Abstract
Objectives
To determine whether a home-based care coordination program focused on medication self-management would affect the cost of care to the Medicare program and whether the addition of technology, a medication-dispensing machine, would further reduce cost.
Design
Randomized, controlled, three-arm longitudinal study.
Setting
Participant homes in a large Midwestern urban area.
Participants
Older adults identified as having difficulty managing their medications at discharge from Medicare Home Health Care (N = 414).
Intervention
A team consisting of advanced practice nurses (APNs) and registered nurses (RNs) coordinated care for two groups: home-based nurse care coordination (NCC) plus a pill organizer group and NCC plus a medication-dispensing machine group.
Measurements
To measure cost, participant claims data from 2005 to 2011 were retrieved from Medicare Part A and B Standard Analytical Files.
Results
Ordinary least squares regression with covariate adjustment was used to estimate monthly dollar savings. Total Medicare costs were $447 per month lower in the NCC plus pill organizer group (P = .11) than in a control group that received usual care. For participants in the study at least 3 months, total Medicare costs were $491 lower per month in the NCC plus pill organizer group (P = .06) than in the control group. The cost of the NCC plus pill organizer intervention was $151 per month, yielding a net savings of $296 per month or $3,552 per year. The cost of the NCC plus medication-dispensing machine intervention was $251 per month, and total Medicare costs were $409 higher per month than in the NCC plus pill organizer group.
Conclusion
Nurse care coordination plus a pill organizer is a cost-effective intervention for frail elderly Medicare beneficiaries. The addition of the medication machine did not enhance the cost effectiveness of the intervention.
Keywords: cost effectiveness, care coordination, self-management
The cost of care of chronically ill individuals is estimated to be more than $1 trillion annually, and it is estimated that it will reach $6 trillion by mid-century if changes are not made in the health system's response to chronic illness,1 but little is known about the cost-effectiveness of the majority of interventions delivered to people who are chronically ill. More than half of all Medicare beneficiaries report being treated for five or more conditions during a year.2 For most chronically ill persons, care is a complicated maze of providers and complex medication regimens that are often difficult to self-manage, especially for frail older adults. Poor care coordination is identified as the heart of the problem in care of chronically ill people,3and the Institute of Medicine has identified it as a priority area for healthcare improvement.4
Care coordination is delivered in a wide variety of approaches in different settings, with different providers and different clinical populations. The challenge in evaluation of care coordination programs is the heterogeneity of the approaches, which makes it difficult to conduct systematic reviews or compare the effectiveness of different strategies.5
Disease management programs, one type of care coordination initiative, have demonstrated mixed results with regard to cost savings. A meta-analysis found a small positive effect for cost savings, which was greater with severely ill persons,6 but another review found that disease management programs improved some health outcomes but did not reduce costs.7
Care coordination programs based in primary care settings have demonstrated significant positive clinical advantages over usual care in functional ability,8 depression,8 satisfaction,9 quality of life,10–12 and mortality.13,14 Utilization outcomes were less impressive, with one study noting reduction in emergency visits 11 and two identifying reductions in hospital use.11,14
Hospital-to-home and transitional care programs have been among the most effective at improving clinical outcomes and reducing utilization, with heart failure being the most prevalent focus of the programs.6,15 The Transitional Care Model, in which advanced practice nurses (APNs) visit individuals in the hospital and follow them after discharge, demonstrated improvement in quality of life, had high levels of satisfaction, and reduced hospital admissions and cost of care.16 A different study that used the Care Transitions Model reported significant reductions in hospitalizations and hospital costs.17
Home-based nurse care coordination (NCC) programs have not led to consistent significant quality or cost improvements. A small to moderate reduction in hospital days related to home-based care coordination was identified in a meta-analysis,18 but a recent review found that home-based programs had no significant influence on outcomes of care.19 Problems in the studies included infrequent contacts with participants, which in some cases were as little as four times during a 12-month period, and older adults most in need of intervention were not necessarily the targets of these programs.
The Centers for Medicare and Medicaid Services (CMS) conducted the Medicare Coordinated Care Demonstration (MCCD) to test whether care coordination and disease management can lower costs and improve outcomes and well-being for Medicare fee-for-service beneficiaries with chronic illnesses.20 Fifteen sites participated and varied widely in how they delivered the care coordination intervention. Of the 15 sites, only three (Health Quality Partners, Georgetown, and Mercy) had treatment groups whose monthly Medicare expenditures were less than those in the control group.
