1. Introduction
Stroke, a leading cause of death and long-term disability, is a public health problem worldwide. Globally, there are an estimated 15 million strokes, leading to nearly 5 million deaths and another 5 million cases of permanent disability per year.1 Because of the increasing size of the elderly population and increasing prevalence of major risk factors such as hypertension and obesity, stroke is predicted to continuously increase.2 Moreover, the mortality rates of stroke have kept increasing in some countries in recent decades.2 Although the US and some European countries experienced decreasing stroke mortality rates in the same period,2-4 the decreasing stroke mortality rate and the increasing size of the elderly population increase the long-term disability among stroke survivors.5
Many studies have found high direct costs associated with stroke, including costs for inpatient stays, outpatient visits, rehabilitation, medications, and nursing home, etc. For example, total annual direct costs were estimated at $22.8 billion in 2009 for the US6 and €26.6 billion in 2010 for the EU plus Iceland, Norway, and Switzerland.7 Far fewer studies have considered the indirect costs of stroke, including productivity loss due to morbidity and mortality, and costs of informal caregiving usually provided by unpaid family members, although the indirect costs has been claimed to be large.8
To better understand the total economic burden of stroke, especially the indirect costs of stroke consisting of productivity loss and informal caregiving costs, we examined peer-reviewed publications of the past two decades, including an analysis of the indirect cost. The information we present here will be useful to decision makers in public health, and researchers for developing strategies for stroke prevention, treatment, and rehabilitation.
2. Methods
We performed a comprehensive literature search of peer-reviewed journal articles published in English between January 1990 and September 2012 by using the databases PubMed, MEDLINE, and EconLit. We augmented the search by using Google Scholar and checking the references of the articles we obtained. Keywords for the search included stroke, cerebrovascular disease, subarachnoid hemorrhage, intracerebral hemorrhage, cost-of-illness, productivity loss, indirect cost, economic burden, and informal caregiving. We investigated two main categories of indirect cost, productivity loss and informal care cost. Productivity loss consisted of loss due to premature death (mortality cost) and the cost of disability because of the reduced productivity of stroke survivors (morbidity cost).9 The cost of informal caregiving is the value of time spent by family members or other caregivers that is not considered to be part of the care given by formal health care providers.10 A cost for care provided by formal health care providers such as a home health aide is considered to be a direct cost. Because the proportion of total cost that was represented by indirect cost is a useful indicator measuring the importance of indirect costs estimation, we included cost-of-illness (COI) studies with sufficient analyses of the indirect cost. COI studies estimate the value of all resources spent or foregone, including health care cost and productivity loss, due to stroke.
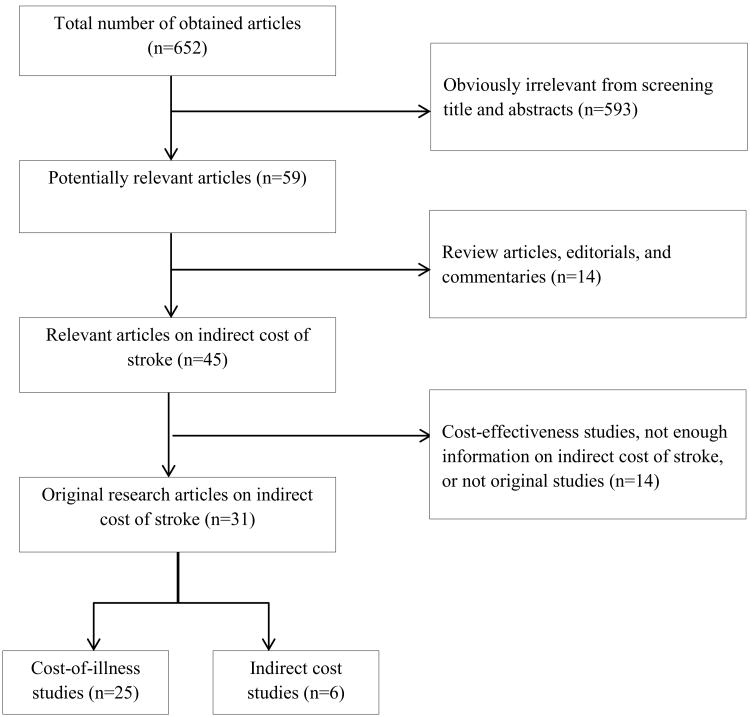
Figure 1 shows the algorithm used for selecting studies for this review. The initial review of titles and abstracts excluded studies that: (a) were not about stroke, (b) assessed the burden of stroke using nonmonetary terms, such as hours of caregiving or emotional distress, or (c) were only about direct medical costs. In addition, we excluded review articles, editorials, and commentaries. We completed full-text review of all articles that passed the initial review and finalized the set of original research articles for this study by further excluding studies that: (a) were focused on cost-effectiveness; (b) used an unspecified indirect cost for stroke within broad disease categories, such as cardiovascular disease or brain disorders; (c) were about direct costs only, such as studies that included the cost of informal caregiving as a part of direct cost and did not specify indirect costs at all; and (d) were not original studies. We included articles on cardiovascular diseases and brain disorders if the indirect costs of stroke were estimated separately.
Figure 1. Diagram for selection of studies on the indirect cost of stroke, 1990-2012.
We investigated three types of study designs. First, we investigated whether a study is a prevalence based or an incidence based study. A prevalence-based study examines the costs incurred during a given time period regardless of the date of stroke onset, while an incidence-based study estimates costs of new onset of stroke within a specific period of time for defined lengths of follow-up (lifetime, one year, or six months).11
Next, for estimating the productivity loss, there are two approaches: the human capital approach (HCA), which estimates forgone earnings due to stroke as the productivity loss,12, 13 and the friction approach (FA), which assumes a friction cost, a cost associated with the replacement of workers including productivity losses due to substitution of workers or the training costs of new employees, as the productivity loss.
