Abstract
Background
Genetic variations in ERBB4 were associated with increased susceptibility for schizophrenia (SCZ) and bipolar disorders (BPD). Structural imaging studies showed cortical abnormalities in adolescents and adults with SCZ or BPD. However, less is known about subclinical cortical changes or the influence of ERBB4 on cortical development.
Methods
971 healthy children (ages 3–20 years old; 462 girls and 509 boys) were genotyped for the ERBB4-rs7598440 variants, had structural MRI, and cognitive evaluation (NIH Toolbox ®). We investigated the effects of ERBB4 variants and family history of SCZ and/or BPD (FH) on cortical measures and cognitive performances across ages 3–20 years using a general additive model.
Results
Variations in ERBB4 and FH impact differentially the age-related cortical changes in regions often affected by SCZ and BPD. The ERBB4-TT-risk genotype children with no FH had subtle cortical changes across the age span, primarily located in the left temporal lobe and superior parietal cortex. In contrast, the TT-risk genotype children with FH had more pronounced age-related changes, mainly in the frontal lobes compared to the non-risk genotype children. Interactive effects of age, FH and ERBB4 variations were also found on episodic memory and working memory, which are often impaired in SCZ and BPD.
Conclusions
Healthy children carrying the risk-genotype in ERBB4 and/or with FH had cortical measures resembling those reported in SCZ or BPD. These subclinical cortical variations may provide early indicators for increased risk of psychiatric disorders and improve our understanding of the effect of the NRG1–ERBB4 pathway on brain development.
Keywords: ERBB4, Brain Development, Family History of Psychiatric Disorders, Surface-based Morphometry, Neuropsychological Tests
INTRODUCTION
The ERBB4 receptor tyrosine-protein kinase gene is involved in the production of different isoforms of the ERBB4 protein through alternative splicing (Steinthorsdottir et al., 2004). ERBB4 binds trophic factors, such as neuregulin-1 (NRG1) or neuregulin-3. In the central nervous system, ERBB4 and its ligand NRG1 play a critical role in neurodevelopment such as glial and neuronal migration, myelination, excitatory neuronal receptor expression and the onset of puberty (S. Chen et al., 2006; Mei & Xiong, 2008). Alterations in the NRG1-ERBB4 signaling results in brain dysfunctions (Law et al., 2006; Mei & Xiong, 2008; Norton et al., 2006), which in turn may lead to processes that might contribute to the pathophysiology of schizophrenia (SCZ) and bipolar disorders (BPD) (Maier, Zobel, & Wagner, 2006; Mei & Xiong, 2008; Stefansson et al., 2002).
In prior epidemiologic studies, genetic variations and risk-haplotypes in ERBB4 were associated with SCZ and BPD (P. Chen et al., 2012; Goes et al., 2011) in several ethnicities (Bae et al., 2012; Buxbaum et al., 2008; Greenwood, Light, Swerdlow, Radant, & Braff, 2012; Hatzimanolis et al., 2013; Lu, Wang, Chen, Lai, & Liou, 2010; Nicodemus et al., 2006; Norton et al., 2006; Shiota et al., 2008; Silberberg, Darvasi, Pinkas-Kramarski, & Navon, 2006; Stefanis et al., 2013). SCZ and BPD share some common symptoms, such as psychosis, and are hypothesized to be neurodevelopmental disorders caused by both genetic and environmental factors (Lewis & Levitt, 2002; Rapoport, Addington, Frangou, & Psych, 2005). Symptoms of SCZ and BPD typically emerge during late adolescence (Buka & Fan, 1999); however, potential markers or predictors are rarely investigated in the pre-symptomatic stages in humans.
Meta-analyses of magnetic resonance imaging (MRI) studies in patients with SCZ and/or BPD showed significant brain abnormalities (Arnone et al., 2009; Beyer, Young, Kuchibhatla, & Krishnan, 2009; Gogtay & Rapoport, 2008). The most consistent brain abnormalities include enlarged lateral ventricle, reduced volume of the left medial temporal lobe, and reduced gray matter in the frontal and latero-temporal lobes (Arnone et al., 2009; Ellison-Wright, Glahn, Laird, Thelen, & Bullmore, 2008; Fornito, Yucel, & Pantelis, 2009; Fornito, Yucel, Patti, Wood, & Pantelis, 2009; Glahn et al., 2008; Honea, Crow, Passingham, & Mackay, 2005; Shenton, Dickey, Frumin, & McCarley, 2001). Widespread cortical thinning across the entire brain was reported in SCZ adults (Goldman et al., 2009; Kuperberg et al., 2003), while BPD patients had only thinning of the frontal and temporo-parietal regions{McIntosh, 2004 #136; Lyoo, 2006 #135;Rimol, 2010 #28;Rimol, 2012 #13;Gutierrez-Galve et al., 2014}. SCZ and BPD adolescents with new onset symptoms also showed thinner frontal and temporal cortices compared to healthy controls(Janssen et al., 2014; Voets et al., 2008). However, adolescents with SCZ had altered cortices in the temporal, parietal and occipital regions compared to BDP adolescents, who had only focal thinning of their frontal regions (Janssen et al., 2014). The lack of age-related cortical thinning observed in SCZ and/or BPD patients may result from an abnormal pruning in those patients compared to healthy individuals (Mattai et al., 2011; Thormodsen et al., 2013). Therefore, an overlap of cortical structure abnormalities exists between SCZ and BPD, suggesting a common pathophysiological process; however, little is known about the effect of age on frontal and temporal abnormalities in SCZ and BDP.
Few neuroimaging studies have evaluated the effect of ERBB4 variations on brain activation and microstructures, primarily in healthy adults. For instance, healthy adults with risk-haplotypes in ERBB4 and NRG1 along with another susceptibility gene for schizophrenia, AKT1 gene, showed less brain activation (Nicodemus et al., 2010). Adults carrying these risk-haplotypes also had less activation in the dorsolateral prefrontal cortex during working memory tasks compared to the non-risk-haplotype carriers, although no group difference in activation was observed in subjects with only one of these risk-genotypes (Nicodemus et al., 2010). Diffusion tensor imaging (DTI) showed that ERBB4-risk-genotype carriers had lower fractional anisotropy (FA) in the left temporal lobe (Konrad et al., 2009) and in the anterior limb of the internal capsule (Zuliani et al., 2011), compared to non-risk genotype carriers. Taken together, these findings suggest that risk variants in ERBB4 in healthy adults are associated with reduced brain function and white matter integrity in the left frontotemporoparietal regions, which are often affected in SCZ patients (Winterer, Coppola, Egan, Goldberg, & Weinberger, 2003) and may indicate increased risk for these disorders. However, the effect of ERBB4 variations on typical brain development before the manifestation of symptoms of SCZ or BPD has not been studied using surface-based morphometry.
The risk-haplotype for schizophrenia includes three variants (rs839523, rs707284 and rs7598440) in ERBB4 that are all associated individually with white matter alterations, although each may have a minor effect on the risk of developing SCZ (Konrad et al., 2009). We focused our analysis on the functional rs7598440 variants since the TT-risk variant for SCZ and BPD was associated with increased expression of the CYT-1 isoforms of ERBB4 (exon 26) in the human SCZ brain (Silberberg et al., 2006), specifically in the hippocampus and the dorsolateral prefrontal cortex (Law, Kleinman, Weinberger, & Weickert, 2007). Healthy TT-risk-carriers at rs7598440 also had higher GABA levels in their dorsal anterior cingulate gyrus (Marenco et al., 2011) and in their cerebrospinal fluid (Luykx et al., 2012) compared to non-risk-carriers. Moreover, the rs7598440-TT-risk variant is commonly found (25–30%) in the general population (Harrison & Weinberger, 2005). Furthermore, studying rs7598440-TT phenotypes in typical children eliminates the potential influence of antipsychotic drugs on the expression of ERBB4 (Wang, Su, Guo, Yang, & Si, 2008) and on brain structures (Torres, Portela-Oliveira, Borgwardt, & Busatto, 2013). Therefore, we investigated the ERBB4 genotype and age-by-genotype interactions on cortical brain morphometry in a large cohort of 3–20 year old children, using a surface-based morphometric analysis. Typically-developing children with the risk-variant in ERBB4 at rs7598440 may exhibit age-related smaller cortices of the frontal and temporal regions resembling those observed in SCZ and BPD patients, even in the absence of clinical symptoms of psychiatric disorders.