The current study evaluated the cost-effectiveness of a home-based care coordination program that targeted older adults with problems self-managing their chronic illnesses. The ability to self-manage a medication regimen was used as a proxy measure for general self-management ability, because medication management is usually a major component of self-management in chronically ill persons. In the intention-to-treat (ITT) analysis of the clinical outcomes over a 12-month period, participants who received the NCC intervention scored significantly better than participants in the control group in depression (P < .001), functional status (P < .001), cognition (P < .001), and quality of life (P < .001). The purpose of this study was to determine whether an NCC program that focused on medication self-management would affect the cost of care to the Medicare program. It also tested whether addition of technology, a medication-dispensing machine, would lead to further reductions in cost to the Medicare program.
Methods
The study was a three-arm randomized controlled trial with a repeated-measures design over a 12-month period. There were three groups: the NCC plus pill organizer group; the NCC plus a medication-dispensing machine group; and the control group, who received usual care. Participants were followed for 1 year, with data collected at baseline and 3, 6, 9, and 12 months. Admissions to the study were from May 2006 to June 2009, with new participants admitted as nurse caseloads permitted. The University of Wisconsin-Milwaukee and Arizona State University institutional review boards approved the study protocol.
The setting was the urban area of a large midwestern city. Discharged home health recipients were chosen because they were a group of older adults who did not meet the Medicare Home Health Criteria but were in need of continued intervention. The focus was on unintentional nonadherence. Eligible participants were recruited at the time of their discharge from three Medicare-certified home health agencies. At discharge, agency home healthcare nurses asked eligible individuals whether they were interested in the study and obtained oral consent for the research staff to contact them. Inclusion criteria included aged 60 and older, Medicare as primary payer, impaired medication management ability as indicated by a score of 1 or higher on Outcome and Assessment Information Set (OASIS) discharge assessment item MO780 (Management of Oral Medications) or impaired cognitive functioning but able to follow directions with prompting as indicated by a score of 1 or 2 on OASIS discharge assessment item MO560 (Cognitive Functioning), and working telephone line and electricity. Exclusion criteria were a terminal diagnosis or hospice care that would suggest that attrition was likely, use of a device for medications (such as a prompt pager), and Medicare through managed care.
Sample size was based on the probability of detecting a 2-point difference on the Medical Outcomes Study 36-item Short-Form Survey (SF-36) Physical Component Summary (PCS) and Mental Component Summary (MCS) 21 in groups with a 5% Type I error rate. Four hundred fifty-six older adults provided oral permission and were randomly assigned to a group using a computer program that the study statistician (FS) designed. After randomization, a research data collector contacted each participant and obtained consent; 414 met eligibility criteria and consented to participant in the study.
Intervention
Because one component of the intervention was loading medications into a pill organizer or the medication-dispensing machine, a pharmacist and APNs conducted a pharmacy screen, which included comparing all medications that the participant identified with corresponding medical diagnoses (when available from the home care record). In the review, they used a program to identify drug interactions and the Beers Criteria for Inappropriate Medication Use in the Elderly.22 All participants underwent the pharmacy screen to remove its influence from participant outcomes. Each participant's prescribing provider(s) received the results of the pharmacy screens. The control group received no interventions beyond the pharmacy screen.
Nurse Care Coordination
APN and RN members of the study intervention team provided NCC, which began with comprehensive admission assessments and plans of care focused on supporting participants’ and their families’ self-management behaviors.23 Participants were visited at least every 2 weeks to fill their pill organizer or medicine-dispensing machine and more frequently if their condition required additional visits. If participants were hospitalized, the study nurse care coordinators visited or were in contact with staff in the hospital and participated in participants’ discharge planning. Study nurse care coordinators also communicated frequently with participants’ physicians, pharmacists, social workers, and other service providers.
Medication-Dispensing Device
Two different devices were used to enhance medication self-management behaviors. In the NCC plus pill organizer group, medications were placed in a pill organizer, a box with separate compartments holding medications to be taken up to four times a day for 1 week. Older adults use pill organizers routinely to assist with organizing and remembering to take their medications.24 A medication-dispensing machine preloaded with medications in reusable plastic cups was used in the NCC plus medicine-dispensing machine group.
Training
Study nurse care coordinators received education related to self-management, care coordination, drug use in elderly adults, the physiology of aging, and medication management. The intervention protocols25 were embedded in the clinical information system26 and were used to document care. A study APN made supervisory visits to monitor intervention fidelity and educate research staff as needed.
