Abstract
Aims
To evaluate real-world clinical outcomes for switching basal insulin analogues [insulin glargine (GLA) and insulin detemir (DET)] among US patients with type 2 diabetes mellitus (T2DM).
Methods
Using the GE Centricity Electronic Medical Records database, this retrospective study examined two cohorts: cohort 1, comprising patients previously on GLA and then either switching to DET (DET-S) or continuing with GLA (GLA-C); and cohort 2, comprising patients previously on DET and then either switching to GLA (GLA-S) or continuing with DET (DET-C). Within each cohort, treatment groups were propensity-score-matched on baseline characteristics. At 1-year follow-up, insulin treatment patterns, glycated haemoglobin (HbA1c) levels, hypoglycaemic events, weight and body mass index (BMI) were evaluated.
Results
The analysis included 13 942 patients: cohort 1: n = 10 657 (DET-S, n = 1797 matched to GLA-C, n = 8860) and cohort 2: n = 3285 (GLA-S, n = 858 matched to DET-C, n = 2427). Baseline characteristics were similar between the treatment groups in each cohort. At 1-year follow-up, in cohort 1, patients in the DET-S subgroup were significantly less persistent with treatment, more likely to use a rapid-acting insulin analogue, had higher HbA1c values, lower HbA1c reductions and lower proportions of patients achieving HbA1c <7.0 or <8.0% compared with patients in the GLA-C subgroup, while hypoglycaemia rates and BMI/weight values and change from baseline were similar in the two subgroups. In cohort 2, overall, there were contrasting findings between patients in the GLA-S and those in the DET-C subgroup.
Conclusions
This study showed contrasting results when patients with T2DM switched between basal insulin analogues, although these preliminary results may be subject to limitations in the analysis. Nevertheless, this study calls into question the therapeutic interchangeability of GLA and DET, and this merits further investigation.
Keywords: adherence, cost, hypoglycaemia, insulin detemir, insulin glargine, persistence, switching, type 2 diabetes
Introduction
Type 2 diabetes mellitus (T2DM) is a progressive disease, meaning that most patients will require the introduction of insulin into their treatment regimen at some point during the continuum of care 1. To facilitate the transition to insulin, numerous clinical and real-world studies have investigated the efficacy and safety of initiating long-acting analogue insulins. In clinical studies, both insulin glargine (GLA; Lantus®, Sanofi US, Inc., Bridgewater, NJ, USA) and insulin detemir (DET; Levemir®, Novo Nordisk A/S, Bagsvaerd, Denmark) have been shown to result in equivalent improvements in glycaemic control with a low incidence of hypoglycaemia when used as part of a basal-bolus regimen 2–4. Although glycaemic outcomes in such trials have been similar for these two basal analogue insulins, it has been noted that higher doses and twice-daily dosing are often required with DET compared with GLA 3,4. A recent trial has suggested that when both DET and GLA are used once daily as an adjunct to metformin, a greater proportion of patients treated with GLA reached glycated haemoglobin (HbA1c) levels <7.0% than did patients treated with DET 5.
Attempts to examine the relative advantages of these insulin analogues in a real-world setting have proved difficult to interpret. Some studies have reported enhanced glycaemic control with GLA compared with DET, along with improved adherence and persistence, with no difference with regard to hypoglycaemia, healthcare costs or weight gain 6–8; however, other studies found no difference in glycaemic control between the two insulin types 9,11, with one suggesting that patients initiating DET were 30% less likely to gain 0.9 kg or more in body weight than GLA users 10. Studies investigating the efficacy and safety of switching from GLA to DET are similarly conflicting in their findings. Although once-daily dosing of GLA and DET has been shown to result in equivalent 24-h glycaemic control, switching from GLA to DET was associated with improved HbA1c levels and fewer hypoglycaemic events, compared with remaining on GLA in an observational study of patients with type 1 diabetes mellitus (T1DM) and T2DM 7,11,12. A retrospective analysis, however, reported that switching from GLA to DET did not improve glycaemic control among a cohort of patients with T2DM 13.
A recent longitudinal study sought to expand on these data by assessing real-world outcomes using a retrospective analysis of two large, independent, national, US databases comprising commercially insured and Medicare populations: the IMPACT® and Humana® cohorts 14. The study found that, at 1-year follow-up, patients who had remained on GLA showed significantly higher persistence with and adherence to treatment compared with those who switched to DET (p < 0.001). In addition, 37% of patients who switched to DET restarted GLA (p < 0.05), compared with only 20% of GLA users returning to DET after having switched to GLA. Overall hypoglycaemia rates were significantly higher for patients continuing on GLA than for patients switching to DET in the Humana cohort (16 vs. 11%; p < 0.05), but overall hypoglycaemia rates were similar in the IMPACT cohort (11 vs. 12%; p = 0.490).
In the present study, to further evaluate the consequences of insulin switching, we used data from patients' medical records to investigate retrospectively the real-world clinical outcomes for patients with T2DM who switched between these two basal insulin analogues.
Methods
The present analysis was a retrospective cohort study using the GE Centricity Electronic Medical Records (EMR) database from 1 July 2005 to 31 December 2012. In 2007, the GE Centricity EMR database contained the medical records of ∼30 million patients in 49 US states. As the analysis was performed on de-indentified data, approval from an ethics committee was not necessary.
Patients
We analysed two cohorts in the present study: cohort 1 included patients who were previously treated with GLA and switched to DET (DET-S) or who remained on GLA (GLA-C); and cohort 2 included patients previously treated with DET and subsequently switched to GLA (GLA-S) or who remained on DET (DET-C). In cohort 1, the patients in the DET-S subgroup were required to have ≥1 physician prescription for GLA <6 months before first DET physician prescription order date (after 1 January 2006). The first DET physician prescription date was designated as the index date. Patients in the GLA-C subgroup were required to have ≥2 GLA physician prescriptions starting from the third-quarter of 2005, without a subsequent physician prescription order of DET, with ≥1 of the orders being <6 months before the index date. The index date was a randomly selected date between the second to the last GLA physician prescription. In cohort 2, patients in the GLA-S subgroup were required to have ≥1 physician prescription for DET <6 months before the first GLA prescription order date (after 1 July 2006). The first GLA physician prescription date was designated as the index date. Patients in the DET-C subgroup were required to have ≥2 DET physician prescriptions starting from the first-quarter of 2006, without a subsequent physician prescription order for GLA. The index date was a randomly selected date between the second and last DET physician prescriptions dates.
