Abstract
Background
The clinical course of individuals exposed to alcohol in utero is influenced by multiple factors, including the social environments of the gravid female and offspring. In the present studies we focused on the effects of prenatal alcohol exposure (PAE) and the prenatal and early-life social environments on the hippocampal formation, as impaired development and functioning of this brain region has been implicated in several of the adverse cognitive outcomes associated with PAE.
Methods
We combined our PAE mouse model with two conditions of housing pregnant dams and their preweanling offspring: the standard nest (SN), in which a dam is individually housed prior to parturition and then remains isolated with her offspring, and the communal nest (CN), in which multiple dams are housed together prior to parturition and then following delivery the moms and their litters share a nest. Mouse dams consumed either 10% (w/v) ethanol in 0.066% (w/v) saccharin (SAC) or 0.066% (w/v) SAC alone using a limited (4-h) access, drinking-in-the-dark paradigm. Immunoblotting techniques were used to measure levels of the glucocorticoid receptor (GR), 11-β-hydroxysteroid dehydrogenase 1, the FK506-binding proteins 51 and 52, and corticotropin-releasing hormone (CRH) receptor type 1 in the hippocampal formation isolated from male adolescent offspring. We also determined the effect of PAE and rearing conditions on context discrimination, a hippocampal-dependent learning/memory task.
Results
SN PAE offspring displayed impaired context discrimination and neurochemical changes in the hippocampal formation consistent with increased GR nuclear localization. These effects of PAE were, in general, ameliorated in mice reared in a CN. The CN also altered neurochemical measures and improved learning/memory in SAC control animals
Conclusions
These studies demonstrate a complex interplay between the effects of PAE and social environments. The findings have important translational implications, as well as highlight the importance of considering rearing conditions in the interpretation of research findings on PAE.
Keywords: prenatal alcohol, glucocorticoid receptor, hippocampus, housing, communal nest
BACKGROUND
The in utero and early postnatal milieus, and the interplay between these two environments, exert long-lasting effects on an organism (Barker, 2004; Gluckman and Hanson, 2004; Monk et al., 2012). Correspondingly, the clinical course of individuals exposed to alcohol in utero is impacted by the physiologic state of the pregnant woman and the early life social environment (Olson et al., 2009; Schneider et al., 2011; Streissguth et al., 2004). Prenatal alcohol exposure (PAE) produces a variety of developmental, cognitive, behavioral, social and physical abnormalities termed Fetal Alcohol Spectrum Disorders (FASD) (Guerri et al., 2009; Kelly et al., 2000). Studies which explore the interactions between PAE and the maternal and early social environments offer the potential to identify interventions that may alter the outcomes of FASD, as well as provide insight on the molecular underpinnings of the effects of PAE.
Animal models of PAE are critical, translational tools in the development of effective treatment strategies, as the long-term consequences of interventions can be assessed on time scales much shorter than are available in clinical populations. In the present studies we employed a mouse model of PAE (Brady et al., 2012) to determine the impact of the maternal and early life (preweaning) social environments on PAE’s effects on measures of hippocampal neurochemistry and learning and memory. We have reported that animals that were exposed to alcohol prenatally and reared in a standard nest (SN) environment, in which a single dam and her litter are present, display alterations in hippocampal formation neurochemistry (Brady et al., 2013; Caldwell et al., 2014), reduced hippocampal slice synaptic plasticity (Brady et al., 2013) and impaired hippocampal formation-dependent learning and memory (Brady et al., 2012; Caldwell et al., 2014). In the present studies, we compared neurochemical measures and learning/memory performance in animals reared under these same SN conditions with those reared in a communal nest (CN), an ethologically relevant housing system in which multiple dams are housed together and maintain their litters in a single nest (Branchi, 2009). Compared to the SN, the CN is characterized by increased maternal-pup interactions (Branchi et al., 2013a; Branchi et al., 2006a; Branchi et al., 2006b) and increased peer interactions prior to weaning (Branchi, 2009; Branchi et al., 2013a).
Although in utero exposure to alcohol is associated with structural and functional alterations in multiple brain regions, the effects on the hippocampal formation are particularly marked (Valenzuela et al., 2012). Corticosteroids (corticosterone in rodents and cortisol in humans), acting through glucocorticoid receptors (GRs), are key regulators of hippocampal formation development, structure and functioning (McEwen, 2007, 2012) and, thus, may be a target of PAE. In the present studies we chose to use our previous demonstrations of PAE-associated alterations in the hippocampal formation GR system and impaired performance on a hippocampal-dependent learning and memory task, context discrimination, in adolescent male mice (Caldwell et al., 2014), as outcome measures for the assessment of the interplay between the maternal and early life social environments and PAE. We found that saccharin (SAC) control and PAE mice responded differently to rearing conditions, demonstrating interaction between PAE and the prenatal and postnatal environments. In addition, our findings underscore the importance of considering rearing conditions when interpreting research studies on the effects of PAE.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the University of New Mexico Institutional Animal Care and Use Committee. C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were maintained in a reverse light/dark (lights off at 0800 h and on at 1800 h), temperature-controlled vivarium. Animals had free access to food and water except for the removal of the water bottle(s) during the alcohol/SAC consumption procedure described below. At the time of tissue collection or at the beginning of behavioral testing, offspring were postnatal day 40–55; ages are noted in figure legends. All tissue was taken between 0730 and 0830 h. All neurochemical and behavioral studies in the offspring used naïve male mice.
Animal housing, mating and prenatal alcohol exposure paradigm
SN females/dams were housed alone prior to and throughout pregnancy. For communal rearing, 3 or 4 females were housed together prior to and throughout pregnancy. The Whitten effect (Heiderstadt et al., 2014; Whitten, 1957) was used to synchronize the estrous cycle and facilitate the efficiency of a limited (two-day) mating paradigm. The mating protocol involved placing either two solo-housed females or all females from a communal cage into the cage of a solo-housed male from 1400 to 0800 h for two successive days. Nine days later all females were weighed to check for pregnancy. If fewer than two pregnant females were present in a communal cage, females were transferred between communal cages; communal cages had dams that delivered litters within five days of each other.
The PAE paradigm has been described previously (Brady et al., 2012). Briefly, female mice had access to either 10% (w/v) ethanol (DLI, King of Prussia, PA) in 0.066% (w/v) SAC (Sigma-Aldrich, St. Louis, MO) or 0.066% (w/v) SAC for four hours (1000 – 1400) each day. A single drinking tube was placed on SN cages, while two tubes were used for CN cages. Drinking was established for 7–10 days prior to mating, maintained throughout gestation, and withdrawn following parturition using a step-down procedure over a six-day period. Offspring were weaned at 21–23 days of age and reared in standard sized cages as like-sex littermates with tap water and laboratory chow available ad libitum.
Determination of alcohol consumption and blood ethanol concentrations
The amount of ethanol consumed was measured and divided by the number of dams in the cage. Blood ethanol concentrations (BECs) were determined as described (Brady et al., 2012) in dams whose pups were not used in any of the studies.
