Abstract
Purpose
Pancreatic ductal adenocarcinoma (PDA) cells are known to produce excessive amounts of reactive oxygen species (ROS), particularly superoxide, which may contribute to the aggressive and refractory nature of this disease. Extracellular superoxide dismutase (EcSOD) is an antioxidant enzyme that catalyzes the dismutation of superoxide in the extracellular environment. The current work tests the hypothesis that EcSOD modulates PDA growth and invasion by modifying the redox balance in PDA.
Experimental Design
We evaluated the prognostic significance of EcSOD in a human tissue microarray of patients with PDA. EcSOD overexpression was performed in PDA cell lines and animal models of disease. The impact of EcSOD on PDA cell lines was evaluated with Matrigel invasion in combination with a superoxide-specific SOD mimic and a nitric oxide synthase inhibitor to determine the mechanism of action of EcSOD in PDA.
Results
Loss of EcSOD expression is a common event in PDA, which correlated with worse disease biology. Overexpression of EcSOD in PDA cell lines resulted in decreased invasiveness that appeared to be related to reactions of superoxide with nitric oxide. Pancreatic cancer xenografts overexpressing EcSOD also demonstrated slower growth and peritoneal metastasis. Over-expression of EcSOD or treatment with a superoxide-specific SOD mimic caused significant decreases in PDA cell invasive capacity.
Conclusions
These results support the hypothesis that loss of EcSOD leads to increased reactions of superoxide with nitric oxide which contributes to the invasive phenotype. These results allow for the speculation that superoxide dismutase mimetics might inhibit PDA progression in human clinical disease.
Keywords: pancreatic cancer, extracellular superoxide dismutase, peroxynitrite, cancer progression, superoxide mimetics
INTRODUCTION
Extracellular superoxide dismutase (EcSOD; SOD3) is a glycosylated enzyme that binds to heparin sulfate moieties in the extracellular environment and catalyzes the dismutation of superoxide (O2•−) to hydrogen peroxide (H2O2) with a rate constant of > 109 M−1 s−1. By limiting O2•− availability and producing H2O2, EcSOD is thought to regulate the redox state of the extracellular environment.(1) By virtue of removing O2•−, EcSOD has also been hypothesized to decrease the formation of highly reactive peroxynitrite (ONOO−) from nitric oxide (•NO) in the extracellular environment and exert a protective role over •NO bioavailability.(2) Compared to SOD1 and SOD2 (CuZnSOD and MnSOD, respectively) expression of EcSOD is highly cell-type specific and can be found most abundantly in the pancreas, lung, kidney, and vasculature.(3)
A common feature in human cancers is an imbalance in the production and removal of reactive oxygen species (ROS) such as O2•− and H2O2 due to both increased fluxes of these ROS as well as alterations in antioxidant capacity. These redox imbalances in both the intracellular as well as extracellular environment have been hypothesized to significantly contribute to cancer cell proliferation and progression to malignancy.(4–13) However, historically, many more studies have focused on imbalances in the intracellular redox environment, relative to the extracellular environment.(4–13) In addition, many studies have not differentiated the effects of specific ROS.
Increased intracellular or extracellular steady-state levels of specific ROS can occur because of a decrease in the activity of antioxidant enzymes such as SODs, catalase, and glutathione peroxidases.(5, 14–16) The loss of EcSOD expression has been reported in several cancers (17–19) suggesting that EcSOD may play an essential role in maintaining an extracellular redox balance that opposes tumor growth and metastasis. This concept was highlighted in previous work showing that human pancreatic ductal adenocarcinoma (PDA) cell lines transduced with adenoviral vectors, enforcing the transient over-expression of EcSOD, demonstrated decreased cell growth and plating efficiency in vitro, as well as slower tumor growth and increased survival in a xenograft model. (17, 19) Furthermore, similar results were obtained in vitro and in vivo with human breast and lung carcinoma cells where EcSOD over-expression not only decreased growth rate and clonogenic survival but also the invasive phenotype of these cancer cells.(18, 20, 21)
Building on the previous findings that EcSOD is lost in PDA cell lines and over-expression inhibits growth and cloning efficiency in vitro and in xenograft models, (17) we hypothesized that decreased levels of EcSOD in human PDA would correlate with poorer outcomes as well as increased aggressiveness of clinical disease progression. We also performed preclinical studies to evaluate the impact of overexpression of EcSOD on PDA tumor cell survival in cell culture and animal models, as well as determine the potential contribution of H2O2 and •NO to any of the observed biological effects of EcSOD on PDA progression. Results from our studies reveal that loss of EcSOD expression is a common event in PDA patients, and that loss of EcSOD predicts a more aggressive tumor phenotype with respect to invasive and metastatic behavior. These findings highlight the important role of specific extracellular ROS in PDA biology and suggest potential utility for increasing EcSOD activity as a therapeutic target for slowing disease progression in PDA patients.
MATERIALS and METHODS
Pancreatic ductal adenocarcinoma tissue microarray
A PDA tissue microarray (TMA) was obtained from US Biomax, Inc. (#PA2081) and IHC performed (EcSOD, AbFontier, #LF-MA1021). Sections were deparaffinized, rehydrated, and subjected to heat-induced epitope retrieval in citrate buffer (pH6). Endogenous peroxidase activity was blocked with 3% H2O2 followed by serum block. After incubation with primary antibody at a dilution of 1:400 for 30 min, the Dako Mouse Envision System was used for detection. Dako DAB Plus was applied and slides were lightly counterstained with hematoxylin.
Immunohistochemistry analysis of pancreatic cancer specimens
University of Iowa institutional review board approval was obtained to retrospectively evaluate tissue from patients with PDA. Tissue blocks from 17 patients treated surgically for PDA were retrieved, and IHC for EcSOD and 3-nitrotyrosine (3-NT) (Millipore; #06284, 1:2000) was performed as above.
ONCOMINE data
ONCOMINE is a cancer microarray database (22) that is publically available at www.oncomine.org. The database was accessed and a search for SOD3 and PDA was performed. There were 7 data sets available that were reported as published on the website.
Pancreatic cancer specimen evaluation using qPCR
EcSOD expression in human normal and PDA samples was examined using a TissueScan™ Real-Time PDA qPCR Panel (I) from ORIGENE Technologies (#PNRT301) and from tissue specimens obtained from patients with PDA at the University of Iowa. EcSOD expression was measured using a TaqMan primer/probe set (Hs00162090_m1) from Applied Biosystems and normalized against β-actin control primers.
Surveillance, Epidemiology and End Results (SEER) TMA analysis and outcomes data
Case identification and selection methods of SEER PDA TMA have been described.(23) The present study includes PDA specimens from 114 patients with Stage I–IV disease. Ethical and transfer of material approvals were obtained from originating sites and the National Cancer Institute. The association of EcSOD expression in the 114 patients with disease stage and pancreatic resection status was evaluated with Chi-square analysis. Survival was defined as months from diagnosis to death and Kaplan-Meier survival plots were constructed to estimate survival as a function of time and log-rank was used to compare survival between patients with and without EcSOD expression. Cox proportional hazards regression was used in multivariate analyses to estimate the impact of loss of EcSOD on survival (SAS 9.3, Cary, NC).
Adenovirus transduction
Cells were plated at a density of 1 × 106 in 60-mm dishes in media containing 10% FBS and allowed to attach for 24 h. Cells were rinsed and Ad.EcSOD or Ad.Empty as this vector has been previously described. (24)
Clonogenic survival
Cells were plated in triplicate into 60-mm dishes at a density of 1×103 (Bx-PC3) or 1 × 102 (Mia-PaCa-2) in complete medium. Dishes were incubated for 2 weeks to allow for colony formation. The colonies were then fixed in ice-cold methanol and stained with 0.5% w/v crystal violet in 25% methanol. Colonies containing > 50 cells were scored as survivors.
