Abstract
Purpose
To assess the relationship between radiation dose and change in a set of mathematical intensity- and texture-based features and to determine the ability of texture analysis to identify patients who develop radiation pneumonitis (RP).
Methods and Materials
A total of 106 patients who received radiation therapy (RT) for esophageal cancer were retrospectively identified under institutional review board approval. For each patient, diagnostic computed tomography (CT) scans were acquired before (0–168 days) and after (5–120 days) RT, and a treatment planning CT scan with an associated dose map was obtained. 32- × 32-pixel regions of interest (ROIs) were randomly identified in the lungs of each pre-RT scan. ROIs were subsequently mapped to the post-RT scan and the planning scan dose map by using deformable image registration. The changes in 20 feature values (ΔFV) between pre- and post-RT scan ROIs were calculated. Regression modeling and analysis of variance were used to test the relationships between ΔFV, mean ROI dose, and development of grade ≥2 RP. Area under the receiver operating characteristic curve (AUC) was calculated to determine each feature’s ability to distinguish between patients with and those without RP. A classifier was constructed to determine whether 2- or 3-feature combinations could improve RP distinction.
Results
For all 20 features, a significant ΔFV was observed with increasing radiation dose. Twelve features changed significantly for patients with RP. Individual texture features could discriminate between patients with and those without RP with moderate performance (AUCs from 0.49 to 0.78). Using multiple features in a classifier, AUC increased significantly (0.59–0.84).
Conclusions
A relationship between dose and change in a set of image-based features was observed. For 12 features, ΔFV was significantly related to RP development. This study demonstrated the ability of radiomics to provide a quantitative, individualized measurement of patient lung tissue reaction to RT and assess RP development.
Introduction
Radiation-induced lung injury is the major dose-limiting factor in thoracic radiation therapy (RT). High doses of radiation delivered to healthy lung tissue result in alveolar damage, which presents acutely as symptomatic radiation pneumonitis (RP) (1). RP symptoms include cough, dyspnea, and fever, which affect patient quality of life and, in severe cases, may result in patient mortality or termination of further cancer treatment (2).
Research of thoracic RT-induced toxicity has aimed at determining factors that contribute to RP development. Because of the observed relationship between RP incidence and dose to an irradiated lung volume, many studies correlate measurements derived from radiation dose maps, such as mean lung dose (MLD) or percent of lung volume irradiated above a specified threshold dose (Vdose), with RP development (3, 4). Although several metrics have appeared promising, results vary across institutions (3), indicating that lung sensitivity to RT may be highly variable across patient populations.
Rather than assume that radiation-induced lung injury will be uniform across patients, imaging-based methods have been developed to measure each patient’s individual reaction to radiation. Several groups have observed a relationship between radiation dose and computed tomography (CT) scan density change following RT (5, 6). Hart et al (7) demonstrated a relationship between uptake of 18F-labeled fluorodeoxyglucose (FDG) in positron emission tomography (PET) scans and both radiation dose and RP development. These studies demonstrated that thoracic imaging can facilitate quantitative measurement of tissue changes following RT, indicating the presence of lung tissue damage and likelihood of RP development.
In this study, we developed a method for quantitative analysis of lung tissue reaction in the CT scans of patients who were treated with RT for esophageal cancer. Rather than measure only density changes in post-RT scans, changes were described by mathematical intensity and texture-based features that characterize image appearance based on pixel values and spatial relationships among pixels (8). This radiomics-based approach, in which quantitative imaging features are extracted from medical images (9) thus facilitated higher-order characterization of complex changes in lung parenchyma due to radiationinduced damage. Several groups have used CT scan–based texture analysis to quantify complex lung disease patterns (10–12). Mattonen et al (13) recently demonstrated the utility of texture analysis for RT treatment assessment by using first-order and co-occurrence matrix features to distinguish radiation-induced fibrosis from tumor recurrence. In the present work, the change in texture feature values (ΔFV) between pre- and post-RT CT scans was calculated to facilitate patient-specific characterization of radiation-induced damage. The goal was to assess the relationship between radiation dose and texture change and determine the utility of texture analysis to identify patients who developed RP.
Methods and Materials
Patient database
The retrospective database consisted of CT scans from each of 115 patients who received curative radiation doses (median: 50.4 Gy) for esophageal cancer at MD Anderson Cancer Center between April 2004 and March 2013. Patients were selected under institution review board approval from over 300 patients because they met the selection criteria. Specifically, each patient underwent 2 high-resolution diagnostic CT scans, the first acquired before RT and the second acquired no more than 4 months (120 days) after RT and prior to any surgical intervention. Diagnostic CT scans were reconstructed using lung convolution and smoothing kernels. If multiple CT scans met these criteria, the 2 scans acquired closest to the RT treatment dates were selected. Nine patients were not included for further analysis due to failure of lung segmentation that resulted from indistinct lung boundaries due to either lung compression or alternate pathology. For each of the remaining 106 patients (Table 1), a treatment planning CT scan with an associated dose map was collected. Dose maps were generated during treatment planning using heterogeneity corrections, using either Pinnacle (Philips Medical Systems, Andover, MA) for photon RT or Eclipse treatment planning system (Varian Medical Systems, Palo Alto, CA) for proton RT. Patients were monitored clinically for at least 6 months after RT. RP incidence was determined retrospectively through consensus of 3 clinicians using all available patient records and imaging from 6 months up to 1 year following RT. RP grade was scored using Common Toxicity Criteria for Adverse Events, version 4, based on the grade at first presentation, with grade ≥2 indicating symptomatic RP development.
