Abstract
Heart failure is associated with the reactivation of a fetal cardiac gene programme that has become a hallmark of cardiac hypertrophy and maladaptive ventricular remodelling, yet the mechanisms that regulate this transcriptional reprogramming are not fully understood. Using mice with genetic ablation of calcium/calmodulin-dependent protein kinase II δ (CaMKIIδ), which are resistant to pathological cardiac stress, we show that CaMKIIδ regulates the phosphorylation of histone H3 at serine-10 during pressure overload hypertrophy. H3 S10 phosphorylation is strongly increased in the adult mouse heart in the early phase of cardiac hypertrophy and remains detectable during cardiac decompensation. This response correlates with up-regulation of CaMKIIδ and increased expression of transcriptional drivers of pathological cardiac hypertrophy and of fetal cardiac genes. Similar changes are detected in patients with end-stage heart failure, where CaMKIIδ specifically interacts with phospho-H3. Robust H3 phosphorylation is detected in both adult ventricular myocytes and in non-cardiac cells in the stressed myocardium, and these signals are abolished in CaMKIIδ-deficient mice after pressure overload. Mechanistically, fetal cardiac genes are activated by increased recruitment of CaMKIIδ and enhanced H3 phosphorylation at hypertrophic promoter regions, both in mice and in human failing hearts, and this response is blunted in CaMKIIδ-deficient mice under stress. We also document that the chaperone protein 14–3–3 binds phosphorylated H3 in response to stress, allowing proper elongation of fetal cardiac genes by RNA polymerase II (RNAPII), as well as elongation of transcription factors regulating cardiac hypertrophy. These processes are impaired in CaMKIIδ-KO mice after pathological stress. The findings reveal a novel in vivo function of CaMKIIδ in regulating H3 phosphorylation and suggest a novel epigenetic mechanism by which CaMKIIδ controls cardiac hypertrophy. © 2014 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Keywords: H3 phosphorylation, CaMKIIδ, cardiac hypertrophy, transcription, 14–3–3, epigenetic
Introduction
Nuclear DNA is wrapped around histone octamers consisting of histone H3, H4, H2A and H2B, which form the nucleosome, considered to be the fundamental unit of chromatin [1]. A primary role of chromatin is to control various cellular processes by manipulation of its architecture. Mechanisms that regulate chromatin structure, commonly known as epigenetic alterations, include histone post-translational modifications occurring by acetylation, methylation, phosphorylation, ubiquitination, sumoylation and ADP-ribosylation [2]. These reversible modifications are the basis of the 'histone code' that controls gene activity, resulting in specific cellular phenotypes.
Changes in histone acetylation and methylation affect cardiac development and cause major cardiac pathologies (reviewed in [3,4]). However, the role of other modifications, such as H3 phosphorylation, remains poorly understood. In organs with high proliferative capacities, H3 phosphorylation at serine 10 (p-H3 S10) is associated with mitosis, chromosome condensation and transcriptional activation [5–10]. In cardiac muscle, p-H3 S10 is detected in proliferating cardiomyocytes during fetal life, during the first week of post-natal life and in cardiomyocytes of young adults [11,12]. After pathological insult, H3 phosphorylation increases in the adult heart [13,14]. However, the role of this modification and the physiological H3 kinases involved have been overlooked.
Heart failure remains a major cause of mortality worldwide [15]. Heart failure is often preceded by a phase of cardiac hypertrophy associated with re-activation of fetal cardiac genes, such as atrial natriuretic peptide (ANP) and β-myosin heavy chain (β-MHC) [16,17]. Mechanisms controlling this switch include the ATP-dependent chromatin remodeller Brg1, which recruits the histone deacetylase HDAC4 and PARP1 [18]. CaMKIIδ, a critical mediator of a variety of cardiovascular disorders [19–22], regulates cardiac hypertrophy by interfering with the activity of HDACs [23,24]. We recently reported that in isolated cardiomyocytes, CaMKIIδ not only signals to HDAC4 but to chromatin itself [25]. In the present study, we address the in vivo function of CaMKIIδ in H3 phosphorylation and the underlying mechanism. Using mice with global deletion of CaMKIIδ [20] and samples from patients in end-stage heart failure, we show that CaMKIIδ controls H3 phosphorylation in the adult heart in response to pressure overload. One underlying mechanism involves binding of the chaperone protein 14–3–3 to phosphorylated H3, which allows transcription elongation of hypertrophy genes by RNA polymerase II (RNAPII). These results reveal a novel function of CaMKIIδ in regulating H3 phosphorylation in response to haemodynamic stress, and suggest a new epigenetic mechanism for the control of growth mechanisms in the diseased heart.
Materials and methods
Detailed methods are provided in Supplementary materials and methods (see supplementary material).
Human tissue dissection, histology and immunofluorescence
All human and animal studies were approved by the institutional review board at King Faisal Specialist Hospital and Research Centre (KFSHRC) and at San Diego State University. Written informed consent was received by each participant prior to inclusion in the research study. Human hearts were obtained from KFSHRC from patients with end-stage heart failure and from donors who died of accidental death (control hearts). Samples from the same region of the left ventricle were dissected from the normal and cardiomyopathic hearts. Frozen and paraffin-embedded cardiac sections were prepared for histology in the Pathology Department of KFSHRC. For indirect immunofluorescence, frozen human heart sections were incubated with the indicated antibodies for 2 h at room temperature and, after washing, sections were incubated with secondary antibodies. Mouse heart sections were subjected to sequential double immunostaining when indicated, using GATA-4 and p-H3S10 antibodies, as described in Supplementary materials and methods (see supplementary material). Primary neonatal rat cardiomyocytes were incubated with primary and secondary antibodies, using standard methods and as reported previously [25].
Mice, thoracic aortic constriction and echocardiography
CaMKIIδ knockout mice (CaMKIIδ-KO) were obtained from Dr Eric Olson [20]. Littermate 8–12 week-old male mice were subjected to thoracic aortic constriction (TAC) or sham operation, as described previously [20]. Left ventricular dimensions and cardiac function were measured at baseline and after sham operation or TAC surgery in mice sedated with 1–2% isofluorane, using a Vivid E9 high-resolution ultrasound system (GE).
Chromatin immunoprecipitation (ChIP) and qPCR
ChIP and quatitative PCR (qPCR) assays were performed from primary neonatal rat cardiomyocytes and from mouse and human heart tissue, as described [25], with some modifications. Briefly, ChIP was performed with the indicated antibodies from approximately 1 × 106 cells or 6 mg tissue after chromatin had been sheared to an average length of 600 bp. The immunoprecipitated DNA was analysed by qPCR with primers specific for the promoter regions of ANP, β-MHC, GATA-4, GAPDH and actin.
In vitro transcription
In vitro transcription and gel shift assays were performed as described previously [25]. In summary, wild-type or mutant H3 S10A were reconstituted as octamers with the other core histones and were incubated with DNA templates carrying Mef2-responsive elements, CaMKIIδB, 14–3–3 and HeLa nuclear extracts, in the presence of γ32P-ATP. Transcription was measured by the formation of RNA corresponding to the transcribed Mef2.
