Abstract
Background
The purpose of this study was to determine whether baseline salivary inflammatory biomarkers could discriminate between different clinical levels of disease and/or predict clinical progression over a 3-week stent-induced biofilm overgrowth (SIBO) period.
Materials and Methods
168 participants were enrolled in a 21-day experimental gingivitis investigation and grouped according to clinical measures of periodontal status of health and diseased individuals representing each of five biofilm gingival interface (BGI) periodontal groups (H, G, P1, P2, P3). Stents were used to prevent plaque removal during brushing over one maxillary and one mandibular posterior dental sextant for 21 days. Clinical periodontal parameters and unstimulated saliva were collected at screening, baseline, and each week during SIBO. Saliva samples were assessed for levels of 13 different biomarkers by multiplex immunoassay.
Results
Higher salivary levels of interleukin (IL)-1β, matrix metalloproteinase (MMP)-3, MMP-8, MMP-9, and neutrophil gelatinase-associated lipocalin (NGAL) were found in diseased groups compared to healthy at baseline. Conversely, higher IL-1 receptor antagonist (ra) levels were found in healthy patients at baseline. In addition, during SIBO MMP-1, tissue inhibitor of metalloproteinase (TIMP)-1, and TIMP-2 levels increased across all participant groups. A stepwise linear regression model using all salivary biomarkers demonstrated that at baseline increased IL-1ra (p=0.0044) and IL-6 (p=0.0093) were the two best predictors of change in probing depths during SIBO.
Conclusions
In summary, this investigation supports salivary levels of IL-1ra and IL-6 as potential indicators for significant probing depth changes during induced gingival inflammation. In addition, participants from BGI-P3 group (severe periodontitis) demonstrated elevated baseline levels of IL-1β, MMP-3, MMP-8, MMP-9, and NGAL compared to other study groups strengthening the relevance of participant's biological phenotype on salivary biomarkers expression
Introduction
The potential application of saliva-based diagnostic tests for periodontal disease represents an exciting new opportunity for chair-side diagnostics based on its non-invasive characteristics. Combining fast turnaround with non-invasive sampling will enable clinicians to stratify patients by risk and allocate treatment accordingly. Recent reports have demonstrated that salivary and biofilm biomarkers offer potential for the identification of periodontal disease progression or stability.1, 2 However, despite advances in research methodology and laboratory assays in order to identify biomarkers associated with chronic periodontal disease, it is still unclear how to effectively utilize this technology for disease diagnosis and detection of disease activity.
Periodontitis is both a polymicrobial and multifactorial disease where clinical measures, such as pocket depth (PD), clinical attachment level (CAL) and bleeding on probing (BOP), provides retrospective history of disease and current status, but has limited predictive value. A cross-sectional data analysis identified putative biomarkers from saliva and anaerobic pathogens that were strongly related to disease status.3 Recently, a longitudinal study evaluating the potential for prediction of periodontal disease progression demonstrated that saliva and biofilm biomarkers offer potential for the identification of periodontal disease activity.1
Given the complex nature of periodontal disease, a precise evaluation of periodontal disease activity becomes a clinical challenge. To date, limited longitudinal studies have been conducted to identify biomarkers that predict disease progression prior to radiographic and clinical manifestations.1, 4, 5 Still seeking to speed the translation of research results into therapies, an alternative methodology to evaluate periodontal disease activity is through the experimental gingivitis design. Contemporary experimental gingivitis studies have incorporated stents to encourage compliance, a method termed stent-induced biofilm overgrowth (SIBO), and to evaluate periodontal disease activity in participants with pre-existing periodontal disease.6-8 It has been reported that gingival crevicular fluid (GCF) analysis can indicate differences within specific inflammatory mediators that reflect the magnitude of the clinical changes seen in this gingivitis induction model.8
The aim of this study was to understand whether salivary biomarkers can be used to discriminate among health, gingivitis, and three levels of chronic periodontitis severity and whether salivary biomarkers can predict periodontal disease activity during SIBO.
Materials & Methods
Patient Population
One hundred sixty eight participants were recruited and inform consent was obtained at the Center for Oral and Systemic Diseases clinic between 2005-2007 (University of North Carolina, Chapel Hill, North Carolina). The study was approved by the University of North Carolina Health Sciences Institutional Review Board. Individuals age 18 years and older were eligible for the study. All individuals were required to have at least four teeth in the functional dentition with a minimal of three adjacent teeth with interproximal papilla in each posterior sextant receiving the SIBO, not received periodontal treatment or antibiotic therapy for medical or dental reasons for 1 month prior to the start of the investigation, and not taking long-term medications affecting periodontal status, including calcium antagonists, anticonvulsives, immunosuppressives, and anti-inflammatory medications. Exclusion criteria included a history of metabolic bone diseases, autoimmune diseases, unstable diabetes, or post-menopausal osteoporosis. Pregnant or lactating women were not allowed to participate in the study. Smokers and individuals with diabetes were not excluded, with no stratification performed on these risk factors.