The heritability of SCZ between twins is about 40–60% in monozygotic twins and 10–20% in first-degree relatives (Agim et al., 2013; Cardno & Gottesman, 2000; McGue & Gottesman, 1991), suggesting a family pre-disposition. We further analyzed the effects of family history of SCZ and/or BDP and their interactions with the ERBB4 variations at rs7598440 on cortical regions shown to be altered in children with the ERBB4 variants or with SCZ and/or BPD, in particular the frontal and temporal regions. Furthermore, we examined whether the rs7598440 variant and family history of SCZ and/or BPD showed independent or combined effects on cognitive domains commonly impaired in SCZ or BPD, including episodic memory, working memory, executive function and processing speed (Schaefer, Giangrande, Weinberger, & Dickinson, 2013). We expect that children with the TT-risk allele and family history of SCZ and BPD will have lower cognitive performance compared to children with the two other genotypes (CC and TC).
MATERIALS AND METHODS
The Pediatric Imaging, Neurocognition, and Genetics (PING) study is a cross-sectional study that enrolled 1,494 typically-developing children ages 3 to 20 years old. Data were collected across ten academic institutions in the United States, and included whole genome single nucleotide polymorphism genotyping, developmental and neuropsychological assessments (NIH Toolbox®), and high-resolution brain MRI. The database is available at http://ping.chd.ucsd.edu. The PING study design, experiments and consent (oral and written) were approved by the institutional review boards involved with human subject research protections at each of the ten institutions.
Subjects
Details on the recruitment and subject characteristics are presented on the PING website (http://ping.chd.ucsd.edu) and have been reported previously (Brown et al., 2012; Douet et al., 2014; Fjell et al., 2012; Walhovd et al., 2012). Briefly, girls and boys of any ethnicity 3 to 20 years old were included in the PING study. They were excluded if they were born preterm (<36 weeks); had any neurological disorders (e.g. cerebral palsy, cerebral palsy, fetal alcohol syndrome, Down’s syndrome, fragile X, cerebral neoplasm, bacterial meningitis, epilepsy, etc.); history of failure to thrive within the first year of life; any diagnosis of psychiatric disorders (autism, schizophrenia, bipolar disorders); history of head trauma with loss of consciousness for more than 30 minutes; pregnancy; or any contraindications for MRI or severe claustrophobia. The three ERBB4 genotypes at rs7598440; TT, TC, and CC, were available in 982 of 1,494 children enrolled. Although being preterm born was an exclusion criteria in the original PING protocol, eleven children were born prematurely and had genotype available (<36 weeks of gestation: 1CC, 3TT and 7 CT; HWE χ2=1.1, p=0.29), but were excluded from our study. A sub-cohort of 136 children (40CC, 65CT, 31TT, HWE χ2=0.49, p=0.78) had a family history of SCZ and/or BPD. Family history included both first-degree and second-degree relatives that share about 25–50% of the participant’s DNA.
Genotyping and Genetic Ancestry Factor
Saliva samples were collected from each participant. Genomic DNA was extracted and analyzed using the Illumina Human660W-Quad BeadChip. Replication and quality control filters (i.e., sample call rate >99%, call rates >95%, minor allele frequency >5%) were used (Bakken et al., 2012). Genetic ancestry factors (GAF) were assessed using multidimensional scaling analysis and actual estimates of local ancestry in admixed populations (LAMP) (Sankararaman, Sridhar, Kimmel, & Halperin, 2008), as previously described (Akshoomoff, C.S., Gruen, O., & A.M. & Jernigan, 2013).
Image acquisition and processing
The neuroimaging techniques, data acquisition and analyses were reported previously (Brown et al., 2012; Fjell et al., 2012; Walhovd et al., 2012) and are available on the PING website (http://ping.chd.ucsd.edu). MRI was performed on 3-Tesla scanners (7 Siemens; 1 General Electric; 2 Philips Medical) and included a 3-D T1-weighted structural scan (MP-RAGE, 1.0 × 1.0 × 1.2 mm3 resolution, 8 minutes acquisition time). The MRI data were transferred to the central processing site (UCSD), and were checked for protocol compliance, based on DICOM header information. After visual inspection for possible artifacts, unacceptable scans were excluded from the study. The MP-RAGE images were analyzed with modified Freesurfer software (http://surfer.nmr.mgh.harvard.edu/) that parceled the brain into fuzzy clusters based on genetics correlations of relative surface area measures (Bakken et al., 2012). 893 of the 971 children genotyped for rs7598440-ERBB4 had data for socioeconomic status (SES), sex, scanner device and GAF, and met quality criteria for cortical measurements (thickness, area, volume). Among children excluded from the study due to missing data (n=78), the genotype distribution was 22CC, 42CT and 14TT (HWE χ2=0.61, p=0.44).
Cognitive performance
The NIH Toolbox® Cognition Battery evaluated 6 major cognitive domains: Executive Function, Attention, Episodic Memory, Processing Speed, Language, and Working Memory (Akshoomoff et al., 2013; Weintraub et al., 2013). However, we focused only on the four domains shown to be affected in SCZ or BPD (Schaefer et al., 2013), which include episodic memory, executive function, processing speed and working memory.
Statistics
Statistical analyses were performed using the Dataportal interface (https://ping-dataportal.ucsd.edu/) (Bartsch, Thompson, Jernigan, & Dale, 2014) that uses a general additive model (GAM) as implemented by the R program (http://www.r-project.or/). GAM is a multiple linear regression model that allows smooth functions of the predictor variables, such as age or covariates, where the degree of smoothness is data-driven rather than pre-specified (Ha, 1986). The Dataportal provided significance maps (vertex model) for effects of interest on cortical volume, area and thickness. Significance maps for the genotype effects and genotype-by-age interactions on cortical structures were generated using age as a smooth independent variable, and included a linear independent term for genotype and a smooth interaction between age and genotype. Significance maps for the family history of SCZ and BP (FH) on cortical structures were analyzed with the vertex model using smooth age as covariate, and a linear term for FH. All statistical models accounted for SES (reflecting the highest parental/guardian education and household income), sex, GAF (Akshoomoff et al., 2013), and scanner device, as needed. Significance maps were thresholded using the false discovery rate (FDR) to correct for multiple comparisons at the 5% level (Benjamini Yoav, 1995). Three-way age-by-FH-by-ERBB4 interactions on cognitive performance were analyzed with ANCOVA, using age (continuous), FH (category), and genotype (category) as independent variables, and a linear interaction between age, FH and the genotypes. Post-hoc analyses of the interactive effects of FH and age on cognitive domains were performed only on domains showing a 3-way interaction (Age-by-FH-by-ERBB4). Corrections for multiple comparisons were performed using Holm-Bonferroni rank order method.
RESULTS
Participants and covariate selection (Table 1)
TABLE 1.
Participant characteristics by genotype (all values are in mean ± standard errors).
| Children Characteristics | ||||
| ERBB4-rs7598440 | CC | TC | TT |
One-Way ANOVA or χ2 p-value |
| Genotype distribution | 289 | 482 | 200 | 0.97 (χ20.44,2) |
| Age (years) | 12.17±0.28 | 12.44±0.23 | 11.03±0.35 | 0.003 (F5.83,970,2) |
| Boys /Girls (%) | 157/132 (54.3/45.7) |
246/236 (51.0/49.0) |
106/94 (53.0/47.0) |
0.66 (χ20.82,2) |
| GAF_Europe | 0.54±0.022 | 0.65±0.017 | 0.68±0.027 | <0.0001 (F11.89,969,2) |
| GAF_Africa | 0.24±0.020 | 0.08±0.009 | 0.04±0.010 | <0.0001 (F53.09,969,2) |
| GAF_American Indian | 0.06±0.008 | 0.04±0.005 | 0.04±0.008 | 0.08 (F2.54,969,2) |
| GAF_East Asia | 0.13±0.017 | 0.20±0.016 | 0.20±0.024 | 0.02 (F3.92,969,2) |
| GAF_Oceania | 0.007±0.002 | 0.007±0.001 | 0.016±0.003 | 0.001 (F6.82,969,2) |
| GAF_Central Asia | 0.03±0.008 | 0.03±0.006 | 0.02±0.006 | 0.52 (F0.66,969,2) |
| Parent/Guardian Characteristics | ||||
| Household Income (1=<$5K, 6= 40K–50K, 12=≥$300K) |
6.26±0.009 | 7.02±0.005 | 6.82±0.005 | 0.0002 (F8.66,927,2) |
| Highest Education (7=Professional, 4=High School Graduate, 1=<7 yrs of school) |
5.60±0.005 | 5.91±0.002 | 5.90±0.002 | 0.0007 (F7.32,937,2) |
The different rs7598440 ERBB4 genotypes differ by the genetic ancestry factor (GAF) and socioeconomic status (SES, which combined household income and highest education of parents/guardians). Both variables (GAF and SES) were therefore included in our statistical models for group comparisons.
