Abstract
Background
The Recipient Epidemiology and Donor Evaluation Study -III (REDS-III) is a 7-year multicenter transfusion safety research initiative launched in 2011 by the National Heart, Lung, and Blood Institute.
Study design
The domestic component involves 4 blood centers, 12 hospitals, a data coordinating center, and a central laboratory. The international component consists of distinct programs in Brazil, China, and South Africa which involve US and in-country investigators.
Results
REDS-III is using two major methods to address key research priorities in blood banking/transfusion medicine. First, there will be numerous analyses of large “core” databases; the international programs have each constructed a donor/donation database while the domestic program has established a detailed research database that links data from blood donors and their donations, the components made from these donations, and data extracts from the electronic medical records of the recipients of these components. Secondly, there are more than 25 focused research protocols involving transfusion recipients, blood donors, or both that are either in progress or scheduled to begin within the next 3 years. Areas of study include transfusion epidemiology and blood utilization; transfusion outcomes; non-infectious transfusion risks; HIV-related safety issues (particularly in the international programs); emerging infectious agents; blood component quality; donor health and safety; and other donor issues.
Conclusions
It is intended that REDS-III serve as an impetus for more widespread recipient and linked donor-recipient research in the US as well as to help assure a safe and available blood supply in the US and in international locations.
Keywords: transfusion medicine research, study design, donor-recipient linkage, blood safety, blood availability
The Recipient Epidemiology and Donor Evaluation Study -III (REDS-III) is a seven-year transfusion safety research initiative launched in 2011 by the National Heart, Lung, and Blood Institute (NHLBI). It includes a domestic component and three distinct international programs in Brazil, China, and South Africa. REDS-III is a successor program to two previous NHLBI multicenter epidemiology programs, the Retrovirus Epidemiology Donor Studies - REDS and REDS-II – which were initiated over two decades ago in response to the HIV epidemic.1,2 The emphasis of REDS-III has shifted to recipient-based research, particularly transfusion epidemiology and outcomes, and to evaluating whether donor factors affect recipient outcomes. Studies in the areas of blood donor safety and availability and the retention of a rapid response capability to evaluate the threat of new emerging infectious agents in the blood supply remain important features of the current program.
The REDS-III international component focuses on donor and laboratory research aimed at characterizing the current HIV epidemic and decreasing HIV transfusion-transmission in non-US settings and in recipients with specific clinical conditions [e.g. obstetrical hemorrhage in South Africa and sickle cell disease (SCD) in Brazil]. Additionally, transfusion-transmitted infections (TTIs) that could potentially threaten the safety of the US blood supply are studied. Whenever possible, an integrated approach across international programs is/will be used, with one goal being to improve the scientific and analytical skills of the people responsible for blood safety in developing countries.
Infrastructure of the REDS-III program
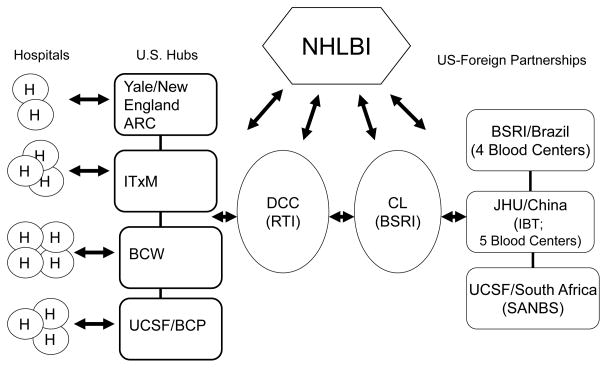
The REDS-III domestic program consists of four blood centers, 12 hospitals (each of which receives components from one of the blood centers), a data coordinating center (DCC), and a central laboratory (CL). Collaborations with external organizations (government, blood banking, research laboratories, and industry) are established as needed. The international program consists of the same DCC and CL along with either a national blood organization (e.g., South African National Blood Services – SANBS – which collects blood in eight of the nine South African provinces) or a consortium of regional blood centers (Brazil and China) with additional participation of selected hospitals in focused research protocols. (See Table 1 for a list of participating institutions and Figure 1 for organizational structure).
Table 1.
Participating domestic and international REDS-III institutions
| Domestic Component - Four participating Hubs |
Blood Center of Wisconsin (BCW), Milwaukee, WI
|
The Institute For Transfusion Medicine (ITXM), Pittsburgh, PA
|
University of California, San Francisco (UCSF), San Francisco, CA
|
Yale University School of Medicine, New Haven, CT
|
| International Component -Three large collaborative programs between U.S. institutions and blood banks in countries seriously affected by HIV/AIDS |
Brazil
|
China
|
South Africa
|
Domestic and International components
|
Figure 1. REDS-III Institutional Infrastructure.
Coordination of institutions participating in REDS -III
Abbreviations: ARC - American Red Cross; BCP – Blood Centers of the Pacific; BCW- BloodCenter of Wisconsin; BSRI - Blood Systems Research Institute; CL- Central Laboratory: DCC - Data Coordinating Center: IBT - Chinese Institute of Blood Transfusion; ITXM - The Institute For Transfusion Medicine; JHU -Johns Hopkins University; NHLBI – National Heart Lung and Blood Institute; RTI – RTI International; SANBS - South African National Blood Service; UCSF - University of California, San Francisco; Yale - Yale University School of Medicine
Contribution to Education and Training
REDS-III strives to foster the development of junior investigators who have an interest in epidemiology and laboratory research in transfusion medicine. To accomplish this, each domestic hub mentors junior investigators and prepares them to apply for NIH career development awards. The international program has several training initiatives, including scientific and mentoring symposia rotating among international sites, attended by senior and junior investigators from all three sites. The most promising overseas junior investigators will also be eligible for 6 to 8 weeks of training at REDS-III institutions in the US. These efforts are meant to encourage development of multi-national analyses and build sustainable relationships between established and new investigators in the US and international sites.
REDS-III Portfolio
The REDS-III portfolio addresses key research priorities in blood banking/transfusion medicine by conducting analyses of large core databases and focused research protocols that involve enrolling study subjects or testing retained biorepository specimens.
The REDS-III domestic program has established for the first time a detailed research database infrastructure that links data from blood donors and their donations, the components made from these donations, and the recipients of these components; i.e., a particular donation can be traced through component production and, if transfused at a participating hospital, to a data extract from the electronic medical record of the transfusion recipient. This permits the conduct of numerous analyses that characterize blood component utilization patterns in diverse settings, inform the design of future clinical trials, and potentially determine blood donor/donation effects on recipients’ clinical outcomes. Table 2 provides the number of blood components annually transfused in participating hospitals.
Table 2.
Annual transfusions at 12 domestic REDS-III hospitals
| Hub | Hospital | RBC | Whole blood platelet (individual units) | Apheresis platelet | Plasma | Cryoprecipitate pools* |
|---|---|---|---|---|---|---|
| 1 | 1 | 6,294 | 0 | 627 | 2,881 | 100 |
| 1 | 2 | 29,000 | 0 | 9,950 | 12,500 | 280 |
| 1 | 3 | 2,055 | 0 | 529 | 662 | 46 |
| 2 | 4 | 22,875 | 20,269 | 232 | 20,645 | 855 |
| 2 | 5 | 20,520 | 25,074 | 1,427 | 9,431 | 347 |
| 2 | 6 | 5,700 | 1,679 | 108 | 1,477 | 10 |
| 3 | 7 | 25,211 | 18,260 | 5,239 | 9,433 | 819 |
| 3 | 8 | 5,926 | 2,220 | 229 | 2107 | 40 |
| 4 | 9 | 21,280 | 0 | 5,891 | 4,830 | 3,349 |
| 4 | 10 | 17,238 | 0 | 3,829 | 5,255 | 926 |
| 4 | 11 | 2,219 | 0 | 99 | 1,020 | 11 |
| 4 | 12 | 6,281 | 0 | 1,133 | 2,647 | 377 |
| Total | 164,599 | 67,502 | 29,293 | 72,888 | 7,160 |
Pools consist of 5 or 6 individual cryoprecipitate units
Another large linked donor-recipient database that will be accessed for focused protocol-driven analyses supported by REDS-III is the ScanDat database, which consists of computerized records of blood donation and transfusion activities dating back to the mid 1960’s in Sweden and the early 1980’s in Denmark.3,4 The database – which through record linkages also can retrieve data from nationwide health data registers for cancer, hospital care and causes of death – includes data on >1.1 million blood donors and >1.3 million transfused patients. It is currently being updated, extending coverage through 2012.
