Abstract
The introduction of enzalutamide and abiraterone has led to improvement in the treatment of metastatic castration-resistant prostate cancer (mCRPC). However, acquired resistance to enzalutamide and abiraterone therapies frequently develops within a short period in many patients. In the present study, we developed enzalutamide resistant prostate cancer cells in an effort to understand the mechanisms of resistance. Global gene expression analysis showed that steroid biosynthesis pathway is activated in enzalutamide resistant prostate cancer cells. One of the crucial steroidogenic enzymes, AKR1C3, was significantly elevated in enzalutamide resistant cells. In addition, AKR1C3 is highly expressed in metastatic and recurrent prostate cancer and in enzalutamide resistant prostate xenograft tumors. Liquid Chromatography-Mass Spectrometry (LC-MS) analysis of the steroid metabolites revealed that androgen precursors such as cholesterol, DHEA and progesterone, as well as androgens are highly up regulated in enzalutamide resistant prostate cancer cells compared to the parental cells. Knock down of AKR1C3 expression by shRNA or inhibition of AKR1C3 enzymatic activity by indomethacin resensitized enzalutamide resistant prostate cancer cells to enzalutamide treatment both in vitro and in vivo. In contrast, overexpression of AKR1C3 confers resistance to enzalutamide. Furthermore, the combination of indomethacin and enzalutamide resulted in significant inhibition of enzalutamide-resistant tumor growth. These results suggest that AKR1C3 activation is a critical resistance mechanism associated with enzalutamide resistance, targeting intracrine androgens and AKR1C3 will overcome enzalutamide resistance and improve survival of advanced prostate cancer patients.
Keywords: prostate cancer, enzalutamide, intracrine androgens, AKR1C3, indomethacin
Introduction
Targeting androgen signaling via androgen deprivation therapy has been the mainstay of clinical interventions in prostate cancer (PCa). While initially effective, the majority of men experience only transient benefit and relapse with castrate-resistant prostate cancer (CRPC), which is currently incurable. Enzalutamide, a second-generation antiandrogen, was recently approved for the treatment of castration resistant prostate cancer (CRPC) in patients. Despite these advances that provide temporary respite, resistance to enzalutamide occurs frequently and the mechanisms that contribute to resistance are still rudimentary and are under intense investigation. Several potential mechanisms of resistance have been revealed such as AR variants expression (1-3), IL6-Stat3-AR axis activation (4), AR F876L mutation (5, 6) and glucocorticoid receptor (GR) overexpression (7, 8).
Intratumoral androgen biosynthesis has been well characterized as a mechanism of CRPC (9-12), but its role in enzalutamide resistance is yet to be understood. Clinical reports have shown that patients treated with enzalutamide have elevated testosterone levels in the bone marrow (13, 14). A cascade of enzymes is involved in the biosynthesis of intratumoral androgens, including CYP17A1, HSD3B and AKR1C3. A gain of function mutation in HSD3B1 (N367T) has been identified in CRPC patients recently and was postulated to confer resistance to enzalutamide (15, 16). Aldo-keto reductase family 1 member C3 (AKR1C3) is a multi-functional enzyme and is one of the most important genes involved in androgen synthesis and metabolism. AKR1C3 facilitates the conversion of weak androgens androstenedione (A’ dione) and 5 α-androstanedione (5α-dione) to the more active androgens testosterone and DHT respectively (17, 18). It catalyzes conversion of steroids and modulates trans-activation of steroid receptors. Elevated expression of AKR1C3 has been associated with PCa progression and aggressiveness (19, 20). The role of AKR1C3 in enzalutamide resistant prostate cancer is unknown.
In the present study, we developed PCa cell lines resistant to enzalutamide and found that intracrine androgen synthesis is activated in enzalutamide resistant prostate cancer cells. Activation of one of the important steroidogenic enzymes, AKR1C3, was identified as a critical mechanism that confers resistance to enzalutamide. Inhibition of AKR1C3 activity using either shRNA or indomethacin resensitized enzalutamide resistant PCa cells to enzalutamide. Furthermore, the combination of indomethacin and enzalutamide resulted in significant inhibition of enzalutamide-resistant PCa xenograft tumor growth.
Materials and Methods
Reagents and Cell Culture
LNCaP, CWR22Rv1, VCaP and HEK293T cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). All experiments with cell lines were performed within 6 months of receipt from ATCC or resuscitation after cryopreservation. ATCC uses Short Tandem Repeat (STR) profiling for testing and authentication of cell lines. C4-2B cells were kindly provided and authenticated by Dr. Leland Chung, Cedars-Sinai Medical Center, Los Angeles, CA. LN-95 cells were kindly provided and authenticated by Dr. Joel Nelson, University of Pittsburg, PA. The cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin and 0.1 mg/ml streptomycin. LNCaP-neo and LNCaP-AKR1C3 cells were generated by stable transfection of LNCaP cells with either empty vector pcDNA3.1 or pcDNA3.1 encoding AKR1C3 and were maintained in RPMI1640 medium containing 300 μg/mL G418. AKR1C3 shRNA (TRCN0000026561 and TRCN0000025694) were purchased from Sigma. Cells resistant to enzalutamide were referred to as C4-2B MDVR (C4-2B enzalutamide resistant) as described previously (2). All cells were maintained at 37°C in a humidified incubator with 5% carbon dioxide.
Sample preparation and analysis of steroids
The steroid extraction and analysis has been described previously (21). Briefly, 50 million C4-2B parental and C4-2B MDVR cells were cultured in serum- and phenol red-free RPMI1640 medium for 5 days, then cells were suspended in 4 mL of a 1:1 water/methanol mixture. The suspension was homogenized, and the resulting homogenate was cooled on ice. The precipitated material was removed by centrifuging at high speed for 5 min, and the supernatant was removed and evaporated in a SpeedVac (Labconco Inc.) followed by lyophilizer (Labconco Inc.). The residue was suspended in 150 μL of CH3OH/H2O (1:1), filtered through a 0.2 μm ultracentrifuge filter (Millipore inc.) and subjected to UPLC/MS-MS analysis. Samples were run in duplicate during UPLC-MS/MS analysis. Samples were placed in an Acquity sample manager which was cooled to 8 °C to preserve the analytes. Pure standards were used to optimize the UPLC-MS/MS conditions prior to sample analysis. Also, the standard mixture was run before the first sample to prevent errors due to matrix effect and day-to-day instrument variations. In addition, immediately after the initial standard and before the first sample, two spiked samples were run to calibrate for the drift in the retention time of all analytes due to the matrix effect. After standard and spiked sample runs, blank was injected to wash the injector and remove carry over effect.
