Abstract
Accurate distance measurement in 3D confocal microscopy is important for quantitative analysis, volume visualization and image restoration. However, axial distances can be distorted by both the point spread function (PSF) and by a refractive-index mismatch between the sample and immersion liquid, which are difficult to separate. Additionally, accurate calibration of the axial distances in confocal microscopy remains cumbersome, although several high-end methods exist. In this paper we present two methods to calibrate axial distances in 3D confocal microscopy that are both accurate and easily implemented. With these methods, we measured axial scaling factors as a function of refractive-index mismatch for high-aperture confocal microscopy imaging. We found that our scaling factors are almost completely linearly dependent on refractive index and that they were in good agreement with theoretical predictions that take the full vectorial properties of light into account. There was however a strong deviation with the theoretical predictions using (high-angle) geometrical optics, which predict much lower scaling factors. As an illustration, we measured the PSF of a correctly calibrated point-scanning confocal microscope and showed that a nearly index-matched, micron-sized spherical object is still significantly elongated due to this PSF, which signifies that care has to be taken when determining axial calibration or axial scaling using such particles.
Keywords: Axial calibration, axial scaling, confocal microscopy, refractive index mismatch
Introduction
Confocal microscopy is a powerful tool for 3D in situ measurements of both structure and dynamics for a wide range of scientific disciplines, such as cell-biology, pharmaceutics and materials science (Wilson, 1990; White & Errington, 2005; Pawley, 2006; Prasad et al., 2007). However, care has to be taken with 3D measurements because not all three dimensions are effected in the same way by both optics and data acquisition software. The inevitable difference in lateral and axial resolution affects the apparent shape of any (sub)micron-sized feature in a 3D measurement (Jenkins & Egelhaaf, 2008). Furthermore, there is often a refractive index (RI) mismatch between immersion fluid and sample. Not only does the RI mismatch deteriorate the point spread function (PSF) with increasing focus depth, and therefore the resolution, it also introduces a decrease in intensity and a shift of the objective focus (Visser et al., 1992; Hell et al., 1993; Sheppard et al., 1994; Visser & Oud, 1994; Sheppard & Török, 1997; Wiersma et al. 1997; de Grauw et al., 1999; Diaspro et al., 2002; Neuman et al., 2005; Egner & Hell, 2006; Shaevitz & Fletcher, 2007). When the RI of the sample is smaller than the immersion liquid used for imaging, axial distances appear more elongated due to the refractive effects on the focus position. A clear distinction can be made between studies that analyse these focal shifts with geometrical optics and studies that take the vectorial properties of light into account. On the basis of geometrical optics, axial elongation up to a factor of three times the actual distance has been predicted for high-aperture oil-immersion imaging in aqueous samples (Visser et al., 1992; Visser & Oud, 1994). It seems likely however that in the mechanism of the axial shift, paraxial rays dominate over the high-angle rays that are used in the geometrical optics approach (Sheppard et al., 1994). Studies that take the vectorial properties of light into account therefore predict significantly smaller axial elongations (Hell et al., 1993; Jacobsen & Hell, 1995; Sheppard & Török, 1997).
There are however still significant differences between the precise values of the axial scaling factors for different vector-based theories (Hell et al., 1993; Jacobsen & Hell, 1995; Sheppard & Török, 1997; Wiersma et al. 1997) and the amount of experimental studies remains limited (Hell et al., 1993; White et al., 1996; Neuman et al., 2005). Also, in most experimental studies on axial distance scaling, little attention is devoted to the axial-distance calibration which is indispensable for precise measurements. Calibration of the lateral distances is both straightforward and accurate, e.g. by using a calibration grid. However, accurate calibration of the axial distances in confocal microscopy remains cumbersome, although several high-precision methods exist (Boddeke et al., 1997; Bornfleth et al., 1998; Jensen et al., 2013).
In this paper, we demonstrate two methods to calibrate axial distances in confocal microscopy that are both accurate and practical to employ. In the first method, we use light interference to accurately measure the height of an empty calibration cell. We filled the cell with four different solvents mixed with fluorescent dye, which enabled the determination of the axial scaling factors as a function of RI for high-aperture 3D confocal-microscopy imaging with an oil-immersion objective. We also demonstrate a second method to accurately calibrate the confocal microscope, which is with large (∼50 μm) spherical particles that only have a thin fluorescent shell (compared to their size). Finally, we show with a correctly z-calibrated confocal microscope that spherical objects of a micrometer or smaller are still significantly elongated due to the PSF and possibly also due to a small RI mismatch between particle and solvent.
Materials and methods
Calibration cell construction and FTIR measurement
To calibrate the axial distances in a point-scanning confocal microscope, we built a custom sample cell with standard glass cover slips (Menzel Gläzer). The glass cover slips had a RI (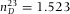 ) close to the RI of the oil-immersion liquid (Type F, Leica,
) close to the RI of the oil-immersion liquid (Type F, Leica, 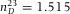 ) used for imaging. We avoided using glass capillaries (Vitrocom), often used in confocal studies on colloidal systems, since they provide lower quality imaging which is partially due to their manufacturing process and also due to the RI (
) used for imaging. We avoided using glass capillaries (Vitrocom), often used in confocal studies on colloidal systems, since they provide lower quality imaging which is partially due to their manufacturing process and also due to the RI (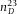 = 1.47). We used a standard No. 1.0 coverslide, which has a thickness between 130 and 160 μm, as specified by the manufacturer (Menzel Gläzer). Although standard confocal microscopy objectives are optimized for a cover slip thickness of 170 μm (Pawley, 2006) and therefore a No. 1.5 cover slip (thickness 160-190 μm) would have been more accurate, we could not however completely image our cell (with a height ∼ 80 μm), due to the limited working distance of the high numerical aperture objectives that we used. As spacers, we used No. 00 cover slips (thickness 55–80 μm) and the individual components of the cell were permanently fixed onto a standard microscopy slide (Menzel Gläzer) with UV glue (Norland 68 Optical Adhesive), see Figure1(A). The resulting height of the cell H was measured with a Fourier Transform Infrared (FTIR) spectrometer, with a selected diameter aperture of 0.25 mm (Vertex 70, Bruker). To avoid additional interference effects from the top cover slip itself, a drop of immersion oil was carefully placed on top of the cell before the measurement. The thickness and irregularities of the much thicker microscopy slide (∼ 1 mm) made it not necessary to correct for its interference effects.