Additionally, each international program has established a donor/donation database which will be used to evaluate TTI incidence and prevalence, donor demographics and return patterns, provide background statistics, and serve as a sampling frame for specific research protocols.
To ensure that the development of focused research protocols encompassed key research priorities in transfusion medicine, an NHLBI-appointed external committee reviewed the general content of the portfolio developed by REDS –III investigators and provided recommendations. Protocol development steps included comprehensive discussions among REDS-III investigators, active support from the scientific community through consultant experts, and review by members of the REDS-III Observational Study Monitoring Board (OSMB). The portfolio includes over 25 protocols. involving transfusion recipients, blood donors, or both; these are either in progress or scheduled to launch within the next three years. Table 3 groups these protocols by domestic and international site and Table 4 by major scientific subject area. Each protocol is briefly summarized below.
Table 3.
REDS-III protocols by programmatic site
| Protocol | |
|---|---|
| Domestic | Retrospective cohort study of plasma use, transfusion related circulatory overload (TACO), and risk associated with use of ABO compatible, non-identical plasma (Epidemiology of plasma transfusion) |
| Population-based study of the detection and prediction of in-hospital transfusion-related adverse events within the Northern California Kaiser Permanente system (Transfusion modeling) | |
| Red cell in the Elderly Transfusion Outcomes (RETRO) | |
| The genetic basis of allo-antibody formation and persistence in blood donors (Leukocyte alloimmunization GWAS) | |
| Severe Transfusion Reactions including Pulmonary Edema (STRIPE) | |
| HIV in sickle cell disease patients in-vitro evaluation (HIV in SCD in-vitro study) | |
| Mechanisms of GB Virus type C reduction in mortality and viral load in HIV-infected patients with transfusion transmitted GBV-C infection in the NHLBI Viral Activation Transfusion Study (GBV-C impact on HIV infection) | |
| Red Blood Cell-Omics (RBC-Omics): Genetic basis of 1) differential RBC storage capacity and 2) iron balance and clinical syndromes in high-intensity blood donors | |
| Cognition, Hemoglobin, and Iron Levels in teen donors (CHILL) | |
| Hemoglobin and Iron Recovery Study (HEIRS) | |
| The effect of TMPRSS6 polymorphisms on hemoglobin and iron stores in high intensity blood donors (TMPRSS6 polymorphisms) | |
| Opinions and perspectives about the current blood donation policy for men who have sex with men (Blood DROPS) | |
| Brazil | Standardizing staging and molecular characterization of HIV-1 strains in REDS-III International programs (Brazil, China, and South Africa) to assure optimal detection of incident infections and classification of genotypes/circulating recombinant forms, drug resistance profiles, and detailed characterization of transmitted/founder viruses (HIV Molecular Surveillance) |
| Establishing a Brazilian sickle cell disease cohort and identifying molecular determinants of response to transfusions, genetic determinants of alloimmunization, and risk factors associated with HIV infection (SCD in Brazil) | |
| Dengue virus incidence and prevalence in Brazilian donors and recipients: Evaluating rates and correlates of transfusion-transmission, and clinical outcomes of infections in recipients (Tx-transmitted DENV in Brazil) | |
| HIV risk factor study | |
| Improving the safety of blood donation in Brazil through an assessment of the effectiveness of HIV notification and counseling, and linkage of HIV positive donors to healthcare (Brazil HIV notification) | |
| China | Standardizing staging and molecular characterization of HIV-1 strains in REDS-III International programs (Brazil, China, and South Africa) to assure optimal detection of incident infections and classification of genotypes/circulating recombinant forms, drug resistance profiles, and detailed characterization of transmitted/founder viruses (HIV Molecular Surveillance) |
| A study of Severe Fever with Thrombocytopenia Syndrome Virus (SFTSV) seroprevalence and rates of asymptomatic viremia in Chinese blood donors from three Chinese regions (SFTSV in China) | |
| REDS-III Blood Utilization study in Chinese hospitals (RBC utilization in China) | |
| HIV case-control study | |
| South Africa | Standardizing staging and molecular characterization of HIV-1 strains in REDS-III International programs (Brazil, China, and South Africa) to assure optimal detection of incident infections and classification of genotypes/circulating recombinant forms, drug resistance profiles, and detailed characterization of transmitted/founder viruses (HIV Molecular Surveillance) |
| Transfusion in Pregnancy in South Africa (TIP in South Africa) | |
| Incident HIV and incident HBV infections in South African blood donors: behavioral risk factors, genotypes and biological characterization of early infections (Incident HIV/HBV in South Africa) | |
| Blood donor recruitment and retention in South Africa (South Africa donor recruitment/retention) | |
| Denmark and Sweden (ScanDat) | Using a linked donor-recipient database (ScanDat) to search for new transfusion-transmitted diseases (Searching for new TTIs) |
| Apheresis donation and bone fracture risk using the ScanDat database (Apheresis donation and bone fractures) |
Table 4.
REDS-III protocols classified by broad scientific subject area and population studied
| Subject area | Population studied | ||
|---|---|---|---|
| Transfusion Recipients | Linked donors and recipients | Blood donors | |
| Transfusion Epidemiology/Blood Utilization |
|
||
| Transfusion Outcomes |
|
||
| Non-infectious Transfusion Risks |
|
|
|
| HIV |
|
|
|
| Emerging infectious agents |
|
|
|
| Blood component quality |
|
||
| Donor Health and Safety |
|
||
| Donor Deferral criteria |
|
||
| Donor Recruitment and Retention |
|
||
Transfusion Epidemiology and Blood Utilization
1. Epidemiology of plasma transfusion
There are few US data on use of fresh frozen plasma or its alternatives (e.g., thawed plasma). This study evaluating plasma transfusion at 11 hospitals in a recent one-year period includes data on ~70,000 units of transfused plasma. Study aims are to characterize plasma use by patient diagnosis, dose, type of component, and laboratory trigger; determine efficacy when ordered to correct coagulation testing abnormalities; and compare adverse events for ABO identical vs. compatible transfusions.5 The data will help design clinical trial(s) to evaluate plasma transfusion strategies in non-trauma settings.6
2. Transfusion modeling
This REDS-III / Kaiser Permanente Northern California collaboration examines patterns of blood product utilization and predictors of RBC transfusion in hospitalized patients within an integrated health care delivery system. This retrospective cohort study utilizes data on over 250,000 transfusions in 450,000 adult hospitalizations over a four-year period. These data are obtained from electronic medical records (EMR) of 3.3 million health plan members (~ 20% of Northern California’s population) receiving care at 21 hospitals and 56 outpatient clinics. EMR data allow for adjustment to control for each patient’s co-morbidities and illness severity.7
Pulmonary complications of transfusion (e.g., TACO and TRALI) cause significant morbidity and are leading causes of transfusion-associated mortality.8,9 Another study objective, utilizing EMR-based approaches for detecting transfusion-related hypoxemia (e.g., vital sign and arterial blood gas data as well as natural language processing of chest x-ray reports to identify pulmonary edema),9,10 is to identify these clinical syndromes and evaluate their prevalence, risk factors, and outcomes in a community hospital setting.
3. RBC utilization in China
This study will retrospectively identify all patients receiving RBC transfusion over a one-year period in three Chinese hospitals and characterize rates of transfusion by demographics. One thousand transfused patients at each hospital will be characterized as to demographics, co-morbidities, surgical procedures, RBC unit(s) characteristics, and pre-transfusion hemoglobin concentration. Patients in frequently transfused diagnostic categories and a diagnoses-matched, non-transfused control group will be evaluated to determine the hemoglobin threshold and other clinical features associated with transfusion. The pre-transfusion HIV infection rate and data on possible transfusion-transmitted HIV cases will be captured.
This study may lead to improved blood use and/or provide data to design clinical trials in China.
Transfusion Outcomes
1. SCD cohort in Brazil
In Brazil, SCD patients receive outpatient care in blood banks (Hemocenters). This project will develop a comprehensive database of clinical, laboratory, and transfusion-exposure information from 3,000 SCD patients treated at the four REDS-III Hemocenters. This cohort will be followed for three years to: define incidence of specific SCD manifestations; characterize blood utilization; identify rates and correlates of transfusion complications including alloimmunization, iron overload, and TTIs such as HIV; determine the effect of chronic transfusion programs on clinical outcomes; describe baseline laboratory parameters and their association with clinical outcomes; and assess rates and causes of mortality.11–14 A biospecimen collection will be established.
