Abstract
Background
Plasmodium and soil transmitted helminth infections (STH) are a major public health problem, particularly among children. There are conflicting findings on potential association between these two parasites. This study investigated the Plasmodium and helminth co-infections among children aged 2 months to 9 years living in Bagamoyo district, coastal region of Tanzania.
Methods
A community-based cross-sectional survey was conducted among 1033 children. Stool, urine and blood samples were examined using a broad set of quality controlled diagnostic methods for common STH (Ascaris lumbricoides, hookworm, Strongyloides stercoralis, Enterobius vermicularis, Trichuris trichura), schistosoma species and Wuchereria bancrofti. Blood slides and malaria rapid diagnostic tests (mRDTs) were utilized for Plasmodium diagnosis.
Results
Out of 992 children analyzed, the prevalence of Plasmodium infection was 13% (130/992), helminth 28.5% (283/992); 5% (50/992) had co-infection with Plasmodium and helminth. The prevalence rate of Plasmodium, specific STH and co-infections increased significantly with age (p < 0.001), with older children mostly affected except for S. stercoralis monoinfection and co-infections. Spatial variations of co-infection prevalence were observed between and within villages. There was a trend for STH infections to be associated with Plasmodium infection [OR adjusted for age group 1.4, 95% CI (1.0–2.1)], which was more marked for S. stercoralis (OR = 2.2, 95% CI (1.1–4.3). Age and not schooling were risk factors for Plasmodium and STH co-infection.
Conclusion
The findings suggest that STH and Plasmodium infections tend to occur in the same children, with increasing prevalence of co-infection with age. This calls for an integrated approach such as using mass chemotherapy with dual effect (e.g., ivermectin) coupled with improved housing, sanitation and hygiene for the control of both parasitic infections.
Author Summary
Parasitic infectious agents rarely occur in isolation and multiparasitism is a norm specifically in children living in endemic areas of Tanzania. We studied the pattern and predictors of Plasmodium and STH co-infections in rural Bagamoyo district, coastal region of Tanzania. Parents/guardians of healthy children aged 2 months to 9 years who were willing to participate in the study were invited from the community. Stool, urine and blood were examined for helminth and Plasmodium parasites. We found that children aged above five years and those who are not schooling had the greatest burden of co-infection with Plasmodium and helminth parasites. The risk of being co-infected with Plasmodium increased with age with all the common types of STH isolated (E. vermicularis, hookworm and S. stercoralis). Younger children had a significantly higher risk of having Plasmodium when co-infected with S. stercoralis. Integrated control approaches including health education, environmental sanitation and hygiene, novel chemoprophylaxis as well as long lasting Impregnated Nets (LLINs) distributions should be implemented considering the pattern and types of infections within the area in order to interrupt transmission of both parasites among young and school-aged children.
Introduction
Parasitic infections such as Plasmodium present a major public health problem among children in Africa [1,2] and its coexistence with Soil Transmitted Helminth (STH) infections is common [3,4]. Multipasitism is a norm among children in developing countries including United Republic of Tanzania [5,6]. It is defined as a concurrent infection in a single host with two or more species whereas monoinfection consists of only one infection from a single species [7]. Variety of environmental and host related factors can influence the structure and dynamics of the parasite communities which make up these multiple infections [8–10]. These conditions include poverty, environmental contamination with infected faeces containing helminth eggs, water bodies, lack of effective preventive measures [11] and immunity of the host. In addition, overlap of Plasmodium infection and other pathogens depends on the conditions that favour multiple parasitic species survival and transmission such as exposure related risk and within host interactions between co-infecting species [3,11,12]. There is mounting evidence indicating that helminth infections increase susceptibility to Plasmodium infection [13–15]. On the other hand, some studies showed that specific STH like Ascaris lumbricoides are protective against Plasmodium disease and its severe manifestations [16]. Previous epidemiological studies have shown coexistence of Plasmodium and helminth infections with spatial heterogeneity in their distribution [3,4,6,17]. In Tanzania, cross-sectional surveys conducted among school and preschool children showed that multiple parasitic infections are common [5,6,17]. Study by Kinung’hi et al showed that the prevalence of malaria parasites tended to increase with increasing number of co-infecting helminth species as compared to helminth free children although the difference was not statistically significant [6].
In Tanzania, global strategies to control malaria are being conducted by the National malaria control program (NMCP) via Long Lasting Insecticide Impregnated Nets (LLINs), Intermittent Preventive Treatment in Pregnancy (IPTp) and prompt treatment with artemether/lumefantrine. The helminth control is done via mass drug administration (MDA), chemotherapy based morbidity control campaigns. The planning for prevention and control program are designed to focus on a single infection approach despite occurrence of co-infections [18]. There is an underestimation of the burden of infection and lack of understanding how these parasitic infections interact [18]. This underlines the importance of investigating the epidemiology of co-infections in different geographical locations where different pattern of infections are expected. In the present study we aimed to determine the relation between Plasmodium and STH co-infections among children aged 2 months to 9 years living in Bagamoyo district, coastal region of Tanzania. Knowledge of the magnitude and on the common risk factors for co-infections should guide the development of focused integrated control programs targeting multiple infections endemic in each country.
Materials and Methods
Reporting of the study follows STROBE checklist (Strengthening the Reporting of Observational studies in Epidemiology) [19].
Ethics statement
The study was conducted under the IDEA study protocol which was approved by the institutional review boards of the Swiss Tropical and Public Health Institute (Swiss TPH; Basel, Switzerland) and the Ifakara Health Institute (IHI; Dar es Salaam, United Republic of Tanzania). The ethical approval for the conduct of the study was granted by the Ethikkomission beider Basel (EKBB; Basel, Switzerland; reference number: 257/08) and the National Institute for Medical Research of Tanzania (NIMR; Dar es Salaam, United Republic of Tanzania; reference number: NIMR/HQ/R.8a/Vol. IX/1098).
The local district, community, school teachers and health authorities were informed during sensitization meetings about the purpose, procedures, risk and benefits of the study prior to the start. Written informed consent was obtained from the parents/guardians of children prior to study procedures after explaining them to the group. Illiterate parents/ guardians were asked to bring witness who participated within the discussion prior to obtaining their thumbprints and witness signature. Participants infected with helminth and/or malaria or other medical conditions received appropriate treatment/referral according to the national treatment guidelines of Tanzania.
Study area
Bagamoyo is a district in the coastal region of Tanzania where Ifakara Health Institute (IHI) through its branch, Bagamoyo Research and Training Centre (BRTC) works in close collaboration with the Bagamoyo District hospital (BDH) officials to ensure quality health care delivery using its research platforms. The BRTC study area covers about 1160 square kilometres. The eastern border of the study area is formed by the Indian Ocean, with the Ruvu river forming part of the western and northern borders. The area extends for approximately 7 km on either side of a road running westwards for 62 kilometres. To the south is an uninhabited forest reserve. According to the 2012 Tanzania National Census, the population of the Bagamoyo District was 311,740 which can be reached by dirt road throughout the year; all are within an hour drive [20].
The main rainy season is from March to May, with a second period from November to December, although occasional rain occurs at all times of the year. Average rainfall is 1200 to 2100 mm per year. There is year round grassland vegetation or subsistence agriculture throughout the study area. According to meteorological statistics the average temperature for the region is about 28°C. Majority of the people are either subsistence farmers who cultivate rice, maize and cassava, or fish from the sea or the Ruvu River and its tributaries. Agriculture employs 76% of the population [20].
The survey was conducted in the western rural area including hamlets within the villages of Kiwangwa, Msata, Mkange and Magomeni. The settlements are located about 20 to 60 km from Bagamoyo town. The inhabitants of the villages are mainly smallholder farmers engaged in food crop production such as pineapples, cassava, maize, vegetables and in fishing and salt mining. The prevalence of malaria within the western study area is still high compared to Bagamoyo town with seasonal variations secondary to malaria interventions through research and Tanzania National Malaria Control program (NMCP) [21]. Research on malaria drugs and vaccine have been conducted in Bagamoyo town since 2005 through IHI and its collaborative partners. The rural water supply is mostly from dams and ponds [22] highly contaminated with fecal coliform bacteria [23]. Eighty four percent of the communities have soil based latrines [22]. The latter resemble to simple pit latrines but without floor nor hygiene cover slab, nor lid covering the hole.