Cell growth
Cells were seeded in duplicate at a density of 1 × 105 in 6-well plates with complete medium and were counted daily for 96 h. Cell population doubling time in hours was determined as previously described.(25)
Matrigel invasion and migration assays
BxPC3/MiaPaCa2 cells were seeded at a density of 2×105 in serum-free medium into Matrigel chambers (8 μm pore size) from the BD Bio-Coat Matrigel Invasion Assay System (BD Biosciences, #354480). The effects of various interventions on invasion were evaluated, including 20 mM N-acetylcysteine (Cumberland Pharmaceuticals, #66220-207-30), 10 U/mL catalase (Sigma-Aldrich, #C30), and 100 μM NG-nitro-L-arginine (Calbiochem, #483120). To evaluate the effects of the SOD mimetic (Galera Therapeutics, #GC4419) on invasion, GC4419 was placed on the cells for 24 h before, as well as in the invasion chambers during the invasion assay. To evaluate the effects of conditioned media on invasion, media (2% FBS) was harvested from Bx-EcSOD or Mia-EcSOD cells and placed in the invasion chambers. In all cases, the transwell chambers were placed into 24-well plates containing medium with 10% FBS and incubated for 22–24 h at 37 °C, 5% CO2. Non-invading cells from the upper chamber were removed. Invading BxPC3 cells were stained by placing transwell chambers into 0.5 mg/mL of a Thiazolyl Blue Tetrazolium Bromide (MTT) solution (Sigma-Aldrich) in RPMI-1640 medium without FBS or phenol red. Inserts were incubated at 37°C for 1h before membranes were removed and incubated in 150 μL of DMSO. DMSO solution was transferred to a 96-well plate, and the absorption at 550 nm was measured immediately and compared to known cell concentrations from a standard curve. Invading MiaPaCa2 cells were fixed in 100% methanol and stained with Giemsa and the number of invading cells counted from 5 random fields. Invasion data is represented as relative invasion except for data containing NG-nitro-L-arginine (L-NNA) and catalase, which are shown as percent invasion (invasion/migration × 100) due to the impact of these chemicals on migration. For transwell migration, cells were seeded at a density of 2×105 in serum-free medium into BD Falcon PET track-etched membrane chambers (8 μm pore size) (BD Biosciences).
Murine subcutaneous tumor growth and survival evaluation
Female 6–10 week old athymic-nu/nu mice were purchased from Harlan Laboratories and handled in accordance with the University of Iowa Animal Care and Use Committee (IACUC). BxPC3 cells (2.5 × 106) stably expressing either EcSOD or Empty vector were injected subcutaneously and tumor volumes were determined using calipers applying the equation Volume=Length (longest diameter)×Width (shortest diameter)2 × 0.5. Animals were sacrificed when the tumors reached 15 mm in diameter.
Murine intraperitoneal metastasis model and bioluminescence imaging (BLI) analyses
1×107 Bx-EcSOD and Bx-Control cells were administered via intraperitoneal (IP) injection into athymic nude mice and tumor formation was monitored by bioluminescent imaging. 15 mg/mL VivoGloTM IP luciferin (Promega) was used, and after 5min were anesthetized by isoflurane inhalation maintained at 3% through a nose and imaged on the AMI-1000 (Spectral Instruments Imaging).
Statistics
Statistical analyses for the in vitro and in vivo studies were examined and analyzed primarily by Student’s t and Mann-Whitney tests. The resulting data from the studies with more than two groups were analyzed using the ANOVA or nonparametric ANOVA (Kruskal-Wallis) test, followed by a Tukey’s or Dunn’s multiple comparisons. Nonparametric tests were chosen when the sample size was small or the data were not normally distributed. All statistical tests were two-sided and assessed for significance at the 0.05 level (SAS 9.2, Cary, NC) and GraphPad Prism 6.0 (San Diego, CA).
SUPPLEMENTARY METHODS
Quantitative real-time reverse transcriptase-PCR
Total RNA for all in vitro studies was extracted using Trizol and the PureLink RNA Mini kit (Life Technologies, #12183018A). Each sample was reverse transcribed and gene expression was measured using Power SYBR PCR master mix (Applied Biosystems, # 4367659). Samples were normalized to 18s control using the ΔΔCt method.
Cells and establishment of stable cell lines
Human cell lines (BxPC3, MiaPaCa2, and HPNE) were obtained directly from ATCC and were passaged actively for less than 6 months from the time of receiving them from ATCC and were not further authenticated and maintained in the recommended medium.
Luciferase-expressing BxPC3 cells were generated by subcloning the firefly luciferase gene, Luc2 (Promega) into a pQCXIP vector. Transfection of the construct was carried out using 3 μg of DNA and Lipofectamine 2000 into Phoenix packaging cells. Retroviral supernatants were collected 48 h after transfection, spun down, and filtered through a 0.45 μm filter. Filtered retroviral supernatant was then incubated with cells for 24 h before changing to complete medium. Transduced cells were selected 24h later with G418 (Geneticin). EcSOD expressing BxPC3 and MiaPaCa-2 cells were generated as previously described.(26)
Western blotting
Protein expression of intracellular SODs was assayed as previously described.(26) Antibodies to MnSOD and CuZnSOD were prepared as previously characterized (17). The EcSOD antibody was a kind gift from Drs. James Crapo and Rebecca Oberley-Deegan.(27)
EcSOD activity determined by Electron Paramagnetic Resonance (EPR) and calormetric assays
The concentration of EcSOD that accumulated in cell culture media was determined by a kinetic analysis of the competition of the reaction of O2•− with 5,5-dimethylpyrroline-N-oxide (DMPO), kDMPO = 19 M−1 s−1 (28), and EcSOD, kEcSOD = 4.0 × 109 M−1 s−1 (29) using xanthine oxidase/hypoxanthine as a source of O2•−. Samples were examined by EPR using a Bruker EMX spectrometer with an HS cavity and AquaX 4-bore sample cell (Bruker Biospin). Bovine CnZnSOD was used as a verified standard (30); 1 unit of activity (50% inhibition of the DMPO-OOH signal) corresponds to 7.3 × 10−10 M of bovine CuZnSOD as dimer. One unit of activity for EcSOD corresponds to a concentration of 2.4 × 10−10 M of fully active EcSOD tetramers.
To quantify EcSOD activity the WST-1 SOD assay kit (Dojindo Molecular Technologies, #S311) was used as previously described. (24) To verify L-NNA inhibited NO-synthase (NOS) activity, a nitrite assay was performed on media samples using a kit as per manufacturer’s instructions (Griess Reagent System, Promega, #TB229). Media from BxPC3-control cells incubated with or without 100 μM L-NNA for 24 hrs were assayed for nitrite concentration using media without FBS or phenol red and compared to a nitrite standard curve for each assay. The data were normalized per cell.
