Abstract
Background
The benefit of a primary prevention implantable cardioverter defibrillator (ICD) among patients with chronic kidney disease (CKD) is uncertain.
Study Design
Meta-analysis of patient-level data from randomized controlled trials.
Setting & Population
Patients with symptomatic heart failure and left ventricular ejection fraction of <35%.
Selection Criteria for Studies
From 7 available randomized control studies with patient level data, we selected studies with available data on important covariates. Studies without patient-level data on baseline eGFR were excluded.
Intervention
Primary prevention ICD versus usual care
Outcomes
Mortality, re-hospitalizations, and effect modification by estimated GFR (eGFR)
Results
We included data from the Multicenter Automatic Defibrillator Implantation Trial I (MADIT-I), MADIT-II, and the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). 2,867 patients were included; 36.3% had at least stage 3 CKD (eGFR<60). The Kaplan-Meier estimate of the probability of death during follow-up was 43.3% among 1,334 patients receiving usual care and 35.8% among 1,533 ICD recipients. After adjustment for baseline differences, there was evidence that the survival benefit of ICDs in comparison to usual care depends on eGFR (posterior probability for null interaction p <0.001). The ICD was associated with survival benefit among patients with an eGFR ≥ 60 (adjusted HR (aHR)=0.49 [95% posterior credible interval (PCI) 0.24-0.95]) but not among patients with eGFR <60 (aHR = 0.80 [95% PCI 0.40-1.53]). eGFR did not modify the association between the ICD and re-hospitalizations.
Limitations
Few patients with eGFR <30 were available. Differences in trial-to-trial measurement techniques may lead to residual confounding.
Conclusions
Reductions in baseline eGFR decrease the survival benefit associated with the ICD. These findings should be confirmed by additional studies specifically targeting patients with varying levels of eGFR.
Introduction
Chronic kidney disease (CKD) affects more than 20 million Americans. Compared with the general population, these patients have a four to twenty fold greater risk of sudden cardiac death (SCD).1,2 Primary-prevention implantable cardioverter-defibrillators (ICDs), used among patients who are at risk for but have not yet had life-threatening ventricular arrhythmias, have been proven to reduce SCD and overall mortality among selected patients with a reduced ejection fraction.3 However, the benefits and risks of this therapy among patients with CKD are not clear. Observational studies have consistently described decreased overall survival and increased complication rates among recipients of primary prevention ICDs who have CKD compared with those with preserved renal function. 4-6 However, the absence of a control group who did not receive primary prevention ICD therapy in these studies makes assessment of risk/benefit in CKD patients uncertain. There is a paucity of randomized trial data in this subset of patients, since all the major clinical trials comparing ICDs to usual care excluded patients with advanced kidney disease.
A collaborative consortium involving the principal investigators of existing ICD trials was established to explore the effectiveness of the ICD in various subgroups. We conducted this pooled patient-level data analysis to assess whether kidney disease as estimated by the glomerular filtration rate modifies the effect of ICD treatment on mortality and rehospitalizations compared to usual care.
Methods
Data Sources and Study Selection
Individual data on patients enrolled in 7 primary prevention ICD trials were provided. We included clinical trials randomizing patients to a primary prevention ICD versus usual care for whom data on kidney function were available at enrollment. Patients from four clinical trials were excluded due to lack of available data on serum creatinine: the Coronary Artery Bypass Graft Patch trial7, the Defibrillators in Non-ischemic Cardiomyopathy Treatment Evaluation trial8, the Defibrillator in Acute Myocardial Infarction Trial9, and the Multicenter Unsustained Tachycardia Trial10. The following 3 trials were included: the Multicenter Automatic Defibrillator Implantation Trial I (MADIT I)11, MADIT II12, and the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT)13. The criteria used by these trials to exclude patients with advanced CKD at baseline were as follows; for MADIT I, BUN >70 mg/dl or creatinine>3 mg/dl; for MADIT 2, creatinine>3 mg/dl; and for SCD-HeFT, creatinine>2.5 mg/dl. For our study, we included patients with symptomatic heart failure (New York Heart Association Class (NYHA) < IV), a left ventricular ejection fraction (LVEF) of ≤ 35%, and assignment to either an ICD or usual care. Patients without heart failure symptoms or with NYHA Class IV symptoms, a LVEF of > 35%, who were missing data on prior MI, who had an MI in the 40 days preceding randomization, or whose time from randomization was unknown were excluded. Patients who were missing important covariates or who were assigned to other treatment arms besides ICD or usual care were also excluded.