Patients aged ≥18 years on the index date were included in the analysis if they had a diagnosis of T2DM [International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes: 250.x0 or 250.x2] with EMR activity data for ≥6 months pre-index (baseline) and 12 months post-index (follow-up). Patients were further required to have: ≥1 baseline HbA1c measure taken during the period 90 days before up to 15 days after the index date; ≥1 baseline body mass index (BMI) measure taken in the period 30 days before up to 15 days after the index date; and ≥1 follow-up HbA1c measure from the second-quarter until 3 months after the 1-year follow-up. In cohort 1, patients with baseline prescriptions for premixed insulin or basal insulins other than GLA [i.e. neutral protamine Hagedorn (NPH) insulin, Lente, or Ultralente] were excluded. Similarly, in cohort 2, patients with baseline prescriptions for premixed insulin or basal insulins other than DET (i.e. NPH insulin, Lente or Ultralente) were excluded.
Measures
Baseline demographic and clinical characteristics included age, gender, region, race, geographic region, insurance type, BMI, weight, individual comorbidities and prescribed diabetes medications. In addition, the Charlson Comorbidity Index (CCI) score was calculated, which is the weighted sum of 19 categories of comorbidity defined using ICD-9-CM diagnoses codes, with a higher score indicating a more severe burden of comorbidity and a higher mortality risk 15,16. Clinical outcome variables were measured 1 year after the index date (follow-up) and included follow-up HbA1c, change in HbA1c from baseline, follow-up hypoglycaemic events, follow-up BMI, change in BMI from baseline follow-up body weight, change in body weight from baseline and follow-up oral antidiabetes drug (OAD) use (including GLA, DET and rapid-acting insulin). Baseline HbA1c measures were defined as those occurring between 90 days before and up to 15 days after the index date; baseline body weight and BMI measures were defined as ≥1 baseline measurement between 30 days before and up to 15 days after the index date. Follow-up HbA1c values were defined as those occurring during the period from the second-quarter until 3 months after 1-year follow-up, and follow-up body weight and BMI values were defined as those occurring during the 30 days before or after the end of the 1-year follow-up period (index date + 360 days). If patients had multiple HbA1c, body weight or BMI results during this period, the value dated closest to the index date was used as the baseline value, and the value dated closest to the end of the follow-up period was used as the follow-up value.
Hypoglycaemia was defined as a healthcare encounter (outpatient, inpatient or emergency department visit) with a primary or secondary ICD-9-CM diagnosis code for hypoglycaemia, based on the algorithm published by Ginde et al. 17. Both the proportion of patients with ≥1 hypoglycaemic event (prevalence rate) and the average number of hypoglycaemic events per patient (event rate) during the baseline and 1-year follow-up period were identified.
Treatment persistence was defined as the patient remaining on the index long-acting insulin (GLA for GLA-C and DET for DET-S for cohort 1; DET for DET-C and GLA for GLA-S for cohort 2) during the 1-year follow-up period without discontinuation after study drug initiation. Based on the published methodology 18, study medication was considered discontinued if the prescription was not re-ordered by the physician within the expected time of medication coverage (the pre-defined 90th percentile of the time between first and second physician prescriptions among patients with ≥1 prescription). Patients who restarted their initial medication after a period of not having used this medication during follow-up were considered non-persistent. Persistence days were measured as the number of days between the index date and the discontinuation date. Sensitivity analyses were also conducted using 75th and 95th percentiles of the expected time of medication coverage.
Data Analysis
Descriptive and multivariate statistical methodologies were used to assess the baseline characteristics and to compare the outcomes over the 1-year follow-up period. To address potential selection bias in the real-world setting, in each cohort, patients from the two subgroups were matched by propensity-score matching, with a 1 up to 5 ratio in cohort 1 and a 1 up to 3 ratio in cohort 2, to balance baseline age, gender, race, geographic region, insurance type, physician specialty, index year, baseline comorbidity, baseline OAD use, concomitant medication, HbA1c and BMI. These ratios and matchings were chosen because our preliminary analysis revealed a prescription imbalance, with significantly more eligible patients in the GLA group when compared with the DET group, and a much lower number of patients in the ‘switcher’ groups versus the ‘continuer’ groups. Additionally, one-to-many matching has previously been validated as a method to increase precision in cohort studies, compared with one-to-one matching, and has also been supported in a recent review assessing the quality of statistical methodologies in matched case–control studies 19,20. Matching was implemented without replacement and any patient without ≥1 match was excluded from the analysis. Between-group covariate balance was evaluated using descriptive t-tests and chi-squared tests (with corresponding p-values) and standardized differences, where a standardized difference <10 indicated adequate balance 21.
Because not every patient in the matched cohorts had follow-up BMI or body weight data, we conducted, as sensitivity analysis, ordinary least squares regressions for BMI and body weight changes, adjusting for potential baseline imbalances. The model used a larger set of covariates, which included patient demographics (age, gender, geographic locations and BMI categories), baseline comorbidities and some basic clinical characteristics (baseline OAD use, statins for baseline concomitant medication use, baseline HbA1c level and baseline hypoglycaemic events).