UPLC-MS/MS analysis of steroid metabolites
All experiments were performed on a Waters Xevo-TQ triple quadruple mass spectrometer (Milford, MA, USA) and MS and MS/MS spectra were recorded using Electro Spray Ionization (ESI) in positive ion (PI) and negative ion (NI) mode, capillary voltage of 3.0 kV, extractor cone voltage of 3 V and detector voltage of 650 V. Cone gas flow was set at 50 L/h and desolvation gas flow was maintained at 600 L/h. Source temperature and desolvation temperatures were set at 150 and 350 °C, respectively. The collision energy was varied to optimize daughter ions. The acquisition range was 20-500 Da. Analytical separations were conducted on the UPLC system using an Acquity UPLC HSS T3 1.8 μm 1 X 150 mm analytical column kept at 50°C and at a flow rate of 0.15 ml/min. The gradient started with 100% A (0.1% formic acid in H2O) and 0% B (0.1% formic acid in CH3CN), after 2min, changed to 80% A over 2 min, then 45% A over 5min, followed by 20% A in 2min. Finally it was changed over 1 min to original 100% A, resulting in a total separation time of 15 min. The elutions from the UPLC column were introduced to the mass spectrometer and resulting data were analyzed and processed using MassLynx 4.1 software.
cDNA microarray analysis
The microarray analysis has been described previously (22). Briefly, twenty-four hours after plating of 5 × 105 C4-2B parental and C4-2B MDVR cells, total RNA was isolated using TRIzol Reagent (Invitrogen) and purified with Eppendorf phase-lock-gel tube. RNA quality of all samples was tested by RNA electrophoresis to ensure RNA integrity. Samples were analyzed by the Genomics Shared Resource (UC Davis Medical Center, Sacramento, CA) using the Affymetrix Human Gene 1.0 ST array. The data was analyzed by Subio platform and Gene Set Enrichment Analysis (GSEA) (23). Microarray data has been deposited in GEO with the accession number GSE64143.
Western blot analysis
Cellular protein extracts were resolved on SDS–PAGE and proteins were transferred to nitrocellulose membranes. After blocking for 1 hour at room temperature in 5% milk in PBS/0.1% Tween-20, membranes were incubated overnight at 4°C with the indicated primary antibodies [AKR1C3 (A6229, Sigma); CYP17A1 (SC-66849, Santa Cruz Biotechnology, Santa Cruz, CA); HSD3B (SC-28206, Santa Cruz Biotechnology, Santa Cruz, CA); AR (SC-815, Santa Cruz Biotechnology, Santa Cruz, CA); Tubulin (T5168, Sigma-Aldrich, St. Louis, MO)]. Tubulin was used as loading control. Following secondary antibody incubation, immunoreactive proteins were visualized with an enhanced chemiluminescence detection system (Millipore, Billerica, MA).
Cell growth assay
C4-2B MDVR, CWR22Rv1 cells were seeded on 12-well plates at a density of 0.5×105 cells/well in RPMI 1640 media containing 10% FBS and transiently transfected with AKR1C3 shRNA or control shRNA following treatment with 20 μM enzalutamide. Total cell numbers were counted after 3 or 5 days. LNCaP–neo, LNCaP-AKR1C3 or LN-95 cells were treated with different concentrations of enzalutamide for 48 hours. Total cell numbers were counted or the cell survival rate (%) was calculated. Cell survival rate (%) = (Treatment group cell number / Control group cell number) ×100%.
Clonogenic Assay
C4-2 parental or C4-2B MDVR cells were treated with DMSO, 10 μM or 20 μM enzalutamide in media containing 10% FBS. CWR22Rv1 cells or C4-2B MDVR cells were treated with 10 μM or 20 μM indomethacin with or without 20 μM enzalutamide, cells were plated at equal density (1500 cells/dish) in 100 mm dishes for 14 days, the medium was changed every 3 days; LNCaP- neo or LNCaP–AKR1C3 cells were treated with DMSO or 10 μM enzalutamide in media containing 10% complete FBS, cells were plated at equal density (10000 cells/dish) in 100 mm dishes for 28 days, the colonies were rinsed with PBS before staining with 0.5% crystal violet/4% formaldehyde for 30 min and the numbers of colonies were counted.
Real-Time quantitative RT-PCR
Total RNAs were extracted using TriZOL reagent (Invitrogen). cDNAs were prepared after digestion with RNase-free RQ1 DNase (Promega). The cDNAs were subjected to real-time reverse transcription-PCR (RT-PCR) using Sso Fast Eva Green Supermix (Bio-Rad) according to the manufacturer's instructions and as described previously (24). Each reaction was normalized by co-amplification of actin. Triplicates of samples were run on default settings of Bio-Rad CFX-96 real-time cycler. Primers used for Real-time PCR are: AKR1C3, 5′-gagaagtaaagctttggaggtcaca-3′ (forward) and 5′-caacctgctcctcattattgtataaatga-3′ (reverse); AKR1C1/2, 5′-ggtcacttcatgcctgtcct-3′ (forward) and 5′-actctggtcgatgggaattg-3′ (reverse); HSD3B1, 5′-agaatctagaccactcttctgtccagcttt-3′ (forward) and 5′-ctttgaattcaactatgtgaaggaatggaa-3′ (reverse); HSD3B2, 5′-cgggcccaactcctacaag-3′ (forward) and 5′-ttttccagaggctcttcttcgt-3′ (reverse); CYP17A1, 5′-gggcggcctcaaatgg-3′ (forward) and 5′-cagcgaaggcgaaggcgataccctta-3′ (reverse); HSD17B3, 5′-tgggacagtgggcagtga-3′ (forward) and 5′-cgagtacgctttcccaattcc-3′ (reverse); SRD5A1, 5′-acgggcatcggtgcttaat-3′ (forward) and 5′-ccaacagtggcataggctttc-3′ (reverse); and Actin, 5′-agaactggcccttcttggagg-3′ (forward) and 5′-gtttttatgttcctctatggg-3′ (reverse).
Measurement of PSA
PSA levels were measured in sera from C4-2B parental or C4-2B MDVR tumor bearing mice using PSA ELISA Kit (KA0208, Abnova, Inc., Walnut, CA) according to the manufacturer’s instructions.
In vivo tumorigenesis assay
C4-2B parental or C4-2B MDVR cells (4 million) were mixed with matrigel (1:1) and injected into the prostates of 6-7 week male SCID mice. When the serum PSA level reached 5 ng/ml, mice were randomized into two groups (4 mice in each group) and treated as follows: (1) vehicle control (0.5% weight/volume (w/v) Methocel A4M p.o), (2) enzalutamide (25 mg/kg, p.o.). Tumors were monitored by PSA level. All tumor tissues were harvested after 3 weeks of treatment.
