Abstract
The biosynthesis pathway of eicosanoids derived from arachidonic acid, such as prostaglandins and leukotrienes, relates to the pathophysiology of diabetes mellitus (DM). A better understanding of how lipid mediators modulate the inflammatory process may help recognize key factors underlying the progression of diabetes complications. Our review presents recent knowledge about eicosanoid synthesis and signaling in DM-related complications, and discusses eicosanoid-related target therapeutics.
1. Introduction
Eicosanoids are biologically active lipid mediators that regulate inflammation [1] and that include prostaglandins (PGs), prostacyclins, thromboxanes (TX), leukotrienes (LT), and lipoxins (LX) (Figure 1) [2–4]. They may amplify or reduce inflammation, which coordinates cytokine production, antibody formation, cell proliferation and migration, and antigen presentation [2, 5, 6]. To prevent great tissue damage, eicosanoids also control the inflammatory resolution and tissue repair process [7, 8]. Imbalances in eicosanoid synthesis have been reported to drive chronic inflammation [1, 9], which deregulates signaling pathways and/or cellular events leading to abnormal immune functions [6, 10]. In particular, circulating and local mediators, such as eicosanoids, interleukin- (IL-) 1β, tumor necrosis factor- (TNF-) α, IL-6, IL-8, macrophage migration inhibitory factor (MIF), and free radicals, create a state of low-chronic inflammation in diabetic patients [5, 10, 11]. Inflammation may lead to diabetes progression, including damage to the kidneys (diabetic nephropathy), eyes (diabetic retinopathy), nerves (diabetic neuropathy), and cardiovascular system [12] (Figure 2).
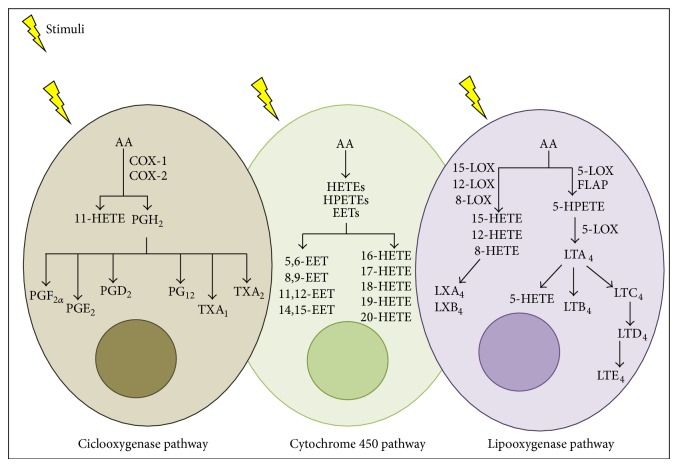
Figure 1.
Eicosanoid synthesis pathways. After cell stimulation, arachidonic acid (AA) can be metabolized by three enzymes: cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP 450). COX catalyzes AA in (prostaglandin) PGG2 and PGH2, and these are converted into PGD2, PGE2, PGF2α, PG12, TXA1, and TXA2. The LOX pathway catalyzes AA into hydroxyeicosatetraenoic acids (HETEs) and diverse hydroperoxyeicosatetraenoic acids (HPETEs). This pathway involves four enzymes: 5-LOX, 8-LOX, 12-LOX, and 15-LOX. 5-LOX interacts with a 5-LOX-activating protein (FLAP), enhancing the interaction of 5-LOX to AA. LTA4 hydrolases convert LTA4 into LTB4, and LTC4 synthase can convert LTA4 to LTC4, whereupon it is then metabolized to LTD4 and LTE4. 5-LOX synthetizes LXA4 and LXB4 using 15-HETE. The pathway of CYP-450 leads to the conversion of HETEs, including 16-, 17-, 18-, 19-, and 20-HETE and epoxyeicosatrienoic acids (EETs): 5,6-, 8,9-, 11,12-, and 14,15-EET.
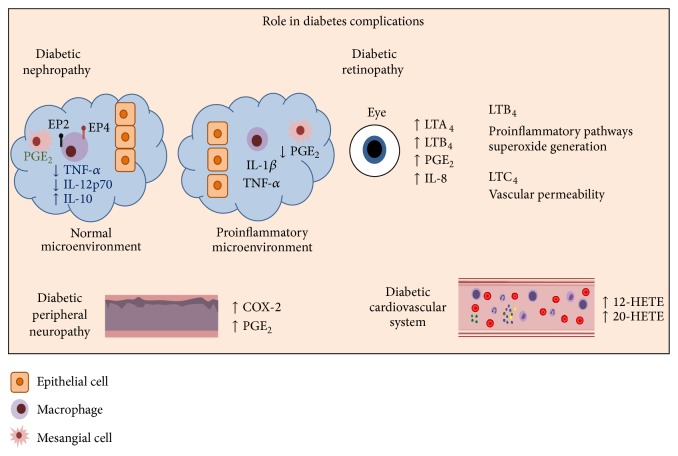
Figure 2.
Eicosanoid compounds affect different organs in diabetes complications. Diabetic nephropathy, one of the most common complications in diabetes, shows low PGE2 levels and altered glomerular hemodynamics. This dilates arteries and increases microvascular permeability. In normal conditions PGE2 downregulates TNF-α production and upregulates IL-10 production through EP2 and EP4 receptor signaling. However, a proinflammatory environment leads to cell permeabilization, low concentrations of PGE2, and mesangial cell proliferation. Diabetic retinopathy is another common complication in diabetes. In diabetes, the environment in the retina has a particular lipid profile, with higher COX-2 and abnormal production of PG. LTA4 and LTB4 are enhanced in addition to IL-8. Diabetic peripheral neuropathy is correlated with high COX-2 and PGE2. In a diabetic's cardiovascular system, PGE2 has an important role in microvascular permeability, and 12-HETE and 20-HETE lower the activity of endothelial progenitor cell (EPC) function.