Results
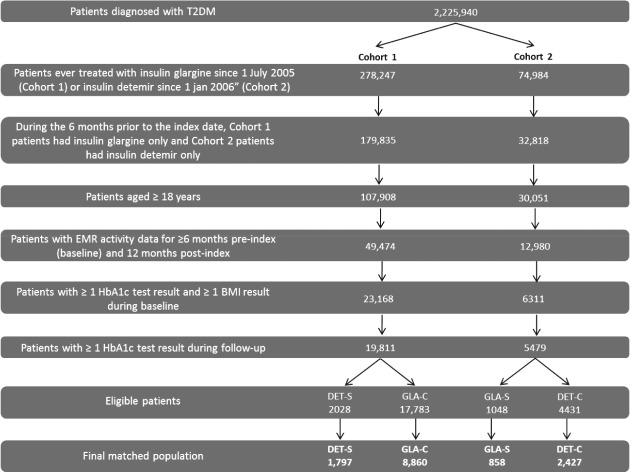
A total of 25 290 eligible patients were identified with 19 811 patients from cohort 1 (GLA-C, n = 17 783 and DET-S, n = 2028) and 5479 from cohort 2 (DET-C, n = 4431 and GLA-S, n = 1048). Patient attrition is shown in Figure 1. Significant baseline differences existed between the subgroups in each cohort (Table S1). In cohort 1, when compared with patients in the DET-S subgroup, patients in the GLA-C subgroup were older, were more likely to be black, were more likely to reside in the Midwest or West, were more likely to be enrolled in Medicare, had higher CCI scores and had lower HbA1c values. No significant differences were found for BMI or body weight between the GLA-C and DET-S subgroups at baseline. In cohort 2, when compared with the DET-C subgroup, patients in the GLA-S subgroup were younger, were more likely to be black, were more likely to reside in the Midwest or West, had higher HbA1c values, and had higher body weight and BMI values. Patients in the GLA-S and DET-C subgroups had similar CCI scores at baseline. For both cohorts, no significant differences were found for baseline hypoglycaemic events between groups.
Figure 1.
Patient attrition. BMI, body mass index; EMR, electronic medical records; HbA1c, glycated haemoglobin; T2DM, type 2 diabetes mellitus.
After propensity-score matching, the final matched study population included 13 942 patients overall, with 10 657 from cohort 1 (GLA-C, n = 8860 and DET-S, n = 1797; female patients 54.7%, age 58.4 years, HbA1c level 8.7%, BMI 35.1 kg/cm2, weight 99.3 kg) and 3285 patients from cohort 2 (DET-C, n = 2427 and GLA-S, n = 858; female 53.5%, age 58.3 years, HbA1c level 8.8%, BMI 35.4 kg/cm2, weight 100.7 kg). The baseline demographic and clinical characteristics were balanced between treatment groups in each cohort (Table1).
Table 1.
Baseline characteristics among matched patients in cohorts 1 and 2
| Cohort 1 |
Cohort 2 |
|||||
|---|---|---|---|---|---|---|
| DET-S | GLA-C | p | GLA-S | DET-C | p | |
| n = 1797 | n = 8860 | n = 858 | n = 2427 | |||
| Demographics | ||||||
| Women, n (%) | 996 (55.4) | 4831 (54.5) | 0.4848 | 477 (55.6) | 1281 (52.8) | 0.1556 |
| Mean (s.d.) age, years | 58.1 (12.1) | 58.4 (12.2) | 0.2461 | 58.0 (12.5) | 58.5 (11.9) | 0.3701 |
| Race, n (%) | 0.9760 | 0.9802 | ||||
| White | 967 (53.8) | 4840 (54.6) | 490 (57.1) | 1383 (57.0) | ||
| Black | 197 (11.0) | 959 (10.8) | 81 (9.4) | 233 (9.6) | ||
| Hispanic | 63 (3.5) | 297 (3.4) | 21 (2.5) | 62 (2.6) | ||
| Other | 47 (2.6) | 222 (2.5) | 23 (2.7) | 56 (2.3) | ||
| Unknown/undetermined | 523 (29.1) | 2542 (28.7) | 243 (28.3) | 693 (28.6) | ||
| US region, n (%) | 0.9293 | 0.9167 | ||||
| Midwest | 414 (23.0) | 1981 (22.4) | 185 (21.6) | 520 (21.4) | ||
| Northeast | 495 (27.5) | 2441 (27.6) | 196 (22.8) | 571 (23.5) | ||
| South | 702 (39.1) | 3502 (39.5) | 383 (44.6) | 1089 (44.9) | ||
| West | 186 (10.4) | 936 (10.6) | 94 (11.0) | 247 (10.2) | ||
| Insurance type, n (%) | 0.9650 | 0.7944 | ||||
| Commercial | 391 (21.8) | 1859 (21.0) | 171 (19.9) | 521 (21.5) | ||
| Medicaid | 51 (2.8) | 254 (2.9) | 12 (1.4) | 38 (1.6) | ||
| Medicare | 636 (35.4) | 3155 (35.6) | 312 (36.4) | 859 (35.4) | ||
| Self-pay | 31 (1.7) | 150 (1.7) | 23 (2.7) | 53 (2.2) | ||
| Unknown | 688 (38.3) | 3442 (38.8) | 340 (39.6) | 956 (39.4) | ||
| Clinical characteristics | ||||||
| Mean (s.d.) HbA1c, % | 8.7 (1.9) | 8.7 (1.9) | 0.4172 | 8.8 (1.8) | 8.8 (1.9) | 0.6951 |
| HbA1c category, n (%) | 0.0667 | 0.4926 | ||||
| <7.0% | 283 (15.7) | 1561 (17.6) | 127 (14.8) | 333 (13.7) | ||
| ≥7.0 to <8.0% | 457 (25.4) | 2398 (27.1) | 215 (25.1) | 658 (27.1) | ||
| ≥8.0 to <9.0% | 403 (22.4) | 1826 (20.6) | 196 (22.8) | 547 (22.5) | ||
| ≥9.0 to <10.0% | 282 (15.7) | 1272 (14.4) | 141 (16.4) | 354 (14.6) | ||
| ≥10.0% | 372 (20.7) | 1803 (20.3) | 179 (20.9) | 535 (22.0) | ||
| Mean (s.d.) BMI, kg/m2 | 35.1 (8.2) | 35.0 (7.9) | 0.5837 | 35.6 (8.0) | 35.4 (7.8) | 0.5149 |
| Mean (s.d.) body weight, kg | 99.5 (25.2) | 99.3 (24.9) | 0.7933 | 100.4 (24.8) | 100.8 (25.1) | 0.6780 |
| Mean (s.d.) CCI score | 1.13 (1.49) | 1.11 (1.49) | 0.5523 | 1.02 (1.45) | 1.01 (1.37) | 0.8216 |
