Abstract
Objective
Rheumatoid arthritis (RA) is associated with increased risk of cardiovascular disease (CVD). High urinary albumin excretion is a risk factor for CVD in the general population, but its role in atherosclerosis in patients with RA is not well defined.
Methods
We determined the urine albumin to creatinine ratio (UACR) in 136 patients with RA and 79 controls. Individuals with diabetes or a clinical history of CVD were excluded. We measured coronary artery calcium (CAC) with electron beam computer tomography and augmentation index (AIX) using pulse wave analysis. In patients with RA, erythrocyte sedimentation rate (ESR) and concentrations of vascular cell adhesion protein-1 (VCAM-1), interleukin-10 (IL-10), C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and cystatin-C were measured and results correlated with UACR.
Results
Patients with RA had higher UACR [median (IQR): 7.6 (4.0-15.5) mg/g than control subjects: 5.6 (3.3-9.0)mg/g, p=0.02]. The presence of CAC was not associated with UACR in RA or control subjects. In patients with RA, UACR was significantly correlated with AIX (rho=0.24, p=0.01), higher levels of VCAM-1 (rho=0.2, p=0.01) and lower levels of IL-10 (rho=-0.2, p=0.02). The association between AIX and higher UACR remained significant in multivariate analysis [β coefficient of 1.9 (95% CI 0.4-3.4), p=0.01 that adjusted for age, sex, and race].
Conclusion
Urinary albumin excretion was higher in RA patients than controls and correlated with increased arterial stiffness, higher VCAM-1, and lower IL-10 concentrations.
Keywords: rheumatoid arthritis, microalbuminuria, arterial stiffness, atherosclerosis
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that is associated with an increased risk for cardiovascular disease (CVD); this increase in risk is not explained by traditional CVD risk factors.(1;2) Therefore, there is a need to identify additional markers of early CVD in this patient population. Urinary albumin excretion is a well-studied marker of CV risk in the general population. The normal rate of albumin excretion is less than 30 mg/day,(3) and albuminuria is defined as excretion of ≥ 30 mg/day.(4) Moderate albuminuria (previously known as microalbuminuria) is currently defined as urinary albumin excretion between 30 to 299 mg/day and appears to be a sign of renal endothelium dysfunction.(5) Macroalbuminuria is defined as urinary albumin excretion of ≥ 300 mg/day. There is a dose-response relationship between urinary albumin excretion and CV risk, with a 30% increase in CV deaths for every 2-fold increase in urinary albumin excretion. Interestingly, this increased risk was observed at concentrations far below the clinically accepted cut-off point of 300 mg/L for macroalbuminuria.(6)
A report, using data from the National Health and Nutrition Examination Survey-3 (NHANES3), estimated that the prevalence of albuminuria in the general population was 7.8%.(7) Albuminuria appears to be frequent in RA; a study in a contemporary cohort of patients with RA, who did not receive treatment with either penicillamine or gold, estimated that 11.9% patients had microalbuminuria.(8) However, there is little information about the relationship between albuminuria and pre-clinical CV disease in patients with RA. Therefore, we hypothesized that urinary albumin excretion may not only be higher in patients with RA but also be a marker of inflammation and increased asymptomatic CV disease.
Materials and Methods
Study Population
In a cross-sectional study, we evaluated 136 patients with RA and 79 control subjects without inflammatory disease, who are enrolled in ongoing studies of CV disease and risk factors. Details of the recruitment procedures and study methods have been previously described.(9) In summary, eligible patients with RA met the 1987 ACR classification criteria for RA, (10) were older than 18 years of age, and had disease duration of over 1 year. Control subjects did not meet the criteria for any autoimmune disease, and were frequency-matched to patients for age, sex and race. Patients were recruited from local rheumatology clinics in Nashville, TN. For this study, patients with RA and controls with a clinical diagnosis of coronary artery disease or diabetes mellitus were excluded. All participating subjects provided a written informed consent prior to enrollment. The study was approved by the Institutional Review Board at Vanderbilt University.