Determination of eGFR group
Kidney function was determined by calculating the estimated glomerular filtration rate (eGFR) at study enrollment. We used the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, which utilizes serum creatinine concentration, age, race, and gender to determine estimated GFR and has been validated across populations with a wide range of GFR. 14 For consistency with prior literature and for simplicity, we dichotomized the cohort into two strata of eGFR: eGFR<60 (stage 3-5 CKD) and eGFR≥60 ml/min1.73 m2. We also examined outcomes by finer categories of eGFR (eGFR<45, 45-59, 60-89, and ≥90 ml/min/1.73m2).
Baseline Characteristics
Patient characteristics at study enrollment were summarized according to eGFR group. The association of eGFR group with categorical and continuous baseline variables was tested using the χ2 tests and tests of equality of means, respectively.
Endpoints
All-cause mortality was the primary endpoint of interest. Re-hospitalization was a secondary endpoint. Re-hospitalization was defined as admission to a hospital for any reason after ICD placement during each trial study period. In addition, we assessed ICD-related complications which were catalogued across trials included pneumothorax, hemothorax, pocket hematoma, lead dislodgement, ICD migration, impending ICD pocket erosion, ICD-related infection, and other clinical events deemed to be directly related to ICD implantation.
Statistical Analysis
To assess the relationship between CKD and ICDs on outcomes, our primary analysis was to examine the interaction between continuously-valued estimated GFR and ICD therapy on mortality. For interaction testing, statistical significance was assessed by computing the two-sided posterior probability of a null interaction. We also examined survival outcomes based on eGFR classification (eGFR>=60 versus eGFR<60) using Kaplan-Meier survival curves. For ICD recipients, we assessed the association between eGFR group and ICD-related complications with the χ2 test.
For the analyses of the primary and secondary endpoints, we used Bayesian Weibull survival and Bayesian logistic regression models, respectively, to combine trial data and address missing data. 15,16 A Bayesian approach models uncertainty with prior distributions and establishes inferences based on posterior probabilities. The use of Bayesian modeling methods in pooled-data analyses is advantageous because it allows for inferences to be made about subgroups that may be under-represented in included trials by borrowing information across studies and subgroups. Variation within and between trials can be better accounted for by taking into account not only parameter uncertainty but also additional complexity such as missing data. 16 (For further discussion on the advantages of Bayesian hierarchical models in pooled data analyses compared to classical Frequentist approaches, see Supplemental Item S1). In our models, missing covariate values were imputed based on their trial-specific empirical distribution. As an additional sensitivity analysis, we also created models that excluded patients who had missing data on any covariates. We adjusted for the following covariates: sex, race, age, LVEF, NYHA class, history of heart failure, history of hypertension, history of diabetes, history of coronary artery bypass grafting (CABG), presence of left bundle branch block, history of ischemic disease, and use of cardiac medications including antiarrhythmic, β-blockers, and ace-inhibitors. Assuming random effects for trial-specific treatment effects and for parameters defining trial-specific baseline hazard functions, a model including the main effects of the ICD and eGFR group as well as the multiplicative interaction of the ICD with eGFR group was fitted. Statistical significance for hazard ratios was established by whether the posterior credible interval (PCI) did not include unity. Analogously, a Bayesian logistic regression model was fitted to re-hospitalizations.
All analyses were performed using R version 3.0.1 and WinBUGS version 1.4.3 (WinBUGS code is provided as a Supplemental Item S2). All data were previously collected as part of the primary trials, and the use of the de-identified dataset was approved by the Duke Institutional Review Board.
Results
Figure 1 depicts a flow diagram summarizing the criteria used to select trials and select patients within included trials. From the 3 included clinical trials (MADIT-I, MADIT-II, and SCDHeFT), a total of 2,867 patients were included in our final study population. Table 1 summarizes the characteristics of the clinical trials and the number of subjects from each trial that were included in this study. Excluded patients differed from included patients largely based on the exclusion criteria (Supplemental Table S1), but there was no significant difference in mortality risk between excluded and included patients. (log rank p=0.2)
Figure 1.
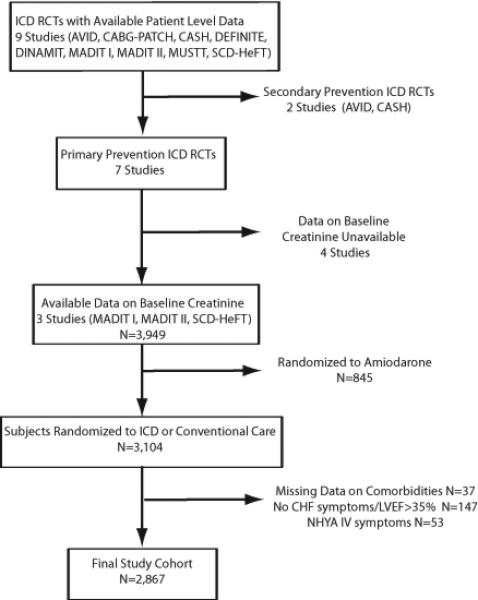
Study selection and patient inclusion within selected studies
Table 1.
