Abstract
Environmental factors are suspected in the rise of obesity and cancer in industrialized countries but poorly understood. Here we used animal models to test how future generations may be affected by Westernized diets. We discover long-term consequences of grandmothers’ in-utero dietary exposures leading to high rates of obesity and frequent cancers of lung and liver in two subsequent generations of mice. Transgenerational effects were transplantable using diet-associated bacteria communities alone. Consequently, feeding of beneficial microbes was sufficient to lower transgenerational risk for cancer and obesity regardless of diet history. Targeting microbes may be a highly effective population-based approach to lower risk for cancer.
Keywords: pregnancy, Western diet, lung cancer, hepatocellular carcinoma, bacteria, microbes
Introduction
Inflammation-associated, metabolic and neoplastic diseases have increased in frequency in the industrialized world due to numerous factors including reduced activity levels, increased total energy intake, environmental carcinogens, and immune status (1,2). Compelling hypotheses aim to explain this phenomenon and re-shape preventive medicine. According to one microbe-immune-centric hypothesis, inhabitants of developed countries have immune systems of reduced lifelong regulatory capacity due to societal practices in the form of refined diets, antibiotics and Caesarian births with insufficient beneficial microbe exposure during perinatal life (1–3). Impaired regulatory capacity leads to uncontrolled immune inflammatory responses, obesity and cancer later in life (2, 3). Our recent studies in mice have shown that Westernized diet (NWD)-associated obesity and cancer coincide with changes in gastrointestinal (GI) tract microbial communities and immune regulatory capacity preventable by dietary enrichment with beneficial bacteria(4–7). We test here whether these postulated effects of gut microbes transcend generations. We discover that mother mice consuming NWD during pregnancy convey detrimental long-term consequences to subsequent generations, in particular high rates of lung and liver cancer with obesity and premature senescence. We show this transgenerational predisposition is mediated by intestinal bacteria, highlighting a key role for microbial flora in reducing the risk of cancer.
Materials and Methods
Animals
Outbred conventional or germ-free CD-1 Swiss stock mice (Charles River; Wilmington, MA) were housed and handled in Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited facilities with diets, experimental methods, and housing as approved by the Institutional Animal Care and Use Committee. A genetically-outbred stock with robust breeding capacity and absent transgenic predilections to cancer was selected for these transgenerational studies. In order to test the transgenerational impact of dietary microbes on progeny, the experimental design was to expose F0 [grandmother] mice to diets starting at the age of eight weeks. Special dietary treatment continued until the birth of their pups. Progeny were later examined to determine health risks to subsequent generations. Euthanasia was performed using carbon dioxide overdose at one year of age, unless otherwise specified. Due to early-life morbidity, F2 progeny (grandchildren) were euthanized at six-months-of-age or younger according to institutional humane criteria and clinical disease. Tissues were collected upon necropsy and then examined histologically. Each experiment included 5–10 animals per sex per treatment group, performed in duplicate, as described in detail below.
Special diets for animals
Outbred Swiss CD-1 mice were placed on experimental diets starting at 8 weeks of age: control AIN-76A (Harlan-Teklad Madison WI), and a New Western diet (NWD) with high fat and low fiber with substandard levels of Vitamins B and D (TD.96096); Harlan-Teklad; Table Supplemental 1) as previously described (7). Subsets of CD-1 mice who had been treated with NWD in-utero subsequently received in their drinking water an anti-inflammatory strain of Lactobacillus reuteri ATCC-PTA-6475 cultivated as described elsewhere (8,9), with live organisms supplied at a starting dosage of 3.5×105 organisms/mouse/day in drinking water (4). Live bacterial counts in water bottles were calculated to be 1.4×106 colony forming units (CFU) per mouse on day 1, 4.1×105 CFU on day 2, and 1.1×105 CFU on day 3, and L reuteri was detectable by PCR in feces and bowel of mice undergoing the dosing regimen, as described in detail in Lakritz et al (2014) (4). Control mice received regular drinking water. Fresh drinking water for both groups of animals was replaced twice weekly throughout the experiments.
Experimental design
Experiment 1
Six eight-week-old CD-1 female mice were fed ad libitum a Westernized (NWD) diet TD.96096 mimicking fast-food starting at 8 weeks of age (Figure 1A). Females who ultimately served as mothers and grandmothers were immediately arranged into breeding pairs to induce pregnancy. Special diets were replaced with control AIN-76A chow upon birth of F1 progeny. To serve as controls, six separate eight-week-old CD-1 female mice were fed ad libitum a control chow AIN-76A and similarly arranged into breeding pairs to produce experimental control progeny animals. All animals had ad libitum access to diets with unrestricted exercise. Their progeny were also examined for obesity and cancer when they reached one-year-of-age including body weights along with tissue collections upon necropsy for histological examination.