In this review, we summarize the role of eicosanoids on the pathogenesis and progression of diabetes. In addition, we review drugs used to treat diabetic complications by acting on compounds of the eicosanoid pathway and speculate on possible future targets to treat diabetes complications.
2. The Role of Eicosanoids in Diabetes
The level of inflammation severity in diabetes is associated with hemoglobin A1 levels [13]. Increased PGE2 levels are related to dysfunction in insulin-regulated glycogen synthesis and gluconeogenesis in the liver [14, 15]. 12- as well as 15-hydroxyeicosatetraenoic acid (HETE) increases inflammatory cytokine expression, such as IL-6, TNF-α, and MCP-1, inducing chronic inflammation and the infiltration of inflammatory cells in adipose tissue [16–18]. In addition, 12-lipoxygenase (LOX) metabolites impair insulin action in adipocytes and can downregulate glucose transport, both of which may lead to insulin resistance [18, 19]. Nimesulide and metformin improved acute inflammation and impaired glucose metabolism [20], suggesting that impairing functions of prostaglandin synthesis are mediated by altered glucose levels [21].
2.1. Diabetic Nephropathy
Diabetic nephropathy is the major cause of diabetes-related death [22]. Renal disorders associated with diabetic nephropathy consist of modifications in renal hemodynamics, glomerular hypertrophy, mesangial cell proliferation, matrix accumulation, and proteinuria [23]. In normal conditions, PGE2 is the major PG in the kidneys and acts in renal physiology, glomerular filtration, and renin release [24, 25]. PGE2 activates kidney EP receptors, such as EP1, EP2, EP3, and EP4 in the collecting duct (except for EP2 whose mRNA has been localized to the outer and inner medulla of the kidney and EP4 which can also be expressed in the glomerulus) [25, 26]. Interactions between resident renal cells and macrophages change the microenvironment to a proinflammatory state, contributing to tissue damage and scarring [27, 28]. Macrophages and T cells infiltrate the glomeruli and interstitium, contributing to chronic renal failure in diabetic patients [27, 29–31].
During inflammation, macrophages release IL-1B and TNF-α, inducing endothelial cell permeability, altering glomerular hemodynamics, and decreasing PGE2 production by mesangial cells [32]. Normal levels of PGE2 suppress Th1 immune responses [33] and downregulate TNF-α production and upregulate IL-10 production through EP2 and EP4 receptor signaling, ending nonspecific inflammation [33–35]. Through an IL-10-dependent mechanism, PGE2 regulates IL-12 secretion by selectively inhibiting IL-12p70 production and stimulating IL-12p40 release [36, 37]. However, PGE2 is reduced in diabetic nephropathy, and this plays an essential role in the evolution of diabetic renal injury, strengthening the conclusion that inflammatory mechanisms have a significant role in both diabetic nephropathy development and progression [38–40]. Knockout podocyte-specific mice are protected against diabetes-induced nephropathy and albuminuria, showing the importance of COX-2 metabolites in the establishment of diabetic nephropathy [41].
2.2. Diabetic Retinopathy
Estimates done between 2005 and 2008 suggest that 28.5% of diabetics over the age of 40 in the United States had diabetic retinopathy and vision-threatening problems [42]. Low-grade chronic inflammation has been implicated in the pathogenesis of diabetic retinopathy [43]. The retina of diabetic individuals has a particular lipid profile [44]. COX-2 increases in the retina of diabetic animals, which contributes to abnormal production of PG [45].
5-LO-derived 5-HETE is the major proinflammatory eicosanoid, being five times higher in the vitreous of diabetics versus nondiabetics patients [46]. Mice null for the 5-LO gene demonstrated a minor inflammatory reaction [47–49]. Mice deficient in 5-LO had significantly less degeneration of retinal capillaries induced by diabetes, less superoxide generation, and less nuclear factor (NF)-kB expression [50]. Therefore, the generation of LTs could contribute to chronic inflammation and retinopathy in diabetes [51].
In addition, a hyperglycemic environment causes the release of 5-LO metabolites, LTA4 and LTB4. Retinas from both nondiabetic and diabetic mice are unable to produce LT or 5-LO mRNA. However, it was demonstrated that transcellular delivery of LTA4, from bone marrow-derived cells to retinal cells, results in the generation of LTB4/LTC4 [52]. LTC4 induces vascular permeability after binding with the retinal microvascular endothelial cells, and LTB4 coordinates proinflammatory pathways and superoxide generation, which may contribute to endothelial cell death and capillary degeneration, in turn contributing to chronic inflammation and diabetic retinopathy development [53].
2.3. Diabetic Peripheral Neuropathy
Estimates suggest 50% of diabetic patients have diabetic peripheral neuropathy, which affects the sensorimotor and autonomic parts of the peripheral nervous system [54–56]. Few studies describe the involvement of the eicosanoid pathway in DPN. In streptozotocin-induced rats, the intrathecal administration of COX-2 inhibitors, but not of COX-1 or COX-3 inhibitors, had an antihyperalgesic effect, supporting the importance of spinal COX-2 in DPN [57]. Pain may be attributed to the action of PGE2 on peripheral sensory neurons and on central sites within the spinal cord and the brain [58].
