Abstract
Magnetic resonance imaging of the human fetal brain has been a clinical tool for many years and provides valuable additional information to compliment more common ultrasound studies. Advances in both MRI acquisition and post processing over the last 10 years have enabled full 3D imaging and the accurate combination of data acquired in different head positions to create improved geometric integrity, tissue contrast and resolution. This research is now motivating the development of new quantitative MRI based techniques for clinical imaging that can more accurately characterize brain development and detect abnormalities. In this paper we will review some of the key areas that are driving changes in our understanding of fetal brain growth using quantitative measures derived from inutero MRI, and possible directions for its increased use in improving the evaluation of pregnancies and the accurate characterization of abnormal brain growth.
1. Introduction
Our knowledge of the dynamic changes within the human brain in utero has been derived mainly from studies of animal fetal brain development [1, 2], postmortem human histology [3, 4] and more recently postmortem MRI [5, 6]. In humans, the use of magnetic resonance imaging (MRI) to examine living human fetal brain development in utero was, until around 10 years ago, focused on using multislice imaging techniques for visual radiological evaluation of morphology. The approach in day to day clinical use relied heavily on fast multi-slice imaging to freeze fetal head movements and provide the radiologist with basic 2D views of the developing brain. This type of imaging presents a significant advance over ultrasound studies that cannot provide the contrast between different brain tissues available to MRI. However, compared to adult brain MRI techniques, these types of studies are still crude. As a result it can be difficult to form reproducible interpretations for diagnoses or quantitative measurements for basic science studies, because of inconsistencies in slice positioning, image contrast and limited resolution. In general, the protocols rely on careful planning of fast 2D slice acquisitions whereby motion is frozen within most slices to an extent that data is amenable to visual inspection by a radiologist. The inherent motion of the fetal head still precludes the routine use of longer 3D imaging techniques, or imaging that requires the collection of multiple images of the same anatomy to form a derived or parametric contrast. As such, evaluation of the brain morphology has been limited to a careful visual evaluation of slices acquired in poorly determined orientations.
Over the last decade, the use of faster snapshot slice acquisition techniques married with post-hoc computer vision approaches to 3D image estimation [7] has opened up a new window into the developing fetal brain. Full 3D imaging has always been possible on some fraction of cases where motion is by chance small during the imaging session. The key contribution has been to enable 3D imaging in the majority of cases to allow realistic studies to begin to be planned and executed. To compliment advances in clinical MRI there have been advances in our understanding of both normal and abnormal brain development that have begun to emerge from animal and postmortem studies. The underlying trend is to use techniques that can ensure accurate 3D imaging [8], and move toward the extraction of quantitative measures of brain development. In this article we will review how this methodology has been evolving to enable increased accuracy and reproducibility for in-utero MRI and highlight how this may impact both neuroimaging research and also the clinical evaluation of pregnancy.
2. Accurate Spatial Quantitation from 3D Structural Imaging
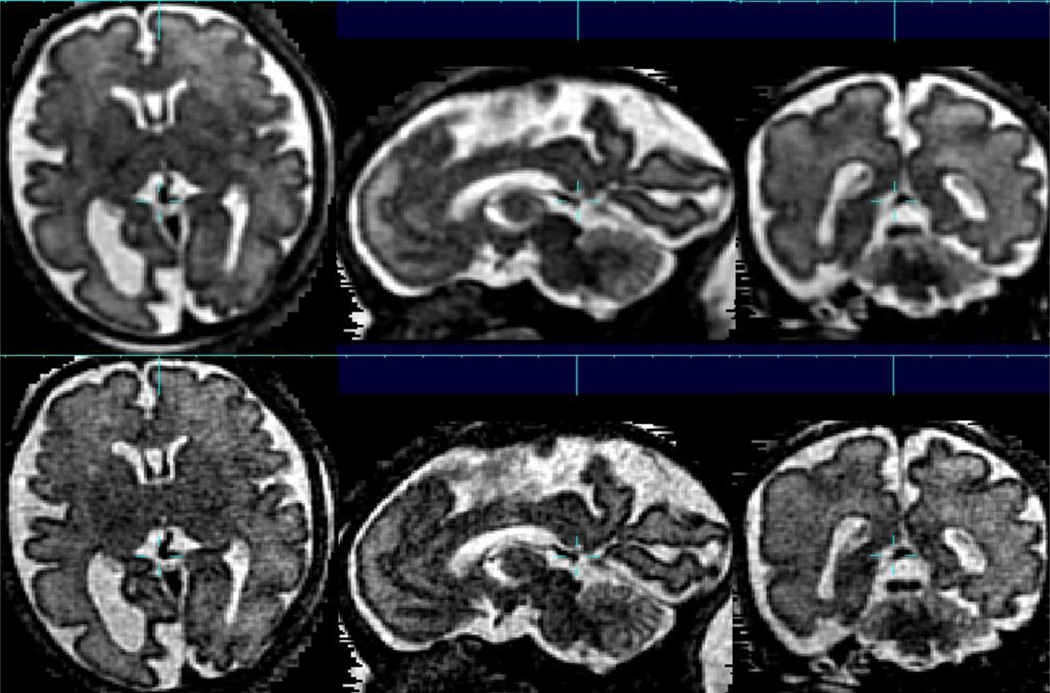
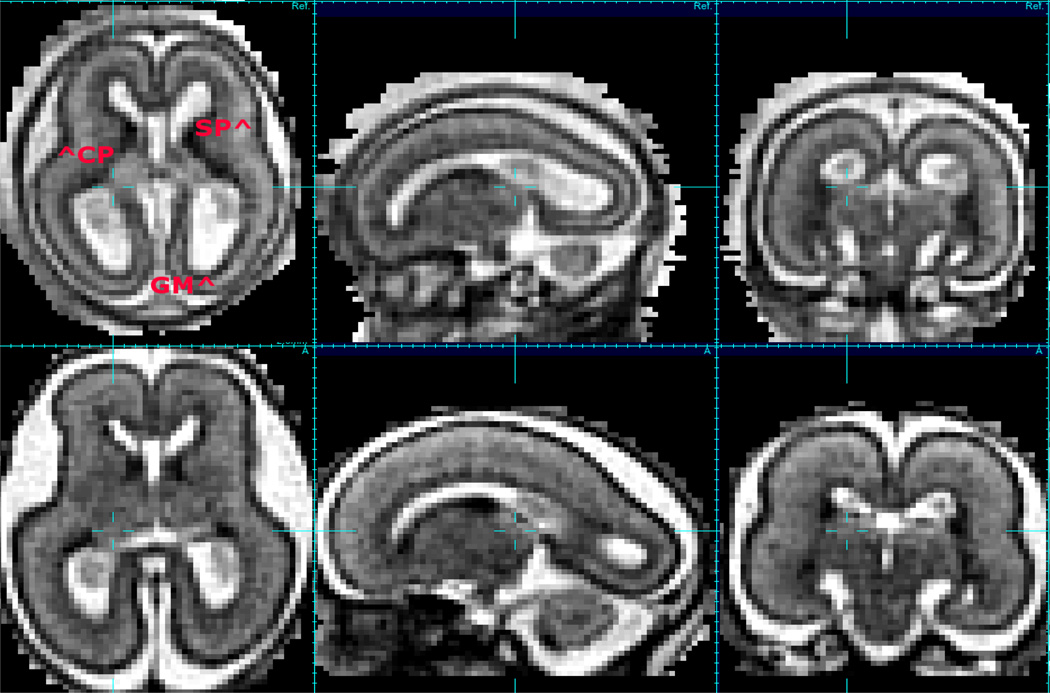