The 971 children were 12.07±4.95 years old (462 girls and 509 boys). Across the three rs7598440-ERBB4 variant groups, children were similar in genotype distribution (Hardy-Weinberg equilibrium, p=0.97) and sex proportion. However, children carrying the TT-risk genotype for SCZ and BPD were younger than C-carriers (post-hoc t-test analysis, p<0.0001). The CC-homozygous children had the lowest socioeconomic status (SES), calculated from the combined household income and parents/guardians’ highest level of education.
No genotype differences were found among children with American Indian and Central Asian genetic ancestry (genetic ancestry factor, GAF). However, the T-risk allele frequency was higher than the CC-homozygous frequency in children with European and East Asian GAF, but lower in children with African GAF. The homozygous TT-children were more prevalent amongst those with Oceania GAF. To account for these group differences, each statistical model included GAF, sex and age as covariates.
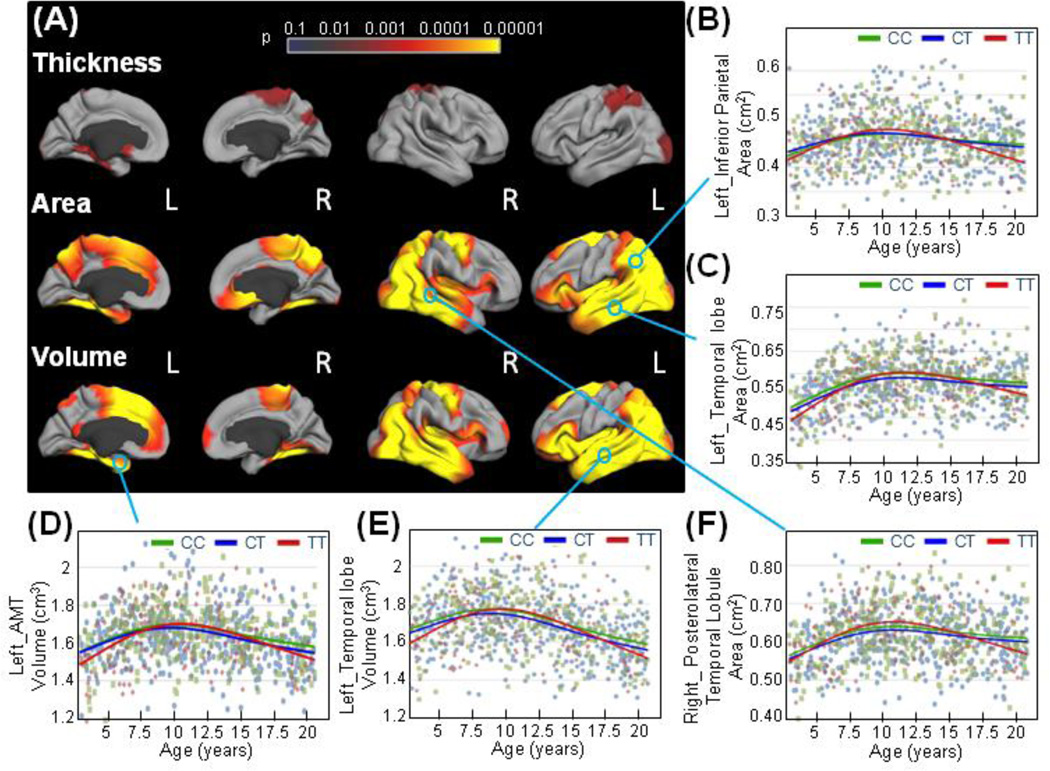
Effects of the ERBB4 variation on cortical structures. (Figure 1)
Figure 1. Effects of the ERBB4 variation on cortical structures.
(a) Statistical maps for the effects of the ERBB4 on cortical thickness, area, and volume at each vertex were generated using the general additive model. Sex, GAF, SES and scanner device were included as covariates, and the models were corrected for multiple comparisons with the false discovery rate (FDR; 0.05). The effects of the ERBB4 genotype on cortical structures were plotted against age. (b) Children with the TT-risk genotype for schizophrenia/bipolar disorder (red) showed a more pronounced inverted U-shaped age-trajectory compared to the non-risk genotype children (CC in green and CT in blue) in cortical areas of the left inferior parietal cortex (shown left, b), left temporal lobe (c) and right posterolateral temporal lobule (f), as well as in volumes of the left anteromedial temporal cortex (d), and in volumes of the left temporal lobe (e).
The anteromedial temporal (AMT) region comprises the fusiform, entorhinal and parahippocampal cortices.
Figure 1 shows statistical probability maps (F-test) of ERBB4 effects on cortical structures. ERBB4 effects on cortical volumes were mostly driven by genotype group differences in cortical surface areas rather than on cortical thickness (Figure 1A); however, we evaluated both area and thickness differences to illustrate these group effects.
Genotype effects on cortical areas and volumes were commonly found in brain regions that are altered in patients with psychotic and schizophrenic symptoms (Rimol et al., 2012) (Friedman et al., 1999; Gutierrez-Galve et al., 2014; Jacobsen et al., 1998; Janssen et al., 2014; Rimol et al., 2010; Thompson et al., 2001). These regions included the temporal and occipital lobes, the cortical part of the limbic system (entorhinal and parahimpoccampal cortices), the precuneus and the inferior parietal cortex in both hemispheres; the superior frontal,and the medial orbito-frontal cortex in the right hemisphere, and the superior frontal cortex in the left hemisphere (Figure 1 A). Overall, areas and volumes in these regions followed an inverted-U shape, peaking around age 10 years, showing the onset of pruning of the gray matter structures as the brain matures (Figures 1B– F). However, children with the TT-risk genotype at rs7598440-ERBB4 (red curves) showed a more pronounced age-related growth of these regions at younger ages, followed by a steeper decline during adolescence compared to the non-risk children (Figures 1B–F).
Cortical thickness was affected by ERBB4 genotype in very few cortical regions, including the superior parietal and parahippocampal cortices and the occipital lobule, in the left hemisphere (Figure 1A). These regions also presented strong genotype effects on area and volumes.
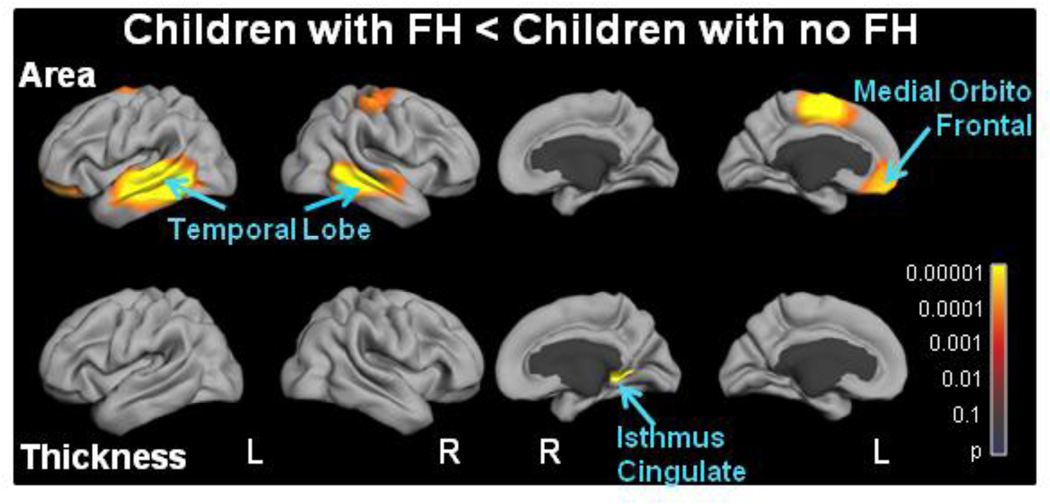
Family history of schizophrenia and bipolar disorders focally affects cortical structures. (Figure 2)
Figure 2. Statistical p-value maps for the effects of Family History of schizophrenia and bipolar disorders on cortical structures.
The effects of family history of schizophrenia (SCZ) and/or bipolar disorders (BPD) on cortical area and thickness were evaluated at each vertex with a general additive model. Sex, GAF, SES and scanner device were included as covariates and models were corrected for multiple comparisons with the false discovery rate (FDR; 0.05). Overall, family history of SCZ and/or BPD (FH) affects cortical areas more than thickness. Children with FH had greater reduced areas in the temporal lobes, the left supramarginal gyrus, the left medial orbito-frontal and the right superior frontal cortices compared to children with no FH.