Table 1.
Patient characteristics and image acquisition parameters
| Parameter | Value |
|---|---|
| No. of patients | 106 |
| Males: (n = 89) | |
| Females: (n = 17) | |
| Median age, y (range) | 63 (29–81) |
| No. with smoking history | 84 |
| Disease stage | I (n = 4) |
| II (n = 34) | |
| III (n = 57) | |
| IV (n = 10) | |
| Recurrent (n = 1) | |
| Histology | Adenocarcinoma (n = 87) |
| Squamous cell carcinoma (n = 16) | |
| Neuroendocrine (n = 2) | |
| Sarcoma (n = 1) | |
| Tumor location | Distal/GE junction (n = 90) |
| Middle (n = 14) | |
| Proximal (n = 2) | |
| RP grade | 0 (n = 38) |
| 1 (n = 48) | |
| 2 (n = 11) | |
| 3 (n = 5) | |
| 4 (n = 3) | |
| 5 (n = 1) | |
| Treatment regimen | Chemoradiation therapy (n = 105) |
| RT only (n = 1) | |
| Treatment modality | Photon 3D–CRT (n = 17) |
| Photon IMRT (n = 56) | |
| Proton therapy (n = 33) | |
| Median dose (range) (Gy) | 50.4 (45–66) |
| Dose per fraction (Gy) | 1.8 (n = 102) |
| 2 (n = 3) | |
| 2.25 (n = 1) | |
| Median treatment time (range) (d) | 38 (35–55) |
| Median time between pre-RT scan and RT start (range) (d) | 27 (0–168) |
| Median time between post-RT scan and RT end (range) (d) | 38 (5–120) |
| Treatment planning scan parameters (n = 106): | |
| Peak kilovoltage (kVp) | 120 |
| Slice spacing (mm) | 2.5 |
| Pixel spacing (mm) | 0.97 |
| Diagnostic scan parameters (n = 212): | |
| Scanner manufacturer and model | GE LightSpeed 16 (n = 97) |
| GE LightSpeed VCT (n = 52) | |
| GE LightSpeed Plus (n = 31) | |
| GE LightSpeed QX/i (n = 18) | |
| GE Discovery CT70 HD (n = 6) | |
| GE Discovery RX (n = 1) | |
| GE BrightSpeed (n = 1) | |
| Philips Brilliance 64 (n = 4) | |
| Hitachi SCENARIA (n = 1) | |
| Toshiba Aquilion (n = 1) | |
| Number of scan pairs acquired with different scanners | Same manufacturer, different model (n = 60) |
| Different manufacturer and model (n = 6) | |
| Peak kilovoltage (kVp) | 120 (n = 209) |
| 140 (n = 3) | |
| Peak kilovoltage difference between paired scans (kVp) | 0 (n = 103) |
| 20 (n = 3) | |
| Mean slice spacing (range) (mm) | 2.5 (2.0–4.0) |
| Mean slice spacing differences between paired scans (range) (mm) | 0.1 (0.0–1.5) |
| Mean pixel spacing (range) (mm) | 0.80 (0.63–0.98) |
| Mean pixel spacing difference between paired scans (range) (mm) | 0.00 (0–0.12) |
Abbreviations: 3D-CRT = 3-dimensional conformal radiation therapy; IMRT = intensity modulated radiation therapy; RP = radiation pneumonitis; RT = radiation therapy.
CT scan preprocessing
To facilitate quantitative analysis of lung tissue, semi-automated lung segmentation was performed on all planning (14) and diagnostic (15) CT scans. Lung segmentation results were reviewed by an experienced thoracic CT researcher (A.C.) and modified manually if necessary. The segmented lungs of each post-RT and planning scan were registered to the lungs of the pre-RT scan by using open source software (version 1.5.12-beta; Plastimatch) (16) for demons deformable registration (17). Each registration generated a displacement vector field that allowed for identification of matched anatomic locations among CT scans.