Clinical Variables
Patient assessment included a detailed review of medical records, a standardized interview, physical examination, and laboratory testing. We collected information about demographics, CVD risk factors, disease duration and activity for patients, medications and smoking history. The presence of hypertension was defined by the use of antihypertensive medications, or a systolic blood pressure of ≥140mmHg or a diastolic blood pressure of ≥90mmHg. The average of 2 blood pressure measurements obtained 5 minutes apart was used. Height and weight were measured and body mass index (BMI) calculated. A fasting blood sample was obtained for laboratory testing for high-density lipoprotein (HDL) and low- density lipoprotein (LDL) cholesterol, triglycerides, glucose and insulin, creatinine (Cr). The glomerular filtration rate was estimated using the Modification of Diet in Renal disease (MDRD) formula.(11) Insulin sensitivity was determined using the homeostasis model assessment of insulin resistance (HOMA) index, defined as: fasting glucose (mmoles/liter) × fasting insulin (μU/ml/22.5) and patients were classified as having metabolic syndrome or not using the modified World Health Organization (WHO) definition, which requires the presence of insulin resistance and two of the following three criteria: central obesity, dyslipidemia, and hypertension.(12) We calculated the Framingham risk score (13) as an index of CV risk prediction.
Inflammatory markers and urinary albumin excretion
In patients with RA, serum concentrations of vascular cell adhesion protein-1 (VCAM-1), interleukin-10 (IL-10), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α)were measured using a multiplex platform (Lincoplex, Inc.). Serum cystatin-C concentrations were measured by ELISA. C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were measured by the Vanderbilt University Medical Center Clinical Laboratory. Prior to 2003, this laboratory reported low concentrations as <3 mg/dl; therefore, in patients with CRP<3 mg/dl, CRP concentrations were re-measured by ELISA (Millipore Corp).
Early morning urine samples were also collected. Urine was stored at -80°C. The kinetic alkaline picrate method was used to measure urine albumin concentrations and the turbidimetric immunoassay to quantify urine creatinine concentrations. Their coefficients of variation were 3.1% and 2.6%, respectively. Tests were done at the Vanderbilt University Medical Center Clinical Laboratory. To standardize the results of urine microalbumin measurements, the urine albumin to creatinine ratio (UACR) was calculated.
The primary analyses were based on results of UACR as a continuous variable, but planned secondary analyses included data categorized by presence or absence of albuminuria, defined as a UACR ratio of ≥30mg/g.(14)
Coronary Artery Calcification
As previously described,(9) patients and control subjects underwent imaging with chest tomography using an Imatron C-150 scanner (GE/Imatron, South San Francisco, CA, USA). Forty slices of 3 mm thickness and 100ms scanning time were obtained during a single breath-holding period from the aortic arch to the level of the diaphragm. The presence of CAC was determined by a single expert investigator (PR), who was blinded to the subjects' clinical status.
Augmentation Index
Augmentation index, a measure of vascular stiffness, was determined using non-invasive applanation tonometry and pulse wave analysis (AtCor Medical, Sydney, Australia), as described before.(15) AIX values were normalized to a heart rate of 75 beats per minute. Results were available in 110 patients.
Statistical Analysis
For continuous variables, data are presented as mean and standard deviation or median with interquartile range, based on the distribution. For categorical variables, data are presented as frequencies and percentages. We compared traditional risk factors, UACR and measures of subclinical CVD between RA and controls using using Fisher's exact test and Wilcoxon rank sum for categorical and continuous variables, respectively. The association of UACR with clinical and laboratory variables was assessed using Spearman's correlation coefficient and Wilcoxon rank sum test, as appropriate. The association between UACR and CAC and AIX was adjusted for age, sex and race using linear or logistic multivariate analyses, as appropriate. These covariates were selected based on their clinical significance while maintaining adequate test power. In patients with RA, we used Fisher's exact test to examine the association of moderate albuminuria (>30 mg/g) with each of the following variables: sex, hypertension, presence of metabolic syndrome, use of NSAIDs, and presence of CAC.
All statistical analyses were conducted using STATA software version 12.1 (StataCorp, College Station, TX). A 2-sided significance level of 5% was used.
Results
Baseline Characteristics
Patients with RA and control subjects had a similar mean age and race and sex distribution (Table 1). There was no significant difference between the two groups in regards to other traditional risk factors for CVD including hypertension, BMI, family history of coronary artery disease, Framingham score or renal function. Fifty-four percent of patients with RA were taking low-doses of prednisone, 74% were taking low-doses of weekly methotrexate, 18% were taking leflunomide, and 21% were receiving an anti-TNF agent.