Study design
The study is part of the IDEA project, an African-European Research initiative, funded by European community, with the aim of dissecting the immunological interplay between poverty related diseases (malaria, TB and HIV) and helminth infections[24]. The present community cross-sectional survey was conducted at the start of the IDEA malaria project to provide baseline data to inform further prospective immune-epidemiological studies of malaria infected individuals.
Participant recruitment and sample collection
Study population included a random sample of healthy children as regarded by their parents/guardians, aged 2 months to 9 years inclusively, whose parents/guardians where informed about the study through Village Health Care Workers (VHCW) and agreed to come for screening at the meeting points. The villages were purposely selected based on the environmental conditions favouring both malaria and helminth survival and transmission. In the malaria arm, a sample size of 100 children with asymptomatic Plasmodium parasitemia was required. The malaria prevalence being 10% within the study area [25], we enrolled about ~1000 children from the community survey [26]. Standardized questionnaires were used to collect information on demographics, vital and clinical signs and symptoms to ensure that they were free of common diseases at that point in time. Parents or guardians where asked about interventions implemented within the Tanzanian National program, namely the use of long-lasting impregnated bednets (LLINs) and prior anti-helminth treatment. Participant recruitment and data collection was done between July 2011 and November 2012 covering an entire year and thus including seasonal variation [26].
All children had a finger prick to obtain about 1ml of blood which was collected in an Ethyl Diamine Tetra acetic Acid (EDTA) tube for malaria slide and full blood count which were performed at the main BRTC laboratory. Malaria rapid diagnostic test (SD BIOLINE, SD standard diagnostics, inc.Korea) and hemoglobin level using HemoCue hemoglobinometer (EKF diagnostic GmbH, Germany) were done in the field for inclusion/exclusion criteria and immediate management of the children with malaria and severe anemia.
Additionally, each participant was provided with i) two clean containers (100mls) for stool and urine samples ii) a plastic pocket with an adhesive tape (50 x 20mm) and a glass slide. All labelled with participant identification number. Parents/guardians were instructed on how to apply the adhesive tape and advised to collect sufficient amount of fresh stool and urine. The filled containers and adhesive tape slide were collected by the VHCW at a predefined meeting point in the village centre, the next day before noon and submitted to the Helminth Unit (HU) of the BRTC where all stool and urine samples were examined by experienced technicians.
Diagnosis of Plasmodium infection
Thick and thin blood films were prepared, air dried and Giemsa stained for detection and quantification of malaria parasites according to the IHI laboratory Standard Operating Procedures (SOP). To detect malaria parasites, 200 fields were examined. Parasite density expressed per μl of blood was calculated by multiplying a factor of 40 to the number of parasites counted, assuming 8,000 leucocytes per μl of blood [27]. All slides were read by two independent qualified technicians. In case of discrepancy between two readers, a third reader was requested. The final result was the geometric mean of the two geometrically closest readings out of the three. For cases of positive/negative discrepancy the majority decision was adopted. If the test results were positive, the final one was then taken as the geometrical mean of the two positive results.
Diagnosis of helminth infection
Duplicate Kato-Katz thick smear slides using a 41.7 mg template, adhesive tape slides, Baermann and FLOTAC methods were used to diagnose intestinal helminth [28]. Microhaematuria was examined using a dipstick (Hemastix; Siemens Healthcare Diagnostics, Eschborn, Germany) and for S. haematobium eggs by urine filtration (hydrophilic polycarbonate membrane filter; pore size 20 micron, diameter 13mm; Sterlitech, Kent, WA, United States of America). Binax NOW Filariasis rapid immunochromatic test (ICT) card (inverness medical professional diagnostics; ME; United States of America) was performed at the HU to detect W. bancrofti antigen using whole blood All Kato-Katz thick smear, adhesive tape and urine filtration slides were stored in boxes and 10% of slides re-examined for quality control by the senior experienced personnel after 3–6 months [28].
Data management and statistical analysis
The helminth species specific results derived by each method were entered into an electronic data base using Microsoft ACCESS 2010. Double entry of the clinical and laboratory data was done using the DMSys software (FDA approved for ICH/GCP clinical trials). The two datasets were transferred into STATA format merged and cleaned. Data analysis was performed using STATA version 11.0 software (Stata Corp LP; College Station, Texas, USA). For duplicate Kato-Katz methods, the average of the two slides multiplied by a factor 24 was done to obtain egg per gram of stool (EPG). S. stercoralis and E. vermicularis intensity expressed as larvae counts and number of eggs counted respectively. Helminth infection intensity was categorized according to WHO criteria [29].
To investigate the relationship between Plasmodium and helminth, only the children who had both Plasmodium and helminth results were included. Table 1 defines the terms used in the analysis for easiness of interpretation. Geographical information system (arcgis 10) was used to map the distribution of Plasmodium and helminth monoinfections/co-infections among children in the coastal region of Bagamoyo. Harmonization of the identified hamlets within the studied villages was achieved using the known registered villages and hamlets/streets names from the Tanzania shape file (http://openmicrodata.wordpress.com/2010/12/16/tanzania-shapefiles-for-eas-villages-districts-and-regions/). Hamlets with low numbers (less than 10) were systematically merged to the nearest hamlet within the same village. Baseline characteristics were presented within age groups (children less than 3 years, preschool children aged 3–5 years and school-aged children from 6–9 years). The categorization was chosen to explore the age dependency variability considering the ongoing malaria and mass drug administration helminth programs with different approaches based on age, mainly focusing on under five and above five years of age. Crude odds ratios (ORs) including 95% confidence interval and p-values were calculated for variables potentially associated with infections/co-infections. To investigate risk factors, different models were explored with all Plasmodium, Plasmodium monoinfection, helminth mono- and mixed infections, and Plasmodium + helminth co-infections. The association between helminth species and Plasmodium infection was explored to further study co-infection patterns. Variables that were associated in bivariate analysis with a p-value level of < 0.05 were considered in multiple logistic regression models. The association between Plasmodium and helminth co-infections was subsequently investigated using negative binomial regression estimation after comparing the conditional means and variances of variables, all variances greater than means signifying over dispersion of data. To further investigate the age dependency relationship, Mantel—Haenszel stratified odds ratios (ORs) were conducted.
Table 1. Definitions of the terms.
| Single helminth infection | Helminth monoinfection, one species only |
| Mixed helminth infection | More than one species of helminth |
| All helminth infections | Single, mixed helminth infection and co-infection |
| Plasmodium monoinfection* | Plasmodium species infection only |
| All Plasmodium infections | Plasmodium monoinfection and co-infection |
| Co-infection | Plasmodium and helminth co-infection |
| Plasmodium and single helminth | |
| Plasmodium and Mixed helminth |
*The term single Plasmodium was not used because only P. falciparum species is considered as the main cause of infection during this analysis [2].
A more detailed description and analysis of the helminth distribution is provided in the published paper [26].The present one focuses on the relationship of soil transmitted helminth (STH) and Plasmodium as prevalence of other helminth like W. bancrofti and S. hematobium were low among the studied children.
Results
Baseline characteristics of the study participants
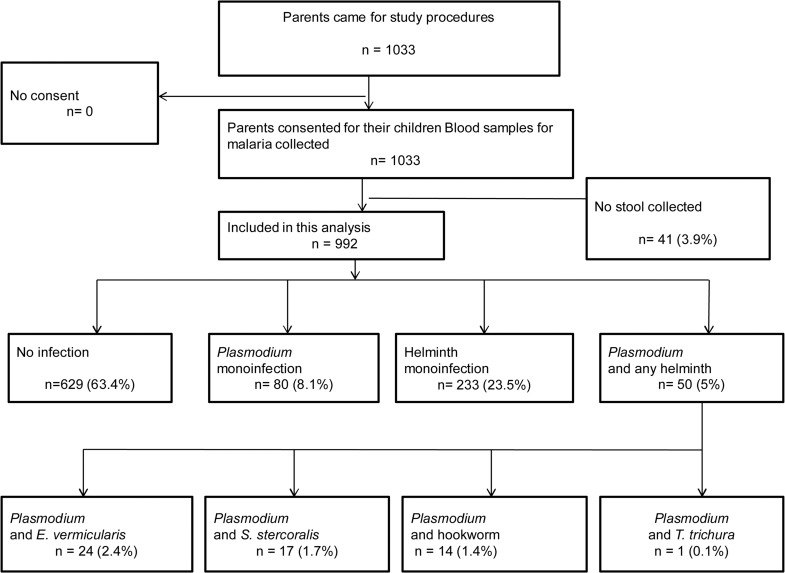
A total of 1033 children were recruited. Of these, 41 (3.9%) did not submit stool samples which left 992 children as study analysis population (Fig 1). Demographic characteristics and intervention coverage are described in Table 2. Overall median age was 4.7 years with 25th of 2.3 and 75th quartiles of 6.5; 459 (46%) were represented by children above five years of age. Among children aged five years and above 185 (40.3%) were not schooling at the time of the survey. 494 (49.8%) were males. Eight hundred and twenty (82.7%) of the study population slept under a long lasting insecticide impregnated net (LLIN) the night before the survey. The parents/guardians reported use of albendazole and mebendazole past six months in 411 (41.4%) and 188 (18.9%) of their children respectively.