Measurement of feature change
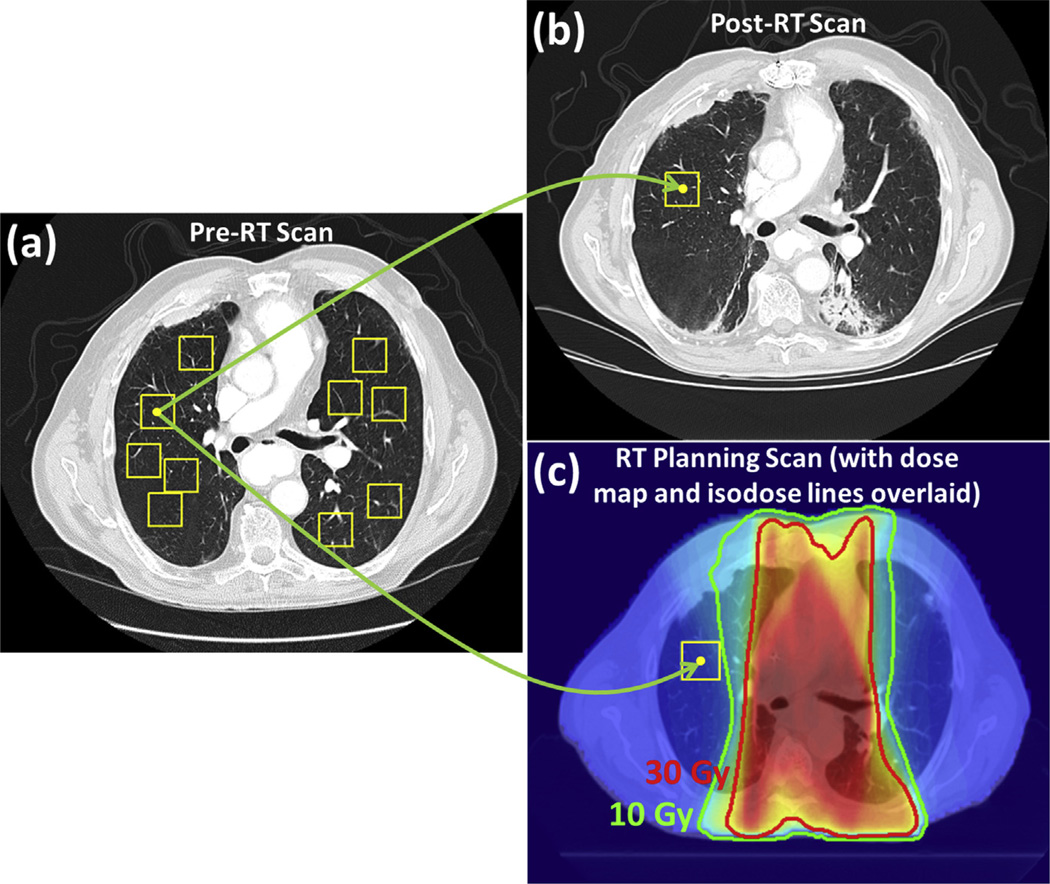
Nonoverlapping 32- × 32-pixel regions of interest (ROIs) were randomly placed in the lungs of each patient’s pre-RT scan by using a fully automated method (Fig. 1a) (17). A maximum of 10 ROIs were placed in each axial section. A previously developed approach was used to identify ROIs in the post-RT scan that were anatomically matched with the pre-RT scan ROIs, facilitating texture analysis on original nondeformed scans (18). Specifically, the center pixel of each ROI was mapped to the post-RT scan using the displacement vector field output by deformable registration, and then a 32- × 32-pixel ROI centered at this mapped center pixel was formed (Fig. 1b). Through deformable registration, all pre-RT scan ROIs were also associated with anatomically corresponding ROIs in the planning scan (and thus the associated dose map) (Fig. 1c) (19). Within each ROI, the average planned physical radiation dose (ie, not the biologically equivalent dose) was calculated.
Fig. 1.
Process for mapping the center of each ROI (yellow box) from the pre-RT CT scan (a) to the post-RT CT scan (b) and the RT planning CT scan with assocated dose map (c). CT scans. CT=computed tomography; ROI=region of interest; RT = radiation therapy. A color version of this figure is available at www.redjournal.org. Abbreviations: RT = radiation therapy.
Twenty texture- and intensity-based features distributed among first-order, fractal, Laws’ filter, and gray level co-occurrence matrix (GLCM) classes were calculated in the ROIs of all pre- and post-RT CT scans. These features were selected from over 140 features because they had been shown previously to vary minimally in the absence of pathologic change (18, 20). First-order features were derived from the gray-level histogram of a region (21, 22). Fractal features characterize the self-similarity of a region at different scales and relate to region detail (22, 23). Laws’ filters emphasize region microstructure, specifically spot, wave, ripple, edge, and level surfaces (24). GLCM features quantify the spatial relationship of gray-level values in a region (25). The absolute ΔFV from pre-RT to post-RT scan ROI was calculated.