Determination of plasma corticosterone concentration
Plasma corticosterone was measured as described (Goggin et al., 2012) using a DetectX Corticosterone EIA Kit (Arbor Assays, Ann Arbor, MI; #K014-H1). Blood was collected at 0800 h, at the start of the dark cycle and 18-hours following removal of the saccharin or saccharin plus ethanol drinking tubes.
Determination of maternal care
Maternal care was evaluated (25 observations, 3 min apart) for 5 consecutive days beginning the day following birth for a total of 125 observations per cage. Visual assessments were made manually in the vivarium in the middle of the dark cycle using red illumination and consisted of noting whether the dam was on or off the nest and noting the presence or absence of the following pup-directed behaviors, which were used as a measure of maternal care: licking and grooming of pups, dam moving pups, and nursing. We did not differentiate between dams in a CN and, thus, the CN cage was designated as the unit of analysis (n).
Isolation and subcellular fractionation of hippocampal formation and hypothalamic tissue
Mice were killed by decapitation without anesthesia. The hippocampal formation and hypothalamus were rapidly dissected, snap-frozen in liquid nitrogen, and stored at −80°C. Nuclear (1000 ×gmax, 6 min, 4°C particulate), post-nuclear (1000 ×gmax, 6 min, 4°C soluble), cytosolic and membrane (soluble and particulate, respectively, fractions obtained following centrifugation of the post-nuclear lysate: 200,000 ×gmax, 30 min, 4°C) subcellular fractions were isolated as described (Allan et al., 2014; Caldwell et al., 2014); when present, the Triton X-100 concentration was 1mM.
Immunoblotting
Gel electrophoresis and immunoblotting were conducted as described (Allan et al., 2014; Caldwell et al., 2014). The amount of protein loaded for each analysis was: nuclear GR (10μg), cytosolic GR (13 μg); 11β-HSD1 (16 μg membrane fraction); FKBP51 (13 μg cytosolic fraction); CRHR1 (16 μg membrane fraction); and CRH (10 μg post-nuclear lysate). Antibodies and dilutions were: anti-GR (1:500; Santa Cruz Biotechnology; Dallas, TX; sc-1004), anti-FKBP51 (1:1,000; Santa Cruz Biotechnology; sc-13983), anti-FKBP52 (1:1,000; Abcam; Cambridge, MA; ab129097), anti-11β-HSD1 (1:250; Santa Cruz Biotechnology; sc-19259); anti-CRH (1:500; Santa Cruz Biotechnology; sc-10718) and anti-CRHR1 (1:500; Santa Cruz Biotechnology; sc-5543). Immunoreactivities were normalized to the amount of protein loaded, as determined by Coomassie staining (Coomassie Brilliant Blue R-250; Bio-Rad Laboratories, Hercules, CA; #161-0400).
Context discrimination
Context discrimination studies were conducted between 0900 and 1300 h under dim red illumination, as described (Caldwell et al., 2014). Each day for 7 consecutive days, the mice are placed in a context that was paired with a footshock (context A) and one where no footshock was delivered (context B). Training in Context A consisted of the following sequence: a 90-sec wait period, a 2-sec 0.8 mA footshock, a 90-sec inter-shock interval, a 2-sec 0.8 mA footshock and a final 60-sec wait period. Training in Context B was of the same duration but no shocks were administered. Freezing behavior (lack of movements other than those associated with respiration) was scored on days 2–7 during the first 90 seconds (which precedes the delivery of the first footshock in context A) within a trial. A discrimination score (freezing Context A - freezing Context B)/(freezing Context A + freezing Context B) was calculated. The ability to discriminate between the safe context (context B) and the footshock-paired context (context A) was demonstrated by greater freezing in context A compared to freezing in context B. For most mice, the ability to discriminate between the two contexts did not develop until day 3 of training.
Data analysis
For the CN cages, the cage of offspring was the unit of analysis and pups were assigned randomly to experimental conditions. For maternal measures, n represents a cage.
Context discrimination data were analyzed by mixed two way ANOVA with prenatal and housing conditions as the between factors and discrimination scores on days 4–6 as repeated measures using SPSS (v.22) followed by Tukey test with Bonferroni correction. Immunoblotting data were analyzed by two-way ANOVA using SPSS (v.22) with between factors of prenatal treatment condition (SAC or PAE) and housing (SN or CN); single post hoc comparisons were performed using Student’s t-tests (GraphPad Prism v.6.04; GraphPad Software; San Diego, CA).
RESULTS
Communal nesting increased maternal care
Compared to the SN commonly used in laboratory rodent breeding, the CN has been reported to reduce the percentage of time that pups are present in an unattended nest (Branchi et al., 2006a) and increase pup care (Branchi et al., 2006a; Branchi et al., 2013b; Cancedda et al., 2004; Sayler and Salmon, 1971). As a validation of our CN paradigm, we compared measures of maternal – neonate interactions (time on nest and pup care) in SN and CN cages. For these studies, the SAC/alcohol exposure paradigm was not employed.
We found that both time on nest (t(9)=3.99, p=.003) and pup care (t(9)=4.00, p=.003) were increased by communal housing compared with standard housing (Figure 1), confirming that our CN procedure yielded results consistent with published studies. Visual observations revealed that all dams took turns caring for the CN. The average number of pups per litter in PAE and SAC standard housing (7.1 ± 0.9 and 6.4 ±0.75, respectively) and the communal housing (7.8 ±1.1 and 7.6 ±0.67, respectively) conditions did not differ. Male-to-female ratios between the prenatal and housing groups were also not different (SN SAC = 1.2; SN PAE = 0.96; CN SAC = 0.90; CN PAE = 1.13). Pup weights at postnatal day 7 did not differ (average pup weights of 4.4± 0.11g for the CN and 4.3 ± 0.10g for the SN), indicating that there were no gross nutritional differences between the groups.
Figure 1. Communal housing increased indices of maternal care.
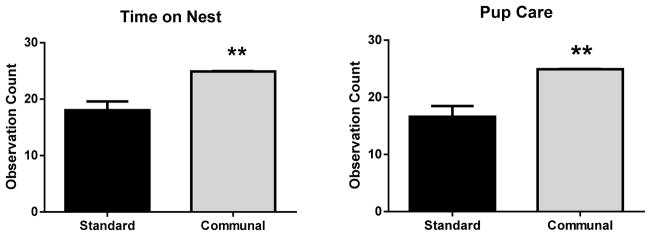
Time spent on nest (left) and pup care (right) were measured using a time-sampling approach, as described in the Materials and Methods. Data are mean +/− SEM from n=5 communal cages and n=6 standard cages. Time on nest (t(9)=3.99), p=.003 and pup care (t(9)=4.00, p=.003) were increased by communal housing compared with standard housing. ** p <.01
Communal housing did not alter the number of alcohol drinking bouts
We considered the possibility that communal housing may alter the amount and/or the pattern of alcohol consumption by the dams. We measured the number of drinking bouts occurring during each hour of the 4 h drinking sessions for SN and CN dams during the first and second week of pregnancy. The two groups of dams did not differ in the number of drinking bouts (Table 1). Additionally, BECs measured at the end of the 4 h consumption period did not differ between SN and CN dams for either the first or second week of pregnancy: SN dams, 81.2 (+/− 13) mg/dL, n=8; CN dams, 85.5 (+/− 10) mg/dL, n=9; n represents a cage; each group housed cage contained 3 pregnant dams and the BECs for all dams were averaged to yield the cage BEC. All dams within the group-housed cages demonstrated significant BECs, with the largest range within a cage being 61–110 mg/dL.