Transfection of cardiomyocytes
Mutant H3 S10A was produced using the GENEART site-directed mutagenesis system (Invitrogen), as described previously [25]. Primary neonatal rat cardiomyocytes isolated using the Worthington Neonatal CardioMyocytes System (Worthington, USA) were co-transfected with a GFP-tagged plasmid and siControl (siCt) or siRNA against 14–3–3 (si14–3–3) from Dharmacon (Thermo Scientific, USA) using lipofectamine (Invitrogen). 24 h post-transfection, total cell lysates were prepared and analysed by western blotting or immunofluorescence.
Statistical analysis
Data are presented as mean ± standard deviation (SD) or standard error of the mean (SEM). Differences between groups were calculated by unpaired two-tailed Student's t-test; p < 0.05 was considered significant.
Results
Increased H3 phosphorylation in human failing hearts
To assess H3 phosphorylation in the adult heart, we obtained samples from the left ventricle of control donor hearts and of patients in end-stage heart failure at the time of cardiac transplantation. Echocardiographic data showed cardiac dilatation and severely compromised cardiac function in patients with end-stage heart failure, with ejection fraction and fractional shortening averaging 16% and 9%, respectively (see supplementary material, Table S1, Figure S1A). Trichrome staining confirmed significant fibrosis in human failing hearts, whereas the hearts of normal individuals displayed almost no signs of pathology (see supplementary material, Figure S1B). Immunoblot analysis revealed a strong increase in p-H3 S10 in dilated human hearts compared to control hearts, which was paralleled by increased expression of CaMKIIδ (Figure 1A, B). As expected, heart failure markers, such as ANP and β-MHC, were up-regulated in failing hearts, whereas total H3 and GAPDH expression remained unchanged (Figure 1A, B). In addition, expression of GATA-4 and c-Myc, which are known to drive cardiac hypertrophy [26], were increased in human failing hearts (Figure 1A–J). Enhanced p-H3 S10 was also detected by indirect immunofluorescence (see supplementary material, Figure S2). Together, these results show a robust increase in p-H3S10 in end-stage human heart failure, associated with increased expression of hypertrophic gene markers as well as transcriptional activators driving cardiac hypertrophy.
Figure 1.
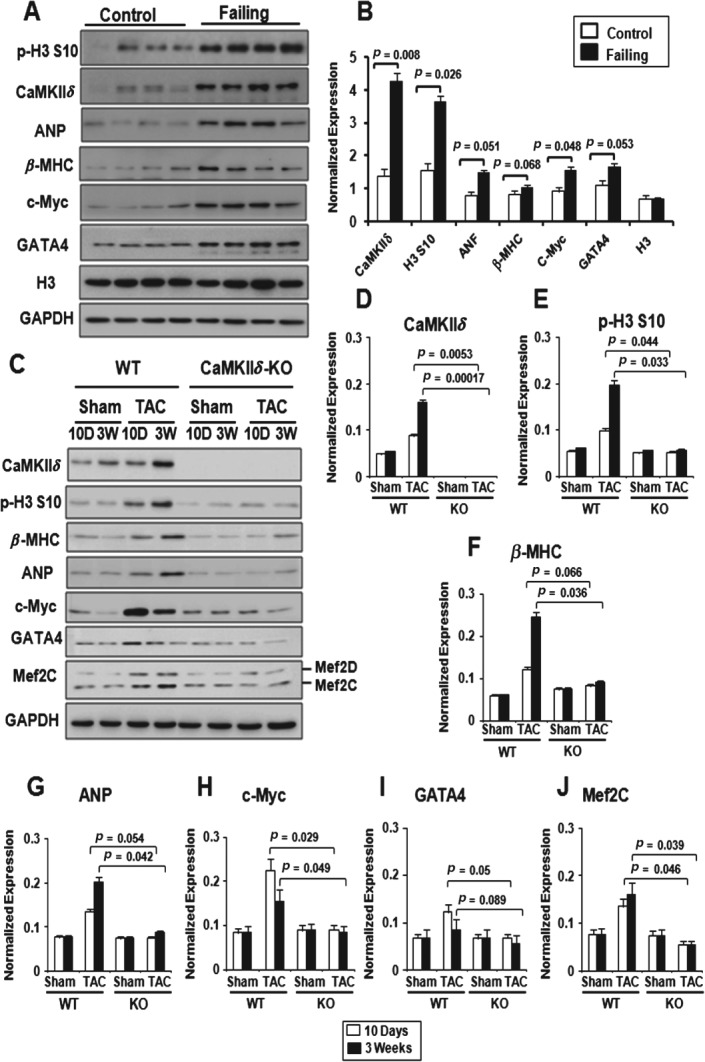
Increased p-H3 S10 in end-stage human heart failure and in rodent heart after pressure overload. (A) Immunoblot of control donor hearts (n = 4) and hearts of patients with end-stage heart failure (n = 4), showing increased p-H3 S10 in dilated human hearts. Consistent with the dilation phenotype, expression of CaMKIIδ, ANP, β-MHC, c-Myc and GATA-4 increased in failing hearts, whereas GAPDH and total H3 expression remained similar. (B) Quantitative analysis of (A); results are corrected for GAPDH and expressed as average ± SD; Student's t-test values are indicated. (C) Representative immunoblots, showing a progressive increase in p-H3 S10 after 10 days and 3 weeks of TAC in CaMKIIδ-WT hearts but not in CaMKIIδ-KO hearts. The hypertrophy marker proteins ANP, β-MHC c-Myc, GATA-4 and Mef2 are induced to different degrees in CaMKIIδ-WT mice subjected to TAC, but not in CaMKIIδ-KO mice after TAC. (D–J) Quantitative analysis of (C), performed in CaMKIIδ-WT and CaMKIIδ-KO mice after sham operation (n = 3) or TAC (n = 3). Values indicate expression of the indicated proteins ± SD, corrected for GAPDH. p values from Student's t-test are indicated
Next, we assessed H3–CaMKIIδ interaction in the diseased human myocardium by immunoprecipitation. Binding of CaMKIIδ to p-H3 S10 was detected in the human myocardium, whereas control IgG gave a lower background signal (see supplementary material, Figure S3). Thus, specific interaction between CaMKIIδ and p-H3 S10 occurs in the human heart.
Lack of H3 hyperphosphorylation in CaMKIIδ knockout mice after pressure overload
To begin to investigate whether CaMKIIδ regulates H3 phosphorylation in vivo, we measured global p-H3 S10 in mice lacking CaMKIIδ (CaMKIIδ-KO), which are resistant to pressure overload hypertrophy [20]. Western blot analysis showed increased p-H3 S10 after 10 days of TAC and a further increase 3 weeks post-TAC. Strikingly, p-H3 S10 remained low 10 days and 3 weeks post-TAC in CaMKIIδ-KO mice (Figure 1C, E). In agreement with their published phenotypes [20], CaMKIIδ-KO mice were protected against pressure overload hypertrophy and pathological cardiac remodelling, as shown by a significant decrease in the heart weight:body weight ratio, interventriculum thickness and reduced fibrosis compared to wild-type mice subjected to TAC (see supplementary material, Figure S4). Consistent with these changes, pressure overload increased the expression of ANP, β-MHC, c-Myc, GATA-4 and Mef2 in wild-type but not CaMKIIδ-KO mice (Figure 1C–J). These results suggest that CaMKIIδ regulates H3 phosphorylation in response to pressure overload.