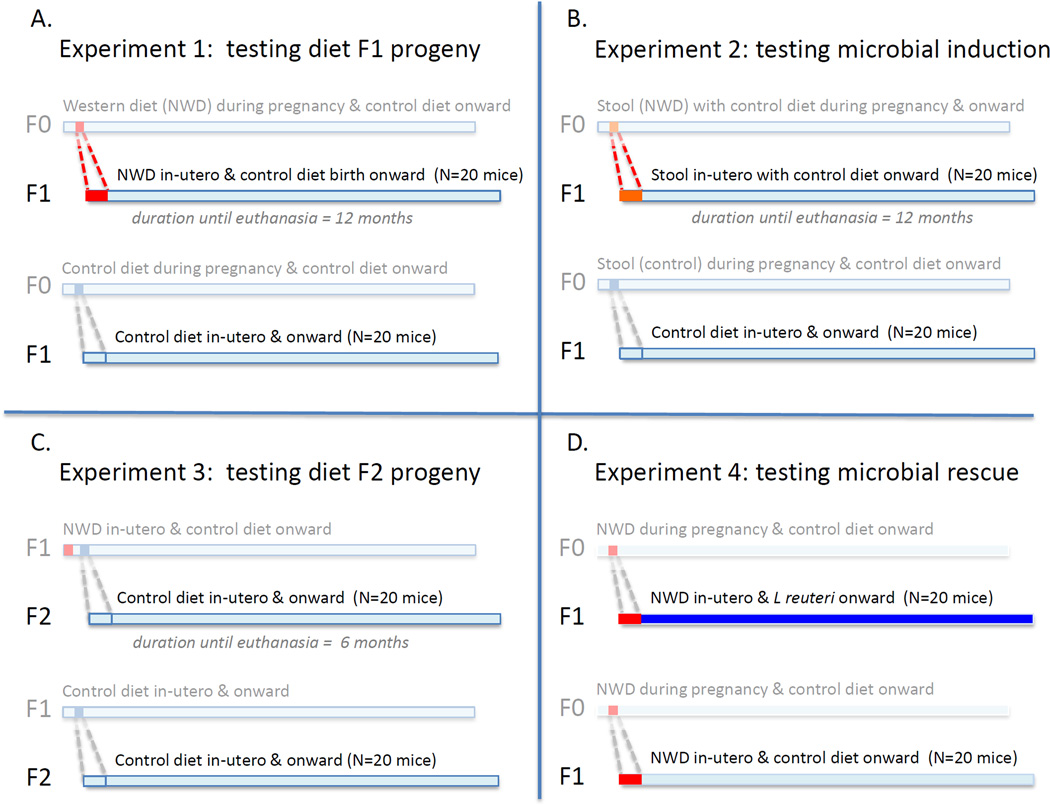
Figure 1. Overview of experimental design.
A In experiment 1, in order to determine the risk of cancer in progeny animals, eight-week-old CD-1 female mice were fed ad libitum a Westernized (NWD) chow mimicking fast-food consumed by humans, and then arranged into breeding pairs to induce pregnancy. Their progeny were examined for obesity and cancer when they reached one-year-of-age. B In experiment 2, germ-free female CD-1 mice were fed by gastric gavage the feces from NWD-fed mice, paired with males and then fed only control chow. Their progeny were examined for cancer at one year of age. C In experiment 3, F1 progeny from NWD-fed mother mice were arranged into breeding pairs to produce offspring (grandchildren) for further observation. Progeny were examined for cancer starting at six months of age. D Three-week-old progeny (F1) from NWD-fed mother mice in Experiment 1 were subdivided and provided in their drinking water Lactobacillus reuteri ATCC-PTA-6475 to test effects of beneficial microbes. Mice receiving NWD in-utero from experiment 1 served as regular-drinking-water controls.
Experiment 2
To test whether gut microbes were sufficient for transgenerational effects, five germ-free female Swiss CD-1 mice were fed by gastric gavage the feces from NWD-fed mother mice in Experiment 1 (Figure 1B). Five germ-free mice received comparable stool collected from control diet-fed moms in Experiment 1 to serve as controls. To achieve this the germ-free mice were dosed three times every-other-day by gastric gavage with 0.05 grams of fresh fecal slurry per mouse per dose. Subsequently both groups of fecal transplant-recipient moms were co-housed with a male mouse in autoclaved caging and fed only control chow AIN-76A diet in order to produce progeny for future examination. Progeny were examined for cancer at one-year-of-age when tissues were collected upon necropsy.
Experiment 3
Six F1 female progeny with in-utero exposure to NWD from Experiment 1 [above] were randomly arranged into breeding pairs at eight-weeks-of-age in order to produce offspring (grandchildren) for further observation (Figure 1C). Six F1 progeny from control diet-fed moms in Experiment 1 were similarly arranged into breeding pairs to serve as controls. Both groups were fed control AIN-76A diet throughout Experiment 3. Unanticipated fertility problems arose in half [3/6] of the matings involving NWD-fed mother mice, whereas control mice had no such issues. Two litters with ancestral exposure to NWD also exhibited a scurfy-like thymic atrophy and failure-to-thrive in 9/20 [45%] infant mice and were euthanized at 8-weeks-of-age. Surviving F2 progeny [N=10 males and N=10 females] were examined for cancer starting at six months of age (earlier than their parents cohort) due to unexpected morbidity requiring euthanasia. Tissues were collected upon necropsy and then examined histologically to confirm diagnoses.
