Abstract
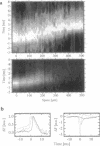
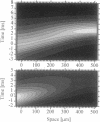
We measured a time-resolved map of electrical activity in a thin straight neurite (1.5 microns thick, 500 microns long) at a resolution of 8 microns and 0.4 ms. The neurite was obtained by guided outgrowth of an identified neuron of the leech on lanes of extracellular matrix protein. The electrical signals were detected by a fluorescent voltage-sensitive dye. We observed the voltage that was caused by an action potential elicited at the soma and by a Gaussian hyperpolarization induced at the soma, respectively. We compared the data with numerical solutions of the cable equation using the Hodgkin-Huxley parametrization. We could attribute the experimental results of depolarization and of hyperpolarization to the propagation of an action potential along an "active" cable and to the spread along a "passive" cable, respectively, if we assigned rather high specific resistances to the cytoplasm (RI = 250 omega.cm) and to the membrane (RM = 22 k omega.cm2). This assignment explained the slow velocity of 150 microns/ms of a pulse by active propagation and the limited range of 200 microns of a pulse by passive spread.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chien C. B., Pine J. Voltage-sensitive dye recording of action potentials and synaptic potentials from sympathetic microcultures. Biophys J. 1991 Sep;60(3):697–711. doi: 10.1016/S0006-3495(91)82099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet M., Masuda-Nakagawa L., Beck K. Attachment to an endogenous laminin-like protein initiates sprouting by leech neurons. J Cell Biol. 1988 Sep;107(3):1189–1198. doi: 10.1083/jcb.107.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Lesher S. Optical monitoring of membrane potential: methods of multisite optical measurement. Soc Gen Physiol Ser. 1986;40:71–99. [PubMed] [Google Scholar]
- Dietzel I. D., Drapeau P., Nicholls J. G. Voltage dependence of 5-hydroxytryptamine release at a synapse between identified leech neurones in culture. J Physiol. 1986 Mar;372:191–205. doi: 10.1113/jphysiol.1986.sp016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshman J. W., Segev I., Burke R. B. Electrotonic architecture of type-identified alpha-motoneurons in the cat spinal cord. J Neurophysiol. 1988 Jul;60(1):60–85. doi: 10.1152/jn.1988.60.1.60. [DOI] [PubMed] [Google Scholar]
- Fromherz P., Gaede V. Exclusive-OR function of single arborized neuron. Biol Cybern. 1993;69(4):337–344. doi: 10.1007/BF00203130. [DOI] [PubMed] [Google Scholar]
- Fromherz P., Müller C. O. Voltage-sensitive fluorescence of amphiphilic hemicyanine dyes in neuron membrane. Biochim Biophys Acta. 1993 Aug 15;1150(2):111–122. doi: 10.1016/0005-2736(93)90079-f. [DOI] [PubMed] [Google Scholar]
- Fromherz P., Schaden H., Vetter T. Guided outgrowth of leech neurons in culture. Neurosci Lett. 1991 Aug 5;129(1):77–80. doi: 10.1016/0304-3940(91)90724-8. [DOI] [PubMed] [Google Scholar]
- Fromherz P., Vetter T. Cable properties of arborized Retzius cells of the leech in culture as probed by a voltage-sensitive dye. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2041–2045. doi: 10.1073/pnas.89.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromherz P., Vetter T. Propagation of voltage transients in arborized neurites of Retzius cells of the leech in culture. Z Naturforsch C. 1991 Jul-Aug;46(7-8):687–696. doi: 10.1515/znc-1991-7-828. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Fine A., Farber I. C., Hildesheim R. Fluorescence monitoring of electrical responses from small neurons and their processes. Biophys J. 1983 May;42(2):195–198. doi: 10.1016/S0006-3495(83)84386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Frostig R. D., Lieke E., Hildesheim R. Optical imaging of neuronal activity. Physiol Rev. 1988 Oct;68(4):1285–1366. doi: 10.1152/physrev.1988.68.4.1285. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Ross W. N., Farber I. Simultaneous optical measurements of electrical activity from multiple sites on processes of cultured neurons. Proc Natl Acad Sci U S A. 1981 May;78(5):3245–3249. doi: 10.1073/pnas.78.5.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D., Loew L. M., Webb W. W. Optical imaging of cell membrane potential changes induced by applied electric fields. Biophys J. 1986 Aug;50(2):339–348. doi: 10.1016/S0006-3495(86)83467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J., Midtgaard J. Dendrite processing in more ways than one . Trends Neurosci. 1989 Sep;12(9):313–315. doi: 10.1016/0166-2236(89)90036-2. [DOI] [PubMed] [Google Scholar]
- Johansen J., Kleinhaus A. L. Differential sensitivity of tetrodotoxin of nociceptive neurons in 4 species of leeches. J Neurosci. 1986 Dec;6(12):3499–3504. doi: 10.1523/JNEUROSCI.06-12-03499.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhaus A. L., Prichard J. W. Differential action of tetrodotoxin on identified leech neurons. Comp Biochem Physiol C. 1983;74(1):211–218. doi: 10.1016/0742-8413(83)90176-7. [DOI] [PubMed] [Google Scholar]
- Koch C., Poggio T., Torre V. Retinal ganglion cells: a functional interpretation of dendritic morphology. Philos Trans R Soc Lond B Biol Sci. 1982 Jul 27;298(1090):227–263. doi: 10.1098/rstb.1982.0084. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. G., Lux H. D. Some electrical measurements of motoneuron parameters. Biophys J. 1970 Jan;10(1):55–73. doi: 10.1016/S0006-3495(70)86285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T. D., Kleinfeld D., Raccuia-Behling F., Salzberg B. M. Optical recording of the electrical activity of synaptically interacting Aplysia neurons in culture using potentiometric probes. Biophys J. 1989 Jul;56(1):213–221. doi: 10.1016/S0006-3495(89)82666-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALL W. Membrane potential transients and membrane time constant of motoneurons. Exp Neurol. 1960 Oct;2:503–532. doi: 10.1016/0014-4886(60)90029-7. [DOI] [PubMed] [Google Scholar]
- Ross W. N., Arechiga H., Nicholls J. G. Optical recording of calcium and voltage transients following impulses in cell bodies and processes of identified leech neurons in culture. J Neurosci. 1987 Dec;7(12):3877–3887. doi: 10.1523/JNEUROSCI.07-12-03877.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. N., Krauthamer V. Optical measurements of potential changes in axons and processes of neurons of a barnacle ganglion. J Neurosci. 1984 Mar;4(3):659–672. doi: 10.1523/JNEUROSCI.04-03-00659.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg B. M., Davila H. V., Cohen L. B. Optical recording of impulses in individual neurones of an invertebrate central nervous system. Nature. 1973 Dec 21;246(5434):508–509. doi: 10.1038/246508a0. [DOI] [PubMed] [Google Scholar]
- Senseman D. M., Horwitz I. S., Salzberg B. M. MSORTV imaging of electrotonic conduction in a syncitium: optical recording of polarization spread in a simple salivary gland. J Exp Zool. 1987 Oct;244(1):79–88. doi: 10.1002/jez.1402440110. [DOI] [PubMed] [Google Scholar]