We next determined whether a family history of SCZ and/or BPD (FH) affected similar cortical brain regions as those affected by the ERBB4 genotype during childhood and adolescence. Children with FH had reduced surface area compared to those with no FH in both temporal lobes, and in the cortices of the left superior frontal and of the left medial orbito-frontal regions. Only one cluster in the extreme posterior part of the right isthmus cingulate cortex showed reduced thickness in children with FH. Interestingly, the bilateral temporal lobes showed both a genotype and an FH effect.
The rs7598440-ERBB4 variations and family history of schizophrenia or bipolar disorders (FH) on cortical structures (Figures 3&4)
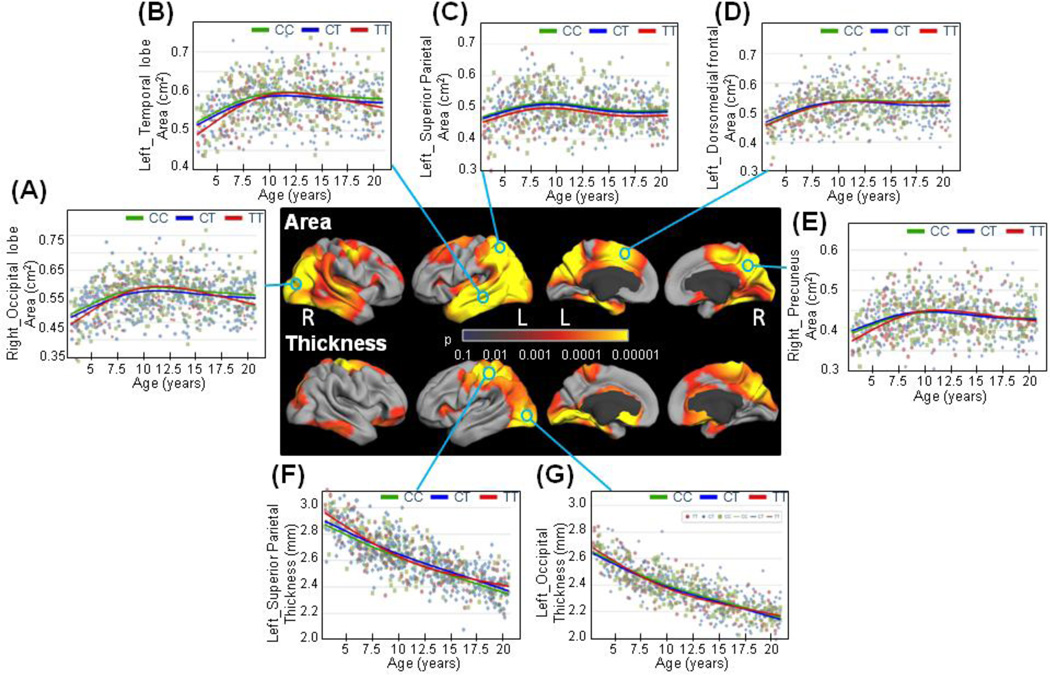
Figure 3.
Statistical significance maps of the ERBB4 variants on cortical structures in children with no family history of schizophrenia (SCZ) and/or bipolar disorders (BPD). The effects of the ERBB4 genotype at rs7598440 were evaluated on cortical area and thickness at each vertex with a general additive model. Sex, GAF, SES and scanner device were included as covariates. Statistical models were corrected for multiple comparisons with the false discovery rate (FDR; 0.05).
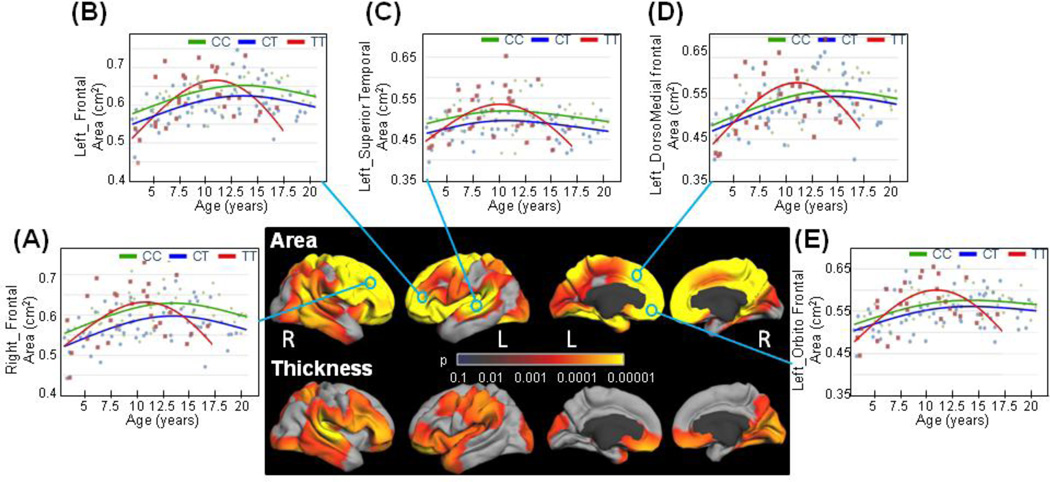
Figure 4.
Statistical significance maps of the ERBB4 variants on cortical structures in children with a family history of schizophrenia (SCZ) and/or bipolar disorders (BPD).
Sex, GAF, SES and scanner device were included as covariates. Statistical models were corrected for multiple comparisons with the false discovery rate (FDR; 0.05).
Since we found specific regions impacted by genotype and by FH, we further evaluated cortical changes in children without FH separately from those with FH (Figures 3&4).
Children without FH exhibited genotype effects (Figure 3A), or subtle but significant genotype-by-age effects (Figures 3B–G) on surface areas for a substantial portion of the cortex. Specifically, children with the TT-risk genotype showed smaller surface areas bilaterally in the occipital and temporal lobes at very young ages (<5 years), but had a more pronounced age-related growth of these regions until 10 years of age, followed by a steeper decline during adolescence compared to the non-risk children (Figures 3 B&C). The TT-risk genotype children had smaller areas for the left superior parietal lobule across the entire age range compared to the C-carriers (Figure 3D). Trajectories for the surface area in the left dorsomedial frontal region diverged at age 13 years between the heterozygous CT children and the other children (Figure 3E), whereas the TT-risk children had smaller surface areas in the right precuneus at younger ages (<10 years) compared to the non-risk children (Figure 3F). Thickness of the left superior parietal lobule, the left occipital lobe and the left lingual and parahippocampal cortices also had a genotype effect in children with no FH (Figure 3A). The cortical thickness in TT-risk carriers showed a slight U-shape curve across the age range in these brain regions (Figures 3G&H) compared to the almost linear age-related decrease in thickness in the non-risk carriers.
Children with FH showed significantly greater and more distinct differences in genotype effects on surface areas compared to those without FH, with pronounced involvement of the frontal lobe (Figures 3&4). In this sub-cohort of children with FH, the TT-risk carriers had steeper cortical surface area growth with age in these frontal as well as temporal regions compared to the non-risk children until early adolescence (<11 years old), but had a greater age-related decrease during adolescence (Figures 4B–F). Note, however, that none of the TT-risk children with a family history of SCZ and/or BPD were older than 17 years old.
These findings show that the effects of the risk allele(s) in rs7598440-ERBB4 on cortical brain regions differ according to the family history of SCZ and/or BPD in these children. For example, the frontal lobe areas differed by the rs7598440-ERBB4 variation across the age range in children with FH, while the temporal lobe surface areas varied with the different rs7598440-ERBB4 genotypes across the age range, but independently of FH. However, smaller regions such as the precuneus, parahippocampal, and entorhinal cortices or the superior temporal gyrus were associated with the rs7598440-ERBB4 variants independently of FH.