Fig 1. Flow of study participants and prevalence of Plasmodium and helminth infections.
Table 2. Demographic characteristics and intervention coverage of study participants by age group.
| Characteristics | < 3 years (n = 297) | 3–5 years (n = 236) | > 5 years (n = 459) | Total (%) N = 992 |
|---|---|---|---|---|
| Median age (25th–75th Quartile) | 1.5 (0.8–2.2) | 4.1 (3.7–4.5) | 6.7 (5.8–7.7) | 4.7 (2.3–6.5) |
| Gender | ||||
| Male | 162 (54.5) | 118 (50.0) | 214 (46.6) | 494 (49.8) |
| Female | 135 (45.5) | 118 (50.0) | 245 (53.4) | 498 (50.2) |
| Education level | ||||
| Too young | 290 (97.7) | 181 (76.7) | 61 (13.3) | 532 (53.6) |
| Preschool | 0 (0.0) | 25 (10.6) | 112 (24.4) | 137 (13.8) |
| Primary school | 0 (0.0) | 0 (0.0) | 145 (31.6) | 147 (14.8) |
| Age to go but doesn't go | 0 (0.0) | 21(8.9) | 124 (27.0) | 145 (14.6) |
| Missed information | 7 (2.3) | 9 (3.8) | 17 (3.7) | 31 (3.1) |
| Bednet information ** | ||||
| Reported to have a bednet | 261 (87.9) | 197 (83.5) | 383 (83.4) | 841 (84.8) |
| Slept under a bednet last night | 261 (87.9) | 196 (83.1) | 373 (81.3) | 830 (83.7) |
| used treated bednet (LLIN) | 259 (87.2) | 191 (80.9) | 370 (80.6) | 820 (82.7) |
| Bednet with holes | 69 (23.2) | 54 (22.9) | 84 (18.3) | 207 (20.9) |
| Reported dewormed past 6 months ** | ||||
| Albendazole | 90 (30.3) | 116 (49.2) | 205 (44.7) | 411 (41.4) |
| Mebendazole | 43 (14.5) | 42 (17.8) | 103 (22.4) | 188 (18.9) |
| Don’t know | 2 (0.7) | 24 (10.2) | 29 (6.3) | 55 (5.5) |
Note: Data are number (%) of participants or infection, unless otherwise indicated.
LLIN = Long Lasting Insecticide impregnated Net.
**The column total doesn’t add up to the specified total age group as the information was collected dependently.
Distribution of infections
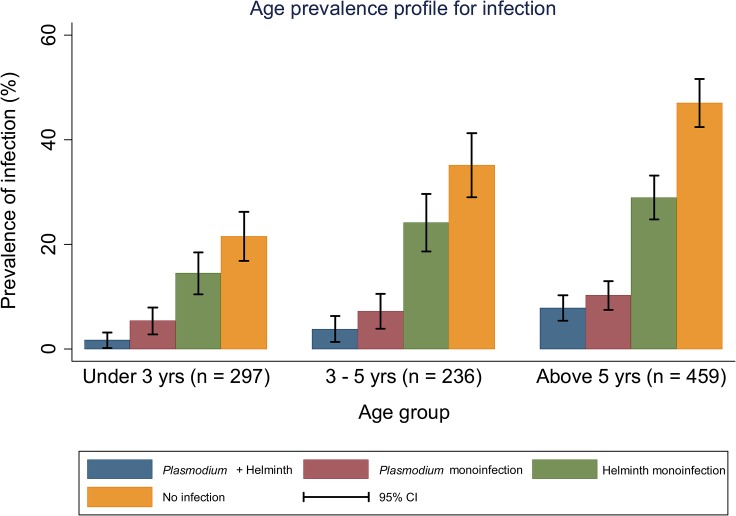
Prevalence of Plasmodium, helminth and co-infections are shown in Fig 1 and Table 3. Out of the 992 children included in the analysis, 130 (13.1%) were infected with Plasmodium species, 283 (28.5%) had helminth infection and 50 (5%) harbored both infections (co-infected). The prevalence of Plasmodium and helminth monoinfection were 8.1% (80/992) and 23.5% (233/992) respectively. E. vermicularis was the most prevalent single helminth infection 116 (11.7%) followed by hookworm 60 (6.1%) and S. stercoralis 42 (4.2%). The prevalence of Plasmodium, STH and co-infections increased with age, older children were mostly affected (Fig 2 and 3A and Table 3). This was especially true for the most prevalent infections, namely E. vermicuralis and hookworm infections, co-infected or not with Plasmodium ( Fig 3B and 3C). The only exception was the prevalence of S. stercoralis monoinfection which was slightly higher in children below five years of age (Table 3 and Fig 3D). Co-infection with Plasmodium and S. hematobium was found in two children (0.2%) and co-infection with Plasmodium and T. trichura in only one child (0.1%), all above five years of age. Two children (0.2%) had positive ICT for W. bancrofti infection.
Table 3. Prevalence of Plasmodium and helminth infections of study participants by age group.
| Characteristics | < 3 years (n = 297) | 3–5 years (n = 236) | > 5 years (n = 459) | Total (%) N = 992 |
|---|---|---|---|---|
| All Plasmodium infection | ||||
| Plasmodium (+ve) | 21 (7.1) | 26 (11.0) | 83 (18.1) | 130 (13.1) |
| Plasmodium (-ve) | 276 (92.9) | 210 (89.0) | 376 (81.9) | 862 (86.9) |
| Geometric mean parasite count (25th–75th Quartile) | 1993 (1200–6740) | 1896 (1260–2680) | 979 (480–1600) | 1227 (560–2200) |
| Plasmodium monoinfection | 16 (5.4) | 17 (7.2) | 47 (10.2) | 80 (8.1) |
| All helminth infection | ||||
| Helminth (+ve) | 48 (16.2) | 66 (28.0) | 169 (36.8) | 283 (28.5) |
| Helminth (-ve) | 249 (83.8) | 170 (72.0) | 290 (63.2) | 709 (71.5) |
| Single helminth infection | ||||
| All single infection | 41 (13.8) | 52 (22.0) | 140 (30.5) | 233 (23.5) |
| E. vermicularis | 17 (5.7) | 25 (10.6) | 74 (16.1) | 116 (11.7) |
| Hookworm | 8 (2.7) | 14 (5.9) | 38 (8.3) | 60 (6.1) |
| S. stercoralis | 13 (4.4) | 11 (4.7) | 18 (3.9) | 42 (4.2) |
| T. trichura | 2 (0.7) | 2 (0.8) | 7 (1.5) | 11 (1.1) |
| S. haematobium | 0 (0.0) | 0 (0.0) | 2 (0.4) | 2 (0.2) |
| W. bancrofti | 1 (0.3) | 0 (0.0) | 1 (0.2) | 2 (0.2) |
| Mixed helminth infection | ||||
| Double helminth species | 7 (2.4) | 12 (5.1) | 24 (5.2) | 43 (4.3) |
| > 2 helminth species | 0 (0.0) | 2 (0.8) | 5 (1.1) | 7 (0.7) |
| Plasmodium and helminth co-infection | ||||
| All Plasmodium + helminth co-infection | 5 (1.7) | 9 (3.8) | 36 (7.8) | 50 (5.0)*** |
| Plasmodium + E. vermicularis | 1 (0.3) | 4 (1.7) | 19 (4.1) | 24 (2.4) |
| Plasmodium + hookworm | 1 (0.3) | 3 (1.3) | 10 (2.2) | 14 (1.4) |
| Plasmodium + S. stercoralis | 4 (1.4) | 3 (1.3) | 10 (2.2) | 17 (1.7) |
| Plasmodium + T. trichura | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Plasmodium + S. hematobium | 0 (0.0) | 0 (0.0) | 2 (0.4) | 2 (0.2) |
*** The total below is more than 5.0% as some specific Plasmodium helminth co-infection have more than one helminth species.