Additionally, to study the impact of transfusion therapy on inflammation, a comprehensive panel of soluble inflammation markers will be measured in serial pre- and post-transfusion samples in 300 SCD patients undergoing acute or chronic transfusion therapy. To investigate the genetic basis of RBC alloimmunization, which has a substantial impact on SCD transfusion management, a genome-wide association study (GWAS) will be performed on DNA from 500 alloimmunized patients and 500 non-alloimmunized controls. Finally, to evaluate the determinants of the apparently low HIV-infection rates in SCD patients, a nested case-control study will quantify HIV risk factors and describe clinical outcomes and laboratory findings in HIV-infected SCD patients.
2. Transfusion in Pregnancy (TIP) in South Africa
In South Africa and other low/middle income countries, obstetric hemorrhage (OH) is a major cause of obstetric morbidity and mortality.15,16 A recently completed REDS-III pilot study showed that although OH incidence in South Africa is not significantly increased compared to the US, the peripartum transfusion rate is ten-fold higher. A positive HIV status was associated with transfusion, even after controlling for age, parity and mode of delivery. We hypothesize that the reasons for this may include antenatal anemia, coagulopathy, and/or variability in institutional and physician practice. To confirm our initial observation of an increased transfusion rate in HIV infected women, a large case-control study of peripartum transfused versus non-transfused women will be conducted using chart review and laboratory testing. Data on OH management, outcomes and co-morbid disease will be captured. Our second study aim (which uses a cross-sectional, observational study design) is to investigate the causes of antenatal anemia in both HIV Ab positive and negative women referred to the specialist antenatal clinics at Chris-Hani Baragwanath Hospital, a major obstetric referral unit in Johannesburg. Finally, the study will characterize antenatal transfusions using a cross-sectional study design with chart review, thereby providing a descriptive evaluation of transfusion risk prior to delivery.
3. Red cell in the Elderly Transfusion Outcomes (RETRO)
This study has two main components. The first uses the REDS-III recipient database to describe RBC transfusion practice in people ≥65, who comprise >60% of US transfusion recipients.17 The database captures demographics, diagnoses, RBC dose and frequency, medications, and hemoglobin-transfusion trigger and response data on inpatients and outpatients at 12 REDS-III hospitals. Questions to be addressed include the hemoglobin threshold for transfusion and whether it varies over time, by hospital, or underlying disease.
Older adults with chronic anemia receive frequent outpatient transfusions to alleviate symptoms such as fatigue or dyspnea.18,19 The second study component, a prospective observational study in which each patient serves as his/ her own control, will determine the impact of transfusion on function and quality of life in 200 outpatients ≥65 years. Enrollees will be evaluated for their performance on a 6-minute walk test and on standardized surveys for fatigue and dyspnea pre- and post-transfusion.
Non-infectious Transfusion Risks
1. Leukocyte alloimmunization GWAS
Alloimmunization, whether to RBC or leukocyte antibodies, may have a host genetic component which could provide insights into how to identify recipients at-risk for this transfusion complication and possibly lead to preventative transfusion strategies.20
This GWAS tests the hypothesis that host genetic polymorphisms increase the risk of leukocyte antibody formation given allogeneic exposure to non-host HLA antigens. Using samples from the REDS-II Leukocyte Antibody Prevalence Study biorepository,21 analyses will compare genotypes of 772 HLA-alloantibody-positive women of European ancestry with those of 772 HLA-alloantibody-negative women with ≥ 2 pregnancies. In parallel, a follow-up study has enrolled 248 of these women and one or more of their children to evaluate the degree of HLA mismatching between them.
These GWAS data may be informative to the planned REDS-III GWAS of RBC alloimmunization in Brazilian SCD patients.
2. Severe Transfusion Reactions including Pulmonary Edema (STRIPE)
This study of severe pulmonary transfusion reactions, with an emphasis on TACO, will be conducted at four hospitals (1/hub). An automated electronic monitoring system will be evaluated for its sensitivity and specificity in TACO diagnosis.10 The automated system will screen real-time data (arterial blood gas, oxygen saturation, and chest radiographs) to identify adult patients with post-transfusion hypoxemia. A nurse coordinator at each hospital will summarize these cases for adjudication by an expert panel of critical care physicians. In addition, these coordinators will perform medical record review (i.e., active hemovigilance) of >40,000 transfusions for cardiopulmonary reactions including TACO, TRALI, sepsis, anaphylaxis, and hypotension. Results will be compared with the rate of routine operational reporting of these reactions.
A year-long case-control study of TACO risk factors will follow. Cases (n=250) will be detected using the validated monitoring system and controls (n=500) will be selected concurrently from transfused, unaffected patients. A natriuretic peptide, NT-pro-BNP, will be measured pre and post-transfusion. Information on predictor variables (e.g., demographics, blood products transfused, infusion rates, fluid balance, coexisting cardiac disease and other medical conditions) and outcomes (e.g., ICU and hospital stay and survival to discharge) will be collected and used to develop a predictive algorithm for TACO.8
HIV Studies
Each international program will calculate HIV prevalence and incidence in donors and residual risk in recipients; conduct molecular surveillance to determine HIV genotypes and drug resistance mutations (DRMs); and evaluate HIV risk factors in HIV-positive donors.22–29 These studies will build on previous REDS-II efforts in Brazil and China but will place an increased emphasis on recently-infected donors who are defined as HIV NAT reactive, HIV antibody negative (i.e., NAT yield); or HIV NAT reactive and HIV antibody positive but negative on the HIV antibody Limiting antigen avidity assay
1. Molecular Surveillance
Coordinated by the REDS-III CL (i.e., BSRI), investigators from the three in-country laboratories have exchanged protocols in order to optimize their methods for HIV amplification and sequencing. The CL, in collaboration with EQAPOL (an NIAID-funded HIV quality control program), has constructed a 50-specimen HIV-test panel to be assayed by each laboratory to ensure sensitivity and accuracy of their of in-country techniques. The panel consists of cultured and highly characterized viruses from recently-transmitted HIV isolates; it includes diverse genotypes, circulating recombinant forms (CRFs) and viruses with DRMs.
To further pursue the aim of global molecular surveillance, the REDS-III CL is performing full-genome deep sequencing of HIV isolates from US, Brazil and SA blood donor specimens collected in the 1990s and recently. Data analysis will evaluate whether the genetic composition of HIV-1 variants that are transmitted to newly infected persons (i.e., so-called transmitter/founder variants) has undergone evolution over the last two decades.30
2. Risk factors in Brazilian donors
This study has two hypotheses. The first is that unprotected MSM (men who disclose having had sex with men) or multiple heterosexual partners and, to a lesser extent, injection drug use will continue as the predominant HIV risk factors among both recent and long-term infected donors. The second is that there will be clinically relevant increases in HIV subtype diversity and primary DRMs among recently infected donors, regardless of specific risk factors. Enrollment is targeted at 25 HIV-positive cases annually at each of four centers over four years. Enrollees will provide a blood sample and complete an audio computer-assisted structured interview (ACASI) about risk factors and motivation to donate blood.
3. Risk factors in Chinese donors
This study has two hypotheses. The first is that high-risk heterosexual behavior including having commercial sex and/or multiple concurrent sex partners will replace IDU and MSM as the leading HIV risk factor for infection among Chinese blood donors, reflecting the major transmission modes in the general population.31 Secondly, the study will evaluate if there is increased HIV subtype diversity and DRMs among recent vs. long-term infected donors.
Target enrollment is 300 cases and 600 controls in five HIV high-prevalence regions over four years. Enrollees will provide a blood sample and complete a mail or online questionnaire about medical/behavioral risk factors and motivation for donation.
4. Incident HIV/HBV in South African donors
This study has several hypotheses: 1a) Risk factors for HIV incident infection will include a recent change in sexual partner, older male/younger female sexual partnership, a greater number of recent sexual partners, unprotected receptive anal intercourse, and lower socioeconomic status; 1b) Risk factors for HBV incident infection will include a greater number of recent sexual partners, recent scarification/tattoo/body markings, and history of personal contact with an HBV-infected person; 2a) DRMs will increase as HAART is administered to a larger percentage of HIV-infected South Africans; and 2b) Incident HBV infections will show an increase of genotype E compared to public health data on prevalent infections.