Measurement
Data on the clinical outcomes of depression (Geriatric Depression Scale,27 (GDS)), cognition (Mini-Mental State Examination28 (MMSE)), functional status (Physical Performance Test29 (PPT)), and quality of life21 (SF-36) were collected in participants’ homes at baseline and every 3 months for 1 year. Data were collected from May 2006 to June 2010. Data collectors were trained on each tool, and interrater reliability was established at 90% and monitored quarterly. Study nurse care coordinators kept time logs related to their care coordination activities. To measure cost, participant claims data were secured from 2005 to 2010 from Medicare Part A and B Standard Analytical Files. Medicare-allowable charges (Medicare payments plus coinsurance and deductibles as they apply to different benefits) were used.
Analysis
Costs were analyzed using the perspective of the payer, in this case, the Medicare program. First, ITT analysis was used that included all participants, including those who did not complete the assigned 12-month intervention. Second, because of the large number of participants who were assigned but did not participate, an analysis was also conducted that included all participants who were in the study for at least 3 months.
For each participant, average monthly Medicare expenditures were calculated for the 12 months after admission or until the participant died. Ordinary least squares regression was used to estimate monthly dollar savings to Medicare, controlling for participant age; sex; race; living arrangements; Medicare expenditures per month over the 12 months before randomization; prior hospitalizations; 10 common chronic conditions;30 and GDS, PPT and MMSE scores measured at admission. A small number of missing GDS and PPT scores (<1%) were addressed using multiple imputation with five imputations. Members who died before 12 months had their case weight reduced proportionately. Separate regressions were performed to compare the NCC plus pill organizer group with the NCC plus medicine-dispensing machine group and the NCC plus pill organizer group with the control group. Data analysis was performed using Proc GLM, Proc MI, and Proc MIANALYZE in SAS version 9.12 (SAS Institute, Inc., Cary, NC).
The cost of the intervention was based on the study nurse care coordinator RN and APN actual salary data and time logs of intervention activities. Mileage reimbursement and the $90/month cost of the medicine-dispensing machine were also included in the determination of the intervention cost according to group. The pill organizer was a one-time cost of $10, or less than $1 per month over the 12-month period.
Results
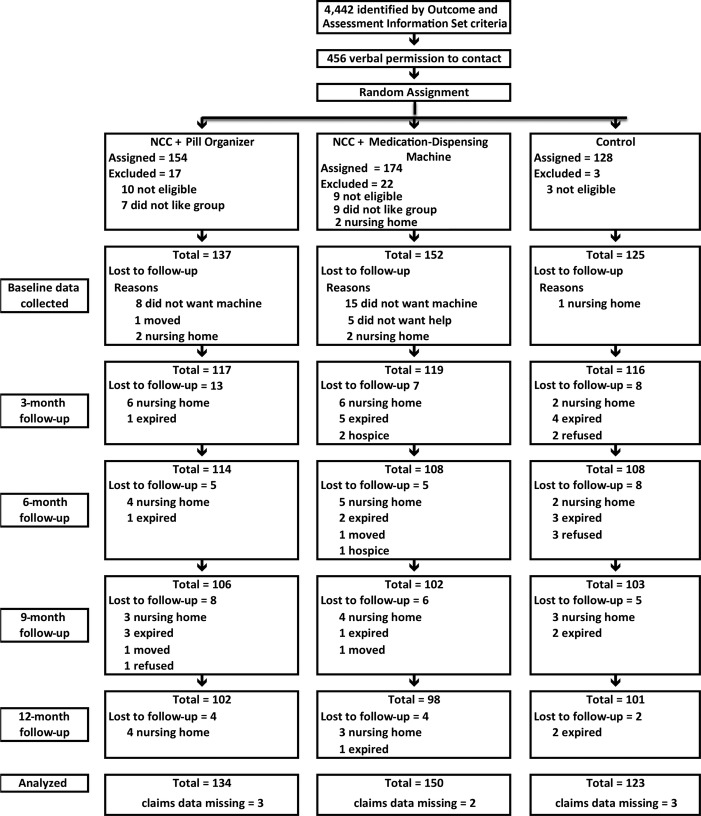
Of the 456 older adults who consented to have study data collection staff contact them, 414 were enrolled and randomized to one of three groups. Medicare claims data were not available for 11 participants (Figure1), so the ITT analysis included 403 participants. At baseline, there were no statistically significant differences in sociodemographic characteristics between the three groups, although there was a lower incidence of dementia (P = .02) and depression (P = .03) in the control group. There also was no statistically significant difference between the groups for the SF-36 PCS; and the control group scored statistically significantly higher (better) on the SF-36 MCS (P = .02) (Table1).