Table 1.
Solo-housed and communal housed dams did not differ in their pattern of alcohol consumption
| Drinking period | First hour | Second hour | Third hour | Fourth hour |
|---|---|---|---|---|
| Solo PAE dams Week 1 | 13.49 (±7.0) | 6.88 (±5.5) | 12.74(±0.9) | 18.69 (±7.4) |
| Solo PAE dams Week 2 | 14.23(±5.2) | 4.05(±1.0) | 14.54(±6.1) | 19.12(±2.4) |
| Communal PAE dams Week 1 | 15.35 (±4.5) | 6.41 (±7.9) | 13.88 (±3.8) | 17.56 (±6.6) |
| Communal PAE dams Week2 | 16.25 (±2.5) | 7.00(±0.8) | 12.75(±3.2) | 15.75(±1.9) |
Data are expressed as the average ± S.E.M. of the number of drinking bouts per mouse during each of the four hours of the alcohol exposure period. The data are from 4 communal cages each containing 4 dams and 6 cages of solo-housed dams. Drinking was assessed during the second week of pregnancy.
Communal housing elevated plasma corticosterone concentrations in dams
Group-housing has been reported to elevate basal plasma corticosterone concentrations in female mice (Arndt et al., 2009; Martin and Brown, 2010). We sought to determine whether dams maintained in the SN and the CN differed in their plasma corticosterone concentrations. Figure 2 shows plasma corticosterone levels in gestational day 14 dams consuming SAC or 10% (w/v/) ethanol in SAC. A two-way analysis of variance of the data revealed that there was a significant effect of housing condition [F(1,23)=7.16, p=.014] but no effect of treatment and no interaction between housing and treatment. Corticosterone levels did not differ between dams consuming SAC and dams consuming ethanol in SAC within a housing condition.
Figure 2. Plasma corticosterone concentrations in gestational day 14 dams were elevated by communal nesting independent of the consumption of saccharin or alcohol; within a housing condition, plasma corticosterone levels did not differ in saccharin and alcohol dams.
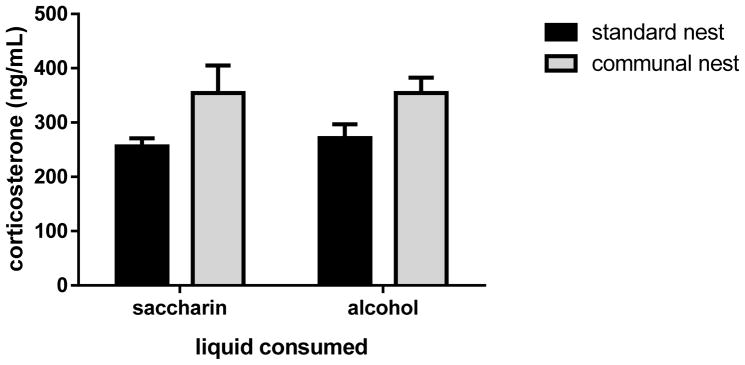
Plasma corticosterone concentrations were measured in solo-housed or communal-housed pregnant females who were consuming 0.066% (w/v) saccharin or 10% (w/v) alcohol in 0.066% (w/v) saccharin, as described in the Materials and Methods. n=7 for all groups except n=6 for solo-housed saccharin dams. A two-way ANOVA revealed that there was a significant effect of housing condition [F(1,23)=7.16, p=.014] without an effect of liquid consumed (saccharin or saccharin plus alcohol) and without an interaction between housing and liquid treatment.
The PAE protocol does not alter maternal care in the communal rearing conditions
We next considered the possibility that our PAE paradigm may impact maternal – neonatal interactions in CN animals. We found no detectable difference in maternal care measures (time on nest and pup care) in CN dams that consumed SAC and those that consumed ethanol in SAC (Figure 3). This finding mirrors our previously reported observation that alcohol consumption does not alter maternal care in the SN (Brady et al., 2012).
Figure 3. Maternal care in the communal nest was not impacted by the prenatal alcohol exposure (PAE) paradigm.
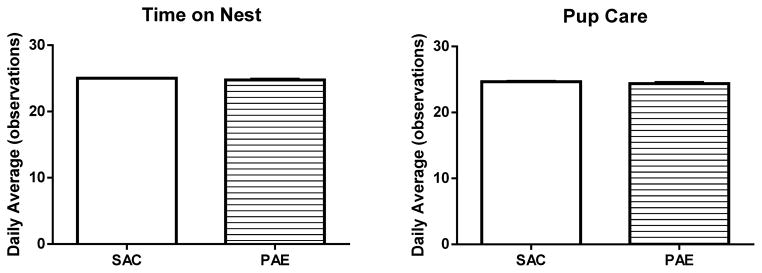
Time spent on nest (left) and pup care (right) were measured in communal nests formed by moms that had consumed saccharin (SAC) and moms that had consumed alcohol (prenatal alcohol exposure (PAE) paradigm) throughout pregnancy using a time-sampling approach, as described in the Materials and Methods. Data are mean +/− SEM. For all measures, SAC n=7, PAE n=10
Communal rearing reversed the detrimental effect of PAE on hippocampal formation-dependent learning and memory
We used our recent demonstration that PAE mice display reduced ability to discriminate between two similar contexts (Caldwell et al., 2014), which depends on hippocampal formation functioning (Sahay et al., 2011), to determine the impact of CN rearing on a PAE-associated learning and memory deficit. As communal nesting has been reported to have no effect on learning and memory in the Lashley III maze (Heiderstadt et al., 2014) or on spatial and reversal learning/memory in a water maze (D’Andrea et al., 2007), we anticipated no improvement in discrimination learning in the CN SAC mice compared to the SN SAC mice.