Statistical analysis
A linear regression model was developed using the lmer package in Revolution R version 6.1 software (Revolution Analytics, Mountain View, CA) to test the individual contribution of several factors (patient, mean dose, and RP status) on ΔFV. ΔFV was modeled for each of the 20 features
| (Equation 1) |
where Patienti represents patient-specific random effects for each patient; MeanDosej is the mean radiation dose to the ROI; RPStatusk represents whether the patient developed RP (grade ≥ 2); and εijkl represents residual error. First, a model was constructed to consider only interpatient variability (Patienti). Mean dose and RP status were then consecutively included in the model. Analysis of variance (ANOVA) was used to determine whether inclusion of each of these 2 terms significantly improved model fit. To maintain a family-wise error rate of α = 0.05, p values for individual tests were modified using the Bonferroni approach (p<0.0025). The effects of treatment modality (photon intensity modulated RT, photon 3D-conformal RT, or proton therapy) and time to post-RT scan on ΔFV were also tested by adding each term individually into the full model (Equation 1) and then performing ANOVA. Statistical analyses were also repeated with grade ≥3 RP considered as an alternative end point. Because a previous study (19) indicated that registration errors between planning and diagnostic CT scans might result in average dose errors of 1 to 7 Gy, analyses were also repeated using a subset of ROIs (86% of ROIs) that were located in regions with low-dose variability (ie, standard deviation of <5 Gy).
Receiver operating characteristic (ROC) analysis (26) was performed to test the ability of each feature to distinguish between patients with and without grade ≥2 RP at various dose levels. For each feature, a patient-specific average ΔFV was calculated in 3 dose regions: 0 to 10 Gy (low dose), 10 to 30 Gy (medium dose), and ≥30 Gy (high dose), allowing for the effects of RP status on ΔFV to be considered independently from the effects of dose. These dose regions were defined based on previous research indicating differences in radiologic and/or symptomatic changes across these dose ranges (27, 28). The slope of the dose versus ΔFV plot fitted using least squares (fitted slope) was also calculated. For each of these 4 dose-dependent measurements, area under the ROC curve (AUC) was calculated using the pROC package in Revolution R software.
RP patient classification
A logistic regression–based classifier was constructed to determine whether combined information from multiple features could improve identification of patients with grade ≥2 RP. Classifier training and testing were performed using 1000 repetitions of random subsampling, with 75% of the RP and non-RP cases randomly selected for classifier training and the remaining 25% retained for testing. The mean AUC across all repetitions was calculated. Each of the 190 possible 2-feature combinations and each of the 1140 3-feature combinations was tested for classifier development. ANOVA and Tukey’s honest significant difference tests (29) were performed to test whether the number of features used to construct the classifier (1, 2, or 3 features) and the dose-dependent measurement (low, medium, or high dose or fitted slope) affected AUC values.
Results
Between 319 and 1014 ROIs were identified in the lungs of each patient’s pre-RT CT scan. Of these ROIs, 6% were discarded because they were subsequently mapped to locations that were not fully contained within the lungs of the post-RT scan, resulting in 268 to 995 (mean: 703) ROI pairs per patient. Most ROIs received mean low-dose radiation, whereas 4474 ROIs (6%) were located in high-dose regions (Table 2).
Table 2.
Distribution of ROIs across radiation dose levels
| Average number of ROI pairs per patient (range) |
||
|---|---|---|
| Mean ROI dose | Photon RT | Proton RT |
| 0–10 Gy | 503 (165–776) | 532 (300–936) |
| 10–30 Gy | 162 (17–398) | 118 (22–294) |
| 30 Gy | 49 (1–154) | 27 (0–90) |
Abbreviations: ROI = region of interest; RT = radiation therapy.
Linear modeling demonstrated a significant relationship between ΔFV and mean ROI dose for all 20 features and between ΔFV and grade ≥2 RP for 12 features (Table 3). When grade ≥3 RP was considered as an alternative endpoint, 5 features remained significant (Table 3). For 17 features, which excluded fractal-based features, a positive relationship existed between dose and ΔFV. The effect of time to post-RT scan or treatment modality on ΔFV was not significant. When the analysis was repeated with ROIs located exclusively in regions with dose standard deviations of <5 Gy, the same 12 features demonstrated significant differences between patients with and without RP, and the increase in ΔFV with increasing dose remained significant for all features. Plots of ΔFV versus dose for 2 representative features demonstrated a monotonic increase or decrease in ΔFV with increasing dose up to 45 Gy and a larger magnitude of ΔFV across all dose ranges for patients with RP (Fig. 2).
Table 3.