| Hypoglycaemia rates | ||||||
| Any hypoglycaemia, n (%) | 24 (1.3) | 92 (1.0) | 0.2683 | 13 (1.5) | 31 (1.3) | 0.6024 |
| Mean (s.d.) number of hypoglycaemic events per patient year | 0.02 (0.14) | 0.01 (0.12) | 0.3674 | 0.02 (0.14) | 0.01 (0.13) | 0.7201 |
| Comorbidities, n (%) | ||||||
| Hypertension | 1385 (77.1) | 6807 (76.8) | 0.8227 | 658 (76.7) | 1884 (77.6) | 0.5729 |
| Hyperlipidaemia | 1429 (79.5) | 7044 (79.5) | 0.9862 | 697 (81.2) | 1971 (81.2) | 0.9876 |
| Neuropathy | 419 (23.3) | 1959 (22.1) | 0.2629 | 183 (21.3) | 490 (20.2) | 0.4773 |
| Nephropathy | 245 (13.6) | 1096 (12.4) | 0.1408 | 103 (12.0) | 260 (10.7) | 0.2995 |
| Retinopathy | 210 (11.7) | 1020 (11.5) | 0.8335 | 63 (7.3) | 212 (8.7) | 0.2056 |
| Mental illness | 841 (46.8) | 4061 (45.8) | 0.4542 | 376 (43.8) | 1086 (44.7) | 0.6398 |
| Severe mental illness | 183 (10.2) | 918 (10.4) | 0.8216 | 88 (10.3) | 217 (8.9) | 0.2538 |
| OAD use, n (%) | ||||||
| Mean (s.d.) number of OADs | 0.78 (0.92) | 0.76 (0.91) | 0.4920 | 0.89 (0.95) | 0.85 (0.92) | 0.3843 |
| Metformin | 627 (34.9) | 3120 (35.2) | 0.7938 | 347 (40.4) | 981 (40.4) | 0.9907 |
| Sulfonylurea | 377 (21.0) | 1721 (19.4) | 0.1306 | 192 (22.4) | 546 (22.5) | 0.9426 |
| Thiazolidinedione | 233 (13.0) | 1072 (12.1) | 0.3068 | 114 (13.3) | 276 (11.4) | 0.1361 |
| Alpha-glucosidase | 5 (0.3) | 28 (0.3) | 0.7927 | 3 (0.3) | 5 (0.2) | 0.4631 |
| Dipeptidyl peptidase-4 inhibitor | 127 (7.1) | 661 (7.5) | 0.5614 | 90 (10.5) | 234 (9.6) | 0.4740 |
| Meglitinide | 27 (1.5) | 137 (1.5) | 0.8907 | 15 (1.7) | 33 (1.4) | 0.4149 |
| Glucagon-like peptide-1 analogue use, n (%) | 90 (5.0) | 454 (5.1) | 0.8388 | 66 (7.7) | 192 (7.9) | 0.8378 |
| Regular insulin use, n (%) | 51 (2.8) | 229 (2.6) | 0.5403 | 16 (1.9) | 28 (1.2) | 0.1194 |
| Rapid-acting insulin use, n (%) | 716 (39.8) | 3553 (40.1) | 0.8391 | 313 (36.5) | 876 (36.1) | 0.8396 |
| Other | ||||||
| Physician specialty, n (%) | 0.8747 | 0.9534 | ||||
| Other | 66 (3.7) | 334 (3.8) | 75 (8.7) | 199 (8.2) | ||
| Endocrinology | 136 (7.6) | 624 (7.0) | 51 (5.9) | 139 (5.7) | ||
| Primary care | 1092 (60.8) | 5388 (60.8) | 496 (57.8) | 1421 (58.5) | ||
| Unknown specialty | 503 (28.0) | 2514 (28.4) | 236 (27.5) | 668 (27.5) | ||
DET, insulin detemir; GLA, insulin glargine; DET-S, patients previously treated with GLA who switched to DET; GLA-C, patients who remained on GLA; GLA-S, patients previously treated with DET who switched to GLA; DET-C, patients who remained on DET; BMI, body mass index; CCI, Charlson Comorbidity Index; HbA1c, glycated haemoglobin; OAD, oral antidiabetes drug; s.d., standard deviation.
Insulin Utilization
During 1-year follow-up, overall, switching from GLA to DET resulted in lower persistence with treatment, whereas switching from DET to GLA resulted in higher persistence. Among patients in cohort 1, those in the DET-S subgroup versus those in the GLA-C subgroup had significantly lower persistence with treatment (52.4 vs. 61.3%, respectively; p < 0.0001) and significantly fewer treatment persistent days (311.6 vs. 331.0 days; p < 0.0001). In cohort 2, however, the GLA-S and the DET-C subgroups had similar levels of treatment persistence (56.5 vs. 58.5%, respectively; p = 0.292) yet fewer treatment persistent days (320.7 vs. 325.3 days; p = 0.015).
Sensitivity analysis using 75th and 95th percentile of the time duration yielded similar results: 75th percentile: cohort 1 GLA-C versus DET-S subgroup, 21.2 versus 35.7% (p < 0.0001); cohort 2 DET-C versus GLA-S subgroup, 24.7 versus 31.6% (p = 0.0001); 95th percentile: cohort 1 GLA-C versus DET-S subgroup, 63.1 versus 68.2% (p < 0.0001); cohort 2 DET-C versus GLA-S subgroup, 67.0 versus 66.0% (p = 0.622).
In cohort 1, the use of rapid-acting insulin was significantly higher among the patients in the DET-S subgroup than among those in the GLA-C subgroup (57.6 vs. 52.5%; p < 0.0001; Figure 2), and 26.9% of patients in the DET-S subgroup restarted GLA. In cohort 2, however, similar proportions of patients used rapid-acting insulin in the DET-C and GLA-S subgroups (50.4 vs. 51.1%; p = 0.712), and 18.6% of patients in the GLA-S subgroup restarted DET.
Figure 2.