Increased p-H3 S10 in ventricular myocytes and non-cardiac cells after pressure overload hypertrophy
P-H3 S10 is a mitotic marker commonly used to assess cellular proliferation [5,27]. Since CaMKIIδ is a critical regulator of cardiac hypertrophy [20], the observation that p-H3 S10 increases in response to pressure overload in wild-type but not CaMKIIδ-KO mice suggests that p-H3 S10 may regulate not only cellular proliferation but also cellular hypertrophy. To test this hypothesis, we assessed p-H3 S10 in ventricular myocytes and in non-cardiac cells in heart sections of control donor hearts and failing human hearts. Double immunofluorescence using p-H3 S10 and α-actinin antibodies showed almost no p-H3 S10 signal in control hearts. In contrast, strong p-H3 S10 was detected in both ventricular myocytes and non-myocytes in human dilated hearts (Figure 2A). Quantitative analysis revealed < 5% of p-H3 S10-positive cells in control hearts, with about half of the cells being myocytes and the other half being non-cardiac cells. In contrast, 85% of the cells stained positive for p-H3 S10 in failing human hearts. Among these, 27% were ventricular myocytes and 73% were non-myocyte cells (Figure 2B). Thus, H3 phosphorylation increases in both ventricular myocytes and non-cardiac cells in human failing hearts. To establish the functional link between H3 S10 phosphorylation and cardiac hypertrophy development, we transfected primary neonatal cardiomyocytes with wild-type-H3 or mutant H3S10A expression vectors and measured the effect on cell size. Double immunofluoroscence with α-actinin was performed to distinguish myocytes from non-cardiac cells, and revealed a significant reduction of myocyte surface area in cells expressing mutant H3 S10A compared to cells transfected with H3 (see supplementary material, Figure S5). These data indicate that H3 S10 phosphorylation plays a role in cellular hypertrophy development.
Figure 2.
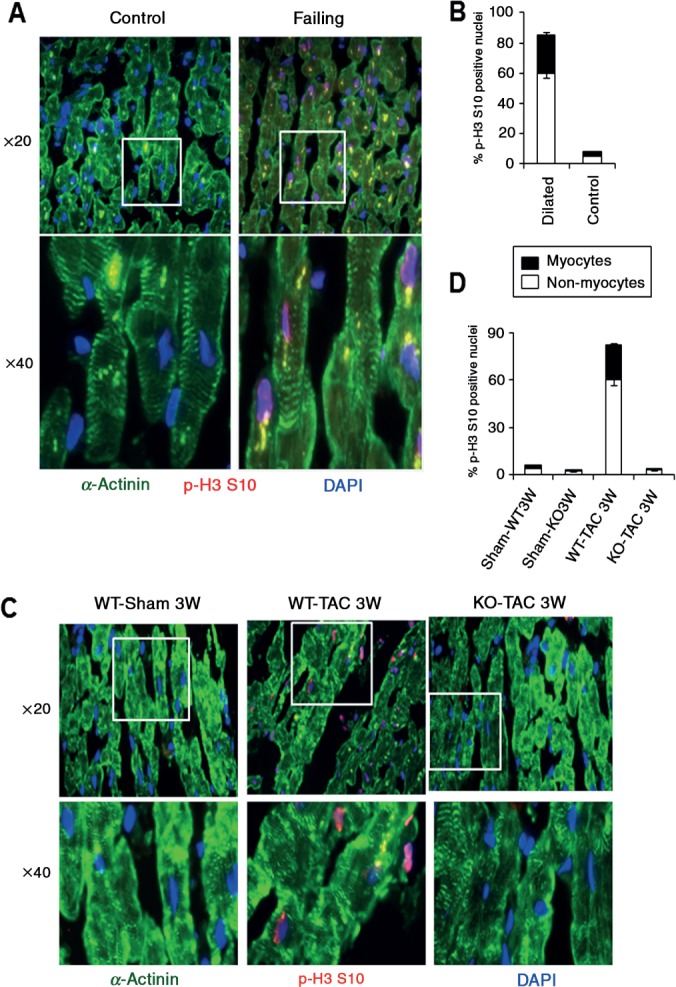
Increased p-H3 S10 in human and rodent ventricular myocytes after pressure overload hypertrophy. (A) Double immunostaining was performed with p-H3 S10 (red) and α-actinin (green) antibody to visualize p-H3 S10 signals in ventricular myocytes, by fluorescence microscopy using a Nikon Eclipse Ti-E microscope with a × 20 or × 40 objective. (B) Quantitative analysis performed in 20 fields selected randomly, showing that ∼30% of ventricular myocytes are positive for p-H3 S10 in dilated human hearts, whereas only ∼8% of myocytes are positive for p-H3 S10 in normal human hearts. (C) Reduced p-H3 S10 in ventricular myocytes of CaMKIIδ-KO mice subjected to TAC compared to wild-type mice subjected to 3 weeks of TAC. (D) Quantitative analysis of (C), performed in 20 fields selected randomly
Next, we investigated whether H3 phosphorylation is an early event in pathological cardiac remodelling. Sequential staining with GATA-4 and p-H3 antibodies revealed no detectable p-H3 S10 in sham-operated hearts, whereas approximately 30% of p-H3 S10 signal was detected in ventricular myocytes at 7 days, 4 weeks and 10 weeks post-TAC (see supplementary material, Figure S6A–E). Remarkably, p-H3 S10 was almost undetectable in both cell types in CaMKIIδ-KO mice subjected to 3 weeks of TAC (Figure 2C, D). Together, these data indicate that CaMKIIδ regulates H3 phosphorylation in a significant number of differentiated adult ventricular myocytes during cardiac hypertrohy.
Loss of CaMKIIδ prevents H3 hyperphosphorylation at fetal cardiac genes
Cardiac hypertrophy is characterized by transcriptional reprogramming of genes normally expressed during embryonic development, such as ANP and β-MHC. To investigate whether H3 phosphorylation plays a role in cardiac hypertrophy, we assessed H3 phosphorylation and CaMKIIδ recruitment at regulatory regions of ANP and β-MHC in control and failing human hearts, using chromatin immunoprecipitation (ChIP) followed by qPCR. Increased p-H3 S10 and enhanced recruitment of CaMKIIδ were observed at both the ANP and β-MHC promoters in failing hearts compared to control hearts. P-H3 S10 and binding of CaMKIIδ remained similar at the α-actin and GAPDH genes, which are not regulated during cardiac hypertrophy (Figure 3A). Furthermore, CaMKIIδ expression and p-H3 S10 significantly correlated with ANP and β-MHC expression (Figure 3B; p < 0.05 for each).
Figure 3.