RESULTS
Reduced expression of EcSOD as a prognostic indicator in patients with PDA
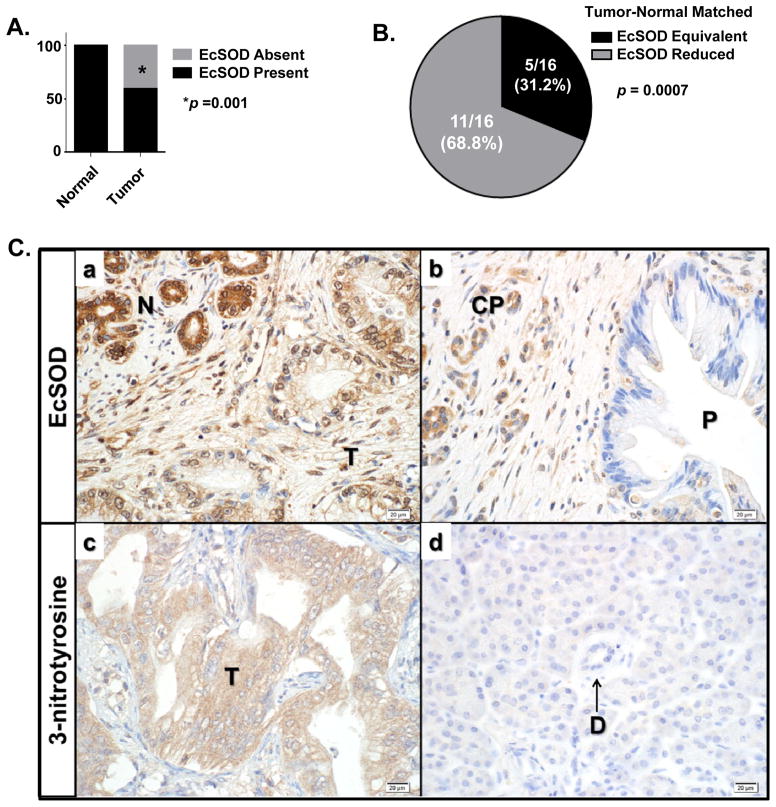
EcSOD has been shown to be highly expressed in several normal human tissues including lung, breast, and pancreas.(17, 18, 20, 21) However, in cancers of the breast, prostate, and lung, EcSOD expression, protein levels, and/or activity are frequently down-regulated compared to non-malignant tissues. (18, 20, 31, 32) To test the hypothesis that EcSOD expression is similarly altered in patients with PDA, a TMA consisting of normal and malignant pancreatic ductal epithelium was evaluated. As shown in Figure 1A, all core biopsies from normal pancreatic ductal epithelium had expression of EcSOD (28/28, 100%), while only 26/44 core biopsies from PDA had EcSOD expression (59%, p < 0.001). Since the core biopsies in the TMA examined were not from matched tumor-normal biopsies, specimens from patients treated with resection were evaluated to determine how EcSOD expression varied between malignant tissue and adjacent, non-diseased pancreatic ductal epithelium. As shown in Figure 1B and Ca, EcSOD expression was reduced in tumor tissue compared to adjacent non-diseased pancreas in 11/16 specimens evaluated (69%, p = 0.0007). A decrease in EcSOD expression was also noted in areas of premalignant pancreatic ductal epithelium (P) (PanIN-3) and chronic pancreatitis (CP) (Figure 1Cb).
Figure 1. EcSOD expression is significantly decreased in patient samples of pancreatic adenocarcinoma (PDA) relative to normal pancreatic ductal epithelium.
(A) Immunohistochemistry (IHC) was performed to evaluate EcSOD expression on a tissue microarray of normal pancreas and PDA which contains the following specimens: 42 PDA, 20 normal pancreas, and 8 chronic pancreatitis, each arrayed in duplicate. EcSOD expression (cytoplasmic in distribution) was scored as present or absent. Among core biopsies from normal pancreatic ductal epithelium, 28/28 (100%) had robust EcSOD expression while 18/44 core biopsies from PDA lacked EcSOD expression (41%). p = 0.001. The sparse stroma of normal pancreas and dense stroma of malignant tumors were devoid of EcSOD expression.
(B) IHC was performed on individual surgical resection specimens from patients with PDA. Staining in tumors was assessed relative to non-neoplastic ductal epithelium and islets of Langerhans, which each demonstrate strong staining. Staining was relative to normal pancreas and was either isointense or reduced intensity. Regions of normal pancreas showed intact EcSOD expression in all cases, whereas adjacent malignant ductal epithelium showed diminished EcSOD expression in 11/16 specimens p = 0.0007.
(C) Immunoreactive EcSOD was reduced and immunoreactive 3-nitrotyrosine was increased in PDA tissue versus normal pancreatic ductal epithelium. Panel Ca) EcSOD was reduced in malignant pancreatic ducts (T) compared to adjacent normal pancreatic ducts (N). Panel Cb) Evaluation of EcSOD in tumor tissue showed that expression was reduced in precursor PanIN lesions (P) and diseased pancreas (chronic pancreatitis; CP). Panel Cc and Cd) IHC for 3-nitrotyrosine (3-NT) showed 12/17 (70.6%) PDA samples demonstrated 3-NT staining in malignant tumor (T) ducts (Panel Cc) compared to 0/17 (0%) of the normal pancreatic ductal (D) epithelium (Panel Cd). Original magnification of all photomicrographs is 400×.
(D) Normal pancreas and pancreatic adenocarcinoma human cDNA samples from a Tissue Scan Real-Time pancreatic cancer qPCR Panel and specimens obtained from surgical resection were assessed for the mRNA expression of EcSOD. Combined there were 13 normal pancreas and 19 PDA samples evaluated. The data were normalized to β-actin expression and are presented as fold-change (log scale) of tumor samples compared to normal. Tumor specimens showed significant reduction of EcSOD expression compared to normal pancreas. p = 0.001.
(E) A thorough analysis of existing Oncomine data revealed a consistent decrease in EcSOD mRNA expression relative to normal pancreas in 5/7 studies; 2 studies were highly significant. p-values represent data provided by the primary study referenced. p < 0.05.
Loss of EcSOD expression is expected to result in a higher steady-state superoxide (O2•−) levels that can be evaluated via biomarkers indicative of oxidative damage to proteins. To evaluate the presence of a protein damage marker associated with the reactions of O2•− with nitric oxide (•NO) to form peroxynitrite (ONOO−) (33) in samples of human PDAs, immunoreactive 3-nitrotyrosine (3-NT) was evaluated in resected specimens of PDA (Figure 1Ccd). None of the non-disease adjacent normal pancreatic ductal epithelium (D) demonstrated significant staining for 3-NT (Figure 1Cd). However, 12/17 (71%) PDA (T) stained positively for 3-NT (p < 0.0001, Figure 1Cc). From these data we conclude that the presence of 3-NT is a common finding in PDA highlighting the reactions of O2•− with •NO that are ongoing as this disease progresses.
To evaluate changes in EcSOD mRNA expression, we evaluated specimens obtained from patients with PDA (n=19) and compared the expression of EcSOD to specimens obtained from normal pancreas (n=9). As shown in Figure 1D, there was a significant reduction in mRNA expression in PDA tissues relative to normal pancreas (p = 0.001). An evaluation of Oncomine data revealed a similar pattern where EcSOD expression was reduced in 5/7 studies evaluated, two of which were highly significant (Figure 1E). From these data we conclude that in addition to protein expression, EcSOD mRNA expression is also reduced in malignant pancreatic tumors. The decrease or absence of EcSOD expression in PDA relative to normal pancreas could explain, at least in part, the elevation of 3-NT seen in these tumor specimens.