Last, for estimating the cost of informal caregiving, we found two methods: the opportunity cost (OC) approach and the replacement approach (RA). The cost of informal caregiving under the OC approach is estimated by using the value of each activity that informal caregivers forego in order to provide informal care.10, 14 In contrast, the RA, also known as the proxy good method, assumes that an informal caregiver substitutes for a paid caregiver who would have provided the same type of caregiving services.10, 14
To compare indirect costs of different countries in different study period, we derived 2012 US dollar value by using consumer price indices of study countries in the years of costs and in 2012 from the World Bank and purchasing power parity (PPP) exchange rate in 2012 from the Organisation for Economic Co-operation and Development (OECD).15
3. Results
In all, 31 original articles were selected for our review. Six of them solely investigated the indirect cost, 10, 14, 16-19 and the remaining 25 were COI studies, which included both the direct and indirect costs(Table 1) 7, 9, 20-42. Among the six studies focusing on indirect costs, four investigated the costs of informal caregiving, one studied mortality cost, and one studied morbidity cost. None of them examined both the productivity loss and the cost of informal caregiving.
Table 1. Type of indirect costs of stroke investigated in the literature, 1990-2012.
| Study type | Country | First author/Year of publication | Type of indirect costs included | ||
|---|---|---|---|---|---|
|
|
|||||
| Mortality | Morbidity | Informal caregiving | |||
| Cost-of-illness | US | Heidenreich 20, 2011 | Yes | Yes | No |
| US | Brown 21, 2006 | Yes | Yes | Yes | |
| US | Taylor 9, 1996 | Yes | Yes | No | |
| US | Wiebers 22, 1992 | Yes | Yes | Yes | |
| Canada | Chan 23, 1998 | Yes | Yes | No | |
|
|
|||||
| Germany | Dodel 24, 2010 | Yes | Yes | Yes | |
| Germany | Rossnagel 25, 2005 | No | Yes | No | |
| Germany | Weimar 26, 2003 | No | Yes | No | |
| Ireland | Smith 27, 2012 | Yes | Yes | Yes | |
| Italy | Pugliatti 28, 2008 | Yes | Yes | Yes | |
| Italy | Gerzeli 29, 2005 | Yes | Yes | Yes | |
| Netherlands | Evers 30, 1997 | Yes | Yes | No | |
| Spain | Lopez-Bastida 31, 2012 | No | Yes | Yes | |
| Spain | Navarrete-Navarro 32, 2007 | Yes | Yes | Yes | |
| Sweden | Persson 33, 2012 | Yes | Yes | Yes | |
| Sweden | Ghatnekar 34, 2004 | Yes | Yes | No | |
| Sweden | Zethraeus 35, 1999 | No | Yes | No | |
| Sweden | Terént 36, 1994 | Yes | Yes | No | |
| UK | Saka 37, 2009 | Yes | Yes | Yes | |
| UK | Luengo-Fernández 38, 2006 | Yes | Yes | Yes | |
| EU* | Gustavsson 7, 2011 | Yes | Yes | Yes | |
| EU | Leal 39, 2006 | Yes | Yes | Yes | |
|
|
|||||
| Australia | Cadilhac 40, 2009 | Yes | Yes | Yes | |
| Australia | Dewey 41, 2001 | Yes | Yes | Yes | |
| New Zealand | Scott 42, 1994 | No | Yes | Yes | |
|
| |||||
| Indirect cost | US | Hickenbottom, 2002 | No | No | Yes |
| US | Fox 16, 1996 | Yes | No | No | |
|
|
|||||
| Sweden | Lindgren 18, 2008 | No | Yes | No | |
| Netherlands | van den Berg 14, 2006 | No | No | Yes | |
|
|
|||||
| Australia | Dewey 10, 2002 | No | No | Yes | |
|
|
|||||
| Thailand | Riewpaiboon 19, 2009 | No | No | Yes | |
Notes: Cost-of-illness (COI) studies included both direct and indirect cost analyses. Indirect cost studies include analysis of indirect costs only. EU: European Union; UK: United Kingdom; US: United States.
European Union plus Iceland, Norway, and Switzerland
As shown in Table 2, which summarized the data sources used to estimate the indirect costs, the US studies relied on national-level survey data, such as Census data or the National Health and Nutrition Examination Survey (NHANES), or area-specific surveillance data for the estimation of incidence or prevalence rates of stroke. To estimate the productivity loss or costs of informal caregiving, government data or national-level survey data were used. Non-US studies used various data sources, such as hospital and local area data and national surveys.
Table 2. Data sources used in the literature of indirect costs of stroke, 1990-2012.
| Country/References | Prevalence/incidence | Productivity loss and informal care cost |
|---|---|---|
| US9, 16, 17, 20-22 |
|
|
| Canada23 |
|
|
| Germany24-26 |
|
|
| Ireland27 |
|
|
| Italy28, 29 |
|
|
| Netherlands14, 30 |
|
|
| Spain 31, 32 |
|
|
| Sweden18, 33-36 |
|
|
| UK37, 38 |
|
|
| Australia 10, 40, 41 |
|
|
| New Zealand42 |
|
|
| Thailand19 |
|
|
Notes: When a data source is previous literature, we did not include it as a data source in this table. UK: United Kingdom; US: United States.
Table 3 presents methods and results of the COI studies, 12 prevalence-based studies and 13 studies based on incidence, while Table 4 summarized studies dealing with indirect costs only.