The development of techniques to robustly and accurately estimate full 3D head movements occurring between the accelerated acquisition of individual slices [9] in a multi-slice imaging study has been the basis for a range of new fetal imaging methodologies and studies. These include reconstruction based [9, 10, 11] and intersection based [12] between slice motion estimation techniques. In addition, techniques to remove the effects of changes in MRI signal strength between slices [13, 14] have enabled improved delineation of subtle developing tissue zones such as the sub-plate visible in T2W MRI. Most recently by ensuring high accuracy correction of motion, it has been possible to employ so-called super-resolution techniques when forming a 3D image from multiple 2D slices scattered by motion [15, 11]. These approaches extend a common technique in photographic image processing (super resolution) to the general problem of 3D imaging from 2D slices. This allows the deconvolution of the coarse through plane slice resolution by combining thick slices with fine in-plane resolution, that have been acquired in different anatomical planes. An example of the capabilities of such an approach are illustrated in figure 1 which compares an interpolation based estimation [9, 10] with an iterative deconvolution based method [16] for 3D image formation. This in particular results in much improved delineation of the complex cortical folding pattern emerging in the third trimester. In addition to resolution, the fusion of signal provides improved contrast that would normally be unavailable because of the imaging time required. Improved contrast to noise is important in the study of the subtle transient tissue zones that are apparent at earlier stages of gestation as illustrated in figure 2. Full 3D imaging in-utero has allowed these zones to be characterized in terms of shape and thickness during the key developmental period between 20 and 27GW [17].
Figure 1.
An example of the advantages of between slice motion correction combined with deconvolution based 3D image reconstruction (lower row) from multi-slice data, allowing a voxel size of 0.75×0.75×0.75mm to be formed from multislice, multiplane acquisitions with original slice thickness of 3.3mm. For comparison, a direct interpolation based estimated is shown in upper row. Such increased spatial resolution is particularly important in studying the cortex in later stages of gestation where the brain is becoming highly folded and complex, with many of the gyri and sulci already formed, while the brain remains relatively small in relation to its final adult size.
Figure 2.
An example of a motion correction 3D image of conventional T2W contrast (motion corrected 1.0 × 1.0 × 1.0mm HASTE imaging) at 20GW and 23GW showing changes in contrast of developmental zones (CP=Cortical Plate, GM=Germinal Matrix, SP=Subplate) over a short time interval with sub-plate visible in the brain at 20GW loosing contrast over the next 3 or so weeks.
2.1. Clinical Applications of Spatial Quantitation
More generally, quantitative MRI measurement techniques also encompass the accurate spatial quantitation of structures within the images, so that image geometric distortions and resolution become important factors in the image formation process. In the fetus early work made use of very careful human based measurements [18]. However, spatial quantitation is also dependent on methods beyond the domain of MRI physics and include higher level image analysis techniques including tissue segmentation [19]. These then allow a range of techniques including volume [20], surface [21], and layer thickness [17] based morphometric analyses.
These tools have been used to examine in 3D common clinical conditions seen in MRI of the human fetus. In particular, one of the most common conditions, ventriculomegaly which has been relatively crudely diagnosed by linear measurements of the ventricular space in 2D slices has recently been examined using motion corrected 3D T2W imaging. Scott et al have shown that in cases of mild ventriculomegaly [22], the shape of the ventricles have characteristic broad differences in shape that go beyond volume and linear dimensions. More interestingly, they were also able to detect significant delays in the cortical folding in mild ventriculomegaly, where there were no differences in overall brain tissue volumes when compared to normally developing fetuses. Such findings may be able to shed more light on the occurrence of delayed cognitive development in some cases of ventriculomegaly. In general ventriculomegaly, where ventricle size can be extreme, 3D image analysis has also shown measurable differences in brain growth [23].