Fig 2. Age prevalence profile for infection (Plasmodium and helminth monoinfections and co-infections) within each age group.
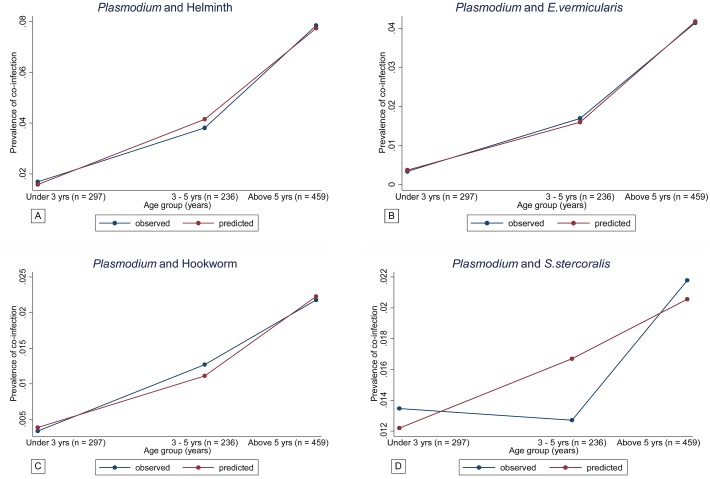
Fig 3. A–D Age prevalence profile of co-infection as predicted from a logistic regression model (Predicted Vs Observed prevalence).
Fig 3A shows Plasmodium and helminth co-infection; 3B Plasmodium and E. vermicularis co-infection; 3C Plasmodium and hookworm co-infection; 3D Plasmodium and S. stercoralis co-infection
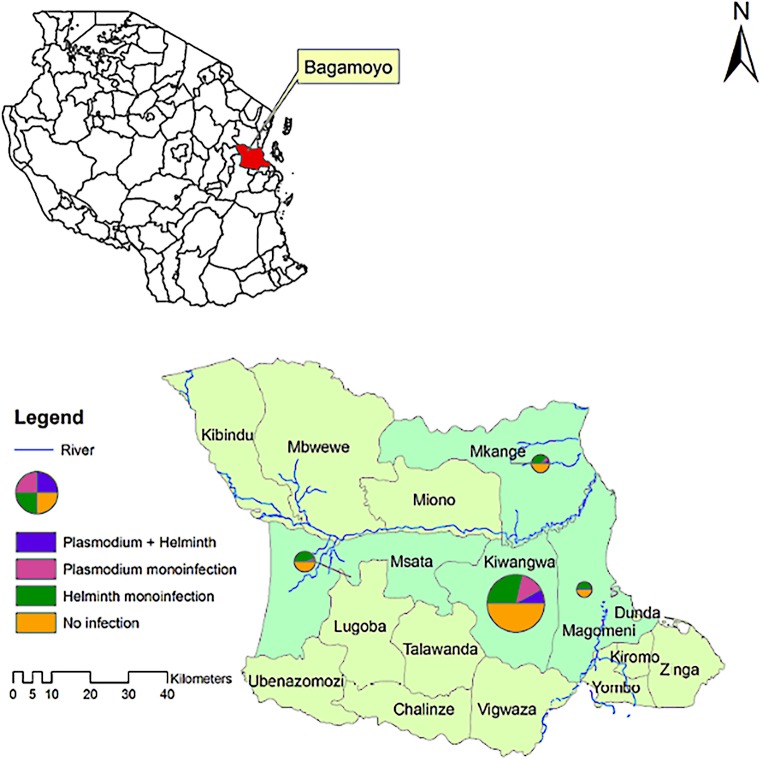
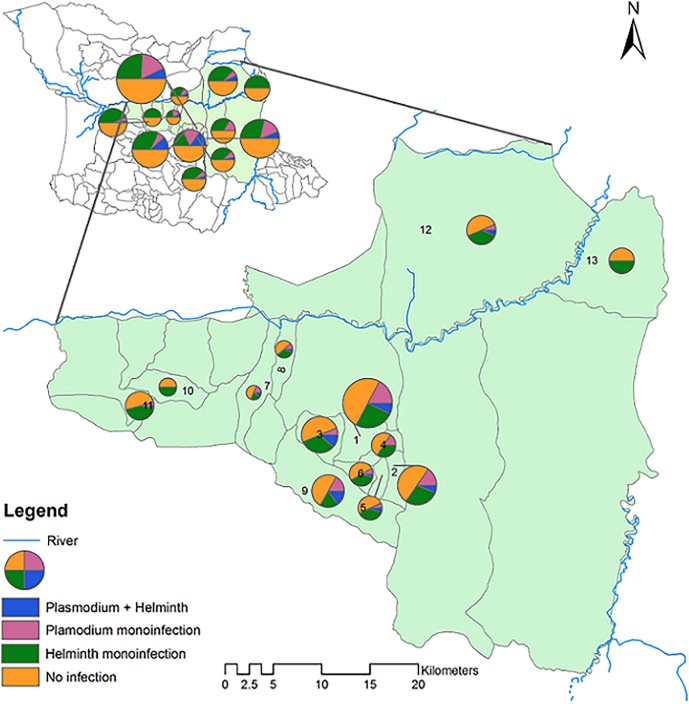
Fig 4 shows administrative map of Tanzania locating Bagamoyo district within coastal region and the spatial distribution of monoinfection/co-infections prevalence in the four villages studied namely Kiwangwa, Mkange, Msata and Magomeni. Spatial heterogeneity of infection prevalence was observed between and within villages. Fig 5 shows the distribution of monoinfection and co-infections among the hamlets of the four studied villages. There were significantly different prevalences of helminth ranging from 44.7% in Mkange to 26.3% in Kiwangwa. The prevalence of Plasmodium infection ranged from 15.4% in Kiwangwa to zero in Magomeni village, with co-infection prevalence being higher in the hamlet of Kiwangwa, Kiwangwa Msinune (p = 0.028), Kiwangwa Bago and Mkange Matipwili (Table 4 and Fig 4 and 5).
Fig 4. Administrative map of Bagamoyo district, coastal region of Tanzania and the spatial distribution of monoinfection and co-infections within four villages, namely Magomeni, Kiwangwa, Msata and Mkange.
The size of the pie is proportional to the sample size contributed by each village/hamlet.
Fig 5. Spatial distribution of monoinfection and co-infection status among hamlets of the four villages within Bagamoyo, coastal region of Tanzania.
The size of the pie is proportional to the sample size contributed by each village/hamlet. 1 = Kiwangwa kiwangwa 2 = Kiwangwa Mwavi 3 = Kiwangwa Bago 4 = Kiwangwa Kibaoni 5 = Kiwangwa Kwambwela 6 = Kiwangwa Pipani 7 = Kiwangwa Masuguru 8 = Kiwangwa Mwetemo 9 = Kiwangwa Msinune 10 = Msata Kihangaiko 11 = Msata Msata 12 = Mkange Matipwili 13 = Magomeni (Makurunge—Kitame).
Table 4. Variables associated with Plasmodium, STH and Plasmodium + STH co-infection using bivariate analysis.