Table 3. Methods and results of cost-of-illness studies for stroke patients, 1990-2012.
| Prevalence-based studies | ||||||||
|
First author/ year of publication/country |
Patients |
Study designb |
Year of cost (Study period) |
Annual indirect cost in millions |
Proportion of total cost represented by indirect cost, %d (Study period) |
|||
| Stroke typea |
Age range |
Local currency (Study period) |
2012 USDc (Study period) |
|||||
| Heindenreich, 2011, US20 | All types (as a part of CVD) | All | HCA | 2008 (2010∼2030) | 25,600 (2010) e 44,400 (2030) e | 27,299 (2010) e 47,347 (2030) e | 47 (2010) 32 (2030) | |
| Brown, 2006, US21 | IS | 45∼64 | HCA, RA | 2005 (2005∼2050) | 1,177,000f (2005∼2050) | 1,383,736f (2005∼2050) | 53 (2005∼2050) | |
| Chan, 1998, Canada (Ontario)23 | All types (CI, ICH, SAH, TIA) | Under 75 | HCA | 1994∼95 | 328e | 381 e | 38 | |
| Smith, 2012, Ireland27 | All types and TIA | All | HCA, OC | 2007 | 143∼248 g | 176∼305 g | 29–31 | |
| Gustavsson, 2011, European countries7 | All types (as a part of brain disorders) | Unclear | Unclear | 2010 | 4,932g, h | N/Aj | 8 | |
| Saka, 2009, UK37 | All types | Under 65 | HCA, RA | 2005 | 3,754 | 6,784 | 42 | |
| Pugliatti, 2008, Italy28 | All types (as a part of brain disorders) | Unclear | HCA, RA | 2004 | 792 g, h | 1,189 g, h | 23 | |
| Leal, 2006, EU39 | All types (as a part of CVD) | All | HCA/FA, OC | 2003 | 13,285 g | 19,508 g | 39 | |
| Luengo-Fernandez, 2006, UK38 | All types (as a part of CVD) | All | HCA/FA, OC | 2004 | 3,328 (HCA) 2,770 (FA) | 6,137 (HCA) 5,108 (FA) | 39 (HCA) 35 (FA) | |
| Evers, 1997, Netherlands30 | All types | 18∼64 | HCA | 1993 | 566e | 1,012e | 22 | |
| Terént, 1994, Sweden36 | All types | Under 65 | HCA | 1991 (1983) | 2,430 e | 379 e | 24 | |
| Scott, 1994, New Zealand42 | IS | 15–64 (n=912) | HCA, OC/RA | 1992 | 6∼14 h | 7∼14 h | 6∼9 | |
| Incidence-based studies | ||||||||
|
First author/ year of publication/ country |
Patients |
Study designb |
Year of cost (Study period) |
Annual indirect cost in millions |
Per patient indirect cost, local currency (2012 USD)c |
Proportion of total cost represented by indirect cost,%d |
||
|
Stroke typea |
Age (sample size) i |
Local currency |
2012 USDc | |||||
| Wiebers, 1992, US22 | Unruptured intracranial aneurysms, aneurysmal SAH | All (n=10,300/17,250) | lifetime, HCA | Unclear (1979,1984,1989) | 309.8 (unruptured aneurysm), 1,242.5 (SAH) | 507 (unruptured aneurysm), 2033 (SAH)k | N/A | 59 (unruptured aneurysm), 71 (SAH) |
| Taylor, 1996, US9 | First SAH, ICH,IS | All (n=N/A) | lifetime, HCA | 1990 | 23,600e | 41,470 e | N/A | 58 |
| Navarrete-Navarro, 2007, Spain32 | First ICH survivors | All (n=425) | lifetime, HCA, RA | 2004 | N/A | N/A | 31,108g (54,067) | 67 |
| Ghatnekar, 2004, Sweden34 | First all type | Under 65 (n=4357) | lifetime, HCA | 2000 (first 6 months in 1997) | N/A | N/A | 163,694 (male)e 86,586 (female) e (22,243/11,765) | 25 (male) 14 (female) |
| Lopez-Bastida, 2012, Spain31 | All type | Under 65 (n=94) | 1 year, HCA, OC | 2004 | N/A | N/A | 12,449 g (21,637) | 71 |
| Persson, 2012, Sweden (VästraGötaland county)33 | First ICH, CI, stroke | All (n=3074) | 1 year, HCA, OC | 2008 | 97 | 11 | N/A | 15 |
| Dodel, 2010, Germany24 | SAH | Under 65 (n=101) | 1 year, HCA, RA | 2009 (2004∼2005) | N/A | N/A | 17,350 g (22,850) | 45 |
| Rossnagel, 2005, Germany25 | All types and TIA | 18∼64 (n=383) | 1 year, HCA | 2002 | N/A | N/A | 2,014e, g (2,960) | 18 |
| Weimar, 2003, Germany26 | ICH | All (n=266) | 1 year, HCA | Unclear (Jan. 1998 ∼ Oct. 1999) | N/A | N/A | 5,537 e, g, k (8,053) | 55 |
| Zethraeus, 1999, Sweden35 | All types (as a part of CVD) | Under 64 (n=12) | 1 year, HCA | 1994 (1993∼1995) | N/A | N/A | 71,731e (10,229) | N/A |
| Gerzeli, 2005, Italy29 | First IS and hemorrhage | Over 18 (n=449) | 6 months, HCA, OC/RA | 1998 | N/A | N/A | 5,026 g (8,691) | 43 |
| Cadilhac,2009, Australia40 | First IS and ICH | Unclear (n=4291/27660) | lifetime, FA | 2004 | 12.8 (ICH)h 46.5 (IS) h | 10 (ICH)h 38 (IS) h | N/A | 5 (ICH) 3 (IS) |
| Dewey, 2001, Australia41 | First CI, ICH, and unclassified stroke | Under 65 (n=165) | 1 year, FA, OC | 1997 (May 1996 ∼April 1997) | 56 | 56 | N/A | 10 |
Notes:
CVD (cardiovascular disease, includes hypertension, coronary heart disease, heart failure, stroke, and all other cardiovascular disease); ICH (intracerebral hemorrhagic stroke); IS (ischemic stroke); SAH (subarachnoid hemorrhage); TIA (transient ischemic attack); CI (cerebral infarction); N/A (not available).
