Abstract
Objective
Some individuals are diagnosed with colorectal cancer (CRC) despite recent colonoscopy. We examined individuals under colonoscopic surveillance for colonic adenomas to assess possible reasons for diagnosing cancer after a recent colonoscopy with complete removal of any identified polyps.
Design
Primary data were pooled from eight large (>800 patients) North American studies in which participants with adenoma(s) had a baseline colonoscopy (with intent to remove all visualised lesions) and were followed with subsequent colonoscopy. We used an algorithm based on the time from previous colonoscopy and the presence, size and histology of adenomas detected at prior exam to assign interval cancers as likely being new, missed, incompletely resected (while previously an adenoma) or due to failed biopsy detection.
Results
9167 participants (mean age 62) were included in the analyses, with a median follow-up of 47.2 months. Invasive cancer was diagnosed in 58 patients (0.6%) during follow-up (1.71 per 1000 person-years follow-up). Most cancers (78%) were early stage (I or II); however, 9 (16%) resulted in death from CRC. We classified 30 cancers (52%) as probable missed lesions, 11 (19%) as possibly related to incomplete resection of an earlier, non-invasive lesion and 14 (24%) as probable new lesions. The cancer diagnosis may have been delayed in three cases (5%) because of failed biopsy detection.
Conclusions
Despite recent colonoscopy with intent to remove all neoplasia, CRC will occasionally be diagnosed. These cancers primarily seem to represent lesions that were missed or incompletely removed at the prior colonoscopy and might be avoided by increased emphasis on identifying and completely removing all neoplastic lesions at colonoscopy.
Introduction
Colorectal cancer (CRC) is the second most common cause of cancer death in the USA and its prevention and early detection are a significant public health concern.1 The United States Preventive Services Task Force and the American Cancer Society in collaboration with the US Multi-Society Task Force, among other organisations, recommend that adults be screened for CRC.2,3 These recommendations are based on high quality evidence that screening can reduce CRC mortality4,5 and incidence6 and recent studies show that nationally CRC incidence and mortality are declining.7
Colonoscopy appears to be a highly effective modality for screening and affords the opportunity to view the entire colorectal mucosa and simultaneously remove premalignant adenomas before they become invasive cancers. While no large randomised controlled trials of screening colonoscopy have been reported, recent observational studies suggest that colonoscopy in the prior 10 years may reduce CRC incidence and mortality by over 60%.8,9
Despite evidence of colonoscopy's effectiveness, some individuals are diagnosed with CRC relatively soon after a colonoscopy that deemed the colon to be free of neoplasia. These ‘interval’ cancers (ie, those that appear to arise between serial colonoscopies) have been observed in studies examining large administrative data sets10,11 and national screening programmes12 as well as in smaller clinical studies.13–16 However, relatively few reports13,17 have explored the possible explanations for these interval cancers. Investigators from the Polyp Prevention Trial (PPT) identified 13 CRCs that occurred during the follow-up phase of that study. Using an algorithm, they estimated that roughly a half were potentially avoidable, being likely missed or the result of a prior incompletely resected polyp.13 At a single Veterans Affairs (VA) centre, records of 45 patients with interval CRCs were examined and 12 (27%) developed cancer in the same segment of the colon from which a prior polyp had been removed.17 Incomplete resection of a prior lesion was felt to be the explanation in those cases. In a clinical series of cases from 20 Indiana hospitals, 47 CRCs were identified within 3 years of a colonoscopy not detecting cancer.16 After review, 27 cases (57%) were felt to be missed cancers.
We used a pooled dataset from eight large prospective studies to assess both the frequency of cancer after complete colonoscopy and the possible reasons for the occurrence of these lesions.
Materials and Methods
Study population
The pooled analyses used patient-level data from eight North American studies18–26 of patients with sporadic colorectal neoplasia. The details regarding the patients enrolled in the individual studies have been reported previously27 and are summarised in table 1. We included studies that met the following criteria: (1) enrolled ≥800 participants; (2) protocol required complete baseline colonoscopy with removal of all visualised lesions, of which at least one was an adenoma; (3) specified the schedule of follow-up colonoscopies; and (4) obtained data regarding the number, size and histopathology of neoplasms detected in follow-up examinations. All of the studies excluded subjects known to be affected by Lynch Syndrome or familial adenomatous polyposis. To our knowledge, we included all studies meeting these criteria that had reported findings by June 2005. Of the 10 021 men and women who were enrolled in the individual studies, we excluded those who had a CRC present at study entry (n=27) and those who had no follow-up colonoscopy performed after their first 6 months of study (n=827) since these likely were not under typical postpolypectomy surveillance (ie, did not undergo follow-up colonoscopy exams during the surveillance period that started 6 months after the baseline exam). Thus, our pooled analyses included data for 9167 (91.5%) of all enrolled patients.