| Risk factors | Plasmodium infection | STH infection | Plasmodium + STH co-infection | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR | p-value | |
| Gender (Female (Ref)) | ||||||
| Male sex | 0.9 (0.6–1.2) | 0.423 | 0.9 (0.7–1.2) | 0.476 | 0.9 (0.6–1.7) | 0.9767 |
| Age | ||||||
| Age in years | 1.2 (1.1–1.3) | < 0.001 | 1.2 (1.1–1.3) | <0.001 | 1.2 (1.1–1.4) | 0.0001 |
| Age group (<3 years (Ref)) | ||||||
| 3–5 years | 1.6 (0.9–3.0) | 0.113 | 2.0 (1.3–3.1) | 0.001 | 2.3 (0.8–7.0) | 0.137 |
| > 5years | 2.9 (1.7–4.8) | < 0.001 | 3.0 (2.1–4.3) | <0.001 | 5.0 (1.9–12.8) | 0.001 |
| Education level (Too young (Ref)) | ||||||
| Preschool | 0.9 (0.5–1.8) | 0.868 | 1.6 (1.1–2.4) | 0.024 | 1.4 (0.6–3.5) | 0.409 |
| Primary | 2.0 (1.2–3.4) | 0.006 | 1.8 (1.2–2.6) | 0.004 | 2.0 (0.9–4.3) | 0.092 |
| Age to go but doesn't | 3.0 (1.9–4.8) | < 0.001 | 1.8 (1.2–2.6) | 0.005 | 2.9 (1.4–5.9) | 0.004 |
| Villages (Hamlets), (Kiwangwa Kiwangwa (Ref)) | ||||||
| Kiwangwa Mwavi | 1.0 (0.5–1.8) | 0.99 | 1.3 (0.8–2.1) | 0.358 | 1.0 (0.3–2.7) | 0.959 |
| Kiwangwa Bago | 0.9 (0.5–1.7) | 0.77 | 2.1 (1.3–3.4) | 0.004 | 2.1 (0.9–5.1) | 0.094 |
| Kiwangwa Kibaoni | 0.5 (0.2–1.5) | 0.248 | 1.0 (0.5–2.0) | 0.931 | Omitted | - |
| Kiwangwa Kwambwela | 0.6 (0.2–1.6) | 0.285 | 2.2 (1.1–4.2) | 0.019 | 0.8 (0.2–4.0) | 0.826 |
| Kiwangwa Pipani | 0.3 (0.1–1.2) | 0.085 | 0.9 (0.4–2.0) | 0.839 | 0.4 (0.1–3.4) | 0.414 |
| Kiwangwa (Mwetemo + Masuguru) | 1.3 (0.6–2.9) | 0.526 | 1.6 (0.8–3.3) | 0.183 | 1.4 (0.4–5.4) | 0.593 |
| Kiwangwa Msinune | 1.7 (0.9–3.1) | 0.07 | 1.3 (0.7–2.2) | 0.413 | 2.7 (1.1–6.7) | 0.028 |
| Msata (Msata + Kihangaiko) | 0.2 (0.1–0.6) | 0.004 | 1.4 (0.8–2.4) | 0.244 | 0.2 (0.0–1.7) | 0.14 |
| Mkange Matipwili | 0.8 (0.4–1.7) | 0.589 | 3.0 (1.7–5.2) | < 0.001 | 1.4 (0.5–4.4) | 0.51 |
| Magomeni (Makurunge—Kitame) | Omitted | - | 2.3 (1.2–4.3) | 0.008 | Omitted | - |
| Did not sleep under bednet last night (Ref) | ||||||
| Slept under bednet last night | 0.5 (0.3–0.9) | 0.0193 | 0.7 (0.4–1.2) | 0.1951 | 1.2 (0.4–4.1) | 0.7206 |
| Did not use antihelminth past 6 months (Ref) | ||||||
| Albendazole | 1.0 (0.7–1.5) | 0.8397 | 1.5 (1.1–2.0) | 0.004 | 1.7 (0.9–3.0) | 0.0679 |
| Mebendazole | 0.9 (0.5–1.4) | 0.6066 | 1.3 (0.9–1.8) | 0.1617 | 0.7 (0.3–1.5) | 0.3444 |
| Helminth negative (Ref) | ||||||
| Any helminth | 1.7 (1.1–2.5) | 0.0072 | ||||
| E. vermicularis monoinfection | 0.8 (0.4–1.5) | 0.43 | ||||
| Hookworm monoinfection | 0.8 (0.4–1.7) | 0.5423 | ||||
| S. stercoralis monoifection | 2.5 (1.2–5.2) | 0.0146 | ||||
| T. trichuris monoinfection | - | 0.1177 | ||||
| Plasmodium density | 1.0 (0.9–1.0) | 0.6971 | 1.0 (1.0–1.0) | <0.001 | ||
| Low density parasitemia | 0.5 (0.1–2.2) | 0.3844 | 3.1 (0.6–16.0) | 0.147 | ||
Note: Density of parasitemia was defined as: Low density parasite <5000/μL and high density ≥5000/ μL
CI, confidence interval; OR, odds ratio
Relationship between Plasmodium and STH infections and predictors of co-infections
There was a pattern for Plasmodium to be associated with helminth infection [OR = 1.7, 95% CI (1.1–2.5)], which was marked for S. stercoralis monoinfection [OR = 2.5, 95% CI (1.2–5.2), p = 0.0146] as shown in Table 4. The effect was statistically significant in a multivariate negative binomial regression model when other factors were considered [IRR = 2.0, 95% CI (1.0–4.00), p = 0.034] as shown in Table 5.
Table 5. Association between Plasmodium and STH infection by multivariate analysis (Negative binomial regression).
| Independent variables | All helminth a | E. vermicularis b | Hookworm c | S. stercoralis d | T. trichura e |
|---|---|---|---|---|---|
| Adjusted IRR (95% CI) | Adjusted IRR (95% CI) | Adjusted IRR (95% CI) | Adjusted IRR (95% CI) | Adjusted IRR (95% CI) | |
| 1.3 (0.9–1.9) | 1.0 (0.5–1.8) | 0.6 (0.3–1.2) | 1.8 (1.0–3.4)* | 0.3 (0.0–1.9) | |
| Age group f | |||||
| < 3 years (Ref) | |||||
| 3–5 years | 1.8 (0.9–3.4) | 2.3 (0.6–8.8) | 2.4 (0.6–9.2) | 2.5 (0.6–9.5) | 2.5 (0.6–9.3) |
| > 5 years | 3.2 (1.6–6.6)* | 4.3 (1.1–16.9)* | 4.5 (1.1–17.9) * | 4.7 (1.2–18.8)* | 4.5 (1.0–17.9)* |
| Education level g | |||||
| Too young (Ref) | |||||
| Preschool | 0.4 (0.2–0.9)* | 0.5 (0.2–1.6) | 0.5 (0.2–1.5) | 0.5 (0.2–1.5) | 0.5 (0.2–1.5) |
| Primary | 0.9 (0.5–1.7) | 0.6 (0.2–1.7) | 0.6 (0.2–1.6) | 0.6 (0.2–1.7) | 0.6 (0.2–1.7) |
| Age to go but doesn’t | 1.3 (0.7–2.3) | 1.1 (0.4–2.9) | 1.1 (0.4–2.8) | 1.2 (0.4–3.1) | 1.0 (0.4–2.8) |
| Village (Hamlets) h | 0.9 (0.9–1.0) | 0.9 (0.9–1.0) | 0.9 (0.9–1.0) | 1.0 (0.9–1.0) | 1.0 (0.9–1.1) |
| Slept under bednet last night i | 0.7 (0.4–1.3) | 2.1 (0.6–7.0) | 1.9 (0.6–6.5) | 2.1 (0.6–6.8) | 2.0 (0.6–6.7) |
| Use of antihelminth past 6 months j | |||||
| Albendazole | 1.0 (0.8–1.3) | 1.0 (0.5–2.0) | 1.0 (0.5–2.0) | 1.0 (0.5–2.0) | 0.9 (0.5–1.8) |
| Mebendazole | 0.8 (0.6–1.1) | 0.5 (0.2–1.3) | 0.4 (0.2–1.2) | 0.5 (0.2–1.3) | 0.5 (0.2–1.2) |
IRR is the incidence rate ratio
All the models a, b, c, d and e were adjusted for f, g, h, i and j.
aReference group was helminth negative
b, c, d, e Reference group were other worm positive species; Variable village (Hamlets)—Report on hamlets categorization was removed as were insignificant when the model was run.
*p values were significant
The risk of Plasmodium, STH and Plasmodium STH co-infections increased with age, with older children (>5 years) being more affected compared to younger children (<3 years and 3–5 years). The differences were statistically significant by bivariate and multivariate negative binomial regression analysis (Tables 4 and 5). Overall there was an age pattern for Plasmodium to be associated with STH [multivariate negative binomial regression [IRR = 2.9, 95% CI (1.7–5.1)], which was more marked for S. stercoralis [IRR = 3.9, 95% CI (1.2–13.1)], and especially in young children. Mantel—Haenszel stratified ORs (Table 6) indicated that the risk of Plasmodium infection with S. stercoralis was higher among younger children aged below 3 years (stratum specific OR = 9.2, 95% CI (0.8–105.5) when exploring for confounding effect of age groups (M-H adjusted OR = 2.2, 95% CI (1.1–4.3), p = 0.0266; homogeneity of ORs, p = 0.3774). Compared to the results with other type of STH, S. stercoralis infection showed increased risk of Plasmodium among younger age group but the homogeneity test suggests no difference in the odds between age groups.