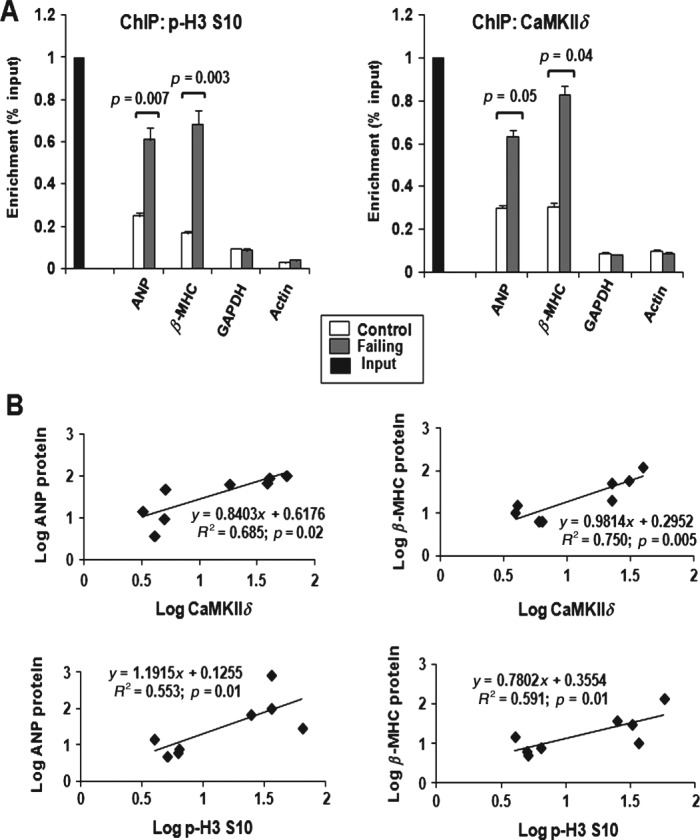
Enhanced p-H3 S10 and CaMKIIδB recruitment at hypertrophic gene loci in end-stage human heart failure. (A) ChIP assay performed with anti-p-H3 S10 or anti-CaMKIIδ antibodies, followed by q-PCR, showing increased p-H3 S10 and CaMKIIδB recruitment at ANF and β-MHC promoters in dilated human hearts compared to control hearts; results are expressed as percentage input over IgG and represent average ± SD (n = 3). (B) Correlations between p-H3 S10 or CaMKIIδ binding and ANP or β-MHC expression in normal and failing human hearts; p values from Student's t-test are indicated
P-H3 S10 progressively increased at the ANP and β-MHC promoters at 10 days and 3 weeks post-TAC in wild-type mice, which paralleled CaMKIIδ recruitment. In contrast, this response was blunted in CaMKIIδ-KO mice subjected to TAC (Figure 4A, B). P-H3 S10 remained similar at the α-actin and GAPDH promoters in wild-type and CaMKIIδ-KO mice after TAC (Figure 4C, D). These data show that H3 phosphorylation fails to increase in response to pressure overload hypertrophy when CaMKIIδ is not expressed in the myocardium. Taken together, these data suggest that p-H3 S10 is important for the re-activation of at least two fetal cardiac genes during cardiac hypertrophy.
Figure 4.
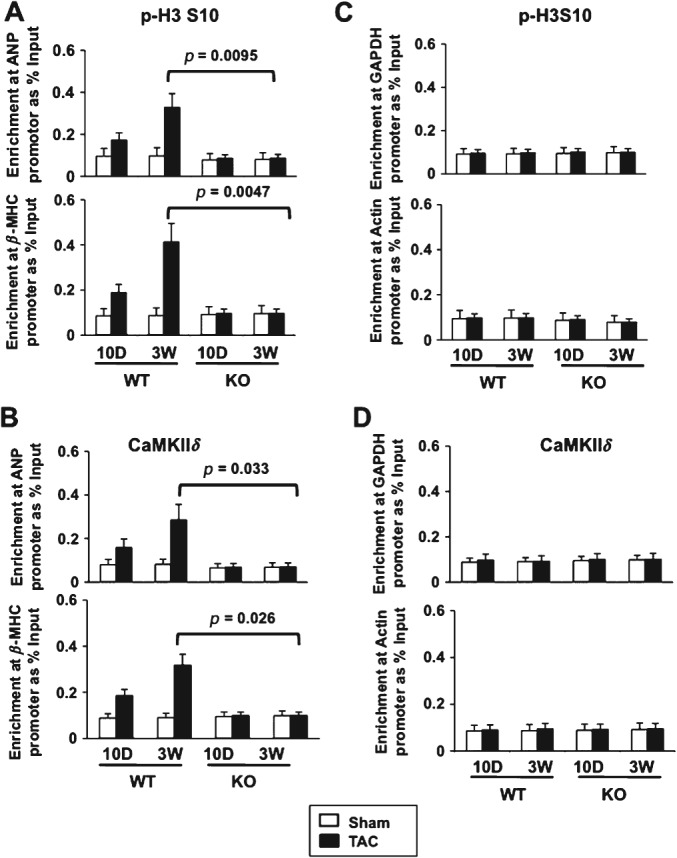
Reduced p-H3 S10 and CaMKIIδB recruitment at hypertrophic gene loci in CaMKIIδ-KO mice after TAC. ChIP–qPCR performed from heart tissue homogenates, showing (A) p-H3 S10 and (B) CaMKIIδ binding at the ANP and β-MHC promoters in WT or CaMKIIδ-KO mice, 10 or 21 days after sham operation or TAC. (C, D) qPCR reactions at GAPDH and α-actin promoters that are not responsive to pressure overload hypertrophy. Results are expressed as percentage input over signals with IgG antibody and represent average ± SD (n = 3). p values from Student's t-test are indicated
Increased binding of 14–3–3 to p-H3 S10 in human failing hearts and enhanced Mef2 transcription in the presence of 14–3–3
14–3–3 is a chaperone protein that binds and is recruited to specific inducible promoters when these are transcriptionally active [28,29]. Based on this, we hypothesized that 14–3–3 may bind phosphorylated H3 at fetal cardiac genes upon hypertrophic stimulation. To test this, we first examined 14–3–3 expression in the normal and failing human heart and saw no significant changes in global expression (Figure 5A). However, increased interaction of 14–3–3 with p-H3 S10 was detected in human failing hearts compared to control hearts (Figure 5B). A gel-shift assay performed with recombinant nucleosomes and purified CaMKIIδB, in the presence or absence of 14–3–3, confirmed that CaMKIIδ and 14–3–3 form a complex in chromatin (see supplementary material, Figure S7A). Binding of 14–3–3 to p-H3 S10 was detected in wild-type mice after 3 weeks of TAC and was reduced in CaMKIIδ-KO TAC animals (Figure 5C). Next we over-expressed wild-type or mutant H3 S10A in cells and assessed 14–3–3/H3 interaction by immunoprecipitation, followed by immunoblotting. Decreased binding of 14–3–3 to mutant H3 S10A was observed, indicating that 14–3–3 preferentially binds to p-H3 S10 (Figure 5D).
Figure 5.