Table 1. Characteristics of pooled studies.
| APPS26 | CPPS20 | AFT21 | PPT24 | WBF19 | NPS25 | VA23 | UDCA18 | |
|---|---|---|---|---|---|---|---|---|
| Intervention | Antioxidants | Calcium | Aspirin/folate | High fibre/low fat diet | Wheat bran fibre | Surveillance interval | None | Ursodiol |
| Recruitment period | 1984–1988 | 1991–1998 | 1994–1998 | 1991–1998 | 1990–1998 | 1980–1990 | 1994–1997 | 1995–1999 |
| # Enrolled/included | 864/837 | 930/913 | 1121/1086 | 2079/2024 | 1429/1304 | 1418/939 | 895*/871 | 1285/1193 |
| Colonoscopy schedule (years) | 1 and 4 | 1 and 4 | 3 | 1 and 4 | 1 and 3 OR 3 | 1 and 3 OR 3 | 2 and 5 OR 5 | 3 |
| % With 1+ colo in the study interval | 96.9 | 98.2 | 96.9 | 97.4 | 91.3 | 81.0 | 97.3 | 92.8 |
| Mean/median follow-up (years) | 3.9/4.1 | 3.9/4.0 | 3.1/3.1 | 4.2/4.3 | 3.5/3.2 | 2.8/3.1 | 4.3/4.9 | 3.5/3.2 |
| Follow-up time (person-years) | 3283.4 | 3564.2 | 3393.4 | 8554.1 | 4516.0 | 2642.3 | 3742.6 | 4139.9 |
Data were from AFT, APPS, CPPS, NPS, PPT, UDCA, VA and WBF studies.
There were 3121 participants in the VA Study; 895 had adenomas at baseline and were included in this pooled analysis.
AFT, Aspirin Folate Trial; APPS, Antioxidant Polyp Prevention Study; CPPS, Calcium Polyp Prevention Study; NPS, National Polyp Study; PPT, Polyp Prevention Trial; UDCA, Ursodoxycholic Acid Study; VA, Veterans Affairs Cooperative Study; WBF, Wheat Bran Fiber Study.
Study endpoints
We identified all participants in the pooled dataset who were diagnosed with invasive cancer after the initial complete colonoscopy and during the main observation period of each study (typically 3–4 years). Most of the descriptive information presented on the cancer cases was derived directly from the pooled data set, including patient demographics, cancer size and location, as well as information on patient outcome (eg, mortality/cause of death). Staff at each study centre also reviewed the endoscopy and pathology reports to confirm the presence of cancer and to abstract data on other clinical details including presenting symptoms (if any), cancer stage and grade where available.
Algorithm to adjudicate cancer cause
Building on prior work by others,13 we developed an algorithm to assign a presumptive explanation for the interval cancer in each case (figure 1 and table 2). Using information regarding the timing and findings on the colonoscopy prior to the one detecting cancer (often but not necessarily the baseline exam), each case was assigned to one of four categories: ‘new cancer,’ ‘missed lesion,’ ‘incomplete adenoma resection’ and ‘failed biopsy detection.’ To be adjudicated as a ‘new cancer’, three or more years must have passed between the prior colonoscopy and the exam diagnosing cancer, and there must have been no significant adenoma detected in the same segment of the colorectum on the prior exam, as described below. Similarly, cancers were assigned as ‘missed lesions’ if they were found within 3 years of the prior colonoscopy and there was no evidence of a significant adenoma in the same segment at that exam. To ascribe a case as ‘incomplete adenoma resection’ there had to be a ‘significant’ adenoma resected from the same segment of the colon during the prior colonoscopy. Given that adenomatous polyps increase in size over time, the definition of what constituted a significant adenoma varied based upon on the time since last colonoscopy. Specifically, if three or more years had passed since the last colonoscopy, then an adenoma ≥5 mm in size, or with villous histology or with high grade dysplasia was considered significant. If fewer than 3 years had passed, then an adenoma ≥1 cm in size, or with villous histology or with high grade dysplasia was considered significant.
Figure 1.
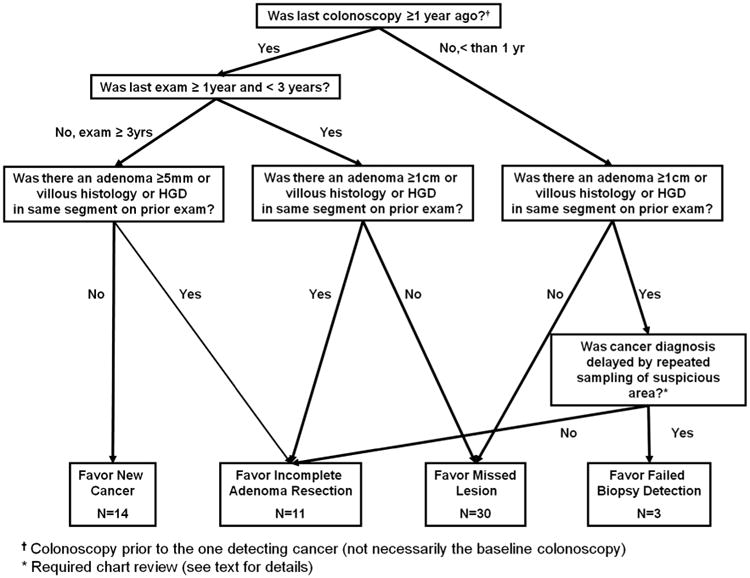
Algorithm used to determine likely cause for early interval cancers and distribution of cancers based upon algorithm review for most likely causes.