EcSOD expression independently predicts survival in patients with PDA
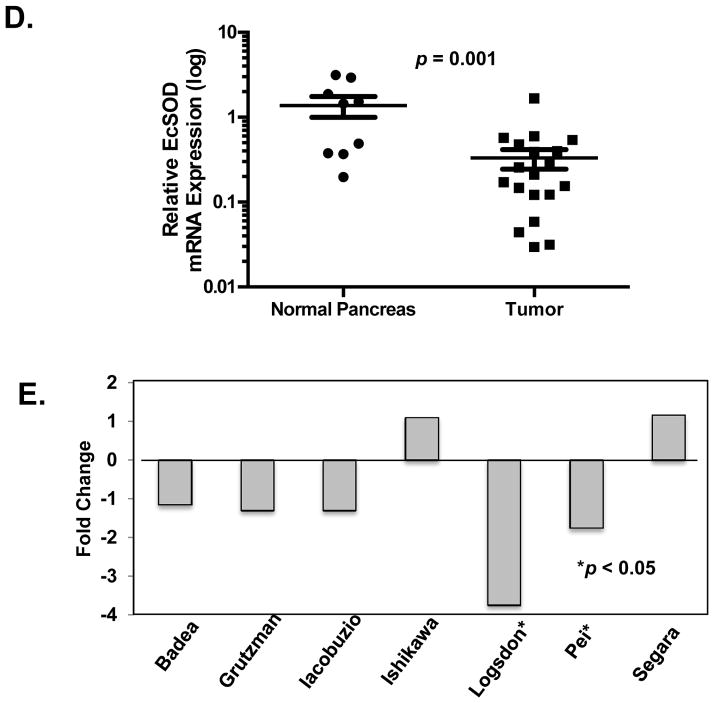
To test the hypothesis that loss of EcSOD expression is predictive for a worse outcome in patients with PDA, a TMA comprised of SEER patients with Stage I – IV PDA was evaluated and clinical parameters and outcome were correlated with EcSOD expression. Among the 114 patients with PDA, EcSOD expression was absent in 41 (36%). Patients with complete loss of EcSOD were more often diagnosed with Stage IV disease (54% vs. 30%, p = 0.019) and less often had surgical resection (39% vs. 67%, p = 0.004) compared to patients with intact EcSOD. In an adjusted proportional hazard model with the 114 PDA patients, female gender and tumor resection were strongly associated with reduced risk for death. Factors independently predictive of increased risk of death included age>65, year of diagnosis, registry, distant disease, and loss of EcSOD expression (HR=1.6, 95% CI = (1.0–2.6, p = 0.04). Kaplan-Meier analysis was performed to compare the overall survival of patients with intact (2+) and with complete loss (0+) of EcSOD expression. The median survival of patients with intact EcSOD expression was 11.0 months compared to 6.5 months for patients with loss of expression. As shown in Figure 2, loss of EcSOD expression was associated with a non-significant trend towards decreased overall survival. From these data we conclude that EcSOD expression is decreased in patients with PDA and that loss of EcSOD expression correlates with worse clinical and pathologic variables and may predict outcome.
Figure 2. Loss of EcSOD expression is associated with decreased long-term survival in patients with PDA.
Biopsies from 76 patients with PDA were evaluated for EcSOD expression using IHC. Patients with no expression (0+) and robust EcSOD expression (2+) in their PDA specimens were compared on univariate analysis. PDA patients with loss of EcSOD expression had a significant reduction in median survival (6.5 months vs. 11.0 months for those with intact EcSOD).
Overexpression of EcSOD reduces pancreatic tumor cell growth and invasion in vitro
To determine the expression of EcSOD in human pancreas and PDA cell lines, EcSOD mRNA was evaluated in three human PDA cell lines relative to HPNE, an immortalized but non-tumorigenic human pancreatic ductal epithelial cell line. Similar to what was shown in clinical specimens, there was a significant reduction in EcSOD mRNA expression in PDA cells relative to HPNE (Supplemental Figure 1A). To determine the impact of EcSOD over-expression on PDA cells, EcSOD was stably overexpressed in two PDA cell lines (Supplemental Figures 1B and 1C) and subsequently characterized. Over-expression of EcSOD resulted in the detection of EcSOD protein in cell lysates as well as in the media, but did not impact expression of either MnSOD or CuZnSOD (Supplemental Figure 1C). SOD activity in the culture media from transduced cells was increased as determined by an indirect colorimetric assay as well as by a direct, quantitative EPR (Supplemental Figure 2A and B). These results show that active EcSOD protein was normally processed and secreted into the media.
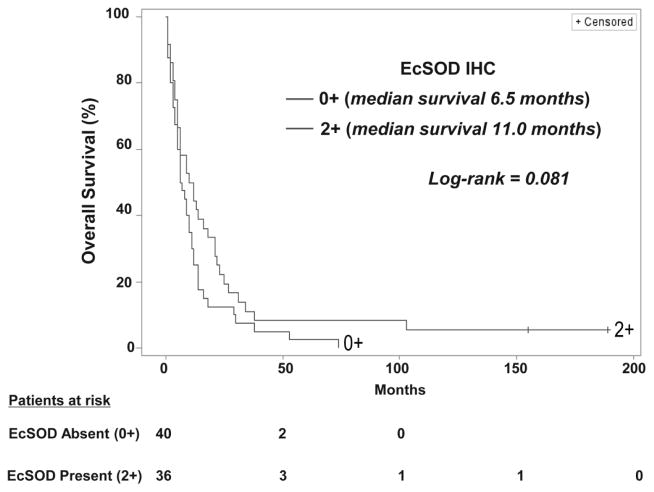
These stably expressing cells were then used to test the hypothesis that overexpression of EcSOD would impact PDA tumor cell growth in culture. As shown in Figure 3A & B, stable overexpression of EcSOD resulted in a significant reduction in clonogenic potential in BxPC3 and MiaPaCa2 PDA cell lines. EcSOD stably expressing cells also displayed a significantly increased doubling time compared to controls (Figure 3C).
Figure 3. Stable overexpression of EcSOD attenuates the malignant phenotype of human pancreatic cancer cell lines.
(A) Stably overexpressing BxPC3 cells and (B) MiaPaCa2 cells were plated to assess colony formation after 2 weeks. Plating efficiency for both Bx-EcSOD and Mia-EcSOD cells decreased compared to control cells. Data represent the relative mean ± SE. p = 0.015 for BxPC3 and 0.044 for MiaPaCa-2.
(C) Growth of overexpressing cells (Bx-EcSOD and Bx-Control) was examined for a total of 96 h. Doubling time in the Bx-EcSOD cells after overexpression increased compared to control cells. Data represent the mean ± SE of cell population doubling time in hours. p = 0.031.
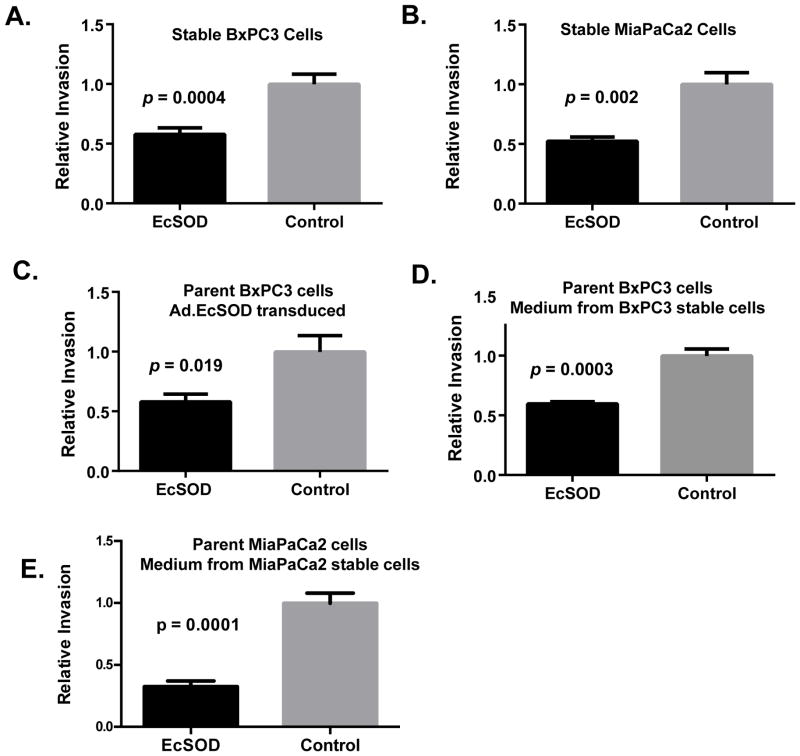
We next evaluated the impact of EcSOD overexpression on tumor cell invasion. BxPC3 and MiaPaCa2 cell lines stably overexpressing EcSOD and empty vector showed no difference in migration capacity at 24h (data not shown). EcSOD overexpressing BxPC3 and MiaPaCa2 cells showed a significant reduction in invasion at 24h compared to controls (Figure 4A and 4B). Parent BxPC3 cells were then transiently transduced with Ad.EcSOD or Ad.Empty for 72h, harvested, and placed on the Matrigel. As shown in Figure 4C, cells transduced with EcSOD adenovirus also showed significantly less invasion compared to controls. To determine whether EcSOD would have a cell-autonomous effect on tumor cell invasion, conditioned media from stably expressing BxPC3 or MiaPaCa2 cells were harvested after 48 h and placed onto the respective parent cells in the Matrigel. Conditioned media from the EcSOD-overexpressing cells, but not the parent cells, significantly reduced tumor cell invasion (Figures 4D and 4E). These findings demonstrate the potent effect of EcSOD on tumor cell invasion both when expressed by cells and when enriched in the tumor microenvironment. These findings support the hypothesis that the more aggressive disease progression seen in patients who have lost EcSOD expression in their tumors may directly relate to less EcSOD activity in the extracellular environment.