= 1.47). We used a standard No. 1.0 coverslide, which has a thickness between 130 and 160 μm, as specified by the manufacturer (Menzel Gläzer). Although standard confocal microscopy objectives are optimized for a cover slip thickness of 170 μm (Pawley, 2006) and therefore a No. 1.5 cover slip (thickness 160-190 μm) would have been more accurate, we could not however completely image our cell (with a height ∼ 80 μm), due to the limited working distance of the high numerical aperture objectives that we used. As spacers, we used No. 00 cover slips (thickness 55–80 μm) and the individual components of the cell were permanently fixed onto a standard microscopy slide (Menzel Gläzer) with UV glue (Norland 68 Optical Adhesive), see Figure1(A). The resulting height of the cell H was measured with a Fourier Transform Infrared (FTIR) spectrometer, with a selected diameter aperture of 0.25 mm (Vertex 70, Bruker). To avoid additional interference effects from the top cover slip itself, a drop of immersion oil was carefully placed on top of the cell before the measurement. The thickness and irregularities of the much thicker microscopy slide (∼ 1 mm) made it not necessary to correct for its interference effects.
Figure 1.
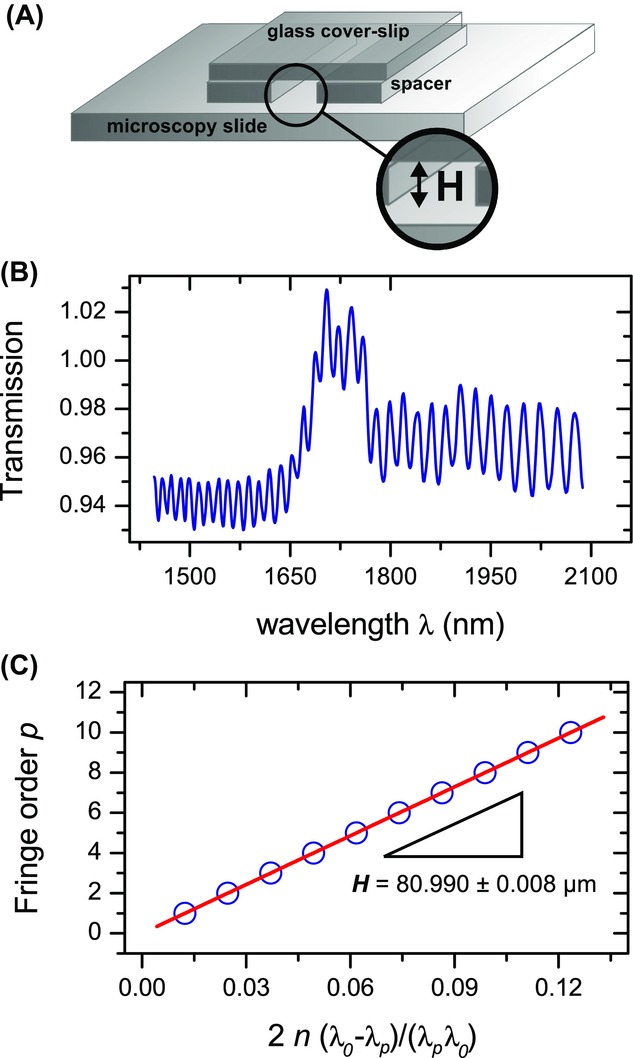
Construction and measurement of a calibration cell. (A) A sample cell with height H was built with glass cover slips and a standard microscopy slide, glued together with UV-glue. (B) When the (empty) cell was placed in a Fourier Transform Infrared (FTIR) spectrometer, Fabry Perrot (FP) fringes were visible in the transmission spectrum. (C) The height of the cavity (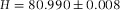 μm) was determined from the spacing between the FP fringes (Jiang et al., 1999). The error-bars on individual points are smaller than the symbol size.
μm) was determined from the spacing between the FP fringes (Jiang et al., 1999). The error-bars on individual points are smaller than the symbol size.
50 μm PMMA spheres
We used large poly(methyl methacrylate) (PMMA) spheres as a second method for calibration. The spheres had an average diameter σ = 50 μm and large polydispersity (> 10%, Altuglas, BS150N). To fluorescently dye the particles, we first prepared (rhodamine isothiocyanate)-aminostyrene (RAS) dye following the method described by Bosma et al. (Bosma et al., 2002). Then, we saturated a quantity of acetone (99%, Merck, USA) with RAS and subsequently centrifuged the saturated acetone at high speed to sediment undissolved dye. The acetone was then added to dodecane (99%, Sigma-Aldrich, USA) to give a 10 wt% solution of acetone. In this mixture, 50 wt% undyed PMMA particles and 0.35 wt% azo-bis-isobutyronitrile (98%, Acros) were suspended in a glass vial. The reaction mixture was heated up to 83°C and left to react for approximately 1 day. During this reaction, RAS molecules become chemically bonded with unreacted PMMA-ends at the surface of the particle. The vial was left open, so acetone could evaporate. The dyed particles were washed with hexane and dried under vacuum. Afterwards, the particles were suspended in a 24 wt% mixture of cis-decahydronaphthalene (cis-decalin, 99%, Sigma-Aldrich) in cyclohexylbromide (CHB, 98%, Sigma-Aldrich). The RI of this mixture was 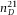 = 1.490, as measured with a refractometer (Atago 3T). This solvent mixture closely matched the RI of the particles, based on the fact that the RI is close to that of the bulk material [
= 1.490, as measured with a refractometer (Atago 3T). This solvent mixture closely matched the RI of the particles, based on the fact that the RI is close to that of the bulk material [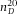 = 1.491 (Kasarova et al., 2007)] and that the particles hardly scattered when viewed under bright-field illumination. As a measure of shape uniformity, we determined the ellipticity of a small ensemble of particles suspended in 24 wt% cis-decalin in CHB. To this end, we fitted an ellipse to a binarized confocal microscopy image of the particles' equator using ImageJ software (Rasband, 1997-2014). We measured the aspect ratio
= 1.491 (Kasarova et al., 2007)] and that the particles hardly scattered when viewed under bright-field illumination. As a measure of shape uniformity, we determined the ellipticity of a small ensemble of particles suspended in 24 wt% cis-decalin in CHB. To this end, we fitted an ellipse to a binarized confocal microscopy image of the particles' equator using ImageJ software (Rasband, 1997-2014). We measured the aspect ratio 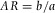 , with b the major and a the minor axis of the ellipse. For a total of 18 particles we found
, with b the major and a the minor axis of the ellipse. For a total of 18 particles we found 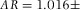 0.002.
0.002.