There is an increasing body of literature showing congenital heart disease (CHD) leads to neurologic dysfunction shortly after birth prior to surgical interventions, in a significant proportion of this high-risk population. Early work in this population had identified abnormalities using spectroscopic imaging and measures of overall brain volume [24]. Using motion corrected 3D imaging of fetuses with with hypoplastic left heart syndrome (HLHS), Clouchoux et al have shown a progressive third trimester reduction in the volume of cortical gray and white matter and subcortical gray matter. These were apparently preceded by cortical folding delays in the frontal, parietal, calcarine, temporal, and collateral regions, indicating that subtle shape changes detectable by 3D imaging may be a more sensitive indicator of developmental problems.
Another key area where improved brain imaging in the fetus is expanding our knowledge of clinical populations is in the study of fetsus that are small for gestational-age. Here there is interest in looking more closely at brain growth. Recent work in this population using fast multi-slice fetal MRI has been able to detect characteristics differences in development in the insular cortex [25] when compared to the brain anatomy of normally developing fetuses.
3. Quantitative Contrast MRI
Within the MRI physics field, the term quantitative MRI generally refers to techniques focusing on the mapping of underlying material properties that induce basic MRI contrast [26]. These properties include Proton Density (PD), Longitudinal Relaxation (T1), Transverse Relaxation (T2), Magnetization Transfer (M T) and Spectroscopic estimation 1H of absolute metabolite concentrations. The term can also extend to include the quantitative measurement of motion of material within the tissues with techniques such as diffusion imaging (random motion of spins in a constrained homogeneous solution), perfusion (amount of blood traveling through capillaries in ml/s/gm of tissue) and flow (bulk motion of fluids such as blood) imaging. Unlike basic radiological imaging of ‘weighted’ image contrast (T1W, T2W) for visual inspection, these techniques are aimed at creating images whose contrast is calibrated and is therefore independent of machine and operator variations. Such methods can be more precisely termed quantitative contrast MRI. Quantitative contrast, although more expensive in requiring longer imaging times has been shown to be able to provide improved sensitivity over conventional weighed contrast [27]. As a result these approaches are becoming more widely used in the context of adult imaging, both for scientific studies and in clinical practice.
A key area of interest for in-utero brain studies is the possibility of better understanding and early detection of fetal brain abnormalities arising from environmental and maternal factors. Within this area, fetal alcohol exposure is the leading cause of environmentally induced birth defects and neuro-developmental mental retardation in the western world [28]. The resulting effects have been studied extensively in children and adults. Exposure inutero is known to be related to impairments in cognitive function and changes in behavior [29], that result in life long impairments [30] that have significant impact on health care and society as a whole. While current criteria for clinical diagnosis of Fetal Alcohol Spectrum Disorder (FASD) are reliable, they are only applicable in mid to late childhood [31]. MR based neuroimaging has been explored for pediatric imaging of brain growth after fetal alcohol exposure with the aim of identifying earlier biomarkers that may enable reliable diagnosis earlier in life [32] and could stimulate the development and use of more powerful treatment strategies as the brain is still developing functionally and structurally.
Neuroimaging studies in children who have been exposed to alcohol in utero have shown measurable differences in brain shape [33, 34]. Postnatally, this work has shown an overall reduction in brain size as well as characteristic shape differences. These include narrowing of the parietal region and reduced growth in the frontal lobe, cerebellar vermis, corpus callosum and caudate nucleus implying that alcohol exposure may be regionally specific.
The work of Zhou et al [35] and Fernandez et al [36] has shown differences in cortical thickness and its change with age in children exposed to alcohol in utero, and raise the question of whether in-utero measurements can be made to better characterize these conditions at an earlier stage. Measurements in fetal animal models of FASD have shown that early exposure results in cell number reductions in the cerebral cortex [37] and detected abnormal microscopic cellular organization [38] and also abnormalities in dendrite development [39].
3.1. Quantitation of Fetal Brain Cortical Microstructure
Most recently these basic cellular level findings have been translated to practical MRI measures by Leigland et al [40]. Specifically it has been shown that quantitative fractional anisotropy measures by MRI in the cerebral cortex remain higher in EtOH exposed fetuses when compared to unexposed fetuses. FA would normally decrease with age reflecting differentiation of neurons [41]. The findings in EtOH exposure indicate that the morphological complexity of axonal and dendritic processes may not be increasing as would be expected during development, when EtOH exposure has occurred [40].
A disadvantage of diffusion MRI for basic science studies is its low resolution, however this can also be used to advantage in that the quantitative measurements made reflect the morphological properties of ensembles of cells. As such the technique can be used to provide a window into a useful scale of tissue property less accessible to single cell and microscopic imaging, that can be related more directly to macroscopic structures such as cortical folds.
3.2. Quantitation of Connectivity from Macroscopic Diffusion Patterns
Diffusion MRI can not only provide macro-structural information on a voxel level, but can be used to explore large scale consistency of microstructure in the form of diffusion based white matter connectivity measures, reflecting the underlying axonal connections [42]. Prior to the use of motion correction in fetal imaging, tractography of the fetal brain has been used in cases of limited motion to explore the formation of white matter connections [43] in-utero. The first work on extending between slice motion correction to fetal imaging [44] illustrated the potential power of the approach for more general studies of the fetus where significant motion was present. These early methods have been developed significantly over recent years. Model free approaches based on a 5D interpolation of diffusion data (3 spatial and two angular) have been explored by [45]. More recently, the work on iterative or deconvolution based image estimation of conventional T2W contrast, has been extended to diffusion data. This uses model based diffusion estimation using a DTI model [46] to form isotropic voxel resolution from multiple lower resolution slice planes. This approach was further developed specifically for fetal brain imaging to allow the combined estimation of slice orientation and isotropic image resolution [47] from multiple slice planes in a so-called unified approach to diffusion weighted slice alignment.