Figure 4. EcSOD overexpression significantly decreases human pancreatic tumor cell invasiveness in vitro.
The effect of EcSOD expression on invasion using Matrigel invasion chambers was examined. In all graphs cells were seeded at 2×105 and allowed to incubate for 22–24 h before being analyzed.
(A) Invasion of cells generated to stably overexpress EcSOD or control vector measured by MTT (Bx-EcSOD and Bx-Control). p = 0.0004.
(B) Invasion of cells generated to stably overexpress EcSOD or control vector measured by cell counting (Mia-EcSOD, mean 135 cells ± 28 cells and Mia-Control, mean 250 cells ± 49 cells, p = 0.002).
(C) Effect of EcSOD transduction (MOI 25 for 72 h) on parent BxPC3 cell invasion measured by MTT. p = 0.019.
(D) Invasion of parent BxPC3 cells incubated with conditioned media harvested after 48 h from stably overexpressing cells measured by MTT (Bx-EcSOD and Bx-Control). p = 0.0003.
(E) Invasion of parent MiaPaCa2 cells incubated with conditioned media harvested after 48h from stably overexpressing cells measured by cell counting (Mia-EcSOD, mean 73 cells ± 11 cells and Mia-Control, mean 227 cells ± 27 cells. p = 0.0001).
The effects of manipulating superoxide and the reactions of hydroperoxides and nitric oxide on the ability of EcSOD to inhibit PDA cell invasive capacity
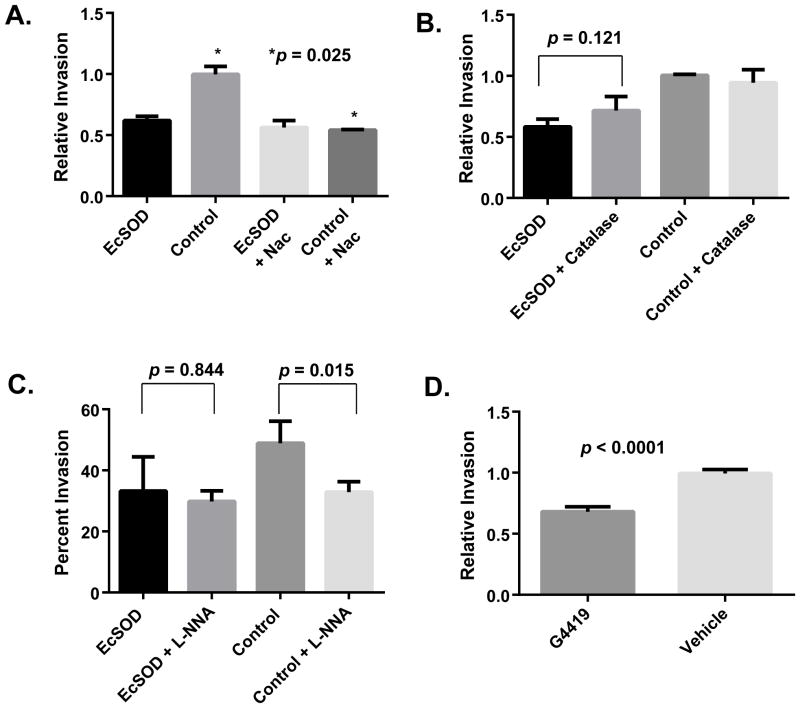
To elucidate the mechanism of action of EcSOD on PDA cell invasion, BxPC3 cells were incubated with 20 mM N-acetylcysteine (NAC), which is a non-specific thiol antioxidant that scavenges a wide variety of reactive oxygen (ROS) and nitrogen (RNS) containing species as well as reducing protein thiols (34). As shown in Figure 5A, NAC significantly reduced invasion in control cells after 24h without a significant impact on the invasion of EcSOD overexpressing PDA cells. These results support the hypothesis that EcSOD overexpression alters the redox balance leading to inhibition of PDA cell invasion. When the experiment was repeated using 10 U/mL catalase, a specific enzymatic scavenger of H2O2 (Figure 5B), catalase did not significantly decrease invasion of either Bx-Control or Bx-EcSOD cells after 24h. These results support the hypothesis that extracellular H2O2 (which is the product of EcSOD reacting with superoxide) was not responsible for the inhibition of invasion caused by EcSOD over expression.
Figure 5. Human pancreatic tumor cell invasion is inhibited by EcSOD activity through inhibition of superoxide and nitric oxide generating radicals.
(A) The effect of N-acetylcysteine (NAC) on stable cell (Bx-EcSOD and Bx-Control) invasion was assessed. 20 mM NAC had no effect on the invasion of Bx-EcSOD cells but showed a significant reduction on invasion of Bx-Control cells. Data represent the mean ± SE of relative invasion compared to control. p = 0.025. Comparison of all groups, p = 0.0014.
(B) The effect of catalase on stable cell (Bx-EcSOD and Bx-Control) invasion was examined. Addition of 10 IU/mL of catalase showed no effect on tumor cell invasion. Data represent the mean ± SE of percent invasion (invasion/migration *100) compared to control. EcSOD vs. EcSOD + Catalase, p = 0.12. Comparison of all groups, p = 0.111.
(C) The effect of NG-nitro-L-arginine (L-NNA) on stable cell invasion was determined. Incubation with 100 μM of LNNA had no effect on Bx-EcSOD invasion (p=0.84) but showed a significant reduction on invasion of Bx-Control cells. p=0.015. Overall statistical evaluation p = 0.004. Data represent the mean ± SE of percent invasion (invasion/migration *100).
(D) 50 μM of EcSOD mimetic GC4419 was examined for effects on the invasion of parent BxPC3 pancreatic cancer cells. 50 μM of GC4419 showed a significant decrease in invasion of BxPC3 cells compared to vehicle. p <0.0001. Data represent the mean ± SE of relative invasion compared to vehicle.