Confocal microscopy measurements
The confocal microscopy measurements were all performed with a Leica SP2 or Leica SP8. All distance measurements were performed on 3D image stacks obtained in xyz-scanmode. Although a (single) vertical scan obtained in xzy-mode is a fast method to view vertical slices through the sample, the obtained distances are in general not accurate and were avoided for any quantitative measurement. Imaging of the empty calibration cell was performed with a 20x/0.7 air-objective (Leica), all other measurements were performed with a 100x/1.4 oil-immersion confocal objective (Leica). The largest measurement error is introduced by the top cover slip being under a small angle with respect to the microscopy glass slide (see Fig.1A), despite careful application of the UV glue. Because we cannot place the sample in exactly the same position after its first measurement, we measured the height gradient in the x- and y-direction and found that the largest slope was 1.9 μm/mm. Assuming that it is possible to place the sample in its original position within 0.3 mm accuracy, a rough estimate of the error on the confocal height measurements is ∼ 0.6μm. We therefore chose our pixel-size in the axial direction to roughly half of this value. For the axial-scaling measurements, we used solvents of increasing RI: immersion oil (Type F, Leica, 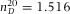 ), cyclohexylchloride (CHC, >98%, Merck,
), cyclohexylchloride (CHC, >98%, Merck, 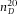 = 1.463), dodecane (>99%, Sigma-Aldrich,
= 1.463), dodecane (>99%, Sigma-Aldrich, 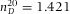 ) and de-ionized water (Millipore system,
) and de-ionized water (Millipore system, 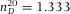 ). The first three (apolar) solvents were saturated with pyrromethene-567 dye (excitation maximum
). The first three (apolar) solvents were saturated with pyrromethene-567 dye (excitation maximum 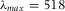 nm, Excition, USA) whereas the water was saturated with fluorescein isothiocyanate (FITC, isomer I, 90%, Sigma-Aldrich). Undissolved dye was removed by centrifugation. Also, a small amount of sterically stabilized PMMA tracer particles (Bosma et al., 2002) (diameter σ = 2.07 μm, polydispersity 3%), which often stick to untreated glass, was added to the apolar solvents to accurately determine the top and bottom of the cell. Because the volume fraction of the PMMA tracer particles is ≪ 1 %, their contribution to the effective RI of the sample can be neglected. Solvents were removed from the sample cell with nitrogen flow and the cell was flushed three times with the new solvent before the sample was carefully placed on the marked area under the confocal microscope to record a new image-stack. The image-stacks of the calibration cell were all recorded on a Leica SP2 with a 488 nm laser and a scan speed of 1000 Hz. The voxel-size of the image stacks was 293 × 293 × 311 nm3. The typical total volume of the images stacks was 38 × 38 × 115 μm3. Images of the large PMMA spheres (σ = 50 μm) were recorded on a Leica SP8 with a 543 nm laser line, voxel-size 51 × 51 × 168 nm3 and total volume 52.8 × 52.8 × 54.1 μm3.
nm, Excition, USA) whereas the water was saturated with fluorescein isothiocyanate (FITC, isomer I, 90%, Sigma-Aldrich). Undissolved dye was removed by centrifugation. Also, a small amount of sterically stabilized PMMA tracer particles (Bosma et al., 2002) (diameter σ = 2.07 μm, polydispersity 3%), which often stick to untreated glass, was added to the apolar solvents to accurately determine the top and bottom of the cell. Because the volume fraction of the PMMA tracer particles is ≪ 1 %, their contribution to the effective RI of the sample can be neglected. Solvents were removed from the sample cell with nitrogen flow and the cell was flushed three times with the new solvent before the sample was carefully placed on the marked area under the confocal microscope to record a new image-stack. The image-stacks of the calibration cell were all recorded on a Leica SP2 with a 488 nm laser and a scan speed of 1000 Hz. The voxel-size of the image stacks was 293 × 293 × 311 nm3. The typical total volume of the images stacks was 38 × 38 × 115 μm3. Images of the large PMMA spheres (σ = 50 μm) were recorded on a Leica SP8 with a 543 nm laser line, voxel-size 51 × 51 × 168 nm3 and total volume 52.8 × 52.8 × 54.1 μm3.
PSF measurement and deconvolution
To suppress the effect of the PSF, we deconvolved the 3D confocal microscopy data-stacks of the spherical particles. All deconvolutions were performed using commercially available software (Huygens Professional 4.4, Scientific Volume Imaging) using the classic maximum likelihood estimation restoration method (van der Voort & Strasters, 1995). For the deconvolution of the image-stack of the large PMMA sphere (σ = 50 μm), we used a depth-dependent theoretical PSF that takes into account the (small) RI-mismatch between sample and immersion fluid (van der Voort & Strasters, 1995). For the deconvolution of the 200 nm and 1040 nm particles, we used a measured PSF, obtained using fluorescent polystyrene spheres with diameter 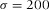 nm, polydispersity 5% and excitation maximum λ = 441 nm (YG Fluoresbrite Microparticles, Polysciences). The polystyrene particles (bulk material
nm, polydispersity 5% and excitation maximum λ = 441 nm (YG Fluoresbrite Microparticles, Polysciences). The polystyrene particles (bulk material 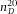 = 1.592 (Kasarova et al., 2007)) were dried on a cover glass (Menzel Gläzer, No. 1.5) and subsequently a drop of immersion oil (Type F, Leica,
= 1.592 (Kasarova et al., 2007)) were dried on a cover glass (Menzel Gläzer, No. 1.5) and subsequently a drop of immersion oil (Type F, Leica, 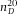 = 1.516) was placed on the glass slide to (nearly) index-match the particles. The sample was then placed on a microscopy slide with glass spacers and sealed with UV glue (Norland 68 Optical Adhesive). Images of the beads were recorded with an inverted confocal microscope (Leica SP8) with a 100x/1.4 oil immersion objective (Leica) in combination with a Hybrid detector. To gain enough statistics, confocal image-stacks of 8 different spheres were recorded with (sub)Nyquist sampling rate (18.2 × 18.2 × 83.9 nm3). Because these particles are only approximate point-sources, the PSF was obtained by iterative deconvolution with a 200 nm bead object (van der Voort & Strasters, 1995). Additionally, we imaged poly(methyl methacrylate) (PMMA) spheres with diameter σ = 1040 nm and a polydispersity δ = 3%, as determined with static light scattering (SLS). The particles were sterically stabilized with poly(12-hydroxystearic acid) (PHS) grafted onto the PMMA backbone which was chemically attached to the core of the particles and covalently labelled with fluorescent 4-methylaminoethylmethacrylate-7-nitrobenzo-2-oxa-1,3-diazol (NBD-MAEM) dye for imaging (Bosma et al., 2002). With the measured PSF, we deconvolved image-stacks of both the fluorescent polystyrene spheres (