Estimation approaches for productivity loss: HCA (the human capital approach), and FA (the friction cost approach). Estimation approaches for the time cost of informal caregiving: OC (the opportunity cost approach), and RA (the replacement approach).
Indirect cost in 2012 USD is estimated by using consumer price indices of study countries in the years of cost and in 2012 from the World Bank (http://data.worldbank.org/indicator/FP.CPI.TOTL) and purchasing power parity (PPP) exchange rate in 2012 from OECD website (http://stats.oecd.org/Index.aspx?datasetcode=SNA_TABLE4).
The proportion of total cost represented by indirect cost = (indirect cost/total cost) × 100. Total cost includes both direct and indirect cost.
Cost of informal caregiving is not included in indirect cost. Also, the total cost does not include the cost of informal caregiving.
Costs are accumulated costs during study periods.
Euro is used as a local currency.
The cost of informal caregiving is included in direct cost and is not separable from that cost. In the calculation of the proportion of total cost represented by indirect cost, the cost of informal caregiving is included in the total cost but not in indirect cost.
When the sample size of the indirect cost is different from the total sample size, the sample size for indirect cost is shown.
Because indirect cost of each country was not provided, we cannot estimate indirect cost in 2012 ISD.
We assume that the year of cost is the publication year.
Table 4. Methods and results of indirect cost studies for stroke patients, 1990- 2012.
| First author/ year of publication/ country |
Patients | Study designc |
Year of cost (Study period) |
Type of indirect cost |
Annual indirect cost in millions |
Annual per-patient indirect cost |
|||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Stroke typea | Age (Sample size)b |
Local currencyc |
2012 USDc, d |
Local currencyc |
2012 USDc, d | ||||
| Fox,1996, US (California)16 | Cerebrovascular disease (as a part of CVD) | All (n=15,222) | Incidence (1 year), HCA | 1991 (1989–1991) | Mortality | 1,082 | 1,824 | 71,081 | 119,829 |
| Hickenbottom, 2002, US17 | All types | Over 70 (n=7,443) | Prevalence, RA | 1999 | Informal care | 6,100 | 8,408 | 3,500–8,200 | 4,824–11,302 |
| Dewey, 2002, Australia10 | First CI, ICH, and unclassified | N/A (n=340) | Incidence (lifetime), OC/RA | 1997 | Informal care | 332 (OC) 171 (RC) | 329 (OC) 170 (RC) | N/A | N/A |
| van den Berg, 2006, Netherlands14 | All types | All (n=218) | Incidence (6 months), OC/RA | 2001 | Informal care | N/A | N/A | 17,482 (OC)e 12,440 (RA)e | 23,451 (OC)e 16,687 (RA)e |
| Lindgren, 2008, Sweden18 | IS | 18–76 (n=275) | Incidence (1 year), HCA | 2006 | Morbidity | N/A | N/A | 120,000 | 14,963 |
| Riewpaiboon 2009, Thailand19 | All types | All (n=149) | Prevalence, OC | 2006 (2001–2005) | Informal care | N/A | N/A | 42,336–68,052 | 904–1,453 |
Notes:
CVD (cardiovascular disease, includes hypertension, coronary heart disease, heart failure, stroke, and all other cardiovascular disease); ICH (intracerebral hemorrhagic stroke); IS (ischemic stroke); SAH (subarachnoid hemorrhage); TIA (transient ischemic attack); CI (cerebral infarction); N/A (not available).
When the sample size of the indirect cost is different from the total sample size, the sample size for indirect cost is shown.
Estimation approaches for productivity loss: HCA (the human capital approach).Estimation approaches for the time cost of informal caregiving: OC (the opportunity cost approach), and RA (the replacement approach).
Indirect cost in 2012 USD is estimated by using consumer price indices of study countries in the years of cost and in 2012 from the World Bank (http://data.worldbank.org/indicator/FP.CPI.TOTL) and purchasing power parity (PPP) exchange rate in 2012 from OECD website (http://stats.oecd.org/Index.aspx?datasetcode=SNA_TABLE4). For Thailand, we use a PPP conversion factor from the World Bank and an exchange rate in 2012 from the webpage of the Federal Reserve Bank of St. Louis, Economic Research, at the following link:http://research.stlouisfed.org/fred2/release?rid=186&soid=1&t=g5a&at=g5a&ob=pv&od=desc.
Euro is used as a local currency.
The “study design” column in Tables 3 and 4 included the methods used for estimating the productivity loss and the cost of informal care. From the HCA and the FA methods estimating the productivity loss, the HCA, which was used by 22 of the 25 studies in Table 3 and two of the six studies in Table 4, was the more common approach. Two studies from Australia used the FA only for estimating productivity loss.40, 41 In addition, two studies used both the FA and the HCA (in these instances for sensitivity analyses).38, 39 Among 13 COI studies including the cost of informal care in Table 3 and four studies of informal care that did so in Table 4, seven studies used the OC approach only, six studies used the RA only, and four studies used both approaches.