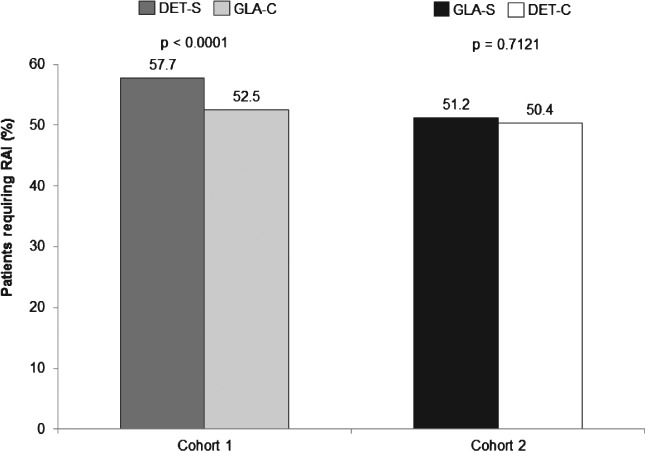
Patients requiring rapid-acting insulin (RAI). DET, insulin detemir; GLA, insulin glargine; DET-S, patients previously treated with GLA who switched to DET; GLA-C, patients who remained on GLA; GLA-S, patients previously treated with DET who switched to GLA; DET-C, patients who remained on DET.
Clinical Outcomes
The baseline mean HbA1c level was similar between the treatment groups in each of the cohorts. In cohort 1, patients in the DET-S subgroup had a significantly higher follow-up HbA1c level (Figure 3A), a significantly lower HbA1c reduction (Figure 3B), and significantly lower proportions of patients achieved HbA1c <7.0% (Figure 3C) and HbA1c <8.0% (Figure 3D) at the end of 1-year follow-up compared with patients in the GLA-C subgroup. In cohort 2, however, compared with patients in the DET-C subgroup, patients in the GLA-S subgroup had a significantly lower follow-up HbA1c (Figure 3A) and a numerically higher (although not statistically significant) HbA1c reduction (Figure 3B), but there were no differences in the proportions of patients achieving an HbA1c level <7.0% (Figure 3C) or <8.0% (Figure 3D).
Figure 3.
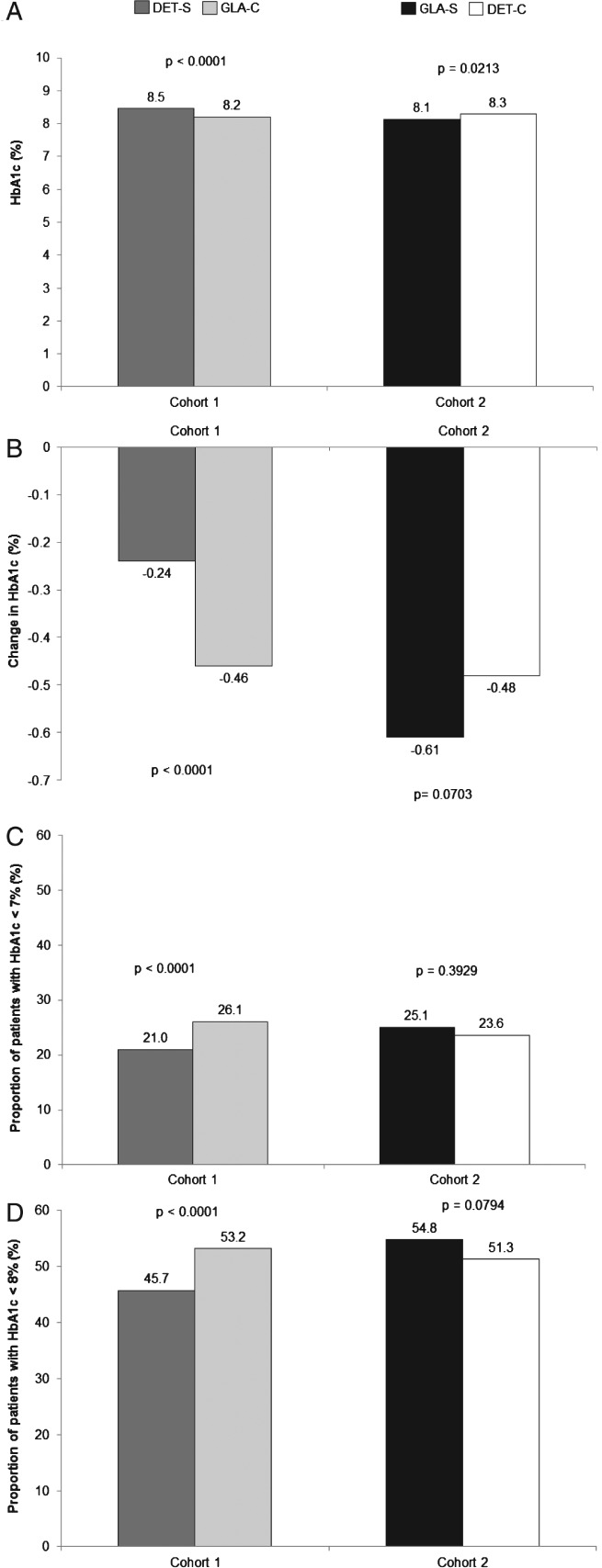
(A) One-year follow-up glycated haemoglobin (HbA1c) values, (B) change in HbA1c from baseline, (C) proportion of patients achieving target HbA1c <7.0%, and (D) proportion of patients achieving target HbA1c <8.0%. DET, insulin detemir; GLA, insulin glargine; DET-S, patients previously treated with GLA who switched to DET; GLA-C, patients who remained on GLA; GLA-S, patients previously treated with DET who switched to GLA; DET-C, patients who remained on DET.
For both prevalence and event rates of any hypoglycaemic events, no significant differences were found after follow-up between the DET-S and GLA-C subgroups in cohort 1 or between the GLA-S and DET-C subgroups in cohort 2 [prevalence rate: 2.0 vs. 2.1%, p = 0.889; event rate (number of events/patient year): 0.023 vs. 0.026, p = 0.4424] or between the GLA-C and DET-S subgroups in cohort 2 (2.3 vs. 1.6%, p = 0.2314; event rate: 0.028 vs. 0.021, p = 0.3727).
In both cohorts, baseline BMI and body weight were similar between the groups (Table1). In cohort 1, BMI at 1-year follow-up was 35.0 kg/m2 for both the DET-S and GLA-C subgroups (p = 0.843). There were also no significant differences in BMI change for patients in the DET-S and GLA-C subgroups after follow-up (Figure 4A). The 1-year follow-up weight was similar for the DET-S and GLA-C subgroups (99.3 vs. 99.4 kg; p = 0.881), with similar weight changes between patients in the DET-S subgroup and those in the GLA-C subgroup (Figure 4B).
Figure 4.