Figure 1.
The majority of participants were hospitalized before nursing home placement or death. Participants were followed for 2 months after hospitalization, rehabilitation, or nursing home placement and then discharged from the study. Examination of the claims data provided information on participants who died or were permanently placed in a nursing home after discharge from the study. NCC = nurse care coordination.
Table 1.
Baseline Characteristics of Study Population
| Characteristic | NCC Plus Pill Organizer, n = 133 | NCC Plus Medicine-Dispensing Machine, n = 148 | Control, n = 122 | P-Value |
|---|---|---|---|---|
| Age, mean ± SD | 79.5 ± 7.6 | 79.5 ± 7.9 | 78.2 ± 7.1 | .31 |
| Female, % | 67.6 | 68.2 | 61.5 | .45 |
| Race and ethnicity, % | ||||
| White | 84.2 | 81.1 | 90.2 | .11 |
| Black | 15.8 | 18.9 | 9.8 | .11 |
| Hispanic | 4.5 | 1.4 | 0.8 | .09 |
| Lives alone, % | 45.9 | 54.7 | 47.5 | .29 |
| Chronic conditions, n (%) | ||||
| Dementia | 29 (14) | 24 (16) | 7 (6) | .02 |
| Atrial fibrillation | 17 (13) | 21 (14) | 17 (14) | .94 |
| Kidney disease | 15 (11) | 13 (9) | 10 (8) | .66 |
| Chronic obstructive pulmonary disease | 20 (15) | 21 (14) | 17 (14) | .97 |
| Depression | 38 (29) | 31 (21) | 18 (15) | .03 |
| Diabetes mellitus | 50 (38) | 57 (39) | 46 (38) | .99 |
| Heart failure | 10 (8) | 10 (7) | 14 (11) | .34 |
| Ischemic heart disease | 19 (14) | 18 (12) | 18 (15) | .80 |
| Osteoporosis | 7 (5) | 7 (5) | 7 (5) | .93 |
| Stroke | 13 (10) | 12 (8) | 7 (5) | .49 |
| Hierarchical Condition Category score, mean ± SD | 3.6 ± 1.9 | 3.3 ± 1.8 | 3.1 ± 1.7 | .69 |
| Medical Outcomes Study 36-item Short-Form Survey score, mean ± SD | ||||
| Physical component subscale | 34.2 ± 9.4 | 33.7 ± 9.4 | 35.7 ± 10.3 | .22 |
| Mental component subscale | 49.4 ± 12.0 | 48.2 ± 12.2 | 54.1 ± 11.3 | >.001 |
| Monthly Medicare cost 12 months before study, $, mean ± SD | 2,800 ± 3,036 | 2,505 ± 2,675 | 2,350 ± 2,210 | .44 |
NCC = nurse care coordination; SD = standard deviation.
All three groups had high monthly Medicare expenditures in the 12 months before enrollment into the study, with the control group averaging the lowest, at $2,350 per month; the NCC plus pill organizer group averaged $2,800 (Table1). During the 12-month study period, mean monthly Medicare expenditures without risk adjustment were higher in the intervention groups than in the control group (Table2). Average monthly Medicare expenditures per participant were approximately four times as high as the 2008 average of $643 for Medicare participants in the study enrollment area.31 The cost of the NCC plus pill organizer intervention was $151 per month, or $1,812 per year. The NCC plus medicine-dispensing machine group cost $271 per month, or $3,252 per year.
Table 2.
Monthly Medicare Expenditures According to Group (Not Risk Adjusted) During 12 Months of Study
| Expenditure | NCC Plus Pill Organizer, n = 134 |
NCC Plus Medication-Dispensing Machine, n = 150 |
Control, n = 123 |
|---|---|---|---|
| Cost, $, Mean ± Standard Deviation | |||
| Total | 1,585 (2,632) | 2,033 (3,380) | 1,521 (2,095) |
| Home health care | 102 (257) | 103 (227) | 106 (238) |
| Durable medical equipment | 56 (127) | 49 (83) | 48 (95) |
| Carrier | 306 (406) | 332 (491) | 302 (391) |
| Inpatient | 756 (1,697) | 1,038 (2,194) | 639 (1,254) |
| Outpatient | 159 (378) | 211 (525) | 208 (452) |
| Skilled nursing facility | 206 (547) | 300 (799) | 218 (674) |
NCC = nurse care coordination.