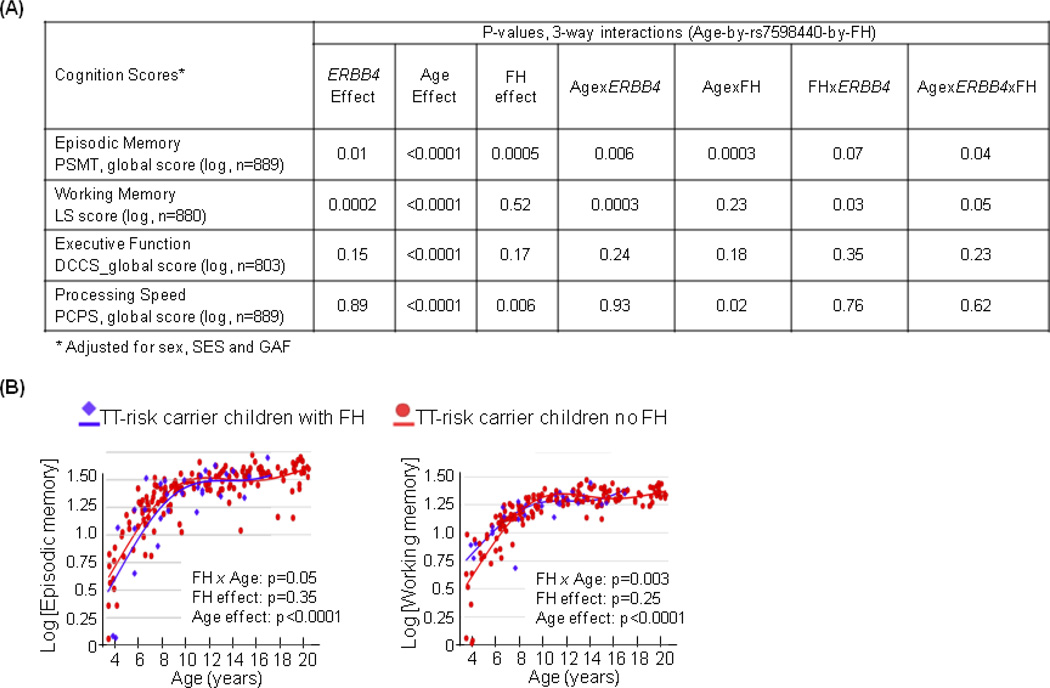
Age-by-genotype interactions on cognitive performance (Figure 5)
Figure 5. Effect of the rs7598440-ERBB4 on cognitive performance.
(A) Three-way Age-by-rs7598440-byFH interactions on the four cognitive domains most consistently impaired in schizophrenia and bipolar disorders: episodic memory (PSMT, Picture Sequence Memory Task), working memory (LS, List Sorting) and executive function (DCCS, Dimensional Change Card Sort) and processing speed (PCPS, Pattern Comparison Processing Speed). Models were performed using a smooth age, a linear term for genotype, sex, SES and GAF, and a smooth interaction between age, genotype and family history of SCZ and/or BPD (FH).
(B) Episodic memory and working memory scores on log scales were plotted against age in children carrying the TT-risk genotype at rs7598440 with (blue) or without (red) a family history of SCZ and/or BPD. Lines are the fitting curves generated using the GAMs by genotype controlling for SES, GAF and sex.
Episodic memory, working memory, executive function and processing speed are cognitive domains often affected in individuals with SCZ and/or BPD (Bora et al., 2014; Fatouros-Bergman, Cervenka, Flyckt, Edman, & Farde, 2014; Schaefer et al., 2013). We examined whether the rs7598440-ERBB4 variant differentially impacts the performance on these cognitive domains, with or without a FH of SCZ and/or BPD, and across the age range. We found 3-way interactions (age-by-rs7598440-ERBB4-by-FH) for episodic memory (p=0.04, Figure 5A) and for working memory (p=0.05, Figure 5A). Specifically, during childhood, TT-risk genotype children with FH performed worse on episodic memory but better on working memory than those without FH (<12 years, Figure 5B). During early adolescence (12–16 years), their performances on both domains became the same in both groups (Figure 5B). In contrast, C-carrier children with FH had similar age-related scores in all four domains compared to those with no FH (p>0.05, data not shown). Executive function showed only an age effect, whereas processing speed presented an interactive effect of age and FH (Figure 5A); children with FH performed worse than those without FH during early childhood, but normalized after puberty (p=0.04, data not shown).
DISCUSSION
To our knowledge, this is the first study describing the independent and interactive effects of the rs7598440-ERBB4 variants and of SCZ and BPD family history on cortical structures in healthy children across an age range that includes puberty, the period of symptom onset for SCZ and BPD. The rs7598440-ERBB4 variations have widespread effects on the temporal and parieto-occipital regions, which are often altered in patients with SCZ or BPD (Friedman et al., 1999; Jacobsen et al., 1998; Konrad et al., 2009; Rimol et al., 2010; Thompson et al., 2001) or in healthy adults carrying the T-risk allele at rs7598440-ERBB4 (Konrad et al., 2009). In contrast, familial history of SCZ and BPD (FH) affects mainly the superior temporal lobe, and the left medial-orbito frontal and left supramarginal regions. Moreover, variations at rs7598440 had a greater impact in various brain regions of children with FH of SCZ and/or BPD, suggesting an additive effect of FH and ERBB4 genotype on cortical development. Lastly, exploratory analysis on cognitive performance showed age-by-genotype-by-FH interactions in episodic memory and working memory; both are cognitive domains most consistently impaired in SCZ or BPD (Bora et al., 2014; Maier et al., 2006; Schaefer et al., 2013). Thus, our observations provide additional evidence for the vulnerability of the risk genotype of ERBB4 in children with subclinical or presymptomatic phenotypes for SCZ and BPD.
Whole-brain analysis showed that variation in ERBB4 affects mainly the development of the temporo-parieto-occipital regions, and the left superior frontal gyrus. Compared to the non-risk C-carrier children, children with the TT-risk genotype for SCZ and/or BPD had smaller volumes and areas in these regions, but had a trend for greater age-related increases in thickness of the left superior parietal lobule and left occipital lobe. These results resemble the pattern of cortical thinning of the parietal and occipital lobes as well as the frontal cortex in SCZ and/or BPD adolescents (Jacobsen et al., 1998; Janssen et al., 2014; Thompson et al., 2001) and adults (Gutierrez-Galve et al., 2014; Rimol et al., 2010). In our study, ERBB4 variations had subtle but significant age-related cortical thinning and surface area shrinkage compared to the typical cortical developement (Sowell, Trauner, Gamst, & Jernigan, 2002). However, the additive effect between ERBB4 variations and family history of SCZ and BPD shows that children with the TT-risk alleles and FH had more pronounced reduction of cortical areas, primarily in frontal regions, than the other two genotypes, suggesting accelerated pruning in these regions. In addition, variations in ERBB4 affected the corticolimbic regions that are impaired in SCZ and/or BPD patients (Diwadkar, Wadehra, et al., 2012). Those regions contain the fronto-temporal fiber tracts and fibers that connect the parieto-occipital regions to the frontal cortex (Oishi K, 2010). The fronto-temporo-parietal regions also showed abnormal activation and reduced functional connectivity in SCZ patients and their relatives (Konrad & Winterer, 2008; Winterer et al., 2003), while the fronto-temporal fiber tract showed lower fractional anisotropy in healthy adults carrying the SCZ-risk-ERBB4 genotype compared to the non-risk carriers (Konrad et al., 2009). Moreover, we found age-by-FH-by-genotype interactions in short-term memory (including episodic and working memory), which is supported by networks spanning frontal and temporal lobes (Metzler-Baddeley, Jones, Belaroussi, Aggleton, & O'Sullivan, 2011). Therefore, our findings suggest that the ERBB4-T-risk alleles may partially contribute to these cognitive deficits by altering primarily age-dependent changes in cortical areas in the brain regions affected in SCZ or BPD.
Differential expression of the ERBB4 receptor, which may lead to imbalance in the NRG1-ERBB4 signaling pathway, may be related to changes in cortical surface area and thickness (Buonanno, 2010; Chong et al., 2008; Konrad et al., 2009; Nicodemus et al., 2010). The variation at rs7598440 results in an increase of the ERBB4 (CYT-1) transcript levels in the hippocampus (Law et al., 2007). Similarly, an increase of ERBB4 protein levels was observed in SCZ patients compared to healthy controls in the dorso-lateral prefrontal cortex (Chong et al., 2008). One could speculate that age-related cortical changes mediated by the ERBB4 genotype are dependent on the actual expression of ERBB4 (CYT-1 up-regulation) in the dorso-lateral prefrontal cortex and the hippocampus of SCZ patients, both regions were implicated in the cortico-limbic system that is impaired in SCZ and BPD patients. Since ERBB4 is involved in progenitor cell control, neuronal and glial migration, dendritic growth and arborization (S. Chen et al., 2006; Mei & Xiong, 2008; Norton et al., 2006), subtle disturbances in each of these factors or a combination of these factors may contribute to the morphometric changes observed in our population. Therefore, intrinsic factors such as genetic variation in ERBB4 may contribute to the pathological processes for SCZ and BPD. However, other risk genes and additional environmental factors are most likely required for the full manifestations of these genetic effects, and ultimately to the development of the diseases.