Table 2. Summary of adjudication strategy based on interval since last colonoscopy and the presence of a significant lesion in the same section of the colon on prior colonoscopy.
| Time interval since last colonoscopy | |||
|---|---|---|---|
|
|
|||
| Significant lesion found in same segment on prior colonoscopy | ≤1 year* | >1 year to <3 years† | ≥3 years‡ |
| Yes | Incomplete adenoma resection Or Failed biopsy detection | Incomplete adenoma Resection | Incomplete adenoma Resection |
| No | Missed lesion | Missed lesion | New cancer |
Cases required hand review to distinguish.
Significant lesion defined as adenoma with advanced histology or ≥1 cm.
Significant lesion defined as adenoma with advanced histology or ≥5 mm.
The label ‘failed biopsy detection’ was used to categorise those cases occurring within 1 year of the previous exam and in the same segment of the bowel as an adenoma and in which the endoscopist was suspicious that cancer may have been present. In these situations, repeated exams might be required to make what is ultimately a delayed diagnosis.13 For this category, we assumed the interval between the colonoscopy diagnosing cancer and the prior colonoscopy would be short (1 year or less) since endoscopists with heightened clinical suspicion for cancer would unlikely recommend follow-up at longer intervals.
To distinguish between incomplete resection (endoscopist indicates no real concern for residual neoplasia) and failed biopsy detection (endoscopist not sure that the lesion was effectively sampled and/or removed), we performed chart review of available data (eg, colonoscopy reports) of all cases that occurred less than 1 year after a colonoscopy in which an adenoma was identified in the same segment of the bowel as the subsequent cancer. Previously published criteria13 were used to guide reviewers (DJR and PL) of those cases. To further clarify how clinical data were used to adjudicate cases, examples are provided in the supplementary material (see online supplementary table S1).
Two reviewers (DJR, DAL) guided by the algorithm independently ascribed a presumptive reason for each interval cancer. If their assessments disagreed, then a third reviewer (SJW) adjudicated the case. At that point, if two of the three reviewers agreed on a classification, that determination was recorded. In one of the 58 cases the assessments of the three reviewers all differed, and a single investigator (DJR) reviewed the primary clinical data for the case, without using the algorithm, and assigned a likely explanation.
Statistical analyses
We used summary statistics to describe the baseline characteristics of the pooled study cohort and t tests and contingency tables to compare the characteristics of patients who developed interval cancer with those who did not. Follow-up time (for incidence calculations) includes time under observation from qualifying colonoscopy until the diagnosis of CRC or through last study colonoscopy for those not detected with CRC. Contingency tables were also used when determining whether the adjudicated explanation for interval cancer was associated with symptomatic presentation or the location of the cancer. Given reports that colonoscopy may be less effective in preventing right-sided cancers,28,29 we examined the data according to whether or not the interval cancer was located proximal to the splenic flexure (ie, right sided). We used κ statistic to assess inter-rater agreement when using the algorithm.
Results
The 9167 studied patients were mostly men (71.2%) and Caucasian (89.1%), and their mean age was 62.0 years. The median clinical follow-up for the entire patient group was 47.2 months (range of medians among studies 36.9–59.0 months) and the majority underwent at least one colonoscopy during that period, ranging among studies from 81% in the National Polyp Study (NPS) to 97% in the VA study.27
A total of 58 patients (0.63%) were diagnosed with invasive CRC following a colonoscopy that had been deemed clear of neoplasia for an incidence rate of interval cancers of 1.71 per 1000 person-years of follow-up. In 46 cases (79%), the indication for the colonoscopy that detected the cancer was routine surveillance. In nine cases, the indication was symptoms suggestive of CRC, and the other three were performed to follow-up an abnormal colonoscopic finding or at patient request. Patients found with cancer were older (mean age=67.4±6.9 years) than those without cancer (mean age=61.9±9.5 years; p=0.0003) and were more likely to be men (86.2% of those with cancer vs 71.1% of those without; p<0.0001).
Interval cancers were detected in each of the eight studies (table 3) and were distributed throughout all sections of the large bowel: 29 (50%) were on the right side of the colon and most (78%) were Stage I or II (see online supplementary material table S2). However, nine of the patients with cancer (15%) subsequently died of CRC during a median follow-up of 4.7 years in these cases. The incidence of interval cancer varied across studies ranging from a low of 1.14 cancers/1000 person-years of follow-up (NPS) to a high of 2.24 cancers per 1000 person-years of follow-up (Calcium Polyp Prevention Study). In four of the eight studies at least one subject died of CRC.