Table 6. Association between Plasmodium and STH infection by Mantel-Haenszel analysis using age group as justification.
| Age group (years) | All helminth | E. Vermicularis | Hookworm | S. stercoralis | T. trichura |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Under 3 years | 1.7 (0.6–4.9) | 0.3 (0.0–3.2) | 0.5 (0.0–5.2) | 9.2 (0.8–105.5) | 0.0 |
| p = 0.3241 | p = 0.3042 | p = 0.5704 | p = 0.0293 | p = 0.6259 | |
| 3–5 years | 1.4 (0.6–3.4) | 0.8 (0.2–3.2) | 0.9 (0.2–3.8) | 1.5 (0.3–7.1) | 0.0 |
| p = 0.4242 | p = 0.7219 | p = 0.8401 | p = 0.5789 | p = 0.2698 | |
| Above 5 years | 1.4 (0.9–2.3) | 0.9 (0.4–1.8) | 0.7 (0.3–1.5) | 1.8 (0.8–4.4) | 0.3 (0.0–2.1) |
| p = 0.1718 | p = 0.6995 | p = 0.3129 | p = 0.1604 | p = 0.178 | |
| Mixed helminth infection | |||||
| Crude OR (95% CI) | 1.7 (1.1–2.5) | 0.8 (0.4–1.5) | 0.7 (0.3–1.3) | 1.9 (1.0–3.7)# | 0.2 (0.0–1.5) |
| p = 0.0072 | p = 0.5392 | p = 0.2784 | p = 0.0588 | p = 0.0811 | |
| Helminth monoinfection | |||||
| Crude OR (95% CI) | - | 0.8 (0.4–1.5) | 0.8 (0.4–1.7) | 2.5 (1.2–5.2) # | 0.0 |
| - | p = 0.43 | p = 0.5423 | p = 0.0146 | p = 0.1177 | |
| M-H adjusted for age group a | |||||
| OR (95% CI) | 1.4 (1.0–2.1) | 0.8 (0.4–1.4) | 0.7 (0.3–1.3) | 2.2 (1.1–4.3) | 0.2 (0.0–1.5) |
| p = 0.0684 | p = 0.4125 | p = 0.2641 | p = 0.0266 | p = 0.0739 | |
| Homogeneity of ORs b | p = 0.9491 | p = 0.7055 | p = 0.9272 | p = 0.3774 | p = 0.8257 |
# Denotes crude ORs. To assess for confounding, crude and adjusted ORs are compared in terms of the difference in relation to magnitude
a The test assesses whether the exposure is significant after adjusting for the age groups
b The test compares whether there is significant difference between age group specific ORs (homogeneity of the stratum ORs), hence whether the overall adjusted OR is valid.
In addition, children who were not attending school although they should have according to their age had increased risk of Plasmodium and co-infections by bivariate analysis although the statistical association was lost in a multivariate analysis. Male and female children were equally affected (Table 4).
The geometric mean Plasmodium parasite count decreased with age as shown in Table 3. Most of the studied children had low intensity helminth infections. Moderate and heavy STH infections intensity were noted among older children. Generally, there were no significant correlation between Plasmodium and STH densities. There was a trend for a negative correlation between Plasmodium parasite density and S. stercoralis larvae count (r = -0.0786, p = 0.8082) and a positive correlation with a hookworm (r = 0.2123, p = 0.5560) and E. vermicularis (r = 0.0418, p = 0.7095) infections.
Discussion
The current study provides baseline epidemiological data of Plasmodium and STH infections in the whole population of children, including the young ones who are rarely surveyed for the latter. To increase sensitivity, different diagnostic methods for helminth infection were used including adhesive tape slides for E. vermicularis and Baermann technique for S. stercoralis. This enabled us to have full range of helminth pattern within the area where malaria transmission occurs throughout the year and National malaria and helminth control programs are undertaken.
Results show that infection with Plasmodium, STH and co-infections are common among children aged below five and above five years living in Bagamoyo district, Tanzania. Despite high coverage of LLINs (above 80%), pockets of Plasmodium infection remain in west side areas like Kiwangwa compared to Magomeni village which is closer to Bagamoyo town where malaria prevalence was documented to be low [21]. This spatial variation could be explained by behavioral factors such as outdoor activities, coverage and effective use of LLINs in the Magomeni village and easy access to health care and hence effective treatment. The geometric mean Plasmodium parasite count decreased with advancing age as expected in most of the malaria endemic countries in relation to development of antimalarial specific immunity [30,31] but the malaria infection prevalence increased with age. These findings may be an indication of a shift of Plasmodium infection towards older age group as observed in Muheza district, Tanzania [32] and other parts of Africa [33–35] where malaria transmission tends to decline and acquisition of immunity is thus delayed. Prevention strategies need thus to take into account the older age group too in the momentum of malaria elimination [36].
The association of STH and Plasmodium infections highlights the extent of the burden of parasites in older age groups. Our results show a definite increase of parasite prevalence, especially STH, with age, as do most of the previous studies [5,6,17,37,38]. The prevalence and pattern of co-infections observed in Bagamoyo differ from those reported in Magu [6] and Mvomero districts [17], Tanzania. Our results show lower prevalence of helminth and Plasmodium helminth co-infections with predominance of E. vermicularis, hookworm and S. stercoralis. The method of detection might be the reason for these differences. Indeed, we used adhesive tape slides for E. vermicularis and Baermann technique for S. stercoralis together with Kato-Katz technique, which detected these specific worms, mostly missed in other surveys. Factors such as exposure and intervention coverage may also explain the types of helminth infection isolated and high prevalence in Magu and Mvomero districts. In Tanzania, published reports on mass drug administration (MDA), mostly under National Lymphatic Filariasis Elimination Program (NLFEP), have shown coverage to fluctuate [39] and its effectiveness to vary depending on the chemoprophylaxis used and duration between cycles [40,41]. The uptake variations could results into persistence of parasites in certain areas and subgroups. In this study, an increased risk of STH, Plasmodium and co-infections was observed among the school-aged children who were not schooling. These may have missed the opportunity to be dewormed, either at school level or within the under-fives program. These children may also be exposed to other risk factors in the environment, or behave differently in terms of sanitation and hygiene. The latter have not been assessed in the present study, which represents a definite limitation. Such a burden in this age group is not only deleterious for them but also acts as a reservoir of infection within the population [42].
Co-infection patterns increased with age as predicted from the age specific prevalence rates by the simple probability model except for S. stercoralis co-infection. The exposure of S. stercoralis and Plasmodium co-infections was significantly different from the other species of helminth. The results show that children with S. stercoralis had twice the risk of Plasmodium infection, with even higher odds among children below 3 years of age, as compared to those with other species of STH. This observation requires further exploration as reports on the age profile for S. stercoralis infection are rare and still conflicting [43–46]. A study done in Côte d’Ivoire showed that hookworm and S. stercoralis had an almost parallel shape of age prevalence pattern [38], but it did not stratify the <5 children into smaller age categories. This could be the reason for the masked trend compared to what was observed in the present study where a negative correlation between S. stercoralis larvae and Plasmodium parasitemia was shown compared to positive correlation with hookworm and E. vermicularis, although not significant. The observed relation between Plasmodium and S. stercoralis infection may arise due to biological associations, whereby S. stercoralis being an early life and chronic infection, starts in the first years and persists through older aged groups promoting establishment and/or survival of Plasmodium infection potentially through Th2 lymphocytes immune modulation [47]. It has been repeatedly shown that hookworm tends to exaggerate Plasmodium infection but knowledge on the immunomodulation with S. stecoralis co-infection is still scarce. Depending on the pro and anti-inflammatory responses mounted within the host, immune response could either promote or inhibit Plasmodium infection [16,47,48]. The equilibrium with S. stercoralis could have been maintained through its intrinsic characteristic to persist for many years in asymptomatic immunocompetent host surviving through low grade autoinfection cycles [47]. In this study, children were not tested for HIV infection which is among the risk factor for S. stercoralis hyperinfection syndrome [49–51]. The immaturity and predominance of Th2 response among younger children could also explain the increased risk of Plasmodium co-infection with S. stercoralis [52].
Heterogeneity of infection prevalence within and between villages indicate that other factors apart from biological association determine the co-infection patterns. Behavioural such as outdoor activities and walking barefooted, sanitation, hygiene and socio-economic factors could explain the variations [38]. Considering the type of STH species isolated and their modes of transmission, exposure factors conducive to both parasites are suspected to be main contributors of Plasmodium and STH co-infections within the studied population [11]. Previous studies done within Bagamoyo district in 2004 suggested that availability of safe water is a serious problem with public health consequences [22]. Up to 40% of people reporting water to be not easily accessible [22] and 70.8% of the water sources were contaminated with fecal coliforms [23]. The situation has not much changed, at least in the rural areas, just few kilometers from the Bagamoyo town. Recent studies conducted within the area showed high rates of water contamination [53,54]. Both hookworm and S. stercoralis are transmitted via skin penetration in poorly maintained latrines and sites of promiscuous defecation. As of E. vermicularis direct transfer of eggs into mouth, inhalation and retroinfection are possible in areas of poor hygiene and scarcity of water [55,56]. All these could contribute to high reinfection rates [57] and persistence of chronic infection post treatment.