Characteristics of Included Clinical Trials
| Clinical Trial | Participating Countries | Publication Year | Mean follow-up (mos) | Eligible LVEF | Factorial Comparison | Total Subjects Enrolled | Subjects included in this study |
|---|---|---|---|---|---|---|---|
| MADIT-I | USA, Italy, Germany |
1996 | 26.8 | ≤ 35% | ICD v. usual medical therapy |
196 | 176 |
| MADIT-II | USA, Netherlands Germany, Israel |
2002 | 20.2 | ≤ 30% | ICD v. usual medical therapy |
1232 | 1080 |
| SCD-HeFT | USA, Canada | 2005 | 40.2 | ≤ 35% | ICD v. placebo v. amiodarone |
2521 | 1611 |
Abbreviations: LVEF, left ventricular ejection fraction; ICD, implantable cardioverter defibrillator.
Table 2 compares the baseline characteristics of subjects with eGFR>60 and eGFR>=60 ml/min/1.73m2. More than one-third (36.3%) had at least stage CKD 3 (eGFR<60) upon study entry, and the mean eGFR of patients with eGFR<60 was 45.4 ml/min/1.73m2 . Consistent with the exclusion criteria of the original trials, there were relatively few subjects with advanced (stage 4 or 5) CKD; only 3.6% of patients had an eGFR <30 ml/min/1.73m2 , and there were no patients with end stage renal disease enrolled in any of the trials. There were no significant differences in the proportion assigned to an ICD between the two eGFR groups. eGFR<60 patients were older (mean age 67 vs. 58 years) and had a higher prevalence of diagnosed heart failure (60.0% vs. 54.5%), prior CABG (47.1% vs. 36.0%), hypertension (59.8% vs. 49.6%), and diabetes (35.5% vs. 27.8%). Patients with eGFR<60 were less likely to be taking a β-blocker (59.0% vs. 66.2%) or an ACE inhibitor (82.9% versus 88.7%) at enrollment. LVEF, the presence of left bundle branch block, and use of antiarrhythmic medications did not differ between groups. Comparison of baseline characteristics by treatment assignment (ICD vs. no ICD) stratified by eGFR group is shown in Supplemental Tables S2 (eGFR>=60) and S3 (eGFR<60).
Table 2.
Baseline Characteristics of eGFR<60 and eGFR>=60 cohorts:
| eGFR < 60 N=1040 | eGFR≥60 N=1827 | P-Value | |
|---|---|---|---|
| Clinical Trial | |||
| MADIT-I (%) | 7.6 | 5.3 | <0.001 |
| MADIT-II (%) | 40.7 | 36.0 | |
| SCD-HeFT (%) | 51.7 | 58.7 | |
| Therapy assignment | |||
| ICD (%) | 52.0 | 54.3 | 0.2 |
| Patient Demographics | |||
| Age (mean, yrs) | 66.6 | 57.9 | <0.001 |
| Female Gender (%) | 20.5 | 18.1 | 0.1 |
| Race | 0.02 | ||
| White (%) | 84.2 | 79.9 | |
| Black (%) | 11.3 | 14.8 | |
| Other (%) | 4.5 | 5.3 | |
| Comorbidities | |||
| History of CHF(%) | 60.0 | 54.5 | 0.005 |
| Hypertension (%) | 59.8 | 49.6 | <0.001 |
| Diabetes (%) | 35.5 | 27.8 | <0.001 |
| Cardiac Disease characteristics | |||
| LVEF % (mean) | 23.5 | 23.8 | 0.2 |
| Prior CABG (%) | 47.1 | 36.0 | <0.001 |
| Left BBB (%) | 21.7 | 19.0 | 0.09 |
| NYHA class | <0.001 | ||
| Class | 15.7 | 17.4 | |
| Class 2(%) | 48.9 | 59.3 | |
| Class 3(%) | 35.4 | 23.3 | |
| Medication use at enrollment | |||
| Antiarrhythmic (%) | 3.7 | 2.4 | 0.06 |
| Beta-blockers (%) | 59.0 | 66.2 | <0.001 |
| ACE inhibitor (%) | 82.9 | 88.7 | <0.001 |
| Estimated GFR | |||
| Mean (ml/min/1.73m2 ) | 44.7 | 78.4 | <0.001 |
| GFR<30 ml/min/1.73m2 (%) | 10.3 | 0 | |
Abbreviations: LVEF, left ventricular ejection fraction; ICD, implantable cardioverter defibrillator; CHF, congestive heart failure; CABG, coronary artery bypass grafting; BBB, bundle branch block; NYHA, New York Heart Association; ACE, angiotension converting enzyme.