A frequency matched case-control study will be conducted. There will be two case groups: incident HIV-infected blood donors (N=300) and incident HBV-infected blood donors (N=150), both to be compared to infectious marker negative controls (N=900). An ACASI questionnaire will be completed. HIV subtype and DRM profiles will be characterized and viral loads determined in all HIV incident cases, as will HBV genotype and viral load in all incident HBV infections. Donors with incident and elite controller HIV infections will be prospectively followed for three additional visits at 2, 3, and 6 months post-index donation.32
5. Brazil HIV notification
In Brazil, blood donors with a reactive infectious disease screening test result receive a non-specific letter requesting their return for follow-up. Approximately 40% do not return, and thus do not undergo confirmatory testing nor receive the counseling and referral information provided by Brazilian blood centers.33
This study will analyze the proportion of donors who are successfully notified of reactive screening results and determine if this varies by type of infection. Additionally, a cohort of HIV-positive former donors who participated in REDS-II and REDS-III HIV case interviews between 2008 and 2013 will participate in ACASI interviews to assess the effectiveness of HIV notification and counseling by evaluating donor follow-up activities (HIV-infection treatment and transmission-prevention behaviors) and to determine ways to improve the disclosure of HIV risks during donor eligibility assessments.34
6. HIV in SCD in-vitro study
To evaluate if there is a biological basis for the apparent lower than expected prevalence of HIV in SCD populations,14 PBMCs from HIV-negative SCD patients and non-SCD control patients will be evaluated for in-vitro susceptibility to HIV-infection. Secondly, the inflammatory cytokine profile in the blood of HIV-negative SCD patients will be compared to that of HIV elite controllers. The epidemiologic (e.g. behavioral risk factor) aspects of HIV infection in SCD patients will be studied as part of the Brazil SCD project (see above).
7. GBV-C impact on HIV infection
GBV-C, a flavivirus discovered in 1995, is highly prevalent but not associated with any disease. Some investigators have observed longer survival among HIV-infected individuals who have active GBV-C co-infection.35 Previous data analyses from the NHLBI Viral Activation Transfusion Study (VATS) looked for an effect of both existing GBV-C infection and acute GBV-C RNA acquisition (presumably through transfusion) on markers of HIV disease progression and all-cause mortality.36,37 Acquisition of GBV-C infection was associated with lower mortality among patients with advanced HIV infection that was independent of HIV risk behaviors, treatment status, HIV viral load, and CD4+ T-cell count before GBV-C infection.
This study will further characterize the impact of GBV-C co-infection on HIV disease progression and identify mechanisms by which co-infection may influence HIV infection outcome. Specifically, this project will evaluate whether the immune response triggered by GBV-C helps to control HIV replication.38 Using a case-control design, the study will compare inflammation and cellular activation markers consistent with HIV infection in 262 VATS specimens from 30 subjects with and 30 subjects without GBV-C co-infection.
Emerging Infectious Agents
1. Transfusion-transmitted Dengue Virus (DENV) in Brazil
DENV is considered a serious emerging infectious threat for the US. Annually, ~390 million DENV infections occur worldwide with ~96 million of these being clinically apparent infections.39 The DENV group consists of four closely related viruses, DENV 1–4.40 Infection with a given DENV results in seropositivity and confers immunity to that virus. However, this does not protect against infection with another DENV; further, these secondary infections are associated with worse clinical outcomes. High viremia rates in blood donors have been documented during epidemic periods and transfusion-transmission has been confirmed in several locations.41
This study has retrospectively tested ~ 20,000 donations from the 2012 dengue transmission season in two Brazilian cities using a highly sensitive dengue RNA assay.42 This test has also been applied to post-transfusion samples from ~ 1,200 enrolled recipients to determine the transfusion-transmission rate from RNA positive donations. Reactive samples are being characterized for viral type, viral load, and DENV IgM, IgG and type-specific antibodies. Clinical outcomes of DENV infection in recipients over a 30-day interval are being evaluated. In addition, a large linked donor-recipient repository for future arbovirus research has been established.
The prevalence of previous DENV infection and the incidence of new infections in donors after the 2012 epidemic season are being determined through IgM and IgG antibody testing. This will allow estimation of the viremic window period detected by the DENV RNA assay, and correlation of seasonal incidence with detection of asymptomatic donor viremia and community reported clinical disease rates.
2. Searching for new TTIs
The ability to rapidly identify and manage emerging TTIs is of vital importance.43 Rapid response capability requires an understanding of the etiology of a transfusion-transmitted disease (TTD) and the nature of the agent. Conceptually, it is possible to study TTDs by searching for risk associations either between blood donors and their respective recipients or within clusters of patients who receive blood from the same donor. This study will use the SCANDAT database to search for such risk correlations either to identify or to exclude new or unknown TTDs. Analyses will first be conducted using retrospective data with subsequent plans to develop methods for prospective blood safety surveillance.
3. Severe Fever with Thrombocytopenia Virus (SFTSV) in China
In 2009, researchers identified a bunyavirus as the probable cause of a newly discovered disease in central and northeast China.44 Documented person-to-person transmission has been attributed to blood contact (and possibly also to droplet contact),45 raising the possibility that infection may be transfusion-transmitted. The prevalence and incidence of SFTSV infection in Chinese blood donors is currently unknown. This cross-sectional study will perform SFTSV antibody and PCR testing of ~15,000 blood donor samples from an endemic region and 3,000 samples from two non-endemic regions. Seroprevalence/viremia rates and demographic characteristics of seropositive and viremic donors will be determined.
Blood Component Quality
1. RBC-Omics Part I: Genetic basis of differential RBC storage capacity
While some elements of the RBC storage lesion are well characterized (e.g., free hemoglobin release, increasing levels of RBC-derived microparticles, loss of RBC deformability and enzymatic activities), little is known about the molecular mechanisms of hemolysis and how hemolysis-propensity varies among individuals and is modulated.46,47 There are >4,000 identified polymorphisms affecting RBC stability and life-span, yet none have been evaluated for their effects on RBC storage parameters.48 Further, these likely represent only a subset of relevant polymorphisms with many as yet undiscovered variants affecting storage hemolysis.
This study will pursue the hypothesis that genetic variation underlies the variable propensity of donors’ erythrocytes for storage hemolysis by evaluating 14,000 distinct donors (including 2,000 black and 2,000 Asian donors) and their RBC donations. Samples from stored leukoreduced-RBC components will be analyzed for spontaneous and stress-induced storage (42 day) hemolysis. Several hundred donors with high/low hemolysis will be recalled for repeat testing and more detailed evaluation of hemolysis kinetics and metabolomics as well as exome sequencing to identify new variants linked to storage hemolysis. Detailed genetic testing employing a GWAS array that will include all known and newly identified polymorphisms will then be conducted on DNA from all 14,000 enrolled and phenotyped donors. A shareable biorepository will be established.
Donor Health and Safety
1. Apheresis donation and bone fracture risk
Exposure to citrate during apheresis platelet (AP) donation triggers changes in ionized serum calcium, parathyroid hormone, and markers of bone metabolism suggesting that persistent alterations in bone density may occur.49–51 However, no data exist on the association of history of apheresis donation and the risk of bone fracture. The SCANDAT database of donation and hospital admissions data for approximately 200,000 Scandinavian apheresis donors will be used to evaluate if frequent apheresis donation increases fracture risk (in particular, osteoporosis fractures). Data will be analyzed as a cohort study with time-dependent exposure data taking into account apheresis donation activity, age, sex, socioeconomic factors, and prescription drug use.