In the ITT analysis, the NCC plus pill organizer group total monthly participant Medicare costs were lower than those of the control group, although the difference was not statistically significant (b = 447.48, P = .11) (Table S1). Total costs (b = 0.22, P = .002) and number of hospitalizations (b = 421.65, P < .001) during the 12 months before study admission were statistically significant predictors of Medicare cost. The NCC plus medicine-dispensing machine group total monthly participant Medicare costs were higher than those of the NCC plus pill organizer group (b = 640.90, P = .08). Once again, total costs (b = 0.19, P = .03) and number of hospitalizations (b = 413.14, P = .002) during the 12 months before study admission were also significant predictors of Medicare cost. Black race was also predictive of higher cost in the NCC plus medicine-dispensing machine group (b = 1,165.28, P = .03) (Table S2).
Examination of participants who were in the study for at least 3 months (n = 344) found that total Medicare costs for the NCC plus pill organizer group were lower than for the control group (b = 491.03, P = .06) (Table S3). Total costs (b = 0.23, P < .001) and number of hospitalizations (b = 353.40, P < .001) during the 12 months before study admission were statistically significant predictors of Medicare cost. In addition, the NCC plus medicine-dispensing machine group total monthly participant Medicare costs were higher than those of the NCC plus pill organizer group (b = 409.41, P = .21). Total Medicare cost in the previous 12 months (b = 0.26, P = .001) and number of hospitalizations before admission (b = 293.17, P = .02) were significant predictors of total Medicare costs after study admission (Table S4). Heart disease was also a negative predictor (b = −962.62, P = .048).
Discussion
The objective of this study was to determine whether the clinical improvements attributed to home-based NCC (better quality of life, better functional status, less depression, better cognition) were associated with lower Medicare costs. Whether adding a medication-dispensing machine to NCC was associated with a reduction in Medicare costs was also tested. Total Medicare costs of the NCC plus pill organizer group were $448 lower than those of participants who received usual care, but the difference was not statistically significant (P = .11). Examination of participants who were in the study at least 3 months revealed a savings of $491 in the NCC plus pill organizer group (P = .06). Results of this study are similar to those of another home-based care coordination program, the Health Quality Partners MCCD site, which reported a $408 (P = .15) savings per member per month in Medicare expenditures in the treatment group compared with a control group.32 Although the reduction in monthly total Medicare costs per participant do not meet the traditional criterion for statistical significance (P < .05), the results are promising and support testing of the model in a larger, longer study. The addition of the medication-dispensing machine did not reduce total Medicare cost, which were actually higher.
It would seem that the active ingredient in the NCC intervention was the home visit. The major component of the intervention was conducted in participants’ homes. Working in participants’ environments provided opportunities to observe barriers to self-management and to create interventions that were more viable for them to use in their self-management practices. In addition, participants were visited at least every 2 weeks, providing time to develop a close therapeutic relationship. When needed, study nurse care coordinators accompanied participants to physician visits or called providers before and after visits to ensure continuity of care. Finally, participants were followed during and after hospitalization, with study nurse care coordinators actively participating in discharge planning. The program included the six practices identified in the more successful programs in the CMS MCCD: frequent in-person contact, occasional in-person meetings with providers, acting as a communication hub among providers, use of evidence-based education to patients, medication management, and timely and comprehensive transitional care after hospitalization.32
The addition of the medication machine did not reduce total Medicare costs; that group actually had higher total Medicare costs. The machine group also required additional visits related to the functioning of the machine, increasing the cost of the intervention. New technology holds promise to support independence in the home, but it often distracts providers in the provision of care. Perhaps the study nurse care coordinators were more engaged with the participants who used the pill organizer because more observation and participant engagement in self-management were needed in their care.
Prospective payment provides incentives for home healthcare providers to deliver care in short episodes versus the continuous care provided at different levels of intensity. Care was started when participants were no longer eligible for the Medicare home health benefit because of the stability of their chronic condition, but frail older adults who demonstrated problems in self-management of their chronic conditions, because of mild cognitive impairment, complex medication regimens or both, were targeted. As a result, participants in the study were high users of Medicare funds. The results of this study support the need to reexamine the medically necessary criteria in the current Medicare home health benefit. The chronically ill older adults in this study were medically stable, but they benefited from additional care to support the self-management of their chronic conditions.