Overall, the results of this study demonstrate that both Plasmodium and STH exhibit marked age dependency in infection patterns. In main land Tanzania, control program against helminth has been implemented through expanded program of immunization (EPI) using mebendazole or albendazole among <5 years children and community based Global Program to Eliminate Lymphatic Filariasis (GPELF) using ivermectin plus albendazole among school-aged children and adult population. Under five years children have been targeted by interventions against malaria via antenatal and later postnatal programs where LLINs are distributed. Generally, school-aged children have been rather neglected and therefore not well covered by both control programs. In the current study, the heaviest load of helminth infection was detected among children aged above five years underlying the importance of deworming program to be focused on this age group as suggested by the WHO in order to reduce morbidity and transmission of helminth [29]. Integrated control approaches emphasizing on health education, improvement of environmental sanitation and hygiene coupled with improved housing and access to water, chemoprophylaxis [29,42] and LLINs distributions are required considering the pattern and types of infections within the area to interrupt transmission of both STH and Plasmodium among both the school-aged children but also the under-fives. Frequent and effective antihelminth administrations at least twice a year with a drug like ivermectin which has shown to reduce both helminth and malaria transmission could be prioritized to reduce the burden of co-infection in school-aged children [58]. Potential safety and additional impact of ivermectin to reduce malaria requires further exploration considering the risk of co-infection early in childhood with S. stercoralis [58]. The risk of Plasmodium with S. stercoralis infection among young children requires more investigation to better understand this singular interaction.
Supporting Information
(DOC)
(ZIP)
Acknowledgments
The authors acknowledge all staff of the IDEA project and all children and their parents who agreed to participate in this study and submitted blood, stool and urine samples to achieve and accomplish what we have in the study. We are particularly obliged to Raymond Singo, Shabani Halfan, Rehema Mangoli, and Tatu Nassor for their help in the field and laboratory work, the data unit of the BRTC for entering the large amount of data and providing ample support. In addition, we acknowledge the Bagamoyo district officials, specifically the district immunization and vaccination officer (DIVO) Farah Mohammed for his great collaboration.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study received financial support from the European Commission (Contract/Grant agreement number: 241642) (http://ec.europa.eu/research/health/infectious-diseases/neglected-diseases/projects/014_en.html) in the frame of the IDEA project “Dissecting the Immunological Interplay between Poverty Related Diseases and Helminth infections: An African-European Research Initiative.” The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. WHO (2013) World malaria report: 2013: World Health Organization. [Google Scholar]
- 2.RBM (2012) (Roll Back Malaria) Partnership: Progress and Impact Series 3, 2012. Focus on Mainland Tanzania. Available at: http://www.rbm.who.int/ProgressImpactSeries/docs/report10-en.pdf, accessed 7 February 2014.
- 3. Brooker SJ, Pullan RL, Gitonga CW, Ashton RA, Kolaczinski JH, et al. (2012) Plasmodium-helminth coinfection and its sources of heterogeneity across East Africa. J Infect Dis 205: 841–852. 10.1093/infdis/jir844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pullan RL, Kabatereine NB, Bukirwa H, Staedke SG, Brooker S (2011) Heterogeneities and consequences of Plasmodium species and hookworm coinfection: a population based study in Uganda. J Infect Dis 203: 406–417. 10.1093/infdis/jiq063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mazigo HD, Waihenya R, Lwambo NJ, Mnyone LL, Mahande AM, et al. (2010) Co-infections with Plasmodium falciparum, Schistosoma mansoni and intestinal helminths among schoolchildren in endemic areas of northwestern Tanzania. Parasit Vectors 3: 44 10.1186/1756-3305-3-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kinung'hi SM, Magnussen P, Kaatano GM, Kishamawe C, Vennervald BJ (2014) Malaria and Helminth Co-Infections in School and Preschool Children: A Cross-Sectional Study in Magu District, North-Western Tanzania. PLoS ONE 9: e86510 10.1371/journal.pone.0086510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steinmann P, Utzinger J, Du Z-W, Zhou X-N (2010) Multiparasitism: a neglected reality on global, regional and local scale. Adv Parasitol 73: 21–50. 10.1016/S0065-308X(10)73002-5 [DOI] [PubMed] [Google Scholar]
- 8. Petney TN, Andrews RH (1998) Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. Int J Parasitol 28: 377–393. [DOI] [PubMed] [Google Scholar]
- 9. Rothman KJ, Greenland S (2005) Causation and causal inference in epidemiology. Am J Public Health 95 Suppl 1: S144–150. [DOI] [PubMed] [Google Scholar]
- 10. Brooker S, Clements AC (2009) Spatial heterogeneity of parasite co-infection: Determinants and geostatistical prediction at regional scales. Int J Parasitol 39: 591–597. 10.1016/j.ijpara.2008.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Booth M (2006) The role of residential location in apparent helminth and malaria associations. Trends in parasitology 22: 359–362. [DOI] [PubMed] [Google Scholar]
- 12. Brooker S, Akhwale W, Pullan R, Estambale B, Clarke SE, et al. (2007) Epidemiology of plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anemia, and prospects for combining control. Am J Trop Med Hyg 77: 88–98. [PMC free article] [PubMed] [Google Scholar]
- 13. Druilhe P, Tall A, Sokhna C (2005) Worms can worsen malaria: towards a new means to roll back malaria? Trends Parasitol 21: 359–362. [DOI] [PubMed] [Google Scholar]
- 14. Nacher M, Singhasivanon P, Yimsamran S, Manibunyong W, Thanyavanich N, et al. (2002) Intestinal helminth infections are associated with increased incidence of Plasmodium falciparum malaria in Thailand. Journal of Parasitology 88: 55–58. [DOI] [PubMed] [Google Scholar]
- 15. Spiegel A, Tall A, Raphenon G, Trape J-F, Druilhe P (2003) Increased frequency of malaria attacks in subjects co-infected by intestinal worms and Plasmodium falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 97: 198–199. [DOI] [PubMed] [Google Scholar]
- 16. Nacher M (2011) Interactions between worms and malaria: Good worms or bad worms? Malar J 10: 259 10.1186/1475-2875-10-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mboera LE, Senkoro KP, Rumisha SF, Mayala BK, Shayo EH, et al. (2011) Plasmodium falciparum and helminth coinfections among schoolchildren in relation to agro-ecosystems in Mvomero District, Tanzania. Acta Trop 120: 95–102. 10.1016/j.actatropica.2011.06.007 [DOI] [PubMed] [Google Scholar]
- 18. Mazigo HD, Ambrose-Mazigo EE (2012) Mono-parasite infection versus co-infections in Tanzania: the need to revise our research focus. Tanzania Journal of Health Research 14. [DOI] [PubMed] [Google Scholar]
- 19. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, et al. (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PLoS Med 4: e297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Bureau of Statistics, NBS (2012). Ministry of Planning, Economy and Empowerment, The United Republic of Tanzania Population and Housing Census 2012. www.nbs.go.tz, accessed 18 November 2014.
- 21.Williams J, Dillip A, Smithson P, Hildon Z (CSS report-ihi, 2013) Comparing changes in morbidity and mortality in under-five year olds in Kilombero and Bagamoyo district hospitals.