CWR22Rv1 cells (4 million) were mixed with matrigel (1:1) and injected subcutaneously into the flanks of 6-7 week male SCID mice. Tumor-bearing mice (tumor volume around 50-100 mm3) were randomized into four groups (5 mice in each group) and treated as follows: (1) vehicle control (5% Tween 80 and 5% ethanol in PBS, i.p.), (2) enzalutamide (25 mg/kg, p.o.), (3) indomethacin (3 mg/kg, i.p.), (4) enzalutamide (25 mg/kg, p.o.) + indomethacin (3 mg/kg, i.p.). Tumors were measured using calipers twice a week and tumor volumes were calculated using length × width2/2. Tumor tissues were harvested after 3 weeks of treatment.
Immunohistochemistry
Tumors were fixed by formalin and paraffin embedded tissue blocks were dewaxed, rehydrated, and blocked for endogenous peroxidase activity. Antigen retrieving was performed in sodium citrate buffer (0.01 mol/L, pH 6.0) in a microwave oven at 1,000 W for 3 min and then at 100 W for 20 min. Nonspecific antibody binding was blocked by incubating with 10% fetal bovine serum in PBS for 30 min at room temperature. Slides were then incubated with anti-Ki-67 (at 1:500; NeoMarker), anti-AKR1C3 (at 1:100; Sigma) at 4°C overnight. Slides were then washed and incubated with biotin-conjugated secondary antibodies for 30 min, followed by incubation with avidin DH-biotinylated horseradish peroxidase complex for 30 min (Vectastain ABC Elite Kit, Vector Laboratories). The sections were developed with the diaminobenzidine substrate kit (Vector Laboratories) and counterstained with hematoxylin. Nuclear staining cells was scored and counted in 5 different vision areas. Images were taken with an Olympus BX51 microscope equipped with DP72 camera.
Statistical Analysis
All data are presented as means ± standard deviation of the mean (SD). Statistical analyses were performed with Microsoft Excel analysis tools. Differences between individual groups were analyzed by one-way analysis of variance (ANOVA) followed by the Scheffé procedure for comparison of means. p < 0.05 was considered statistically significant.
Results
Identification of AKR1C3 activation in enzalutamide resistant prostate cancer cells
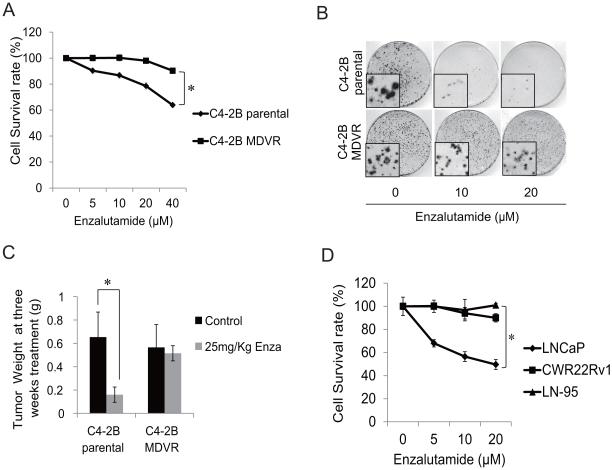
In our previous study, we generated enzalutamide resistant prostate cancer cells, named C4-2B MDVR, by chronic culture of C4-2B cells in media containing enzalutamide (2). As shown in Fig.1A and 1B, enzalutamide significantly inhibited proliferation and clonogenic ability of C4-2B parental cells but had little effect on C4-2B MDVR cells. We also examined the effects of enzalutamide treatment on C4-2B MDVR cells in vivo. As shown in Fig.1C, C4-2B MDVR xenografts were resistant to enzalutamide. Tumor weights of C4-2B xenograft were significantly inhibited by enzalutamide after 3 weeks treatment with enzalutamide, while tumor weights of treated C4-2B MDVR group were comparable to those of non-treated control group. These results suggest that C4-2B-MDVR cells are resistant to enzalutamide both in vitro and in vivo. We also characterized several other prostate cancer cell lines in response to enzalutamide treatment. As shown in Fig.1D, LNCaP cells are sensitive to enzalutamide, while CWR22Rv1 and LN-95 cells are resistant to enzalutamide treatment, consistent with previously published studies (25-27).
Figure 1.
C4-2B MDVR cells are resistant to enzalutamide in vitro and in vivo. A, C4-2B parental and C4-2B MDVR cells were treated with different concentrations of enzalutamide for 48 hours, total cell numbers were counted and cell survival rate was calculated. B, The clonogenic ability of C4-2B parental and C4-2B MDVR cells treated with 10 μM or 20 μM enzalutamide was analyzed. Enzalutamide significantly inhibited clonogenic ability of C4-2B parental cells. . C, C4-2B parental and C4-2B MDVR cells were injected orthotopically into the prostates of SCID mice and treated with 25mg/Kg enzalutamide or vehicle control. Tumors were harvested and weighed at 3 weeks. D, LNCaP, LN-95 and CWR22Rv1 cells were treated with different concentrations of enzalutamide for 2 days, total cell numbers were counted and cell survival rate (%) was calculated. * p>0.05.
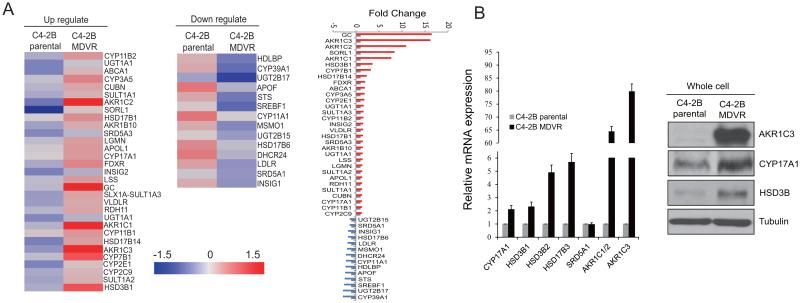
Intratumoral androgen biosynthesis has been well characterized as a mechanism of CRPC (9-12, 28), but its role in enzalutamide resistance remains unknown. To further understand potential mechanisms that underlie enzalutamide resistance, we performed microarray analysis of the enzalutamide resistant -C4-2B-MDVR vs. C4-2B parental cells. Expression of transcripts encoding for steroid hormone biosynthesis was analyzed by gene set enrichment. Among 45 genes involved in hormone biosynthesis, 31 genes were up regulated while 14 genes were down regulated in C4-2B MDVR cells. As shown in Fig.2A, we found increased expression of AKR1C3, AKR1C1, AKR1C2, HSD3B1, CYP17A1 and SRD5A3, and decreased expression of UGT2B15, UGT2B17, CYP39A1, HSD17B6 and SRD5A1 in C4-2B MDVR cells compared to C4-2B parental cells. To verify the gene expression data, CYP17A1, HSD3B1, HSD3B2, HSD17B3, SRD5A1, AKR1C1/2 and AKR1C3 mRNA levels were measured using specific primers by qRT-PCR. As shown in Fig.2B left, the levels of mRNA expression were consistent with the microarray data. We also confirmed the results by western blot, as shown in Fig.2B right, C4-2B MDVR cells express significantly higher levels of AKR1C3, HSD3B and CYP17A1 proteins compared to C4-2B parental cells. These results suggested that acquired androgen synthesis signaling was upregulated in enzalutamide resistant prostate cancer cells.