Table 3. Description of CRC cases by study.
| Study (N) | APPS (837) | CPPS (913) | AFT (1086) | PPT (2024) | WBF (1304) | NPS (939) | VA (871) | UDCA (1193) | Total |
|---|---|---|---|---|---|---|---|---|---|
| CRC, N (%/N study) | 5 (0.6) | 8 (0.9) | 6 (0.6) | 13 (0.6) | 8 (0.6) | 3 (0.3)* | 8 (0.9) | 7 (0.6) | 58 (0.6) |
| Cancer/1000 person-years follow-up | 1.52 | 2.24 | 1.77 | 1.52 | 1.77 | 1.14* | 2.14 | 1.69 | 1.71 |
| Stage I or II CRC N | 5 | 6 | 5 | 8 | 8 | 3 | 5 | 5 | 45 |
| Right sided N (%) | 2 | 4 | 4 | 5 | 5 | 3 | 5 | 1 | 29 |
| Died CRC N (%) | 0 | 2 | 0 | 2 | 2 | 0 | 3 | 0 | 9 |
Does not include two cancers detected after the first 3 years of this study during a longer period of clinical follow-up as reported elsewhere.15
AFT, Aspirin Folate Trial; APPS, Antioxidant Polyp Prevention Study; CPPS, Calcium Polyp Prevention Study; CRC, colorectal cancer; NPS, National Polyp Study; PPT, Polyp Prevention Trial; UDCA, Ursodoxycholic Acid Study; VA, Veterans Affairs Cooperative Study; WBF, Wheat Bran Fiber Study.
The clinical reviewers were generally consistent in ascribing potential explanations to the interval cancers. Of the 58 cases with cancer, four were excluded from the κ statistic calculation because chart review was required by protocol (three cases), or all reviewers agreed that chart review was necessary (one case). For the other 54 cases, there was 78% agreement between the primary and secondary reviewers (κ =0.64). The putative reason for the interval cancers was missed lesions for 30 cases (52%), incomplete adenoma resection for 11 cases (19.0%) and new cancer for 14 (24%); the remaining three cases (5%) were categorised as failed biopsy detection. Of the nine patients who died of CRC, seven were ascribed to missed lesions and two to new lesions.
Interval cancers on the right side of the colon were statistically significantly more likely to be classified as missed (19/29) than those on the left side (11/29) (p=0.04, table 4). In 16% of the cases, clinical symptoms were the indication for the exam that detected cancer (table 5). Interval cancers detected on these exams were more likely to be adjudicated as missed lesions (8/9) than those detected at routine surveillance (20/46) (p=0.01).
Table 4. Association of proposed explanation for interval cancer and location of interval cancer.
| Proposed explanation for interval cancer | Median time from baseline colonoscopy (years) | Location of interval cancer | |
|---|---|---|---|
|
|
|||
| Right (N=29)* N (%) | Left (N=29) N (%) | ||
| Missed (N=30) | 2.3 | 19 (65.5) | 11 (37.9) |
| Incomplete removal (N=11) | 2.9 | 4 (13.8) | 7 (24.1) |
| New (N=14) | 3.5 | 4 (13.8) | 10 (34.5) |
| Failed biopsy detection (N=3) | 2.0 | 2 (6.9) | 1 (1.2) |
Right colon defined as proximal to the splenic flexure.
Table 5. Clinical details of nine interval cancers detected on colonoscopy exams with clinical symptoms as the indications for the procedure.
| Age/gender | Indication† | Location | Time since prior colo (years) | Adenomas prior colo (size cm) | Stage | Died of CRC |
|---|---|---|---|---|---|---|
| 67 M | Blood in stool | Rectum | 3.5 | None | I | No |
| 75 M | Colovesical fistula | Sigmoid | 1.5 | AC (0.4) | IV | Yes |
| 74 M | Blood in stool | Cecum | 0.5 | AC (0.9) | I | No |
| 73 M | Abdominal pain | Proximal* | 2.0 | AC (0.5) and SC (0.5) | I | Yes |
| 72 M | Anaemia | Ascending | 2.2 | SC (0.4) | II | No |
| 71 M | Bleeding | Rectum | 2.4 | AC (0.25) and TC (1.0) | I | No |
| 61 M | Change in bowels | Recto-sigmoid | 2.4 | None | IV | No |
| 70 M | T1 symptoms | Hepatic flexure | 1.2 | SC (12.5) and 3 Ce (0.5) | IV | Yes |
| 71 M | Weight loss; haematochezia | Hepatic flexure | 2.5 | None | II | Yes |
Precise location unspecified.
Indication field is derived from the pooled data set and was the indication abstracted from the indication field of the colonoscopy report.
AC, ascending colon; Ce, cecum; CRC, colorectal cancer; SC, sigmoid colon; TC, transverse colon.
Discussion
Using pooled data on 9167 adenoma patients from eight large North American studies, we identified 58 individuals diagnosed with CRC in a short interval (median follow-up 4 years) after a complete colonoscopy and polypectomy that was intended to have left the colon free of neoplasia. Upon review, nearly three-quarters of these cancers were judged to be the possible result of a missed lesion, incomplete adenoma resection or failed biopsy detection, and thus were potentially preventable or detectable at an earlier stage.