Performance of patient-averaged values of individual feature changes to separate RP patients from non-RP patients
| AUC | ||||
|---|---|---|---|---|
| Feature/dose-dependent measurement | Low dose | Medium dose | High dose | Fitted slope |
| First-order features | ||||
| 70% Quantile*,† | 0.72 | 0.77 | 0.77 | 0.77 |
| Median*,† | 0.70 | 0.73 | 0.75 | 0.75 |
| Mean*,† | 0.68 | 0.73 | 0.75 | 0.76 |
| Binned entropy* | 0.68 | 0.74 | 0.75 | 0.68 |
| 30% quantile* | 0.67 | 0.69 | 0.72 | 0.74 |
| Unbinned entropy | 0.66 | 0.69 | 0.71 | 0.69 |
| 5% Quantile | 0.61 | 0.62 | 0.65 | 0.69 |
| Minimum | 0.58 | 0.56 | 0.63 | 0.58 |
| Fractal features | ||||
| Brownian dimension* | 0.66 | 0.72 | 0.78 | 0.78 |
| Box-counting dimension | 0.49 | 0.54 | 0.60 | 0.67 |
| Fine box-counting dimension | 0.49 | 0.49 | 0.52 | 0.54 |
| Laws’ filter features | ||||
| E5L5 entropy* | 0.63 | 0.72 | 0.75 | 0.77 |
| R5L5 entropy* | 0.63 | 0.73 | 0.75 | 0.77 |
| S5L5 entropy* | 0.62 | 0.71 | 0.74 | 0.76 |
| W5L5 entropy* | 0.62 | 0.71 | 0.73 | 0.76 |
| GLCM features | ||||
| Sum average*,† | 0.69 | 0.74 | 0.76 | 0.77 |
| Sum of squares variance*,† | 0.70 | 0.75 | 0.76 | 0.77 |
| Sum entropy | 0.68 | 0.70 | 0.73 | 0.68 |
| Difference entropy | 0.62 | 0.65 | 0.68 | 0.66 |
| Entropy | 0.59 | 0.60 | 0.60 | 0.50 |
| Average AUC across features | 0.64 | 0.68 | 0.71 | 0.71 |
Abbreviations AUC = Area under the receiver operating characteristic curve; E5L5 = edge-level filter; GLCM = gray-level co-occurrence; RP = radiation pneumonitis; R5L5 = ripple-level filter; S5L5 = spot-level filter; W5L5 = wave-level filter.
Significant (P<0.0025) relationship between change in feature value (ΔFV) and grade ≥2 RP.
Significant (P<0.0025) relationship between DFV and grade ≥3 RP.
Fig. 2.
Plots of ΔFV versus mean physical dose with 95% confidence intervals for 2 features. Average values were calculated on a per-patient basis in 5-Gy dose bins.
AUC values ranged from 0.49 to 0.78 when single features were used to separate RP patients from non-RP patients (Table 3). When multiple features were combined in a classifier, mean AUC values increased by 0.01 to 0.04 (Table 4). AUC was significantly different among all dose-dependent measurements and increased significantly from 1 to 2 features (Table 4). The change in AUC from 2 to 3 features was not significant.
Table 4.
Comparison of single-feature performance to distinguish patients with RP compared with a classifier composed of each 2- or 3-feature combination
| No. of features/dose-dependent measurement |
Mean AUC (range) | |||
|---|---|---|---|---|
| Low dose | Medium dose | High dose | Fitted slope | |
| 1 Feature | 0.64 (0.49–0.72) | 0.68 (0.49–0.77) | 0.71 (0.52–0.78) | 0.71 (0.50–0.78) |
| 2 Features | 0.66 (0.59–0.74) | 0.71 (0.59–0.78) | 0.73 (0.59–0.78) | 0.74 (0.59–0.84) |
| 3 Features | 0.66 (0.59–0.75) | 0.72 (0.59–0.79) | 0.72 (0.60–0.79) | 0.75 (0.61–0.83) |
Abbreviations: AUC = area under the receiver operating characteristic curve; RP = radiation pneumonitis.
Discussion
This study demonstrated that a relationship exists between radiation dose and change in image-based features, using pre- and post-RT CT scans for an esophageal cancer patient cohort. Furthermore, RP patients can be differentiated from patients without clinically significant RP, based on ΔFV. All 20 features investigated demonstrated a significant increase or decrease in ΔFV with increasing dose. Twelve features demonstrated significant increases in dose-dependent FV change for patients with grade ≥2 RP (Table 3). AUC values were highest using measurements derived from high-dose regions and dose-dependent feature changes (fitted slope). Although AUC was lower in low- and medium-dose regions, AUC values (Table 3) indicated that differences between RP and non-RP patients existed even in regions where the magnitude of visible change was likely to be low (27, 28). When features were combined in a classifier, AUC values increased significantly using 2 features but showed no additional increase when a third feature was included (Table 4). Due to the small number of positive cases (n=20), inclusion of more than 2 features may have led to overfitting during classifier training, resulting in no significant improvements in classifier performance (30, 31). Although all feature combinations were tested, we could not conclusively determine an optimal feature set and model due to the small number of RP cases. In a larger database, patients could be subdivided into 3 cohorts for feature selection, classifier training, and classifier testing, facilitating identification of a small set of features. Although this study demonstrates that texture analysis can distinguish between patients with and without symptomatic RP, the choice of features and model coefficients remains to be determined using a larger database and/or an independent database.
Combined features were able to classify RP and non-RP patients with moderate performance (AUC values from 0.59 to 0.84). Inclusion of MLD and V20 information in classifiers resulted in no significant increase in AUC compared with the values obtained using single features (Table 3), and no significant relationship was observed between RP development and either MLD or V20 in our dataset compared with other existing reports of RP. It is possible that a nonlinear classifier such as a neural network (32, 33) may further improve classification accuracy, but that approach remains to be investigated with a larger database.