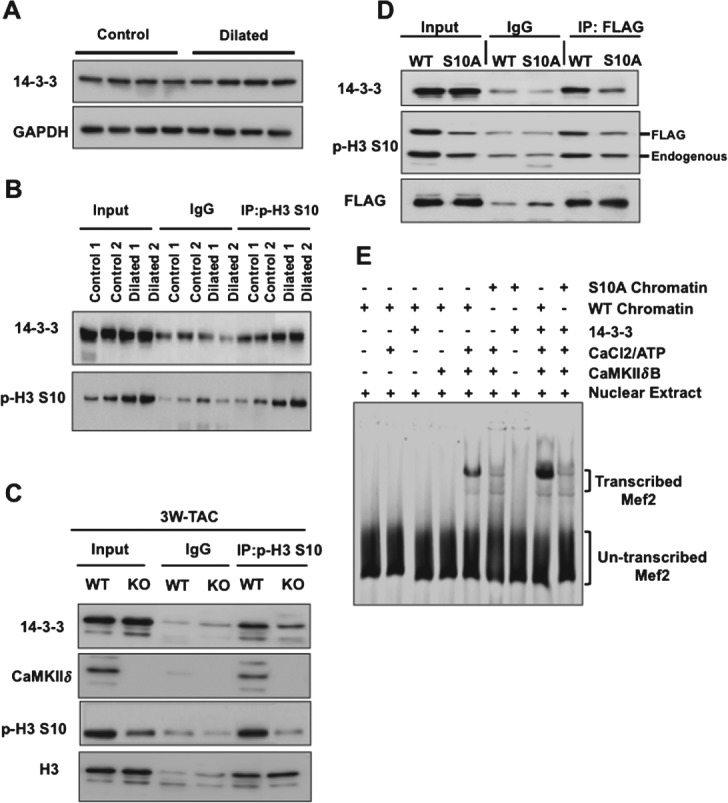
Increased interaction of 14–3–3 with p-H3 S10 after pathological stress and enhanced transcription of Mef2 in the presence of 14–3–3. (A) Immunoblot analysis from control (n = 4) and failing human hearts (n = 4) showing similar expression of 14–3–3. (B) Immunoprecipitation with anti-p-H3 S10 antibody or control IgG followed by immunoblotting showing increased interaction between 14–3–3 and p-H3 S10 in human dilated hearts. (C) Reduced binding between 14–3–3 and p-H3 S10 in CaMKIIδ-KO mice, 3 weeks after TAC. (D) Immunoprecipitation performed with anti-Flag antibody, followed by immunoblotting using the indicated antibodies from Cos-7 cells expressing wild-type or mutant Flag-H3S10A, showing reduced binding of 14–3–3 to mutant p-H3 S10A. (E) In vitro transcription assay from DNA templates harbouring multimerized Mef2-binding sites and chromatin reconstituted with wild-type H3 or mutant H3 S10A [25] after addition of recombinant CaMKIIδB, 14–3–3 and nuclear extracts. CaMKIIδB-mediated chromatin transcription requires p-H3 S10 and 14–3–3 enhances the rate of Mef-2 transcription
Mef2 is a transcription factor present at regulatory regions of many cardiac-specific genes and is a point of convergence of many hypertrophic signalling pathways [15]. We reported that S10 phosphorylation by CaMKIIδ is required for Mef2 to be transcribed from chromatin templates [25]. Thus, we measured the amount of Mef2 transcribed when chromatin is reconstituted with wild-type H3 or mutant H3 S10A in the presence or absence of 14–3–3; the latter increased the rate of Mef2 transcription with wild-type H3 but not with mutant H3 S10A (Figure 5E; see also supplementary material, Figure S7B). All together, these data show that 14–3–3 binds to p-H3 S10 and that this interaction increases Mef2 transcription from chromatin templates.
14–3–3 binding to chromatin is required for transcriptional elongation of fetal cardiac genes
To establish the functional link between H3 phosphorylation, 14–3–3 recruitment and the transcriptional reprogramming of fetal cardiac genes, we assessed 14–3–3 binding at fetal cardiac genes in CaMKIIδ wild-type and KO mice subjected to sham operation or TAC. 14–3–3 binding increased significantly at the ANP and β-MHC promoters and exonic region after stress in wild-type mice, but this effect was significantly reduced in CaMKIIδ-KO mice (Figure 6A). Phosphorylation of RNAPII at serine 2 (p-RNAPII S2) is associated with transcription elongation, which requires 14–3–3 [30]. Therefore, we assessed p-RNAPII S2 at the promoter and exonic region of fetal cardiac genes and of GATA-4 in neonatal rat cardiomyocytes transfected with control siRNA (siCT) or siRNA against 14–3–3 (si14–3–3) after stimulation with the hypertrophic agonist phenylephrine (Figure 6B). As previously reported [30], 14–3–3 knockdown reduced p-RNAPII S2 (Figure 6B). p-RNAPII S2 was relatively low at the promoters of ANP, β-MHC and GATA-4 in both siCtr- and si14–3–3-transfected cells (Figure 6C). p-RNAPII S2 increased at ANP, β-MHC and GATA-4 exonic regions in cells with normal expression of 14–3–3, but not in cells with reduced 14–3–3 levels (Figure 6C). We also observed increased p-RNAPII S2 at ANP and β-MHC exonic regions, while it decreased at the promoter region of both genes in wild-type mice subjected to TAC, but not in CaMKIIδ-KO mice after TAC (Figure 6D, E). Taken together, these results suggest that 14–3–3 binding to phosphorylated H3 is important for the recruitment of RNAPII and elongation of fetal cardiac genes and GATA-4 during cardiac hypertrophy.
Figure 6.
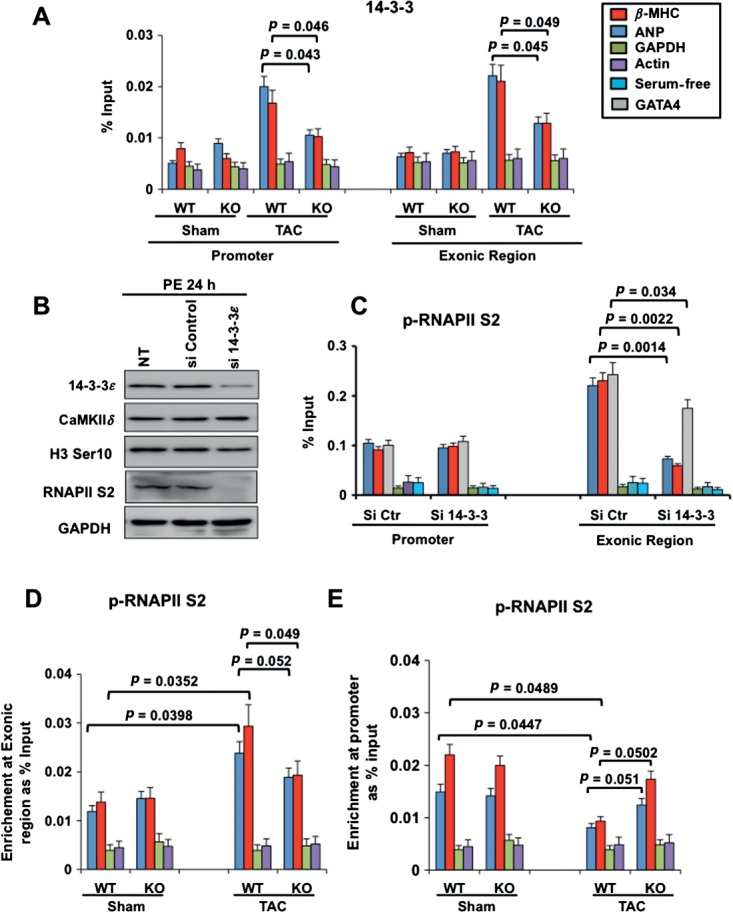
14–3–3 recruitment to chromatin is important for transcriptional elongation of GATA-4 and of fetal cardiac genes. (A) ChIP–qPCR performed from mouse heart tissues showing 14–3–3 binding at the ANP and β-MHC promoters in CaMKIIδ-WT or CaMKIIδ-KO mice 21 days after sham operation or TAC. qPCR reactions were also performed at GAPDH and α-actin promoters that are not responsive to pressure overload hypertrophy: results are expressed as percentage input over signals with IgG antibody and represent average ± SD (n = 3); p values from Student's t test are indicated. (B) Immunoblotting of cardiomyocytes expressing reduced 14–3–3 (si14–3–3) or normal 14–3–3 (siControl). (C) ChIP–qPCR, showing p-RNAPII S2 at the promoter and internal exon of ANP, β-MHC, GATA-4, GAPDH and actin in primary neonatal rat cardiomyocytes with siCt or si14–3–3 and stimulated with phenylephrine (PE) for 24 h. (D) ChIP–qPCR assay from mouse heart chromatin, showing p-RNAPII S2 at the ANP, β-MHC, actin and GAPDH exonic region in CaMKIIδ-WT or CaMKIIδ-KO animals, 21 days after sham operation or TAC. (E) ChIP–qPCR assay, showing p-RNAPII S2 at the ANP, β-MHC, actin and GAPDH promoter regions in CaMKIIδ-WT or CaMKIIδ-KO animals, 21 days after sham operation or TAC: results are expressed as percentage input over signals with IgG antibody, and represent average ± SD (n = 3); p values from Student's t-test are indicated
Discussion
CaMKII enzyme is central to pathological hypertrophic pathways, structural heart disease, arrhythmia and diabetes [19–22,31,32]. We recently reported that in isolated cells, CaMKIIδ interacts with H3 to increase p-H3 S10, and that the S10 site is critical for chromatin-dependent transcription of Mef2 [25]. In the present study, we show that H3 phosphorylation is regulated by CaMKIIδ in vivo, and that this modification controls fetal cardiac gene expression in response to pressure overload. Mechanistically, fetal cardiac genes are activated by binding of 14–3–3 to phosphorylated H3, which facilitates the transcription elongation by RNAPII (Figure 7).