Table 1. Baseline characteristics of RA patient and matched controls.
| Characteristics | RA | Controls | P value |
|---|---|---|---|
|
| |||
| Age | 52.9 ± 12.1 | 51.6 ± 10.5 | 0.52 |
|
| |||
| Sex n (% female) | 100 (73.5) | 54 (68.4) | 0.44 |
|
| |||
| Race n (% Caucasian) | 120 (88.2) | 67 (84.8) | 0.24 |
|
| |||
| Smoking | |||
| Current n (%) | 36 (26.5) | 7 (8.9) | 0.002 |
| Past n (%) | 60 (44.1) | 28 (35.4) | 0.25 |
|
| |||
| Family history of early CAD n (%) | 38 (27.9) | 21 (26.6) | 0.88 |
|
| |||
| Weight (kg) | 81.8 ± 19.2 | 79.2 ± 17.4 | 0.35 |
|
| |||
| Height (m) | 1.7 ± 0.1 | 1.7 ± 0.1 | 0.75 |
|
| |||
| BMI (kg/m2) | 29.0 ± 6.3 | 28.1 ± 5.5 | 0.37 |
|
| |||
| Waist Circumference (cm) | 93.9 ± 15.5 | 87.7 ± 12.5 | 0.003 |
|
| |||
| Hypertension n (%) | 66 (48.5 | 27 (34.2) | 0.07 |
| Systolic BP (mmHg) | 131.1 ±19.8 | 127.1 ± 16.1 | 0.15 |
| Diastolic BP (mmHg) | 74.2 ± 10.6 | 72.2 ± 9.1 | 0.24 |
|
| |||
| Use of antihypertensives, n (%) | 42 (30.9) | 17 (21.5) | 0.11 |
|
| |||
| Use of angiotensin-converting enzyme inhibitors (ACEi), n (%) | 18 (13.6) | 9 (11.5) | 0.42 |
|
| |||
| Use of angiotensin II receptor blockers (ARBs), n (%) | 7 (5.1) | 3 (3.9) | 0.46 |
|
| |||
| HDL cholesterol (mg/dL) | 47.4 ± 14.1 | 47.2 ± 12.4 | 0.88 |
|
| |||
| LDL cholesterol (mg/dL) | 113.2 ± 32.6 | 123.4 ± 31.8 | 0.02 |
|
| |||
| Triglycerides (mg/dL) | 125.0 ± 68.8 | 111.3 ± 51.5 | 0.20 |
|
| |||
| Total cholesterol (mg/dL) | 185.7 ± 35.4 | 192.9 ± 34.4 | 0.13 |
|
| |||
| Statin use n (%) | 11 (8.1) | 7 (8.9) | 0.82 |
|
| |||
| Serum creatinine, mg/dl | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.66 |
|
| |||
| Glomerular rate filtration, mL/min per 1.73 m2 | 89.6 ± 25.5 | 88.0 ± 17.7 | 0.66 |
|
| |||
| Insulin resistance – HOMA-IR index | 1.9 ± 1.9 | 0.8 ± 0.7 | < 0.001 |
|
| |||
| Metabolic syndrome n (%) | 39 (28.7) | 13 (16.5) | 0.05 |
|
| |||
| Framingham risk score, units | 11.5 ± 6.1 | 10.4 ± 5.4 | 0.21 |
|
| |||
| Coronary artery calcium score (Agatston units) | 0 (0-68.0) | 0 (0-4.8) | 0.03 |
|
| |||
| Augmentation Index*, % | 29.9 ± 9.8 | 25.6 ± 10.2 | 0.01 |
|
| |||
| DAS28 | 3.6 ± 1.6 | N/A | |
Data presented as number (%), mean ± standard deviation, and median (interquartile range)
Augmentation index is normalized to a heart rate of 75 beats per minute
As reported previously in patients from this cohort,(9;15) patients with RA had higher prevalence of CAC (p=0.03) and higher augmentation index (p=0.01) than control subjects. (Table 1)
Urinary albumin excretion in RA and control subjects
Patients with RA had higher urinary excretion of albumin [median (interquartile range) UACR 7.6 (4.0-15.5) mg/g] than control subjects [5.6 (3.3-9.1) mg/g, p = 0.02)]. (Figure 1) None of the study participants had severe chronic kidney disease. Their mean creatinine was 0.8±0.2 mg/dl and their mean glomerular filtration rate (GFR) was 89±23 (range:28-161) mL/min per 1.73 m2. Four study participants (three patients with RA and one control subject) had an estimated GFR <45 mL/min per 1.73 m2. Excluding them from the analysis did not change our results (p=0.02).