The retrospective nature of RP diagnosis makes it possible that RP grade was incorrectly identified in some patients, as symptomatic changes characteristic of RP can also be caused by other comorbidities (27, 34). In the present study, RP grade was determined based on the consensus of 3 clinicians after review of all patient imaging and clinical records. To further increase the reliability of RP diagnosis, future prospective studies should be performed. Retrospective data collection also resulted in differences in technical parameters across CT scans, which may affect feature values. In a previous study, changes in 140 features in the low-dose (<5 Gy) regions of the CT scans used in this study were compared with feature changes in scans acquired from healthy patients at a different institution (20). Despite differences in CT scanner type, scan resolution, and patient health status, the 20 features used in the present study remained stable, indicating that these features may be useful for data analysis from a variety of CT scanners.
Errors in CT scan coregistration may have occurred, resulting in incorrect identification of matched ROIs between diagnostic scans. In previous work in our laboratory, landmark matching was used to evaluate the registration accuracy between pre- and post-RT CT scans of 24 lung cancer patients, using Plastimatch demons. Average diagnostic CT scan registration error was 2.4 mm across patients who did not have radiation-induced changes and 4.6 mm across patients with moderate or severe changes. In the current database, the degree of physiologic change among scans was smaller than in the lung cancer patient database due to the smaller volume that received high radiation dose and the absence of lung tumors, which could undergo volume changes due to RT or disease progression. The applicability of our findings in a lung cancer patient cohort remains to be investigated. The registration algorithm and parameters used in this study were selected because of previous research that showed high registration accuracy among diagnostic CT scans (17). It is likely, however, that further improvements in image registration could improve the findings of this study by facilitating improved alignment accuracy between ROIs in pre- and post-RT scans.
Previous studies have observed changes in CT scan density with increasing radiation dose for patients treated with conventionally fractionated RT (5) or stereotactic radiation therapy (6), which is consistent with our finding that ΔFV for a wide variety of features increased significantly with increasing dose. Phernambucq et al (35) found no relationship between CT scan density changes and increasing dose or RP status, although the authors attributed this result in part to the small sample size (7 patients with RP). To our knowledge, the present study is the first to demonstrate that texture changes following thoracic RT are associated with patient RP status. Although most texture analysis research quantifies lung disease status at a single time point, this study used deformable registration to relate pre- and post-RT scans, facilitating measurement of treatment-related change. When feature values were measured directly in post-RT scans, feature value variability increased, significantly (p<0.05), decreasing AUC values for individual features (Table 3) by up to 0.14 (mean: 0.03).
Although several of the first-order features analyzed were strongly related to mean CT density changes (eg, median pixel value, 70% quantile, and 30% quantile), higher-order texture features were also used to measure the patterns of radiation-induced damage. The correlation between changes in mean pixel value and changes in 9 of the 19 remaining feature values was low (|r|<0.5), indicating the potential for uncorrelated features to provide complimentary information to mean CT density. Specifically, these features could capture aspects of image appearance that characterize the patchy appearance of acute RT-induced injury but are independent of changes in mean pixel value.
The post-RT CT scans in our study were acquired at various time points from 1 week to 4 months following RT (Table 1), which is a time frame that is typical for RP development. Due to the retrospective nature of RP diagnosis, we were unable to determine the date of RP onset or whether the measured texture changes occurred prior to RP development. Future prospective studies should investigate whether texture changes in CT scans acquired after RT but before RP incidence could predict RP development. Identification of patients who are at high risk for future RP development would allow for closer monitoring, facilitating earlier treatment and improved outcomes for these patients (36). Alternative measurements including elevated serum levels (37), dose-volume histogram data (3, 4), and functional information derived from SPECT (38) or PET (39, 40) scans show promise for predicting RP. Future studies may benefit from combining measurements derived from these various methods with texture analysis of CT scans.
Conclusions
This study demonstrated that quantitative measurement of dose-dependent texture changes between pre- and post-RT CT scans can differentiate between patients with clinical (grade ≥2) RP and those patients without RP. Twelve intensity- and texture-based features demonstrated significantly increased changes for patients with RP. In general, individual features could be used to discriminate between patients with and without RP with moderate performance. When multiple features were combined in a classifier, AUC increased significantly (AUC values from 0.59 to 0.84). This study demonstrates the potential ability of radiomics to provide a quantitative, individualized approach to measure patient lung tissue reaction to RT and assess radiation pneumonitis.
Summary.
For 106 esophageal cancer patients treated with radiation therapy (RT), the relationship among changes in 20 image-based features in post-RT computed tomography scans, radiation doses, and development of radiation pneumonitis (RP) was tested. All features changed significantly with increasing doses. Twelve features were significantly related to RP development. Patients with RP could be distinguished by using a texture-based classifier. These methods provide a quantitative, individualized approach to measure lung tissue reaction to RT and assess RP development.
Acknowledgments
The authors acknowledge Thomas Guerrero, MD, PhD, Ngoc Pham, MD, and Heath Skinner, MD, PhD, for performing retrospective radiation pneumonitis grading for all patients; and Mary Martel, PhD, for facilitating cross-institutional collaboration; Christopher Straus, MD, for help interpreting CT scans; and Nick Gruszauskas, PhD, and Jonathan Marino for their guidance during CT scan anonymization.