By catalyzing the dismutation of O2•−, EcSOD inhibits the reaction of nitric oxide (•NO) with O2•− to form RNS such as ONOO− (35), and therefore the potential roles of RNS derived from reactions of •NO with O2•− were examined. Bx-EcSOD and Bx-Control cells were analyzed in a Matrigel assay that included the arginine analog NG-nitro-L-arginine (100 μM L-NNA), a competitive inhibitor of NO-synthase activity. L-NNA was found to significantly inhibit the accumulation of nitrite (which is derived from the reaction of •NO with O2) in the media of BxPC3 cells supporting the conclusion that NO-synthase activity was inhibited by L-NNA (Supplemental Figure 3). Similar to what was seen with NAC, L-NNA did not significantly impact the invasive capacity of the Bx-EcSOD cells, while causing a significant inhibition of the invasive capacity of the Bx-Control cell line (Figure 5C). These results support the hypothesis that the decrease in tumor cell invasion seen with the overexpression of EcSOD was due to the removal of O2•−, reducing the reactions of •NO with O2•−, and limiting the formation of ONOO−.
Superoxide-specific SOD mimetics have also been shown to limit the formation of ONOO− induced damage to proteins, as assayed by the formation of immunoreactive 3-nitrotyrosine in the livers from irradiated animals (36). Therefore the effects of the small molecule superoxide dismutase mimetic, GC4419, on PDA cell invasion were determined. GC4419 is reported to be highly selective in its dismutation of O2•− (and its protonated form, hydroperoxyl radical, HOO•), while not catalyzing reactions with other ROS under physiologic conditions, making it a suitable chemical probe to look at the specific role of O2•− in this biology. As shown in Figure 5D, 50 μM of GC4419 resulted in a significant reduction of tumor cell invasion. These results further support the hypothesis that excess extracellular O2•− significantly accelerates PDA cell invasion. GC4419 is currently being evaluated in a Phase 1b clinical trial (NCT01921426) in head and neck cancer patients receiving chemoradiation therapy, highlighting the potential translational impact of these findings.
Stable overexpression of EcSOD reduces PDA xenograft tumor growth and peritoneal growth in vivo
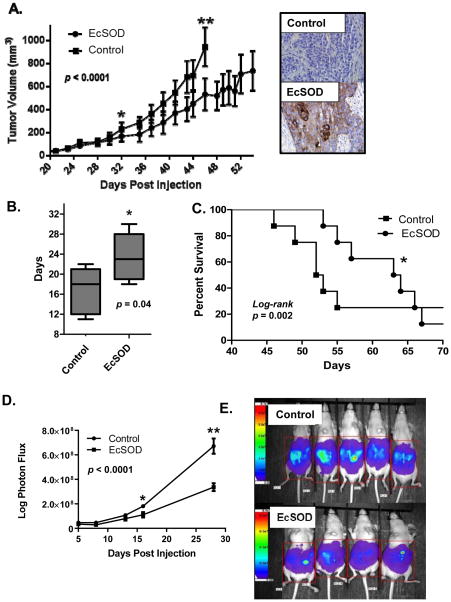
To test the hypothesis that stable EcSOD expression would reduce tumor growth in vivo, Bx-EcSOD and Bx-Control xenografts were generated in athymic nude mice. Overexpression of EcSOD effectively suppressed tumor growth compared to controls (Figure 6A). Bx-EcSOD tumors also took significantly longer time to double in size and mice bearing EcSOD-expressing BxPC3 tumors had prolonged median survival compared to mice bearing empty vector tumors (Figure 6B & C). To examine the impact of EcSOD overexpression on peritoneal tumor formation, Bx-EcSOD and Bx-Control cells were delivered via I.P. injection into athymic nude mice and monitored as a function of time with bioluminescence. The peritoneal cavity provides an environment more similar to clinical PDA than the xenograft tumor model. The bioluminescence signal was equivalent during the first 5 days post-injection but at 16 and 28 days post-injection, the animals bearing EcSOD stably overexpressing cells formed less peritoneal tumor growth as compared to controls (Figures 6D and E).
Figure 6. Stable overexpression of EcSOD reduces tumor xenograft growth, doubling time, animal survival, and peritoneal growth in athymic nude mice.
BxPC3 cells stably expressing EcSOD or Empty vector (Control) were injected into athymic nude mice hind limbs (8 animals per group) on day 1. When maximum tumor diameter exceeded 15 mm mice were sacrificed.
(A) Tumor growth curves demonstrate that EcSOD tumor volumes were significantly smaller (p = 0.03) starting on day 32 and remained significantly smaller until day 46 (p = 0.0001) when the first mouse met criteria for euthanasia. Overall comparison of growth, p <0.0001. IHC was performed in 5 tumors to confirm EcSOD protein expression was present in the EcSOD cell lines compared to empty vector control (Inset).
(B) Box and whiskers plot demonstrating the minimum, maximum and mean days it took for the tumor to double from a diameter of 6 mm to 12 mm. EcSOD tumors took significantly longer time to double (23 vs. 17 days, p=0.04,).
(C) Kaplan-Meier survival plot demonstrates that mice baring EcSOD expressing BxPC3 tumors had prolonged median survival compared to mice baring Empty vector tumors (63 vs. 52 days p = 0.002, log-rank).
(D) Following intraperitoneal injection of 1 × 107 Bx-EcSOD and Bx-Control cells animals were imaged starting 5 days post-injection up to 28 days when the control group met criteria for sacrifice. A region of interest was placed around each mouse image and total photon flux was quantified (AMIView software) and data reported as mean ±SE of total photon flux (photons per second). Values obtained show statistically significant differences in growth between cell types at 16 and 28 days post-injection. *p = 0.015, **p = 0.003. Overall statistical comparison between curves, p < 0.0001.
(E) Representative image of BLI of mice 28 days post-intraperitoneal injections of luciferase-expressing Bx-EcSOD or Bx-Control cells. A region of interest (as shown) was placed around each mouse and total photon flux was quantified. Mice injected with luciferized Bx-EcSOD cells showed statistically less tumor burden than control mice.
DISCUSSION
Oxidative stress as a contributor to malignant disease progression has been extensively studied and proposed to play a role in several solid tumors refractory to conventional therapies.(13, 18, 20, 31, 37) PDA is currently the fourth leading cause of cancer deaths in the U.S. and remains highly lethal due to a poor understanding of the biology of the disease and resistance to available, conventional therapies.(38) Prior evaluations of SODs in PDA have demonstrated potential for antioxidant therapy as a treatment for this disease, however the mechanism underlying the anti-cancer effects of this treatment and translational avenues to the clinic remain relatively unexplored.(17, 19)
The current studies describe both analyses of clinical specimens and preclinical in vitro and in vivo studies, testing the hypothesis that the loss of EcSOD leads to an increase in the progression and invasiveness of PDA. These investigations are also the first to provide an in-depth investigation correlating EcSOD with clinical outcomes in PDA patients. In addition, these studies examined the effect of EcSOD activity on invasion and metastasis in in vitro and in vivo models of PDA using multiple methods of EcSOD overexpression.
The results using patient samples indicate that loss of EcSOD expression is a common feature of PDAs that is accompanied by an increase in 3-NT formation,(39) which may indicate increased protein nitration mediated by ONOO−. The loss of EcSOD expression in patient samples correlated with more advanced disease and a trend towards shorter overall survival. These results support the hypothesis that loss of EcSOD expression in PDAs may lead to an increase in ONOO− in the extracellular environment that could contribute to a more aggressive disease phenotype. Since the primary route of ONOO− formation is via reaction of O2•− with •NO, either increases in O2•− or •NO would be expected to give rise to increase ONOO− formation, which we propose would be exacerbated by loss of EcSOD expression.
In support of the aforementioned hypothesis we also found in preclinical cell culture models that both the stable and transient overexpression of EcSOD in PDA cells decreased cloning efficiency and increased the doubling time of PDA cells. This was true for both cell-directed expression and using conditioned media. In addition, in vitro overexpression of EcSOD or treatment with the O2•−-specific small molecule dismutase mimetic (GC4419) also caused significant decreases in PDA cell invasion.