Unadjusted Outcomes
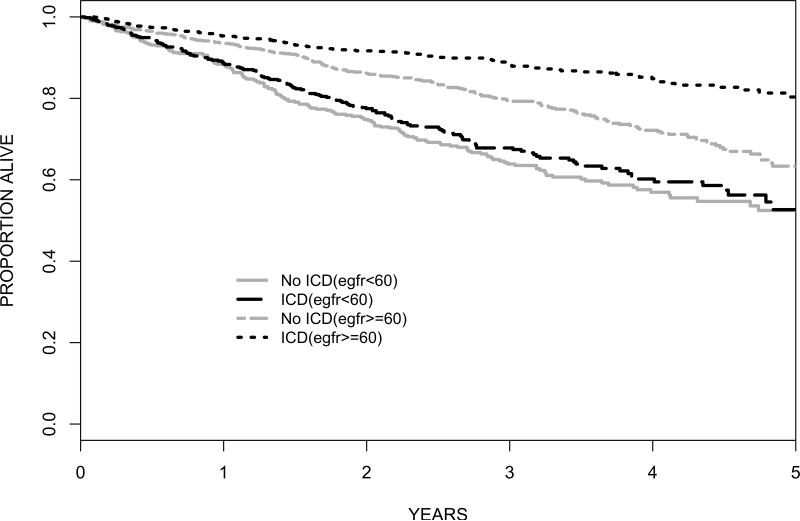
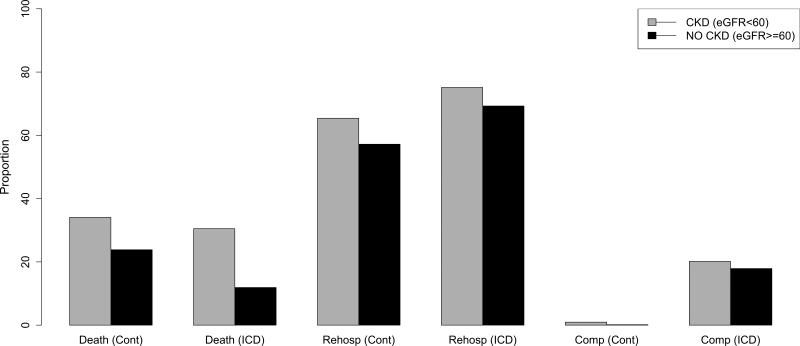
Overall, among 2,867 patients and 2.7 years of average follow-up (standard deviation = 1.5 years), the Kaplan-Meier estimate of the probability of death during follow-up was 43.3% in the 1,334 patients receiving usual care as compared with 35.8% in the 1,533 ICD recipients. Figure 2 illustrates the survival curves for unadjusted all-cause mortality by treatment assignment stratified by eGFR grouping, showing the absence of a significant survival advantage with ICD assignment among patients with eGFR<60 ml/min/1.73m2 (HR=0.92, 95% CI 0.74-1.14). A total of 1,805 (66.2%) patients were re-hospitalized at least once. Figure 3 shows the overall rates of re-hospitalization and ICD-related complications. Patients who received an ICD were more likely to be re-hospitalized. Comparison of re-hospitalizations according to CKD categories showed that eGFR<60 patients in either treatment arm were more likely to be hospitalized. ICD-related complications in the ICD-treatment arms were also slightly higher among eGFR<60 patients, but the difference was not statistically significant (22.1% overall in the eGFR<60 group versus 18.8% in the eGFR>=60 group, p=0.1).
Figure 2.
Kaplan-Meier Survival Curves in ICD Recipients vs. Non-recipients According to eGFR. Unadjusted HR for mortality benefit of ICD: eGFR>=60 HR=0.53, 95% CI 0.42-0.67; eGFR<60 HR=0.92, 95% CI 0.74-1.14.
Figure 3.
Proportion (%) of Study Participants with Adverse Outcomes by eGFR group.