This work was supported in part by U.S. National Institutes of Health (NIH) grants S10 RR021039 and P30 CA14599 and a pilot award from the Institute for Translational Medicine, The University of Chicago. Dr Castillo was supported in part by NIH research scientist development award K01CA181292.
Conflict of interest: Dr Armato has received royalties and licensing fees through The University of Chicago for computer-aided diagnosis technology. Dr Al-Hallaq has received royalties for computer-aided diagnosis software for breast cancer detection, licensed from The University of Chicago.
References
- 1.Abratt RP, Morgan GW. Lung toxicity following chest irradiation in patients with lung cancer. Lung Cancer. 2002;35:103–109. doi: 10.1016/s0169-5002(01)00334-8. [DOI] [PubMed] [Google Scholar]
- 2.Inoue A, Kunitoh H, Sekine I, et al. Radiation pneumonitis in lung cancer patients: A retrospective study of risk factors and the long-term prognosis. Int J Radiat Oncol Biol Phys. 2001;49:649–655. doi: 10.1016/s0360-3016(00)00783-5. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues G, Lock M, D’Souza D, et al. Prediction of radiation pneumonitis by dose-volume histogram parameters in lung cancer―A systematic review. Radiother Oncol. 2004;71:127–138. doi: 10.1016/j.radonc.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Yorke ED, Jackson A, Rosenzweig KE, et al. Dose-volume factors contributing to the incidence of radiation pneumonitis in non-small-cell lung cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2002;54:329–339. doi: 10.1016/s0360-3016(02)02929-2. [DOI] [PubMed] [Google Scholar]
- 5.Ma J, Zhang J, Zhou S, et al. Regional lung density changes after radiation therapy for tumors in and around the thorax. Int J Radiat Oncol Biol Phys. 2010;76:116–122. doi: 10.1016/j.ijrobp.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Palma DA, Van Sornsen de Koste J, Verbakel WR, et al. Lung density changes after stereotactic radiotherapy: A quantitative analysis in 50 patients. Int J Radiat Oncol Biol Phys. 2011;81:974–978. doi: 10.1016/j.ijrobp.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 7.Hart JP, McCurdy MR, Ezhil M, et al. Radiation pneumonitis: Correlation of toxicity with pulmonary metabolic radiation response. Int J Radiat Oncol Biol Phys. 2008;71:967–971. doi: 10.1016/j.ijrobp.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castellano G, Bonilha L, Li LM, et al. Texture analysis of medical images. Clin Radiol. 2004;59:1061–1069. doi: 10.1016/j.crad.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Kumar V, Gu Y, Basu S, et al. Radiomics: The process and the challenges. Magn Reson Imaging. 2012;30:1234–1248. doi: 10.1016/j.mri.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chabat F, Yang G-Z, Hansell DM. Obstructive lung diseases: Texture classification for differentiation at CT. Radiology. 2003;228:871–877. doi: 10.1148/radiol.2283020505. [DOI] [PubMed] [Google Scholar]
- 11.Uchiyama Y, Katsuragawa S, Abe H, et al. Quantitative computerized analysis of diffuse lung disease in high-resolution computed tomography. Med Phys. 2003;30:2440–2454. doi: 10.1118/1.1597431. [DOI] [PubMed] [Google Scholar]
- 12.Uppaluri R, Hoffman EA, Sonka M, et al. Computer recognition of regional lung disease patterns. Am J Respir Crit Care Med. 1999;160:648–654. doi: 10.1164/ajrccm.160.2.9804094. [DOI] [PubMed] [Google Scholar]
- 13.Mattonen SA, Palma DA, Haasbeek CJA, et al. Early prediction of tumor recurrence based on CT texture changes after stereotactic ablative radiotherapy (SABR) for lung cancer. Med Phys. 2014;41:033502. doi: 10.1118/1.4866219. [DOI] [PubMed] [Google Scholar]
- 14.Castillo R, Pham N, Ansari S, et al. Pre-radiotherapy FDG PET predicts radiation pneumonitis in lung cancer. Radiat Oncol. 2014;9:74–83. doi: 10.1186/1748-717X-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sensakovic WF, Armato SG, Straus C, et al. Computerized segmentation and measurement of malignant pleural mesothelioma. Med Phys. 2011;38:238–244. doi: 10.1118/1.3525836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharp GC, Kandasamy N, Singh H, et al. GPU-based streaming architectures for fast cone-beam CT image reconstruction and demons deformable registration. Phys Med Biol. 2007;52:5771–5783. doi: 10.1088/0031-9155/52/19/003. [DOI] [PubMed] [Google Scholar]