Figure 2.
Intracrine androgen synthesis pathway activated in enzalutamide resistant prostate cancer cells. A, Expression of transcripts encoding genes involved in steroid hormone biosynthesis was analyzed by gene set enrichment. Genes that were regulated 1.3 fold between C4-2B parental cells and C4-2B MDVR cells were enriched and heat map was generated by Subio platform. B, C4-2B parental cells and C4-2B MDVR cells were cultured in RPMI 1640 media containing 10% FBS for 3 days, total RNAs were extracted and CYP17A1, HSD3B1, HSD3B2, HSD17B3, SRD5A1, AKR1C1/2 or AKR1C3 mRNA levels were analyzed by qRT-PCR. AKR1C3, HSD3B and CYP17A1 protein levels were examined by western blot (right panel).
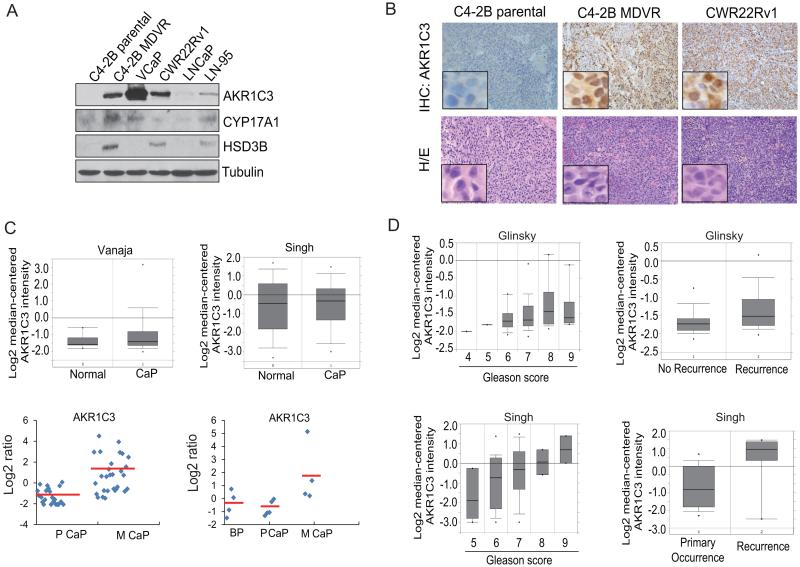
AKR1C3 is highly expressed in metastatic and recurrent prostate cancer and enzalutamide resistant prostate xenograft tumors
We found that AKR1C3 was up regulated by more than 16-fold in enzalutamide resistant C4-2B MDVR cells compared to the C4-2B parental cells. We examined AKR1C3 expression in different prostate cancer cell lines including VCaP, CWR22Rv1, LNCaP, LN-95, C4-2B and C4-2B-MDVR cells. C4-2B MDVR, CWR22Rv1, and LN-95 cells are resistant to enzalutamide while C4-2B and LNCaP cells are sensitive to enzalutamide. As shown in Fig.3A, C4-2B MDVR, VCaP, CWR22Rv1 and LN-95 cells all express significantly higher levels of AKR1C3; C4-2B MDVR, CWR22Rv1 and LN-95 cells express higher levels of HSD3B; C4-2B MDVR and LN-95 cells also expressed higher levels of CYP17A1. We also examined AKR1C3 expression in tumor xenografts by IHC, as shown in Fig.3B, C4-2B MDVR and CWR22Rv1 tumors express higher levels of AKR1C3 compared to C4-2B parental tumors. We performed data-mining using the Oncomine and GEO data bases to compare the expression of AKR1C3 in normal prostate and prostate cancer. Primary prostate cancer and normal prostate express similar AKR1C3 levels in two independent prostate datasets, while AKR1C3 was significantly elevated in metastatic prostate cancer in GEO datasets (Fig.3C), which is consistent with the previous reports (29, 30). We further examined the correlation between AKR1C3 and prostate cancer disease progression. As shown in Fig.3D, AKR1C3 was significantly correlated with Gleason score and recurrence status in prostate cancer patients in two independent prostate datasets in Oncomine. Collectively, these results demonstrate that AKR1C3 is highly expressed in late stage prostate cancer.
Figure 3.
AKR1C3 is highly expressed in metastatic prostate tumors and enzalutamide resistant xenografts . A, C4-2B parental, C4-2B MDVR, VCaP, CWR22Rv1 , LNCaP and LN-95 cells were harvested and whole lysates were subjected to Western blotting. B, AKR1C3 expression level was analyzed by IHC staining in C4-2B parental, C4-2B MDVR and CWR22Rv1 xenografts. C, Gene expression analysis using the Oncomine database showing the relative expression levels of AKR1C3 in two datasets comparing normal prostate tissue and prostate cancer. Vanaja: normal, n = 8; cancer, n = 32. Singh: normal, n = 50; cancer, n = 52. Data are presented as mean ± S.E. of normalized expression units according to Oncomine output (upper). AKR1C3 gene expression analysis using the GEO database in two datasets comparing benign, primary or metastatic prostate cancer. GSE27616: Benign, n = 4; primary prostate cancer, n = 5; and metastatic prostate cancer, n = 4; GSE32269: primary prostate cancer, n = 22; and metastatic prostate cancer, n = 29. Data were extracted and analyzed by Subio platform (bottom). D, Gene expression analysis using the Oncomine database showed that AKR1C3 expression was correlated with prostate cancer progression and recurrence in two independent datasets (Glinsky and Singh prostate).