Figure 7.
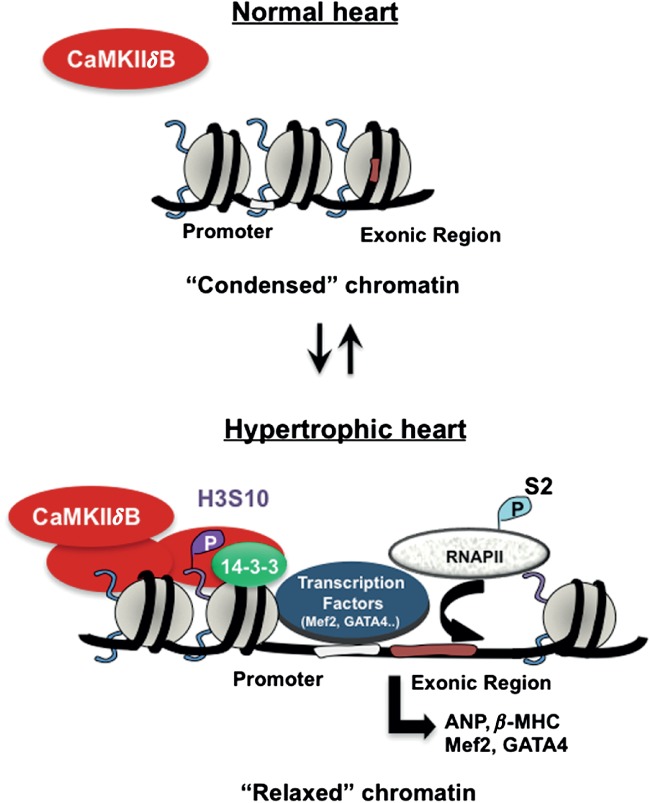
Hypothetical model showing the regulation of H3 phosphorylation by CaMKIIδ in response to haemodynamic stress. Pressure overload hypertrophy increases CaMKIIδ, which is subsequently recruited to chromatin regions to phosphorylate H3 at S10. H3 hyperphosphorylation relaxes chromatin, allowing binding of 14–3–3 and recruitment of transcription factors (ie GATA-4, Mef2) to promote transcriptional elongation of hypertrophic genes by RNAPII
Our study documents that CaMKIIδ controls H3 phosphorylation during the development of cardiac hypertrophy in the animal, and that similar changes occur in end-stage human heart failure. Indeed, we observed a robust phosphorylation of H3 in mouse hearts subjected to pressure overload hypertrophy and in human dilated hearts. H3 phosphorylation appears to be an early event coinciding with up-regulation of c-Myc and GATA-4, which are early drivers of cardiac hypertrophy. These changes occur prior to the decline in cardiac function characteristic of cardiac decompensation and heart failure. Strikingly, the increased H3 phosphorylation and expression of proteins driving early hypertrophic signalling in response to pathological stress do not occur in CaMKIIδ-KO mice. Primary cardiomyocytes expressing mutant H3 S10A that cannot be phosphorylated have reduced size compared to cells expressing wild-type H3. Collectively, these results indicate that CaMKIIδ regulates H3 phosphorylation in the stressed myocardium and that this modification is critical for cellular hypertrophy.
Our data showing that CaMKIIδ deletion protects against cardiac hypertrophy and fibrosis are consistent with results of the Olson laboratory [20]. However, another group showed that CaMKIIδ deficiency protects from dilatation and systolic dysfunction but does not confer antihypertrophic effects [31]. Differences may be due to different gene-targeting strategies; alternatively, the discrepancy may be explained by different surgical procedures or different genetic backgrounds. Thus, it will be interesting to investigate whether loss of H3 phosphorylation occurs in both models of CaMKIIδ deficiency. While global deletion of CaMKIIδ protects against heart diseases [32], nuclear CaMKIIδB exerts cardioprotective effects by increased transcription of heat-shock genes and increased binding of GATA-4 to the Bcl2 promoter [33,34]. Thus, CaMKII splice variants may have antagonistic roles, due to their different subcellular localizations. Alternatively, CaMKII isoforms may be regulated differently in various models of heart disease. In any case, the broad distribution of CaMKII enzyme in various tissues and its implication in a wide range of pathologies suggest that CaMKIIδ signalling to H3 is likely to affect a broad range of transcriptional programmes.
H3S10 is a 'classical' mitotic marker [8]. In heart muscle, H3 phosphorylation occurs in cardiomyocytes during fetal and post-natal life, and in young adults, due to cellular proliferation that still exists at these two developmental stages [11,12]. Recent studies indicate that cardiomyocyte mitosis and proliferation can be induced in the adult myocardium by manipulation of specific pathways [35–38]. In spite of this, the proliferative capacity of the normal adult myocardium is very low. Increased H3 phosphorylation has been reported in the adult heart after pathological stress [13,14,39]. However, the cellular kinases regulating this modification, and whether it exists in different cells populating the myocardium, remain unclear. Since CaMKIIδ knockout mice are resistant to pathological cardiac hypertrophy [20], our observation that H3 phosphorylation fails to increase in CaMKIIδ-KO mice after pressure overload implies that in heart muscle, p-H3 S10 has a function in cellular proliferation but also in cellular hypertrophy. In support of this, H3 phosphorylation is induced not only in non-cardiac cells but also in ventricular myocytes after pressure overload hypertrophy in wild-type mice, but not in CaMKIIδ-deficient mice. Hypertrophic promoters are hyperphosphorylated after pressure overload, and this response coincides with CaMKIIδ recruitment at the very same regions, while H3 phosphorylation remains low at promoter regions of hypertrophic genes in CaMKIIδ-KO mice after haemodynamic stress. Thus, in ventricular myocytes, H3 phosphorylation likely regulates a subset of genes, those implicated in early hypertrophic signalling, such as c-Myc and GATA-4, and also fetal cardiac genes. Because H3 phosphorylation is totally blunted in CaMKIIδ-KO mice, our data also suggest that H3 phosphorylation by CaMKIIδ may regulate cardiac fibroblast proliferation. Future assessment of fibroblast turnover and collagen synthesis should address this possibility. The finding that H3 phosphorylation plays a role in cellular hypertrophy, although initially surprising, is perhaps not so unexpected. Many common mechanisms are shared between the fetal and the diseased heart. For instance, the chromatin remodeller Brg1 regulates cardiomyocyte proliferation in the embryonic heart and hypertrophy in the adult myocardium [18] by assembly of different proteins and chromatin remodellers during cardiac development and in disease. Experiments are ongoing to attempt to identify mega-protein complexes formed in chromatin in response to pathological cardiac stress.