2.4. Diabetic Cardiovascular System
Impaired endothelial function is described in diabetes [59–61]. COX-2 expression and dilator prostaglandin synthesis increase in the coronary arterioles of diabetic patients [62]. Venous smooth muscle cells express more COX-2 and release more PGE2 when stimulated by a mix of inflammatory cytokines [63]. PGE2 causes pyrexia, hyperalgesia, and arterial dilation [58, 64]. PGE2 may act as a mediator of active inflammation, promoting first local vasodilatation, then the recruitment and activation of neutrophils, macrophages, and mast cells [65–68]. Deregulation of PGE2 synthesis leads to a wide range of pathological conditions [69]. In a normal cardiovascular system, PG12 acts as a potent vasodilator and TXA2 as a vasoconstrictor [70, 71]. The presence of both PGI2 and TXA2 maintains the normal physiology of the circulatory system [72]. In addition, the myocardium of diabetic and healthy rats does not differ in PG12 and PGE2 [73].
CYP-450-derived eicosanoids 12-HETE and 20-HETE, along with other inflammatory components in diabetic patients, lower the activity of endothelial progenitor cell function. Diabetic vascular complications are associated with reduced vascular regenerative potential and nonfunctional endothelial progenitor cell [79].
In sum, imbalanced levels of eicosanoids can induce modification of the microenvironment in the kidneys, eyes, nerves, and cardiovascular system and contribute to the progression of diabetes pathogenesis. Eicosanoid compounds have been studied as targets for drug development to control diabetes progression (Table 1). Thus, we reviewed drugs based on lipid mediators that are involved in diabetes complications.
Table 1.
Eicosanoid compounds as targets for drug development to control diabetes progression.
| Drug | Target | Condition | Consideration | Reference |
|---|---|---|---|---|
| Celecoxibe | COX-2 inhibitor | Diabetes nephropathy | Female patients received higher dose of PGs vasodilator to maintain blood vessel function than male patients. | [74] |
| Aspirin | Nonselective COX inhibitor |
Diabetes retinopathy | Delay in development of retinal microaneurysms in DR. | [75] |
| Celecoxibe | COX-2 inhibitor | Diabetes retinopathy | Reduction of vascular leakage. | [76] |
| Latanoprost | PGF2α
agonist |
Diabetes retinopathy | Reduces the diameter of dilated retinal arterioles. | [77] |
| Ketorolac tromethamine | Nonselective COX inhibitor |
Diabetes retinopathy | Patients with suspected or visible fibrovascular proliferation demonstrated a reduction in IL-8 and platelet-derived growth factor levels in vitreous humor. | [78] |
3. Lipid Mediators in Modulation of Diabetes Complications
When celecoxib, a COX-2 inhibitor, was administered as therapy for diabetic nephropathy in a type 1 diabetes (T1DM) population, COX-2-dependent factors neutralized the angiotensin II effect in the renal microcirculation; further, this effect was greater in women with uncomplicated T1DM than in men [74]. These gender differences could be explained by higher plasma prostanoid found in female animals, an effect that may be estrogen mediated [80–83].
Lower modified levels of PGE2 relate to changes in the kidney microenvironment and the progression of diabetic nephropathy; thus, PGE2 and its action are also important targets for drug development [84]. The PGE2-EP4 pathway contributes to the progression of tubule interstitial fibrosis, and the chronic administration of EP4-agonist in mice, exacerbated inflammation via IL-6, and consequently albuminuria and fibrosis [85]. Additionally, EP4-agonist mediates hyperfiltration in the glomerulus in the early stages of diabetes [86, 87]. Diabetes inflammatory state and chemokine production also increased when mice (T1DM model) were treated with an EP4 agonist [85] and upregulated the development of immune responses Th1 and Th17 [88]. On the other hand, EP receptor antagonists inhibited Th1 and Th17 response [89, 90]. In summary, the activation of the EP4 receptor exacerbates albuminuria levels, inflammation, and fibrosis. COX-2 inhibition reduces albuminuria in renal disease in rats [91]. Recently, using PGE1 in diabetic nephropathy patients in different disease stages decreased proteinuria and albuminuria [92].
Treating diabetic rats with 50 mg/Kg of aspirin plus 2 mg/Kg of meloxicam (a COX-2 inhibitor) reduced leukocyte adhesion and suppression of the blood-retinal barrier breakdown. This combined dose also reduced retinal ICAM-1 expression, and aspirin alone reduced the expression of C11a, CD11b, and CD18. Together, aspirin and meloxicam reduced the level of TNF-α [93]. Among diabetic patients, 330 mg of aspirin significantly slowed the development of retinal microaneurysms in diabetic retinopathy [75]. Another controlled trial showed that celecoxib reduced vascular leakage in diabetic patients with diabetic retinopathy [76].
Topical administration of nonsteroidal anti-inflammatory drugs (NSAIDs) compared to nontopical administration minimizes systemic exposure to the drug, such that topical NSAIDs can help enhance intraocular penetration. Diabetic patients exhibited elevated plasma IL-8 and elevated vitreous PGE2 and IL-8 [78, 94]. Exposure to PGE2 induces IL-8 gene transcription in human T cells [95]. The binding of IL-1β, TNF-α, and IFN-γ also stimulates human retinal pigment epithelial cells to express IL-8 [96]. One study provides direct clinical evidence that topical ocular ketorolac tromethamine (0.45% NSAID) reduces vitreous IL-8 in patients with proliferative diabetic retinopathy [97].
One study found that latanoprost (a PGF2α agonist) used topically significantly reduced dilation of retinal arterioles in type I diabetes patients with diabetic retinopathy, whereas topical diclofenac had no significant effect [77]. In diabetic rats, celecoxib lowered the synthesis of PGE2 in the retina (a result attributed to selective COX-2 inhibition, since COX-1 inhibitor did not have this effect) [98]. In addition, another COX inhibitor, nepafenac, inhibits increased retinal PG production and leukocyte adhesion in the retinal vessels of diabetes-induced rats [51].