Abbreviations: Cont=control arm; Rehosp= rehospitalizations; Comp=complications
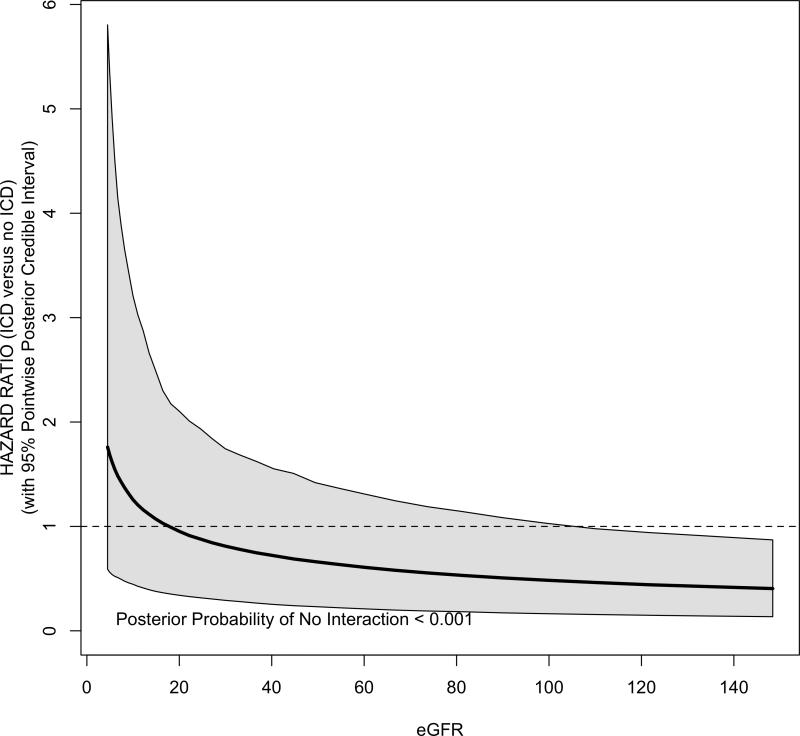
Adjusted Outcomes
Figure 4 shows the covariate–adjusted mortality hazard ratio of ICD versus usual care across the spectrum of eGFR. Overall, there was a significant interaction between eGFR and the benefit of ICD for all-cause mortality (posterior probability p <0.001). The survival benefit of ICD declines in proportion with declining eGFR; however, the 95% PCI does not exclude the possibility of benefit among those with low eGFR. There was no evidence of interactions between eGFR and re-hospitalizations (Supplemental Figure S1) or ICD-related complications.
Figure 4.
Bayesian-Weibull Covariate-Adjusted Hazard Ratio for Mortality Benefit of ICD as a function of baseline eGFR. Shaded areas reflect 95% pointwise posterior credible intervals.
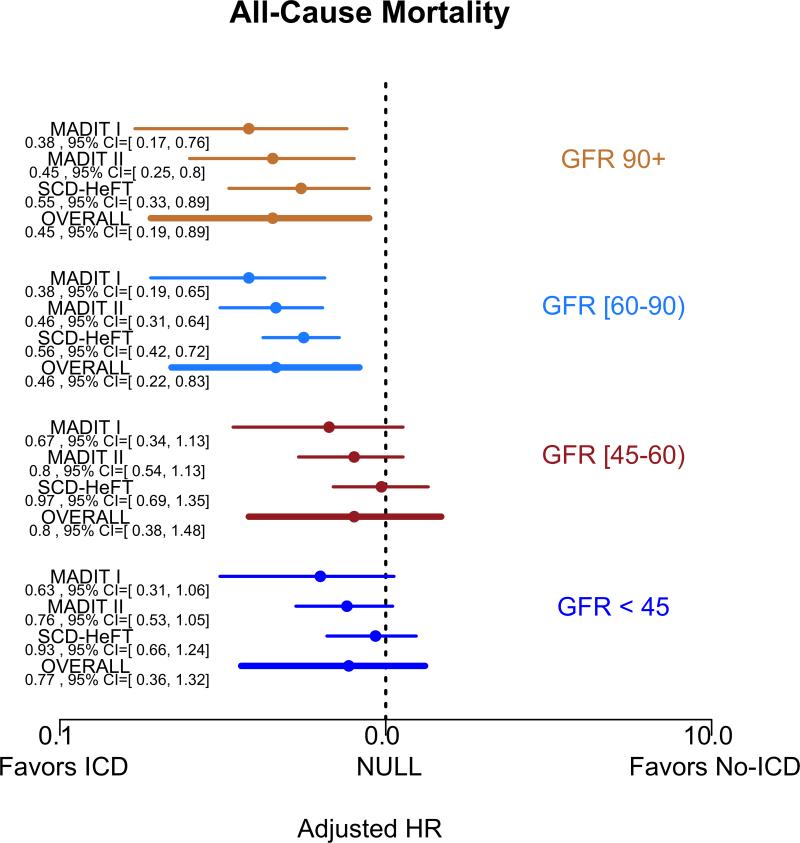
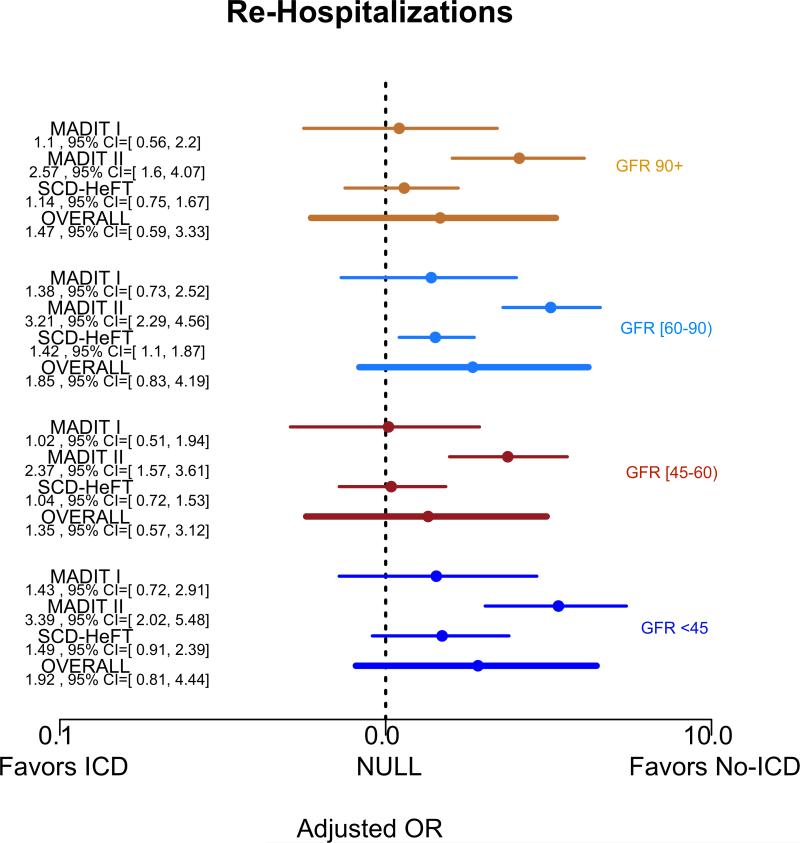
Figure 5 summarizes the covariate-adjusted point estimates for the relative benefit of the ICD on outcomes according to four eGFR groups (eGFR<30, 30-59, 60-90, and >90 ml/min/1.73m2). The effect of the ICD in comparison to usual care on all-cause mortality for all eGFR groups >=60 ml/min/1.73m2 was consistent with a survival benefit (eGFR >90 adjusted HR=0.45 95% posterior credible interval (PCI) 0.19-0.89; eGFR 60-90 aHR=0.46 [95% PCI 0.22-0.83]). We did not observe a significant benefit of the ICD in either of the eGFR subgroups <60 ml/min/1.73m2 (eGFR 30-59 aHR = 0.8 [95% PCI 0.38-1.48]; eGFR<30 aHR=0.77 [95% PCI 0.36-1.32]). The risk of re-hospitalization between ICD and usual care arms did not differ substantially when compared according to eGFR subgroups (Figure 6).
Figure 5.
Forest Plots of covariate-adjusted HR and 95% PCI for A) all-cause mortality and B) re-hospitalizations by clinical trial and eGFR group, with x-axis displayed in the log-scale.