Interestingly, in parent BxPC3 cells but not BxPC3 cells overexpressing EcSOD, in vitro invasion was inhibited by a thiol antioxidant (NAC) and a •NO-synthase inhibitor (L-NNA). NAC is a non-specific thiol antioxidant that can react directly with several ROS/RNS, react to keep protein thiols reduced, as well as be taken up by cells to increase levels of small molecular weight thiols such as cysteine, γ-glutamylcysteine, and glutathione (34, Metabolic oxidative stress activates signal transduction and gene expression during glucose deprivation in human tumor cells.(40) Since NAC only had an effect on invasion in the absence of EcSOD overexpression, these results support the conclusion that redox imbalances reversed by NAC in the absence of EcSOD are contributing to the invasive properties of BxPC3 cells. Furthermore, the finding that L-NNA also only inhibited invasion in the absence of EcSOD overexpression, supports the hypothesis that reactions of •NO with O2•− only contribute to invasion of BxPC3 cells when EcSOD is minimally expressed. Taken together these results support the hypothesis that loss of EcSOD in PDA leads to increased reactions of •NO with O2•− that contribute to alterations in the extracellular redox balance leading to the invasive phenotype. This idea is further supported by the increases in 3-NT seen in human PDA specimens. Finally these results support the speculation that the formation of ONOO− may contribute to the aggressive progression of clinical PDAs.
While we have not addressed the precise mechanism of how ONOO− could contribute to invasiveness and progression of PDA in the current work, a previous report found that extracellular lecithinized superoxide dismutase (PC-SOD) inhibited metastasis in a lung cancer animal model by a mechanism that might involve the inhibition of ONOO− formation leading to less damage the extracellular matrix and reduced tumor metastasis.(41) Future studies will seek to identify the targets of reactive nitrogen species such as ONOO− that may contribute to increase disease progression in PDA.
Here we have shown that loss of EcSOD expression in human PDA may be associated with more aggressive disease progression and poorer patient outcomes; however the mechanism underlying silencing of EcSOD in PDA remains unknown. In human lung and breast cancers, EcSOD is lost at the transcriptional level in association with a distinct gain of aberrant cytosine methylation in the EcSOD promoter region (18, 20), so this mechanism may also extend to PDA and will be the subject of future inquiry. Overall, our results support the hypothesis that increasing SOD activity in the extracellular space could provide a useful target for limiting disease progression in PDA. Furthermore these results continue to support the hypothesis that small molecule SOD mimetics could be useful adjuvants to therapies designed to limit disease progression.
Supplementary Material
Statement of Translational Relevance.
Tumor cells exhibit imbalances in redox metabolism that contribute to their abnormal growth and invasive capacity. The results of this study demonstrate that the loss of Extracellular Superoxide Dismutase, an extracellular antioxidant enzyme, contributes to the invasive phenotype seen in pancreatic ductal adenocarcinoma. Mechanistic studies reveal that this appears to occur through the formation of reactive species derived from reactions of superoxide and nitric oxide. These findings reveal potential targets for therapy with superoxide dismutase mimics to limit pancreatic cancer progression.
Acknowledgments
Financial Support: This work was supported by the American Surgical Association Foundation Fellowship (JJM); the American Cancer Society Seed Grant through the Holden Comprehensive Cancer Center (JJM); NIH grant T32CA148062 (JJM); generous gifts from the families of John Van Sickel and Jack Kloet and grateful patient donations to the Holden Comprehensive Cancer Center for pancreatic cancer research. Radiation and Free Radical Research Core in the Holden Comprehensive Cancer Center at The University of Iowa core laboratory support through NIH P30 CA086862. DRS and MAF were supported by R01 CA182804, R01CA133114, and R21 CA161182; GRB and BAW were supported by R01 CA169046; FED was supported by RO1 CA115438; and MDH was supported by RO1 CA130916. Construction of the pancreatic TMA was supported by NCI contract numbers N01-PC-35143 (Iowa), N01-PC-35137 (Hawaii), and N01-PC-35139 (Los Angeles).
The authors thank Dr. James Crapo and Dr. Rebecca Oberley-Deegan (National Jewish Medical and Research Center, Denver, CO, USA) for providing antibodies to EcSOD. We also thank Dr. Apollina Goel (Free Radical and Radiation Biology Program, The University of Iowa, IA, USA) for the use of a Tecan plate reader and Dr. Nalin Goonesekere for assistance with Oncomine data gathering and interpretation.
Footnotes
Conflicts of interest: Dr. Beardsley serves as a member of the boards of directors of Galera, Euclises Pharmaceuticals and Epigenetx, serves as a paid consultant to Confluence Life Sciences, and holds equity positions in Galera, Euclises, Epigenetx, and Confluence. The remaining authors have no conflicts of interest to disclose.
References
- 1.Carlsson LM, Jonsson J, Edlund T, Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(14):6264–8. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circulation research. 2003;93(7):622–9. doi: 10.1161/01.RES.0000092140.81594.A8. [DOI] [PubMed] [Google Scholar]
- 3.Marklund SL. Extracellular superoxide dismutase in human tissues and human cell lines. The Journal of clinical investigation. 1984;74(4):1398–403. doi: 10.1172/JCI111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ridnour LA, Oberley TD, Oberley LW. Tumor suppressive effects of MnSOD overexpression may involve imbalance in peroxide generation versus peroxide removal. Antioxidants & redox signaling. 2004;6(3):501–12. doi: 10.1089/152308604773934260. [DOI] [PubMed] [Google Scholar]
- 5.Oberley LW, Oberley TD, Buettner GR. Cell division in normal and transformed cells: the possible role of superoxide and hydrogen peroxide. Medical hypotheses. 1981;7(1):21–42. doi: 10.1016/0306-9877(81)90018-9. [DOI] [PubMed] [Google Scholar]
- 6.Oberley LW, Oberley TD, Buettner GR. Cell differentiation, aging and cancer: the possible roles of superoxide and superoxide dismutases. Medical hypotheses. 1980;6(3):249–68. doi: 10.1016/0306-9877(80)90123-1. [DOI] [PubMed] [Google Scholar]
- 7.Oberley LW, Oberley TD. Role of antioxidant enzymes in cell immortalization and transformation. Molecular and cellular biochemistry. 1988;84(2):147–53. doi: 10.1007/BF00421049. [DOI] [PubMed] [Google Scholar]
- 8.Chaiswing L, Zhong W, Cullen JJ, Oberley LW, Oberley TD. Extracellular redox state regulates features associated with prostate cancer cell invasion. Cancer research. 2008;68(14):5820–6. doi: 10.1158/0008-5472.CAN-08-0162. [DOI] [PubMed] [Google Scholar]
- 9.Chaiswing L, Zhong W, Liang Y, Jones DP, Oberley TD. Regulation of prostate cancer cell invasion by modulation of extra- and intracellular redox balance. Free radical biology & medicine. 2012;52(2):452–61. doi: 10.1016/j.freeradbiomed.2011.10.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis A, Du J, Liu J, Ritchie JM, Oberley LW, Cullen JJ. Metastatic progression of pancreatic cancer: changes in antioxidant enzymes and cell growth. Clinical & experimental metastasis. 2005;22(7):523–32. doi: 10.1007/s10585-005-4919-7. [DOI] [PubMed] [Google Scholar]