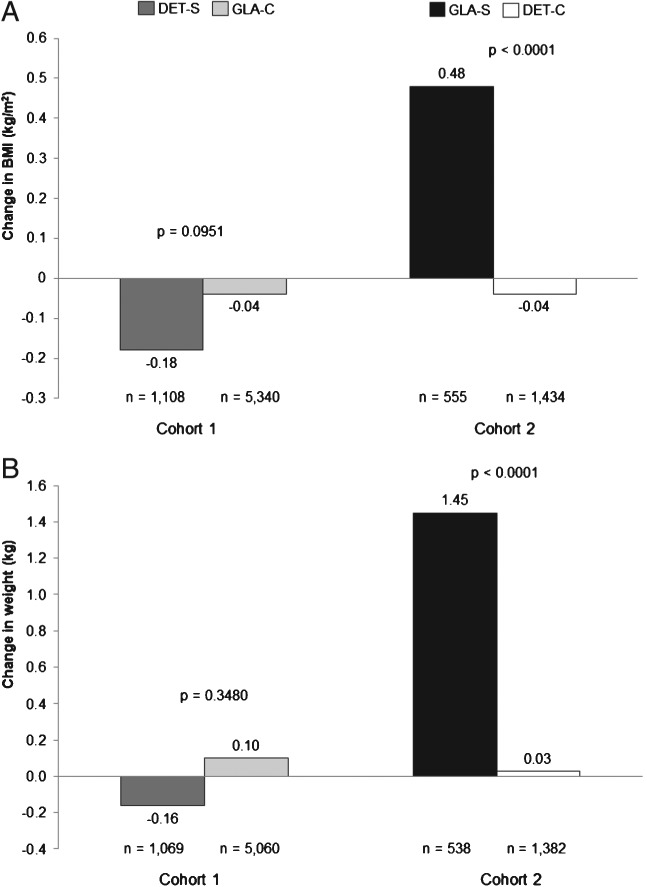
Changes in (A) body mass index (BMI) and (B) body weight at 1-year follow-up. DET, insulin detemir; GLA, insulin glargine; DET-S, patients previously treated with GLA who switched to DET; GLA-C, patients who remained on GLA; GLA-S, patients previously treated with DET who switched to GLA; DET-C, patients who remained on DET.
In cohort 2, at the end of 1-year follow-up, patients in the GLA-S subgroup had a higher BMI (36.3 vs. 35.5 kg/m2; p = 0.052), but this was not statistically significant. Change in BMI was significantly different (Figure 4A). There were no significant differences at 1-year follow-up for body weight between patients in the GLA-S and DET-C subgroups (102.2 vs. 100.9 kg; p = 0.299); however, patients in the GLA-S subgroup had a greater change from baseline in body weight compared with those in the DET-C subgroup (Figure 4B).
Sensitivity analyses using ordinary least squares regressions yielded similar results: adjusted weight change: cohort 1 DET-S versus GLA-C subgroup, −0.18 versus +0.10 kg (p = 0.329); cohort 2 GLA-S versus DET-C subgroup, +1.48 versus +0.02 kg (p < 0.001); adjusted BMI change: cohort 1 GLA-C versus DET-S subgroup, −0.18 versus −0.04 kg (p = 0.122); cohort 2 GLA-S versus DET-C subgroup, +0.48 versus − 0.05 kg (p < 0.001).
Discussion
The aim of the present study was to evaluate retrospectively the real-world clinical outcomes of switching between basal insulin analogues, compared with continuing on previous basal insulin, among patients with T2DM identified from the US GE Centricity EMR database. Overall, contrasting findings were observed when patients switched from GLA to DET, as compared with switching from DET to GLA.
In cohort 1, compared with patients in the DET-S subgroup, patients in the GLA-C subgroup had better clinical outcomes at 1-year follow-up, including lower HbA1c levels and significantly higher HbA1c reductions. In addition, significantly more patients in the GLA-C subgroup achieved the targeted HbA1c levels of <7.0% or <8.0% and those in the DET-S subgroup had significantly higher rapid-acting insulin use. In addition, no significant differences in hypoglycaemia rates and weight/BMI were observed between the two groups. These results could be attributable to lower persistence/adherence as patients might not be taking their full medication. In cohort 2, however, patients in the GLA-S subgroup had lower HbA1c levels and a numerically higher (but non-significant) HbA1c reduction; similar proportions of patients achieved HbA1c levels of <7.0 or <8.0%, and similar hypoglycaemia rates were seen in the two groups. Although 1-year follow-up body weight and BMI were similar, patients in the DET-C subgroup had a decreased BMI and a lower body weight increase, whereas patients in the GLA-S subgroup had increased BMI and significantly greater body weight increase. Although the patients in the GLA-S subgroup gained weight, the increase was <1% which, it is suggested, is not clinically relevant 22, and may reflect differences in follow-up plasma glucose levels or other factors. Furthermore, the increase in weight with GLA could be taken to be a sign of better compliance. If a switch between insulin analogues is of a ‘disruptive nature’, it will result in decreased compliance. For example, if a regimen becomes more complex (from once daily to twice daily, adding a rapid-acting insulin etc.), it could lead to less of the typical weight gain associated with insulin treatment.
In cohort 1, the patients in the DET-S subgroup, who switched from GLA to DET, had lower persistence than those who remained on GLA (robust in sensitivity analyses). This could be attributable to the fact that most patients on DET administer twice daily, whereas most patients on GLA administer once daily 13,23. Unfortunately, this information is not captured in the GE Centricity EMR database. By contrast, in cohort 2, data on patients in the GLA-S subgroup switching from DET to GLA suggested similar overall persistence with treatment. Approximately 27% of the patients in the DET-S subgroup restarted GLA during the follow-up period. Of patients in the GLA-S subgroup, however, only 19% restarted DET during the follow-up period. Although the reasons for restarting the former insulin are not captured in the GE Centricity EMR database, these findings are consistent with a previous IMPACT and Humana database study 14. Unlike randomized controlled trials, where patients are required to be ‘persistent’ with their therapy, real-world patients and physicians do not follow a stringent protocol and, therefore, any differences in patients' persistence with their insulin treatment can translate into differences in clinical and economic outcomes 17.