Experiment 4
To further test the microbe-centric hypothesis, three-week-old progeny (F1) from NWD-fed CD-1 mother mice in Experiment 1 were randomly subdivided into groups of twenty mice [ten males and ten females per treatment] and received in their drinking water Lactobacillus reuteri ATCC-PTA-6475 as described elsewhere (8,9) continuously until one-year-of-age (Figure 1D). Mice from Experiment 1 that received NWD while in-utero that then got regular drinking water served as controls. Tissues were collected upon necropsy at one-year-of-age.
Stool microbiome analyses
Genomic DNA was extracted from stool samples using the Qiagen QIAamp DNA Stool Mini Kit. Samples for paired-end Illumina sequencing were constructed using a two-step PCR amplicon approach targeting the V4 region of the 16S rRNA gene (U515F and E786R) and reads were quality filtered and clustered into operational taxonomic units (OTUs) at 97% nucleotide identity as previously described (7).
Histopathology and immunohistochemistry
For histologic evaluation, formalin-fixed tissues were embedded in paraffin, cut at 5 μm, and stained with hematoxylin or immunohistochemistry as previously described (3,10). Primary antibodies for IHC included rabbit antibodies against myeloperoxidase, p53 (ThermoFisher Scientific/Lab Vision, Fremont, CA), IL-17 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), Thyroid transcription factor 1 and ki-67 (TTF1, Abcam, Cambridge, UK). Heat-induced antigen retrieval was performed with citrate buffer, pH 6, for myeloperoxidase, with EDTA buffer, pH 8, for TTF1 and p53 detection or with CC1 epitope retrieval solution (Ventana Medical Systems, Inc., Tucson, AZ) for ki-67 and IL-17. Primary antibody binding was detected with goat anti-rabbit polymer HRP (ZytoChem Plus, Berlin, Germany). Color was developed with DAB substrate-chromogen system (ThermoFisher Scientific/Lab Vision) and tissues were counterstained with hematoxylin.
Statistical analyses
The Mann-Whitney U test was used for whole body and thymic weight analyses. Tumor incidence in experimental groups was compared with the Fisher’s exact test. All analyses were performed with the Graphpad Prism version 5.0 for windows, GraphPad software, San Diego, CA.A p-value < 0.05 was statistically significant.
Results
Offspring of mice consuming Western diet (NWD) have high risk for cancer
Lifestyle changes including Westernized diet appear to underlie many of the chronic inflammatory diseases including obesity, diabetes, heart disease, autoimmune diseases and cancer (11). To test whether Westernized (NWD) diet-induced obesity (7) and neoplasia (4) may be passed to subsequent generations, we fed NWD to mother mice (F0) during pregnancy and then examined health outcomes in their children reared eating a regular control chow diet (Figure 1a). For these studies we used outbred white Swiss CD-1 stock without additional exposure to carcinogens. Mother mice of generation F0 consuming NWD for several weeks during pregnancy were not themselves affected by cancer and obesity in this study. However, we found that F1 progeny of moms consuming NWD during pregnancy had more frequent cancers when compared with offspring of mother mice eating control diet during pregnancy [Table 1].
Table 1.
Frequency of cancers diagnosed in vital organs of progeny animals.
| generation | treatment |
age at necropsy |
group ID | organ type examined |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| lung | liver | lymph | intestine | pancreas | mammary | ovary | ||||
| Experiment #1: testing whether in-utero exposure to NWD increases risk of cancer in first generation progeny | ||||||||||
| F1 | control | 12 months | all females males |
0/20 0/10 0/10 |
0/20 0/10 0/10 |
5% (1/20) 1/10 0/10 |
0/20 0/10 0/10 |