Having a family history of SCZ and/or BPD involves both shared genetic inheritance and likely shared environmental factors. Our children with a FH of SCZ and/or BPD indeed showed regional age-related changes that primarily affected areas in the left superior temporal lobe as well as bilaterally the superior frontal cortex, independent of the ERBB4 genotype effect. However, while the temporo-parietal regions showed ERBB4-genotype interactions in children with no FH, the frontal lobe including the dorso-lateral prefrontal cortex and the superior temporal lobe exhibited interactions only in children with FH. Our findings are consistent with prior studies of SCZ patients that consistently found cortical shrinkage n prefrontal and temporal cortices (Jacobsen et al., 1998; Janssen et al., 2014; Mattai et al., 2011; Rimol et al., 2012), suggesting abnormal pruning and/or synaptic reorganization. In SCZ, reduced connectivity between temporal lobe regions was shown previously, and involved memory and language functions and the dorsolateral prefrontal cortex, which is required for executive control (Diwadkar, Pruitt, et al., 2012; Eisenberg & Berman, 2010; Kane & Engle, 2002). In our pediatric population, we only found episodic memory and working memory to be affected in younger children with the TT-risk genotype children and a family history of SCZ and/or BPD.
Lastly, our study provides additional support for the role of ERBB4 in the pathophysiology of SCZ and BPD, and its differential effects with age on cortical structures and short-term memory (working memory and episodic memory) performance. The finding that these effects are further enhanced in the children with a family history of these disorders support the heritable characteristics, or other shared environmental risk factors, for SCZ and BPD.
Limitations
Our cross-sectional study did not include the entire lifespan, but included the period when SCZ or BPD symptoms may emerge. The age-by-genotype and age-by-genotype-by-FH-related changes on cortical structures in this large sample of subjects are unlikely caused by a cohort effect; however, longitudinal studies are required to confirm the validity of these findings over time and their relation to changes in the clinical phenotypes (Kraemer, Yesavage, Taylor, & Kupfer, 2000).
Schizophrenia and bipolar disorders are polygenic diseases whereby each of multiple gene variations contributes minor fractions to the overall risk for disease. On the other hand, structural brain abnormalities observed in SCZ and BPD are also found in other disorders, such as substance use disorders (Marshal et al., 2008). Therefore, combining brain imaging and genetics (i.e. imaging genetics) not only allow identification of vulnerability for SCZ or BPD, but also provide the identification of prodomal markers for developing psychotic symptoms. Given our preliminary results on the differential effects of ERBB4 genotypes on cortical changes in children with and without family history of SCZ and/or BPD, it would be interesting to further investigate brain differences in children and adolescents with new-onset psychosis or BPD symptoms, and determine whether the cortical changes we observed are similar to those with a higher risk of developing SCZ or BPD. However, it is important to note that cortical changes mediated by the ERBB4 risk variant is likely to play only a subtle role in the total vulnerability to these disorders, since other risk genes likely contribute independently or interactively with this variant to the pathophysiology. For instance, NRG1 genotype variation is also associated with increased risk for SCZ and/or BPD and with altered brain development in the same pediatric cohort (Douet et al., 2014).
Our interpretation is limited to typically developing children, some of whom may have a higher risk for SCZ and/ BPD as well as other psychiatric disorders (Jeppesen et al., 2014). Longitudinal follow-up would validate and assess the progression of the regional brain changes in these psychiatric disorders, while eliminating the potential effects of medication or treatments on cortical measures (Torres et al., 2013), or on ERBB4 expression (Wang et al., 2008).
Conclusion
The specific functional variation rs7598440 in ERBB4 has age-specific effects on cortical structures in a large cohort of healthy children ages 3–20 years old. Age-by-genotype interactions were found primarily in the fronto-temporal lobes of children with a family history of SCZ and/or BPD, but only in the temporo-parieto-occipital regions in children with no family history of these disorders. These cortical changes were observed in children without any cognitive or clinical manifestations of psychiatric disorders. Nevertheless, many of the changes in children with the T-risk-allele of ERBB4 and a family history of SCZ and/or BPD occurred in brain regions that are also altered in patients with SCZ or BPD; therefore, these findings may indicate increased risk of SCZ or BPD in these healthy children.
Further replication studies involving clinical samples as well as longitudinal follow-up studies are needed to confirm whether these prodromal brain alterations would evolve in relation to clinical symptoms. These subclinical cortical changes may provide early indicators or predict the development of psychiatric disorders and improve our understanding of the biological effects of the NRG1–ErBB4 signaling cascade in human brain.
Supplementary Material
ACKNOWLEDGMENTS
We thank all of the children and their families that have participated in our study, the physicians who referred some of the participants and all the clinical and technical staff from the research teams in the PING consortium. Data collection and sharing for this project was performed by the Pediatric Imaging, Neurocognition and, Genetics (PING) consortium. PING is co-funded by the National Institute on Drug Abuse and the Eunice Kennedy Shriver National Institute of Child Health & Human Development (RC2DA029475). PING data are disseminated by the PING Coordinating Center at the Center for Human Development, University of California, San Diego.
Additional Grant Support from the National Institutes of Health: 2K24-DA016170, U54NS56883, G12-MD007601.
Footnotes
Data used in preparation of this article were obtained from the Pediatric Imaging, Neurocognition and Genetics Study (PING) database (http://ping.chd.ucsd.edu). As such, the investigators within PING contributed to the design and implementation of PING and/or provided data, but did not participate in analysis or writing of this report. A complete listing of PING investigators can be found at https://ping-dataportal.ucsd.edu/sharing/Authors10222012.pdf.
FINANCIAL DISCLOSURES
All the authors declare no conflict of interest.
REFERENCES
- Agim ZS, Esendal M, Briollais L, Uyan O, Meschian M, Martinez LA, Ozcelik H. Discovery, validation and characterization of Erbb4 and Nrg1 haplotypes using data from three genome-wide association studies of schizophrenia. [Research Support, N.I.H., Extramural. Research Support, Non-U.S. Gov't] PLoS One. 2013;8(1):e53042. doi: 10.1371/journal.pone.0053042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akshoomoff N, Newman E, Thompson WK, McCabe C, Bloss CSCL, Amaral DG, Casey BJ, Ernst TM, Frazier JA, Gruen JR, Kaufmann WE, Kenet T, Kennedy DN, Libiger OMS, Murray SS, Sowell ER, Schork N, Dale AM, Jernigan TL. The NIH Toolbox Cognition Battery: Results from a large normative Developmental Sample (PING) Neuropsychology, in press. 2013 doi: 10.1037/neu0000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. [Meta-Analysis Research Support, Non-U.S. Gov't Review] Br J Psychiatry. 2009;195(3):194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- Bae JS, Pasaje CF, Park BL, Cheong HS, Kim JH, Kim JY, Woo SI. Genetic association analysis of ERBB4 polymorphisms with the risk of schizophrenia and SPEM abnormality in a Korean population. [Research Support, Non-U.S. Gov't] Brain Res. 2012;1466:146–151. doi: 10.1016/j.brainres.2012.05.029. [DOI] [PubMed] [Google Scholar]
- Bakken TE, Roddey JC, Djurovic S, Akshoomoff N, Amaral DG, Bloss CS, Carlson H. Association of common genetic variants in GPCPD1 with scaling of visual cortical surface area in humans. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] Proc Natl Acad Sci U S A. 2012;109(10):3985–3990. doi: 10.1073/pnas.1105829109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch H, Thompson WK, Jernigan TL, Dale AM. A web-portal for interactive data exploration, visualization, and hypothesis testing. Front Neuroinform. 2014;8:25. doi: 10.3389/fninf.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Yoav HY. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Vol. 1995. Wiley; 1995. [Google Scholar]
- Beyer JL, Young R, Kuchibhatla M, Krishnan KR. Hyperintense MRI lesions in bipolar disorder: A meta-analysis and review. [Meta-Analysis Research Support, N.I.H., Extramural Review] Int Rev Psychiatry. 2009;21(4):394–409. doi: 10.1080/09540260902962198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Lin A, Wood SJ, Yung AR, McGorry PD, Pantelis C. Cognitive deficits in youth with familial and clinical high risk to psychosis: a systematic review and meta-analysis. [Research Support, Non-U.S. Gov't] Acta Psychiatr Scand. 2014;130(1):1–15. doi: 10.1111/acps.12261. [DOI] [PubMed] [Google Scholar]