Overall, contrasting outcomes were observed when patients were switched from GLA to DET, when compared with switching from DET to GLA. This raises questions about the therapeutic interchangeability of these two basal analogue insulins in clinical practice, although further studies are required to confirm this non-interchangeability. The results of the present GE Centricity EMR switching analysis, however, were highly consistent with the previously performed claims database analysis 14.
The present study has a number of strengths. The GE Centricity EMR database is a large and comprehensive source of data to understand clinical practice and outcomes in the real-world setting. This database contains rich clinical information, such as HbA1c level, weight and BMI, that is not available in insurance claim databases. Overall 25 000 patients were identified in this study, and almost 15 000 were propensity-score-matched and analysed, including those switching from DET to GLA and those switching from GLA to DET during the same time period. In previous observational studies on switching from GLA to DET 11,13,24, conflicting results were shown with relatively low numbers of patients included (30–1300 patients) and including either only patients with T1DM, or a mixed group of patients with T1DM and T2DM. Our previous study used administrative claims data 14, and the results described in the present study not only validate our previous findings with a different data source but also strengthen them with additional data on weight. One of the key limitations of the present study, however, is that the EMR analysis was carried out on primary care physician prescription data, rather than on claims databases. Prescription orders do not necessarily mean that the prescriptions were filled and taken as directed. In addition, certain data were not available, including glucose levels, the duration of treatment with the initial insulin, the duration of T2DM, and the reason for switching insulin (e.g. insurance required switching to other insulin). Although propensity-score matching was used to balance the baseline data between treatment groups, the differences between the groups were quite significant (Table S1) and the potential residual confounding might therefore still be present, particularly unobserved selection bias. In a real-world setting, switching patients to another treatment regimen may introduce confounding by indication, an important limitation to observational studies reporting on patients changing their treatment regimens, including the study described here. It is difficult to amend for this by analyses. Other potential sources of bias and confounding include the range of time from measurement of HbA1c to index date, differences in time for follow-up HbA1c and follow-up BMI, and patient/physician preference. In addition, the dose of insulin at the time of switching was not available in the GE Centricity EMR database and might have impacted on clinical outcomes, especially in patients who had recently switched insulin analogues and had not had the opportunity to titrate their dosage. Specifically, the present study was designed to compare between patients who switched to a different basal insulin and those who continued on the previous basal insulin. In the case of comparing patients in the DET-S with those in the GLA-C subgroup, patients in the latter subgroup may already be fully titrated, while it takes time for patients switching to DET to titrate. This may lead to an overestimation of the benefits of GLA compared with DET, representing a further limitation of this analysis. The same limitation applies to the comparison of the GLA-S with the DET-C subgroup. One approach to account for confounding by indication is to compare patients in the GLA-S subgroup with those in the DET-S subgroup directly; however, these patients had different baseline doses of basal insulin, and based on a real-world study among US patients initiating GLA or DET, it may be that higher insulin doses were used in the DET-treated patients 6. It is not possible to establish causality from the differences in outcomes, nor are the data necessarily representative of all patients with T2DM. Patients could drop out for various reasons and thus selection may occur. In the present study, more patients treated with DET than those treated with GLA dropped out from the study population (56 vs. 35%) when 6-month baseline and 12-month follow-up were required. Similarly, only 8% of patients on DET treatment compared with 40% on GLA, dropped out when being aged >18 years was required (Figure 1). Sufficient data on HbA1c levels and BMI were missing for half of the patients in this analysis, and this lack of completeness should be considered as a major limitation of EMR databases. When interpreting the findings of the present study, it should be remembered that, because of its limitations, the methodology used may have introduced bias or affected the ‘real-world’ representativeness of the results. Additionally, because of the real-world nature of the present study, patients did not have their HbA1c levels or BMI evaluated regularly and thus were excluded if they did not have baseline and/or follow-up HbA1c/BMI values. Overall, recognizing the inherent limitations of real-world data analyses, the findings of the present study alone should not be used to guide clinicians in the decision to switch insulin, but the findings do merit further investigation. Future studies should look more closely at a number of other factors, including the dose, duration of treatment, reasons for and cost-effectiveness of switching in patients with T2DM. In addition, although previous studies have suggested that in patients with T1DM DET was associated with a higher dose and more frequent twice-daily dosing than GLA 13,24, large cohort comparative studies in patients with T1DM should be conducted to confirm these findings.
In conclusion, the results of the present study suggest that switching patients with T2DM from GLA to DET might lead to decreases in treatment persistence alongside having a negative effect on glycaemic outcomes. Maintaining patients on GLA or switching from DET to GLA, however, might be beneficial, although may result in minor weight gain. This suggests that the two basal insulin analogues might not be therapeutically interchangeable, and further studies are required to investigate this hypothesis.
Acknowledgments
This study was funded by Sanofi US, Inc. The authors received writing/editorial support in the preparation of this manuscript from Pim Dekker, PhD, of Excerpta Medica, funded by Sanofi US, Inc.
Conflict of Interest
P. L. is a member of the advisory panel for Sanofi US, Inc. and Novo Nordisk, Inc., is a consultant for Novo Nordisk, Inc., Eli Lilly and Co. and Sanofi US, Inc., has received research support from Eli Lilly and Co., Sanofi US, Inc., Novo Nordisk, Inc., Amylin Pharmaceuticals, Inc. and Boehringer Ingelheim Pharmaceuticals, Inc., and serves on speaker's bureaus for Eli Lilly and Co., Novo Nordisk, Inc., Amylin Pharmaceuticals, Inc. and Boehringer Ingelheim Pharmaceuticals, Inc. W. W., R. M., F. Y. and J. G. are employees of Sanofi US, Inc. L. X. and O. B. are employees of STATinMED, which received funding to carry out this work from Sanofi US, Inc.
P. L. co-developed the concept, interpreted the results of the analyses, reviewed manuscript outline and drafts and provided comments. W. W. proposed and co-developed the concept, co-developed the analysis plan, interpreted the results of the analyses, reviewed manuscript outline and drafts and provided comments. R. M. co-developed the concept, co-developed the analysis plan, interpreted the results of the analyses, reviewed manuscript outline and drafts and provided comments. F. Y. collected the data, co-developed the analysis plan, conducted statistical analyses and interpreted the results, reviewed manuscript outline and drafts and provided comments. L. X. collected the data, co-developed the analysis plan, conducted statistical analyses and interpreted the results, reviewed manuscript outline and drafts and provided comments. O. B. interpreted the results of the analyses, reviewed manuscript outline and drafts and provided comments. J. G. developed the concept, co-developed the analysis plan, interpreted the results of the analyses, reviewed manuscript outline and drafts and provided comments.