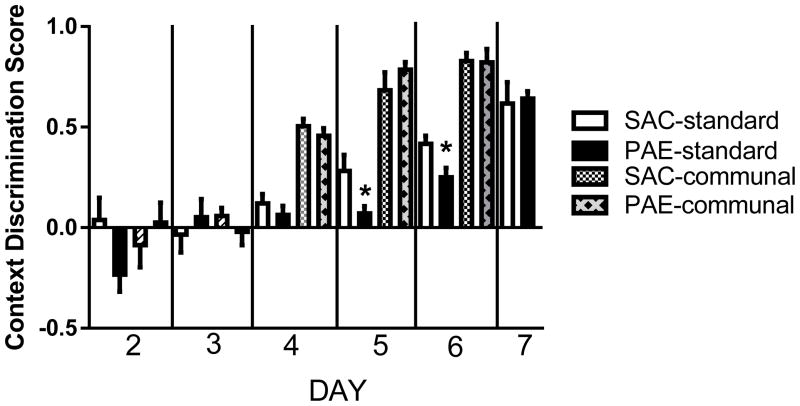
Positive discrimination scores, indicating learning, were obtained for all groups on days 4–6; thus, we analyzed scores from days 4–6 using a two way mixed ANOVA. Analysis revealed a significant effect of prenatal treatment [F(1,18)= 4.2, p=.05] and a significant effect of housing condition [F(1,19) 169.5, p <.0001]. There was a significant interaction between housing and prenatal treatment [F(1,18)= 5.90, p = .025]; the source of this was evident in the post hoc comparisons where SN PAE displayed a deficit in context discrimination on Day 5 (p = .0425) and Day 6 (p = .027) of testing, whereas there was not a difference between CN SAC and PAE mice on these days. The performance of all groups increased with days of training [F (2,36) = 37.6, p= .0001]. Day 7 was not included in the ANOVA since the communally housed PAE and SAC were not run on this day, as they were able to discriminate by Day 6.
Communal housing altered neurochemical measures in both SAC and PAE mice
We focused our neurochemical studies on the GR and on corticotropin releasing hormone (CRH), as we have shown these to be impacted by PAE (Caldwell et al., 2014).
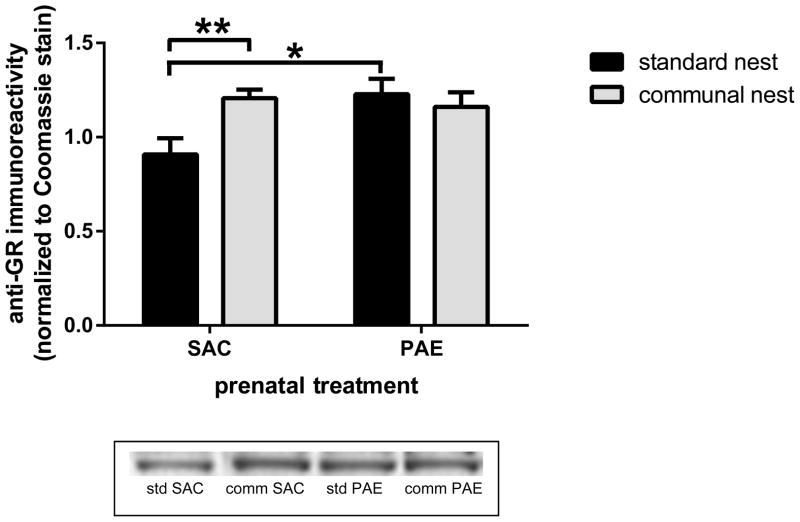
The CN increased hippocampal formation nuclear GR levels in SAC but not PAE offspring
We have reported that nuclear levels of the GR were elevated in the hippocampal formation of PAE mice reared under SN conditions, whereas cytosolic levels of the receptor remained unchanged (Caldwell et al., 2014). We measured anti-GR immunoreactivities associated with cytosolic and nuclear subcellular fractions isolated from the hippocampal formation of postnatal day 40–50 SAC and PAE mice reared in either an SN or CN. Cytosolic anti-GR immunoreactivities were not altered by housing or prenatal treatment, and there was no interaction between these factors (data not shown). A two-way ANOVA of the data for nuclear GR levels (Figure 5) revealed a significant prenatal treatment X housing condition interaction [F(1,30)=5.83, p<.05]. Post hoc analysis of the data confirmed our previous finding (Caldwell et al., 2014): PAE was associated with an increase in nuclear GR levels (p<.02) in SN mice. In contrast, there were no differences in nuclear GR levels in SAC and PAE mice reared in a CN. Compared to the SN, the CN was associated with elevated nuclear GR levels in SAC (p<.01) but not PAE mice.
Figure 5. Communal housing selectively elevated hippocampal formation nuclear glucocorticoid receptor (GR) levels in saccharin (SAC) control offspring.
Anti-GR immunoreactivities were measured in nuclear fractions prepared from the hippocampal formation of postnatal day 40–50 SAC and prenatal alcohol exposure (PAE) offspring that had been reared under standard or communal nest conditions, as described in the Materials and Methods. Nuclear GR levels were higher in PAE than is SAC offspring reared in standard nest conditions; (* p<.02). Nuclear GR levels in SAC offspring reared in communal nests were higher than in SAC offspring reared in standard nest conditions; (** p<.01).
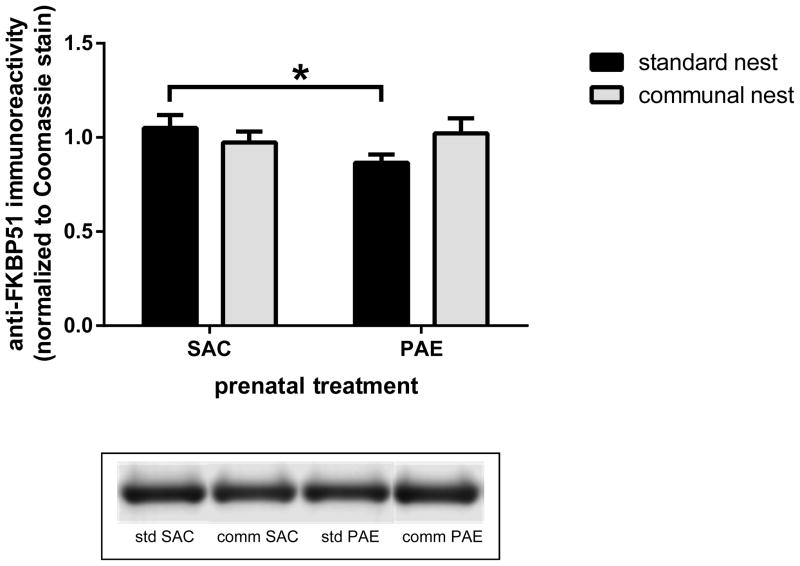
The CN was associated with decreased FKBP51 and elevated 11β-HSD1 levels in the hippocampal formation of PAE mice
Trafficking of the GR between the cytosolic and nuclear compartments is regulated by chaperone and co-chaperone proteins, including the FK506-binding proteins 51 (FKBP51; FKBP5) and 52 (FKBP52, FKBP4) (Vandevyver et al., 2012). We have reported that cytosolic FKBP51 levels were reduced in PAE mice reared in SN conditions (Caldwell et al., 2014). In the present studies we measured of anti-FKBP51 immunoreactivities in the cytosolic fraction isolated from the hippocampal formation of SN and CN SAC and PAE mice (Figure 6). Two-way ANOVA of the data revealed no effects of prenatal treatment and housing and no interaction between these two factors. A pre-planned comparison of the data for the SN animals revealed that our previous observation of reduced FKBP51 levels in PAE mice was replicated (p=.0454). FKBP52 levels were not affected by housing or by prenatal treatment and there was no interaction between these factors (data not shown).