We also performed additional sensitivity analyses removing patients with missing covariate data, and this analysis did not significantly alter the study findings.
Reported Mode of Death
We compared the trial-reported mode of death for the eGFR<60 and eGFR>=60 groups, focusing on the proportion of arrhythmia related vs. non-arrhythmic deaths. 24% of deaths were classified as cardiac deaths that could not be further classified as arrhythmic or nonarrhythmic. There was a higher proportion of arrhythmia-related death among patients with eGFR>=60 compared to patients with eGFR<60 (35.0% vs 23.8%, p=0.002). There was slightly greater proportion of non-arrhythmic deaths among eGFR<60 patients (48.4% vs. 40.7%) but this did not reach statistical significance (p=0.06). In the eGFR>=60 group, ICD treatment was associated with a significant reduction in arrhythmic death (unadjusted HR=0.22, 95%CI 0.14-0.35, P=0.001), whereas ICD treatment was not associated with a significant reduction in arrhythmic death in the eGFR<60 group (HR 0.68, 95% CI 0.44-1.07, p=0.3).
Discussion
To our knowledge, this meta-analysis of 2,867 subjects enrolled in primary prevention ICD trials is the largest collection of prospective data on the risks and benefits of a primary prevention ICD among patients with CKD, and the first meta-analysis to compare ICD outcomes against usual care in this patient population. In summary, we found that the mortality reduction associated with the ICD compared to control is significantly impacted by baseline kidney function, with decreasing benefit as GFR declines. As a group, patients with a GFR < 60 ml/min/1.73m2 had a higher risk of re-hospitalization, but we did not observe a significant interaction between level of kidney function (as measured by the estimated GFR) and the risk of re-hospitalizations. There was no significant difference in the risk of ICD-related complications according to eGFR group.