Eight COI studies (Table 3), all based on incidence, provided a per-patient indirect costs. Five of them used one year as the follow-up period, one used six months, and the other two used lifetime. In 2012 US dollars (shown in parentheses in the table) the lowest per-patient indirect cost among one-year follow-up studies was $2,960 in a German study, which did not include informal caregiving cost,25 while for lifetime follow-up the costs were $22,243 per male and $11,765 per female in a Swedish study, which did not include informal caregiving cost, 34 and $54,067 per patient, including informal caregiving cost, in a study from Spain32. Informal caregiving cost in the study from Spain was around 80% of the total indirect cost.32 The study from Spain focused on intracerebral hemorrhage,32 while the Swedish study used all type of stroke34.
The proportion of total cost that was represented by indirect cost for COI studies ranged from a low of 3% for ischemic stroke in a study from Australia40 to a high of 71%in two studies, one from Spain31 and the other from the US in which this figure was for subarachnoid hemorrhage only22 (Table 3). The median and mean proportions of total cost represented by indirect cost were 32% and 33%, respectively (not shown in table).
Among the six studies that were confined to indirect cost (Table 4), three provided a per-patient estimate of the annual cost of informal caregiving. These costs ranged from a low of $904–$1,453 in a study from Thailand19 to a high of $16,687-$23,451 in a study from the Netherlands14 (all figures in 2012 US dollars). The annual morbidity cost in Sweden (in 2012 US dollars) was estimated to be $14,963 per patient in 2006.
4. Discussion
4.1. Indirect cost of stroke around the world
In this review, six of the 31 studies were from the US (Table 1). Of these six, two focused solely on indirect costs; the other four were COI studies. Of the two that focused entirely on indirect costs, one was a national-level study that examined informal caregiving for elderly stroke patients17; this report estimated the annual cost of such care (in 2012 US dollars) to be$8.4 billion in 1993. The other study, which was limited to the state of California,16 estimated the annual cost of lost productivity associated with stroke mortality in that state to be US$1.8 billion in 1991.
Of the four US studies of COI, two used prevalence approach, and the other two used incidence approach. In one of the prevalence-based studies, Brown and colleagues estimated that the indirect cost of ischemic stroke (in 2012 US dollars) was $1,384 billion and indirect costs would account for 53% of the total costs of ischemic stroke for the period of 2005-2050.21 Brown and coworkers suggested that indirect costs will continue to increase, especially among African Americans and Hispanics, because of increasing salaries among those two groups.21 More recently, Heidenreich and coworkers, also a prevalence-based study, predicted that annual indirect cost (in 2012 US dollars) will increase from $27 billion in 2010 to $47 billion in 2030.20 However, the proportion of total cost made up of indirect cost was predicted to decrease from 47% to 32% over the 20-year period because of rapidly increasing direct medical costs.20
In the more recent of the two incidence-based studies in the US, Taylor and associates found that indirect costs accounted for 58% of the total cost of first-ever stroke, including subarachnoid hemorrhage, intracerebral hemorrhage, and ischemic stroke.9 The authors estimated the lifetime indirect costs of the combination of these three major types of stroke to be $41.5 billion (in 2012 US dollars) in 1990.9 Another incidence-based study compared the lifetime costs of unruptured intracranial aneurysms and aneurysmal subarachnoid hemorrhage.22 One-year productivity losses due to unruptured intracranial aneurysms were estimated to be $507 million; the comparable figure for aneurysmal subarachnoid hemorrhage was $2,033 million (in 2012 US dollars).22 The estimated proportions of total cost made up of indirect cost were 59% for unruptured intracranial aneurysms and 71% for aneurysmal subarachnoid hemorrhage.22
In the four US COI studies overall, therefore, the proportion of total cost represented by indirect cost ranged from 32% to 71%, confirming that the burden of indirect cost in stroke patients, as expected, was quite considerable.
Among the 31 studies reviewed, 19 were from European countries, four from Oceania, and one each from Canada and Thailand. Among the 19 European studies, the proportion of total cost represented by indirect cost varied considerably. Large wage variations within Europe may affect the variation of the proportion of total cost represented by indirect cost as well as the variation of indirect cost level. Even within one country, the variation in this parameter could be considerable. The proportion of total cost represented by indirect cost ranged from 14% to 25% in Sweden.33-36 Studies in Australia and New Zealand revealed a lower proportion of indirect cost as a percentage of total cost comparing to other studies (3–10% in Australia, 6–9% in New Zealand).40-42 Because the study from Thailand was not a COI study, indirect cost as a percentage of total cost was not available. Notably, the annual per-patient cost (in 2012 US dollars) in the Thai study ($904 to 1,453) was much lower than the estimate from a national study in the US ($4,823 to 11,301).17, 19 This big difference can be partly explained by the much lower wages in Thailand.
4.2. Factors affecting the estimation of indirect costs
In this report, several major factors affected the estimation of indirect cost. First, the levels of indirect cost depended on the cost categories included in the studies. For 11 of the 25 COI studies, either one or two of the three categories in Table 1 (mortality, morbidity, and informal caregiving) had to be marked as “No” in the table either because the study did not include that category or did not clearly delineated it. Because the total indirect costs in COI studies were treated as the sum of the three indirect cost categories, the size of indirect cost depended on the indirect cost categories that were included in the studies.