Figure 6. Communal housing reversed the reduction in hippocampal formation FK506-binding proteins 51 (FKBP51) levels observed in prenatal alcohol exposure (PAE) mice reared in standard housing conditions.
Anti-FKBP51 immunoreactivities were measured in cytosolic fractions isolated from the hippocampal formation of postnatal day 40–50 PAE and saccharin (SAC) control mice that had been reared in standard nesting or communal nesting conditions. In mice reared under standard conditions, PAE was associated with a reduction in FKBP51 levels (*, p<.05). There was a trend toward an increase in PAE mice reared in a communal nest compared to PAE mice reared in a standard nest (p=.123). n=8 for all groups except n=7 for standard nest PAE.
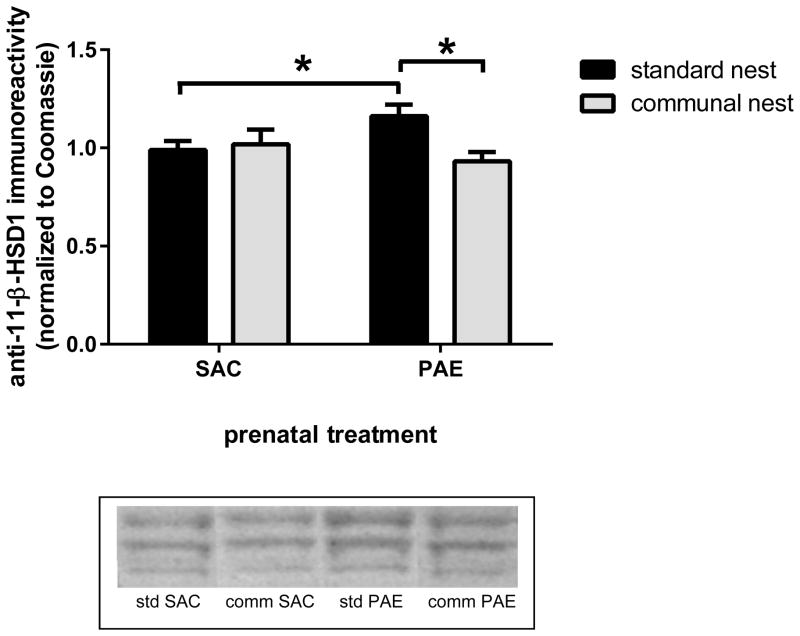
In the hippocampus, an important regulator of intracellular corticosteroid levels is 11-β-hydroxysteroid dehydrogenase 1 (11β-HSD1) (Yau et al., 2011), which catalyzes the formation of corticosterone from the inactive 11-dehydrocorticosterone (Wyrwoll et al., 2011). We previously reported that PAE was associated with elevated anti-11β-HSD1 immunoreactivity in the hippocampal formation of adolescent mice reared in the SN (Caldwell et al., 2014). In the present study, we measured anti-11β-HSD1 immunoreactivities associated with membrane fractions prepared from postnatal day 45 SAC and PAE, SN and CN, male mice (Figure 7). The antibody recognizes 3 isoforms of 11β-HSD1 and all three bands were quantified in the analysis. Two-way ANOVA of the data showed an interaction between prenatal treatment and housing [F(1,24)=6.00, p=.022] and a nearly significant effect of housing [F(1,24)=4.10, p=.054]. We replicated our previous finding, as SN PAE offspring had increased 11β-HSD1 levels compared SN SAC offspring (p=.040). In contrast, we found that 11β-HSD1 levels did not differ between SAC control and PAE offspring that were reared in CNs. 11β-HSD1 levels were significantly lower in PAE mice reared in a CN than in SN (p=.013).
Figure 7. Communal nesting lowered 11-β-hydroxysteroid dehydrogenase 1 (11β-HSD1) levels in the hippocampal formation of prenatal alcohol exposure (PAE) but not saccharin (SAC) control offspring and reversed the PAE-associated elevation of 11β-HSD1 levels that was observed in offspring reared in standard conditions.
Anti-11β-HSD1 immunoreactivities were determined in a membrane fraction prepared from postnatal day 45 SAC control and PAE male offspring reared in standard or communal nests, as described in the Materials and Methods. 11β-HSD1 levels were elevated in PAE offspring reared in standard conditions compared to levels found in SAC controls reared under the same conditions (p=.040). 11β-HSD1 levels were significantly lower in PAE mice reared in communal housing than in standard housing (p=.013). *, p<.05. n=7 for all groups except n=6 for communal nest PAE.
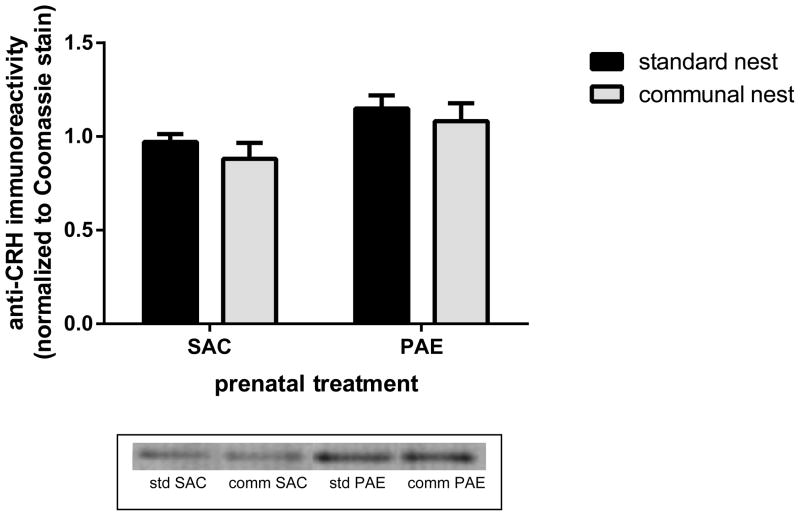
PAE was associated with significant elevations in hypothalamic CRH levels
Hypothalamic corticotropin-releasing hormone (CRH) is under inhibitory control from the hippocampal formation (Herman et al 2005). We previously reported that, in animals reared in SN conditions, hypothalamic CRH levels are higher in PAE mice (Caldwell et al. 2014). Figure 8 shows the results of analyses of CRH levels in cytosolic fractions prepared from the hypothalamus of postnatal day 45 mice that had been reared in SNs or CNs. Two-way ANOVA revealed that there was an effect of prenatal treatment [F(1,32)=6.42, p=.016] but no effect of housing and no interaction between housing and prenatal treatment. As previously reported, hypothalamic CRH levels were increased in PAE mice reared under standard conditions.
Figure 8. Prenatal alcohol exposure (PAE) produced elevations of hypothalamic corticotropin releasing hormone (CRH) levels.