There were several limitations of this study. First, accessing frail older adults was difficult. Study data collectors and nurse care coordinator RNs and APNs were university employees. Although agency home care nurses assisted by obtaining oral permission from potential participants for the data collection staff to contact them, the older adults were often reluctant to participate because they did not have a relationship with the data collection or nurse care coordinator staff of the study. During the pilot, it was found that randomizing after collecting baseline data often confused participants. Several participants were visibly upset when they were assigned to the control group. Because of the vulnerability of the participants, it was decided to randomize after oral consent. After oral consent, the study coordinator used a computer program to assign the older adult to a study group. If the older adults did not want to be in their assigned group, they were not eligible for any other group in the study. After group assignment, research data collection staff visited participants in their home and collected baseline data. It is likely that healthier older adults were less likely to participate in the two intervention groups and more likely to consent to be in the control group, which had only quarterly visits for data collection. Delays also were encountered in starting the intervention for the two treatment groups. Physician orders were required before the study nurse care coordinators could load the pill organizers or the medicine-dispensing machine machines. There were more than 400 prescribing providers, with an average of 2.5 per participant. Several participants were hospitalized before the intervention could be started because of delays in obtaining orders for their medications. Another problem encountered was the size of the medicine-dispensing machine. At first, nurses brought the machine into participants’ home in its large packing box. They learned that participants were more accepting if the machine was removed from the box before being brought into the home. Given these limitations, many participants assigned to the interventions groups never received any intervention from the study nurse care coordinators. Because of the delay, some participants in the ITT analysis had left the study before receiving any of the research intervention because of hospitalization or transfer to a nursing home. To address this problem, it was decided to conduct a second analysis of participants who were in the study at least 3 months, to ensure that the effect of the intervention could be evaluated.
The limitations experienced are not uncommon in community-based interventions for frail elderly adults. Care was delivered based on a home care model. Many other programs have used a primary care model, in which the majority of care management occurs in the outpatient setting. It is likely that the program would have been more successful if specific primary care providers had been worked with, similar to the approach used in the Guided Care program.33 In addition, because the physicians on call were unfamiliar with participants’ medical history, they often sent participants to the emergency department when there was a change in the participants’ condition. Once physician practices were familiar with the project, it was possible to suggest alternatives to visits to the emergency department when there was a change in participant status. RNs conducted the intervention in this study, and an APN led it. The APNs in this study did not prescribe medications. An enhancement to the program would be to expand the role of the APNs to deliver a portion of primary care in the home, eliminating the need for participants to make frequent visits to the outpatient clinic, given the exhausting nature of such a visit for these frail individuals.
The debate regarding the preferred site for delivery of chronic care is not new. One of the major problems is the financial incentive in the current healthcare system that reimburses hospitals and physicians through admissions or visits to providers. The recently implemented penalty to hospitals for admissions within 30 days of discharge for specific diagnoses provides some recognition of the consequences of a broken system. The use of Accountable Care Organizations also holds promise to reduce the incentive to provide institutional care when lower-cost alternatives are available.
Delivering cost-effective care to frail older adults is challenging. A home-based care coordination program provides a venue to support a self-management system for frail older adults. An interdisciplinary approach to care is critical. In this study, participants were able to manage their medication regimens in their homes with NCC that focused on medication management. The intervention facilitated communication between physicians, pharmacists, and frail older adults.
Perhaps, how health care is delivered to many chronically ill older adults needs to be rethought. Care provision in the home with frequent communication with primary care providers, pharmacists, and other healthcare providers may be a more cost-effective alternative in this population. Given the cost of care for chronically ill individuals and the consequences of care mismanagement, investment in systems to support self-management is essential.
Acknowledgments
Components of this paper were presented at the National State of the Science Congress on Nursing Research, Washington, District of Columbia, October 4, 2008.
Conflict of Interest: This research was funded by National Institute of Nursing Research Grant 5R01NR008911, University of Wisconsin-Milwaukee Self-Management Science Center Grant 1P20NR010674, and Arizona State University Grant 1T32NR012718 Transdisciplinary Training in Health Disparities Science.