Regarding the switch of MHC isoforms during cardiac hypertrophy, increased expression of β-MHC in a small pool of cardiomyocytes located in the base of the heart near large coronary arteries and towards the right ventricle was recently reported to confer resistance to cardiac hypertrophy [40]. Since our human tissue samples were systematically collected from the lower left ventricle, the increase in β-MHC in human cardiomyopathic hearts is likely not associated with cardioprotective effects. Assessment of β-MHC and α-MHC together with phosphorylated H3 in different regions of the murine myocardium should reveal whether β-MHC and H3 are co-expressed in the same cell, and mark cardiomyocytes that are resistant or sensitive to pressure overload hypertrophy. In either case, the in vivo regulation of H3 phosphorylation by CaMKIIδ reveals a new function of CaMKIIδ in chromatin modification.
14–3–3 proteins are scaffolding proteins that interact with chromatin-modifying proteins and with H3 to regulate a variety of processes, such as transcription [28,29]. On this basis, we hypothesized that 14–3–3 may bind to p-H3 S10 in the adult heart as an underlying mechanism for hypertrophic gene activation. Our mouse and human data show increased interaction of 14–3–3 with phosphorylated H3 after pressure overload hypertrophy. Reduced interaction is observed in CaMKIIδ-deficient mice and in cells where S10 in H3 is mutated. Functionally, interaction of 14–3–3 with p-H3 S10 is important for Mef2-dependent transcription of hypertrophic targets. These results indicate that 14–3–3–H3 interaction specializes in the transcription of hypertrophic genes. To further establish this, we assessed p-RNAPII S2, which is required for RNAPII release from proximal promoters and for transcription elongation [41]. RNAPII S2 level was higher at exonic regions of ANP, β-MHC and GATA-4 compared to their promoter region and reducing 14–3–3 decreased RNAPII S2 levels. After pressure overload, p-RNAPII S2 increased at ANP, β-MHC and GATA-4 exonic regions in wild-type mice, but not in CaMKIIδ-KO mice, indicating that productive elongation is impaired in mice deficient for CaMKIIδ. Thus, 14–3–3 localizes with actively transcribed hypertrophic genes, allowing RNAPII transcription elongation, and this localization is dependent on phosphorylated H3.
CaMKIIδ regulates cardiac hypertrophy by acetylation of histones. This mechanism occurs after CaMKIIδ selectively phosphorylates HDAC4, which becomes exported outside the nucleus with other class II HDACs and can no longer repress Mef2 [23,24]. However, the absence of a cardiac phenotype in HDAC4 knockout mice suggests the possibility of another layer of regulation [42]. Our results suggest that CaMKIIδ signalling to H3 represents an additional regulatory mechanism. Since the 'histone code' hypothesis predicts a crosstalk between the various histone markers, and phosphorylation of H3 is known to influence H3 acetylation [43,44], it is likely that both markers co-exist and are implicated in cardiac hypertrophy development. Also, HDAC4 has recently been shown to control H3 methylation during pathological cardiac stress, possibly through a CaMKIIδ-dependent mechanism [45]. This raises the exciting possibility that H3 phosphorylation may be an early signal regulating H3 acetylation and methylation status. The bigger question, of course, relates to the sequence of these events and how they occur chronologically to alter specific transcriptional programmes.
In summary, our data show that chromatin regulation by CaMKIIδ-mediated H3 phosphorylation is an important mechanism for fetal cardiac gene activation during cardiac hypertrophy. With the recent development of large-scale epigenetic studies, assessment of H3 phosphorylation and binding of CaMKIIδ across the genome, using ChIP-seq, should provide a deeper understanding of genome-wide functional consequences of H3 modification by CaMKIIδ. The future identification of protein complexes formed in chromatin and other H3 post-translational modifications in the normal and diseased myocardium should also help to understand how growth mechanisms are regulated in the normal and diseased heart. This may help identify new points of control at the level of chromatin to develop novel strategies for the treatment of major cardiac diseases.
Acknowledgments
CP thanks all the members of her laboratory for their hard work. We thank Haytham El Zein, Ahmed Abusaleem, Khalid Al-Khatib and other members of the Heart Transplant Team at KFSHRC for coordinating the transfer of the explanted hearts to the Cardiovascular Research Programme. We also thank the Comparative Medicine Department staff for their help in various aspects of this project. We thank Eric Olson for generously providing CaMKIIδ knockout mice. This study was supported by King Abdulaziz City for Science and Technology (Grant Nos KACST 10-BIO 1350–20 and 13-MED456-20, to CP).
Author contributions
SA and CP designed the experiments; SA, KMAA-H, PQ, MK, NA-Y, SFM, AA and WA-H performed the experiments; QM and FS performed experiments and statistical analysis; FA-D, GS, DB and MS contributed reagents; and SA and CP wrote the paper.