Compared to saccharin (SAC) control mice PAE mice had elevated anti-CRH immunoreactivities in cytosolic fractions prepared from the hypothalamus of postnatal day 45 male mice n=10 for standard nest SAC; n=8 for standard nest PAE; n=9 for both communal nest SAC and communal nest PAE.
Effects of PAE and housing on hippocampal formation CRH receptor 1 levels
The effects of CRH are mediated by two types of receptors: CRH receptor type 1 (CRHR1) and CRH receptor type 2 (CRHR2). Intrahippocampal injection of CRH enhances context-dependent fear conditioning via a CRHR1-dependent mechanism (Radulovic et al., 1999); long-term potentiation is reduced in hippocampal slices prepared from CRHR1 knock-out mice (Schierloh et al., 2007). Thus, we reasoned that the impaired contextual fear conditioning (Brady et al., 2012) and hippocampal slice long-term potentiation (Brady et al., 2013) seen in SN PAE mice may be associated with reduced hippocampal formation CRHR1 levels. We are unaware of any reports comparing hippocampal CRHR1 levels in animals reared in the SN and the CN.
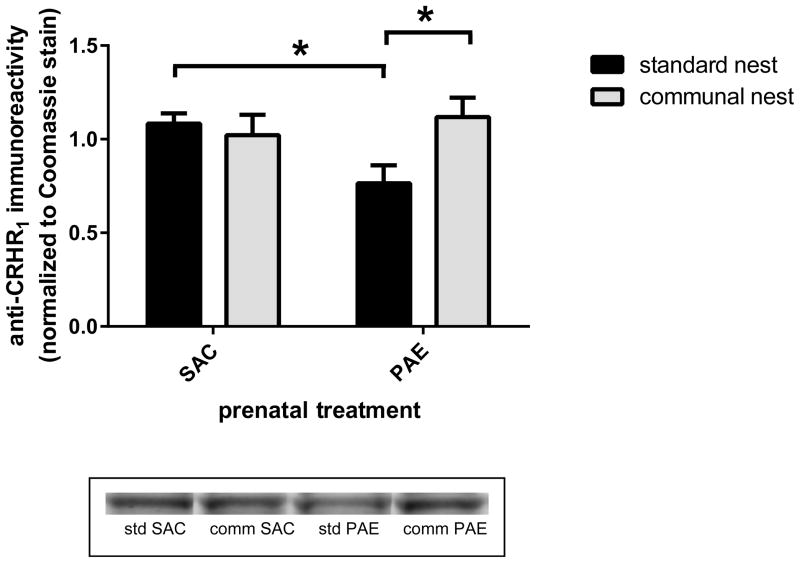
Two-way ANOVA of immunoblotting data revealed an interaction between prenatal treatment and housing [F(1,27)=4.98, p=.034] but no overall effect of prenatal treatment or housing (Figure 9). Post hoc comparison of the SN animals found that anti-CRHR1 immunoreactivities were significantly lower in PAE than SAC hippocampal formation (p=.013). Additionally, the CN was associated with significantly elevated anti-CRHR1 immunoreactivities in PAE mice (p=.026).
Figure 9. Communal nesting reversed the prenatal alcohol exposure (PAE)-associated reduction in hippocampal formation anti-corticotropin-releasing hormone receptor type 1 (CRHR1) immunoreactivity that was observed in offspring reared in standard conditions.
Anti-CRHR1 immunoreactivities were determined in membrane fractions prepared from postnatal day 45 saccharin (SAC) control and PAE male offspring reared in standard or communal nests, as described in the Materials and Methods. For animals reared in the standard nest, anti-CRHR1 immunoreactivities were lower in PAE than SAC samples (p=.013). Compared to PAE mice reared in the standard nest, PAE mice from communal nests had elevated anti-CRHR1 immunoreactivities (p=.026). *, p<.05. n=8 for all groups except n=9 for communal nest PAE mice.
DISCUSSION
These studies demonstrate that the maternal and early life social environments significantly influence the sequelae of PAE. In SN animals, PAE was associated with alterations in several neurochemical measures in the hippocampal formation and impaired hippocampal-dependent learning/memory. In contrast, in animals that were reared in CN conditions, we found no significant differences between PAE and SAC mice on several neurochemical measures and the damaging effects of PAE on a hippocampal formation learning/memory were eliminated. However, as discussed below, in some instances, the reversal of the neurochemical difference was due to an effect of the environment on the SAC animals and in other cases was due to an effect on the PAE animals. These findings are consistent with studies showing that the prenatal and postnatal environments have significant impact on the development of an organism (Barker, 2004; Gluckman and Hanson, 2004; Monk et al., 2012) and on the clinical course of disorders associated with PAE in human populations (Olson et al., 2009; Schneider et al., 2011; Streissguth et al., 2004).
Several studies have found that, in general, maternal care is increased in the CN compared to the SN (Branchi et al., 2013a; Branchi et al., 2006a; Branchi et al., 2013b; Sayler and Salmon, 1971). Similarly, our CN procedure yielded increased maternal care. The finding that maternal care was similar in CN SAC and PAE cages indicates that the mitigating effects of the CN on PAE-associated neurochemical and behavioral abnormalities are not likely due to altered maternal care. However, it is possible that our methods of observation were not sensitive enough to detect subtle differences in maternal care. Another limitation to the present study is that there could be physiological effects of communal housing on the dams which were not assessed. While we observed a similar elevation in plasma corticosterone in both the PAE and SAC CN dams compared to SN dams, other physiological effects on the dams due to communal housing were not assessed and could have been different between the groups. Communal housing could present a greater energy demand on the dams for greater milk production during each feeding bout. Nursing CN dams have been reported to have increased mammary gland development compared to nursing SN dams, while caloric content of milk is similar in the groups (Sayler and Salmon, 1971). Additionally, the CN temperature is likely to be increased and the structure more difficult to maintain with the larger litter. Any of these changes in the energy expectations could have a differential effect on the PAE offspring. Finally, it is worth noting that the BECs reported in this study were measured in a set of dams whose offspring were not used in the behavioral and neurochemical studies. This design was used in order to avoid introducing an additional confound of maternal stress. Although it is possible that the BECs in the dams whose offspring were used in the behavioral and neurochemical studies were different from these values, we do not believe that this is likely the case, as the levels measured in the SN mice (81.2 (+/− 13) mg/dL) are consistent with levels reported from our previous studies (88.3 ± 11.5 mg/dL (Brady et al., 2012); 90.5 ± 11.61 mg/dL (Brady et al., 2013)), in which dams were housed in an SN environment.
Previous studies on the effects of communal nesting on brain neurochemistry (Branchi et al., 2013a; Branchi et al., 2006a; Branchi et al., 2006b; Branchi et al., 2011; Cirulli et al., 2010) have not assessed the interaction between the CN and other treatment variables (e.g., PAE). CN increased CRHR1 levels and reduced 11β-HSD1 levels in PAE mice without altering levels in SAC animals. In contrast, we found that the CN was associated with increased GR nuclear localization in SAC control, but not PAE, mice. Thus, there is a complex interplay between the programming effects of the fetal environment, the maternal and early postnatal social environments and hippocampal neurochemistry.