- 17.Cunliffe AR, Al-Hallaq HA, Labby ZE, et al. Lung texture in serial thoracic CT scans: Assessment of change introduced by image registration. Med Phys. 2012;39:4679–4690. doi: 10.1118/1.4730505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunliffe AR, Armato SG, Fei XM, et al. Lung texture in serial thoracic CT scans: Registration-based methods to compare anatomically matched regions. Med Phys. 2013;40:061906e1–061906e9. doi: 10.1118/1.4805110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunliffe AR, Contee C, Armato SG, et al. Effect of deformable registration on the dose calculated in radiation therapy planning CT scans of lung cancer patients. Med Phys. 2015 Jan;42:391. doi: 10.1118/1.4903267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunliffe A, Armato S, Castillo R, et al. Quantitative texture features calculated in lung tissue from CT scans demonstrate consistency between two databases from different institutions. Med Phys. 2014;41:450. [Google Scholar]
- 21.Pratt WK. Digital Image Processing. 3rd ed. New York: John Wiley & Sons, Inc; 2001. Image feature extraction; pp. 509–550. [Google Scholar]
- 22.Li H, Giger ML, Huo Z, et al. Computerized analysis of mammographic parenchymal patterns for assessing breast cancer risk: Effect of ROI size and location. Med Phys. 2004;31:549–555. doi: 10.1118/1.1644514. [DOI] [PubMed] [Google Scholar]
- 23.Chen C-C, DaPonte JS, Fox MD. Fractal feature analysis and classification in medical imaging. IEEE Trans Med Imaging. 1989;8:133–142. doi: 10.1109/42.24861. [DOI] [PubMed] [Google Scholar]
- 24.Laws KI. USCIPI Technical Report No. 940. Los Angeles: University of Southern California; 1980. Textured image segmentation. [Google Scholar]
- 25.Haralick RM, Shanmugam S, Dinstein I. Texture features for image classification. IEEE Trans Syst Man Cybern B Cybern. 1973;3:610–621. [Google Scholar]
- 26.Obuchowski NA. Receiver operating characteristic curves and their use in radiology. Radiology. 2003;229:3–8. doi: 10.1148/radiol.2291010898. [DOI] [PubMed] [Google Scholar]
- 27.Movsas B, Raffin TA, Epstein AH, et al. Pulmonary radiation injury. Chest. 1997;111:1061–1076. doi: 10.1378/chest.111.4.1061. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins P, Welsch A. Computed tomography appearance of early radiation injury to the lung: Correlation with clinical and dosimetric factors. Int J Radiat Oncol Biol Phys. 2011;81:97–103. doi: 10.1016/j.ijrobp.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Christensen R. Analysis of Variance, Design, and Regression: Applied Statistical Methods. Boca Raton, FL: Chapman and Hall; 1996. Multiple comparison methods; pp. 146–165. [Google Scholar]
- 30.Harrell FE. Regression Modeling Strategies With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer-Verlag; 2001. Multivariable modeling strategies; pp. 53–81. [Google Scholar]
- 31.Hua J, Xiong Z, Lowey J, et al. Optimal number of features as a function of sample size for various classification rules. Bioinformatics. 2004;21:1509–1515. doi: 10.1093/bioinformatics/bti171. [DOI] [PubMed] [Google Scholar]
- 32.Dreiseitl S, Ohno-Machado L. Logistic regression and artificial neural network classifications models: A methodology review. J Biomed Inform. 2002;35:352–359. doi: 10.1016/s1532-0464(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 33.Ayer T, Chhatwal J, Alagoz O, et al. Informatics in radiology: Comparison of logistic regression and artificial neural network models in breast cancer risk estimation. Radiographics. 2010;30:13–22. doi: 10.1148/rg.301095057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kocack Z, Evans ES, Zhou S-M, et al. Challenges in defining radiation pneumonitis in patients with lung cancer. Int J Radiat Oncol Biol Phys. 2005;62:635–638. doi: 10.1016/j.ijrobp.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Phernambucq ECJ, Palma DA, Vincent A, et al. Time and dose-related changes in radiological lung density after concurrent chemoradiotherapy for lung cancer. Lung Cancer. 2011;74:451–456. doi: 10.1016/j.lungcan.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Rubin P, Casarett GW. Clinical Radiation Pathology. Vol. 1. Philadelphia: WB Saunders; 1968. Respiratory system; pp. 423–470. [Google Scholar]
- 37.Hartsell WF, Scott CB, Dundas GS, et al. Can serum markers be used to predict acute and late toxicity in patients with lung cancer? Analysis of RTOG 91-03. Am J Clin Oncol. 2007;30:368–376. doi: 10.1097/01.coc.0000260950.44761.74. [DOI] [PubMed] [Google Scholar]
- 38.Hoover DA, Reid RH, Wong E, et al. SPECT-based functional lung imaging for the prediction of radiation pneumonitis: A clinical and dosimetric correlation. J Med Imaging Radiat Oncol. 2014;58:14–22. doi: 10.1111/1754-9485.12145. [DOI] [PubMed] [Google Scholar]
- 39.McCurdy MR, Castillo R, Martinez J, et al. [18F]-FDG uptake dose response correlates with radiation pneumonitis in lung cancer patients. Radiother Oncol. 2012;104:52–57. doi: 10.1016/j.radonc.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castillo R, Pham N, Ansari S, et al. Pre-radiotherapy FDG PET predicts radiation pneumonitis in lung cancer. Radiat Oncol. 2014;9:74–83. doi: 10.1186/1748-717X-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]