Fig. 1. UACR in rheumatoid arthritis patients and controls.
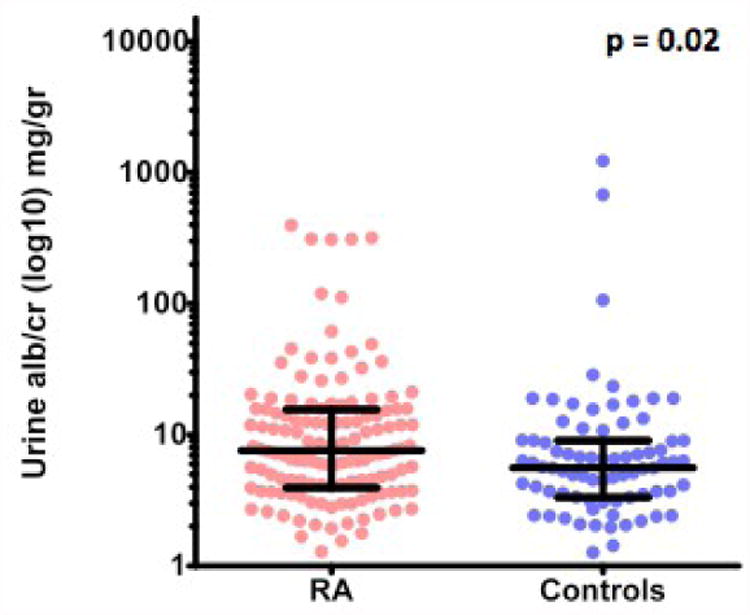
Log10 UACR was presented in a dot plot graph with the median and interquartile range shown in dark lines. The p-value was estimated using Wilcoxon rank-sum test.
The prevalence of albuminuria (UACR >30 mg/g) was also higher in patients with RA (11.8%) than in control subjects (3.8%), p = 0.04.
Traditional CVD Risk Factors and Urinary Albumin Excretion in patients with RA
The association between UACR and traditional CVD risk factors was assessed in patients with RA. No association was observed between UACR and multiple other CVD risk factors including age, gender, BMI, LDL cholesterol, insulin resistance, or Framingham score (Table 2). The association of the presence of albuminuria (UACR > 30 mg/g) and CVD risk factors was also assessed in patients with RA. We found a significant association between albuminuria and HTN (p-value = 0.01), but not with the metabolic syndrome.
Table 2. The association between CVD risk factors and markers of inflammation with UACR in Patients with RA.
| Spearman rho | P-value | |
|---|---|---|
| Cardiovascular risk factors | ||
| Age | 0.16 | 0.07 |
| Cumulative Smoking | -0.11 | 0.19 |
| BMI | -0.11 | 0.21 |
| Waist Circumference | -0.08 | 0.38 |
| Systolic BP | 0.07 | 0.44 |
| Diastolic BP | -0.09 | 0.28 |
| LDL | 0.02 | 0.80 |
| Total cholesterol | 0.04 | 0.63 |
| Creatinine | -0.13 | 0.13 |
| Insulin resistance – HOMA-IR | 0.0 | 0.80 |
| Framingham Risk Score | 0.07 | 0.40 |
| Inflammatory Markers | ||
| ESR | 0.13 | 0.13 |
| CRP | 0.07 | 0.31 |
| IL-6 | -0.02 | 0.87 |
| TNF-α | -0.01 | 0.88 |
| VCAM-1* | 0.22 | 0.01 |
| IL-10* | -0.20 | 0.02 |
| Cystatin-C | -0.03 | 0.72 |
p<0.05
Urinary Albumin Excretion and Inflammatory markers in RA
In patients with RA, we found a significant association between UACR and VCAM-1 with a rho of 0.2 and p-value of 0.01 (Table 2). There was also a significant association between UACR and IL-10 with a rho of - 0.2 and p-value of 0.02. No association was observed with other studied inflammatory markers including ESR, CRP, IL-6, TNF-α, and cystatin-C.