Study Design
This prospective cohort study involved the induction of experimental biofilm overgrowth using stents as recently described.8 All teeth were assessed for periodontal clinical measures by calibrated examiners. Clinical parameters including PD, CAL, and BOP, were measured at six sites per tooth. Other clinical assessments included measures of plaque accumulation (PI)9 and gingival inflammation index (GI). 10Based on clinical assessments, patients were enrolled in one of the following five categories: (1) Biofilm Gingival Interface (BGI) health (H), all PD<3mm, BOP<10%; (2) BGI-gingivitis (G), all PD<3mm, BOP≥10%; (3) BGI-P1 (P1), 1+ site with PD>3mm, BOP≤10%; (4) BGI-P2 (P2), 1+ site with PD>3mm, BOP>10% but BOP≤50%; (5) BGI-P3 (P3), 1+ site with PD>3mm, BOP>50%. These five categories are clearly different from traditional definitions of health, gingivitis, mild periodontitis, moderate periodontitis, and severe periodontitis as they considered use BOP and PD only. The BGI categories display a similar gradient with increasing severity and extent of clinical signs from BGI-H through BGI-P3 categories and reflect current periodontal status rather than historical levels of disease activity.11
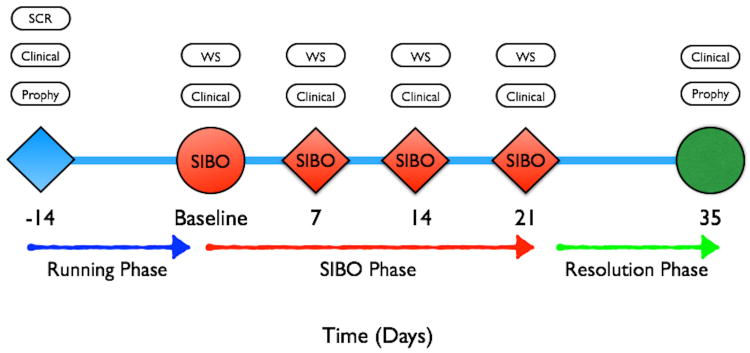
Figure 1 illustrates the timeline of the study. After a prophylaxis at (−) 2weeks, participants received acrylic stents at the baseline visit to be worn only during their oral hygiene routine in order to abstained from brushing and flossing teeth in one maxillary and one mandibular posterior sextant during a three- week period. The sextants selected were the right maxillary and mandibular sextants, except when fewer than four teeth were present in one posterior sextant. If there were fewer than 4 teeth in a right posterior sextant, the opposing posterior sextant was used for the stent placement. Patients were monitored for safety every week and after the induction of experimental biofilm overgrowth through 21 days, participants reinstituted normal full mouth oral hygiene and daily plaque control. Participants were followed for the next four weeks during gingivitis resolution. Prophylaxis with scaling and root planing were performed at completion of the study. The investigation was a single masked study. Participants were excluded from the study or analysis if any of the following conditions applied: (1) changes in the participant's medical status or medications that were not negligible; (2) use of antibacterial rinses; (3) use of dentifrices, toothbrush, dental floss, or any irrigation device during the non-hygiene phase of the study; (4) participant's inability or noncompliance to wear their stents or shields over one mandibular and one maxillary sextant during daily brushing procedures;(5) use of oral antibiotics and non-steroidal anti-inflammatory drugs; (6) participants requiring treatment for an acute medical or dental condition during the study were withdrawn from the investigation. Any site undergoing PD increase of > 2 mm from the baseline measurement was deemed as “progressing” and participant was exited from the study and given scaling and root planing treatment as a rescue therapy.
Figure 1.
Study timeline illustrating data collection and treatment delivery during running and resolution phase. SCR: screening, Prophy: adult prophylaxis or scaling and root planing, SIBO: stent-induced biofilm overgrowth, Clinical: probing depth, clinical attachment level, bleeding on probing, plaque index, gingival index, WS: whole saliva.
Whole Saliva Collection and Analysis
Unstimulated whole saliva was collected at the beginning of each study visit with passive drooling into sterile plastic tubes from all patients.12 At each time point, approximately 3 mL of unstimulated saliva were collected into a 15 mL plastic conical tube. Participants refrained from eating, drinking, chewing gum, breath mints, or performing oral hygiene procedures for at least one hour prior to saliva collection. Samples were further placed on ice and aliquoted prior to storage at -80°C. Whole saliva samples were tested for the presence of interleukin (IL)-1β, IL-1 receptor antagonist (ra), IL-8, monocyte chemoattractant protein-1 (MCP-1), matrix metalloproteinase (MMP)-1, MMP-3, MMP-8, MMP-9, tissue inhibitor of metalloproteinase (TIMP)-1, TIMP-2, TIMP-3, and TIMP-4.