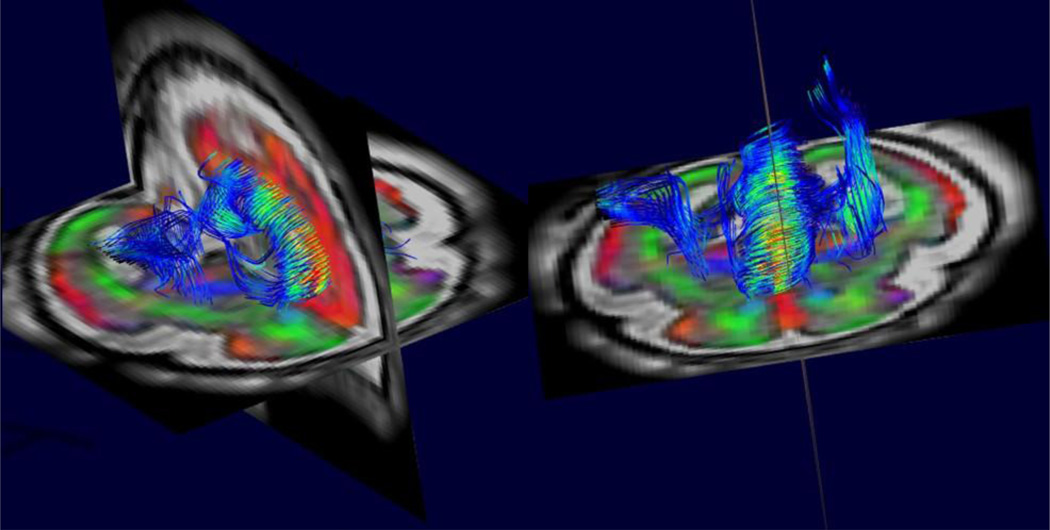
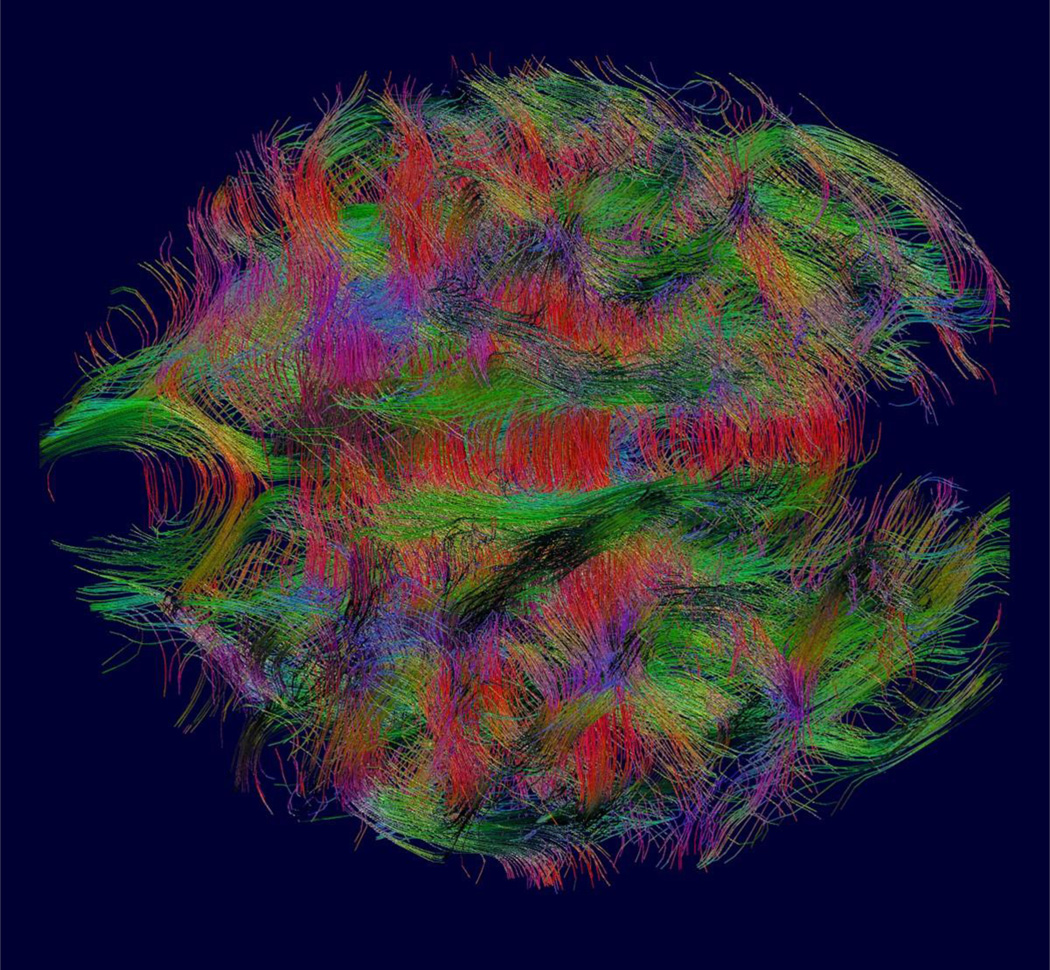
These types of approach, providing both motion robustness and increased spatial resolution, have enabled more powerful computational techniques to begin to be deployed to study the developing connectivity in the human brain as illustrated in figures 3 and 4. These techniques include global tractography (Figure 5), probabilistic tractography [45] and graph analysis [48]. In particular, early work has shown the ability of graph based metrics to detect the emergence of small world properties in the fetal brain before birth [49]. These more powerful motion-corrected based imaging and analysis techniques have also begun to be applied to clinical cases to identify new features of abnormal connectivity development [50] in utero.
Figure 3.