= 1.516) was placed on the glass slide to (nearly) index-match the particles. The sample was then placed on a microscopy slide with glass spacers and sealed with UV glue (Norland 68 Optical Adhesive). Images of the beads were recorded with an inverted confocal microscope (Leica SP8) with a 100x/1.4 oil immersion objective (Leica) in combination with a Hybrid detector. To gain enough statistics, confocal image-stacks of 8 different spheres were recorded with (sub)Nyquist sampling rate (18.2 × 18.2 × 83.9 nm3). Because these particles are only approximate point-sources, the PSF was obtained by iterative deconvolution with a 200 nm bead object (van der Voort & Strasters, 1995). Additionally, we imaged poly(methyl methacrylate) (PMMA) spheres with diameter σ = 1040 nm and a polydispersity δ = 3%, as determined with static light scattering (SLS). The particles were sterically stabilized with poly(12-hydroxystearic acid) (PHS) grafted onto the PMMA backbone which was chemically attached to the core of the particles and covalently labelled with fluorescent 4-methylaminoethylmethacrylate-7-nitrobenzo-2-oxa-1,3-diazol (NBD-MAEM) dye for imaging (Bosma et al., 2002). With the measured PSF, we deconvolved image-stacks of both the fluorescent polystyrene spheres (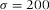 nm) and of the larger PMMA spheres (σ = 1040 nm) that were dried on a glass cover slip (Menzel Glazer, No. 1.5) and subsequently immersed in immersion oil (Type F, Leica). The particles were imaged within one hour of sample preparation. We acquired images stacks with voxel-size 5.4 × 5.4 × 41.96 nm3 and 18.75 × 18.75 × 83.9 nm3, respectively, using a 100x/1.4 oil objective and a 488 nm laser-line selected from a white light laser.
nm) and of the larger PMMA spheres (σ = 1040 nm) that were dried on a glass cover slip (Menzel Glazer, No. 1.5) and subsequently immersed in immersion oil (Type F, Leica). The particles were imaged within one hour of sample preparation. We acquired images stacks with voxel-size 5.4 × 5.4 × 41.96 nm3 and 18.75 × 18.75 × 83.9 nm3, respectively, using a 100x/1.4 oil objective and a 488 nm laser-line selected from a white light laser.
Results
Calibration cell and distance measurements
The sample cell used for calibration is shown in Figure1(A). When placed in a spectrometer, light reflecting from the front and back of the inside of the sample cell resulted in oscillations in the transmission spectrum, known as Fabry Perrot (FP) fringes, and shown in Figure1(B).
We determined the height of the cell from the spacing between the maxima of the FP fringes with the formula (Jiang et al., 1999)
| 1 |
with λ0 the longest wavelength, p the fringe order of subsequent maxima at wavelength 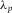 and n the RI of the medium (air). In Figure1(C) the fringe order p is plotted as a function of
and n the RI of the medium (air). In Figure1(C) the fringe order p is plotted as a function of 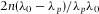 . The slope of the linear fit directly gives the height of the cell
. The slope of the linear fit directly gives the height of the cell 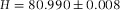 μm.
μm.
In Figure2(A), we show a confocal micrograph of the empty calibration cell, imaged in reflection mode with a 20x/0.7 air objective and 488 nm laser. The image clearly shows the reflections at the glass-air interfaces, which we assumed to be positioned at the highest pixel-intensity. We measured the height at the same position as was done with the spectrometer (for four different times), which resulted in a mean value of H = 80.8 ± 0.3 μm. This value is in good agreement with the spectrometer measurement (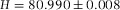 μm) and thus confirms proper calibration of the microscope in the axial direction.
μm) and thus confirms proper calibration of the microscope in the axial direction.
Figure 2.
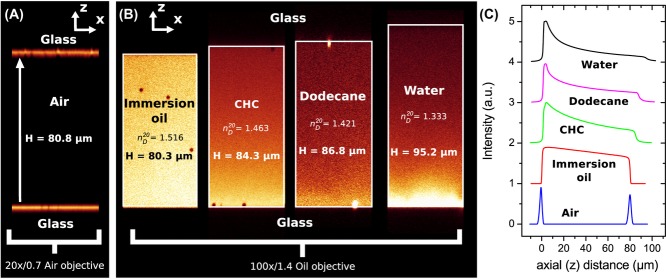
Axial distances measured with confocal microscopy. (A) The empty calibration cell with 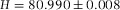 μm was measured in confocal reflection mode (Leica SP2) with a 20x/0.7 air-objective (Leica), which resulted in
μm was measured in confocal reflection mode (Leica SP2) with a 20x/0.7 air-objective (Leica), which resulted in 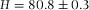 μm. (B) The cell filled with immersion oil, pyrromethene dye and poly(methyl methacrylate) (PMMA) tracer particles (left). The sample was imaged with an 100x/1.4 oil objective (Leica) and a similar height was measured (
μm. (B) The cell filled with immersion oil, pyrromethene dye and poly(methyl methacrylate) (PMMA) tracer particles (left). The sample was imaged with an 100x/1.4 oil objective (Leica) and a similar height was measured (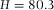 μm). However, when the cell was re-filled with solvents that had a RI mismatch with the oil-objective, deviating axial-distances were found, as indicated in the figure. (C) Intensity profiles along the axial (z) direction show the increase in (apparent) axial distance as well as decrease of intensity deeper in the sample. The profiles where normalized and shifted for better visualization.
μm). However, when the cell was re-filled with solvents that had a RI mismatch with the oil-objective, deviating axial-distances were found, as indicated in the figure. (C) Intensity profiles along the axial (z) direction show the increase in (apparent) axial distance as well as decrease of intensity deeper in the sample. The profiles where normalized and shifted for better visualization.
Figure2(B) shows the same cell, this time filled with solvents of decreasing RI, as indicated in the figure. The tracer particles were used to measure the height of the sample. When the cell was filled with immersion oil (Fig.2B, left) a single value of H = 80.30 μm was obtained. After removal of the oil, the empty cell was measured again with an air objective which resulted in a value of H = 80.92 μm. From these measurements, we can conclude that the confocal was accurately calibrated and that filling the cell with solvent did not alter the height significantly.