In this study, 3-ml unstimulated saliva was analyzed in duplicate for samples collected at Days 0 (Baseline) and 21 (peak induction) for IL-1β, IL-1ra, IL-8, MCP-1, MMP-1, MMP-3, MMP-8, MMP-9, TIMP-1-4 using a Bio-Plex 2000 multiplex format.§ Neutrophil gelatinase-associated lipocalin (NGAL) levels were measured by ELISA.§ After thawing, each sample was re-centrifuged again and diluted with assay buffer according to the manufacturer's recommendations for serum sample analysis. Local levels of inflammatory biomarkers were determined following methods described by Offenbacher et al.8 The following list of biomarkers were assayed followed by the mean minimum detection level in picograms per milliliter shown in brackets []: IL-1β [0.27], IL-8 [5.3], MCP-1 [0.16], MMP-1 [4.4], MMP-3 [1.3], MMP-8 [8.9], MMP-9 [7.4], TIMP-1 [230], TIMP-2 [730], TIMP-3 [2300], TIMP-4 [120], IL-1ra [2.06] and NGAL [7.8]. Volumes of 100 μl were used for each panel of mediators. All mediator values were corrected for assay dilution and expressed as a saliva concentration. Mean values and standard deviations were computed for stent sites for each patient at each visit. Log mean values of mediator concentrations were computed for comparisons, as the mean values were not normally distributed.
Statistical Methods
Statistical analyses and data management were performed using SAS║. Statistical significance was set at p<0.05, and the unit of analysis was the individual participant. A pre-study power analysis indicated a minimum sample size of eight test/control pairs to yield power of 90% with alpha of 5% for IL-1β changes. In four pairs of samples tested in replicate, the mean inter-group difference in IL-1β expression was 10.7 with a standard deviation of 16.0. Therefore, the intended sample of 30 participants per group would be sufficient to detect differences in the expression of individual inflammatory markers of interest from baseline to induction of SIBO. The expected magnitude of the changes and the variance in other biomarkers were unknown. For this reason, a minimum of thirty three participants were enrolled per group for a total of 168 participants. Statistical comparisons were made using chi-square for categorical variables and ANOVA for continuous variables. Clinical and biochemical changes were analyzed across groups using a mixed-models analysis. For mediators that showed overall significance between groups, generalized linear modeling allowed analysis of each mediator independent of changes of the others. Changes in clinical signs were used to identify high and low responders for each of the BGI conditions based upon the clinical response to biofilm overgrowth dichotomizing on the median change in clinical sign at 21 days as compared to baseline.
Results
A total of 299 patients were screened for study eligibility; of these, 170 patients met all study criteria and were evenly distributed among the five BGI categories. During the study two participants, both from the H and P1 group respectively, withdrew before study completion. Demographics baseline characteristics of this cohort have been described in Table 1. The mean age for participants differed significantly between H and G compared to P1, P2, and P3 (p=0.01). Sixty- seven percent overall were female. Only participants in group G were of normal weight by body mass index (BMI<25kg/m2); all the other BGI groups were overweight, particularly P2 and P3. Overall 60.1% were African-Americans. Twelve percent of the population were current cigarette smokers, and they were evenly distributed in all BGI groups. Only 4% of the population were diabetic and no significant difference was detected in the distribution among the BGI groups. The clinical presentation of the study participants at baseline is described in Table 2. As expected, at baseline, plaque scores and BOP were significantly lower in H, and P1 than in the other BGI groups. As anticipated, P2 and P3 presented with significantly higher GI scores than P1. Similarly, P1, P2, and P3 presented with increased mean CAL ≥ 3mm and PD ≥ 4mm in comparison to H and G. No participants were exited due to increasing of PD > 2mm. Participants with increasing PD during the SIBO were found to respond to therapy at exit with PD returning to baseline or improving from baseline. No participants experienced irreversible loss of attachment.
Table 1.
Study demographics.
| H | G | P1 | P2 | P3 | p-value | |
|---|---|---|---|---|---|---|
| Female (n) | 23 | 23 | 23 | 23 | 20 | |
| Male (n) | 10 | 11 | 10 | 11 | 14 | 0.87 |
| African American (n) | 21 | 26 | 23 | 16 | 15 | |
| Caucasian (n) | 7 | 6 | 7 | 17 | 14 | |
| Other (n) | 5 | 2 | 3 | 1 | 4 | 0.03 |
| Diabetic (n) | 3 | 0 | 1 | 1 | 2 | |
| No (n) | 30 | 34 | 32 | 33 | 32 | 0.41 |
| Current Smoker (n) | 3 | 5 | 7 | 3 | 3 | |
| No (n) | 30 | 29 | 26 | 31 | 30 | 0.47 |
| Mean (StdErr) Age | 30.3 (1.9) | 29.6 (1.9) | 36.5 (1.9) | 37.0 (1.9) | 34.4 (1.9) | 0.01 |
| Mean (StdErr) BMI | 28.1 (1.3) | 24.5 (1.2) | 27.4 (1.3) | 30.7 (1.2) | 29.6 (1.2) | 0.008 |
BGI-Healthy: H; BGI-Gingivitis: G; BGI-P1: P1; BGI-P2: P2; BGI-P3: P3; n: number of patients; StdErr: Standard error; BMI: Body mass index.