An example of between slice motion corrected high resolution 3D diffusion tensor imaging from combined multi-slice and multi-plane DWI. This allows tractography based analysis to quantify the development of white matter connections within the fetal brain based on the estimates of water diffusion over the brain. Note also the color coded direction map of the surrounding anatomy that highlights the highly radial structure of the cortex at this early stage of development. This contrasts with the cortical microstructure of the adult cortex which provides no directional constraints on the diffusion of water..
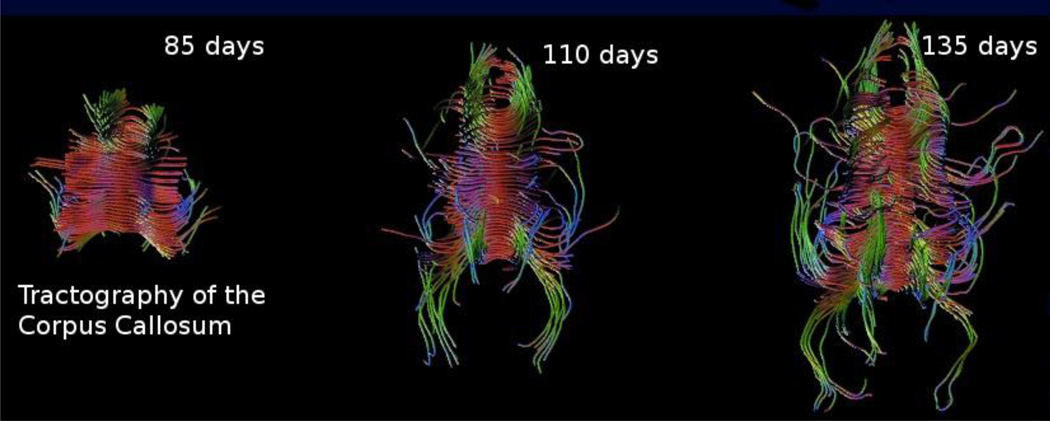
Figure 4.
For basic science studies of how the human brain develops, a key advantage of MRI is the ability to study normal brain anatomy over time. Here we see the development of the corpus callosum as imaged by motion corrected DTI [47] and mapped by tractography.
Figure 5.
Global tractography of fetal white matter can be used on motion corrected whole brain diffusion imaging studies to create a summary of interconnections between cortical and subcortical grey matter. Combined with graph analysis methods, this type of data allows us to study the development of small world connectivity [49] in the developing fetus
3.3. Relaxation Properties of Developing Tissues
Quantitative T*2 measurements of early brain development using MRI of babies born prematurely [51] has shown values are elevated when compared to those seen in adults. This raises the question of whether those measurements are normal or represent some indication of injury after premature birth. Measuring such values in-utero will provide an age equivalent reference against which these values can be compared. In addition there has been considerable interest in the use of functional imaging to study early brain growth using Blood Oxygen Level Dependence (BOLD) contrast. BOLD contrast and its measurement are dependent on the underlying relaxation properties of the tissue being studied. Given the very different nature of, in particular, mid gestation neuroanatomy, the optimum imaging parameters are not known for any functional MRI study.
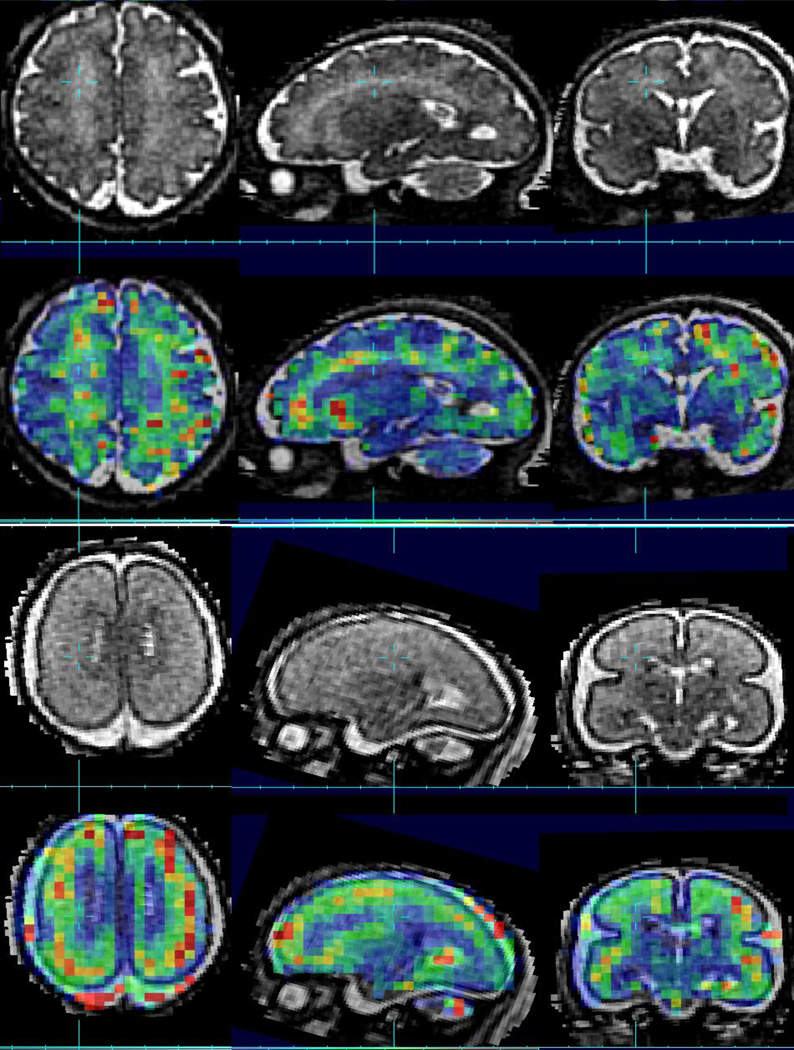
For contrast based quantitation, in fetal MRI studies, the techniques are still in their infancy. This is primarily due to the requirement for multiple measurements in most quantitative MRI contrast techniques. Even relatively efficient techniques [52] used in adult studies requiring minimal image sets for parameter estimation are in-practical for fetal studies. As a result, the first fetal studies have recently focused on faster echo planar techniques. The first R*2 measurements made in-utero were as part of motion robust functional imaging [53] which collected multi-slice time series data using only two-echoes. Later work by Vasylechko [54] has used multiple echo times to form a more stable estimate of T*2 in the context of basic parametric imaging, using slice based measurements only. The technique acquired multiple echoes at each slice location before proceeding to imaging the next slice location. Such an approach minimizes motion between the subsequent echoes, but makes it less feasible to recover between-slice motion in order to reconstruct a 3D volume, because of the time between spatially neighboring slices. The recent findings based on this approach have indicated that there may be differences between the premature-neonatal and in-utero relaxation properties of tissues. Figure 6 illustrates the changes in T* maps with age as the fetal brain develops over time. These are overlaid onto conventional T2W images. We show the un-interpolated voxel dimensions to highlight the difference in resolution. Although much lower resolution, the T* contrast between different developing tissue regions appears much more significant than that seen in basic T2W imaging (eg figure 2).