We also measured the effect of RI on the axial distances, indicated by the intensity profiles shown in Figure2(C). Not only does the (apparent) axial distance change as a function of RI, also the intensity becomes nonlinearly dependent on the axial distance, which is described in detail elsewhere (Hell et al., 1993). We compared the data obtained from Figure2(C) with a theoretical model for the scaling factor of axial distances 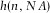 , based on geometrical optics, given by (Visser et al., 1992; Visser & Oud, 1994)
, based on geometrical optics, given by (Visser et al., 1992; Visser & Oud, 1994)
| 2 |
with n the RI of the suspension, 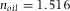 the RI of the oil immersion liquid and NA the numerical aperture of the objective. For low NA-objectives, Eq. (2) simplifies to an expression of the focal shift in the paraxial limit
the RI of the oil immersion liquid and NA the numerical aperture of the objective. For low NA-objectives, Eq. (2) simplifies to an expression of the focal shift in the paraxial limit
| 3 |
We also compared our measurement to two theoretical studies that take the full vectorial properties of light into account (Hell et al., 1993; Sheppard & Török, 1997). A summary of these scaling factors is shown in Figure3. The (black) circles are our measurement points, which are connected with a linear fit (dashed black line). The (green) continuous and (green) dashed-dotted lines are from the theoretical prediction of Eq. (2), for NA = 0.7 and NA = 1.4, respectively. The (pink) square is based on a theoretical study by Sheppard et al. (Sheppard & Török, 1997) for NA = 1.4 and the (blue) diamonds show calculations based on a study by Hell et al. for NA = 1.3 (Hell et al., 1993), both at a wavelength around 500 nm. The reason for choosing a lower NA in the latter study is that due to total internal reflection at the glass/water interface, a numerical aperture of 1.4 becomes effectively 1.3 (Hell et al., 1993).
Figure 3.
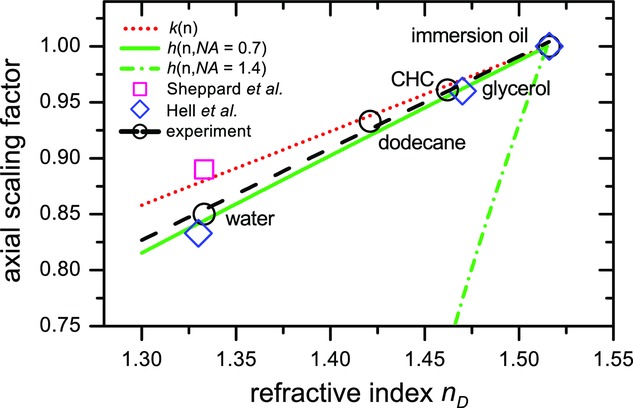
Axial scaling factors as a function of the sample refractive index 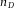 . Our measurements are indicated with black open circles, which were fitted with the dashed (black) line. The (green) continuous and (green) dashed-dotted lines are from the high-angle geometrical prediction of Eq. (2), for NA = 0.7 and NA = 1.4, respectively, and the (red) dotted line is from the paraxial limit of Eq. (3). The (pink) square is based on a theoretical study by Sheppard et al. (Sheppard & Török, 1997) for NA = 1.4 and the (blue) diamonds show calculations based on a study by Hell et al. for NA = 1.3 (Hell et al., 1993), both at a wavelength around 500 nm.
. Our measurements are indicated with black open circles, which were fitted with the dashed (black) line. The (green) continuous and (green) dashed-dotted lines are from the high-angle geometrical prediction of Eq. (2), for NA = 0.7 and NA = 1.4, respectively, and the (red) dotted line is from the paraxial limit of Eq. (3). The (pink) square is based on a theoretical study by Sheppard et al. (Sheppard & Török, 1997) for NA = 1.4 and the (blue) diamonds show calculations based on a study by Hell et al. for NA = 1.3 (Hell et al., 1993), both at a wavelength around 500 nm.
The calculations by Hell et al. seem to agree best with our measurements (black circles). It is also clear from Figure3 that the formula based on geometrical optics (Eq. ) is highly dependent on NA and that our measurements do not correspond at all with the theoretical predictions for NA = 1.4. This is a confirmation that indeed the paraxial rays dominate the mechanism of axial shift instead of the high-angle rays used in geometrical optics. Interestingly though, if we assume an ‘effective NA’ of 0.7 (continuous green line), Eq. (2) fits our data remarkably well.
We also measured the axial shift when the calibration cell was filled with CHC and imaged with a 100x oil-immersion objective with variable NA between 0.7 and 1.4 (not shown here). This resulted in an increase in axial distance of 2% from NA = 0.7 to NA = 1.4, whereas Eq. (2) predicts an increase of 31%. This result is however again in good agreement with the theoretical prediction and experimental measurement reported by Hell et al. (Hell et al., 1993).
From a linear fit to our measurement points, we obtained the empirical formula
| 4 |
with the coefficient of correlation 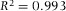 indicating a strong linear correlation. This empirical formula could be used to predict (or estimate) the axial scaling factor for 3D images acquired with an oil-immersion objective (NA = 1.4) for any RI between 1.3 and 1.5.
indicating a strong linear correlation. This empirical formula could be used to predict (or estimate) the axial scaling factor for 3D images acquired with an oil-immersion objective (NA = 1.4) for any RI between 1.3 and 1.5.