0/20 0/10 0/10 |
0/10 0/10 nd |
0/10 0/10 nd |
| F1 | NWD in-utero | 12 months | all females males |
70% (14/20) 7/10 7/10 |
66% (13/20) 6/10 7/10 |
55% (11/20) 7/10 4/10 |
5% (1/20) 1/10 0/10 |
0/20 0/10 0/10 |
20% (2/10) 2/10 nd |
30% (3/10) 3/10 nd |
| Experiment #1 Fisher's exact p value | all | p<0.0001 | p<0.0001 | p<0.003 | ns | ns | ns | ns | ||
| Experiment #2: testing whether transplantable microbes are sufficient for increased cancer risk in first generation progeny | ||||||||||
| F1 | control feces | 12 months | all females males |
1/20 1/10 0/10 |
1/20 0/10 1/10 |
3/20 2/10 1/10 |
0/20 0/10 0/10 |
0/20 0/10 0/10 |
0/10 0/10 nd |
0/10 0/10 nd |
| F1 | NWD feces | 12 months | all females males |
70% (14/20) 8/10 6/10 |
70% (14/20) 7/10 7/10 |
66% (13/20) 8/10 5/10 |
5% (1/20) 1/10 0/10 |
5% (1/20) 1/10 0/10 |
20% (2/10) 2/10 nd |
20% (2/10) 2/10 nd |
| Experiment # 2 Fisher's exact p value | all | p<0.0001 | p<0.0001 | p<0.003 | ns | ns | ns | ns | ||
| Experiment #3: testing whether in-utero exposure to NWD increases risk of cancer in second generation [grandchildren] progeny | ||||||||||
| F2 | control in utero | 6 months | all females males |
0/20 0/10 0/10 |
0/20 0/10 0/10 |
1/20 1/10 0/10 |
0/20 0/10 0/10 |
0/20 0/10 0/10 |
0/10 0/10 nd |
0/10 0/10 nd |
| F2 | NWD in-utero | 6 months | all females males |
75% (15/20) 8/10 7/10 |
80% (16/20) 8/10 8/10 |
50% (10/20) 7/10 3/10 |
5% (1/20) 0/10 1/10 |
5% (1/20) 0/10 1/10 |
20% (2/10) 2/10 nd |
30% (3/10) 3/10 nd |
| Experiment #3 Fisher's exact p value | all | p<0.0001 | p<0.0001 | p<0.003 | ns | ns | ns | ns | ||
| Experiment #4: testing whether treatment of first generation progeny with beneficial bacteria inhibits cancer risk after in-utero NWD exposure | ||||||||||
| F1 | oral LR tx of NWD in-utero |
12 months | all females males |
5% (1/20) 1/10 0/10 |
5% (1/20) 0/10 1/10 |
( 20%) 4/20 3/10 1/10 |
0/20 0/10 0/10 |
0/20 0/10 0/10 |
0/10 0/10 nd |
0/10 0/10 nd |
| F1 | NWD in-utero | 12 months | all females males |
70% (14/20) 7/10 7/10 |
66% (13/20) 6/10 7/10 |
50% (10/20) 6/10 4/10 |
10% (2/20) 1/10 1/10 |
5% (1/20) 1/10 0/10 |
20% (2/10) 2/10 nd |
30% (3/10) 3/10 nd |
| Experiment #4 Fisher's exact p value | all | p<0.0001 | p<0.0001 | p<0.04 | ns | ns | ns | ns | ||
Specifically, the most profoundly increased cancer types arising in mice with in-utero exposure to NWD included pulmonary adenoma (3/14) and adenocarcinoma (11/14) [14/20; p<0.0001], liver hepatocellular carcinoma [12/20; p<0.0001], and spleen or mesenteric lymph node lymphoma [11/20; p<0.003] (Table 1, Figure 2A-C). Other types of neoplasia were increased, although to a lesser extent, including mammary carcinoma (Figure 2D), reproductive organ malignancies such as ovarian theca and granulosa cell tumors and cystadenoma (Figure 2D), and primary hepatic hemangiosarcoma, small intestine adenoma and islet cell adenoma of pancreas (Figure Supplemental 1). Additionally, progeny of NWD-fed moms were more likely to suffer obesity than their counterparts whose mothers had eaten a control diet (Figure 3A), earlier linked with high levels of cytokine IL-17 (7). Using IL-17A-specific immunohistochemistry (IHC) we discovered extensive fat pyogranulomas displaying high levels of IL-17 protein in situ in offspring when their mothers had eaten the fast-food-style NWD chow [Figure 3A]. We also found higher abundance of Firmicutes and lower abundance of Bacteroidetes in NWD-treated mice consistent with our previous findings and also those of other groups who’ve studied obesity and metabolic syndrome (Figure S2) (7,12). Knowing that GI tract microbial communities change within days after eating NWD (7), we hypothesized that microbial dysbiosis in pregnant mother mice placed their infants at increased risk for cancer.