Several studies 13–16,30–38 have reported on incident cancers in patients under colonoscopic surveillance, including four of the trials included in this report.13–15,38 Overall, there is some heterogeneity in the incidence rates reported across these studies and some have proposed explanations35 for these observed differences. Study specific factors such as the composition of the population (ie, whether individuals with large polyps were excluded) and whether or not participant follow-up ended with surveillance endoscopy are likely important. For example, studies that exclude patients with very large polyps15,31 tend to report lower incidence rates of cancer. The frequency of colonoscopic examination may also be an important factor since more frequent colonoscopy affords greater opportunity to detect small asymptomatic cancers. Of the eight studies included in our analyses, the one with the highest observed cancer incidence20 also had the largest percentage of participants with one or more colonoscopies during the study period. Conversely, the study with the lowest observed cancer incidence25 had the lowest percentage of participants with one or more colonoscopies during the study period.
To our knowledge, only one prior study has categorised early interval cancers as to the reasons for their occurrence.13 The PPT investigators examined 13 interval cancers, all of whom are also included in our current report, and estimated that seven were potentially ‘avoidable’ because they were categorised as incomplete removals or missed lesions. We employed similar methodology, but we required a slightly longer dwell time for a lesion to be adjudicated as a new cancer (36 vs 30 months) and did not consider the stage of the detected cancer in our determination. Also, unlike the PPT, we judged most cases based solely upon colonoscopy data (eg, polyp size, location, histology) recorded in the pooled data set—not the actual endoscopy and pathology reports. Only for potential cases of failed biopsy detection was the primary data reviewed to distinguish these cases from cases of incomplete resection. Overall, compared with the PPT report, we ascribed a higher percentage of interval cancers as missed lesions (52% vs 23%) and a lower percentage as ‘failed biopsy detection’ cases (5% vs 23%). We did not allow ‘failed biopsy detection’ as an explanation if more than 1 year had transpired between colonoscopies (based on our assumption that a shorter interval would have been recommended if there were concerns about missed cancer). Two of the three cases adjudicated as ‘failed biopsy detection’ in the PPT report occurred in cases where more than 1 year transpired between colonoscopies, and thus we assigned a different putative explanation for similar cases. Had we assumed, like the PPT investigators, that more advanced stage lesions were likely missed, we would have further increased the number of our cancers adjudicated as ‘missed’. These differences attest to the difficulty of ascertaining the reasons for the occurrence of interval cancers.
While it is impossible to know the exact frequency of important missed lesions during colonoscopy, they unarguably occur. In one study, using a tandem colonoscopy design, adenoma miss rates of 6% for lesions ≥1 cm were observed,39 and a meta-analysis of tandem colonoscopy studies estimated that 2% of large adenomas are missed.40 In studies of CT colonography that used segmental unblinding, optical colonoscopy missed 11%–17% of lesions ≥1 cm.41–43 More than a half of the interval cancers in this pooled analysis were adjudicated as likely missed lesions. Also, in our analysis, symptomatic cancers were more likely to be classified as missed on the prior examination.
Inadequate bowel preparation for colonoscopy is one plausible explanation for missing significant neoplasia at the time of colonoscopy. One study, using data from the Clinical Outcomes Research Initiative database, examined the association of bowel preparation quality and adenoma detection rates in 93 004 colonoscopies and found that adequate preparation was associated with a higher rate of colorectal lesion detection (OR=1.21 for detection of one or more lesions; 95% CI 1.16 to 1.25).44 In the NPS, the baseline colonoscopy was deemed inadequate in 13% of the cases and therefore was repeated. This factor may have contributed to the lower rates of subsequent cancer incidence relative to other studies, although none of the studies in our pooled analysis included subjects whose colonoscopy preparation was known to be inadequate. After the first year of follow-up, cancer incidence rates observed in the other studies included in our pooled analysis were similar to those observed in the NPS, supporting the importance of the quality of the baseline clearing colonoscopy.45
The contribution of flat and depressed colorectal polyps to the occurrence of interval cancers, likely through the mechanism of missed lesions, has been a focus of intense interest and debate.46 While these lesions have long been described in the Japanese literature, there has been controversy about the frequency of their occurrence in Western populations. One US study analysing a veteran population suggested that non-polypoid colorectal neoplasms accounted for nearly 10% of the adenomas removed and that these lesions were more likely to contain in situ or submucosal carcinoma than their polypoid counterparts (OR, 11.1; 95% CI 4.98 to 24.8).47 However, a study using NPS data48 concluded that such lesions have long been recognised, but often termed ‘sessile’ rather than flat. When all baseline ‘sessile’ polyps in the NPS were recategorised based upon review of pathology specimens, 27% would have met formal definition as a ‘flat’ adenoma. Patients with these flat lesions were not at a significantly higher risk for advanced adenomas at baseline or at the 3-year follow-up exam. We cannot specifically comment on the role of flat polyps, if any, in the current pooled study as polyp shape was not a variable abstracted into the pooling study data repository.