Calibration with a 50 μm PMMA sphere
As a second method to calibrate the axial distance in a confocal microscope, we exploited the well-defined 3D geometry of large spherical PMMA particles (average diameter σ = 50 μm and polydispersity larger than 10%), dyed with a thin fluorescent shell (∼ 500 nm). We used these particles to determine the z-calibration of a point-scanning confocal microscope (Leica SP8). We first confirmed correct calibration of the xy-distances of the microscope by imaging a calibration grid (Ted Pella, grid spacing 0.01 mm) in reflection mode using a 100x/1.4 oil immersion objective (Leica). Then we imaged a single particle in 3D using the same objective. Figure4(A) shows a 3D image-stack of a particle dispersed in an RI-matching mixture of 24 wt% cis-decalin/CHB. In Figure4(B), a single xy-image shows that the diameter of the particle in the x- and y-direction is equal. However, a reconstructed xz-view of the particle (Fig.4C) shows that there is an elongation in the z-direction. From the intensity profiles, shown in Figure4(D), we determined the diameter of the particle in the x-, y- and z-direction, and found an elongation of 5.8% in the z-direction. We also deconvolved the 3D image stack with a theoretical depth-dependent PSF. The resulting intensity profile in the z-direction is indicated with the (blue) dashed line in Figure4(D). The deconvolution resulted in a decrease of the width of both peaks, however, there was no significant change in the distance between them. Additionally, we acquired images for different scan-speeds and different image-sizes and found similar results. Due to the (small) RI mismatch between the suspension (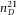 = 1.490) and the immersion oil (
= 1.490) and the immersion oil (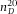 = 1.516) we expected, based on Eq. (4), an axial scaling factor in the z-direction of only f(1.49) = 0.98. We therefore conclude that there is a small but significant elongation in the z-direction of 3.7%, which is most likely due to an incorrect calibration of the microscope. To confirm this statement, we measured the height of our calibration cell when it was filled with immersion-oil (Fig.2B) with the same microscope and objective as used for the image-stack in Figure4, and found a distance of
= 1.516) we expected, based on Eq. (4), an axial scaling factor in the z-direction of only f(1.49) = 0.98. We therefore conclude that there is a small but significant elongation in the z-direction of 3.7%, which is most likely due to an incorrect calibration of the microscope. To confirm this statement, we measured the height of our calibration cell when it was filled with immersion-oil (Fig.2B) with the same microscope and objective as used for the image-stack in Figure4, and found a distance of 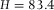 μm. This indicated a similar deviation of 3.0% in the axial direction.
μm. This indicated a similar deviation of 3.0% in the axial direction.
Figure 4.
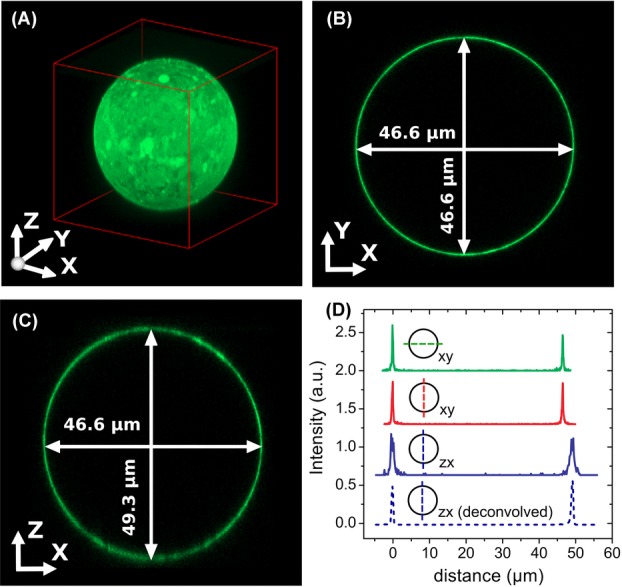
A fluorescent PMMA sphere dispersed in an index matching mixture of 24 wt% cis-decalin in CHB, recorded with a confocal microscope (Leica SP8). (A) 3D view constructed from a XYZ image stack. (B) A single XY image shows that x and y distances are equal. (C) The reconstructed XZ view of the image shows that there is a small (5.8%) elongation in the z-direction. Due to the refractive index mismatch between the suspension (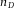 = 1.49) and the oil immersion (
= 1.49) and the oil immersion (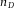 = 1.52) an elongation in the z-direction of 2% was expected. (D) Intensity profiles along different lines trough the sphere, as indicated in the figure. The profiles were normalized and shifted for better visualization.
= 1.52) an elongation in the z-direction of 2% was expected. (D) Intensity profiles along different lines trough the sphere, as indicated in the figure. The profiles were normalized and shifted for better visualization.
Because the calibration of the xy-distances in confocal microscopy is simple and straightforward (e.g. with a calibration grid), the fluorescent PMMA spherical particles described above can be used to measure absolute axial-distance deviations within ∼ 1–2%. An additional benefit is that these particles hardly display thermal motion, even when dispersed in a solvent with viscosity ∼1 cP, which is due to their large size.
PSF measurement and imaging of single fluorescent beads
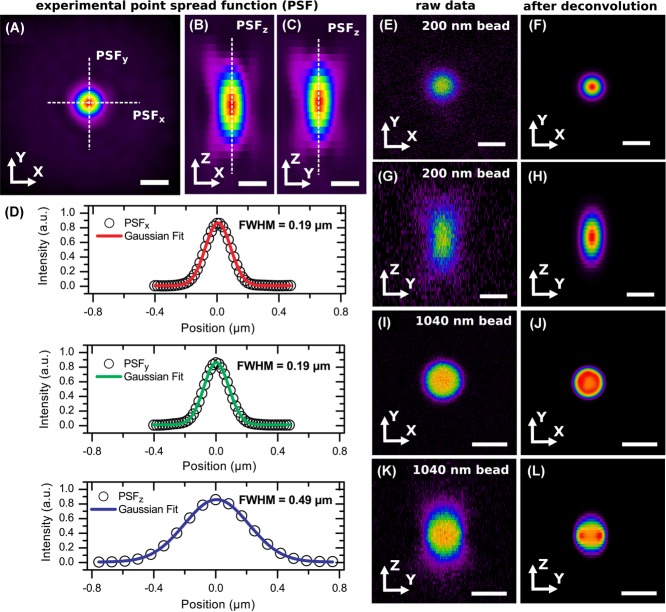
In Figure5, we show examples of an experimental measurement of the PSF and its effect on confocal microscopy measurements of fluorescent particles. In Figure5(A)–(C), we show images of the PSF of an accurately calibrated point-scanning confocal microscope (Leica SP8) equipped with a 100x/1.4 oil-immersion objective (Leica). The intensity profiles of the PSF in the x, y and z-direction could be well fitted with Gaussian functions (Fig.5 D). From the full width at half maximum (FWHM) of these Gaussian fits, we obtained a measure of the resolution of the microscope. The values that we obtained are 190 nm in the lateral and 490 nm in the axial direction, which is close to the maximum resolution possible for a conventional point-scanning confocal microscope, which is around 178 nm in the lateral and 459 nm in the axial direction for this setup (Wilhelm et al., 1997; Cole et al., 2011). Also, the symmetry of the PSF in all three directions is high, indicating little optical aberration. In Figure5(E)–(L), we demonstrate the effect of the PSF on the geometry of two (nearly) index-matched spherical particles. In Figure5(E)–(H), orthogonal views are shown of a polystyrene bead with a diameter of 200 nm that was immersed in immersion oil (Type F, Leica) before and after deconvolution. It is clear from Figure5(G) that its dimensions in the axial direction were stretched. Deconvolution (Figs.5F,H) reduced the apparent size of the particle, however, anisotropy in the particle shape still remained. In Figure5(I)–(L), orthogonal views are shown of a PMMA sphere (diameter 1040 nm), before and after deconvolution. Despite its larger size, the particle still seems elongated in the axial direction (Fig.5K), however, deconvolution almost recovered the spherical shape of the particle (Fig.5L).