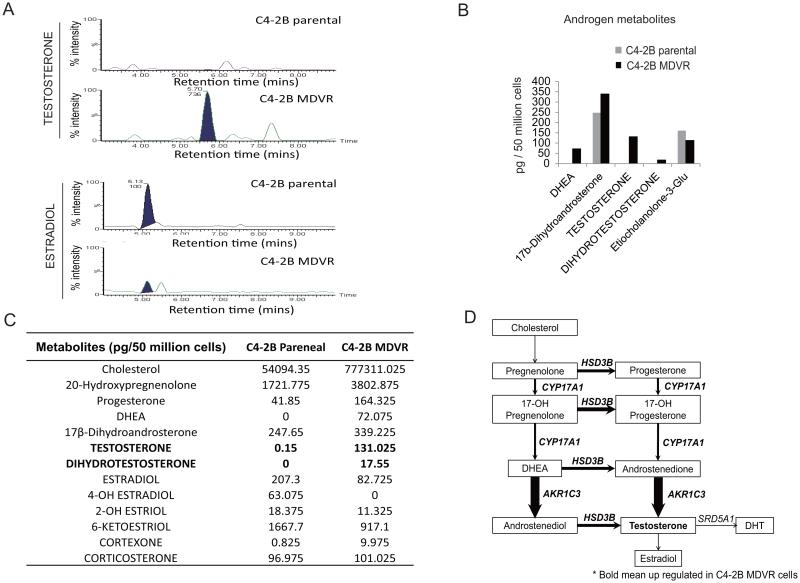
Intracrine androgens are elevated in enzalutamide resistant prostate cancer cells
AKR1C3 (also named 17βHSD5) is one of the most important genes involved in androgen synthesis and metabolism. AKR1C3 facilitates the conversion of weak androgens androstenedione (A’ dione) and 5 α-androstanedione (5α-dione) to the more active androgens, testosterone and DHT respectively. To further confirm that intracellular androgen synthesis was acquired by C4-2B MDVR cells, steroid metabolism in C4-2B parental and C4-2B MDVR cells was analyzed by Liquid Chromatography-Mass Spectrometry (LC-MS). C4-2B parental and C4-2B MDVR cells were cultured in serum free and phenol red free medium for 5 days, and steroid metabolites were extracted from 50x106 cells and subjected to LC-MS analysis. As shown in Fig.4A, 4B and 4C, C4-2B MDVR cells synthesize extremely high levels of testosterone (131.025 vs. 0.15 pg/50 million cells), dihydrotestosterone (17.55 vs. 0 pg/50 million cells), and DHEA (72.075 vs. 0 pg/50 million cells), compared to C4-2B parental cells. Intriguingly, the active estrogen metabolite estradiol was significantly reduced (82.725 vs. 207.3 pg/50 million cells) in C4-2B MDVR cells, suggesting that the biosynthesis of androgens was activated while transformation of estrogen from androgens was suppressed in C4-2B MDVR cells. Of note, the precursors involved in intracrine androgen synthesis such as cholesterol, DHEA and progesterone are also elevated in C4-2B MDVR cells compared to C4-2B parental cells (Fig.4C). The steroidogenic enzymes involved in androgen synthesis and metabolism are illustrated in Fig. 4D, bold arrows and bold font indicate upregulation in enzalutamide resistant prostate cancer cells compared to C4-2B parental cells (Fig.4D). Collectively, these results suggest that intracrine acquired androgen synthesis was elevated in prostate cancer cells resistant to enzalutamide.
Figure 4.
Intracrine androgens were up regulated in enzalutamide resistant prostate cancer cells. A, C4-2B parental and C4-2B MDVR cells were cultured in serum free and phenol red free RPMI1640 medium for 5 days, and levels of steroids in the cell extracts were analyzed by LC-MS. Representative testosterone and estradiol chromatograms generated by MassLynx 4.1 software are shown. B, the difference between levels of androgen metabolites in C4-2B parental and C4-2B MDVR cells was analyzed and quantified. C, the represented steroid metabolites between C4-2B parental and C4-2B MDVR cells were quantified. D, the androgen metabolism pathway was up regulated in enzalutamide resistant prostate cancer cells.
AKR1C3 confers resistance to enzalutamide in prostate cancer cells
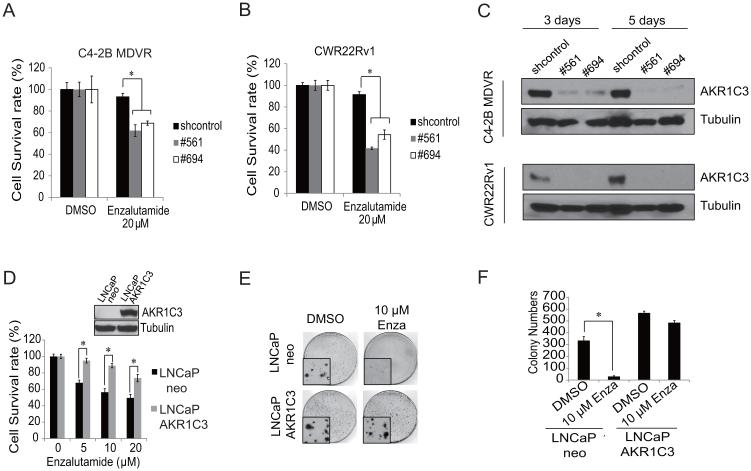
Having demonstrated that AKR1C3 is upregulated in enzalutamide resistant prostate cancer cells and in late stage prostate cancer patients, we next examined whether AKR1C3 could confer resistance to enzalutamide. We found that AKR1C3 was sufficient to confer resistance to enzalutamide in prostate cancer cells. CWR22Rv1 cells or C4-2B MDVR cells were transiently transfected with control shRNA or AKR1C3 shRNA following treatment with enzalutamide for three days. As shown in Fig.5A and B, CWR22Rv1 and C4-2B MDVR cells are resistant to enzalutamide, while knock down of AKR1C3 expression by two independent shRNAs (#561 and #694) restored their sensitivity to enzalutamide. The down regulation of AKR1C3 by shRNA was confirmed by western blot (Fig.5C). We also generated LNCaP cells stably expressing AKR1C3 (LNCaP-AKR1C3) to test whether exogenous expression of AKR1C3 induces enzalutamide resistance. LNCaP-AKR1C3 and LNCaP-neo vector control cells were treated with different concentrations of enzalutamide for 48 hours and cell numbers were counted. As shown in Fig.5D, LNCaP-AKR1C3 cells exhibited greater resistance to enzalutamide than LNCaP-neo cells. These results were also confirmed by clonogenic ability assay. LNCaP-AKR1C3 cells showed significantly more clonogenic ability than the control LNCaP-neo cells in response to enzalutamide treatment (Fig.5E, F). Collectively, these results demonstrate that overexpression of AKR1C3 confers resistance to enzalutamide, while down regulation of AKR1C3 resensitizes enzalutamide resistant prostate cancer cells to enzalutamide treatment.
Figure 5.