The cost of informal caregiving was the most common missing component in estimating the indirect costs. Among the 11 COI studies that were missing at least one of the three categories of indirect cost, nine did not include the cost of informal care. Another concern was that some studies did not report the cost of informal care separately even though those studies included that cost. This problem can be explained in part by the absence of a consensus whether or not informal caregiving cost is an established category of indirect cost. In the present review, when a study treated the cost of informal care as a part of direct nonmedical cost, sometimes it did not report the cost of informal care separately. For those studies, the cost of informal care could not be included as a part of indirect cost but was included as a part of direct cost for calculating the proportions under the last column head in Table 3. For instance, Cadilhac and coworkers40, Gustavsson and associates7, Pugliatti and colleagues28, and Scott and Scott42 included the cost of informal care as a part of direct cost, and thus in Table 3 the figure only productivity loss was left as the indirect cost and used in calculating the proportion of total costs represented by indirect costs.
Another concern was that although mortality and morbidity costs were included in almost every COI study in Table 1, the subcategories of morbidity and mortality cost were not fully addressed in all the studies. In addition to the cost of informal caregiving, the productivity loss in nonmarket production, such as in housekeeping or volunteer work, and the morbidity cost of decreased market production because of sick leave were the most common missing components in deriving the indirect cost.
Beyond the categories of indirect cost, the criteria for including direct costs affected the proportion of total stroke-related costs represented by indirect costs. For example, the cost of staying in a nursing home, which should be treated as a part of direct cost, has been found to constitute a significant proportion of the total cost of stroke (18–19%)8 but it was often missed in the COI studies we reviewed. Those studies, which did not include the cost of nursing homes, underestimated the direct cost and leading to an overestimation (Table 3) of the proportion of total cost represented by indirect cost.
Estimating the indirect cost depended on study designs. The choice of the length of follow-up and whether prevalence or incidence was used influenced the levels of indirect cost. The direct medical cost was incurred primarily within one year of stroke onset, while indirect costs might be amassed over the course of many years. Thus, when the incidence-based method was used, the proportion of total costs represented by indirect costs was higher when lifetime follow-up was used than when one year follow-up was used. The proportion of total stroke cost represented by indirect costs when a prevalence-based approach was used lies between those two approaches.
Another issue to consider was that the methods chosen to estimate the productivity loss greatly affected the estimation of indirect cost. In 2001, Dewey and coworkers (2001) found that the estimation of productivity loss to mortality using the FA was only about 10% of the estimated cost using the HCA.41 In Table 3, the proportion of total cost represented by indirect cost was 3% (ischemic stroke) or 5% (intracerebral hemorrhage) and 10%, respectively, in two studies from Australia 40, 41 using the FA, while indirect cost was between 6% 42 and 71% 22, 31 of total cost for studies using the HCA. Also, the choice between the opportunity cost approach (OC in the present paper) and the replacement approach (RA) was known to considerably affecting the levels of indirect cost.14
In addition to cost categories and study design, characteristics of the stroke patients, such as the type of stroke and the patient's age, and country-specific factors, such as the structure of the health care system and income level, affected the level of indirect cost. Although we adjusted the indirect cost to 2012 US dollars, the year that costs were incurred also affected the level of indirect cost, as mortality and long-term disability rates after the incidence of stroke changed over time, as they were highly related to technological advances in stroke treatment and the population profile.
4.3. Potential research areas
Although the literature treated the estimation of indirect cost with various methods, study designs, stroke types, and study settings, some potential research areas remained in studies of indirect cost of stroke. First, there was an underestimation of the indirect cost if nonmarket productivity loss was not considered. As was stated above, nonmarket productivity loss was not included in current studies of indirect cost. In addition, estimation of the indirect cost was limited to those younger than 65 years because nonmarket productivity loss was not treated as a part of the indirect cost of stroke. We found relevant information about the nonmarket productivity loss was lacking, and this shortcoming lead to an underestimation of the indirect cost.
In general, the quality of data used for estimating the cost of informal care was poor. Information about types of activities forgone because of caregiving using the opportunity cost approach or types of informal caregiving activities for the replacement cost approach was not available at the national level. Some special surveys designed for informal care studies included more detailed information about the types of activities provided by a caregiver or the types of activities foregone due to caregiving 14, 19, but such studies are scarce.
Another concern with the data quality of informal care was the lack of information to verify the informal care costs of stroke patients that were not due to stroke but instead were due to other health conditions. Because most stroke patients were elderly, it was possible that the patients would use informal caregiving services even without stroke. However, data that provided the reasons for using informal care were not available. Only one study compared the use of informal care during “pre-stroke” and “post-stroke” periods as a way to resolve this issue.35 As an alternative, Hickenbottom and coworkers used regression analysis to control for comorbidities, which might increase the use of informal caregiving among stroke patients.17
Finally, research on the indirect cost of stroke in developing countries was lacking. Among the 31 studies reviewed, only one study was about a developing country, Thailand. Because some components of indirect cost, such as informal caregiving cost, were more important in developing countries where were often lacking formal care facilities or nursing home services for stroke survivors, studies on informal caregiving in developing countries should have public health significance in improving the quality of life for stroke patients.
5. Conclusions
This study found that the indirect costs of stroke varied from 3% to 71% of the total cost of that event. The level of the indirect cost depended on the length of study periods, methods, study design, types of stroke, and cost components. Regardless, the level of indirect cost was considerable, and in the present review the median proportion of indirect cost was 32% of the total cost of stroke. The indirect cost will increase even further with the aging population and improving survival rate of stroke patients. To better quantify the economic burden of stroke, developing proper methods to study indirect costs, and establishing relevant data sources for those studies are in critical need. More-refined studies will facilitate the development of interventions for stroke prevention to reduce the health and economic burden associated with stroke.
Acknowledgments
The findings and conclusions of this article are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention (CDC).