Urinary Albumin Excretion and pre-clinical atherosclerosis in RA
In patients with RA, there was a significant association between log transformed UACR and AIX with a β coefficient of 1.9 (95% C.I., 0.4-3.4) and a p-value of 0.01 (Table 3). This association remained significant after adjusting for age, sex and race (p-value 0.03). Additional multivariate analyses confirmed that our primary finding was robust. First, a model including age, sex, race, hypertension, and current use of hydroxychloroquine (β coef. 1.55, p=0.026). Second, a model where age, sex, race, other traditional CVD risk factors including smoking, HTN, ESR, and disease duration were also adjusted for (β coef. 1.62, p=0.024), showed that the significant association between UACR and AIX in this group remained significant. The association between UACR and RA also remained significant after adjustment for smoking status and abdominal circumference (p=0.038). This association was not observed in controls.
Table 3. The association between UACR and subclinical cardiovascular disease.
| UACR | |||||
|---|---|---|---|---|---|
| Unadjusted | Adjusted for age, sex, and race | ||||
| β coef. (95% CI) | P value | β coef. (95% CI) | P value | ||
| AIX | RA | 1.9 (0.4-3.4) | 0.01 | 1.5 (0.1-2.8) | 0.03 |
| Controls | -1.3 (-3.6-1.0) | 0.26 | -0.9 (-2.8-1.0) | 0.34 | |
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| CAC | RA | 0.9 (0.7-1.2) | 0.64 | 0.7 (0.5-1.1) | 0.13 |
| Control | 0.6 (0.4-1.1) | 0.11 | 0.7 (0.4-1.3) | 0.31 | |
There was also no significant association between UACR and CAC in either group of subjects (data not shown).
Medications and Urinary Albumin Excretion in RA
In patients with RA, UACR was not associated with the use of angiotensin converting enzyme inhibitors (ACEi), angiotensin II receptor blockers (ARBs), steroids, non-steroidal anti-inflammatory drugs (NSAIDs), methotrexate, or anti-TNF. However, there was a trend towards a lower UACR in RA patients taking hydroxychloroquine [median 5.7 (3.0-8.6)] compared to those who were not [9.0 (4.2-15.8) mg/g] p= 0.05].
An exploratory analysis suggests that patients with microalbuminuria (n=16) were older and had higher sedimentation rates than those without microlbuminuria (n=120).
Discussion
There are three main findings of this study. First, patients with RA have higher urinary albumin excretion and a higher prevalence of albuminuria than control subjects. Second, high urinary albumin excretion was associated with arterial stiffness in patients with RA. Third, in patients with RA, urinary albumin excretion correlated positively with VCAM and negatively with IL-10 concentrations.
There are several reasons why patients with RA can develop albuminuria. Initial studies attributed the presence of albuminuria to either side effects of medications (primarily gold and penicillamine), (16) or to glomerular or tubular nephropathies. However, Niederstadt et al. found that albuminuria in RA patients was secondary to either drug therapy or to vasculitis in only 25% of patients.(17) And later, a study in a more contemporary cohort of patients with RA, found that –in the absence of treatment with gold or penicillamine - microalbuminuria was associated with cardiovascular risk factors, including hypertension and insulin resistance.(8) Thus, rather than just being a manifestation of primary renal diseases, albuminuria also emerged as a potential marker of diffuse vascular injury.
Consistent with the notion that albuminuria is a marker of diffuse vascular injury, our results indicate that higher urinary albumin excretion correlates with augmentation index, a marker of arterial stiffness. These results are also consistent with findings in other populations showing that hypertensive patients with increased urinary albumin excretion had significantly increased AIX independent of other risk factors.(18) In patients with type I diabetes mellitus, there was also a significant association between increased albumin excretion rate and higher augmentation pressure, another measure of arterial stiffness.(19) Increased arterial stiffness can occur as a result of functional changes, for example endothelial dysfunction, or because of structural changes, such as atherosclerosis.