- 22. Kusiluka L, Mlozi M, Munishi P, Karimuribo E, Luoga E, et al. (2004) Preliminary observations on accessibility and utilisation of water in selected villages in Dodoma Rural and Bagamoyo Districts, Tanzania. Physics and Chemistry of the Earth, Parts A/B/C 29: 1275–1280. [Google Scholar]
- 23. Kusiluka L, Karimuribo E, Mdegela R, Luoga E, Munishi P, et al. (2005) Prevalence and impact of water-borne zoonotic pathogens in water, cattle and humans in selected villages in Dodoma Rural and Bagamoyo districts, Tanzania. Physics and Chemistry of the Earth, Parts A/B/C 30: 818–825. [Google Scholar]
- 24. Willyard C (2009) Large trial to examine parasites' influence on global killers. Nat Med 15: 1097–1097. 10.1038/nm1009-1097 [DOI] [PubMed] [Google Scholar]
- 25. Tanzania HIV/AIDS and Malaria Indicator Survey (2012) ICF International Calverton, Maryland USA, 2011–2012 [Google Scholar]
- 26. Salim N, Schindler T, Abdul U, Rothen J, Genton B, et al. (2014) Enterobiasis and strongyloidiasis and associated co-infections and morbidity markers in infants, preschool-and school-aged children from rural coastal Tanzania: a cross-sectional study. BMC Infect Dis 14: 644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. WHO (2009) Malaria microscopy quality assurance manual: World Health Organization. [Google Scholar]
- 28.Knopp S, Salim N, Schindler T, Karagiannis Voules DA, Rothen J, et al. (2014) Diagnostic Accuracy of Kato–Katz, FLOTAC, Baermann, and PCR Methods for the Detection of Light-Intensity Hookworm and Strongyloides stercoralis Infections in Tanzania. The American Journal of Tropical Medicine and Hygiene. [DOI] [PMC free article] [PubMed]
- 29. WHO (2011) Helminth control in school age children: a guide for managers of control programmes Second edition Geneva, Switzerland: World Health Organization. [Google Scholar]
- 30. Doolan DL, Dobano C, Baird JK (2009) Acquired immunity to malaria. Clin Microbiol Rev 22: 13–36, Table of Contents. 10.1128/CMR.00025-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Woolhouse M (1998) Patterns in parasite epidemiology: the peak shift. Parasitology today 14: 428–434. [DOI] [PubMed] [Google Scholar]
- 32. Winskill P, Rowland M, Mtove G, Malima RC, Kirby MJ (2011) Malaria risk factors in north-east Tanzania. Malar J 10: 98 10.1186/1475-2875-10-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Meara WP, Mwangi TW, Williams TN, McKenzie FE, Snow RW, et al. (2008) Relationship between exposure, clinical malaria, and age in an area of changing transmission intensity. Am J Trop Med Hyg 79: 185–191. [PMC free article] [PubMed] [Google Scholar]
- 34. Carneiro I, Roca-Feltrer A, Griffin JT, Smith L, Tanner M, et al. (2010) Age-patterns of malaria vary with severity, transmission intensity and seasonality in sub-Saharan Africa: a systematic review and pooled analysis. PLoS One 5: e8988 10.1371/journal.pone.0008988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mawili-Mboumba DP, Akotet MKB, Kendjo E, Nzamba J, Medang MO, et al. (2013) Increase in malaria prevalence and age of at risk population in different areas of Gabon. Malaria journal 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nankabirwa J, Brooker SJ, Clarke SE, Fernando D, Gitonga CW, et al. (2014) Malaria in school-age children in Africa: an increasingly important challenge. Tropical Medicine & International Health 19: 1294–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brooker S, Akhwale W, Pullan R, Estambale B, Clarke SE, et al. (2007) Epidemiology of Plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anemia and prospects for combining control. The American journal of tropical medicine and hygiene 77: 88 [PMC free article] [PubMed] [Google Scholar]
- 38. Becker SL, Sieto B, Silué KD, Adjossan L, Koné S, et al. (2011) Diagnosis, Clinical Features, and Self-Reported Morbidity of Strongyloides stercoralis and Hookworm Infection in a Co-Endemic Setting. PLoS Negl Trop Dis 5: e1292 10.1371/journal.pntd.0001292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parker M, Allen T (2013) Will mass drug administration eliminate lymphatic filariasis? Evidence from northern coastal Tanzania. J Biosoc Sci 45: 517–543. 10.1017/S0021932012000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simonsen PE, Derua YA, Kisinza WN, Magesa SM, Malecela MN, et al. (2013) Lymphatic filariasis control in Tanzania: effect of six rounds of mass drug administration with ivermectin and albendazole on infection and transmission. BMC infectious diseases 13: 335 10.1186/1471-2334-13-335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Simonsen PE, Pedersen EM, Rwegoshora RT, Malecela MN, Derua YA, et al. (2010) Lymphatic filariasis control in Tanzania: effect of repeated mass drug administration with ivermectin and albendazole on infection and transmission. PLoS neglected tropical diseases 4: e696 10.1371/journal.pntd.0000696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Campbell SJ, Savage GB, Gray DJ, Atkinson J-AM, Soares Magalhães RJ, et al. (2014) Water, Sanitation, and Hygiene (WASH): A Critical Component for Sustainable Soil-Transmitted Helminth and Schistosomiasis Control. PLoS Negl Trop Dis 8: e2651 10.1371/journal.pntd.0002651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Steinmann P, Zhou X-N, Du Z-W, Jiang J-Y, Wang L-B, et al. (2007) Occurrence of Strongyloides stercoralis in Yunnan Province, China, and comparison of diagnostic methods. PLoS Negl Trop Dis 1: e75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Egido J, De Diego J, Penin P (2001) The prevalence of enteropathy due to strongyloidiasis in Puerto Maldonado (Peruvian Amazon). Brazilian Journal of Infectious Diseases 5: 119–123. [DOI] [PubMed] [Google Scholar]
- 45. Lindo JF, Robinson RD, Terry SI, Vogel P, Gam AA, et al. (1995) Age-prevalence and household clustering of Strongyloides stercoralis infection in Jamaica. Parasitology 110: 97–102. [DOI] [PubMed] [Google Scholar]
- 46. Glinz D, N'Guessan NA, Utzinger J, N'Goran EK (2010) High prevalence of Strongyloides stercoralis among school children in rural Côte d'Ivoire. Journal of Parasitology 96: 431–433. 10.1645/GE-2294.1 [DOI] [PubMed] [Google Scholar]
- 47. Concha R, Harrington WJ, Rogers AI (2005) Intestinal Strongyloidiasis: Recognition, Management, and Determinants of Outcome. Journal of Clinical Gastroenterology 39: 203–211. [DOI] [PubMed] [Google Scholar]
- 48. Knowles SC (2011) The effect of helminth co-infection on malaria in mice: a meta-analysis. International journal for parasitology 41: 1041–1051. 10.1016/j.ijpara.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 49. Keiser PB, Nutman TB (2004) Strongyloides stercoralis in the Immunocompromised Population. Clin Microbiol Rev 17: 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marcos LA, Terashima A, DuPont HL, Gotuzzo E (2008) Strongyloides hyperinfection syndrome: an emerging global infectious disease. Transactions of the Royal Society of Tropical Medicine and Hygiene 102: 314–318. 10.1016/j.trstmh.2008.01.020 [DOI] [PubMed] [Google Scholar]
- 51. Olsen A, van Lieshout L, Marti H, Polderman T, Polman K, et al. (2009) Strongyloidiasis–the most neglected of the neglected tropical diseases? Transactions of the Royal Society of Tropical Medicine and Hygiene 103: 967–972. 10.1016/j.trstmh.2009.02.013 [DOI] [PubMed] [Google Scholar]
- 52. PrabhuDas M, Adkins B, Gans H, King C, Levy O, et al. (2011) Challenges in infant immunity: implications for responses to infection and vaccines. Nature immunology 12: 189 10.1038/ni0311-189 [DOI] [PubMed] [Google Scholar]
- 53. Mattioli MC, Boehm AB, Davis J, Harris AR, Mrisho M, et al. (2014) Enteric Pathogens in Stored Drinking Water and on Caregiver’s Hands in Tanzanian Households with and without Reported Cases of Child Diarrhea. PloS one 9: e84939 10.1371/journal.pone.0084939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mattioli MC, Pickering AJ, Gilsdorf RJ, Davis J, Boehm AB (2012) Hands and water as vectors of diarrheal pathogens in Bagamoyo, Tanzania. Environmental science & technology 47: 355–363. [DOI] [PubMed] [Google Scholar]
- 55. Knight R (1982) Parasitic disease in man: Edinburgh, UK; Churchill Livingstone. [Google Scholar]
- 56. Cook G (1994) Enterobius vermicularis infection. Gut 35: 1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brooker S, Bethony J, Hotez PJ (2004) Human hookworm infection in the 21st century. Adv Parasitol 58: 197–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Slater HC, Walker PG, Bousema T, Okell LC, Ghani AC (2014) The potential impact of adding ivermectin to a mass treatment intervention to reduce malaria transmission: a modelling study. J Infect Dis 210: 1972–1980. 10.1093/infdis/jiu351 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.