Despite the fact that all published clinical trials to date excluded patients with severe kidney disease, more than one-third (36.3%) of the patients in our study had at least moderate CKD. Current guidelines do not provide any special consideration for eGFR group or level of kidney function in the decision to implant a primary prevention ICD.3 While there have been no clinical trials specifically designed to address the question of ICD benefit among CKD patients, a prior post hoc analysis of a small group of patients with GFR ≤ 35 (N=60) enrolled in the MADITII study suggested that patients with did not derive a statistically significant benefit from an ICD (all-cause mortality hazard ratio 1.09, p = 0.8).17 Our study confirms and further extends these findings by showing that the beneficial effect of the ICD on mortality continuously declines with declining kidney function. Although our study population included a large number of patients with CKD, the small number of patients with advanced CKD enrolled in these trials prohibits a detailed assessment of a “threshold” eGFR at which the mortality advantage of ICD compared to usual care is abrogated. However, these data should provide the impetus for further controlled clinical trials specifically designed to address the efficacy of the ICD in this patient population.
There are several lines of evidence that could explain a decreased efficacy of the ICD among CKD patients. First, CKD patients with heart failure may experience a higher proportion of non-arrhythmic death compared to arrhythmic death, thus limiting the ICD efficacy. 18 A secondary analysis of the SOLVD study showed that the risk of non-arrhythmic death increases proportionally with worsening CKD, and the influence of CKD on the risk of non-arrhythmic death was most pronounced among patient with advanced heart failure. 19 In the MADIT-II study, the small subgroup with GFR <35 showed a much higher risk of non-arrhythmic mortality compared to those with GFR>35%. These data support the notion that competing risks of nonarrhythmic mortality in CKD patients may overwhelm potential benefit from prevention of arrhythmic death. In support of this theory, we found that CKD patients experienced a lower proportion of arrhythmic deaths compared to eGFR>=60 patients. Second, multiple studies have documented markedly decreased survival time among ICD-recipients with CKD in comparison to their non-CKD counterparts. 4,6,20 Decreased ICD exposure time because of decreased survival may also reduce opportunity of ICDs to prevent of arrhythmic death and reduce overall mortality. Finally, higher defibrillation thresholds observed in CKD patients might contribute to decreased ICD effectiveness in preventing arrhythmic death.21 Among 95 patients with a broad spectrum of baseline kidney function, Wase et. al. described a trend towards increasing defibrillation thresholds with worsening kidney function (mean defibrillation thresholds for stage 1–2 CKD, stages 3–4 CKD and ESRD/stage 5 CKD were 11.96 ± 4.56 J, 14.51 ±5.16 J, and 16.33 ±5.3 J, respectively.) Although our analysis was hampered by a significant proportion of cardiac deaths which could not be distinguished as either arrhythmic or nonarrhythmic, ICD treatment was only associated with reduction in arrhythmic death among patients with eGFR>=60, whereas no significant reduction was seen among patients with eGFR<60.
Our study has some limitations. First, although we provide the most robust published data on the efficacy of the ICD in patients with moderate CKD, we had limited data on patients with eGFR < 30 ml/min/1.73m2 and no data on patients with end-stage kidney disease. A significant number of sudden deaths in CKD patients occur among those without decreased LVEF and those without CHF symptoms, and these patients were excluded from our study cohort. Therefore, we cannot generalize our conclusions to these important subgroups of patients at risk. Second, since we do not have information on the creatinine assay methodology used in the primary trials, we cannot exclude the possibility of measurement error introduced by the use of non-standardized assays, or the possibility that the CKD-EPI estimating equation may not be accurate among patients with CHF since it has never been validated in this population. Third, although we accounted for a large set of measured covariates that were available across trials, we cannot exclude the possibility that differences in trial-to-trial measurement techniques and unmeasured covariates contribute to residual confounding. Fourth, our analysis assumes that missing data occurred at random with missing covariate values imputed based on the trial-specific empirical distribution, which may or may not reflect the actual process leading to missing values. Finally, our data on mode of death is limited by non-uniformity of definitions across the included trials and missing data. A larger study of mode of death among ICD recipients with CKD would be productive in order to better understand the relationship between competing risks of death and ICD efficacy.
In conclusion, our study provides evidence that the severity of CKD proportionally attenuates the benefit of the ICD. In the absence of randomized trial data geared towards addressing the efficacy of ICDs in this subgroup, clinicians should carefully counsel potential primary prevention ICD recipients with CKD about the likelihood of reduced benefit and increased risk compared to estimates generated from clinical trials enrolling predominantly patients with preserved renal function. While small pilot clinical trials are underway among patients with end stage kidney disease22, the uncertainty of the benefit of primary prevention ICD in the broader population of patients with CKD should provide the rationale for further investigation into appropriate risk stratification methods.
Supplementary Material
Supplemental Figure 1: Bayesian-Weibull Covariate-Adjusted Odds Ratio for Risk of Rehospitalization associated with ICD as a function of baseline eGFR. Shaded areas reflect 95% pointwise posterior credible intervals.