AKR1C3 confers resistance to enzalutamide in prostate cancer cells. A, CWR22Rv1 cells were transiently transfected with control shRNA or AKR1C3 shRNA (#561 and #694), following treatment with 20 μM enzalutamide and cell numbers were determined on 3 days. B, C4-2B MDVR cells were transiently transfected with control shRNA or AKR1C3 shRNA (#561 and #694), following treatment with 20 μM enzalutamide and cell numbers were determined on 3 days. C, CWR22Rv1 or C4-2B MDVR cells were transiently transfected with control shRNA or AKR1C3 shRNA (#561 and #694), cells were collected on 3 or 5 days, and whole cell lysates were subjected to Western blotting. D, LNCaP-neo or LNCaP-AKR1C3 cells were treated with different concentrations of enzalutamide for 2 days, total cell numbers were counted and cell survival rate (%) was calculated. E, LNCaP-neo or LNCaP-AKR1C3 cells were treated with 10 μM enzalutamide and clonogenic assay was performed, the colony size pictures were taken under a microscope. F, Colonies were counted and results are presented as means ± SD of 2 experiments performed in duplicate. * p<0.05. Enza: Enzalutamide.
Indomethacin, an inhibitor of AKR1C3 activity, overcomes enzalutamide resistance
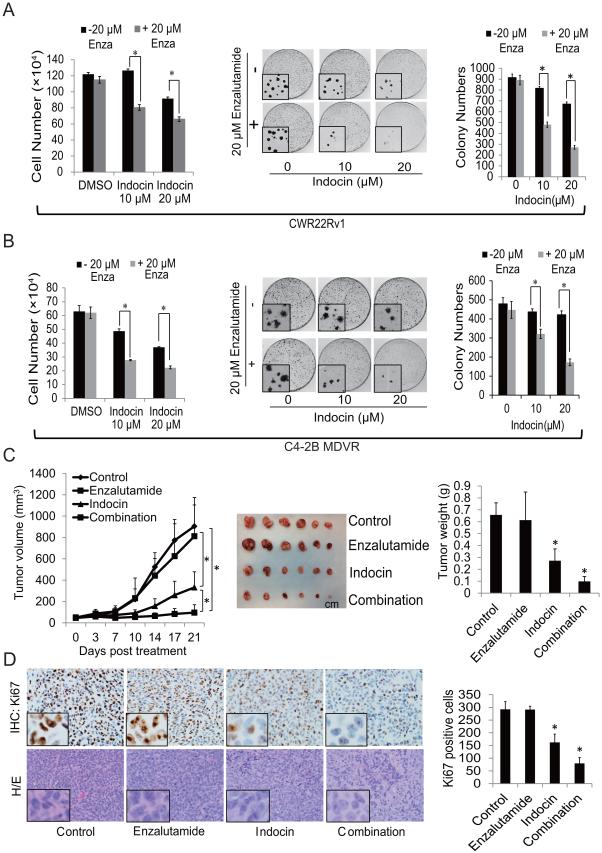
Indomethacin, a non-steroidal anti-inflammatory drug (NSAID) used for reducing fever, pain and inflammation, has been shown to be able to inhibit AKR1C3 activity (9, 31, 32). To further examine the role of AKR1C3 in enzalutamide resistance, we used indomethacin to hinder AKR1C3 activation and examined the effects on the response of PCa cells to enzalutamide treatment in vitro and in vivo. As shown in Fig.6A left, indomethacin did not have an effect on CWR22Rv1 cell growth at 10 μM but inhibited cell growth marginally at 20 μM. However, combination of indomethacin with enzalutamide significantly inhibited the growth of enzalutamide resistant CWR22Rv1 cells. The results were also confirmed by clonogenic assay. As shown in Fig.6A right, combination of indomethacin with enzalutamide significantly inhibited colony numbers and reduced colony size in CWR22Rv1 cells. Similar results were also obtained in C4-2B MDVR cells (Fig.6B). To test whether inhibition of AKR1C3 by indomethacin overcomes resistance to enzalutamide treatment in vivo, CWR22Rv1 xenograft model was used. As shown in Fig. 6C, while CWR22Rv1 tumors were resistant to enzalutamide treatment, indomethacin significantly inhibited tumor growth. Combination of indomethacin with enzalutamide further inhibited tumor growth of CWR22Rv1 xenografts. Immunohistochemical staining of Ki67 showed that cell proliferation was significantly inhibited by indomethacin, and further inhibited by the combination treatment (Fig.6D). Collectively, these results suggest that inhibition of AKR1C3 by indomethacin reduced enzalutamide-resistant tumor growth, and that combination of enzalutamide with indomethacin further reduced the tumor growth of enzalutamide-resistant prostate cancer. These results indicate that inhibition of AKR1C3 by indomethacin potentiates the cell killing effect of enzalutamide.
Figure 6.
Indomethacin, an inhibitor of AKR1C3activity, overcomes enzalutamide resistance. A, CWR22Rv1 cells were treated with 10 μM or 20 μM indomethacin with or without 20 μM enzalutamide for 2 days, total cell numbers were counted (left), and clonogenic assay was performed, the colony size pictures were taken under a microscope (middle). Colonies were counted and results are presented as means ± SD of 2 experiments performed in duplicate (right). B, C4-2B MDVR cells were treated with 10 μM or 20 μM indomethacin with or without 20 μM enzalutamide for 2 days, total cell numbers were counted (left), and clonogenic assay was performed; the colony size pictures were taken under a microscope (middle). Colonies were counted and results are presented as means ± SD of 2 experiments performed in duplicate (right). C, Mice bearing CWR22Rv1 xenografts were treated with vehicle control, enzalutamide (25 mg/Kg p.o), Indomethacin (3mg/Kg i.p) or their combination for 3 weeks, tumor volumes were measured twice weekly and the tumors were collected and weighed. D, IHC staining of Ki67 and H/E staining in each group was performed and quantified as described in methods. * p<0.05. Enza: Enzalutamide. Indocin: Indomethacin.