In peripheral arterial diseases, the goal of treatment is to improve symptoms and prevent cardiovascular events [99]. Beraprost sodium is an analogue active PG12 with antiplatelet and vasodilating properties [100, 101]. Oral administration of beraprost sodium to diabetic patients improved sensations described as burning/hot, electric, sharp, achy, and tingling [100]. Beraprost improves symptoms by dilating peripheral vessels and increasing blood flow to the skin [102], and it can also improve painful peripheral neuropathy over a period of 8 weeks [103].
4. Future Perspectives on Eicosanoids
Components of the eicosanoid pathway have a fundamental role in the development of inflammation. As seen in this review, several studies have established that they participate in the progression of diabetes and its complications. Eicosanoids may act as pro- or anti-inflammatory. Currently, PG agonist and COX-1 and/or COX-2 inhibitors are the most promising tools to control diabetes complications, showing good results and promise for the future. Future studies should aim to unveil the function of specific receptors and enzymes acting in more specific targets available only in certain organs, such as the kidneys, eyes, vessels, or nerves.
Acknowledgments
We thank Sabrina S. Ferreira for assistance with Figure 2. The authors are supported by grant 2010/02272-0 from São Paulo Research Foundation (FAPESP), grant 470523/2013-1 from National Counsel of Technological and Scientific Development (CNPq, Projeto Universal 2013), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Pró-reitoria de Pesquisa da Universidade de São Paulo (PRP/USP, Projeto I and Novos Docentes), Brazil. The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
Conflict of Interests
The authors declare that there is no conflict of interests that would prejudice the impartiality of this scientific work.
References
- 1.Serhan C. N. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? The American Journal of Pathology. 2010;177(4):1576–1591. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris S. G., Padilla J., Koumas L., Ray D., Phipps R. P. Prostaglandins as modulators of immunity. Trends in Immunology. 2002;23(3):144–150. doi: 10.1016/S1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 3.Levin G., Duffin K. L., Obukowicz M. G., et al. Differential metabolism of dihomo-γ-linolenic acid and arachidonic acid by cyclo-oxygenase-1 and cyclo-oxygenase-2: implications for cellular synthesis of prostaglandin E1 and prostaglandin E2 . Biochemical Journal. 2002;365(2):489–496. doi: 10.1042/BJ20011798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wada M., DeLong C. J., Hong Y. H., et al. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. The Journal of Biological Chemistry. 2007;282(31):22254–22266. doi: 10.1074/jbc.M703169200. [DOI] [PubMed] [Google Scholar]
- 5.Odegaard J. I., Chawla A. Alternative macrophage activation and metabolism. Annual Review of Pathology: Mechanisms of Disease. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harizi H., Corcuff J.-B., Gualde N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends in Molecular Medicine. 2008;14(10):461–469. doi: 10.1016/j.molmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Russell C. D., Schwarze J. The role of pro-resolution lipid mediators in infectious disease. Immunology. 2013;141(2):166–173. doi: 10.1111/imm.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao C.-M., Breyer M. D. Roles of lipid mediators in kidney injury. Seminars in Nephrology. 2007;27(3):338–351. doi: 10.1016/j.semnephrol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Meirer K., Steinhilber D., Proschak E. Inhibitors of the arachidonic acid cascade: interfering with multiple pathways. Basic and Clinical Pharmacology and Toxicology. 2014;114(1):83–91. doi: 10.1111/bcpt.12134. [DOI] [PubMed] [Google Scholar]
- 10.Graves D. T., Kayal R. A. Diabetic complications and dysregulated innate immunity. Frontiers in Bioscience. 2008;13(4):1227–1239. doi: 10.2741/2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odegaard J. I., Chawla A. Connecting type 1 and type 2 diabetes through innate immunity. Cold Spring Harbor Perspectives in Medicine. 2012;2(3) doi: 10.1101/cshperspect.a007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Diabetes Federation. IDF Diabetes Atlas. 6th. Brussels, Belgium: International Diabetes Federation; 2013. http://www.idf.org/diabetesatlas. [PubMed] [Google Scholar]
- 13.Cipollone F., Iezzi A., Fazia M., et al. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation. 2003;108(9):1070–1077. doi: 10.1161/01.CIR.0000086014.80477.0D. [DOI] [PubMed] [Google Scholar]
- 14.Puschel G. P., Kirchner C., Schroder A., Jungermann K. Glycogenolytic and antiglycogenolytic prostaglandin E2 actions in rat hepatocytes are mediated via different signalling pathways. European Journal of Biochemistry. 1993;218(3):1083–1089. doi: 10.1111/j.1432-1033.1993.tb18468.x. [DOI] [PubMed] [Google Scholar]
- 15.Henkel J., Neuschäfer-Rube F., Pathe-Neuschäfer-Rube A., Püschel G. P. Aggravation by prostaglandin E2 of interleukin-6-dependent insulin resistance in hepatocytes. Hepatology. 2009;50(3):781–790. doi: 10.1002/hep.23064. [DOI] [PubMed] [Google Scholar]
- 16.Wen Y., Gu J., Chakrabarti S. K., et al. The role of 12/15-lipoxygenase in the expression of interleukin-6 and tumor necrosis factor-α in macrophages. Endocrinology. 2007;148(3):1313–1322. doi: 10.1210/en.2006-0665. [DOI] [PubMed] [Google Scholar]