Further supporting the connection between albuminuria and endothelial dysfunction, our data showed a significant association between UACR and VCAM-1. These findings are consistent with previous reports indicating that VCAM-1 levels are associated with atherosclerosis, as determined by carotid media thickness and carotid plaque (20) and with endothelial dysfunction and inflammation in patients with RA. (21-23)
VCAM-1 is expressed on the endothelium and serves as a marker of endothelial cell activation and vascular inflammation. It mediates the adhesion of inflammatory cells to the endothelium and their penetration into areas of inflammation and has been implicated in numerous conditions, including atherosclerosis and heart failure.(24) A similar association between microalbuminuria and VCAM-1 was previously observed in patients with type I and type II diabetes.(25;26) On the other hand, we found a significant inverse association between UACR and IL-10, a molecule with anti-inflammatory properties.(27) Additional studies are needed to further investigate the role of IL-10 and the other inflammatory cytokines in atherosclerosis in patients with RA.
In contrast to our findings with AIX, no significant association was observed between UACR or albuminuria with CAC in patients with rheumatoid arthritis. A previous report by Sammut et al. (2011 ACR annual meeting abstract) showed an association between albuminuria and CAC, however, this association lost statistical significance after adjusting for other cardiovascular risk factors.
Our study has a few limitations. Because it is cross-sectional, we cannot establish a temporal sequence. Also, only one measure of albumin-to-creatinine ratio was obtained. Since the urine albumin excretion is highly dependable on multiple factors including exercise within 24 hours, fever, pyuria, and hyperglycemia, the prevalence of microalbuminuria might have been over-estimated. However, we excluded patients with diabetes and no patient reported fever or urinary symptoms.
In summary, urinary albumin concentrations were higher in RA patients than controls. Urine albumin correlated with increased arterial stiffness, higher VCAM-1, and lower IL-10 concentrations providing further evidence to the hypothesis that albuminuria is a marker of atherosclerotic risk.
Acknowledgments
This study was supported by grants (P60AR056116, K23AR064768, and GM5M01-RR00095) from the National Institutes of Health, Grant UL1 RR024975-01, now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06, and the Vanderbilt Physician Scientist Development Award.
References
- 1.Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110(3):420–4. doi: 10.1016/j.amjcard.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung CP, Oeser A, Avalos I, Gebretsadik T, Shintani A, Raggi P, et al. Utility of the Framingham risk score to predict the presence of coronary atherosclerosis in patients with rheumatoid arthritis. Arthritis Res Ther. 2006;8(6):R186. doi: 10.1186/ar2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–30. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 4.Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33(5):1004–10. doi: 10.1016/s0272-6386(99)70442-7. [DOI] [PubMed] [Google Scholar]
- 5.Satchell SC, Tooke JE. What is the mechanism of microalbuminuria in diabetes: a role for the glomerular endothelium? Diabetologia. 2008;51(5):714–25. doi: 10.1007/s00125-008-0961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de ZD, van Veldhuisen DJ, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106(14):1777–82. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 7.Jones CA, Francis ME, Eberhardt MS, Chavers B, Coresh J, Engelgau M, et al. Microalbuminuria in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2002;39(3):445–59. doi: 10.1053/ajkd.2002.31388. [DOI] [PubMed] [Google Scholar]
- 8.Daoussis D, Panoulas VF, John H, Toms TE, Antonopoulos I, Treharne G, et al. Microalbuminuria in rheumatoid arthritis in the post penicillamine/gold era: association with hypertension, but not therapy or inflammation. Clin Rheumatol. 2011;30(4):477–84. doi: 10.1007/s10067-010-1446-y. [DOI] [PubMed] [Google Scholar]