2. Cognition, Hemoglobin, and Iron Levels in teen donors (CHILL)
The clinical significance of iron deficiency in blood donors is unclear. Teenage blood donors may be a particularly vulnerable population since there is a high rate of iron deficiency in young women52,53 and both genders are still undergoing neurocognitive and physical development.54
This study will longitudinally examine whether 16- to 18-year-old donors experience neurocognitive deficits or clinically relevant fatigue, and if so, whether these are related to hemoglobin, iron status, and blood donation activity. A cohort of ~400 high school students will be randomly assigned to “Donation” or “No-Donation” groups, with an equal number of males and females in each. Pre-donation neurocognitive status will be assessed using tests of short-term memory, attention, and processing speed. A brief self-administered questionnaire covering physical, mental, and emotional domains of fatigue and vigor will be administered. Study participants will be encouraged to return to subsequent blood drives for follow-up donations (or non-donation visits) and neurocognitive and fatigue assessments. Hematological and iron status will be compared to 19- to 29-year-old donors enrolled in the REDS-II RISE study.55
3. Hemoglobin and Iron Recovery Study (HEIRS)
This prospective randomized clinical trial (ClinicalTrials.gov; Identifier: NCT01555060) will determine the time to recovery of hemoglobin and iron stores in iron-replete and -depleted blood donors after whole blood donation and whether iron replacement therapy improves hemoglobin recovery in iron-depleted individuals. These data will contribute to decisions about required intervals between donations and the value of iron supplementation to optimize blood donor iron health.56,57
This study will assess daily iron supplementation over 24 weeks, more than twice as long as other trials, to test the hypothesis that post-donation iron supplementation will reduce mean hemoglobin recovery time by more than 50%.58 Secondary objectives are to examine recovery times of hemoglobin and iron stores in relation to donors’ gender and age. The study has enrolled 200 donors into four cohorts based on their ferritin values (≤or >26) and administration of iron supplementation over the course of the study. Hemoglobin and ferritin testing were performed at 7 study visits spanning the 24 week post-donation interval.
4. TMPRSS6 polymorphisms
Although a substantial percentage of repeat blood donors develop iron deficiency anemia, some do not.55 Whereas behavioral factors such as iron supplementation can account for some of this variation, genetic factors - particularly polymorphisms in genes regulating dietary iron absorption - likely also contribute. Several GWAS have linked a common single nucleotide polymorphism (SNP) in TMPRSS6, a key iron regulatory protein, with differences in hemoglobin concentration and transferrin saturation.59–61 This polymorphism (SNP), rs855791, is a valine-to-alanine amino acid change. Using a retrospective cohort design, a SNP analysis will evaluate this TMPRSS6 polymorphism among ~900 repository samples from the REDS-II RISE study; relate this to existing data for ferritin, soluble transferrin receptor, and hemoglobin; evaluate whether this polymorphism is associated with higher baseline hemoglobin and greater iron stores in first-time blood donors; and evaluate whether donors with this polymorphism are less likely to become iron deficient when subjected to the stress of repeated blood donation. Cohorts include male and female first-time donors, male donors who were iron deficient on at least one donation attempt, and high-intensity donors (e.g., five to six annual donations without developing iron deficiency).
5. RBC-Omics part II: Iron balance and clinical syndromes in high-intensity blood donors
A second arm of the RBC-Omics study will evaluate the genetic basis of iron balance in a specially-recruited subgroup of 2,000 high-intensity donors who have maintained adequate hemoglobin levels despite very frequent donation.62 The same GWAS described under RBC-Omics part I will investigate whether iron balance, donation frequency, and the iron-dependent conditions of restless leg syndrome and pica (using data obtained via questionnaire) are associated with specific genetic polymorphisms.63,64
Donor Deferral Criteria
1. Blood DROPS
Many countries are re-examining the policy of deferring blood donations from MSM and are conducting risk analyses of current policies and their potential modifications.65 In the US, there is a paucity of data to address important aspects of the policy such as why some MSM donors fail to self-defer, although this information is central to potential changes in the policy.66
This study will assess motivations for blood donation in the US MSM population and compliance with the current policy. Focus groups will be conducted with 16 key informants in each of the four communities where REDS-III blood centers are located. MSM with no history of IDU who believe or know themselves to be negative for HIV, HCV, and HBV will be asked to comment on: (1) perceptions of the current donor deferral policy, (2) suggestions of changes to the screening process, and (3) estimates of their own risk as blood donors. In the next stage of the study, a web-based survey will assess the frequency of compliance with current policy, donation motivations, and intended compliance with potential modified MSM policies; enrollment goals are 1,600 MSM community members and 3,200 male blood donors from REDS-III blood centers. Those MSM who report a history of blood donation will be asked to participate in qualitative telephone interviews to assess their motivations.
Donor Recruitment and Retention
1. South Africa donor recruitment/retention
In Africa, < 40% of the blood needed for transfusion is currently available and African blood services must diversify and expand their pool of donors.67 South Africa has a skewed distribution of blood donors with only 26% of units collected from the majority black population. This is due in part to historical practices now abandoned as well as other factors including cultural obstacles to marketing, socioeconomic influences, and educational differences.68
This study investigates how to increase donation and return rates among black donors. As an initial step in understanding donor motivators/deterrents, secondary analyses of focus group data collected by SANBS has been performed. Next, using a prospective cohort design with “exposure” defined by motivator/deterrent profiles determined by an operational questionnaire administered to black donors by SANBS soon after first donation, the outcome of return for a second donation will be assessed through analysis of the REDS-III donor database. Finally, SANBS recruitment staff will use these findings to develop recruitment messages and strategies (e.g., cell phone contact and text messaging) for increasing donor return rates. Using a randomized study design, researchers will determine if donor return is increased by exposure to SANBS marketing campaigns with varied content and/or by educational interventions aimed at reducing donor fear.
Summary/conclusions
The infrastructure and goals of REDS-III will contribute significantly to stronger recipient and linked donor-recipient research in the US and will address key blood banking and transfusion safety concerns, influence policy development with regard to blood donor screening and counseling, provide quantitative evaluation of relevant issues, and help assure a safe and available blood supply. To broaden its scientific perspectives, REDS-III has established collaborations with several scientific research laboratories, has convened protocol-specific external expert advisory committees, and is interfacing with government agencies and private industry. In addition, the REDS-III portfolio has been designed to be both hypothesis-driven and hypothesis-generating by including the development of new basic and translational research projects that should be highly informative to the fields of hematology and immunology. Finally, REDS-III actively encourages the development of new research collaborations which seek to leverage its infrastructure and the use of its data and/or biospecimens.
Acknowledgments
Contract support
REDS-III is supported by NHLBI contracts NHLBI HHSN2682011-00001I, -00002I, -00003I, -00004I, -00005I, -00006I, -00007I, -00008I, and - 00009I
We would like to thank the following: 1) REDS-III investigators who prepared synopses of one or more protocols for inclusion in this manuscript: Walter Bialkowski, Dr. Evan Bloch, Dr. Brian Custer, Dr. Gustaf Edgren, Dr. Shannon Kelly, Dr. Joseph E. Kiss, Dr. Emily Liu, Dr. Alan E. Mast, Dr. Nareg Roubinian, Bryan Spencer, Dr. Elizabeth St. Lezin, Dr. Darrell J. Triulzi; 2) Members of the REDS-III Observational Study Monitoring Board for reviewing and monitoring the REDS-III protocols [Neil Blumberg (co-chair), Merlyn Sayers (co-chair), Marion Danis Harold Kaplan, and Sharina Person]; 3) The external panel of experts [Harvey Klein (chair), Mark Fung, Louis Katz, Karen Quillen, Lynne Uhl]. for their thoughtful review of REDS-III’s scientific priorities; and 4) Julia Ann Miller, formerly of RTI International for organizational and editorial assistance.
The NHLBI Recipient Epidemiology Donor Evaluation Study - III domestic component is the responsibility of the following persons
Hubs
A.E. Mast and J.L. Gottschall, BloodCenter of Wisconsin (BCW), Milwaukee, WI
D.J. Triulzi and J.E. Kiss, The Institute For Transfusion Medicine (ITXM), Pittsburgh, PA
E.L. Murphy, University of California, San Francisco (UCSF) and Blood Centers of the Pacific (BCP), San Francisco, CA and E.W. Fiebig, University of California, San Francisco (UCSF), San Francisco, CA
E.L. Snyder Yale University School of Medicine, New Haven, CT and R.G Cable, American Red Cross Blood Services, Farmington CT
Data coordinating center
D. J. Brambilla and M. T. Sullivan, RTI International, Rockville, MD
Central laboratory
M.P. Busch and P.J. Norris, Blood Systems Research Institute, San Francisco, CA
Publication Committee Chairman
R. Y. Dodd, American Red Cross, Holland Laboratory, Rockville, MD
Steering Committee Chairman
S. H. Kleinman, University of British Columbia, Victoria, BC, Canada
National Heart, Lung, and Blood Institute, National Institutes of Health
S. A. Glynn and A.M. Cristman
The NHLBI Recipient Epidemiology Donor Evaluation Study - III international component, is the responsibility of the following persons
International Sites
US-Brazil collaboration
M.P. Busch, REDS-III U.S. Principal Investigator; and B. S. Custer, REDS-III U.S. co-Principal Investigator, Blood Systems Research Institute, San Francisco, CA.