- 17.Wen Y., Gu J., Vandenhoff G. E., Liu X., Nadler J. L. Role of 12/15-lipoxygenase in the expression of MCP-1 in mouse macrophages. The American Journal of Physiology—Heart and Circulatory Physiology. 2008;294(4):H1933–H1938. doi: 10.1152/ajpheart.00260.2007. [DOI] [PubMed] [Google Scholar]
- 18.Chakrabarti S. K., Cole B. K., Wen Y., Keller S. R., Nadler J. L. 12/15-Lipoxygenase products induce inflammation and impair insulin signaling in 3t3-l1 adipocytes. Obesity. 2009;17(9):1657–1663. doi: 10.1038/oby.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alpert E., Gruzman A., Totary H., Kaiser N., Reich R., Sasson S. A natural protective mechanism against hyperglycaemia in vascular endothelial and smooth-muscle cells: role of glucose and 12-hydroxyeicosatetraenoic acid. Biochemical Journal. 2002;362, part 2:413–422. doi: 10.1042/0264-6021:3620413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yapakçi E., Uysal O., Demirbilek H., Olgar S., Naçar N., Özen H. Hypoglycaemia and hypothermia due to nimesulide overdose. Archives of Disease in Childhood. 2001;85(6):p. 510. doi: 10.1136/adc.85.6.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coll T., Palomer X., Blanco-Vaca F., et al. Cyclooxygenase 2 inhibition exacerbates palmitate-induced inflammation and insulin resistance in skeletal muscle cells. Endocrinology. 2010;151(2):537–548. doi: 10.1210/en.2009-0874. [DOI] [PubMed] [Google Scholar]
- 22.Broumand B. Diabetes: changing the fate of diabetics in the dialysis unit. Blood Purification. 2006;25(1):39–47. doi: 10.1159/000096396. [DOI] [PubMed] [Google Scholar]
- 23.Molitch M. E., DeFronzo R. A., Franz M. J., et al. Diabetic nephropathy. Diabetes Care. 1998;21(1):S50–S53. [Google Scholar]
- 24.Breyer M. D., Jacobson H. R., Breyer R. M. Functional and molecular aspects of renal prostaglandin receptors. Journal of the American Society of Nephrology. 1996;7(1):8–17. doi: 10.1681/ASN.V718. [DOI] [PubMed] [Google Scholar]
- 25.Breyer M. D., Breyer R. M. Prostaglandin receptors: their role in regulating renal function. Current Opinion in Nephrology and Hypertension. 2000;9(1):23–29. doi: 10.1097/00041552-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Jensen B. L., Stubbe J., Hansen P. B., Andreasen D., Skøtt O. Localization of prostaglandin E2 EP2 and EP4 receptors in the rat kidney. American Journal of Physiology—Renal Physiology. 2001;280(6):F1001–F1009. doi: 10.1152/ajprenal.2001.280.6.F1001. [DOI] [PubMed] [Google Scholar]
- 27.Lim A. K. H., Tesch G. H. Inflammation in diabetic nephropathy. Mediators of Inflammation. 2012;2012:12. doi: 10.1155/2012/146154.146154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shikata K., Makino H. Microinflammation in the pathogenesis of diabetic nephropathy. Journal of Diabetes Investigation. 2013;4(2):142–149. doi: 10.1111/jdi.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chow F., Ozols E., Nikolic-Paterson D. J., Atkins R. C., Tesch G. H. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney International. 2004;65(1):116–128. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 30.Wong C. K., Szeto C. C., Chan M. H. M., Leung C. B., Li P. K. T., Lam C. W. K. Elevation of pro-inflammatory cytokines, C-reactive protein and cardiac troponin T in chronic renal failure patients on dialysis. Immunological Investigations. 2007;36(1):47–57. doi: 10.1080/08820130600745505. [DOI] [PubMed] [Google Scholar]
- 31.Galkina E., Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. Journal of the American Society of Nephrology. 2006;17(2):368–377. doi: 10.1681/ASN.2005080859. [DOI] [PubMed] [Google Scholar]
- 32.Pfeilschifter J., Pignat W., Vosbeck K., Marki F. Interleukin 1 and tumor necrosis factor synergistically stimulate prostaglandin synthesis and phospholipase A2 release from rat renal mesangial cells. Biochemical and Biophysical Research Communications. 1989;159(2):385–394. doi: 10.1016/0006-291X(89)90003-X. [DOI] [PubMed] [Google Scholar]
- 33.Stafford J. B., Marnett L. J. Prostaglandin E2 inhibits tumor necrosis factor-alpha RNA through PKA type I. Biochemical and Biophysical Research Communications. 2008;366(1):104–109. doi: 10.1016/j.bbrc.2007.11.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinomiya S., Naraba H., Ueno A., et al. Regulation of TNFα and interleukin-10 production by prostaglandins I2 and E2: studies with prostaglandin receptor-deficient mice and prostaglandin E-receptor subtype-selective synthetic agonists. Biochemical Pharmacology. 2001;61(9):1153–1160. doi: 10.1016/S0006-2952(01)00586-X. [DOI] [PubMed] [Google Scholar]
- 35.Wang M.-T., Honn K. V., Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer and Metastasis Reviews. 2007;26(3-4):525–534. doi: 10.1007/s10555-007-9096-5. [DOI] [PubMed] [Google Scholar]
- 36.Kaliriski P., Vieira P. L., Schuitemaker J. H. N., de Jong E. C., Kapsenberg M. L. Prostaglandin E2 is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97(11):3466–3469. doi: 10.1182/blood.V97.11.3466. [DOI] [PubMed] [Google Scholar]
- 37.Harizi H., Juzan M., Pitard V., Moreau J. F., Gualde N. Cyclooxygenase-2-issued prostaglandin E2 enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. Journal of Immunology. 2002;168(5):2255–2263. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- 38.Tuttle K. R. Linking metabolism and immunology: diabetic nephropathy is an inflammatory disease. Journal of the American Society of Nephrology. 2005;16(6):1537–1538. doi: 10.1681/ASN.2005040393. [DOI] [PubMed] [Google Scholar]