- 11.Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, Goswami PC. Redox control of the cell cycle in health and disease. Antioxidants & redox signaling. 2009;11(12):2985–3011. doi: 10.1089/ars.2009.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menon SG, Coleman MC, Walsh SA, Spitz DR, Goswami PC. Differential susceptibility of nonmalignant human breast epithelial cells and breast cancer cells to thiol antioxidant-induced G(1)-delay. Antioxidants & redox signaling. 2005;7(5–6):711–8. doi: 10.1089/ars.2005.7.711. [DOI] [PubMed] [Google Scholar]
- 13.Jorgenson TC, Zhong W, Oberley TD. Redox imbalance and biochemical changes in cancer. Cancer research. 2013;73(20):6118–23. doi: 10.1158/0008-5472.CAN-13-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oberley LW, Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer research. 1979;39(4):1141–9. [PubMed] [Google Scholar]
- 15.Gius D, Spitz DR. Redox signaling in cancer biology. Antioxidants & redox signaling. 2006;8(7–8):1249–52. doi: 10.1089/ars.2006.8.1249. [DOI] [PubMed] [Google Scholar]
- 16.Behrend L, Henderson G, Zwacka RM. Reactive oxygen species in oncogenic transformation. Biochemical Society transactions. 2003;31(Pt 6):1441–4. doi: 10.1042/bst0311441. [DOI] [PubMed] [Google Scholar]
- 17.Teoh ML, Sun W, Smith BJ, Oberley LW, Cullen JJ. Modulation of reactive oxygen species in pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(24):7441–50. doi: 10.1158/1078-0432.CCR-07-0851. [DOI] [PubMed] [Google Scholar]
- 18.Teoh-Fitzgerald ML, Fitzgerald MP, Jensen TJ, Futscher BW, Domann FE. Genetic and epigenetic inactivation of extracellular superoxide dismutase promotes an invasive phenotype in human lung cancer by disrupting ECM homeostasis. Molecular cancer research : MCR. 2012;10(1):40–51. doi: 10.1158/1541-7786.MCR-11-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sibenaller ZA, Welsh JL, Du C, Witmer JR, Schrock HE, Du J, et al. Extracellular superoxide dismutase suppresses hypoxia-inducible factor-1alpha in pancreatic cancer. Free radical biology & medicine. 2014;69:357–66. doi: 10.1016/j.freeradbiomed.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teoh-Fitzgerald ML, Fitzgerald MP, Zhong W, Askeland RW, Domann FE. Epigenetic reprogramming governs EcSOD expression during human mammary epithelial cell differentiation, tumorigenesis and metastasis. Oncogene. 2014;33(3):358–68. doi: 10.1038/onc.2012.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teoh ML, Fitzgerald MP, Oberley LW, Domann FE. Overexpression of extracellular superoxide dismutase attenuates heparanase expression and inhibits breast carcinoma cell growth and invasion. Cancer research. 2009;69(15):6355–63. doi: 10.1158/0008-5472.CAN-09-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6(1):1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takikita M, Altekruse S, Lynch CF, Goodman MT, Hernandez BY, Green M, et al. Associations between selected biomarkers and prognosis in a population-based pancreatic cancer tissue microarray. Cancer research. 2009;69(7):2950–5. doi: 10.1158/0008-5472.CAN-08-3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu Y, Iida S, Lund DD, Weiss RM, DiBona GF, Watanabe Y, et al. Gene transfer of extracellular superoxide dismutase reduces arterial pressure in spontaneously hypertensive rats: role of heparin-binding domain. Circulation research. 2003;92(4):461–8. doi: 10.1161/01.RES.0000057755.02845.F9. [DOI] [PubMed] [Google Scholar]
- 25.Cullen JJ, Weydert C, Hinkhouse MM, Ritchie J, Domann FE, Spitz D, et al. The role of manganese superoxide dismutase in the growth of pancreatic adenocarcinoma. Cancer research. 2003;63(6):1297–303. [PubMed] [Google Scholar]
- 26.Case AJ, Mezhir JJ, O’Leary BR, Hrabe JE, Domann FE. Rational design of a secreted enzymatically inactive mutant of extracellular superoxide dismutase. Redox report : communications in free radical research. 2012;17(6):239–45. doi: 10.1179/1351000212Y.0000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao H, Arunachalam G, Hwang JW, Chung S, Sundar IK, Kinnula VL, et al. Extracellular superoxide dismutase protects against pulmonary emphysema by attenuating oxidative fragmentation of ECM. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(35):15571–6. doi: 10.1073/pnas.1007625107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkelstein E, Rosen GM, Rauckman EJ. Spin Trapping - Kinetics of the Reaction of Superoxide and Hydroxyl Radicals with Nitrones. J Am Chem Soc. 1980;102(15):4994–9. [Google Scholar]
- 29.Marklund SL. Human copper-containing superoxide dismutase of high molecular weight. Proceedings of the National Academy of Sciences of the United States of America. 1982;79(24):7634–8. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein S, Fridovich I, Czapski G. Kinetic properties of Cu, Zn-superoxide dismutase as a function of metal content--order restored. Free radical biology & medicine. 2006;41(6):937–41. doi: 10.1016/j.freeradbiomed.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 31.Chaiswing L, Zhong W, Oberley TD. Increasing discordant antioxidant protein levels and enzymatic activities contribute to increasing redox imbalance observed during human prostate cancer progression. Free radical biology & medicine. 2014;67:342–52. doi: 10.1016/j.freeradbiomed.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svensk AM, Soini Y, Paakko P, Hiravikoski P, Kinnula VL. Differential expression of superoxide dismutases in lung cancer. American journal of clinical pathology. 2004;122(3):395–404. doi: 10.1309/A45Q-HB0Q-RRX6-CT9A. [DOI] [PubMed] [Google Scholar]
- 33.Tsikas D, Duncan MW. Mass spectrometry and 3-nitrotyrosine: Strategies, controversies, and our current perspective. Mass spectrometry reviews. 2013 doi: 10.1002/mas.21396. [DOI] [PubMed] [Google Scholar]
- 34.Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochimica et biophysica acta. 2013;1830(8):4117–29. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Obal D, Dai S, Keith R, Dimova N, Kingery J, Zheng YT, et al. Cardiomyocyte-restricted overexpression of extracellular superoxide dismutase increases nitric oxide bioavailability and reduces infarct size after ischemia/reperfusion. Basic research in cardiology. 2012;107(6):305. doi: 10.1007/s00395-012-0305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coleman MC, Olivier AK, Jacobus JA, Mapuskar KA, Mao G, Martin SM, et al. Superoxide mediates acute liver injury in irradiated mice lacking sirtuin 3. Antioxidants & redox signaling. 2014;20(9):1423–35. doi: 10.1089/ars.2012.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wheeler MD, Smutney OM, Samulski RJ. Secretion of extracellular superoxide dismutase from muscle transduced with recombinant adenovirus inhibits the growth of B16 melanomas in mice. Molecular cancer research : MCR. 2003;1(12):871–81. [PubMed] [Google Scholar]
- 38.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. The New England journal of medicine. 2013;369(18):1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vickers SM, MacMillan-Crow LA, Green M, Ellis C, Thompson JA. Association of increased immunostaining for inducible nitric oxide synthase and nitrotyrosine with fibroblast growth factor transformation in pancreatic cancer. Archives of surgery. 1999;134(3):245–51. doi: 10.1001/archsurg.134.3.245. [DOI] [PubMed] [Google Scholar]
- 40.Blackburn RV, Spitz DR, Liu X, Galoforo SS, Sim JE, Ridnour LA, et al. Metabolic oxidative stress activates signal transduction and gene expression during glucose deprivation in human tumor cells. Free radical biology & medicine. 1999;26(3–4):419–30. doi: 10.1016/s0891-5849(98)00217-2. [DOI] [PubMed] [Google Scholar]
- 41.Takenaga M, Igarashi R, Ochiai A, Mizushima Y. Effect of lecithinized superoxide dismutase (PC-SOD) on experimental pulmonary metastasis in mice. Free radical biology & medicine. 1999;26(9–10):1117–25. doi: 10.1016/s0891-5849(98)00301-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.