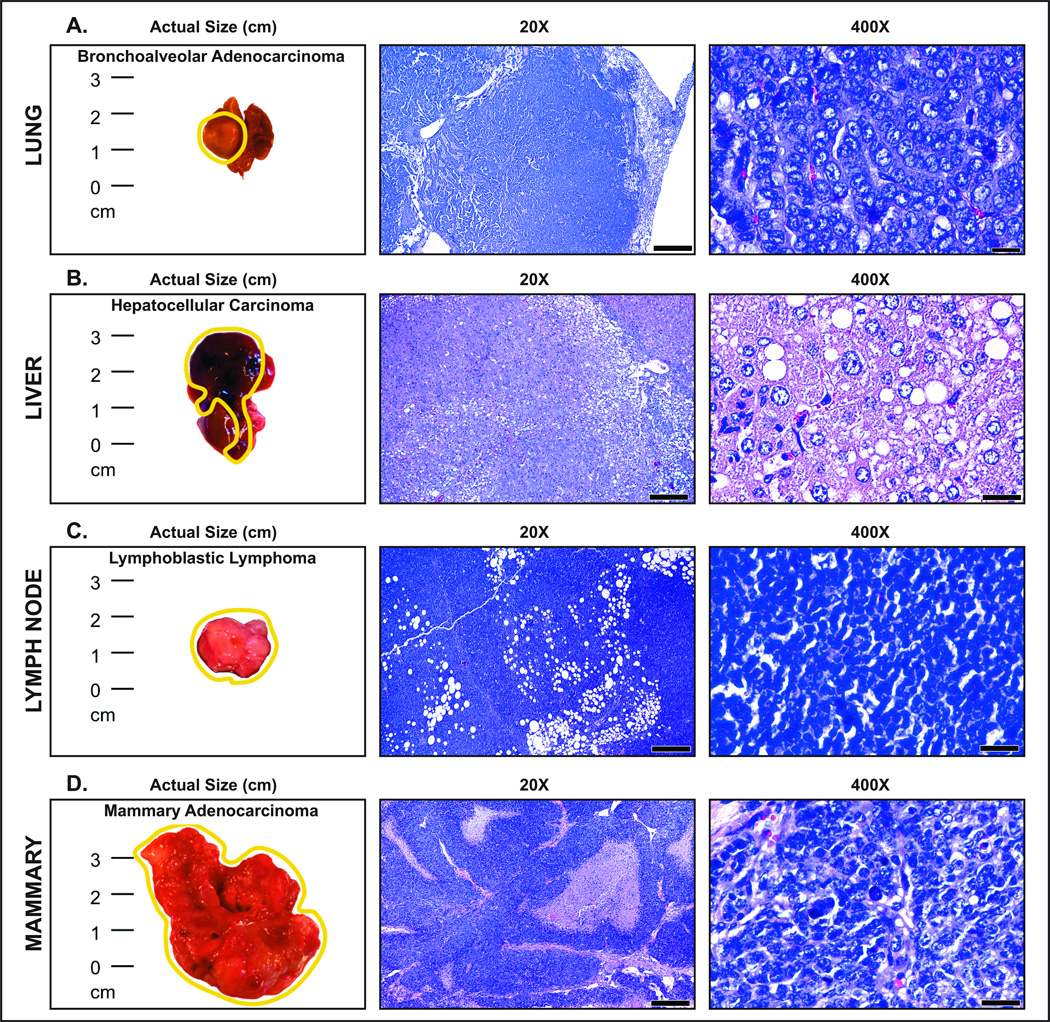
Figure 2. Neoplastic phenotypes encountered in progeny of Swiss CD-1 mice eating Western-style (NWD) chow during pregnancy.
(A) Pulmonary adenocarcinoma. A large raised mass occupies the major part of the left lobe. The expansile, unencapsulated mass shows a mixed acinar and papillary histologic growth pattern with closely-packed lobules and cords supported by sparse stromal elements. Alveolar septa-like structures are lined by atypical cuboidal cells with eosinophilic cytoplasm and large, round euchromatic nuclei. (B) Hepatocellular carcinoma. A large irregular mass with superficial focal necroses obliterates normal liver shape and occupies approximately two thirds of the organ. The large well-circumscribed liver tumor compresses the normal liver parenchyma on the right and displays a solid growth pattern. The diagnosis is moderately well-differentiated hepatocellular carcinoma with large neoplastic hepatocytes and a high degree of nuclear pleomorphism. (c) Lymphoma. White lobulated mesenteric mass comprised of solid sheets of neoplastic cells that infiltrate the mesenteric fat. The uniform, medium-sized lymphocytes have a scant cytoplasm and round nuclei with fine chromatin. Their histomorphology is consistent with that of lymphoblastic lymphoma. (D) Large, irregular, lobulated mammary tumor. Solid prominent cords and large nests of neoplastic cells are separated by moderate amounts of fibrovascular stroma. Occasional nests show central area necrosis (commedo-like pattern). Neoplastic mammary epithelial cells show marked atypia and pleomorphism and abundant mitotic figures, including abnormal ones. Hematoxylin and Eosin. Scale bars: 500 μm (middle column); 25 μm (right column)
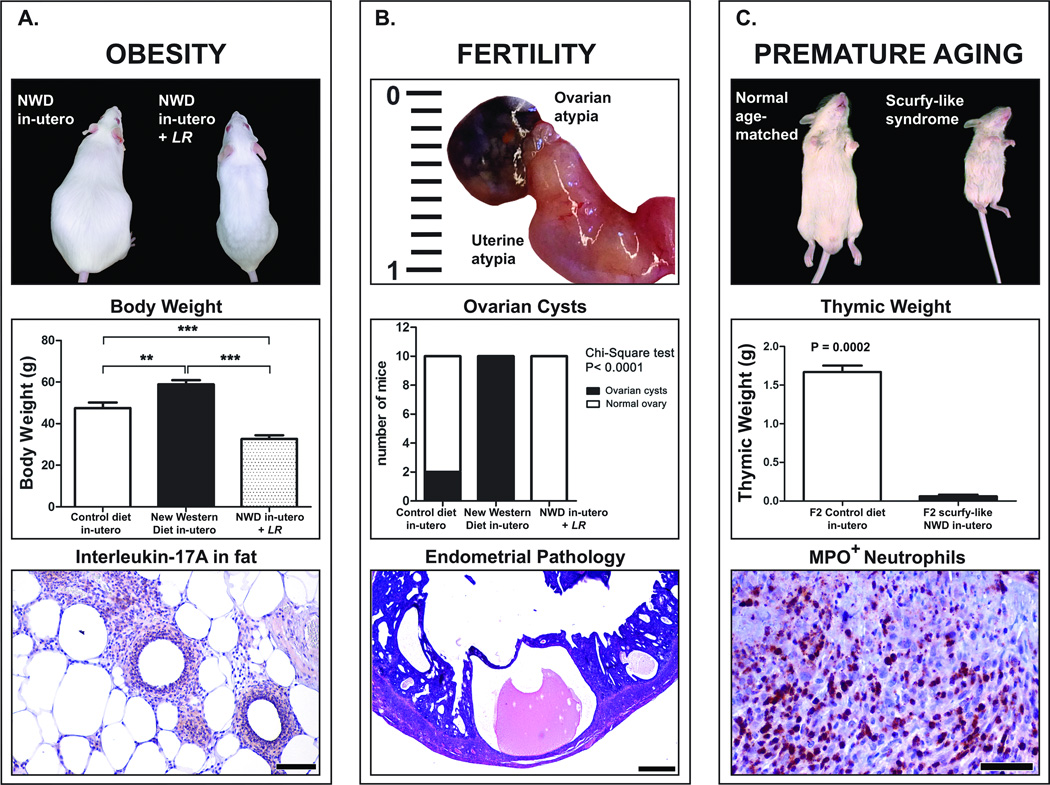
Figure 3. Non-neoplastic pathologies observed in the offspring of CD-1 mice consuming Western ( NWD) chow during pregnancy.
(A). Side-by side comparison of F1 mice derived from mothers consuming NWD during pregnancy after enrichment of their gut flora with L. reuteri LR-treated lean mouse on right) or not (obese mouse on the left). Edible L. reuteri bacteria negate the transgenerational NWD-induced effect otherwise leading to significantly increased body weight. Transgenerational obesity is characterized by typical adipose tissue pathology including IL-17-rich focal pyogranulomatous inflammatory lesions in the ependymal fat. (B) Markedly enlarged uterine horn with a cystically-dilated ovary. The presence of ovarian cysts was a consistent finding in mice exposed to NWD in-utero, but not in those offspring of control diet-fed mice. Enlarged uteri showed cystic endometrial hyperplasia. Note markedly dilated endometrial glands. (C) Eight-week-old F2 progeny of NWD-diet fed grandmother mice exhibited a scurfy-like syndrome with smaller in stature, sparse