Another potential explanation for early interval cancer is incomplete resection of prior adenomas, and we judged that 11 (19%) of our interval cancers were in this category. Our findings are generally consistent with a retrospective study from a single VA centre in which 12 of 45 interval cancers (27%) occurred in the same segment where a polyp had been found on the prior exam.17 Incomplete resection is likely to be particularly relevant to large lesions. Our research group has previously reported that the risk of advanced neoplasia was higher in patients with baseline adenomas ≥20 mm, highlighting the importance of this factor.27
Current guidelines suggest short term follow-up (eg, 3–6 months) for sessile lesions removed piecemeal to assure complete resection.49 The importance of incomplete resection is increasingly being recognised. In one recent study, 345 adenomatous polyps were removed and margins were biopsied to assess adequacy of resection; 10% showed evidence of residual adenoma.50 One reason for the relatively low cancer incidence observed in the NPS could be that the study excluded patients with large (>3 cm) polyps, which are the ones most likely to be incompletely resected. The parent clinical trials composing the pooled cohort required that each participant's colon be believed free of residual neoplasia at study entry. Those individuals recently undergoing resection of large sessile polyps where there was concern of residual neoplasia were not included. Our observed rate of interval neoplasia would likely be higher had such individuals been enrolled into the studies.
A limitation of our study is that the adjudication of the reasons for the interval cancers relied on our algorithm, which necessarily made assumptions about the course of colorectal carcinogenesis (eg, the dwell time from adenoma to cancer). Thus, it is impossible to know the actual natural history of the interval cancers in our study, and we likely have misclassified some cases. For example, we adjudicated many cancers as due to missed lesions only because they were found within 3 years of the prior colonoscopy at which no adenoma was identified in the same segment; however, a new cancer could have developed from visually-normal mucosa during that time. Adenoma dwell time (ie, from adenoma incidence to cancer) has recently been estimated in three microsimulation models51 and our time window of 3 years is lower than those estimates which range from 7.6 to 24.2 years. To the extent that we have underestimated adenoma dwell time in developing our algorithm, we have reduced the number of cases explained by ‘missed’ and increased those explained as ‘new’. Similarly, we cannot exclude that some cancers adjudicated as incomplete resection represent new cancers growing in the area where a prior adenoma was removed. Also, to be consistent with terminology used in prior studies we based our algorithm on one previously published.13 Distinguishing cases of ‘failed biopsy detection’ from ‘incomplete resection’ is difficult by record review, since the determination relies partly on the endoscopist's suspicion of residual cancer being present at the site of a prior biopsy. One could argue that a truly ‘failed biopsy’ would detect only benign tissue, but for consistency with previous research, we retained the prior definition. Although the use of an algorithm to assign possible explanation for the observed cancers has limitations, the algorithm was developed prior to review, and adjudication of the cases was done independently with good agreement between reviewers.
Also it is important to note that we have not tested tumour tissue for either microsatellite instability (MSI) or CpG island methylator phenotype (CIMP) and cannot determine whether these mechanistic pathways are playing a role in the cases described here. In patients with hereditary non-polyposis CRC, cancers with loss of mismatch repair gene function have accelerated tumour growth.52 It is possible that this extends to sporadic MSI-high cancer. One study has reported that early interval cancers are three times more likely to have evidence of MSI-high MSI as compared with non-interval cancers.53 There is also evidence that interval cancers are more likely to demonstrate CIMP than non-interval cancers.54
A final limitation of our work relates to the composition of the pooled cohort itself. All participants were enrolled in clinical studies and the colonoscopy exams may have been performed differently than those in general practice. In fact, our absolute rate of observed cancer (0.6%) after colonoscopy is significantly less than an estimate based on Medicare administrative data (7.2%).55 Also, the trials enrolling these participants were performed many years ago prior to improvements in endoscope technology (eg, high definition endoscopes) and emphasis on issues of colonoscopy quality (eg, adenoma detection rate, withdrawal time). Finally, our cohort was not equally balanced with respect to gender (71% male). There is a prior study that demonstrated missed lesions, particularly on the right side of the colon, occur more frequently in women. Overall, we found that male gender was associated with an increased risk for postcolonoscopy CRC. That study largely included individuals without prior colonoscopy, and so their findings may differ from ours in part because of this.
In conclusion, we estimate that approximately 6/1000 individuals were diagnosed with interval cancer within an average of 4 years following a complete colonoscopy with removal of ‘all’ polyps. Our review suggests that many of the interval cancers might have been found earlier or perhaps excised while still an adenoma at the prior colonoscopy exam. The large pooled patient population drawn from studies with prospectively collected data and high rates of complete colonoscopy follow-up all enhance the value of the results reported here. These results emphasise the importance of performing colonoscopic examinations with meticulous attention to the identification and complete removal of all suspected neoplasms.
Supplementary Material
Significance of this study.
What is already known on this subject?
Colonoscopy is highly sensitive for the detection of polyps and cancer.
However, colorectal cancer has been detected in relatively short intervals after colonoscopy that deemed the colon free of cancer.