Table 2. Baseline clinical parameters of the five BGI group participants by extent scores.
| H | G | P1 | P2 | P3 | p-value | |
|---|---|---|---|---|---|---|
| Mean % plaque score ≥ 1 (StErr) | 47.7 (3.7) | 63 (3.9) | 52.2 (4) | 76.7 (3.8) | 79.2 (3.9) | <0.0001 |
| Mean % GI ≥ 1(StErr) | 65.7 (3.5) | 74.7 (3.7) | 72.1 (3.8) | 94.3(3.6) | 93.8 (3.7) | <0.0001 |
| Mean % BOP(StErr) | 5.7 (1.3) | 22.6 (1.4) | 6.9 (1.5) | 30 (1.4) | 59.5 (1.4) | <0.0001 |
| Mean #sites with CAL ≥3mm (StErr) | 0.2 (0.7) | 1.3 (0.8) | 1.8 (0.8) | 2.3 (0.8) | 5.1 (0.8) | <0.0001 |
| Mean #sites with PD ≥4mm (StErr) | 0 | 0 | 2.2 (0.7) | 4.1 (0.6) | 7.8 (0.6) | <0.0001 |
p-value <0.0001
StErr: Standard error
Baseline Results Comparing Salivary Biomarkers
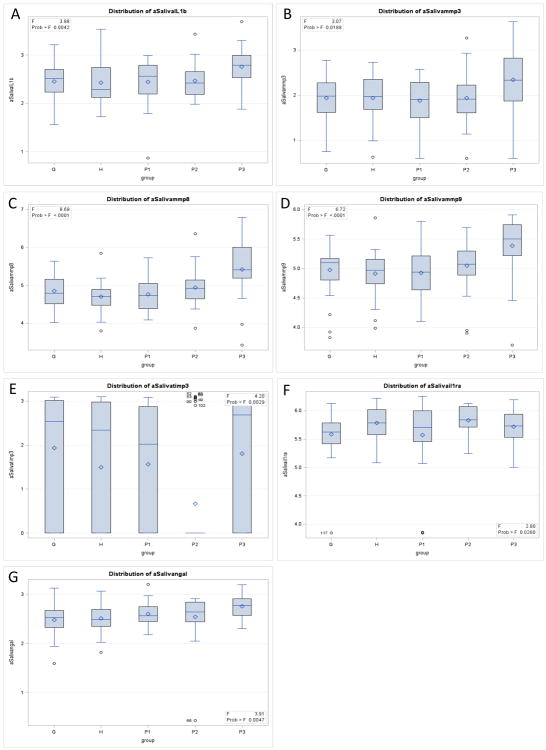
The baseline log10 mean (StdErr) values by BGI category of salivary biomarker levels comparing the five groups are shown in Table 3. An analysis of variance (ANOVA) was performed to detect differences between groups adjusted by Bonferroni correction. Significant differences were found at baseline between groups for IL-1β, MMP-3, MMP-8, MMP-9, TIMP-3, and NGAL. BGI P3 participants had significantly higher log mean values compared to other groups for IL-1β, MMP-3, MMP-8, MMP-9, and NGAL. Compared to H participants, P3 participant levels were more than two-fold elevated for IL-1β, MMP-3, MMP-9, and TIMP-3, and more than five-fold elevated for MMP-8. Mean TIMP-3 was significantly lower for P2 compared to other groups.
Table 3.
Baseline log10 mean (StdErr) saliva mediator by BGI category.
| Health | G | P1 | P2 | P3 | Overall p-value | |
|---|---|---|---|---|---|---|
| IL-1β | 2.43 (0.07) | 2.46 (0.07) | 2.45 (0.07) | 2.47 (0.07) | 2.76 (0.07)a | 0.004 |
| IL-8 | 2.75 (0.06) | 2.80 (0.06) | 2.77 (0.06) | 2.78 (0.06) | 2.98 (0.06) | 0.07 |
| MCP-1 | 2.28 (0.06) | 2.44 (0.06) | 2.45 (0.06) | 2.49 (0.06) | 2.48 (0.06) | 0.07 |
| MMP-1 | 1.50 (0.17) | 1.35 (0.17) | 1.59 (0.18) | 1.18 (0.15) | 1.13 (0.17) | 0.24 |
| MMP-3 | 1.94 (0.11) | 1.94 (0.11) | 1.88 (0.11) | 1.94 (0.10) | 2.34 (0.11)a | 0.02 |
| MMP-8 | 4.70 (0.10) | 4.85 (0.10) | 4.76 (0.10) | 4.94 (0.09) | 5.42 (0.10)a | <0.0001 |
| MMP-9 | 4.92 (0.07) | 4.98 (0.07) | 4.93 (0.08) | 5.05 (0.08) | 5.39 (0.08) a | <0.0001 |
| TIMP-1 | 5.26 (0.05) | 5.24 (0.05) | 5.18 (0.05) | 5.08 (0.05) | 5.21 (0.05) | 0.15 |
| TIMP-2 | 4.53 (0.04) | 4.78 (0.04) | 4.50 (0.04) | 4.45 (0.04) | 4.62 (0.04) | 0.06 |
| TIMP-3 | 1.49 (0.24) | 1.93 (0.24) | 1.56 (0.24) | 0.67 (0.24) b | 1.81 (0.24) | 0.003 |
| TIMP-4 | 1.11 (0.04) | 1.11 (0.04) | 1.08 (0.04) | 1.05 (0.04) | 1.14 (0.04) | 0.67 |
| IL-1ra | 5.79 (0.07) | 5.58 (0.07) | 5.57 (0.07) | 5.83 (0.07) | 5.72 (0.07) | 0.03 |
| NGAL | 2.51 (0.05) | 2.48 (0.05) | 2.60 (0.05) | 2.54 (0.05) | 2.75 (0.05) a | 0.005 |
StdErr = Standard error
= Statistically different from groups H, G, P1, and P2
= Statistically different from groups H, G, P1, and P3
Clinical and Salivary Biomarker Responsiveness to Biofilm Overgrowth
Overall, participants showed significant increases in PI, GI, and BOP across all groups during the stent induction period (Table 4) (p < 0.05). The group G participants also showed a slight but statistically significant increase in attachment loss (p=0.03) during SIBO. Similarly, P1 participants showed a small, but significant increase in PD measurements (p=0.047). When grouped together, H and G participants showed significant increased PI, GI, BOP, and PD (p < 0.05). By contrast, P1-P3 grouped together only demonstrated significant increased PI, GI, and BOP during SIBO (p < 0.05). P2 and P3 showed no changes in probing depths or attachment levels.