- 39.Mora C., Navarro J. F. Inflammation and diabetic nephropathy. Current Diabetes Reports. 2006;6(6):463–468. doi: 10.1007/s11892-006-0080-1. [DOI] [PubMed] [Google Scholar]
- 40.Hotamisligil G. S. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 41.Cheng H., Fan X., Moeckel G. W., Harris R. C. Podocyte COX-2 exacerbates diabetic nephropathy by increasing podocyte (pro)renin receptor expression. Journal of the American Society of Nephrology. 2011;22(7):1240–1251. doi: 10.1681/ASN.2010111149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X., Saaddine J. B., Chou C.-F., et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. The Journal of the American Medical Association. 2010;304(6):649–656. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joussen A. M., Poulaki V., Le M. L., et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. The FASEB Journal. 2004;18(12):1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 44.Tikhonenko M., Lydic T. A., Wang Y., et al. Remodeling of retinal fatty acids in an animal model of diabetes: a decrease in long-chain polyunsaturated fatty acids is associated with a decrease in fatty acid elongases Elovl2 and Elovl4. Diabetes. 2010;59(1):219–227. doi: 10.2337/db09-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abu El-Asrar A. M., Missotten L., Geboes K. Expression of cyclo-oxygenase-2 and downstream enzymes in diabetic fibrovascular epiretinal membranes. British Journal of Ophthalmology. 2008;92(11):1534–1539. doi: 10.1136/bjo.2008.142182. [DOI] [PubMed] [Google Scholar]
- 46.Schwartzman M. L., Iserovich P., Gotlinger K., et al. Profile of lipid and protein autacoids in diabetic vitreous correlates with the progression of diabetic retinopathy. Diabetes. 2010;59(7):1780–1788. doi: 10.2337/db10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X.-S., Sheller J. R., Johnson E. N., Funk C. D. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994;372(6502):179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- 48.Funk C. D., Chen X.-S. 5-Lipoxygenase and leukotrienes: transgenic mouse and nuclear targeting studies. American Journal of Respiratory and Critical Care Medicine. 2000;161(2):S120–S124. doi: 10.1164/ajrccm.161.supplement_1.ltta-24. [DOI] [PubMed] [Google Scholar]
- 49.Tang J., Kern T. S. Inflammation in diabetic retinopathy. Progress in Retinal and Eye Research. 2011;30(5):343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gubitosi-Klug R. A., Talahalli R., Du Y., Nadler J. L., Kern T. S. 5-Lipoxygenase, but not 12/15-lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes. 2008;57(5):1387–1393. doi: 10.2337/db07-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kern T. S. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Experimental Diabetes Research. 2007;2007:p. 95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Talahalli R., Zarini S., Sheibani N., Murphy R. C., Gubitosi-Klug R. A. Increased synthesis of leukotrienes in the mouse model of diabetic retinopathy. Investigative Ophthalmology and Visual Science. 2010;51(3):1699–1708. doi: 10.1167/iovs.09-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talahalli R., Zarini S., Tang J., et al. Leukocytes regulate retinal capillary degeneration in the diabetic mouse via generation of leukotrienes. Journal of Leukocyte Biology. 2013;93(1):135–143. doi: 10.1189/jlb.0112025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boulton A. J. M. Management of diabetic peripheral neuropathy. Clinical Diabetes. 2005;23(1):9–15. doi: 10.2337/diaclin.23.1.9. [DOI] [Google Scholar]
- 55.Callaghan B. C., Cheng H. T., Stables C. L., Smith A. L., Feldman E. L. Diabetic neuropathy: clinical manifestations and current treatments. The Lancet Neurology. 2012;11(6):521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tesfaye S., Boulton A. J., Dyck P. J., et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatment. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsunaga A., Kawamoto M., Shiraishi S., et al. Intrathecally administered COX-2 but not COX-1 or COX-3 inhibitors attenuate streptozotocin-induced mechanical hyperalgesia in rats. European Journal of Pharmacology. 2007;554(1):12–17. doi: 10.1016/j.ejphar.2006.09.072. [DOI] [PubMed] [Google Scholar]
- 58.Funk C. D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 59.Schalkwijk C. G., Stehouwer C. D. A. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clinical Science. 2005;109(2):143–159. doi: 10.1042/CS20050025. [DOI] [PubMed] [Google Scholar]
- 60.Ceriello A. Basal insulin and cardiovascular and other outcomes. The New England Journal of Medicine. 2012;367(18):1762–1763. doi: 10.1056/NEJMc1210553. [DOI] [PubMed] [Google Scholar]
- 61.Guo Z., Su W., Allen S., et al. COX-2 Up-regulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice. Cardiovascular Research. 2005;67(4):723–735. doi: 10.1016/j.cardiores.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 62.Szerafin T., Erdei N., Fülöp T., et al. Increased cyclooxygenase-2 expression and prostaglandin-mediated dilation in coronary arterioles of patients with diabetes mellitus. Circulation Research. 2006;99(5):e12–e17. doi: 10.1161/01.RES.0000241051.83067.62. [DOI] [PubMed] [Google Scholar]