Figure 5.
Experimental measurement of the point spread function (PSF) and imaging of single fluorescent beads. Images were recorded with a 100x/1.4 oil immersion objective. (A) The PSF in the XY plane. Intensity profiles were recorded along the indicated cross-sections. (B–C) The PSF in the z-direction clearly shows the expected elongation, due to the more limited resolution in the axial direction. The scale bars in (A-C) are 300 nm. (D) Recorded intensity profiles from the images in (A) and (B). The FWHMs that we obtained were 190 nm in the lateral and 490 nm in the axial direction. (E–H) Orthogonal views of a polystyrene bead with a diameter of 200 nm, before and after deconvolution. The scale bar is 300 nm. (I–L) Orthogonal views of a PMMA sphere with diameter 1040 nm, again before and after deconvolution. The scale bar is 1 μm.
These measurements demonstrate that even a micron-sized spherical object that was nearly RI-matched seemed elongated in the axial direction due to the anisotropy of the PSF (and possibly to a lesser extent due to a subtle difference in RI between particle and solvent). This demonstrates that care has to be taken when using single, micron-sized features to determine if the microscope is correctly calibrated in the axial direction, even when the sample is almost RI-matched.
Discussion
With the calibration cell described in this paper, we measured the scaling of axial distances as a function of RI mismatch. We found for an aqueous sample dyed with FITC (excitation wavelength 488 nm) imaged with an oil-immersion objective with NA = 1.4, an axial scaling factor of 0.85. This value is in good agreement with the theoretical calculations of Hell et al. (Hell et al., 1993), who found a value of 0.83 and to reasonable extent to the value of 0.89 calculated by Sheppard et al. (Sheppard & Török, 1997). The linear slope fitted to our data was however much smaller than the slope predicted from the high-angle geometrical optics Eq. (2), which predicts a scaling factor of 0.36 for NA = 1.3, and is slightly higher than the slope for the paraxial limit 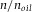 . Our experimental values are however in good agreement with other experimental measurements that use a fluorescent ‘sea’ between two cover slips (Hell et al., 1993; White et al., 1996). Theoretical expressions that take the vectorial properties of light into account found almost linear scaling in axial shift as a function of axial distance, and also found no strong dependence on excitation wavelength (around 500 nm) (Hell et al., 1993; Jacobsen & Hell, 1995; Sheppard & Török, 1997), which extends the applicability of these results.
. Our experimental values are however in good agreement with other experimental measurements that use a fluorescent ‘sea’ between two cover slips (Hell et al., 1993; White et al., 1996). Theoretical expressions that take the vectorial properties of light into account found almost linear scaling in axial shift as a function of axial distance, and also found no strong dependence on excitation wavelength (around 500 nm) (Hell et al., 1993; Jacobsen & Hell, 1995; Sheppard & Török, 1997), which extends the applicability of these results.
Our measurements deviate considerably however from experimental studies on micron-sized particles that are immersed in a solvent with an RI mismatch, where scaling factors of 0.4–0.7 are reported for aqueous samples (Visser et al., 1992; Visser & Oud, 1994; White et al., 1996). In the case of an RI mismatch between the sample and the immersion liquid, both the width of the PSF increases (Hell et al., 1993; Shaevitz & Fletcher, 2007), as well as the apparent axial distance (due to the focal shift). These two effects are hard to separate for micron-sized particles and has led to overestimation of axial distance scaling in previous studies, as described further in Wiersma et al. (1997). The overestimated axial scaling obtained by measuring particles of a few micron in diameter corresponds however approximately to the incorrect axial scaling distances predicted by the geometrical optics model (Eq.).
This does not mean that micron-sized spheres are not useful for calibration samples. On the contrary, regular 3D colloidal crystals of fluorescent micro-spheres can act as an ideal calibration sample, because of the well defined (periodic) 3D distances of the crystal lattice. The particles can be immobilized by post-treatment of the sample and the lattice distances can be measured with complementary methods such as light scattering or X-ray diffraction (Thijssen et al., 2006). Such 3D colloidal crystals are especially worth exploring because a complete theory exists on how to correct for refraction index differences between the micro-spheres and the surrounding medium. Presently we are using such samples to test effective medium theories that are used to arrive at approximate effective refractive indices for the combined particle-solvent system. Furthermore, if the particles have, e.g. a small gold core, the sample can at the same time be used to measure the PSF (in reflection mode).
Conclusion
We demonstrated two methods to calibrate axial distances in confocal microscopy that are both accurate and practical to employ. The first method consists of a sample cell built from ordinary glass cover slips. From the Fabry-Perrot fringes in the transmission spectrum of the empty cell, we could accurately measure its height. We filled the cell with four different solvents mixed with fluorescent dye, which enabled the determination of the axial scaling factors as a function of RI for high-aperture confocal-microscopy imaging. We found that our scaling factors are almost completely linearly dependent on the RI and therefore we determined an empirical formula that provides the axial scaling factor for confocal microscopy images acquired with an oil-immersion objective (NA = 1.4) for any RI between 1.3 and 1.5. Our results are in good agreement with theories that take the full vectorial properties of light into account, and consequently, there was a strong deviation with the high-angle theoretical prediction of geometrical optics, which predicts much lower scaling factors. The prediction in the paraxial limit (considered only valid for low NA) resulted in only slightly higher scaling factors compared to our measurements, which is in agreement with the assertion that paraxial rays dominate in the mechanism of axial shift. Using a straightforward calibration of the lateral distances of a confocal microscope with a calibration grid, we showed that large (∼ 50 μm) spherical particles that only have a fluorescent shell, can conveniently be used to measure axial-distance deviations ∼ 1–2%. As an illustration, we demonstrated with a correctly calibrated confocal microscope that spherical objects of only a micrometer or smaller were still significantly elongated due to the PSF, and possibly due to a small RI mismatch, which signifies that care has to be taken when determining axial calibration or axial scaling using such particles.
Acknowledgments
We thank Hans van der Voort, Hans Gerritsen and Job Thijssen for useful discussion. We thank Johan Stiefelhagen for particle synthesis. This research was carried out partially (THB) under project number M62.7.08SDMP25 in the framework of the Industrial Partnership Program on Size Dependent Material Properties of the Materials innovation institute (M2i) and the Foundation of Fundamental Research on Matter (FOM), which is part of the Netherlands Organisation for Scientific Research (NWO). Part of the research leading to these results has received funding from the European Research Council under the European Unions Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement no. [291667].