Discussion
The second generation androgen antagonist enzalutamide represents an improvement in therapy options for late stage metastatic CRPC (33, 34). However, the initial responders develop resistance inevitably. The potential mechanisms associated with enzalutamide resistance have been the focus of intense investigation. We previously identified several novel mechanisms involved in enzalutamide resistance including activation of NF-κB2/p52 (27, 35), AR-V7 (2, 27), Stat3 (4), and induction of autophagy (36). In this study, we have identified AKR1C3 activation and elevated intracrine androgens as potential mechanisms contributing to enzalutamide resistance. We demonstrate that AKR1C3 is overexpressed in enzalutamide resistant prostate cancer cells. Overexpression of AKR1C3 confers resistance to enzalutamide, while down regulation of AKR1C3 sensitizes PCa cells to enzalutamide treatment. In addition, overexpression of AKR1C3 has been demonstrated in clinical metastatic prostate cancer and correlated with disease progression. We also demonstrated that intracrine steroids including androgens are elevated in enzalutamide resistant cells, possibly through increased expression of steroidogenic enzymes such as AKR1C3. We further demonstrated that indomethacin, a potent inhibitor of AKR1C3, could be used to overcome enzalutamide resistance. The discovery of elevated intracrine androgen synthesis and enhanced AKR1C3 activation in enzalutamide resistant cells reveal a novel mechanism for the development and progression of resistant CRPC. Co-targeting AKR1C3 will provide proof-of-concept experiments to overcome resistance and achieve durable responses in men with second-generation antiandrogen treatment.
Intracrine androgen biosynthesis has been well characterized as a mechanism of CRPC (9-12, 28). Many enzymes are involved in androgen synthesis, including CYP17A1, AKR1C3 and HSD3B. CYP17A1 can be inhibited by abiraterone in clinical treatments (37, 38). AKR1C3 is a steroidogenic enzyme involved in steroid biosynthesis and mediates the last step of testosterone biosynthesis from androstenedione. It catalyzes conversion of steroids and modulates trans-activation of steroid receptors. Elevated expression of AKR1C3 has been associated with PCa progression and aggressiveness (19, 20). AKR1C3 has also been identified as an AR co-activator (39). In this study, we used gene enrichment analysis to compare enzalutamide resistant cells to enzalutamide sensitive cells, and found that the steroid biosynthesis genes were highly enriched in C4-2B MDVR cells, and several important genes involved in androgen synthesis, such as AKR1C3, HSD3B and CYP17A1 were up regulated in enzalutamide resistant cells. In another de novo enzalutamide resistant cell line CWR22Rv1, AKR1C3 was highly expressed compared to C4-2B or LNCaP cells, suggesting that AKR1C3 might play a pivotal role in enzalutamide resistance. To further confirm that intracrine androgen synthesis was acquired by C4-2B MDVR cells, steroid levels in C4-2B parental and C4-2B MDVR cells were determined by Liquid Chromatography-Mass Spectrometry (LC-MS). In addition to the higher levels of testosterone and DHT in enzalutamide resistant cells, the levels of the precursors of testosterone such as cholesterol, DHEA and progesterone were all elevated in C4-2B-MDVR cells compared to C4-2B parental cells. These results demonstrate that AKR1C3 was significantly elevated in enzalutamide resistant prostate cancer cells which potentially resulted in higher levels of testosterone and DHT in enzalutamide resistant cells. The de novo intratumoral steroid synthesis has also been shown as a potential mechanism contributing to abiraterone resistance (9). Long term treatment of abiraterone in patients resulted in an increase in steroids biosynthesis through deregulated steroid enzymes, such as AKR1C3 (40).
Several inhibitors have been developed to target AKR1C3 activation including indomethacin (32). Indomethacin is a non-steroidal anti-inflammatory drug (NSAID) used for reducing fever, pain and inflammation. Several studies revealed indomethacin might have the potential to increase the sensitivity of cancer cells to anticancer agents, such as that of human melanoma cells to TRAIL-induced apoptosis (41), and of colon cancer cells to cisplatin (42). Indomethacin also has the ability to inhibit PSA and ERG protein expression and decreased testosterone and DHT levels in relapsed VCaP xenograft tumors (9). In the present study, we showed that inhibition of AKR1C3 enzyme activity by indomethacin restored enzalutamide sensitivity in enzalutamide resistant prostate cancer cells both in vitro and in vivo. Furthermore, the combination of indomethacin and enzalutamide resulted in significantly greater inhibition of enzalutamide-resistant tumor growth. Our data suggest that inhibition of AKR1C3 holds promise as a sensitizing strategy to restore anti-tumor effects in patients resistant to enzalutamide.
Taken together, we found that AKR1C3 activation and the resultant intracrine androgen synthesis confers resistance to enzalutamide in prostate cancer cells. Inhibition of AKR1C3 by shRNA or indomethacin overcomes resistance to enzalutamide. Furthermore, the combination of indomethacin and enzalutamide resulted in significant inhibition of enzalutamide-resistant tumor growth. Targeting AKR1C3 may provide an effective treatment strategy for patients resistant to enzalutamide.
Acknowledgments
This work was supported in part by grants NIH/NCI CA140468, CA168601, and CA179970.
References
- 1.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer research. 2013;73:483–9. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu C, Lou W, Zhu Y, Nadiminty N, Schwartz CT, Evans CP, et al. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clin Cancer Res. 2014;20:3198–210. doi: 10.1158/1078-0432.CCR-13-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. The New England journal of medicine. 2014;371:1028–38. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu C, Zhu Y, Lou W, Cui Y, Evans CP, Gao AC. Inhibition of constitutively active Stat3 reverses enzalutamide resistance in LNCaP derivative prostate cancer cells. The Prostate. 2014;74:201–9. doi: 10.1002/pros.22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S, et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide) Cancer discovery. 2013;3:1030–43. doi: 10.1158/2159-8290.CD-13-0142. [DOI] [PubMed] [Google Scholar]
- 6.Joseph JD, Lu N, Qian J, Sensintaffar J, Shao G, Brigham D, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer discovery. 2013;3:1020–9. doi: 10.1158/2159-8290.CD-13-0226. [DOI] [PubMed] [Google Scholar]