- 63.Leung J. Y. T., Pang C. C. Y. Effects of nimesulide, a selective COX-2 inhibitor, on cardiovascular function in two rat models of diabetes. Journal of Cardiovascular Pharmacology. 2014;64(1):79–86. doi: 10.1097/FJC.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 64.Boniface K., Bak-Jensen K. S., Li Y., et al. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. The Journal of Experimental Medicine. 2009;206(3):535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu Y., Chadee K. Prostaglandin E2 stimulates IL-8 gene expression in human colonic epithelial cells by a posttranscriptional mechanism. The Journal of Immunology. 1998;161(7):3746–3752. [PubMed] [Google Scholar]
- 66.Nakayama T., Mutsuga N., Yao L., Tosato G. Prostaglandin E2 promotes degranulation-independent release of MCP-1 from mast cells. Journal of Leukocyte Biology. 2006;79(1):95–104. doi: 10.1189/jlb.0405226. [DOI] [PubMed] [Google Scholar]
- 67.Wang X. S., Lau H. Y. A. Prostaglandin E2 potentiates the immunologically stimulated histamine release from human peripheral blood-derived mast cells through EP1/EP3 receptors. Allergy: European Journal of Allergy and Clinical Immunology. 2006;61(4):503–506. doi: 10.1111/j.1398-9995.2006.01043.x. [DOI] [PubMed] [Google Scholar]
- 68.Weller C. L., Collington S. J., Hartnell A., et al. Chemotactic action of prostaglandin E2 on mouse mast cells acting via the PGE2 receptor 3. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(28):11712–11717. doi: 10.1073/pnas.0701700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Legler D. F., Bruckner M., Uetz-von Allmen E., Krause P. Prostaglandin E2 at new glance: Novel insights in functional diversity offer therapeutic chances. International Journal of Biochemistry and Cell Biology. 2010;42(2):198–201. doi: 10.1016/j.biocel.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 70.Vanhoutte P. M., Shimokawa H., Tang E. H. C., Feletou M. Endothelial dysfunction and vascular disease. Acta Physiologica. 2009;196(2):193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 71.Vanhoutte P. M. COX-1 and vascular disease. Clinical Pharmacology and Therapeutics. 2009;86(2):212–215. doi: 10.1038/clpt.2009.108. [DOI] [PubMed] [Google Scholar]
- 72.Kawabe J.-I., Ushikubi F., Hasebe N. Prostacyclin in vascular diseases—recent insights and future perspectives. Circulation Journal. 2010;74(5):836–843. doi: 10.1253/circj.CJ-10-0195. [DOI] [PubMed] [Google Scholar]
- 73.Przygodzki T., Talar M., Watala C. COX-2-derived prostaglandins do not contribute to coronary flow regulation in diabetic rats: distinct secretion patterns of PGI2 and PGE 2. European Journal of Pharmacology. 2013;700(1–3):86–92. doi: 10.1016/j.ejphar.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 74.Cherney D. Z. I., Scholey J. W., Nasrallah R., et al. Renal hemodynamic effect of cyclooxygenase 2 inhibition in young men and women with uncomplicated type 1 Diabetes mellitus . American Journal of Physiology: Renal Physiology. 2008;294(6):F1336–F1341. doi: 10.1152/ajprenal.00574.2007. [DOI] [PubMed] [Google Scholar]
- 75.The Damad Study Group. Effect of aspirin alone and aspirin plus dipyridamole in early diabetic retinopathy. A multicenter randomized controlled clinical trial. Diabetes. 1989;38(4):491–498. doi: 10.2337/diab.38.4.491. [DOI] [PubMed] [Google Scholar]
- 76.Chew E. Y., Kim J., Coleman H. R., et al. Preliminary assessment of celecoxib and microdiode pulse laser treatment of diabetic macular edema. Retina. 2010;30(3):459–467. doi: 10.1097/IAE.0b013e3181bcf1a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tilma K. K., Bek T. Topical treatment for 1 week with latanoprost but not diclofenac reduces the diameter of dilated retinal arterioles in patients with type 1 diabetes mellitus and mild retinopathy. Acta Ophthalmologica. 2012;90(8):750–755. doi: 10.1111/j.1755-3768.2011.02185.x. [DOI] [PubMed] [Google Scholar]
- 78.Schoenberger S. D., Kim S. J., Sheng J., Rezaei K. A., Lalezary M., Cherney E. Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy, and correlation with VEGF and inflammatory cytokines. Investigative Ophthalmology & Visual Science. 2012;53(9):5906–5911. doi: 10.1167/iovs.12-10410. [DOI] [PubMed] [Google Scholar]
- 79.Issana Y., Hochhausera E., Guod A., et al. Elevated level of pro-inflammatory eicosanoids and EPC dysfunction in diabetic patients with cardiac ischemia. Prostaglandins & Other Lipid Mediators. 2013;100-101:15–21. doi: 10.1016/j.prostaglandins.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bayorh M. A., Socci R. R., Eatman D., Wang M., Thierry-Palmer M. The role of gender in salt-induced hypertension. Clinical and Experimental Hypertension. 2001;23(3):241–255. doi: 10.1081/CEH-100102663. [DOI] [PubMed] [Google Scholar]
- 81.Eatman D., Wang M., Socci R. R., Thierry-Palmer M., Emmett N., Bayorh M. A. Gender differences in the attenuation of salt-induced hypertension by angiotensin (1–7) Peptides. 2001;22(6):927–933. doi: 10.1016/S0196-9781(01)00404-1. [DOI] [PubMed] [Google Scholar]
- 82.Orshal J. M., Khalil R. A. Gender, sex hormones, and vascular tone. The American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 2004;286(2):R233–R249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 83.Sullivan J. C., Sasser J. M., Pollock D. M., Pollock J. S. Sexual dimorphism in renal production of prostanoids in spontaneously hypertensive rats. Hypertension. 2005;45(3):406–411. doi: 10.1161/01.HYP.0000156879.83448.93. [DOI] [PubMed] [Google Scholar]