Supporting Information
Additional Supporting Informationmay be found in the online version of this article:
Baseline characteristics among prematched patients of cohorts 1 and 2.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55:1577–1596. doi: 10.1007/s00125-012-2534-0. et al. [DOI] [PubMed] [Google Scholar]
- 2.Hollander P, Cooper J, Bregnhøj J, Pedersen CB. A 52-week, multinational, open-label, parallel-group, noninferiority, treat-to-target trial comparing insulin detemir with insulin glargine in a basal-bolus regimen with mealtime insulin aspart in patients with type 2 diabetes. Clin Ther. 2008;30:1976–1987. doi: 10.1016/j.clinthera.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;7:CD006383. doi: 10.1002/14651858.CD006383.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia. 2008;51:408–416. doi: 10.1007/s00125-007-0911-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meneghini L, Kesavadev J, Demissie M, Nazeri A, Hollander P. Once-daily initiation of basal insulin as add-on to metformin: a 26-week, randomized, treat-to-target trial comparing insulin detemir with insulin glargine in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:729–736. doi: 10.1111/dom.12083. [DOI] [PubMed] [Google Scholar]
- 6.Xie L, Wei W, Pan C, Du J, Baser O. A real-world study of patients with type 2 diabetes initiating basal insulins via disposable pens. Adv Ther. 2011;28:1000–1011. doi: 10.1007/s12325-011-0074-5. [DOI] [PubMed] [Google Scholar]
- 7.King AB. Once-daily insulin detemir is comparable to once-daily insulin glargine in providing glycaemic control over 24 h in patients with type 2 diabetes: a double-blind, randomized, crossover study. Diabetes Obes Metab. 2009;11:69–71. doi: 10.1111/j.1463-1326.2008.01014.x. [DOI] [PubMed] [Google Scholar]
- 8.Davis KL, Tangirala M, Meyers JL, Wei W. Real-world comparative outcomes of US type 2 diabetes patients initiating analog basal insulin therapy. Curr Med Res Opin. 2013;29:1083–1091. doi: 10.1185/03007995.2013.811403. [DOI] [PubMed] [Google Scholar]
- 9.Borah BJ, Darkow T, Bouchard J, Aagren M, Forma F, Alemayehu B. A comparison of insulin use, glycemic control, and health care costs with insulin detemir and insulin glargine in insulin-naive patients with type 2 diabetes. Clin Ther. 2009;31:623–631. doi: 10.1016/j.clinthera.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 10.McAdam-Marx C, Bouchard J, Aagren M, Nelson R, Brixner D. Analysis of glycaemic control and weight change in patients initiated with human or analog insulin in an US ambulatory care setting. Diabetes Obes Metab. 2010;12:54–64. doi: 10.1111/j.1463-1326.2009.01128.x. [DOI] [PubMed] [Google Scholar]
- 11.Yenigun M, Honka M. Switching patients from insulin glargine-based basal-bolus regimens to a once daily insulin detemir-based basal-bolus regimen: results from a subgroup of the PREDICTIVE study. Int J Clin Pract. 2009;63:425–432. doi: 10.1111/j.1742-1241.2008.01973.x. [DOI] [PubMed] [Google Scholar]
- 12.Lüddeke HJ, Sreenan S, Aczel S. PREDICTIVE-a global, prospective observational study to evaluate insulin detemir treatment in types 1 and 2 diabetes: baseline characteristics and predictors of hypoglycaemia from the European cohort. Diabetes Obes Metab. 2007;9:428–434. doi: 10.1111/j.1463-1326.2006.00677.x. et al. [DOI] [PubMed] [Google Scholar]
- 13.Bryant GA, McDanel DL, Horner KE, Farris KB, Newkirk EN. Evaluation of dosing and clinical outcomes in patients undergoing conversion of insulin glargine to insulin detemir. Pharmacotherapy. 2013;33:56–62. doi: 10.1002/phar.1168. [DOI] [PubMed] [Google Scholar]
- 14.Wei W, Zhou S, Miao R. Much ado about nothing? A real-world study of patients with type 2 diabetes switching basal insulin analogs. Adv Ther. 2014;31:539–560. doi: 10.1007/s12325-014-0120-1. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Quan H, Sundararajan V, Halfon P. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. et al. [DOI] [PubMed] [Google Scholar]
- 17.Ginde AA, Blanc PG, Lieberman RM, Camargo CA., Jr Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord. 2008;8:4. doi: 10.1186/1472-6823-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei W, Pan C, Xie L, Baser O. Real-world insulin treatment persistence among patients with type 2 diabetes. Endocr Pract. 2014;20:52–61. doi: 10.4158/EP13159.OR. [DOI] [PubMed] [Google Scholar]
- 19.Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21:69–80. doi: 10.1002/pds.3263. (Suppl. 2): [DOI] [PubMed] [Google Scholar]
- 20.Niven DJ, Berthiaume LR, Fick GH, Laupland KB. Matched case-control studies: a review of reported statistical methodology. Clin Epidemiol. 2012;4:99–110. doi: 10.2147/CLEP.S30816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. Int J Obes (Lond) 2006;30:391–399. doi: 10.1038/sj.ijo.0803175. [DOI] [PubMed] [Google Scholar]
- 23.Abalı S, Turan S, Atay Z, Güran T, Haliloğlu B, Bereket A. Higher insulin detemir doses are required for the similar glycemic control: comparison of insulin detemir and glargine in children with type 1 diabetes mellitus. Pediatr Diabetes. 2014 doi: 10.1111/pedi.12167. ; DOI: 10.1111/pedi.12167. [DOI] [PubMed] [Google Scholar]
- 24.Kabadi UM. Deleterious outcomes after abrupt transition from insulin glargine to insulin detemir in patients with type 1 diabetes mellitus. Clin Drug Investig. 2008;28:697–701. doi: 10.2165/00044011-200828110-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics among prematched patients of cohorts 1 and 2.