References
- 1.World Health Organization. The world health report 2002. Midwifery. 2003;19:72–73. doi: 10.1054/midw.2002.0343. [DOI] [PubMed] [Google Scholar]
- 2.Redon J, Olsen MH, Cooper RS, Zurriaga O, Martinez-Beneito MA, Laurent S, et al. Stroke mortality and trends from 1990 to 2006 in 39 countries from europe and central asia: Implications for control of high blood pressure. European Heart Journal. 2011;32:1424–1431. doi: 10.1093/eurheartj/ehr045. [DOI] [PubMed] [Google Scholar]
- 3.Gillum RF, Kwagyan J, Obisesan TO. Ethnic and geographic variation in stroke mortality trends. Stroke. 2011;42:3294–3296. doi: 10.1161/STROKEAHA.111.625343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunst AE, Amiri M, Janssen F. The decline in stroke mortality: Exploration of future trends in 7 western european countries. Stroke. 2011;42:2126–2130. doi: 10.1161/STROKEAHA.110.599712. [DOI] [PubMed] [Google Scholar]
- 5.Chiu L, Shyu WC, Chen TR. A cost-effectiveness analysis of home care and community-based nursing homes for stroke patients and their families. Journal of Advanced Nursing. 1997;26:872–878. doi: 10.1046/j.1365-2648.1997.00410.x. [DOI] [PubMed] [Google Scholar]
- 6.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E, et al. Cost of disorders of the brain in europe 2010. European neuropsychopharmacologyy. 2011;21:718–779. doi: 10.1016/j.euroneuro.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Demaerschalk BM, Hwang HM, Leung G. Us cost burden of ischemic stroke: A systematic literature review. The American Journal of Managed Care. 2010;16:525–533. [PubMed] [Google Scholar]
- 9.Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. Stroke. 1996;27:1459–1466. doi: 10.1161/01.str.27.9.1459. [DOI] [PubMed] [Google Scholar]
- 10.Dewey HM, Thrift AG, Mihalopoulos C, Carter R, Macdonell RA, McNeil JJ, et al. Informal care for stroke survivors: Results from the north east melbourne stroke incidence study (NEMESIS) Stroke. 2002;33:1028–1033. doi: 10.1161/01.str.0000013067.24300.b0. [DOI] [PubMed] [Google Scholar]
- 11.Ekman M. Economic evidence in stroke: A review. The European Journal of Health Economics. 2004;5(Suppl 1):S74–83. doi: 10.1007/s10198-005-0292-3. [DOI] [PubMed] [Google Scholar]
- 12.Kang HY, Lim SJ, Suh HS, Liew D. Estimating the lifetime economic burden of stroke according to the age of onset in south korea: A cost of illness study. BMC Public Health. 2011;11:646. doi: 10.1186/1471-2458-11-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng H, Ehrlich F, Amin J. Productivity loss resulting from coronary heart disease in australia. Applied Health Economics and Health Policy. 2010;8:179–189. doi: 10.2165/11530520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.van den Berg B, Brouwer W, van Exel J, Koopmanschap M, van den Bos GA, Rutten F. Economic valuation of informal care: Lessons from the application of the opportunity costs and proxy good methods. Social Science & Medicine (1982) 2006;62:835–845. doi: 10.1016/j.socscimed.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 15.Grieve R, Hutton J, Bhalla A, Rastenyte D, Ryglewicz D, Sarti C, et al. A comparison of the costs and survival of hospital-admitted stroke patients across europe. Stroke. 2001;32:1684–1691. doi: 10.1161/01.str.32.7.1684. [DOI] [PubMed] [Google Scholar]
- 16.Fox P, Gazzaniga J, Karter A, Max W. The economic costs of cardiovascular disease mortality in california, 1991: Implications for public health policy. Journal of Public Health Policy. 1996;17:442–459. [PubMed] [Google Scholar]
- 17.Hickenbottom SL, Fendrick AM, Kutcher JS, Kabeto MU, Katz SJ, Langa KM. A national study of the quantity and cost of informal caregiving for the elderly with stroke. Neurology. 2002;58:1754–1759. doi: 10.1212/wnl.58.12.1754. [DOI] [PubMed] [Google Scholar]
- 18.Lindgren P, Glader EL, Jonsson B. Utility loss and indirect costs after stroke in sweden. European Journal of Cardiovascular Prevention and Rehabilitation. 2008;15:230–233. doi: 10.1097/HJR.0b013e3282f37a22. [DOI] [PubMed] [Google Scholar]
- 19.Riewpaiboon A, Riewpaiboon W, Ponsoongnern K, Van den Berg B. Economic valuation of informal care in asia: A case study of care for disabled stroke survivors in thailand. Social Science & Medicine (1982) 2009;69:648–653. doi: 10.1016/j.socscimed.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 20.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. Forecasting the future of cardiovascular disease in the united states: A policy statement from the american heart association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 21.Brown DL, Boden-Albala B, Langa KM, Lisabeth LD, Fair M, Smith MA, et al. Projected costs of ischemic stroke in the united states. Neurology. 2006;67:1390–1395. doi: 10.1212/01.wnl.0000237024.16438.20. [DOI] [PubMed] [Google Scholar]
- 22.Wiebers DO, Torner JC, Meissner I. Impact of unruptured intracranial aneurysms on public health in the united states. Stroke. 1992;23:1416–1419. doi: 10.1161/01.str.23.10.1416. [DOI] [PubMed] [Google Scholar]