- 84.Sreeramkumar V., Fresno M., Cuesta N. Prostaglandin e 2 and T cells: friends or foes. Immunology and Cell Biology. 2012;90(6):579–586. doi: 10.1038/icb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mohamed R., Jayakumar C., Ramesh G. Chronic administration of EP4-selective agonist exacerbates albuminuria and fibrosis of the kidney in streptozotocin-induced diabetic mice through IL-6. Laboratory Investigation. 2013;93(8):933–945. doi: 10.1038/labinvest.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nasrallah R., Robertson S. J., Hébert R. L. Chronic COX inhibition reduces diabetes-induced hyperfiltration, proteinuria, and renal pathological markers in 36-week B6-Ins2Akita mice. American Journal of Nephrology. 2009;30(4):346–353. doi: 10.1159/000229304. [DOI] [PubMed] [Google Scholar]
- 87.Sakata D., Yao C., Narumiya S. Prostaglandin E2, an immunoactivator. Journal of Pharmacological Sciences. 2010;112(1):1–5. doi: 10.1254/jphs.09R03CP. [DOI] [PubMed] [Google Scholar]
- 88.Yao C., Sakata D., Esaki Y., et al. Prostaglandin E2-EP4 signaling promotes immune inflammation through TH1 cell differentiation and TH17 cell expansion. Nature Medicine. 2009;15(6):633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 89.Chizzolini C., Chicheportiche R., Alvarez M., et al. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood. 2008;112(9):3696–3703. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Q., Muramoto K., Masaaki N., et al. A novel antagonist of the prostaglandin E 2 EP 4 receptor inhibits Th1 differentiation and Th17 expansion and is orally active in arthritis models. British Journal of Pharmacology. 2010;160(2):292–310. doi: 10.1111/j.1476-5381.2010.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang J. L., Cheng H. F., Shappell S., Harris R. C. A selective cyclooxygenase-2 inhibitor decreases proteinuria and retards progressive renal injury in rats. Kidney International. 2000;57(6):2334–2342. doi: 10.1046/j.1523-1755.2000.00093.x. [DOI] [PubMed] [Google Scholar]
- 92.Li P.-F., Mu Y.-R., Xin Y., Qu Y., Liao L. Therapeutic effect of prostaglandin E1 on diabetic nephropathy: a one-year follow-up study. Journal of Southern Medical University. 2010;30(3):482–485. [PubMed] [Google Scholar]
- 93.Joussen A. M., Poulaki V., Mitsiades N., et al. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-α suppression. The FASEB Journal. 2002;16(3):438–440. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- 94.Funk M., Schmidinger G., Maar N., et al. Angiogenic and inflammatory markers in the intraocular fluid of eyes with diabetic macular edema and influence of therapy with bevacizumab. Retina. 2010;30(9):1412–1419. doi: 10.1097/IAE.0b013e3181e095c0. [DOI] [PubMed] [Google Scholar]
- 95.Caristi S., Piraino G., Cucinotta M., Valenti A., Loddo S., Teti D. Prostaglandin E2 induces interleukin-8 gene transcription by activating C/EBP homologous protein in human T lymphocytes. Journal of Biological Chemistry. 2005;280(15):14433–14442. doi: 10.1074/jbc.M410725200. [DOI] [PubMed] [Google Scholar]
- 96.Elner V. M., Burnstine M. A., Strieter R. M., Kunkel S. L., Elner S. G. Cell-associated human retinal pigment epithelium interleukin-8 and monocyte chemotactic protein-1: immunochemical and in-situ hybridization- analyses. Experimental Eye Research. 1997;65(6):781–789. doi: 10.1006/exer.1997.0380. [DOI] [PubMed] [Google Scholar]
- 97.Schoenberger S. D., Kim S. J., Shah R., Sheng J., Cherney E. Reduction of interleukin 8 and platelet-derived growth factor levels by topical ketorolac, 0.45%, in patients with diabetic retinopathy. JAMA Ophthalmology. 2014;132(1):32–37. doi: 10.1001/jamaophthalmol.2013.6203. [DOI] [PubMed] [Google Scholar]
- 98.Ayalasomayajula S. P., Kompella U. B. Retinal delivery of celecoxib is several-fold higher following subconjunctival administration compared to systemic administration. Pharmaceutical Research. 2004;21(10):1797–1804. doi: 10.1023/B:PHAM.0000045231.51924.e8. [DOI] [PubMed] [Google Scholar]
- 99.Vane J. R., Botting R. M. Pharmacodynamic profile of prostacyclin. American Journal of Cardiology. 1995;75(3):3A–10A. doi: 10.1016/S0002-9149(99)80377-4. [DOI] [PubMed] [Google Scholar]
- 100.Nony P., Ffrench P., Girard P., et al. Platelet-aggregation inhibition and hemodynamic effects of beraprost sodium, a new oval prostacyclin derivative: a study in healthy male subjects. Canadian Journal of Physiology and Pharmacology. 1996;74(8):887–893. doi: 10.1139/cjpp-74-8-887. [DOI] [PubMed] [Google Scholar]
- 101.Demolis J.-L., Robert A., Mouren M., Funck-Brentano C., Jaillon P. Pharmacokinetics and platelet antiaggregating effects of beraprost, an oral stable prostacyclin analogue, in healthy volunteers. Journal of Cardiovascular Pharmacology. 1993;22(5):711–716. doi: 10.1097/00005344-199311000-00007. [DOI] [PubMed] [Google Scholar]
- 102.Yoon H. S., Choi W. J., Sung I. H., Lee H. S., Chung H. J., Lee J. W. Effects of Beraprost Sodium on subjective symptoms in diabetic patients with peripheral arterial disease. Clinics in Orthopedic Surgery. 2013;5(2):145–151. doi: 10.4055/cios.2013.5.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shin S., Kim K. J., Chang H.-J., et al. The effect of oral prostaglandin analogue on painful diabetic neuropathy: a double-blind, randomized, controlled trial. Diabetes, Obesity and Metabolism. 2013;15(2):185–188. doi: 10.1111/dom.12010. [DOI] [PubMed] [Google Scholar]