Figure 6.
Example Conventional T2W contrast (1.0 × 1.0 × 1.0mm HASTE imaging) with a color overlay of quantitative T*2 imaging estimates (3.0 × 3.0 × 3.0 mm dual echo EPI) in the same fetus imaged at 20GW (lower row) and 35GW (upper row)..
4. Summary
The last ten years has seen a number of methodological advances that have improved our ability to image the growing human fetal brain. Initial work focused on 3D image formation in the presence of motion during fast multi-slice imaging. This in particular moved 3D MRI imaging as a tool for use in a small subset of fetuses where there is limited motion, to a tool that can be generally applied for practical clinical and basic science studies. These methods have since been further developed to provide both improved contrast and improved spatial resolution for conventional T2W anatomical imaging. The same basic idea has been generalized to create model based quantitative multi-slice imaging methods that make use of between slice motion correction to combine MRI measures needed for quantitative contrast imaging. Current examples include 3D diffusion and relaxometry based imaging. These techniques promise to dramatically expand our understanding of normal and abnormal brain development in-utero and provide a range of new tools for the clinician when evaluating pregnancies.
Acknowledgments
Disclosure
The author would like to thank the NIH for research support (NS 061957,NS 055064, EB 017133, AA 021981) during the preparation of this manuscript and the many contributions from the postdoctoral scholars and students who have worked in his lab.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. Journal of Comparative Neurology. 1972;145(1):61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- 2.Kroenke CD, Van Essen DC, Inder TE, Rees S, Bretthorst GL, Neil JJ. Microstructural changes of the baboon cerebral cortex during gestational development reflected in magnetic resonance imaging diffusion anisotropy. J Neurosci. 2007 Nov;27(46):12506–12515. doi: 10.1523/JNEUROSCI.3063-07.2007. Available from: http://www.hubmed.org/display.cgi?uids=18003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kostović I, Judaš M, Radoš M, Hrabač P. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cerebral Cortex. 2002;12(5):536–544. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]
- 4.Huang H, Vasung L. Gaining Insight of Fetal Brain Development with Diffusion MRI and Histology. International Journal of Developmental Neuroscience. 2013 doi: 10.1016/j.ijdevneu.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang H, Xue R, Zhang J, Ren T, Richards LJ, Yarowsky P, et al. Anatomical Characterization of Human Fetal Brain Development with Diffusion Tensor Magnetic Resonance Imaging. The Journal of Neuroscience. 2009 Apr;29(13):4263–4273. doi: 10.1523/JNEUROSCI.2769-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang H, Jeon T, Sedmak G, Pletikos M, Vasung L, Xu X, et al. Coupling diffusion imaging with histological and gene expression analysis to examine the dynamics of cortical areas across the fetal period of human brain development. Cerebral cortex. 2013;23(11):2620–2631. doi: 10.1093/cercor/bhs241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Studholme C. Mapping Fetal Brain Development In Utero Using Magnetic Resonance Imaging: The Big Bang of Brain Mapping. Annual Review of Biomedical Engineering. 2011;13:345–368. doi: 10.1146/annurev-bioeng-071910-124654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Limperopoulos C, Clouchoux C. Advancing fetal brain MRI: targets for the future. Semin Perinatol. 2009 Aug;33(4):289–298. doi: 10.1053/j.semperi.2009.04.002. Available from: http://www.hubmed.org/display.cgi?uids=19631089. [DOI] [PubMed] [Google Scholar]
- 9.Rousseau F, Glenn OA, Iordanova B, Rodriguez-Carranza C, Vigneron DB, Barkovich JA, et al. Registration-based approach for reconstruction of high-resolution in utero fetal MR brain images. Academic radiology. 2006;13(9):1072–1081. doi: 10.1016/j.acra.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Jiang S, Xue H, Glover A, Rutherford M, Rueckert D, Hajnal JV. MRI of moving subjects using multislice snapshot images with volume reconstruction (SVR): application to fetal, neonatal, and adult brain studies. IEEE Trans Med Imaging. 2007;26:967–980. doi: 10.1109/TMI.2007.895456. [DOI] [PubMed] [Google Scholar]
- 11.Gholipour A, Estroff JA, Warfield SK. Robust Super-Resulution Volume Reconstruction From Slice Acquisitions: Application to fetal Brain MRI. IEEE transactions on Medical Imaging. 2010 Oct;29(10):1739–1758. doi: 10.1109/TMI.2010.2051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim K, Habas PA, Rousseau F, Glenn OA, Barkovich AJ, Studholme C. Intersection based motion correction of multislice MRI for 3-D in utero fetal brain image formation. IEEE Trans Med Imaging. 2010;29(1):146–158. doi: 10.1109/TMI.2009.2030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim K, Habas PA, Rajagopalan V, Scott JA, Corbett-Detig JM, Rousseau F, et al. Bias field inconsistency correction of Motion-Scattered multislice MRI for improved 3D image reconstruction. IEEE Transactions on Medical Imaging. 