- 9.Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: Relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52(10):3045–53. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 10.Arnett FC, Edworthy SM, Bloch DA, Mc Shane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 11.Botev R, Mallie JP, Couchoud C, Schuck O, Fauvel JP, Wetzels JF, et al. Estimating glomerular filtration rate: Cockcroft-Gault and Modification of Diet in Renal Disease formulas compared to renal inulin clearance. Clin J Am Soc Nephrol. 2009;4(5):899–906. doi: 10.2215/CJN.05371008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reilly MP, Wolfe ML, Rhodes T, Girman C, Mehta N, Rader DJ. Measures of insulin resistance add incremental value to the clinical diagnosis of metabolic syndrome in association with coronary atherosclerosis. Circulation. 2004;110(7):803–9. doi: 10.1161/01.CIR.0000138740.84883.9C. [DOI] [PubMed] [Google Scholar]
- 13.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 14.Standards of medical care in diabetes. Diabetes Care. 2005;28(Suppl 1):S4–S36. [PubMed] [Google Scholar]
- 15.Avalos I, Chung CP, Oeser A, Gebretsadik T, Shintani A, Kurnik D, et al. Increased augmentation index in rheumatoid arthritis and its relationship to coronary artery atherosclerosis. J Rheumatol. 2007;34(12):2388–94. [PubMed] [Google Scholar]
- 16.Pedersen LM, Nordin H, Svensson B, Bliddal H. Microalbuminuria in patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54(3):189–92. doi: 10.1136/ard.54.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niederstadt C, Happ T, Tatsis E, Schnabel A, Steinhoff J. Glomerular and tubular proteinuria as markers of nephropathy in rheumatoid arthritis. Rheumatology (Oxford) 1999;38(1):28–33. doi: 10.1093/rheumatology/38.1.28. [DOI] [PubMed] [Google Scholar]
- 18.Tsioufis C, Tzioumis C, Marinakis N, Toutouzas K, Tousoulis D, Kallikazaros I, et al. Microalbuminuria is closely related to impaired arterial elasticity in untreated patients with essential hypertension. Nephron Clin Pract. 2003;93(3):c106–c111. doi: 10.1159/000069546. [DOI] [PubMed] [Google Scholar]
- 19.Prince CT, Secrest AM, Mackey RH, Arena VC, Kingsley LA, Orchard TJ. Augmentation pressure and subendocardial viability ratio are associated with microalbuminuria and with poor renal function in type 1 diabetes. Diab Vasc Dis Res. 2010;7(3):216–24. doi: 10.1177/1479164110375297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dessein PH, Joffe BI, Singh S. Biomarkers of endothelial dysfunction, cardiovascular risk factors and atherosclerosis in rheumatoid arthritis. Arthritis Res Ther. 2005;7(3):R634–R643. doi: 10.1186/ar1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dessein PH, Joffe BI. Suppression of circulating interleukin-6 concentrations is associated with decreased endothelial activation in rheumatoid arthritis. Clin Exp Rheumatol. 2006;24(2):161–7. [PubMed] [Google Scholar]
- 22.Dessein PH, Solomon A, Woodiwiss AJ, Norton GR, Tsang L, Gonzalez-Gay MA. Marked independent relationship between circulating interleukin-6 concentrations and endothelial activation in rheumatoid arthritis. Mediators Inflamm. 2013;2013:510243. doi: 10.1155/2013/510243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Gay MA, Garcia-Unzueta MT, de Matias JM, Gonzalez-Juanatey C, Garcia-Porrua C, Sanchez-Andrade A, et al. Influence of anti-TNF-alpha infliximab therapy on adhesion molecules associated with atherogenesis in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24(4):373–9. [PubMed] [Google Scholar]
- 24.Ley K, Huo Y. VCAM-1 is critical in atherosclerosis. J Clin Invest. 2001;107(10):1209–10. doi: 10.1172/JCI13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clausen P, Jacobsen P, Rossing K, Jensen JS, Parving HH, Feldt-Rasmussen B. Plasma concentrations of VCAM-1 and ICAM-1 are elevated in patients with Type 1 diabetes mellitus with microalbuminuria and overt nephropathy. Diabet Med. 2000;17(9):644–9. doi: 10.1046/j.1464-5491.2000.00347.x. [DOI] [PubMed] [Google Scholar]
- 26.Bruno CM, Valenti M, Bertino G, Ardiri A, Bruno F, Cunsolo M, et al. Plasma ICAM-1 and VCAM-1 levels in type 2 diabetic patients with and without microalbuminuria. Minerva Med. 2008;99(1):1–5. [PubMed] [Google Scholar]
- 27.Girndt M, Kohler H. Interleukin-10 (IL-10): an update on its relevance for cardiovascular risk. Nephrol Dial Transplant. 2003;18(10):1976–9. doi: 10.1093/ndt/gfg311. [DOI] [PubMed] [Google Scholar]