Table 4. Mean (StdErr) delta clinical sign (Day 21-Day 0) during SIBO by BGI group.
| BGI Category | Mean (StdErr) | p value |
|---|---|---|
| BGI (Health) | ||
| Plaque | 0.85 (0.11) | <0.0001 |
| Gingival Index | 0.44 (0.06) | <0.0001 |
| Bleeding On Probing | 19.4 (4.03) | <0.0001 |
| Probing Depth (mm) | 0.05 (0.06) | 0.46 |
| Attachment Level (mm) | -0.08 (0.05) | 0.12 |
| BGI (Gingivitis) | ||
| Plaque | 0.79 (0.09) | <0.0001 |
| Gingival Index | 0.29 (0.05) | <0.0001 |
| Bleeding On Probing | 20.5 (3.49) | <0.0001 |
| Probing Depth (mm) | 0.18 (0.09) | 0.052 |
| Attachment Level (mm) | -0.11 (0.05) | 0.03 |
| BGI (P1) | ||
| Plaque | 0.70 (0.10) | <0.0001 |
| Gingival Index | 0.21 (0.05) | 0.0001 |
| Bleeding On Probing | 21.0 (4.47) | <0.0001 |
| Probing Depth (mm) | 0.11 (0.05) | 0.047 |
| Attachment Level (mm) | 0.01 (0.05) | 0.91 |
| BGI (P2) | ||
| Plaque | 0.82 (0.10) | <0.0001 |
| Gingival Index | 0.28 (0.06) | 0.0001 |
| Bleeding On Probing | 15.7 (4.05) | 0.0005 |
| Probing Depth (mm) | 0.03 (0.06) | 0.67 |
| Attachment Level (mm) | 0.03 (0.06) | 0.63 |
| BGI (P3) | ||
| Plaque | 0.65 (0.08) | <0.0001 |
| Gingival Index | 0.15 (0.04) | 0.002 |
| Bleeding On Probing | 8.30 (4.47) | 0.07 |
| Probing Depth (mm) | -0.06 (0.06) | 0.30 |
| Attachment Level (mm) | 0.02 (0.08) | 0.79 |
| BGI (Health + Gingivitis) | ||
| Plaque | 0.82 (0.07) | <0.0001 |
| Gingival Index | 0.37 (0.04) | <0.0001 |
| Bleeding On Probing | 19.9 (2.67) | <0.0001 |
| Probing Depth (mm) | 0.11 (0.05) | 0.04 |
| Attachment Level (mm) | -0.09 (0.03) | 0.008 |
| BGI (P1-P3) | ||
| Plaque | 0.73 (0.05) | <0.0001 |
| Gingival Index | 0.22 (0.03) | <0.0001 |
| Bleeding On Probing | 15.8 (2.51) | <0.0001 |
| Probing Depth (mm) | 0.02 (0.03) | 0.48 |
| Attachment Level (mm) | 0.02 (0.04) | 0.66 |
StdErr = Standard error
During SIBO, pooling all participants, there were significant increases in salivary concentrations from baseline to peak induction for MMP-1, TIMP-1, and TIMP-2 (p < 0.0038) (Table 5). After adjusting by Bonferroni analysis, TIMP-4 did not meet statistical significance. When subdivided by disease category there were no significant changes in salivary biomarker levels during SIBO induction. In addition, no mediator showed a significant decrease during the biofilm overgrowth phase.
Table 5.