Supplementary Material on the Internet
The following supplementary material may be found in the online version of this article:
Supplementary materials and methods
Representative echocardiography and Masson's trichrome staining
Increased H3 S10 in failing human hearts
Interaction of CaMKIIδ and phosphorylated H3 in dilated human heart
Reduced hypertrophy in CaMKIIδ-KO mice after TAC
Reduced average cell surface in H3S10A-transfected cardiomyocytes
Time-course analysis of p-H3 S10 in mouse heart
Gel-shift and in vitro transcription data for 14–3–3
Demographic and echocardiography data of controls and patients
References
- 1.Kornberg RD, Lorch Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell. 1999;98:285–294. doi: 10.1016/s0092-8674(00)81958-3. [DOI] [PubMed] [Google Scholar]
- 2.Zentner GE, Henikoff S. Regulation of nucleosome dynamics by histone modifications. Nat Struct Mol Biol. 2013;20:259–266. doi: 10.1038/nsmb.2470. [DOI] [PubMed] [Google Scholar]
- 3.Chang CP, Bruneau BG. Epigenetics and cardiovascular development. Annu Rev Physiol. 2012;74:41–68. doi: 10.1146/annurev-physiol-020911-153242. [DOI] [PubMed] [Google Scholar]
- 4.Mahmoud SA, Poizat C. Epigenetics and chromatin remodeling in adult cardiomyopathy. J Pathol. 2013;231:147–157. doi: 10.1002/path.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung P, Allis CD, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell. 2000;103:263–271. doi: 10.1016/s0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 6.Bode AM, Dong Z. Inducible covalent posttranslational modification of histone H3. Sci STKE. 2005;2005 doi: 10.1126/stke.2812005re4. : re4. [DOI] [PubMed] [Google Scholar]
- 7.Mahadevan LC, Willis AC, Barratt MJ. Rapid histone H3 phosphorylation in response to growth factors, phorbol esters, okadaic acid, and protein synthesis inhibitors. Cell. 1991;65:775–783. doi: 10.1016/0092-8674(91)90385-c. [DOI] [PubMed] [Google Scholar]
- 8.Thomson S, Clayton AL, Hazzalin CA, et al. The nucleosomal response associated with immediate-early gene induction is mediated via alternative MAP kinase cascades: MSK1 as a potential histone H3/HMG-14 kinase. EMBO J. 1999;18:4779–4793. doi: 10.1093/emboj/18.17.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sassone-Corsi P, Mizzen CA, Cheung P, et al. Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3. Science. 1999;285:886–891. doi: 10.1126/science.285.5429.886. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto Y, Verma UN, Prajapati S, et al. Histone H3 phosphorylation by IKKα is critical for cytokine-induced gene expression. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- 11.Porrello ER, Mahmoud AI, Simpson E, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mollova M, Bersell K, Walsh S, et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci USA. 2013;110:1446–1451. doi: 10.1073/pnas.1214608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res. 1998;83:1–14. doi: 10.1161/01.res.83.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Liew CC, Sole MJ. Studies of nuclear proteins in the heart of the cardiomyopathic Syrian hamster – phosphorylation of histones. J Mol Cell Cardiol. 1978;10:847–855. doi: 10.1016/0022-2828(78)90393-0. [DOI] [PubMed] [Google Scholar]
- 15.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 16.Abraham WT, Gilbert EM, Lowes BD, et al. Coordinate changes in myosin heavy chain isoform gene expression are selectively associated with alterations in dilated cardiomyopathy phenotype. Mol Med. 2002;8:750–760. [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer K, Lubo Z. Fetal programming of cardiac function and disease. Reprod Sci. 2007;14:209–216. doi: 10.1177/1933719107302324. [DOI] [PubMed] [Google Scholar]
- 18.Hang CT, Yang J, Han P, et al. Chromatin regulation by Brg1 underlies heart muscle development and disease. Nature. 2010;466:62–67. doi: 10.1038/nature09130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang T, Brown JH. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc Res. 2004;63:476–486. doi: 10.1016/j.cardiores.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Backs J, Backs T, Neef S, et al. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci USA. 2009;106:2342–2347. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swaminathan PD, Purohit A, Hund TJ, et al. Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ Res. 2012;110:1661–1677. doi: 10.1161/CIRCRESAHA.111.243956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erickson JR, Pereira L, Wang L, et al. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–376. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Backs J, Song K, Bezprozvannaya S, et al. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest. 2006;1–12 doi: 10.1172/JCI27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Little GH, Bai Y, Williams T, et al. Nuclear calcium/calmodulin-dependent protein kinase IIδ preferentially transmits signals to histone deacetylase 4 in cardiac cells. J Biol Chem. 2007;282:7219–7231. doi: 10.1074/jbc.M604281200. [DOI] [PubMed] [Google Scholar]
- 25.Awad S, Kunhi M, Little GH, et al. Nuclear CaMKII enhances histone H3 phosphorylation and remodels chromatin during cardiac hypertrophy. Nucleic Acids Res. 2013;41:7656–7672. doi: 10.1093/nar/gkt500. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Izumo S, Nadal-Ginard B, Mahdavi V. Proto-oncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci USA. 1988;85:339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goto H, Tomono Y, Ajiro K, et al. Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J Biol Chem. 1999;274:25543–25549. doi: 10.1074/jbc.274.36.25543. [DOI] [PubMed] [Google Scholar]
- 28.Macdonald N, Welburn JP, Noble ME, et al. Molecular basis for the recognition of phosphorylated and phosphoacetylated histone h3 by 14–3–3. Mol Cell. 2005;20:199–211. doi: 10.1016/j.molcel.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 29.Walter W, Clynes D, Tang Y, et al. 14–3–3 interaction with histone H3 involves a dual modification pattern of phosphoacetylation. Mol Cell Biol. 2008;28:2840–2849. doi: 10.1128/MCB.01457-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karam CS, Kellner WA, Takenaka N, et al. 14–3–3 mediates histone cross-talk during transcription elongation in Drosophila. PLoS Genet. 2010;6:e1000975. doi: 10.1371/journal.pgen.1000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling H, Zhang T, Pereira L, et al. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Little GH, Saw A, Bai Y, et al. Critical role of nuclear calcium/calmodulin-dependent protein kinase IIδB in cardiomyocyte survival in cardiomyopathy. J Biol Chem. 2009;284:24857–24868. doi: 10.1074/jbc.M109.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng W, Zhang Y, Zheng M, et al. Cardioprotection by CaMKII-δB is mediated by phosphorylation of heat shock factor 1 and subsequent expression of inducible heat shock protein 70. Circ Res. 2010;106:102–110. doi: 10.1161/CIRCRESAHA.109.210914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xin M, Kim Y, Sutherland LB, et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4 doi: 10.1126/scisignal.2002278. : ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Gise A, Lin Z, Schlegelmilch K, et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci USA. 2012;109:2394–2399. doi: 10.1073/pnas.1116136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xin M, Kim Y, Sutherland LB, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci USA. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahmoud AI, Kocabas F, Muralidhar SA, et al. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497:249–253. doi: 10.1038/nature12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nadal-Ginard B, Kajstura J, Leri A, et al. Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res. 2003;92:139–150. doi: 10.1161/01.res.0000053618.86362.df. [DOI] [PubMed] [Google Scholar]
- 40.Lopez JE, Myagmar BE, Swigart PM, et al. β-Myosin heavy chain is induced by pressure overload in a minor subpopulation of smaller mouse cardiac myocytes. Circ Res. 2011;109:629–638. doi: 10.1161/CIRCRESAHA.111.243410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivaldi MS, Karam CS, Corces VG. Phosphorylation of histone H3 at Ser10 facilitates RNA polymerase II release from promoter-proximal pausing in Drosophila. Genes Dev. 2007;21:2818–2831. doi: 10.1101/gad.1604007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vega RB, Matsuda K, Oh J, et al. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119:555–566. doi: 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 43.Baek SH. When signaling kinases meet histones and histone modifiers in the nucleus. Mol Cell. 2011;42:274–284. doi: 10.1016/j.molcel.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 44.Lo WS, Trievel RC, Rojas JR, et al. Phosphorylation of serine 10 in histone H3 is functionally linked in vitro and in vivo to Gcn5-mediated acetylation at lysine 14. Mol Cell. 2000;5:917–926. doi: 10.1016/s1097-2765(00)80257-9. [DOI] [PubMed] [Google Scholar]
- 45.Hohl M, Wagner M, Reil JC, et al. HDAC4 controls histone methylation in response to elevated cardiac load. J Clin Invest. 2013;123:1359–1370. doi: 10.1172/JCI61084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative echocardiography and Masson's trichrome staining
Increased H3 S10 in failing human hearts
Interaction of CaMKIIδ and phosphorylated H3 in dilated human heart
Reduced hypertrophy in CaMKIIδ-KO mice after TAC
Reduced average cell surface in H3S10A-transfected cardiomyocytes
Time-course analysis of p-H3 S10 in mouse heart
Gel-shift and in vitro transcription data for 14–3–3
Demographic and echocardiography data of controls and patients