dull fur, and thymic atrophy. Thymic weight was significantly reduced when compared with age-matched controls. Arteritis in a mesenteric vessel involved high numbers of MPO-positive neutrophils infiltrating the vessel wall and local tissues. Hematoxylin and Eosin (lower panel of B). IHC; Diaminobenzidine chromogen, Hematoxylin counterstain. (lower panels of A and C). Scale bars: 500 μm (lower panel of B); 100 μm (lower panel of A); 50 μm (lower panel of C). Numbers on the y-axis of bar graphs correspond to the mean±SEM of the parameters assessed. *p<0.05, ** p<0.001, ***p<0.0001.
Fecal transplant from NWD-fed donors is sufficient for cancer in progeny
To test our microbe-centric hypothesis, we used fresh fecal slurry containing gut bacteria from NWD-fed mother mice delivered by gastric gavage to recipient eight-week-old germ-free Swiss female mice subsequently arranged in matings (Figure 1B). Control germ-free mice received comparable stool collected from control diet-fed moms. Subsequently both groups of stool-recipient moms ate only the standard control chow diet. When progeny were examined one year later we found that mice receiving NWD-donor microbiota had higher rates of lung [p<0.0001] and liver [p<0.0001] cancers, at levels similar to those in offspring from NWD-consuming moms [Table 1]. Conversely, offspring of germ-free mothers receiving control-stool gavage rarely exhibited neoplasms in the target organs. It remains to be determined whether in-utero activities of specific microorganisms result entirely from increased inflammatory tone or other mechanisms (13). Nonetheless, these findings demonstrated that transplantable fecal microbes were sufficient for increased risk of cancer in progeny mice.
F2 progeny display increased cancer and accelerated aging
Recognizing extensive pathology existing in F1 mice, we next tested whether subsequent generations are also at increased risk for cancer arising from ancestral in-utero dietary indiscretions. Thus we performed mating experiments among the F1 children of mother mice who had consumed NWD or control chow during pregnancy (Figure 1C). Initially, infertile mating of Swiss F1 progeny [3/6 (50%) versus 0/6; ns] made it difficult to produce the necessary F2 grandchildren from NWD-fed grandmother mice. Upon further examination of this generation, F1 female nulliparous littermate progeny exhibited not only obesity but also polycystic ovaries [p<0.001] and endometrial disease (Figure 3B). In these same female mice, elongated anogenital distances (data not shown) and urogenital atypia suggestive of masculinization were not extensively characterized. Another feature in daughters of NWD-fed mothers, hepatocellular carcinoma, is more typical of male animals with high levels of IL-6 (14). Specific causes of impaired fertility in these mice remain to be investigated.