Mixed models: Log10 mean (StdErr) saliva mediator by time point.
| Baseline | Peak | p-value | |
|---|---|---|---|
| IL-1b | 2.51 (0.03) | 2.50 (0.03) | 0.60 |
| IL-8 | 2.81 (0.03) | 2.82 (0.03) | 0.73 |
| MCP-1 | 2.43 (0.03) | 2.44 (0.03) | 0.39 |
| MMP-1 | 1.34 (0.08) | 1.51 (0.08) | 0.002 |
| MMP-3 | 2.01 (0.05) | 1.97 (0.04) | 0.39 |
| MMP-8 | 4.94 (0.05) | 4.89 (0.05) | 0.20 |
| MMP-9 | 5.05 (0.04) | 5.07 (0.04) | 0.55 |
| TIMP-1 | 5.20 (0.02) | 5.28 (0.02) | 0.0002 |
| TIMP-2 | 4.52 (0.02) | 4.60 (0.02) | <0.0001 |
| TIMP-3 | 1.50 (0.11) | 1.56 (0.11) | 0.12 |
| TIMP-4 | 1.10 (0.02) | 1.15 (0.02) | 0.03 |
| IL-1ra | 5.70 (0.03) | 5.72 (0.02) | 0.42 |
| NGAL | 2.58 (0.02) | 2.57 (0.02) | 0.76 |
Baseline Salivary Biomarkers Predictors of Clinical Changes
A stepwise linear regression model was used to evaluate the ability of salivary biomarkers to estimate changes in clinical periodontal parameters. From all salivary biomarkers analyzed in the study, IL-1ra (p=0.0044) and IL-6 (p=0.0093) were the two best predictors of change in probing depths during the SIBO, with an overall r2 value of 0.37. The effect slope for IL-1ra was positive (0.675) and negative for IL-6 (-0.246). The results from this study demonstrated no significant salivary predictors of changes in BOP, GI, or CAL.
Discussion
In this study we sought to examine the ability of salivary biomarkers to discriminate different periodontal clinical phenotypes and to evaluate a patient's risk of active periodontal disease as related to PD changes during SIBO. Results demonstrated significant differences in baseline salivary biomarkers among participant groups, but no differences in response to SIBO. This finding differs from other SIBO studies of cytokine changes in gingival crevicular fluid, suggesting that saliva, although a convenient sample, is not as sensitive to clinical changes as GCF. This is logical, since the salivary cytokines arguably represent a pooled GCF sample, which is rich in cytokines. Moreover, mucosal and salivary gland tissues can contribute to salivary cytokines.13 The BGI classification11, adopted in this study, was intended to segregate participants into disease categories with more homogenous biological phenotypes. This was partially achieved, as there were differences in salivary biomarkers at baseline across groups. However, it is interesting to note that significant clinical changes were only observed among the H and G participants that experienced transient increases in PD. Conversely, there were changes in cytokine response that related to clinical changes which occurred across categories.
As previously described by our group, stent-induced biofilm overgrowth in a model of experimental gingivitis is associated with marked, but reversible increases in the concentrations of cytokines and concomitant suppression of multiple chemokines present in gingival crevicular fluid.8 In this investigation we did not see those cytokine and chemokine changes in saliva samples. An experimental gingivitis study reported by Lee and colleagues14 also identified specific salivary biomarker and microbial signatures that are associated with gingival inflammation. However, only participants with healthy periodontal clinical phenotype and no history of periodontal tissue loss were included in the study. To the best of our knowledge, this study is unique in that it provides an analysis of host-response biomarker signatures associated with five different biological phenotypes during stent-induced biofilm overgrowth and its association with active inflammatory processes in a longitudinal study.
During periodontal disease, host inflammatory cells are recruited, and inflammatory cytokines are released from fibroblasts, macrophages, connective tissue, and junctional epithelial cells. Consequently, host-derived enzymes, such as MMP-8 and MMP-9, are released by PMNs and osteoclasts, leading to connective tissue and alveolar bone degradation. Currently, studies have demonstrated the association of host-response salivary biomarkers with periodontal disease.3, 15, 16 Our results showed obvious differences at baseline between P3 group and all other participants (Table 3). The finding of significant differences in IL-1β, MMP-3, MMP-8, MMP-9, TIMP-3, and NGAL between groups is in contrast to Teles and collaborators 17, who found no significant differences in salivary granulocyte-macrophage colony-stimulating factor, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IFNγ, or TNF-α between healthy and periodontitis participants. Though, those participants were grouped by periodontal clinical phenotype rather than BGI classification. Conversely, our results are in accordance with several other studies showing higher levels of IL-1β5, 15, 18-21, MMP-83, 22, 23, MMP-9 23, and NGAL24 in the saliva of gingivitis and periodontitis participants.
Several mediators, including MMP-1, TIMP-1, and TIMP-2 increased significantly from baseline to peak of induction at day 21 (Table 5). To our knowledge, this is the first human study demonstrating changes in TIMPs during experimental gingivitis. The increased levels of TIMPs may be due to a local response to dampen inflammation and prevent protein degradation. MMPs are generally produced in an inactive (preMMP) form and must be cleaved to become functional. It is possible that a collagenolytic activity assay of saliva would be more useful than one measuring collagenase concentration alone. In an attempt to validate a soluble biotinylated-collagen assay, Mancini and collaborators 25 found significant differences in GCF MMP-8 activity from periodontitis patients at baseline, re-evaluation, and maintenance visits. In a different study, the same group showed that the activity levels of MMPs did not correlate significantly with Western blot analysis of zymogen versus active form.26 Our results demonstrated that MMP-3, MMP-8, and MMP-9 did not significantly increase during induced gingivitis. A Possible explanation is that initial changes are mostly expressed by enzymes being cleaved into their active forms. In addition, a three-week study may be too short to induce measurable changes in the amounts of these enzymes.