E.C. Sabino, REDS-III Brazil Principal Investigator, the Fundacao Faculdade de Medicina and Hospital das Clinicas of the Medical School of the University of Sao Paulo, Brazil; and A. F. Carneiro Proietti, REDS-III Brazil Co-Principal Investigator, Fundacao Hemominas, Bello Horizonte, Brazil.
US-China collaboration
H. Shan, REDS-III U.S. Principal Investigator; and P. M. Ness, REDS-III U.S. co-Principal Investigator, The Johns Hopkins University, Baltimore, MD.
J.X. Wang, REDS-III China Principal Investigator, The Chinese Institute of Blood Transfusion (IBT), China.
US-South Africa collaboration
E.L. Murphy, REDS-III U.S. Principal Investigator, University of California, San Francisco (UCSF), San Francisco, CA; and E. M. Bloch, REDS-III U.S. co-Principal Investigator, Blood Systems Research Institute, San Francisco, CA.
C. Ingram, REDS-III South Africa Principal Investigator (phase 2), R. Crookes, REDS-III South Africa Principal Investigator (phase 1), and R. Reddy, REDS-III South Africa, Co-Principal Investigator, the South African National Blood Service (SANBS), South Africa.
Data coordinating center
D. J. Brambilla and M. T. Sullivan, RTI International, Rockville, MD
Central laboratory
M.P Busch and P.J. Norris, Blood Systems Research Institute, San Francisco, CA
Publication Committee Chairman
R. Y. Dodd, American Red Cross, Holland Laboratory, Rockville, MD
Oversight Steering Committee Chairman
S. H. Kleinman, University of British Columbia, Victoria, BC, Canada
National Heart, Lung, and Blood Institute, National Institutes of Health
S. A. Glynn and A.M. Cristman
Footnotes
Conflict of Interest statement
Each of the authors declares that he/she has no conflicts of interest regarding any of the work presented in this publication
References
- 1.Kleinman S, King MR, Busch MP, Murphy EL, Glynn SA. The National Heart, Lung, and Blood Institute retrovirus epidemiology donor studies (Retrovirus Epidemiology Donor Study and Retrovirus Epidemiology Donor Study-II): twenty years of research to advance blood product safety and availability. Transfus Med Rev. 2012;26(4):281–304. e1–2. doi: 10.1016/j.tmrv.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuck TF, Thomson RA, Schreiber GB, Gilcher RO, Kleinman SH, Murphy EL, Ownby HE, Williams AE, Busch MP, Smith JW, et al. The Retrovirus Epidemiology Donor Study (REDS): rationale and methods. Transfusion. 1995;35(11):944–51. doi: 10.1046/j.1537-2995.1995.351196110900.x. [DOI] [PubMed] [Google Scholar]
- 3.Edgren G, Hjalgrim H, Tran TN, Rostgaard K, Shanwell A, Titlestad K, Jakobsson L, Gridley G, Wideroff L, Jersild C, Adami J, Melbye M, Reilly M, Nyren O. A population-based binational register for monitoring long-term outcome and possible disease concordance among blood donors and recipients. Vox Sang. 2006;91(4):316–23. doi: 10.1111/j.1423-0410.2006.00827.x. [DOI] [PubMed] [Google Scholar]
- 4.Edgren G, Hjalgrim H, Reilly M, Tran TN, Rostgaard K, Shanwell A, Titlestad K, Adami J, Wikman A, Jersild C, Gridley G, Wideroff L, Nyren O, Melbye M. Risk of cancer after blood transfusion from donors with subclinical cancer: a retrospective cohort study. Lancet. 2007;369(9574):1724–30. doi: 10.1016/S0140-6736(07)60779-X. [DOI] [PubMed] [Google Scholar]
- 5.Shanwell A, Andersson TM, Rostgaard K, Edgren G, Hjalgrim H, Norda R, Melbye M, Nyren O, Reilly M. Post-transfusion mortality among recipients of ABO-compatible but non-identical plasma. Vox Sang. 2009;96(4):316–23. doi: 10.1111/j.1423-0410.2009.01167.x. [DOI] [PubMed] [Google Scholar]
- 6.Stanworth SJ, Brunskill SJ, Hyde CJ, McClelland DB, Murphy MF. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. Br J Haematol. 2004;126(1):139–52. doi: 10.1111/j.1365-2141.2004.04973.x. [DOI] [PubMed] [Google Scholar]
- 7.Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232–9. doi: 10.1097/MLR.0b013e3181589bb6. [DOI] [PubMed] [Google Scholar]
- 8.Murphy EL, Kwaan N, Looney MR, Gajic O, Hubmayr RD, Gropper MA, Koenigsberg M, Wilson G, Matthay M, Bacchetti P, Toy P. Risk factors and outcomes in transfusion-associated circulatory overload. Am J Med. 2013;126(4):357, e29–38. doi: 10.1016/j.amjmed.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toy P, Gajic O, Bacchetti P, Looney MR, Gropper MA, Hubmayr R, Lowell CA, Norris PJ, Murphy EL, Weiskopf RB, Wilson G, Koenigsberg M, Lee D, Schuller R, Wu P, Grimes B, Gandhi MJ, Winters JL, Mair D, Hirschler N, Sanchez Rosen R, Matthay MA. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119(7):1757–67. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clifford L, Singh A, Wilson GA, Toy P, Gajic O, Malinchoc M, Herasevich V, Pathak J, Kor DJ. Electronic health record surveillance algorithms facilitate the detection of transfusion-related pulmonary complications. Transfusion. 2012 Aug 31; doi: 10.1111/j.1537-2995.2012.03886.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yazdanbakhsh K, Ware RE, Noizat-Pirenne F. Red blood cell alloimmunization in sickle cell disease: pathophysiology, risk factors, and transfusion management. Blood. 2012;120(3):528–37. doi: 10.1182/blood-2011-11-327361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Josephson CD, Su LL, Hillyer KL, Hillyer CD. Transfusion in the patient with sickle cell disease: a critical review of the literature and transfusion guidelines. Transfus Med Rev. 2007;21(2):118–33. doi: 10.1016/j.tmrv.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Treml A, King KE. Red blood cell alloimmunization: lessons from sickle cell disease. Transfusion. 2013;53(4):692–5. doi: 10.1111/trf.12146. [DOI] [PubMed] [Google Scholar]
- 14.Obaro S. Does sickle cell disease protect against HIV/AIDS? Sexually transmitted Infections. 2012 doi: 10.1136/sextrans-2012-050613. [DOI] [PubMed] [Google Scholar]
- 15.Bates I, Chapotera GK, McKew S, van den Broek N. Maternal mortality in sub-Saharan Africa: the contribution of ineffective blood transfusion services. BJOG. 2008;115(11):1331–9. doi: 10.1111/j.1471-0528.2008.01866.x. [DOI] [PubMed] [Google Scholar]
- 16.Lalonde A, Daviss BA, Acosta A, Herschderfer K. Postpartum hemorrhage today: ICM/FIGO initiative 2004–2006. Int J Gynaecol Obstet. 2006;94(3):243–53. doi: 10.1016/j.ijgo.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Rogers MA, Blumberg N, Heal JM, Langa KM. Utilization of blood transfusion among older adults in the United States. Transfusion. 2011;51(4):710–8. doi: 10.1111/j.1537-2995.2010.02937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menis M, Burwen DR, Holness L, Anderson SA. Blood use in the ambulatory setting among elderly in the United States. Transfusion. 2009;49(6):1186–94. doi: 10.1111/j.1537-2995.2009.02114.x. [DOI] [PubMed] [Google Scholar]
- 19.Platzbecker U, Hofbauer LC, Ehninger G, Holig K. The clinical, quality of life, and economic consequences of chronic anemia and transfusion support in patients with myelodysplastic syndromes. Leuk Res. 2012;36(5):525–36. doi: 10.1016/j.leukres.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 20.van de Watering L, Hermans J, Witvliet M, Versteegh M, Brand A. HLA and RBC immunization after filtered and buffy coat-depleted blood transfusion in cardiac surgery: a randomized controlled trial. Transfusion. 2003;43:765–71. doi: 10.1046/j.1537-2995.2003.00390.x. [DOI] [PubMed] [Google Scholar]