Grandchildren (F2 progeny) emerging from fertile F1 mating ultimately exhibited lung [p<0.0001] and liver [p<0.0001] cancers with neoplasms arising at younger ages than those seen in their parents (Table 1). Similar early-onset transgenerational phenomena have been described in F2 progeny of rats undergoing experimental stressors while in- utero (15), raising the possibility that in-utero microbial ‘stress’ is linked with underlying epigenetic mechanisms. Ancestral exposures to NWD were also manifested as failure-to-thrive [9/20 vs 0/20; p<0.05] in eight-week-old F2 progeny (Figure 3C), affecting both male [50% (5/10)] and female [40% (4/10)] animals. The syndrome included thymic involution and inflammatory infiltrates throughout vital organs resembling ‘scurfy’ mice with immune regulatory deficits (16).
Recognizing that pathogenic gut bacteria trigger accumulations of neutrophils (17), we examined non-neoplastic tissues from descendants with gut dysbiosis, and found increased numbers of intravascular and extravasated neutrophils, an observation supported by myeloperoxidase (MPO)-specific IHC in liver, adipose tissue pyogranulomas, and mesenteric arteries (Figure 3C). By contrast, the presence of neutrophils in control diet- and LR-treated mouse tissues was minimal. It was previously shown that reciprocal relationships exist between neutrophils and host regulatory capacity (10,18). Additional studies are needed to ascertain whether perinatal changes in microbe communities serve to modulate immune regulatory capacity later in life. Taken together these findings led us to conclude that microbial dysbiosis leads to transgenerational effects including cancer, obesity, and accelerated aging. Knowing NWD-associated obesity and cancer are preventable by dietary enrichment with beneficial bacteria (4,7), we hypothesized that oral microbe therapy may similarly reduce transgenerational cancer burdens.
Early life exposure to beneficial microbes inhibits transgenerational cancer
Finally, to further test our microbe-centric hypothesis involving detrimental NWD-induced changes in GI tract microbe communities, we applied a dietary enrichment strategy using beneficial bacteria originally isolated from human breast milk. Experiment 4 used F1 progeny of NWD-fed mothers (Figure 1D), such that upon weaning at three-weeks-of-age experimental mice consumed a model microbial organism Lactobacillus reuteri continuously in their drinking water. Control mice consumed regular water throughout the study. Upon later examination at one-year-of-age, F1 children consuming L reuteri had significantly lower risk for obesity (Figure 3A) and cancer than did their untreated control counterparts (Table 1; Figure S3). It is promising but unknown to what extent other microbe cocktails or life stage interventions may be effective, although oral administration of purified L rhamnosus was previously shown to suppress obesity in human adults (19). Mouse models and diets do not precisely mimic human conditions; however, these findings further supported a gut microbe-immune-centric hypothesis and the possibility that diet-associated bacteria may be engineered to mitigate emerging inflammatory disease epidemic in Westernized societies.
Discussion
In summary, we show that in-utero effects of gut microbes may transcend generations. We discover that mother mice consuming a Westernized diet during pregnancy convey to their children and grandchildren detrimental long-term consequences including high risk of obesity and cancer. In humans, there is precedent for maternal dietary folate (vitamin B9) deficiency leading to leukemia in offspring (20); although the cancer outcome in the present study was reproducible using microbes alone, and mothers of the most severely affected F2 progeny were actually eating a nutritionally-balanced control AIN76A diet during pregnancy. Finding polycystic ovaries, endometrial disease, and infertility in F1 female mice implicates sex hormones together with immune dysregulation in transgenerational effects; however, this remains to be explicitly tested. Importantly, fecal microbiome transplant was sufficient for the transgenerational cancer effect. Grandchildren were most severely affected, exhibiting a premature aging scurfy-like syndrome and high rates of cancer at a young age, when their grandmother had consumed Western diet during her pregnancy. The scurfy-like syndrome suggests immune T regulatory (Treg) cell insufficiency (16) previously implicated in obesity and cancer (4–7). Taken together, our data suggests that dietary stress leads to gut microbe dysbiosis that elevates the risk of heritable cancers. Intestinal bacteria were sufficient to remedy the transgenerational effect, pointing to novel microbe-based personal or public health preventative medicine strategies. Intestinal bacteria were sufficient to remedy the transgenerational effect observed in mice, and further study of novel microbe-based mechanisms in the role of human cancer and obesity prevention are warranted.