Increases in plaque, gingival index, and bleeding on probing across all groups are to be expected in an experimental gingivitis study. Our results, in accordance with the literature 8, 27, demonstrated significant increases in PI, GI, and BOP at day 21 compared to baseline (Table 4). participants under the P3 classification did not demonstrate significant changes regarding GI and BOP at day 21. This can be expected by the fact that to be classified under this biologic phenotype category, participants must have more than 50% BOP sites at baseline.
In our study, baseline IL-1ra and IL-6 levels were found to be significant predictors of change in PD. IL-1ra is the antagonist for IL-1β receptor, blocking its action. As IL-1β is a pro-inflammatory molecule, high levels of its receptor antagonist could indicate a compensatory mechanism of negative feedback or the release of molecules that are normally tissue-bound. IL-6 is known to be a pro-inflammatory molecule that shifts the immune response towards a cell-mediated reaction. A study demonstrated that IL-6 is elevated in participants with chronic periodontitis and decreases after periodontal therapy.28 Our results showed that IL-6 was not significantly different between groups at baseline. However, high IL-6 levels at baseline were able to predict an increase in PD. Recently, Giannobile and collaborators demonstrated that patients with high baseline levels of salivary IL-6 and MMP-1 are at higher risk for developing a heightened gingival inflammatory response compared to individuals displaying low levels of these biomarkers.14
In summary, this investigation supports salivary levels of IL-1ra and IL-6 as potential indicators for significant probing depth changes during induced gingival inflammation. In addition, P3 participants demonstrated elevated baseline levels of IL-1β, MMP-3, MMP-8, MMP-9, and NGAL compared to other study groups strengthening the relevance of participant's biological phenotype on salivary biomarkers expression. Clinical implications include improved patient monitoring and control of disease activity. Future large study populations will be needed to validate the use of salivary biomarkers to identify susceptible patients in order to provide them preventive therapy to avoid irreversible periodontal breakdown.
Figure 2.
Acknowledgments
The authors declare that there are no conflicts of interest in this study. This study was funded by Philips Oral Healthcare, Inc., and NIH grants RR00046 and UL1RR025747. JTM was supported by the Carolina Postdoctoral Program for Faculty Diversity at the University of North Carolina at Chapel Hill. SJK was supported by T90 DE021986 NIH training grant. NY was supported by R90DE021986 NIH training grant. MBA and MW are employees of Philips Oral Healthcare Inc., Dental & Scientific Affairs.
Footnotes
(R&D Systems, Minneapolis, MN, USA)
(SAS Institute, Inc., Cary, NC, USA, version 9.1.3)
References
- 1.Kinney JS, Morelli T, Braun T, et al. Saliva/pathogen biomarker signatures and periodontal disease progression. J Dent Res. 2011;90:752–758. doi: 10.1177/0022034511399908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinney JS, Morelli T, Oh M, et al. Crevicular fluid biomarkers and periodontal disease progression. J Clin Periodontol. 2014;41:113–120. doi: 10.1111/jcpe.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramseier CA, Kinney JS, Herr AE, et al. Identification of pathogen and host-response markers correlated with periodontal disease. J Periodontol. 2009;80:436–446. doi: 10.1902/jop.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fine DH, Markowitz K, Furgang D, et al. Macrophage inflammatory protein-1alpha: a salivary biomarker of bone loss in a longitudinal cohort study of children at risk for aggressive periodontal disease? J Periodontol. 2009;80:106–113. doi: 10.1902/jop.2009.080296. [DOI] [PubMed] [Google Scholar]
- 5.Sexton WM, Lin Y, Kryscio RJ, Dawson DR, 3rd, Ebersole JL, Miller CS. Salivary biomarkers of periodontal disease in response to treatment. J Clin Periodontol. 2011;38:434–441. doi: 10.1111/j.1600-051X.2011.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burrell RC, Walters JD. Distribution of systemic clarithromycin to gingiva. J Periodontol. 2008;79:1712–1718. doi: 10.1902/jop.2008.080013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Offenbacher S, Barros SP, Paquette DW, et al. Gingival transcriptome patterns during induction and resolution of experimental gingivitis in humans. J Periodontol. 2009;80:1963–1982. doi: 10.1902/jop.2009.080645. [DOI] [PubMed] [Google Scholar]