- 21.Triulzi DJ, Kleinman S, Kakaiya RM, Busch MP, Norris PJ, Steele WR, Glynn SA, Hillyer CD, Carey P, Gottschall JL, Murphy EL, Rios JA, Ness PM, Wright DJ, Carrick D, Schreiber GB. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion. 2009;49(9):1825–35. doi: 10.1111/j.1537-2995.2009.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabino EC, Goncalez TT, Carneiro-Proietti AB, Sarr M, Ferreira JE, Sampaio DA, Salles NA, Wright DJ, Custer B, Busch M. Human immunodeficiency virus prevalence, incidence, and residual risk of transmission by transfusions at Retrovirus Epidemiology Donor Study-II blood centers in Brazil. Transfusion. 2012;52(4):870–9. doi: 10.1111/j.1537-2995.2011.03344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Liu J, Yao F, Wen G, Li J, Huang Y, Lu Y, Wen X, Wright D, Yu Q, Guo N, Ness P, Shan H. Prevalence, incidence, and residual risks for transfusion-transmitted human immunodeficiency virus Types 1 and 2 infection among Chinese blood donors. Transfusion. 2012 Nov 1; doi: 10.1111/j.1537-2995.2012.03940.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Busch MP, Glynn SA, Stramer SL, Strong DM, Caglioti S, Wright DJ, Pappalardo B, Kleinman SH. A new strategy for estimating risks of transfusion-transmitted viral infections based on rates of detection of recently infected donors. Transfusion. 2005;45(2):254–64. doi: 10.1111/j.1537-2995.2004.04215.x. [DOI] [PubMed] [Google Scholar]
- 25.Alencar C, Sabino E, Carvalho S, Leao S, Carneiro-Proietti A, Capuani L, Oliveira C, Carrick D, Birch R, Goncalez T, Keating S, Swanson P, Hackett J, Jr, Busch M. HIV genotypes and primary drug resistance among HIV seropositive blood donors in Brazil: role of infected blood donors as sentinel populations for molecular surveillance of HIV. J Acquir Immune Defic Syndr. 2013 Mar 15; doi: 10.1097/QAI.0b013e31828ff979. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng P, Wang J, Huang Y, Guo X, Li J, Wen G, Yang T, Yun Z, He M, Liu Y, Yuan Y, Schulmann J, Glynn S, Ness P, Jackson JB, Shan H. The human immunodeficiency virus-1 genotype diversity and drug resistance mutations profile of volunteer blood donors from Chinese blood centers. Transfusion. 2012;52(5):1041–9. doi: 10.1111/j.1537-2995.2011.03415.x. [DOI] [PubMed] [Google Scholar]
- 27.de Almeida-Neto C, Goncalez TT, Birch RJ, de Carvalho SM, Capuani L, Leao SC, Miranda C, Rocha PC, Carneiro-Proietti AB, Johnson BR, Wright DJ, Murphy EL, Custer B. Risk factors for human immunodeficiency virus infection among Brazilian blood donors: a multicentre case-control study using audio computer-assisted structured interviews. Vox Sang. 2013 Mar 20; doi: 10.1111/vox.12028. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blatyta PF, Custer B, Goncalez TT, Birch R, Lopes ME, Lopes Ferreira MI, Carneiro Proietti AB, Sabino EC, Page K, de Almeida-Neto C. Undisclosed human immunodeficiency virus risk factors identified through a computer-based questionnaire program among blood donors in Brazil. Transfusion. 2013 Mar 22; doi: 10.1111/trf.12166. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Liu J, Huang Y, Yang T, Yao F, Dong X, Wen G, Bi X, Zhao M, Wen X, Huang M, Lu Y, Ma H, Yu Q, Wright D, Guo N, Ness P, Shan H. An analysis of risk factors for human immunodeficiency virus infection among Chinese blood donors. Transfusion. 2013 Jan 10; doi: 10.1111/trf.12062. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bunnik EM, Euler Z, Welkers MRA, Boeser-Nunnink BDM, Marlous L, Grijsen ML, Prins JM, Schuitemaker H. Adaptation of HIV-1 envelope gp120 to humoral immunity at a population level. Nature Medicine. 2010;16:995–997. doi: 10.1038/nm.2203. [DOI] [PubMed] [Google Scholar]
- 31.Chinese Ministry of Health, Joint United Nations Programme on HIV/AIDS, World Health Organization. 2011 estimates for the HIV/AIDS epidemic in China. Beijing, China: Chinese Ministry of Health, Joint United Nations Programme on HIV/AIDS, and World Health Organization; 2011. [cited 2013 Apr 4]. Available from: http://www.chinaids.org.cn/n1971/n2151/n777994.files/n777993.pdf. [Google Scholar]
- 32.Vermeulen M, Coleman C, Mitchel J, Reddy R, van Drimmelen H, Fickett T, Busch M, Lelie N. Comparison of human immunodeficiency virus assays in window phase and elite controller samples: viral load distribution and implications for transmission risk. Transfusion. 2013 Feb 27; doi: 10.1111/trf.12117. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Germano FN, da Silva TM, Mendoza-Sassi R, Martinez AM. High prevalence of users who did not return to the Testing and Counseling Center (TCC) for knowing their serological status: Rio Grande, RS, Brazil. Cien Saude Colet. 2008;13(3):1033–40. doi: 10.1590/s1413-81232008000300026. [DOI] [PubMed] [Google Scholar]
- 34.Goncalez T, Sabino E, Sales N, Chen YH, Chamone D, Busch M, Murphy E, Custer B, McFarland W. Human immunodeficiency virus test-seeking blood donors in a large blood bank in Sao Paulo, Brazil. Transfusion. 2010;50(8):1806–14. doi: 10.1111/j.1537-2995.2010.02650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang W, Chaloner K, Tillmann HL, Williams CF, Stapleton JT. Effect of early and late GB virus C viraemia on survival of HIV-infected individuals: a meta-analysis. HIV Med. 2006;7(3):173–80. doi: 10.1111/j.1468-1293.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- 36.Vahidnia F, Petersen M, Stapleton JT, Rutherford GW, Busch M, Custer B. Acquisition of GB virus type C and lower mortality in patients with advanced HIV disease. Clin Infect Dis. 2012;55(7):1012–9. doi: 10.1093/cid/cis589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vahidnia F, Petersen M, Rutherford G, Busch M, Assmann S, Stapleton JT, Custer B. Transmission of GB virus type C via transfusion in a cohort of HIV-infected patients. J Infect Dis. 2012;205(9):1436–42. doi: 10.1093/infdis/jis209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiang J, George SL, Wunschmann S, Chang Q, Klinzman D, Stapleton JT. Inhibition of HIV-1 replication by GB virus C infection through increases in RANTES, MIP-1alpha, MIP-1beta, and SDF-1. Lancet. 2004;363(9426):2040–6. doi: 10.1016/S0140-6736(04)16453-2. [DOI] [PubMed] [Google Scholar]
- 39.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013 doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10(12 Suppl):S98–109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 41.Linnen JM, Vinelli E, Sabino EC, Tobler LH, Hyland C, Lee TH, Kolk DP, Broulik AS, Collins CS, Lanciotti RS, Busch MP. Dengue viremia in blood donors from Honduras, Brazil, and Australia. Transfusion. 2008;48(7):1355–62. doi: 10.1111/j.1537-2995.2008.01772.x. [DOI] [PubMed] [Google Scholar]
- 42.Lanteri MC, Busch MP. Dengue in the context of “safe blood” and global epidemiology: to screen or not to screen? Transfusion. 2012;52(8):1634–9. doi: 10.1111/j.1537-2995.2012.03747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glynn SA, Busch MP, Dodd RY, Katz LM, Stramer SL, Klein HG, Simmons G, Kleinman SH, Shurin SB. Emerging infectious agents and the nation’s blood supply: responding to potential threats in the 21st century. Transfusion. 2013;53(2):438–54. doi: 10.1111/j.1537-2995.2012.03742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bao CJ, Qi X, Wang H. A novel bunyavirus in China. N Engl J Med. 2011;365(9):862–3. doi: 10.1056/NEJMc1106000. author reply 4–5. [DOI] [PubMed] [Google Scholar]