- 23.Chan BBH. Cost of stroke in ontario, 1994/95. Canadian Medical Association Journal. 1998;159:S2–S8. [Google Scholar]
- 24.Dodel R, Winter Y, Ringel F, Spottke A, Gharevi N, Muller I, et al. Cost of illness in subarachnoid hemorrhage: A German longitudinal study. Stroke. 2010;41:2918–2923. doi: 10.1161/STROKEAHA.110.586826. [DOI] [PubMed] [Google Scholar]
- 25.Rossnagel K, Nolte CH, Muller-Nordhorn J, Jungehulsing GJ, Selim D, Bruggenjurgen B, et al. Medical resource use and costs of health care after acute stroke in Germany. European Journal of Neurology. 2005;12:862–868. doi: 10.1111/j.1468-1331.2005.01091.x. [DOI] [PubMed] [Google Scholar]
- 26.Weimar C, Weber C, Wagner M, Busse O, Haberl RL, Lauterbach KW, et al. Cerebrovascular diseases. Vol. 15. Basel, Switzerland: 2003. Management patterns and health care use after intracerebral hemorrhage. A cost-of-illness study from a societal perspective in Germany; pp. 29–36. [DOI] [PubMed] [Google Scholar]
- 27.Smith S, Horgan F, Sexton E, Cowman S, Hickey A, Kelly P, et al. The cost of stroke and transient ischaemic attack in Ireland: A prevalence-based estimate. Age and Ageing. 2012;41:332–338. doi: 10.1093/ageing/afr141. [DOI] [PubMed] [Google Scholar]
- 28.Pugliatti M, Sobocki P, Beghi E, Pini S, Cassano GB, Altamura AC, et al. Cost of disorders of the brain in Italy. Neurological Sciences. 2008;29:99–107. doi: 10.1007/s10072-008-0868-7. [DOI] [PubMed] [Google Scholar]
- 29.Gerzeli S, Tarricone R, Zolo P, Colangelo I, Busca MR, Gandolfo C. The economic burden of stroke in Italy. The eclipse study: Economic longitudinal incidence-based project for stroke evaluation. Neurological Sciences. 2005;26:72–80. doi: 10.1007/s10072-005-0439-0. [DOI] [PubMed] [Google Scholar]
- 30.Evers SM, Engel GL, Ament AJ. Cost of stroke in the Netherlands from a societal perspective. Stroke. 1997;28:1375–1381. doi: 10.1161/01.str.28.7.1375. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Bastida J, Oliva Moreno J, Worbes Cerezo M, Perestelo Perez L, Serrano-Aguilar P, Monton-Alvarez F. Social and economic costs and health-related quality of life in stroke survivors in the Canary islands, Spain. BMC health services research. 2012;12:315. doi: 10.1186/1472-6963-12-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navarrete-Navarro P, Hart WM, Lopez-Bastida J, Christensen MC. The societal costs of intracerebral hemorrhage in Spain. European Journal of Neurology. 2007;14:556–562. doi: 10.1111/j.1468-1331.2007.01756.x. [DOI] [PubMed] [Google Scholar]
- 33.Persson J, Ferraz-Nunes J, Karlberg I. Economic burden of stroke in a large county in Sweden. BMC health services research. 2012;12:341. doi: 10.1186/1472-6963-12-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghatnekar O, Persson U, Glader EL, Terent A. Cost of stroke in Sweden: An incidence estimate. International Journal of Technology Assessment in Health Care. 2004;20:375–380. doi: 10.1017/s0266462304001217. [DOI] [PubMed] [Google Scholar]
- 35.Zethraeus N, Molin T, Henriksson P, Jonsson B. Costs of coronary heart disease and stroke: The case of Sweden. Journal of Internal Medicine. 1999;246:151–159. doi: 10.1046/j.1365-2796.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 36.Terent A, Marke L, Asplund K, Norrving B, Jonsson E, Wester P. Costs of stroke in Sweden: A national paerspective. Stroke. 1994;25:2363–2369. doi: 10.1161/01.str.25.12.2363. [DOI] [PubMed] [Google Scholar]
- 37.Saka O, McGuire A, Wolfe C. Cost of stroke in the United Kingdom. Age and Ageing. 2009;38:27–32. doi: 10.1093/ageing/afn281. [DOI] [PubMed] [Google Scholar]
- 38.Luengo-Fernandez R, Leal J, Gray A, Petersen S, Rayner M. Cost of cardiovascular diseases in the United Kingdom. Heart (British Cardiac Society) 2006;92:1384–1389. doi: 10.1136/hrt.2005.072173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leal J, Luengo-Fernandez R, Gray A, Petersen S, Rayner M. Economic burden of cardiovascular diseases in the enlarged European Union. European Heart Journal. 2006;27:1610–1619. doi: 10.1093/eurheartj/ehi733. [DOI] [PubMed] [Google Scholar]
- 40.Cadilhac DA, Carter R, Thrift AG, Dewey HM. Estimating the long-term costs of ischemic and hemorrhagic stroke for australia: New evidence derived from the North East Melbourne Stroke Incidence Study (NEMESIS) Stroke. 2009;40:915–921. doi: 10.1161/STROKEAHA.108.526905. [DOI] [PubMed] [Google Scholar]
- 41.Dewey HM, Thrift AG, Mihalopoulos C, Carter R, Macdonell RA, McNeil JJ, et al. Cost of stroke in australia from a societal perspective: Results from the North East Melbourne Stroke Incidence Study (NEMESIS) Stroke. 2001;32:2409–2416. doi: 10.1161/hs1001.097222. [DOI] [PubMed] [Google Scholar]
- 42.Scott WG, Scott H. Ischaemic stroke in New Zealand: An economic study. The New Zealand Medical Journal. 1994;107:443–446. [PubMed] [Google Scholar]