ACKNOWLEDGEMENT
This project was funded under grant number 5R01HS018505 from the Agency for Heathcare Research and Quality (AHRQ), U.S. Department of Health and Human Services. The opinions expressed in this document are those of the authors and do not reflect the official position of AHRQ or the U.S. Department of Health and Human Services.
Footnotes
Financial Disclosure: None
REFERENCES
- 1.Pun PH, Smarz TR, Honeycutt EF, Shaw LK, Al-Khatib SM, Middleton JP. Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int. 2009 Sep;76(6):652–658. doi: 10.1038/ki.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deo R, Lin F, Vittinghoff E, Tseng ZH, Hulley SB, Shlipak MG. Kidney dysfunction and sudden cardiac death among women with coronary heart disease. Hypertension. 2008 Jun;51(6):1578–1582. doi: 10.1161/HYPERTENSIONAHA.107.103804. [DOI] [PubMed] [Google Scholar]
- 3.Russo AM, Stainback RF, Bailey SR, et al. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. Heart rhythm : the official journal of the Heart Rhythm Society. 2013 Apr;10(4):e11–58. doi: 10.1016/j.hrthm.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Sakhuja R, Keebler M, Lai TS, McLaughlin Gavin C, Thakur R, Bhatt DL. Meta-analysis of mortality in dialysis patients with an implantable cardioverter defibrillator. Am J Cardiol. 2009 Mar 1;103(5):735–741. doi: 10.1016/j.amjcard.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Charytan DM, Patrick AR, Liu J, et al. Trends in the use and outcomes of implantable cardioverter-defibrillators in patients undergoing dialysis in the United States. Am J Kidney Dis. 2011 Sep;58(3):409–417. doi: 10.1053/j.ajkd.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 6.Cuculich PS, Sanchez JM, Kerzner R, et al. Poor prognosis for patients with chronic kidney disease despite ICD therapy for the primary prevention of sudden death. Pacing Clin Electrophysiol. 2007 Feb;30(2):207–213. doi: 10.1111/j.1540-8159.2007.00651.x. [DOI] [PubMed] [Google Scholar]
- 7.Bigger JT., Jr. Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. Coronary Artery Bypass Graft (CABG) Patch Trial Investigators. N Engl J Med. 1997 Nov 27;337(22):1569–1575. doi: 10.1056/NEJM199711273372201. [DOI] [PubMed] [Google Scholar]
- 8.Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004 May 20;350(21):2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 9.Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverterdefibrillator after acute myocardial infarction. N Engl J Med. 2004 Dec 9;351(24):2481–2488. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 10.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999 Dec 16;341(25):1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 11.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996 Dec 26;335(26):1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 12.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002 Mar 21;346(12):877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 13.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005 Jan 20;352(3):225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010 Apr;55(4):622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibrahim JG CM, Sinha D. Bayesian survival analysis. Springer-Verlag; 2001. [Google Scholar]
- 16.Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res. 2001 Aug;10(4):277–303. doi: 10.1177/096228020101000404. [DOI] [PubMed] [Google Scholar]
- 17.Goldenberg I, Moss AJ, McNitt S, et al. Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol. 2006 Aug 15;98(4):485–490. doi: 10.1016/j.amjcard.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 18.Kent DM, Alsheikh-Ali A, Hayward RA. Competing risk and heterogeneity of treatment effect in clinical trials. Trials. 2008;9:30. doi: 10.1186/1745-6215-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alsheikh-Ali AA, Trikalinos TA, Ruthazer R, et al. Risk of arrhythmic and nonarrhythmic death in patients with heart failure and chronic kidney disease. Am Heart J. 2011 Jan;161(1):204–209. e201. doi: 10.1016/j.ahj.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Eckart RE, Gula LJ, Reynolds MR, Shry EA, Maisel WH. Mortality following defibrillator implantation in patients with renal insufficiency. J Cardiovasc Electrophysiol. 2006 Sep;17(9):940–943. doi: 10.1111/j.1540-8167.2006.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wase A, Basit A, Nazir R, et al. Impact of chronic kidney disease upon survival among implantable cardioverter-defibrillator recipients. J Interv Card Electrophysiol. 2004 Dec;11(3):199–204. doi: 10.1023/B:JICE.0000048570.43706.34. [DOI] [PubMed] [Google Scholar]
- 22.de Bie MK, Lekkerkerker JC, van Dam B, et al. Prevention of sudden cardiac death: rationale and design of the Implantable Cardioverter Defibrillators in Dialysis patients (ICD2) Trial--a prospective pilot study. Curr Med Res Opin. 2008 Aug;24(8):2151–2157. doi: 10.1185/03007990802237343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Bayesian-Weibull Covariate-Adjusted Odds Ratio for Risk of Rehospitalization associated with ICD as a function of baseline eGFR. Shaded areas reflect 95% pointwise posterior credible intervals.