2011;30(9):1704. doi: 10.1109/TMI.2011.2143724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuklisova-Murgasova M, Quaghebeur G, Rutherford MA, Hajnal JV, Schnabel JA. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Medical Image Analysis. 2012 doi: 10.1016/j.media.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rousseau F, Kim K, Studholme C, Koob M, Dietemann J. On super-resolution for fetal brain MRI. In: Medical Image Computing and Computer-Assisted Intervention–MICCAI 2010. LNCS: Springer Verlag; 2010. pp. 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fogtmann M, Chapman T, Kim K, Seshamani S, Studholme C. A unified approach for motion-estimation and super-resolution reconstruction from structural Magnetic Resonance on moving subjects. Proceedings of the MICCAI 2012 Workshop on Perinatal and Paediatric Imaging: PaPI. 2012;2012 p. –. [Google Scholar]
- 17.Corbett-Detig JM, Habas PA, Scott JA, Kim K, Rajagopalan V, Mc-Quillen PS, et al. 3-D global and regional patterns of human fetal subplate growth determined in utero. Brain Struct Funct. 2011;215(3–):255–263. doi: 10.1007/s00429-010-0286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grossman R, Hoffman C, Mardor Y, Biegon A. Quantitative MRI measurements of human fetal brain development in utero. Neuroimage. 2006;33(2):463–470. doi: 10.1016/j.neuroimage.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Habas PA, Kim K, Corbett-Detig JM, Rousseau F, Glenn OA, Barkovich AJ, et al. A spatiotemporal atlas of MR intensity, tissue probability and shape of the fetal brain with application to segmentation. Neuroimage. 2010 Nov;53(2):460–470. doi: 10.1016/j.neuroimage.2010.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajagopalan V, Scott J, Habas PA, Kim K, Rousseau F, Glenn OA, et al. Mapping directionality specific volume changes using tensor based morphometry: An application to the study of gyrogenesis and lateralization of the human fetal brain. NeuroImage. 2012;63(2):947–958. doi: 10.1016/j.neuroimage.2012.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habas PA, Scott JA, Roosta A, Rajagopalan V, Kim K, Rousseau F, et al. Early folding patterns and asymmetries of the normal human brain detected from in utero MRI. Cerebral Cortex. 2012;22(1):13–25. doi: 10.1093/cercor/bhr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott JA, Habas PA, Rajagopalan V, Kim K, Barkovich AJ, Glenn OA, et al. Volumetric and surface-based 3D MRI analyses of fetal isolated mild ventriculomegaly : Brain morphometry in ventriculomegaly. Brain Struct Funct. 2012 May; doi: 10.1007/s00429-012-0418-1. Available from: http://www.hubmed.org/display.cgi?uids=22547094. [DOI] [PubMed] [Google Scholar]
- 23.Gholipour A, Akhondi-Asl A, Estroff JA, Warfield SK. Multi-atlas multishape segmentation of fetal brain MRI for volumetric and morphometric analysis of ventriculomegaly. Neuroimage. 2012 Apr;60(3):1819–1831. doi: 10.1016/j.neuroimage.2012.01.128. Available from: http://www.hubmed.org/display.cgi?uids=22500924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL, et al. Brain Volume and Metabolism in Fetuses With Congenital Heart Disease Evaluation With Quantitative Magnetic Resonance Imaging and Spectroscopy. Circulation. 2010;121(1):26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egaña-Ugrinovic G, Sanz-Cortes M, Figueras F, Couve-Perez C, Gratacòs E. Fetal MRI Insular Cortical Morphometry and its Association with Neurobehavior in Late-Onset Small For Gestational Age Fetuses. Ultrasound in Obstetrics & Gynecology. 2014;44:322–329. doi: 10.1002/uog.13360. [DOI] [PubMed] [Google Scholar]
- 26.Tofts P. Quantitative MRI of the brain: measuring changes caused by disease. John Wiley & Sons. 2005 [Google Scholar]
- 27.Weiskopf N, Suckling J, Williams G, Correia MM, Inkster B, Tait R, et al. Quantitative multi-parameter mapping of R1, PD*, MT, and R2* at 3T: a multi-center validation. Frontiers in neuroscience. 2013;7 doi: 10.3389/fnins.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiner IB, Craighead WE. The corsini encyclopedia of psychology. Vol. 4. John Wiley & Sons; 2010. [Google Scholar]
- 29.Guerri C, Bazinet A, Riley EP. Foetal alcohol spectrum disorders and alterations in brain and behaviour. Alcohol and Alcoholism. 2009;44(2):108–114. doi: 10.1093/alcalc/agn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. Journal of Developmental & Behavioral Pediatrics. 2004;25(4):228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Elliott EJ, Payne J, Morris A, Haan E, Bower C. Fetal alcohol syndrome: a prospective national surveillance study. Archives of Disease in Childhood. 2008;93(9):732–737. doi: 10.1136/adc.2007.120220. [DOI] [PubMed] [Google Scholar]