- 45.Gai Z, Liang M, Zhang Y, Zhang S, Jin C, Wang SW, Sun L, Zhou N, Zhang Q, Sun Y, Ding SJ, Li C, Gu W, Zhang F, Wang Y, Bian P, Li X, Wang Z, Song X, Wang X, Xu A, Bi Z, Chen S, Li D. Person-to-person transmission of severe fever with thrombocytopenia syndrome bunyavirus through blood contact. Clin Infect Dis. 2012;54(2):249–52. doi: 10.1093/cid/cir776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gkoumassi E, Dijkstra-Tiekstra MJ, Hoentjen D, de Wildt-Eggen J. Hemolysis of red blood cells during processing and storage. Transfusion. 2012;52(3):489–92. doi: 10.1111/j.1537-2995.2011.03298.x. [DOI] [PubMed] [Google Scholar]
- 47.Glynn SA. The red blood cell storage lesion: a method to the madness. Transfusion. 2010;50(6):1164–9. doi: 10.1111/j.1537-2995.2010.02674.x. [DOI] [PubMed] [Google Scholar]
- 48.Delaunay J. The molecular basis of hereditary red cell membrane disorders. Blood Rev. 2007;21(1):1–20. doi: 10.1016/j.blre.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Bolan CD, Greer SE, Cecco SA, Oblitas JM, Rehak NN, Leitman SF. Comprehensive analysis of citrate effects during plateletpheresis in normal donors. Transfusion. 2001;41(9):1165–71. doi: 10.1046/j.1537-2995.2001.41091165.x. [DOI] [PubMed] [Google Scholar]
- 50.Amrein K, Katschnig C, Sipurzynski S, Stojakovic T, Lanzer G, Stach E, Pieber TR, Dobnig H. Apheresis affects bone and mineral metabolism. Bone. 2010;46(3):789–95. doi: 10.1016/j.bone.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 51.Dettke M, Buchta C, Bieglmayer C, Kainberger F, Macher M, Hocker P. Short- and long-term effects of citrate on bone metabolism and bone mineral density in healthy plateletpheresis donors [abstract of the 24th Annual Meeting of the American Society for Apheresis, Squaw Valley, California, May 7–10, 2003] J Clin Apher. 2003;18(2):87. [Google Scholar]
- 52.Cogswell ME, Looker AC, Pfeiffer CM, Cook JD, Lacher DA, Beard JL, Lynch SR, Grummer-Strawn LM. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003–2006. Am J Clin Nutr. 2009;89(5):1334–42. doi: 10.3945/ajcn.2008.27151. [DOI] [PubMed] [Google Scholar]
- 53.Recommendations to prevent and control iron deficiency in the United States. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47(RR-3):1–29. [PubMed] [Google Scholar]
- 54.Jahanshad N, Kohannim O, Hibar DP, Stein JL, McMahon KL, Zubicaray GI, Medland SE, Montgomery GW, Whitfield JB, Martin NG, Wright MJ, Toga AW, Thompson PM. Brain structure in healthy adults is related to serum transferrin and the H63D polymorphism in the HFE gene. PNAS. 2012 doi: 10.1073/pnas.1105543109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cable RG, Glynn SA, Kiss JE, Mast AE, Steele WR, Murphy EL, Wright DJ, Sacher RA, Gottschall JL, Tobler LH, Simon TL. Iron deficiency in blood donors: the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2012;52(4):702–11. doi: 10.1111/j.1537-2995.2011.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bryant BJ, Yau YY, Arceo SM, Daniel-Johnson J, Hopkins JA, Leitman SF. Iron replacement therapy in the routine management of blood donors. Transfusion. 2012;52(7):1566–75. doi: 10.1111/j.1537-2995.2011.03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Popovsky MA. Anemia, iron depletion, and the blood donor: it’s time to work on the donor’s behalf. Transfusion. 2012;52(4):688–92. doi: 10.1111/j.1537-2995.2012.03562.x. [DOI] [PubMed] [Google Scholar]
- 58.Pottgiesser T, Specker W, Umhau M, Dickhuth HH, Roecker K, Schumacher YO. Recovery of hemoglobin mass after blood donation. Transfusion. 2008;48(7):1390–7. doi: 10.1111/j.1537-2995.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- 59.Du X, She E, Gelbart T, Truksa J, Lee P, Xia Y, Khovananth K, Mudd S, Mann N, Moresco EM, Beutler E, Beutler B. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320(5879):1088–92. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chambers JC, Zhang W, Li Y, Sehmi J, Wass MN, Zabaneh D, Hoggart C, Bayele H, McCarthy MI, Peltonen L, Freimer NB, Srai SK, Maxwell PH, Sternberg MJ, Ruokonen A, Abecasis G, Jarvelin MR, Scott J, Elliott P, Kooner JS. Genome-wide association study identifies variants in TMPRSS6 associated with hemoglobin levels. Nat Genet. 2009;41(11):1170–2. doi: 10.1038/ng.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benyamin B, Ferreira MA, Willemsen G, Gordon S, Middelberg RP, McEvoy BP, Hottenga JJ, Henders AK, Campbell MJ, Wallace L, Frazer IH, Heath AC, de Geus EJ, Nyholt DR, Visscher PM, Penninx BW, Boomsma DI, Martin NG, Montgomery GW, Whitfield JB. Common variants in TMPRSS6 are associated with iron status and erythrocyte volume. Nat Genet. 2009;41(11):1173–5. doi: 10.1038/ng.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mast AE, Schlumpf KS, Wright DJ, Custer B, Spencer B, Murphy EL, Simon TL. Demographic correlates of low hemoglobin deferral among prospective whole blood donors. Transfusion. 2010;50(8):1794–802. doi: 10.1111/j.1537-2995.2010.02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spencer BR, Kleinman S, Wright DJ, Glynn SA, Kiss JE, Mast AE, Cable RG. Restless legs syndrome, pica, and iron status in blood donors. Transfusion. doi: 10.1111/trf.12260. forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stefansson H, Rye DB, Hicks A, Petursson H, Ingason A, Thorgeirsson TE, Palsson S, Sigmundsson T, Sigurdsson AP, Eiriksdottir I, Soebech E, Bliwise D, Beck JM, Rosen A, Waddy S, Trotti LM, Iranzo A, Thambisetty M, Hardarson GA, Kristjansson K, Gudmundsson LJ, Thorsteinsdottir U, Kong A, Gulcher JR, Gudbjartsson D, Stefansson K. A genetic risk factor for periodic limb movements in sleep. N Engl J Med. 2007;357(7):639–47. doi: 10.1056/NEJMoa072743. [DOI] [PubMed] [Google Scholar]
- 65.Benjamin RJ, Bianco C, Goldman M, Seed CR, Yang H, Lee J, Keller AJ, Wendel S, Biagini S, Murray J, Devine DV, Zhu Y, Turek P, Moftah FM, Kullaste R, Pillonel J, Danic B, Bigey F, Follea G, Seifried E, Mueller MM, Lin CK, Makroo RN, Grazzini G, Pupella S, Velati C, Tadokoro K, Bravo Lindoro A, D’Artote Gonzalez A, Giner VT, Flanagan P, Olaussen RW, Letowska M, Rosiek A, Poglod R, Zhiburt E, Mali P, Rozman P, Gulube S, Castro Izaguirre E, Ekermo B, Barnes SM, McLaughlin L, Eder AF, Panzer S, Reesink HW. Deferral of males who had sex with other males. Vox Sang. 2011;101(4):339–67. doi: 10.1111/j.1423-0410.2011.01489.x. [DOI] [PubMed] [Google Scholar]
- 66.Martucci J. Negotiating exclusion: MSM, identity, and blood policy in the age of AIDS. Soc Stud Sci. 2010;40(2):215–41. doi: 10.1177/0306312709346579. [DOI] [PubMed] [Google Scholar]
- 67.Tagny CT, Owusu-Ofori S, Mbanya D, Deneys V. The blood donor in sub-Saharan Africa: a review. Transfus Med. 2010;20(1):1–10. doi: 10.1111/j.1365-3148.2009.00958.x. [DOI] [PubMed] [Google Scholar]
- 68.deConing D. Challenges facing donor recruitment in South Africa. Vox Sang. 2002;83 (Suppl 1):237–41. doi: 10.1111/j.1423-0410.2002.tb05310.x. [DOI] [PubMed] [Google Scholar]