- Brown TT, Kuperman JM, Chung Y, Erhart M, McCabe C, Hagler DJ, Jr, Dale AM. Neuroanatomical assessment of biological maturity. [Research Support, N.I.H., Extramural] Curr Biol. 2012;22(18):1693–1698. doi: 10.1016/j.cub.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buka SL, Fan AP. Association of prenatal and perinatal complications with subsequent bipolar disorder and schizophrenia. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. Review] Schizophr Res. 1999;39(2):113–119. doi: 10.1016/s0920-9964(99)00109-7. discussion 160–111. [DOI] [PubMed] [Google Scholar]
- Buonanno A. The neuregulin signaling pathway and schizophrenia: from genes to synapses and neural circuits. [Research Support, N.I.H., Intramural Review] Brain Res Bull. 2010;83(3–4):122–131. doi: 10.1016/j.brainresbull.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Georgieva L, Young JJ, Plescia C, Kajiwara Y, Jiang Y, O'Donovan MC. Molecular dissection of NRG1-ERBB4 signaling implicates PTPRZ1 as a potential schizophrenia susceptibility gene. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] Mol Psychiatry. 2008;13(2):162–172. doi: 10.1038/sj.mp.4001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardno AG, Gottesman II. Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. [Review] Am J Med Genet. 2000;97(1):12–17. [PubMed] [Google Scholar]
- Chen P, Chen J, Huang K, Ji W, Wang T, Li T, Shi Y. Analysis of association between common SNPs in ErbB4 and bipolar affective disorder, major depressive disorder and schizophrenia in the Han Chinese population. [Comparative Study Research Support, Non-U.S. Gov't] Prog Neuropsychopharmacol Biol Psychiatry. 2012;36(1):17–21. doi: 10.1016/j.pnpbp.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Chen S, Velardez MO, Warot X, Yu ZX, Miller SJ, Cros D, Corfas G. Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function. [Research Support, N.I.H., Extramural] J Neurosci. 2006;26(12):3079–3086. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong VZ, Thompson M, Beltaifa S, Webster MJ, Law AJ, Weickert CS. Elevated neuregulin-1 and ErbB4 protein in the prefrontal cortex of schizophrenic patients. [Comparative Study Research Support, Non-U.S. Gov't] Schizophr Res. 2008;100(1–3):270–280. doi: 10.1016/j.schres.2007.12.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Pruitt P, Zhang A, Radwan J, Keshavan MS, Murphy E, Zajac-Benitez C. The neural correlates of performance in adolescents at risk for schizophrenia: inefficiently increased cortico-striatal responses measured with fMRI. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] J Psychiatr Res. 2012;46(1):12–21. doi: 10.1016/j.jpsychires.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Wadehra S, Pruitt P, Keshavan MS, Rajan U, Zajac-Benitez C, Eickhoff SB. Disordered corticolimbic interactions during affective processing in children and adolescents at risk for schizophrenia revealed by functional magnetic resonance imaging and dynamic causal modeling. [Research Support, N.I.H., Extramural] Arch Gen Psychiatry. 2012;69(3):231–242. doi: 10.1001/archgenpsychiatry.2011.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douet V, Chang L, Pritchett A, Lee K, Keating B, Bartsch H, Ernst T. Schizophrenia-risk variant rs6994992 in the neuregulin-1 gene on brain developmental trajectories in typically developing children. [Research Support, N.I.H., Extramural] Transl Psychiatry. 2014;4:e392. doi: 10.1038/tp.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DP, Berman KF. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. [Research Support, N.I.H., Intramural Review] Neuropsychopharmacology. 2010;35(1):258–277. doi: 10.1038/npp.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. [Meta-Analysis Research Support, N.I.H., Extramural] Am J Psychiatry. 2008;165(8):1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatouros-Bergman H, Cervenka S, Flyckt L, Edman G, Farde L. Meta-analysis of cognitive performance in drug-naive patients with schizophrenia. Schizophr Res. 2014;158(1–3):156–162. doi: 10.1016/j.schres.2014.06.034. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Brown TT, Kuperman JM, Chung Y, Hagler DJ, Jr, Genetics S. Multimodal imaging of the self-regulating developing brain. [Multicenter Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] Proc Natl Acad Sci U S A. 2012;109(48):19620–19625. doi: 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Yucel M, Pantelis C. Reconciling neuroimaging and neuropathological findings in schizophrenia and bipolar disorder. [Research Support, Non-U.S. Gov't Review] Curr Opin Psychiatry. 2009;22(3):312–319. doi: 10.1097/YCO.0b013e32832a1353. [DOI] [PubMed] [Google Scholar]
- Fornito A, Yucel M, Patti J, Wood SJ, Pantelis C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. [Research Support, Non-U.S. Gov't] Schizophr Res. 2009;108(1–3):104–113. doi: 10.1016/j.schres.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Friedman L, Findling RL, Kenny JT, Swales TP, Stuve TA, Jesberger JA, Schulz SC. An MRI study of adolescent patients with either schizophrenia or bipolar disorder as compared to healthy control subjects. [Comparative Study Research Support, Non-U.S. Gov't] Biol Psychiatry. 1999;46(1):78–88. doi: 10.1016/s0006-3223(98)00351-5. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, Fox PT. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. [Research Support, N.I.H., Extramural] Biol Psychiatry. 2008;64(9):774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goes FS, Rongione M, Chen YC, Karchin R, Elhaik E, Bipolar Genome S, Potash JB. Exonic DNA sequencing of ERBB4 in bipolar disorder. [Research Support, N.I.H., Extramural] PLoS One. 2011;6(5):e20242. doi: 10.1371/journal.pone.0020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Rapoport JL. Childhood-onset schizophrenia: insights from neuroimaging studies. [Review] J Am Acad Child Adolesc Psychiatry. 2008;47(10):1120–1124. doi: 10.1097/CHI.0b013e31817eed7a. [DOI] [PubMed] [Google Scholar]
- Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, Chen Q, Meyer-Lindenberg A. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov't] Arch Gen Psychiatry. 2009;66(5):467–477. doi: 10.1001/archgenpsychiatry.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Light GA, Swerdlow NR, Radant AD, Braff DL. Association analysis of 94 candidate genes and schizophrenia-related endophenotypes. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] PLoS One. 2012;7(1):e29630. doi: 10.1371/journal.pone.0029630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Galve L, Chu EM, Leeson VC, Price G, Barnes TR, Joyce EM, Ron MA. A longitudinal study of cortical changes and their cognitive correlates in patients followed up after first-episode psychosis. Psychol Med. 2014:1–12. doi: 10.1017/S0033291714001433. [DOI] [PubMed] [Google Scholar]
- Ha T. General additive model. 1986;1 [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. [Research Support, Non-U.S. Gov't Review] Mol Psychiatry. 2005;10(1):40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- Hatzimanolis A, McGrath JA, Wang R, Li T, Wong PC, Nestadt G, Avramopoulos D. Multiple variants aggregate in the neuregulin signaling pathway in a subset of schizophrenia patients. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] Transl Psychiatry. 2013;3:e264. doi: 10.1038/tp.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. [Comparative Study Meta-Analysis Research Support, Non-U.S. Gov't Review] Am J Psychiatry. 2005;162(12):2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Giedd JN, Castellanos FX, Vaituzis AC, Hamburger SD, Kumra S, Rapoport JL. Progressive reduction of temporal lobe structures in childhood-onset schizophrenia. [Comparative Study] Am J Psychiatry. 1998;155(5):678–685. doi: 10.1176/ajp.155.5.678. [DOI] [PubMed] [Google Scholar]
- Janssen J, Aleman-Gomez Y, Schnack H, Balaban E, Pina-Camacho L, Alfaro-Almagro F, Desco M. Cortical morphology of adolescents with bipolar disorder and with schizophrenia. Schizophr Res. 2014;158(1–3):91–99. doi: 10.1016/j.schres.2014.06.040. [DOI] [PubMed] [Google Scholar]
- Jeppesen P, Larsen JT, Clemmensen L, Munkholm A, Rimvall MK, Rask CU, Skovgaard AM. The CCC2000 Birth Cohort Study of Register-Based Family History of Mental Disorders and Psychotic Experiences in Offspring. Schizophr Bull. 2014 doi: 10.1093/schbul/sbu167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. [Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S. Review] Psychon Bull Rev. 2002;9(4):637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Konrad A, Vucurevic G, Musso F, Stoeter P, Dahmen N, Winterer G. ErbB4 genotype predicts left frontotemporal structural connectivity in human brain. Neuropsychopharmacology. 2009;34(3):641–650. doi: 10.1038/npp.2008.112. [DOI] [PubMed] [Google Scholar]