Author Contributions: Marek: study design, implementation, analysis, and manuscript preparation. Stetzer, Adams: study design, data analysis and interpretation, manuscript preparation. Bub, Schlidt: study design, implementation, manuscript preparation. Colorafi: data interpretation, manuscript preparation.
Sponsor's Role: The sponsor had no role in study design, methods, subject recruitment, data collections, analysis, or preparation of paper.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell is not responsible for the content, accuracy, errors, or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Table S1.Control versus NCC + Mediplanner: Regression model estimates for each parameter for total Medicare Cost n = 407.
Table S2. NCC + Mediplanner versus NCC + MD2: Regression model estimates for each parameter for total Medicare Cost n = 407.
Table S3. Control versus NCC + Mediplanner: Regression model estimates for each parameter for total Medicare Cost for those in the study at least 3 months n = 344.
Table S4. NCC + Mediplanner vs. NCC + MD2: Regression model estimates for each parameter for total Medicare Cost n = 344.
References
- 1.DeVol R, Bedroussian A, Charuworn A, et al. An Unhealthy America: The Economic Burden of Chronic Disease. Santa Monica, CA: Milken Institute; 2007. [Google Scholar]
- 2.Thorpe KE, Howard DH. The rise in spending among Medicare beneficiaries: The role of chronic disease prevalence and changes in treatment intensity. Health Aff (Millwood) 2006;25:w378–w388. doi: 10.1377/hlthaff.25.w378. [DOI] [PubMed] [Google Scholar]
- 3.Dentzer S. Reform chronic illness care? Yes, we can. Health Aff (Millwood) 2009;28:12–13. doi: 10.1377/hlthaff.28.1.12. [DOI] [PubMed] [Google Scholar]
- 4.Institute of Medicine. Future Directions for the National Healthcare Quality and Disparities Reports. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 5.McDonald K, Schultz E, Albin L, et al. Care Coordination Atlas Version 3 (Contract No. 11–0023-EF) Rockville, MD: AHRQ; 2010. [Google Scholar]
- 6.Krause DS. Economic effectiveness of disease management programs: A meta-analysis. Dis Manag. 2005;8:114–134. doi: 10.1089/dis.2005.8.114. [DOI] [PubMed] [Google Scholar]
- 7.Gonseth J, Guallar-Castillon P, Banegas JR, et al. The effectiveness of disease management programmes in reducing hospital re-admission in older patients with heart failure: A systematic review and meta-analysis of published reports. Eur Heart J. 2004;25:1570–1595. doi: 10.1016/j.ehj.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Boult C, Boult LB, Morishita L, et al. A randomized clinical trial of outpatient geriatric evaluation and management. J Am Geriatr Soc. 2001;49:351–359. doi: 10.1046/j.1532-5415.2001.49076.x. [DOI] [PubMed] [Google Scholar]
- 9.Boult C, Reider L, Frey K, et al. Early effects of “Guided Care” on the quality of health care for multimorbid older persons: A cluster-randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2008;63A:321–327. doi: 10.1093/gerona/63.3.321. [DOI] [PubMed] [Google Scholar]
- 10.Cohen HJ, Feussner JR, Weinberger M, et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med. 2002;346:905–912. doi: 10.1056/NEJMsa010285. [DOI] [PubMed] [Google Scholar]
- 11.Counsell SR, Callahan CM, Clark DO, et al. Geriatric care management for low-income seniors: A randomized controlled trial. JAMA. 2007;298:2623–2633. doi: 10.1001/jama.298.22.2623. [DOI] [PubMed] [Google Scholar]
- 12.Stock R, Mahoney ER, Reece D, et al. Developing a senior healthcare practice using the chronic care model: Effect on physical function and health-related quality of life. J Am Geriatr Soc. 2008;56:1342–1348. doi: 10.1111/j.1532-5415.2008.01763.x. [DOI] [PubMed] [Google Scholar]
- 13.Dorr DA, Wilcox AB, Brunker CP, et al. The effect of technology-supported, multidisease care management on the mortality and hospitalization of seniors. J Am Geriatr Soc. 2008;56:2195–2202. doi: 10.1111/j.1532-5415.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- 14.Schraeder C, Shelton P, Sager M. The effects of a collaborative model of primary care on the mortality and hospital use of community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2001;56A:M106–M112. doi: 10.1093/gerona/56.2.m106. [DOI] [PubMed] [Google Scholar]