- 8.Offenbacher S, Barros S, Mendoza L, et al. Changes in gingival crevicular fluid inflammatory mediator levels during the induction and resolution of experimental gingivitis in humans. J Clin Periodontol. 2010;37:324–333. doi: 10.1111/j.1600-051X.2010.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silness J, Loe H. Periodontal Disease in Pregnancy. Ii. Correlation between Oral Hygiene and Periodontal Condtion. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 10.Loe H, Silness J. Periodontal Disease in Pregnancy. I. Prevalence and Severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 11.Offenbacher S, Barros SP, Singer RE, Moss K, Williams RC, Beck JD. Periodontal disease at the biofilm-gingival interface. J Periodontol. 2007;78:1911–1925. doi: 10.1902/jop.2007.060465. [DOI] [PubMed] [Google Scholar]
- 12.Mandel ID, Wotman S. The salivary secretions in health and disease. Oral Sci Rev. 1976:25–47. [PubMed] [Google Scholar]
- 13.Border MB, Schwartz S, Carlson J, et al. Exploring salivary proteomes in edentulous patients with type 2 diabetes. Mol Biosyst. 2012;8:1304–1310. doi: 10.1039/c2mb05079j. [DOI] [PubMed] [Google Scholar]
- 14.Lee A, Ghaname CB, Braun TM, et al. Bacterial and salivary biomarkers predict the gingival inflammatory profile. J Periodontol. 2012;83:79–89. doi: 10.1902/jop.2011.110060. [DOI] [PubMed] [Google Scholar]
- 15.Gursoy UK, Kononen E, Uitto VJ, et al. Salivary interleukin-1beta concentration and the presence of multiple pathogens in periodontitis. J Clin Periodontol. 2009;36:922–927. doi: 10.1111/j.1600-051X.2009.01480.x. [DOI] [PubMed] [Google Scholar]
- 16.Teles R, Sakellari D, Teles F, et al. Relationships among gingival crevicular fluid biomarkers, clinical parameters of periodontal disease, and the subgingival microbiota. J Periodontol. 2010;81:89–98. doi: 10.1902/jop.2009.090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teles RP, Likhari V, Socransky SS, Haffajee AD. Salivary cytokine levels in subjects with chronic periodontitis and in periodontally healthy individuals: a cross-sectional study. J Periodontal Res. 2009;44:411–417. doi: 10.1111/j.1600-0765.2008.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller CS, King CP, Jr, Langub MC, Kryscio RJ, Thomas MV. Salivary biomarkers of existing periodontal disease: a cross-sectional study. J Am Dent Assoc. 2006;137:322–329. doi: 10.14219/jada.archive.2006.0181. [DOI] [PubMed] [Google Scholar]
- 19.Tobon-Arroyave SI, Jaramillo-Gonzalez PE, Isaza-Guzman DM. Correlation between salivary IL-1beta levels and periodontal clinical status. Arch Oral Biol. 2008;53:346–352. doi: 10.1016/j.archoralbio.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Scannapieco FA, Ng P, Hovey K, Hausmann E, Hutson A, Wactawski-Wende J. Salivary biomarkers associated with alveolar bone loss. Ann N Y Acad Sci. 2007;1098:496–497. doi: 10.1196/annals.1384.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Syndergaard B, Al-Sabbagh M, Kryscio RJ, et al. Salivary Biomarkers Associated with Gingivitis and Response to Therapy. J Periodontol. 2014 doi: 10.1902/jop.2014.130696. published online ahead of print Feb 6, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa PP, Trevisan GL, Macedo GO, et al. Salivary interleukin-6, matrix metalloproteinase-8, and osteoprotegerin in patients with periodontitis and diabetes. J Periodontol. 2010;81:384–391. doi: 10.1902/jop.2009.090510. [DOI] [PubMed] [Google Scholar]
- 23.Isaza-Guzman DM, Arias-Osorio C, Martinez-Pabon MC, Tobon-Arroyave SI. Salivary levels of matrix metalloproteinase (MMP)-9 and tissue inhibitor of matrix metalloproteinase (TIMP)-1: a pilot study about the relationship with periodontal status and MMP-9(-1562C/T) gene promoter polymorphism. Arch Oral Biol. 2011;56:401–411. doi: 10.1016/j.archoralbio.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Westerlund U, Ingman T, Lukinmaa PL, et al. Human neutrophil gelatinase and associated lipocalin in adult and localized juvenile periodontitis. J Dent Res. 1996;75:1553–1563. doi: 10.1177/00220345960750080601. [DOI] [PubMed] [Google Scholar]
- 25.Mancini S, Romanelli R, Laschinger CA, Overall CM, Sodek J, McCulloch CA. Assessment of a novel screening test for neutrophil collagenase activity in the diagnosis of periodontal diseases. J Periodontol. 1999;70:1292–1302. doi: 10.1902/jop.1999.70.11.1292. [DOI] [PubMed] [Google Scholar]
- 26.Romanelli R, Mancini S, Laschinger C, Overall CM, Sodek J, McCulloch CA. Activation of neutrophil collagenase in periodontitis. Infect Immun. 1999;67:2319–2326. doi: 10.1128/iai.67.5.2319-2326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loe H, Theilade E, Jensen SB. Experimental Gingivitis in Man. J Periodontol. 1965;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- 28.Offenbacher S, Beck JD. A perspective on the potential cardioprotective benefits of periodontal therapy. Am Heart J. 2005;149:950–954. doi: 10.1016/j.ahj.2005.01.046. [DOI] [PubMed] [Google Scholar]