References
- Boddeke FR, Vliet LJV. Young IT. Calibration of the automated z-axis of a microscope using focus functions. J. Microsc. 1997;186:270–274. [Google Scholar]
- Bornfleth H, Saetzler K, Eils R. Cremer C. Distance measurements and volume-conserving segmentation of objects near and below the resolution limit in three-dimensional confocal fluorescence microscopy. J. Microsc. 1998;189:118–136. [Google Scholar]
- Bosma G, Pathmamanoharan C, de Hoog EHS, Kegel WK, van Blaaderen A. Lekkerkerker HNW. Preparation of monodisperse, fluorescent PMMA-latex colloids by dispersion polymerization. J. Colloid Interface Sci. 2002;245:292–300. doi: 10.1006/jcis.2001.7986. [DOI] [PubMed] [Google Scholar]
- Cole RW, Jinadasa T. Brown CM. Measuring and interpreting point spread functions to determine confocal microscope resolution and ensure quality control. Nat. Protoc. 2011;6:1929–1941. doi: 10.1038/nprot.2011.407. [DOI] [PubMed] [Google Scholar]
- de Grauw CJ, Vroom JM, van der Voort HT. Gerritsen HC. Imaging properties in two-photon excitation microscopy and effects of refractive-index mismatch in thick specimens. Appl. Opt. 1999;38:5995–6003. doi: 10.1364/ao.38.005995. [DOI] [PubMed] [Google Scholar]
- Diaspro A, Federici F. Robello M. Influence of refractive-index mismatch in high-resolution three-dimensional confocal microscopy. Appl. Opt. 2002;41:685–690. doi: 10.1364/ao.41.000685. [DOI] [PubMed] [Google Scholar]
- Egner A. Hell S. Aberrations in confocal and multi-photon fluorescence microscopy induced by refractive index mismatch. In: Pawley JB, editor; Handbook of biological confocal microscopy. New York: Springer; 2006. pp. 404–413. (ed. by ), chap. 20 &. [Google Scholar]
- Hell S, Reiner G, Cremer C. Stelzer EHK. Aberrations in confocal fluorescence microscopy induced by mismatches in refractive index. J. Microsc. 1993;169:391–405. [Google Scholar]
- Jacobsen H. Hell S. Effect of the specimen refractive index on the imaging of a confocal fluorescence microscope employing high aperture oil immersion lenses. Bioimaging. 1995;3:39–47. [Google Scholar]
- Jenkins MC. Egelhaaf SU. Confocal microscopy of colloidal particles: towards reliable, optimum coordinates. Adv. Colloid Interface Sci. 2008;136:65–92. doi: 10.1016/j.cis.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Jensen KE, Weitz DA. Spaepen F. Note: a three-dimensional calibration device for the confocal microscope. Rev. Sci. Instrum. 2013;84:016108. doi: 10.1063/1.4776672. [DOI] [PubMed] [Google Scholar]
- Jiang P, Bertone JF, Hwang KS. Colvin VL. Single-crystal colloidal multilayers of controlled thickness. Chem. Mater. 1999;11:2132–2140. [Google Scholar]
- Kasarova SN, Sultanova NG, Ivanov CD. Nikolov ID. Analysis of the dispersion of optical plastic materials. Opt. Mater. 2007;29:1481–1490. [Google Scholar]
- Neuman KC, Abbondanzieri EA. Block SM. Measurement of the effective focal shift in an optical trap. Opt. Lett. 2005;30:1318–1320. doi: 10.1364/ol.30.001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawley JE. Handbook of Biological Confocal Microscopy. 3rd. Berlin: Springer; 2006. [Google Scholar]
- Prasad V, Semwogerere D. Weeks ER. Confocal microscopy of colloids. J. Phys. Cond. Matter. 2007;19:113102. [Google Scholar]
- Rasband WS. 1997. ImageJ, National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/
- Shaevitz JW. Fletcher DA. Enhanced three-dimensional deconvolution microscopy using a measured depth-varying point-spread function. J. Opt. Soc. Am. A. 2007;24:2622–2627. doi: 10.1364/josaa.24.002622. [DOI] [PubMed] [Google Scholar]
- Sheppard CJR, Gu M, Brain K. Zhou H. Influence of spherical aberration on axial imaging of confocal reflection microscopy. Appl. Opt. 1994;33:616–624. doi: 10.1364/AO.33.000616. [DOI] [PubMed] [Google Scholar]
- Sheppard CJR. Török P. Effects of specimen refractive index on confocal imaging. J. Microsc. 1997;185:366–374. [Google Scholar]
- Thijssen JHJ, Petukhov AV, 't Hart DC, Imhof A, van der Werf CHM, Schropp REI. van Blaaderen A. Characterization of photonic colloidal single crystals by microradian x-ray diffraction. Adv. Mater. 2006;18:1662–1666. [Google Scholar]
- van der Voort HTM. Strasters KC. Restoration of confocal images for quantitative image analysis. J. Microsc. 1995;178:165–181. [Google Scholar]
- Visser TD. Oud JL. Volume measurements in three-dimensional microscopy. Scanning. 1994;16:198–200. [Google Scholar]
- Visser TD, Oud JL. Brakenhoff GJ. Refractive index and axial distance measurements in 3-D microscopy. Optik. 1992;90:17–19. [Google Scholar]
- White NS. Errington RJ. Fluorescence techniques for drug delivery research: theory and practice. Adv. Drug Delivery Rev. 2005;57:17–42. doi: 10.1016/j.addr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- White NS, Errington RJ, Fricker MD. Wood JL. Aberration control in quantitative imaging of botanical specimens by multidimensional fluorescence microscopy. J. Microsc. 1996;181:99–116. [Google Scholar]
- Wiersma SH, Török P, Visser TD. Varga P. Comparison of different theories for focusing through a plane interface. J. Opt. Soc. Am. A. 1997;14:1482. doi: 10.1364/JOSAA.35.000593. [DOI] [PubMed] [Google Scholar]
- Wilhelm S, Gröbler B, Gluch M. Heinz H. Carl Zeiss Principles. Carl Zeiss; 1997. &, Germany. [Google Scholar]
- Wilson T. Confocal Microscopy. Academic Press, United States; 1990. [Google Scholar]