The observed increase in GR levels in the CN compared to the SN SAC offspring may be the result of their responding to the increased maternal care that occurs in the CN; Meaney and colleagues (Weaver et al., 2004) reported that increased maternal care is associated with increased anti-GR immunoreactivity in the hippocampus of adult offspring. The mechanism underlying the increase in nuclear GR in the CN SAC animals does not appear to involve CN-dependent effects on FKBP51 or 11β-HSD1 levels, as they were minimally affected by nesting conditions in SAC animals. The lack of an effect of the CN on nuclear GR levels in the PAE is consistent with proposals that we (Allan et al., 2014; Caldwell et al., 2014) and Weinberg and colleagues (Gabriel et al., 2005) have made that PAE impairs the proper programming and development of the GR system in the extended hypothalamic-pituitary-adrenal (HPA) axis (i.e., the frontal cortex – hippocampus - HPA axis).
There were minimal effects of communal housing on hypothalamic CRH protein levels in PAE and SAC mice. CRH levels were higher in PAE than in SAC mice, confirming our previous observation (Caldwell et al., 2014). Hypothalamic CRH levels trended higher in CN PAE than CN SAC animals. Although Liu et al. (Liu et al., 1997) reported that hippocampal GR mRNA expression was elevated, while CRH mRNA levels in the PVN were decreased, in adult offspring of mothers that displayed high vs. low levels of licking/grooming and arched-back nursing (LG-ABN) of their pups, there was no such association between hippocampal formation nuclear GR protein levels and hypothalamic CRH protein levels in the SAC mice. Thus, our results indicate that CN-associated factors in addition to maternal care regulate the relationship between hippocampal nuclear GR and hypothalamic CRH protein levels.
We found that communal housing ameliorated the deficit in context discrimination learning and memory in PAE mice. In addition, we observed a housing-associated increase [(F(1,9)= 31.3 p=.001)] in learning in SAC animals. However, the beneficial effects of communal housing on cognitive processes appear to be task-specific. Whereas communal nesting of mice has been shown to improve performance of mice in a visual discrimination task (Cancedda et al., 2004), it has been reported to not affect offspring learning and memory in the Lashley III maze (Heiderstadt et al., 2014), water maze (D’Andrea et al., 2007), and radial arm maze (Opitz et al., 1997).
Several studies indicate the preweaning environment influences outcome measures of the effects of PAE. Clausing and colleagues (Opitz et al., 1997) reported that communal nesting prevents PAE-associated deficits in conditioned taste aversion in mice. The housing and communal nesting paradigm that these investigators employed differs from ours. Ogilvie & Rivier reported that there was an interaction between PAE, cross-fostering, and postnatal handling on adrenocorticotropic hormone levels in weanling rats (Ogilvie and Rivier, 1997). Weinberg and colleagues have reported that postnatal handling reverses PAE-associated deficits in response inhibition in a step-down passive avoidance task (Gallo and Weinberg, 1982) but does not reverse PAE-associated alterations in hypothalamic CRH mRNA levels (Gabriel et al., 2005). Highlighting the complexity of the interaction between PAE and the postnatal environment, Weinberg and colleagues have also shown that PAE animals that were handled postnatally displayed reduced conditioned taste aversion (Gabriel and Weinberg, 2001) and a deficit in spatial learning in the Morris water maze (Gabriel et al., 2002), whereas non-handled PAE animals exhibited conditioned taste aversion levels and spatial learning similar to control animals.
Conclusions
Communal housing normalized several, but not all, of the effects of PAE that had been previously observed in solo-housed mice. However, the basis for this normalization varied with the outcome measure and did not produce a generalized “correction” of the effects of PAE. That is, in some cases the normalization occurred due to an effect on SAC, rather than PAE, offspring. These findings have important translational implications, as well as highlight the importance of considering rearing conditions in the interpretation of research findings on PAE. The present studies in a mouse model of PAE are consistent with studies showing that the prenatal and postnatal environments have significant impact on the clinical course of PAE in human populations (Olson et al., 2009; Schneider et al., 2011; Streissguth et al., 2004) and, thus, further validate the translational utility of the mouse model for the study of PAE and FASD. A limitation in the current study is that only male offspring were assessed. It is possible that female PAE mice will not show a similar response to communal rearing. Future studies will have to explore the impact of rearing on female offspring.
Figure 4. Communal housing eliminated the deficit in the rate of context discrimination learning observed in standard housed prenatal alcohol exposure (PAE) compared to saccharin (SAC) control mice.
Mean ± SEM Context Discrimination Score data are shown for Days 4–7 of the training/testing; * p<.04 (see text for statistics). Postnatal day 40–55 mice were used for the studies; n=5–7 for each group.
Acknowledgments
This work was funded by National Institutes of Health grant # 1R01AA017449 (AMA), P20AA017068 (KKC, AMA) and P50AA022534 (KKC, AMA). We thank Michael Riblett for computer and equipment support.