- 15.Gwadry-Sridhar FH, Flintoft V, et al. A systematic review and meta-analysis of studies comparing readmission rates and mortality rates in patients with heart failure. Arch Intern Med. 2004;164:2315–2320. doi: 10.1001/archinte.164.21.2315. [DOI] [PubMed] [Google Scholar]
- 16.Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: A randomized clinical trial. JAMA. 1999;281:613–620. doi: 10.1001/jama.281.7.613. [DOI] [PubMed] [Google Scholar]
- 17.Coleman EA, Parry C, Chalmers S, et al. The care transitions intervention: Results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 18.Hughes SL, Ulasevich A, Weaver FM, et al. Impact of home care on hospital days: A meta analysis. Health Serv Res. 1997;32:415–432. [PMC free article] [PubMed] [Google Scholar]
- 19.Bouman A, van Rossum E, Nelemans P, et al. Effects of intensive home visiting programs for older people with poor health status: A systematic review. BMC Health Serv Res. 2008;8:74. doi: 10.1186/1472-6963-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown R, Peikes D, Chen A, et al. 15-site randomized trial of coordinated care in Medicare FFS. Health Care Financ Rev. 2008;30:5–25. [PMC free article] [PubMed] [Google Scholar]
- 21.Ware JE., Jr SF-36 health survey update. Spine. 2000;25:3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 22.Fick DM, Cooper JW, Wade WE, et al. Updating the Beers criteria for potentially inappropriate medication use in older adults: Results of a US consensus panel of experts. Arch Intern Med. 2003;163:2716–2724. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 23.Marek KD, Stetzer F, Ryan PA, et al. Nurse care coordination and technology effects on health status of frail older adults via enhanced self-management of medication: Randomized clinical trial to test efficacy. Nurs Res. 2013;62:269–278. doi: 10.1097/NNR.0b013e318298aa55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman PH, Totten AM, Foust J, et al. Medication management: Evidence brief. Center for Home Care Policy & Research. Home Healthc Nurse. 2009;27:379–386. doi: 10.1097/01.NHH.0000356831.11843.16. [DOI] [PubMed] [Google Scholar]
- 25.Marek KD, Antle L. Medication management of the community-dwelling older adult. In: Hughes RG, editor. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Rockville, MD: Agency for Healthcare Research and Quality; 2008. In:, editor. (Publication No. 08–0043) [PubMed] [Google Scholar]
- 26.CareFacts Information Systems. 2012. [on-line]. Available at http://www.carefacts.com Accessed October 1, 2014.
- 27.de Craen AJ, Heeren TJ, Gussekloo J. Accuracy of the 15-item Geriatric Depression Scale (GDS-15) in a community sample of the oldest old. Int J Geriatr Psychiatry. 2003;18:63–66. doi: 10.1002/gps.773. [DOI] [PubMed] [Google Scholar]
- 28.Kurlowicz L, Wallace M. The Mini Mental State Examination (MMSE) Director. 1999;7:62. [PubMed] [Google Scholar]
- 29.Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. The Physical Performance Test. J Am Geriatr Soc. 1990;38:1105–1112. doi: 10.1111/j.1532-5415.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 30. Condition Categories [on-line]. Available at https://www.ccwdata.org/web/guest/condition-categories Accessed October 4, 2014.
- 31.Centers for Medicare and Medicaid Services. Statistical Supplement, 2008 Edition [on-line]. Available at http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareMedicaidStatSupp/2008.html Accessed August 4, 2014.
- 32.Brown RS, Peikes D, Peterson G, et al. Six features of Medicare coordinated care demonstration programs that cut hospital admissions of high-risk patients. Health Aff (Millwood) 2012;31:1156–1166. doi: 10.1377/hlthaff.2012.0393. [DOI] [PubMed] [Google Scholar]
- 33.Boyd CM, Boult C, Shadmi E, et al. Guided care for multimorbid older adults. Gerontologist. 2007;47:697–704. doi: 10.1093/geront/47.5.697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.Control versus NCC + Mediplanner: Regression model estimates for each parameter for total Medicare Cost n = 407.
Table S2. NCC + Mediplanner versus NCC + MD2: Regression model estimates for each parameter for total Medicare Cost n = 407.
Table S3. Control versus NCC + Mediplanner: Regression model estimates for each parameter for total Medicare Cost for those in the study at least 3 months n = 344.
Table S4. NCC + Mediplanner vs. NCC + MD2: Regression model estimates for each parameter for total Medicare Cost n = 344.