- 32.Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, et al. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2009;33(10):1671–1689. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spadoni AD, McGee CL, Fryer SL, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Neuroscience & Biobehavioral Reviews. 2007;31(2):239–245. doi: 10.1016/j.neubiorev.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norman AL, Crocker N, Mattson SN, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Developmental disabilities research reviews. 2009;15(3):209–217. doi: 10.1002/ddrr.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou D, Lebel C, Lepage C, Rasmussen C, Evans A, Wyper K, et al. Developmental cortical thinning in fetal alcohol spectrum disorders. Neuroimage. 2011;58(1):16–25. doi: 10.1016/j.neuroimage.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 36.Fernández-Jaén A, Fernández-Mayoralas DM, Quiñones Tapia D, Calleja-Pérez B, García-Segura JM, Arribas SL, et al. Cortical thickness in fetal alcohol syndrome and attention deficit disorder. Pediatric neurology. 2011;45(6):387–391. doi: 10.1016/j.pediatrneurol.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Burke MW, Palmour RM, Ervin FR, Ptito M. Neuronal reduction in frontal cortex of primates after prenatal alcohol exposure. Neuroreport. 2009;20(1):13–17. doi: 10.1097/WNR.0b013e32831b449c. [DOI] [PubMed] [Google Scholar]
- 38.Granato A. Altered organization of cortical interneurons in rats exposed to ethanol during neonatal life. Brain research. 2006;1069(1):23–30. doi: 10.1016/j.brainres.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 39.Cui ZJ, Zhao KB, Zhao HJ, Yu DM, Niu YL, Zhang JS, et al. Prenatal alcohol exposure induces long-term changes in dendritic spines and synapses in the mouse visual cortex. Alcohol and alcoholism. 2010;45(4):312–319. doi: 10.1093/alcalc/agq036. [DOI] [PubMed] [Google Scholar]
- 40.Leigland LA, Budde MD, Cornea A, Kroenke CD. Diffusion MRI of the developing cerebral cortical gray matter can be used to detect abnormalities in tissue microstructure associated with fetal ethanol exposure. NeuroImage. 2013;83:1081–1087. doi: 10.1016/j.neuroimage.2013.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leigland LA, Kroenke CD. Animal Models of Behavioral Analysis. Springer; 2011. A comparative analysis of cellular morphological differentiation within the cerebral cortex using diffusion tensor imaging; pp. 329–351. [Google Scholar]
- 42.Kostovic I, Jovanov-Milosevic N. The development of cerebral connections during the first 20–45 weeks’ gestation. 2006;11(6):415–422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Kasprian G, Brugger PC, Weber M, Krssak M, Krampl E, Herold C, et al. In utero tractography of fetal white matter development. neuroimage. 2008;43:213–224. doi: 10.1016/j.neuroimage.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 44.Jiang S, Xue H, Counsell S, Anjari M, Allsop J, Rutherford M, et al. Diffusion tensor imaging (DTI) of the brain in moving subjects: Application to in-utero fetal and ex-utero studies. Magnetic Resonance in Medicine. 2009;62(3):645–655. doi: 10.1002/mrm.22032. [DOI] [PubMed] [Google Scholar]
- 45.Oubel E, Koob M, Studholme C, Dietemann JL, Rousseau F. Reconstruction of Scattered Data in Fetal Diffusion MRI. 2012;16(1):28–37. doi: 10.1016/j.media.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scherrer B, Gholipour A, Warfield SK. Super-resolution reconstruction to increase the spatial resolution of diffusion weighted images from orthogonal anisotropic acquisitions. Med Image Anal. 2012 Jun; doi: 10.1016/j.media.2012.05.003. Available from: http://www.hubmed.org/display.cgi?uids=22770597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fogtmann M, Seshamani S, Kroenke C, Cheng X, Chapman T, Wilm J, et al. A unified approach to diffusion direction sensitive slice registration and 3D DTI reconstruction from moving fetal brain anatomy. IEEE Transactions on Medical Imaging. 2014;N(N):272–289. doi: 10.1109/TMI.2013.2284014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Cheng X, Wilm J, Seshamani S, Fogtmann M, Kroenke C, Studholme C. Adapting Parcellation Schemes to Study Fetal Brain Connectivity in Serial Imaging Studies. Proceedings IEEE EMBS. 2013 doi: 10.1109/EMBC.2013.6609440. [DOI] [PubMed] [Google Scholar]
- 50.Koob M, Weingertner AS, Gasser B, Oubel E, Dietemann JL. Thick corpus callosum: a clue to the diagnosis of fetal septopreoptic holoprosencephaly? Pediatr Radiol. 2011 Oct; doi: 10.1007/s00247-011-2260-7. Available from: http://www.hubmed.org/display.cgi?uids=22006531. [DOI] [PubMed] [Google Scholar]
- 51.Rivkin M, Wolraich D, Als H, McAnulty G, Butler S, Conneman N, et al. Prolonged T* 2 values in newborn versus adult brain: implications for fMRI studies of newborns. Magnetic resonance in medicine. 2004;51(6):1287–1291. doi: 10.1002/mrm.20098. [DOI] [PubMed] [Google Scholar]
- 52.Baudrexel S, Volz S, Preibisch C, Klein JC, Steinmetz H, Hilker R, et al. Rapid single-scan T 2*-mapping using exponential excitation pulses and image-based correction for linear background gradients. Magnetic Resonance in Medicine. 2009;62(1):263–268. doi: 10.1002/mrm.21971. [DOI] [PubMed] [Google Scholar]
- 53.Seshamani S, Gatenby C, Fogtmann M, Cheng X, Dighe M, Studholme C. Combining R2* Maps and Slice Registration for fMRI Analysis of Moving Subjects. Proceedings ISMRM. 2013;2013 [Google Scholar]
- 54.Vasylechko S, Malamateniou C, Nunes RG, Fox M, Allsop J, Rutherford M, et al. T2* relaxometry of fetal brain at 1.5 Tesla using a motion tolerant method. Magnetic Resonance in Medicine. 2014 doi: 10.1002/mrm.25299. [DOI] [PubMed] [Google Scholar]