- 7.Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–22. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isikbay M, Otto K, Kregel S, Kach J, Cai Y, Vander Griend DJ, et al. Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Hormones & cancer. 2014;5:72–89. doi: 10.1007/s12672-014-0173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai C, Chen S, Ng P, Bubley GJ, Nelson PS, Mostaghel EA, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer research. 2011;71:6503–13. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishizaki F, Nishiyama T, Kawasaki T, Miyashiro Y, Hara N, Takizawa I, et al. Androgen deprivation promotes intratumoral synthesis of dihydrotestosterone from androgen metabolites in prostate cancer. Scientific reports. 2013;3:1528. doi: 10.1038/srep01528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer research. 2008;68:6407–15. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 12.Mohler JL, Titus MA, Bai S, Kennerley BJ, Lih FB, Tomer KB, et al. Activation of the androgen receptor by intratumoral bioconversion of androstanediol to dihydrotestosterone in prostate cancer. Cancer research. 2011;71:1486–96. doi: 10.1158/0008-5472.CAN-10-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efstathiou ETM, Tsavachidou D, Hoang A, Karlou M, Wen S, et al. MDV3100 effects on androgen receptor (AR) signaling and bone marrow testosterone concentration modulation: Apreliminary report. ASCO Meeting Abstracts. 2011;29:4501. [Google Scholar]
- 14.Efstathiou E, Titus M, Wen S, Hoang A, Karlou M, Ashe R, et al. Molecular Characterization of Enzalutamide-treated Bone Metastatic Castration-resistant Prostate Cancer. European urology. 2014 doi: 10.1016/j.eururo.2014.05.005. 10.1016/j.eururo.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang KH, Li R, Kuri B, Lotan Y, Roehrborn CG, Liu J, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154:1074–84. doi: 10.1016/j.cell.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang KH, Ercole CE, Sharifi N. Androgen metabolism in prostate cancer: from molecular mechanisms to clinical consequences. British journal of cancer. 2014;111:1249–54. doi: 10.1038/bjc.2014.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labrie F, Luu-The V, Lin SX, Labrie C, Simard J, Breton R, et al. The key role of 17 beta-hydroxysteroid dehydrogenases in sex steroid biology. Steroids. 1997;62:148–58. doi: 10.1016/s0039-128x(96)00174-2. [DOI] [PubMed] [Google Scholar]
- 18.Bauman DR, Steckelbroeck S, Williams MV, Peehl DM, Penning TM. Identification of the major oxidative 3alpha-hydroxysteroid dehydrogenase in human prostate that converts 5alpha-androstane-3alpha,17beta-diol to 5alpha-dihydrotestosterone: a potential therapeutic target for androgen-dependent disease. Molecular endocrinology. 2006;20:444–58. doi: 10.1210/me.2005-0287. [DOI] [PubMed] [Google Scholar]
- 19.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer research. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 20.Wako K, Kawasaki T, Yamana K, Suzuki K, Jiang S, Umezu H, et al. Expression of androgen receptor through androgen-converting enzymes is associated with biological aggressiveness in prostate cancer. Journal of clinical pathology. 2008;61:448–54. doi: 10.1136/jcp.2007.050906. [DOI] [PubMed] [Google Scholar]
- 21.Gaikwad NW. Ultra performance liquid chromatography-tandem mass spectrometry method for profiling of steroid metabolome in human tissue. Analytical chemistry. 2013;85:4951–60. doi: 10.1021/ac400016e. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Y, Liu C, Nadiminty N, Lou W, Tummala R, Evans CP, et al. Inhibition of ABCB1 expression overcomes acquired docetaxel resistance in prostate cancer. Molecular cancer therapeutics. 2013;12:1829–36. doi: 10.1158/1535-7163.MCT-13-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C, Nadiminty N, Tummala R, Chun JY, Lou W, Zhu Y, et al. Andrographolide targets androgen receptor pathway in castration-resistant prostate cancer. Genes & cancer. 2011;2:151–9. doi: 10.1177/1947601911409744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu R, Lu C, Mostaghel EA, Yegnasubramanian S, Gurel M, Tannahill C, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer research. 2013;72:3457–62. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer research. 2008;68:5469–77. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadiminty N, Tummala R, Liu C, Yang J, Lou W, Evans CP, et al. NF-kappaB2/p52 induces resistance to enzalutamide in prostate cancer: role of androgen receptor and its variants. Molecular cancer therapeutics. 2013;12:1629–37. doi: 10.1158/1535-7163.MCT-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fankhauser M, Tan Y, Macintyre G, Haviv I, Hong MK, Nguyen T, et al. Canonical Androstenedione Reduction is the Predominant Source of Signalling Androgens in Hormone Refractory Prostate Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 doi: 10.1158/1078-0432.CCR-13-3483. doi: 10.1158/1078-0432.CCR-13-3483. [DOI] [PubMed] [Google Scholar]
- 29.Mitsiades N, Sung CC, Schultz N, Danila DC, He B, Eedunuri VK, et al. Distinct patterns of dysregulated expression of enzymes involved in androgen synthesis and metabolism in metastatic prostate cancer tumors. Cancer research. 2012;72:6142–52. doi: 10.1158/0008-5472.CAN-12-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer research. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liedtke AJ, Adeniji AO, Chen M, Byrns MC, Jin Y, Christianson DW, et al. Development of potent and selective indomethacin analogues for the inhibition of AKR1C3 (Type 5 17beta-hydroxysteroid dehydrogenase/prostaglandin F synthase) in castrate-resistant prostate cancer. Journal of medicinal chemistry. 2013;56:2429–46. doi: 10.1021/jm3017656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flanagan JU, Yosaatmadja Y, Teague RM, Chai MZ, Turnbull AP, Squire CJ. Crystal structures of three classes of non-steroidal anti-inflammatory drugs in complex with aldo-keto reductase 1C3. PloS one. 2012;7:e43965. doi: 10.1371/journal.pone.0043965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437–46. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 35.Cui Y, Nadiminty N, Liu C, Lou W, Schwartz CT, Gao AC. Upregulation of glucose metabolism by NF-kappaB2/p52 mediates enzalutamide resistance in castration-resistant prostate cancer cells. Endocrine-related cancer. 2014;21:435–42. doi: 10.1530/ERC-14-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen HG, Yang JC, Kung HJ, Shi XB, Tilki D, Lara PN, Jr., et al. Targeting autophagy overcomes Enzalutamide resistance in castration-resistant prostate cancer cells and improves therapeutic response in a xenograft model. Oncogene. 2014;33:4521–30. doi: 10.1038/onc.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. The New England journal of medicine. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. The New England journal of medicine. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yepuru M, Wu Z, Kulkarni A, Yin F, Barrett CM, Kim J, et al. Steroidogenic enzyme AKR1C3 is a novel androgen receptor-selective coactivator that promotes prostate cancer growth. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:5613–25. doi: 10.1158/1078-0432.CCR-13-1151. [DOI] [PubMed] [Google Scholar]
- 40.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:5913–25. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tse AK, Cao HH, Cheng CY, Kwan HY, Yu H, Fong WF, et al. Indomethacin Sensitizes TRAIL-Resistant Melanoma Cells to TRAIL-Induced Apoptosis through ROS-Mediated Upregulation of Death Receptor 5 and Downregulation of Survivin. The Journal of investigative dermatology. 2013;134:1397–407. doi: 10.1038/jid.2013.471. [DOI] [PubMed] [Google Scholar]
- 42.Brunelli C, Amici C, Angelini M, Fracassi C, Belardo G, Santoro MG. The non-steroidal anti-inflammatory drug indomethacin activates the eIF2alpha kinase PKR, causing a translational block in human colorectal cancer cells. The Biochemical journal. 2012;443:379–86. doi: 10.1042/BJ20111236. [DOI] [PubMed] [Google Scholar]