The likely explanation for these postcolonoscopy cancers is not known.
What are the new findings?
Approximately 6/1000 individuals were diagnosed with interval cancer within an average of 4 years following a complete colonoscopy.
Missed lesions and incompletely resected lesions appeared to account for about 70% of postcolonoscopy cancers in our series.
How might it impact on clinical practice in the foreseeable future?
Meticulous colonoscopy technique including mucosal inspection and complete lesion resection are likely the most important factors in reducing the frequency of early interval cancer.
Acknowledgments
The authors are indebted to all the patients, staff and clinical investigators at the individual study sites. The authors thank Erin Ashbeck and Fang Wang for their contributions to the preparation of the manuscript.
Funding This work was supported by Public Health Service grants CA-41108, CA-23074, CA95060, CA37287, CA104869, CA23108, CA59005 and CA26852 from the National Cancer Institute. Funding for the Veteran's Affairs Study was supported by the Cooperative Studies Program, Department of Veterans Affairs. Dr Robertson's work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development Career Development Award.
Footnotes
Contributors DJR: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content. DAL and JAB: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding. SJW and ERG: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; technical, or material support; study supervision. DJA: study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. AS: study concept and design; acquisition of data; analysis and interpretation of data. AJC and PL: acquisition of data; critical revision of the manuscript for important intellectual content. AGZ: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; technical, or material support. TRC: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. MEM: study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content; obtained funding.
Competing interests DJR: Given Imaging (Advisory Board); DAL, SJW, DJA, JAB, AS, AJC, AGZ, TRC, PL, ERG and MEM: none.
Disclaimer The contents of this work do not represent the views of the Department of Veterans Affairs or the US Government.
Ethics approval UCSD IRB.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 3.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 4.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 5.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–71. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 6.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343:1603–7. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 7.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baxter NN, Warren JL, Barrett MJ, et al. Association between colonoscopy and olorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. J Clin Oncol. 2012;30:2664–9. doi: 10.1200/JCO.2011.40.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154:22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 10.Bressler B, Paszat LF, Chen Z, et al. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007;132:96–102. doi: 10.1053/j.gastro.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Baxter NN, Sutradhar R, Forbes SS, et al. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011;140:65–72. doi: 10.1053/j.gastro.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 362:1795–803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 13.Pabby A, Schoen RE, Weissfeld JL, et al. Analysis of colorectal cancer occurrence during surveillance colonoscopy in the dietary Polyp Prevention Trial. Gastrointest Endosc. 2005;61:385–91. doi: 10.1016/s0016-5107(04)02765-8. [DOI] [PubMed] [Google Scholar]
- 14.Robertson DJ, Greenberg ER, Beach M, et al. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology. 2005;129:34–41. doi: 10.1053/j.gastro.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 16.Haseman JH, Lemmel GT, Rahmani EY, et al. Failure of colonoscopy to detect colorectal cancer: evaluation of 47 cases in 20 hospitals. Gastrointest Endosc. 1997;45:451–5. doi: 10.1016/s0016-5107(97)70172-x. [DOI] [PubMed] [Google Scholar]
- 17.Farrar WD, Sawhney MS, Nelson DB, et al. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol. 2006;4:1259–64. doi: 10.1016/j.cgh.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Alberts DS, Martinez ME, Hess LM, et al. Phase III trial of ursodeoxycholic acid to prevent colorectal adenoma recurrence. J Natl Cancer Inst. 2005;97:846–53. doi: 10.1093/jnci/dji144. [DOI] [PubMed] [Google Scholar]
- 19.Alberts DS, Martinez ME, Roe DJ, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. N Engl J Med. 2000;342:1156–62. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- 20.Baron JA, Beach M, Mandel JS, et al. Calcium supplements for the prevention of colorectal adenomas. N Engl J Med. 1999;340:101–7. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 21.Baron JA, Cole BF, Sandler RS, et al. A aandomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg ER, Sporn MB. Antioxidant Vitamins, Cancer, and Cardiovascular Disease. N Engl J Med. 1996;334:1189–90. doi: 10.1056/NEJM199605023341810. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman DA, Weiss DG, Bond JH, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans affairs cooperative study group 380. N Engl J Med. 2000;343:162–8. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 24.Schatzkin A, Lanza E, Corle D, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. N Engl J Med. 2000;342:1149–55. doi: 10.1056/NEJM200004203421601. [DOI] [PubMed] [Google Scholar]