References
- Allan AM, Goggin SL, Caldwell KK. Prenatal alcohol exposure modifies glucocorticoid receptor subcellular distribution in the medial prefrontal cortex and impairs frontal cortex-dependent learning. PLoS One. 2014;9:e96200. doi: 10.1371/journal.pone.0096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt SS, Laarakker MC, van Lith HA, et al. Individual housing of mice--impact on behaviour and stress responses. Physiol Behav. 2009;97:385–93. doi: 10.1016/j.physbeh.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93:26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Brady ML, Allan AM, Caldwell KK. A limited access mouse model of prenatal alcohol exposure that produces long-lasting deficits in hippocampal-dependent learning and memory. Alcohol Clin Exp Res. 2012;36:457–66. doi: 10.1111/j.1530-0277.2011.01644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady ML, Diaz MR, Iuso A, Everett JC, Valenzuela CF, Caldwell KK. Moderate prenatal alcohol exposure reduces plasticity and alters NMDA receptor subunit composition in the dentate gyrus. J Neurosci. 2013;33:1062–7. doi: 10.1523/JNEUROSCI.1217-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I. The mouse communal nest: investigating the epigenetic influences of the early social environment on brain and behavior development. Neurosci Biobehav Rev. 2009;33:551–9. doi: 10.1016/j.neubiorev.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Branchi I, Curley JP, D’Andrea I, Cirulli F, Champagne FA, Alleva E. Early interactions with mother and peers independently build adult social skills and shape BDNF and oxytocin receptor brain levels. Psychoneuroendocrinology. 2013a;38:522–32. doi: 10.1016/j.psyneuen.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I, D’Andrea I, Fiore M, Di Fausto V, Aloe L, Alleva E. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol Psychiatry. 2006a;60:690–6. doi: 10.1016/j.biopsych.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Branchi I, D’Andrea I, Sietzema J, et al. Early social enrichment augments adult hippocampal BDNF levels and survival of BrdU-positive cells while increasing anxiety- and “depression”-like behavior. J Neurosci Res. 2006b;83:965–73. doi: 10.1002/jnr.20789. [DOI] [PubMed] [Google Scholar]
- Branchi I, Karpova NN, D’Andrea I, Castren E, Alleva E. Epigenetic modifications induced by early enrichment are associated with changes in timing of induction of BDNF expression. Neurosci Lett. 2011;495:168–72. doi: 10.1016/j.neulet.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Branchi I, Santarelli S, D’Andrea I, Alleva E. Not all stressors are equal: early social enrichment favors resilience to social but not physical stress in male mice. Horm Behav. 2013b;63:503–9. doi: 10.1016/j.yhbeh.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Caldwell KK, Goggin SL, Tyler CR, Allan AM. Prenatal alcohol exposure is associated with altered subcellular distribution of glucocorticoid and mineralocorticoid receptors in the adolescent mouse hippocampal formation. Alcohol Clin Exp Res. 2014;38:392–400. doi: 10.1111/acer.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda L, Putignano E, Sale A, Viegi A, Berardi N, Maffei L. Acceleration of visual system development by environmental enrichment. J Neurosci. 2004;24:4840–8. doi: 10.1523/JNEUROSCI.0845-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli F, Berry A, Bonsignore LT, et al. Early life influences on emotional reactivity: evidence that social enrichment has greater effects than handling on anxiety-like behaviors, neuroendocrine responses to stress and central BDNF levels. Neurosci Biobehav Rev. 2010;34:808–20. doi: 10.1016/j.neubiorev.2010.02.008. [DOI] [PubMed] [Google Scholar]
- D’Andrea I, Alleva E, Branchi I. Communal nesting, an early social enrichment, affects social competences but not learning and memory abilities at adulthood. Behav Brain Res. 2007;183:60–6. doi: 10.1016/j.bbr.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Gabriel KI, Glavas MM, Ellis L, Weinberg J. Postnatal handling does not normalize hypothalamic corticotropin-releasing factor mRNA levels in animals prenatally exposed to ethanol. Brain Res Dev Brain Res. 2005;157:74–82. doi: 10.1016/j.devbrainres.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Gabriel KI, Johnston S, Weinberg J. Prenatal ethanol exposure and spatial navigation: effects of postnatal handling and aging. Dev Psychobiol. 2002;40:345–57. doi: 10.1002/dev.10023. [DOI] [PubMed] [Google Scholar]
- Gabriel KI, Weinberg J. Effects of prenatal ethanol exposure and postnatal handling on conditioned taste aversion. Neurotoxicol Teratol. 2001;23:167–76. doi: 10.1016/s0892-0362(01)00117-9. [DOI] [PubMed] [Google Scholar]
- Gallo PV, Weinberg J. Neuromotor development and response inhibition following prenatal ethanol exposure. Neurobehav Toxicol Teratol. 1982;4:505–13. [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–6. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Goggin SL, Labrecque MT, Allan AM. Perinatal exposure to 50 ppb sodium arsenate induces hypothalamic-pituitary-adrenal axis dysregulation in male C57BL/6 mice. Neurotoxicology. 2012;33:1338–45. doi: 10.1016/j.neuro.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C, Bazinet A, Riley EP. Foetal Alcohol Spectrum Disorders and alterations in brain and behaviour. Alcohol Alcohol. 2009;44:108–14. doi: 10.1093/alcalc/agn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiderstadt KM, Vandenbergh DJ, Gyekis JP, Blizard DA. Communal nesting increases pup growth but has limited effects on adult behavior and neurophysiology in inbred mice. J Am Assoc Lab Anim Sci. 2014;53:152–60. [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22:143–9. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Martin AL, Brown RE. The lonely mouse: verification of a separation-induced model of depression in female mice. Behav Brain Res. 2010;207:196–207. doi: 10.1016/j.bbr.2009.10.006. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The ever-changing brain: cellular and molecular mechanisms for the effects of stressful experiences. Dev Neurobiol. 2012;72:878–90. doi: 10.1002/dneu.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Spicer J, Champagne FA. Linking prenatal maternal adversity to developmental outcomes in infants: the role of epigenetic pathways. Dev Psychopathol. 2012;24:1361–76. doi: 10.1017/S0954579412000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie KM, Rivier C. Prenatal alcohol exposure results in hyperactivity of the hypothalamic-pituitary- adrenal axis of the offspring: modulation by fostering at birth and postnatal handling. Alcohol Clin Exp Res. 1997;21:424–9. doi: 10.1111/j.1530-0277.1997.tb03786.x. [DOI] [PubMed] [Google Scholar]
- Olson HC, Oti R, Gelo J, Beck S. “Family matters:” fetal alcohol spectrum disorders and the family. Dev Disabil Res Rev. 2009;15:235–49. doi: 10.1002/ddrr.65. [DOI] [PubMed] [Google Scholar]
- Opitz B, Mothes HK, Clausing P. Effects of prenatal ethanol exposure and early experience on radial maze performance and conditioned taste aversion in mice. Neurotoxicol Teratol. 1997;19:185–90. doi: 10.1016/s0892-0362(96)00225-5. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19:5016–25. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–8. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayler A, Salmon M. Ethological Analysis of Communal Nursing by House Mouse (Mus-Musculus) Behaviour. 1971;40:62. [Google Scholar]
- Schierloh A, Deussing J, Wurst W, Zieglgansberger W, Rammes G. Corticotropin-releasing factor (CRF) receptor type 1-dependent modulation of synaptic plasticity. Neurosci Lett. 2007;416:82–6. doi: 10.1016/j.neulet.2007.01.047. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Adkins MM. The effects of prenatal alcohol exposure on behavior: rodent and primate studies. Neuropsychol Rev. 2011;21:186–203. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25:228–38. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Morton RA, Diaz MR, Topper L. Does moderate drinking harm the fetal brain? Insights from animal models. Trends Neurosci. 2012;35:284–92. doi: 10.1016/j.tins.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandevyver S, Dejager L, Libert C. On the trail of the glucocorticoid receptor: into the nucleus and back. Traffic. 2012;13:364–74. doi: 10.1111/j.1600-0854.2011.01288.x. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Whitten MK. Effect of exteroceptive factors on the oestrous cycle of mice. Nature. 1957;180:1436. doi: 10.1038/1801436a0. [DOI] [PubMed] [Google Scholar]
- Wyrwoll CS, Holmes MC, Seckl JR. 11β-hydroxysteroid dehydrogenases and the brain: from zero to hero, a decade of progress. Front Neuroendocrinol. 2011;32:265–86. doi: 10.1016/j.yfrne.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JL, Noble J, Seckl JR. 11β-hydroxysteroid dehydrogenase type 1 deficiency prevents memory deficits with aging by switching from glucocorticoid receptor to mineralocorticoid receptor-mediated cognitive control. J Neurosci. 2011;31:4188–93. doi: 10.1523/JNEUROSCI.6145-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]