- Konrad A, Winterer G. Disturbed structural connectivity in schizophrenia primary factor in pathology or epiphenomenon? [Review] Schizophr Bull. 2008;34(1):72–92. doi: 10.1093/schbul/sbm034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't. P.H.S.] Am J Psychiatry. 2000;157(2):163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. [Comparative Study Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H. S.] Arch Gen Psychiatry. 2003;60(9):878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. [Comparative Study Research Support, N.I.H., Intramural] Hum Mol Genet. 2007;16(2):129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, Weinberger DR. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5' SNPs associated with the disease. Proc Natl Acad Sci U S A. 2006;103(17):6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. [Research Support, U.S. Gov't. P.H.S. Review] Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Lu CL, Wang YC, Chen JY, Lai IC, Liou YJ. Support for the involvement of the ERBB4 gene in schizophrenia: a genetic association analysis. [Research Support, Non-U.S. Gov't] Neurosci Lett. 2010;481(2):120–125. doi: 10.1016/j.neulet.2010.06.067. [DOI] [PubMed] [Google Scholar]
- Luykx JJ, Vinkers CH, Bakker SC, Visser WF, van Boxmeer L, Strengman E, Ophoff RA. A common variant in ERBB4 regulates GABA concentrations in human cerebrospinal fluid. [Multicenter Study] Neuropsychopharmacology. 2012;37(9):2088–2092. doi: 10.1038/npp.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W, Zobel A, Wagner M. Schizophrenia and bipolar disorder: differences and overlaps. [Comparative Study Review] Curr Opin Psychiatry. 2006;19(2):165–170. doi: 10.1097/01.yco.0000214342.52249.82. [DOI] [PubMed] [Google Scholar]
- Marenco S, Geramita M, van der Veen JW, Barnett AS, Kolachana B, Shen J, Law AJ. Genetic association of ErbB4 and human cortical GABA levels in vivo. [Comparative Study Research Support, N.I.H., Intramural] J Neurosci. 2011;31(32):11628–11632. doi: 10.1523/JNEUROSCI.1529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshal MP, Friedman MS, Stall R, King KM, Miles J, Gold MA, Morse JQ. Sexual orientation and adolescent substance use: a meta-analysis and methodological review. [Meta-Analysis Research Support, N.I.H., Extramural Review] Addiction. 2008;103(4):546–556. doi: 10.1111/j.1360-0443.2008.02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattai AA, Weisinger B, Greenstein D, Stidd R, Clasen L, Miller R, Gogtay N. Normalization of cortical gray matter deficits in nonpsychotic siblings of patients with childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2011;50(7):697–704. doi: 10.1016/j.jaac.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Gottesman II. The genetic epidemiology of schizophrenia and the design of linkage studies. [Review] Eur Arch Psychiatry Clin Neurosci. 1991;240(3):174–181. doi: 10.1007/BF02190760. [DOI] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review] Nat Rev Neurosci. 2008;9(6):437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Jones DK, Belaroussi B, Aggleton JP, O'Sullivan MJ. Frontotemporal connections in episodic memory and aging: a diffusion MRI tractography study. [Research Support, Non-U.S. Gov't] J Neurosci. 2011;31(37):13236–13245. doi: 10.1523/JNEUROSCI.2317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemus KK, Law AJ, Radulescu E, Luna A, Kolachana B, Vakkalanka R, Weinberger DR. Biological validation of increased schizophrenia risk with NRG1, ERBB4, and AKT1 epistasis via functional neuroimaging in healthy controls. [Research Support, N.I.H., Intramural] Arch Gen Psychiatry. 2010;67(10):991–1001. doi: 10.1001/archgenpsychiatry.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemus KK, Luna A, Vakkalanka R, Goldberg T, Egan M, Straub RE, Weinberger DR. Further evidence for association between ErbB4 and schizophrenia and influence on cognitive intermediate phenotypes in healthy controls. [Clinical Trial Comparative Study Letter] Mol Psychiatry. 2006;11(12):1062–1065. doi: 10.1038/sj.mp.4001878. [DOI] [PubMed] [Google Scholar]
- Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, O'Donovan MC. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] Am J Med Genet B Neuropsychiatr Genet. 2006;141B(1):96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- Oishi KFA, Van Zijl P, Mori S. MRI Atlas of Human White Matter. 2nd Edition. Elsevier; 2010. (09 Nov 2010 ed.) [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. [Review] Mol Psychiatry. 2005;10(5):434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Hartberg CB, Nesvag R, Fennema-Notestine C, Hagler DJ, Jr, Pung CJ, Agartz I. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. [Research Support, Non-U.S. Gov't] Biol Psychiatry. 2010;68(1):41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Nesvag R, Hagler DJ, Jr, Bergmann O, Fennema-Notestine C, Hartberg CB, Dale AM. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. [Research Support, Non-U.S. Gov't] Biol Psychiatry. 2012;71(6):552–560. doi: 10.1016/j.biopsych.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Sankararaman S, Sridhar S, Kimmel G, Halperin E. Estimating local ancestry in admixed populations. [Comparative Study] Am J Hum Genet. 2008;82(2):290–303. doi: 10.1016/j.ajhg.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer J, Giangrande E, Weinberger DR, Dickinson D. The global cognitive impairment in schizophrenia: consistent over decades and around the world. [Research Support, N.I.H., Intramural] Schizophr Res. 2013;150(1):42–50. doi: 10.1016/j.schres.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S. Review] Schizophr Res. 2001;49(1–2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota S, Tochigi M, Shimada H, Ohashi J, Kasai K, Kato N, Sasaki T. Association and interaction analyses of NRG1 and ERBB4 genes with schizophrenia in a Japanese population. [Comparative Study] J Hum Genet. 2008;53(10):929–935. doi: 10.1007/s10038-008-0332-9. [DOI] [PubMed] [Google Scholar]
- Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: association and expression studies. [Comparative Study Research Support, Non-U.S. Gov't] Am J Med Genet B Neuropsychiatr Genet. 2006;141B(2):142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. [Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S.] Dev Med Child Neurol. 2002;44(1):4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Stefanis NC, Hatzimanolis A, Smyrnis N, Avramopoulos D, Evdokimidis I, van Os J, Weinberger DR. Schizophrenia candidate gene ERBB4: covert routes of vulnerability to psychosis detected at the population level. [Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov't] Schizophr Bull. 2013;39(2):349–357. doi: 10.1093/schbul/sbr169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71(4):877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinthorsdottir V, Stefansson H, Ghosh S, Birgisdottir B, Bjornsdottir S, Fasquel AC, Gulcher JR. Multiple novel transcription initiation sites for NRG1. [Comparative Study] Gene. 2004;342(1):97–105. doi: 10.1016/j.gene.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, Rapoport JL. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. [Research Support, U.S. Gov't, P.H.S.] Proc Natl Acad Sci U S A. 2001;98(20):11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thormodsen R, Rimol LM, Tamnes CK, Juuhl-Langseth M, Holmen A, Emblem KE, Agartz I. Age-related cortical thickness differences in adolescents with early-onset schizophrenia compared with healthy adolescents. Psychiatry Res. 2013;214(3):190–196. doi: 10.1016/j.pscychresns.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Torres US, Portela-Oliveira E, Borgwardt S, Busatto GF. Structural brain changes associated with antipsychotic treatment in schizophrenia as revealed by voxel-based morphometric MRI: an activation likelihood estimation meta-analysis. [Meta-Analysis Research Support, Non-U.S. Gov't] BMC Psychiatry. 2013;13:342. doi: 10.1186/1471-244X-13-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets NL, Hough MG, Douaud G, Matthews PM, James A, Winmill L, Smith S. Evidence for abnormalities of cortical development in adolescent-onset schizophrenia. [Research Support, Non-U.S. Gov't] Neuroimage. 2008;43(4):665–675. doi: 10.1016/j.neuroimage.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Brown TT, Kuperman JM, Chung Y, Hagler DJ, Jr, Genetics S. Long-term influence of normal variation in neonatal characteristics on human brain development. [Comparative Study Multicenter Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] Proc Natl Acad Sci U S A. 2012;109(49):20089–20094. doi: 10.1073/pnas.1208180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD, Su YA, Guo CM, Yang Y, Si TM. Chronic antipsychotic drug administration alters the expression of neuregulin 1beta, ErbB2, ErbB3, and ErbB4 in the rat prefrontal cortex and hippocampus. [Research Support, Non-U.S. Gov't] Int J Neuropsychopharmacol. 2008;11(4):553–561. doi: 10.1017/S1461145707008371. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, Gershon RC. Cognition assessment using the NIH Toolbox. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.] Neurology. 2013;80(11 Suppl 3):S54–S64. doi: 10.1212/WNL.0b013e3182872ded. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Coppola R, Egan MF, Goldberg TE, Weinberger DR. Functional and effective frontotemporal connectivity and genetic risk for schizophrenia. [Comparative Study] Biol Psychiatry. 2003;54(11):1181–1192. doi: 10.1016/s0006-3223(03)00532-8. [DOI] [PubMed] [Google Scholar]
- Zuliani R, Moorhead TW, Bastin ME, Johnstone EC, Lawrie SM, Brambilla P, McIntosh AM. Genetic variants in the ErbB4 gene are associated with white matter integrity. [Research Support, Non-U.S. Gov't] Psychiatry Res. 2011;191(2):133–137. doi: 10.1016/j.pscychresns.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.