling G, Gola H, Campolongo P, Morath J, Roozendaal B, et al. Plasma concentrations of endocannabinoids and related primary fatty acid amides in patients with post-traumatic stress disorder. PLoS One. 2013;8(5):e62741 10.1371/journal.pone.0062741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wong A, Sagar DR, Ortori CA, Kendall DA, Chapman V, Barrett DA. Simultaneous tissue profiling of eicosanoid and endocannabinoid lipid families in a rat model of osteoarthritis. J Lipid Res. 2014;55(9):1902–13. 10.1194/jlr.M048694 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gatta-Cherifi B, Matias I, Vallee M, Tabarin A, Marsicano G, Piazza PV, et al. Simultaneous postprandial deregulation of the orexigenic endocannabinoid anandamide and the anorexigenic peptide YY in obesity. Int J Obes (Lond). 2012;36(6):880–5. 10.1038/ijo.2011.165 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
B in the table refers to the unstandardized coefficients, and these are only shown for analyses with significant ANOVA values.
(DOCX)
B in the table refers to the unstandardized coefficients, and these are only shown for CSF 2-AG, gender and the constant; the other variables did not reach significance in any case (P > 0.3).
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.