- 25.Winawer SJ, Zauber AG, O'Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med. 1993;328:901–6. doi: 10.1056/NEJM199304013281301. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg ER, Baron JA, Tosteson TD, et al. A clinical trial of antioxidant vitamins to prevent colorectal adenoma. Polyp prevention study group. N Engl J Med. 1994;331:141–7. doi: 10.1056/NEJM199407213310301. [DOI] [PubMed] [Google Scholar]
- 27.Martinez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832–41. doi: 10.1053/j.gastro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 29.Brenner H, Hoffmeister M, Arndt V, et al. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2010;102:89–95. doi: 10.1093/jnci/djp436. [DOI] [PubMed] [Google Scholar]
- 30.Bertario L, Russo A, Sala P, et al. Predictors of metachronous colorectal neoplasms in sporadic adenoma patients. Int J Cancer. 2003;105:82–7. doi: 10.1002/ijc.11036. [DOI] [PubMed] [Google Scholar]
- 31.Citarda F, Tomaselli G, Capocaccia R, et al. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 2001;48:812–15. doi: 10.1136/gut.48.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jorgensen OD, Kronborg O, Fenger C, et al. Influence of long-term colonoscopic surveillance on incidence of colorectal cancer and death from the disease in patients with precursors (adenomas) Acta Oncol. 2007;46:355–60. doi: 10.1080/02841860600897918. [DOI] [PubMed] [Google Scholar]
- 33.Kahi CJ, Imperiale TF, Juliar BE, et al. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2009;7:770–5. doi: 10.1016/j.cgh.2008.12.030. quiz 11. [DOI] [PubMed] [Google Scholar]
- 34.Leung K, Pinsky P, Laiyemo AO, et al. Ongoing colorectal cancer risk despite surveillance colonoscopy: the Polyp Prevention Trial Continued Follow-up Study. Gastrointest Endosc. 2010;71:111–17. doi: 10.1016/j.gie.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loeve F, van Ballegooijen M, Snel P, et al. Colorectal cancer risk after colonoscopic polypectomy: a population-based study and literature search. Eur J Cancer. 2005;41:416–22. doi: 10.1016/j.ejca.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Lund JN, Scholefield JH, Grainge MJ, et al. Risks, costs, and compliance limit colorectal adenoma surveillance: lessons from a randomised trial. Gut. 2001;49:91–6. doi: 10.1136/gut.49.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meagher AP, Stuart M. Does colonoscopic polypectomy reduce the incidence of colorectal carcinoma? Aust N Z J Surg. 1994;64:400–4. doi: 10.1111/j.1445-2197.1994.tb02238.x. [DOI] [PubMed] [Google Scholar]
- 38.Lieberman DA, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077–85. doi: 10.1053/j.gastro.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–8. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 40.van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343–50. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 41.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 42.van Gelder RE, Florie J, Stoker J. Colorectal cancer screening and surveillance with CT colonography: current controversies and obstacles. Abdom Imaging. 2005;30:5–12. doi: 10.1007/s00261-004-0249-5. [DOI] [PubMed] [Google Scholar]
- 43.Van Gelder RE, Nio CY, Florie J, et al. Computed tomographic colonography compared with colonoscopy in patients at increased risk for colorectal cancer. Gastroenterology. 2004;127:41–8. doi: 10.1053/j.gastro.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 44.Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76–9. doi: 10.1067/mge.2003.294. [DOI] [PubMed] [Google Scholar]
- 45.Zauber AG, Winawer SJ. High-quality colonoscopies must be an integral part of screening and surveillance programs. Gastroenterology. 2006;130:620–1. doi: 10.1053/j.gastro.2006.01.014. author reply 1–2. [DOI] [PubMed] [Google Scholar]
- 46.Lau PCP, Sung JJY. Flat adenoma in colon: Two decades of debate. J Dig Dis. 2010;11:201–7. doi: 10.1111/j.1751-2980.2010.00439.x. [DOI] [PubMed] [Google Scholar]
- 47.Soetikno RM, Kaltenbach T, Rouse RV, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027–35. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 48.O'Brien MJ, Winawer SJ, Zauber AG, et al. Flat adenomas in the national polyp study: is there increased risk for high-grade dysplasia initially or during surveillance? Clin Gastroenterol Hepatol. 2004;2:905–11. doi: 10.1016/s1542-3565(04)00392-1. [DOI] [PubMed] [Google Scholar]
- 49.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin. 2006;56:143–59. doi: 10.3322/canjclin.56.3.143. quiz 84–5. [DOI] [PubMed] [Google Scholar]
- 50.Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy—results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74–80. doi: 10.1053/j.gastro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 51.Kuntz KM, Lansdorp-Vogelaar I, Rutter CM, et al. A systematic comparison of microsimulation models of colorectal cancer: the role of assumptions about adenoma progression. Med Decis Making. 2011;31:530–9. doi: 10.1177/0272989X11408730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vasen HF, Nagengast FM, Khan PM. Interval cancers in hereditary non-polyposis colorectal cancer (Lynch syndrome) Lancet. 1995;345:1183–4. doi: 10.1016/s0140-6736(95)91016-6. [DOI] [PubMed] [Google Scholar]
- 53.Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology. 2006;131:1700–5. doi: 10.1053/j.gastro.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 54.Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol. 105:1189–95. doi: 10.1038/ajg.2009.699. [DOI] [PubMed] [Google Scholar]
- 55.Cooper GS, Xu F, Barnholtz Sloan JS, et al. Prevalence and predictors of interval colorectal cancers in Medicare beneficiaries. Cancer. 2012;118:3044–52. doi: 10.1002/cncr.26602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