In conclusion, it is now clear that profound changes in our environment have contributed to a societal health crisis. Our previous data have built upon the perinatal microbe-driven concept to include neoplastic diseases (3,5). Our current data expand this microbe-centric view further to include mammalian progeny and suggest that the microbial flora of a mother animal affects the predisposition of her progeny to cancer. We have shown here that a key mediator of diet-induced transgenerational disease predisposition is the gut microbial flora. Consequently, the effect was reversible by enriching the progeny gut flora with beneficial bacteria. Another well-substantiated transgenerational hypothesis suggests that westernized dietary habits during pregnancy shape disease susceptibility profiles of her descendants via epigenomics (21–23). It remains unproven that maternal microbes contribute to epigenetic changes upon their unborn progeny, and how results from an animal model in a highly controlled environment translate into free-living human populations. Our data also suggest that the dietary indiscretions of earlier generations may be reversible by targeting more healthful gut microbes early in life. This suggests new possibilities for decreasing the risk of cancer, obesity and other pathologies that appear more prevalent in modern societies, using targeted gut flora enrichment strategies. This suggests new possibilities for decreasing the risk of cancer, obesity and other pathologies, using targeted gut flora enrichment strategies that remain to be validated in humans.
Supplementary Material
Novelty and impact.
Causes for skyrocketing rates of obesity and cancer in industrialized countries are poorly understood. We report long-term consequences of grandmothers’ in-utero dietary exposures leading to high rates of obesity and cancers of lung and liver in two subsequent generations. We discover diet-associated microbes are sufficient for these transgenerational effects, highlighting novel strategies to re-shape preventative medicine.
Acknowledgements
We thank James Versalovic for the gift of ATCC-PTA-6475 Lactobacillus reuteriand special thanks to James G. Fox for encouragement and support.
This work was supported by National Institutes of Health grants P30-ES002109 (pilot project award to S.E.E and E.J.A), RO1CA108854 (to S.E.E), and U01 CA164337 (S.E.E. and E.J.A.).
References
- 1.Rook GA, Dalgleish A. Infection, immunoregulation, and cancer. Immunol Rev. 2011;240:141–159. doi: 10.1111/j.1600-065X.2010.00987.x. [DOI] [PubMed] [Google Scholar]
- 2.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erdman SE, Poutahidis T. Cancer inflammation and regulatory T cells. Int J Cancer. 2010;127:768–779. doi: 10.1002/ijc.25430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakritz JR, Poutahidis T, Levkovich T, Varian BJ, Ibrahim YM, Chatzigiagkos A, et al. Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice. Int J Cancer. 2014;135:529–540. doi: 10.1002/ijc.28702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erdman SE, Poutahidis T. The microbiome modulates the tumor macroenvironment. Oncoimmunology. 2014;3:e28271–e28271. doi: 10.4161/onci.28271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poutahidis T, Kleinewietfeld M, Erdman SE. Gut microbiota and the paradox of cancer immunotherapy. Front Immunol. 2014;5:157. doi: 10.3389/fimmu.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poutahidis T, Kleinewietfeld M, Smillie C, Levkovich T, Perrotta A, Bhela S, et al. Microbial reprogramming inhibits Western diet-associated obesity. PLoS One. 2013;8:e68596. doi: 10.1371/journal.pone.0068596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saulnier DM, Santos F, Roos S, Mistretta TA, Spinler JK, Molenaar D, et al. Exploring metabolic pathway reconstruction and genome-wide expression profiling in Lactobacillus reuteri to define functional probiotic features. PLoS One. 2011;6:e18783. doi: 10.1371/journal.pone.0018783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levkovich T, Poutahidis T, Smillie C, Varian BJ, Ibrahim YM, Lakritz JR, et al. Probiotic bacteria induce a 'glow of health'. PLoS One. 2013;8:e53867. doi: 10.1371/journal.pone.0053867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poutahidis T, Kearney SM, Levkovich T, Qi P, Varian BJ, Lakritz JR, et al. Microbial Symbionts Accelerate Wound Healing via the Neuropeptide Hormone Oxytocin. PLoS One. 2013;8:e78898. doi: 10.1371/journal.pone.0078898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manzel A, Muller DN, Hafler DA, Erdman SE, Linker RA, Kleinewietfeld M. Role of "Western diet" in inflammatory autoimmune diseases. Current allergy and asthma reports. 2014;14:404. doi: 10.1007/s11882-013-0404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.David LA, Materna AC, Friedman J, Campos-Baptista MI, Blackburn MC, Perrotta A, et al. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014;15:R89. doi: 10.1186/gb-2014-15-7-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 15.Yao Y, Robinson AM, Zucchi FCR, Robbins JC, Babenko O, Kovalchuk O, et al. Ancestral exposure to stress epigenetically programs preterm birth risk and adverse maternal and newborn outcomes. Bmc Med. 2014;12 doi: 10.1186/s12916-014-0121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramsdell F, Ziegler SF. Foxp3 and scurfy: how it all began. Nature Rev Immunol. 2014;14:343–349. doi: 10.1038/nri3650. [DOI] [PubMed] [Google Scholar]
- 17.Erdman SE, Rao VP, Poutahidis T, Rogers AB, Taylor CL, Jackson EA, et al. Nitric oxide and TNF-alpha trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc Natl Acad Sci U S A. 2009;106:1027–1032. doi: 10.1073/pnas.0812347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powrie F, Maloy KJ. Immunology. Regulating the regulators. Science. 2003;299:1030–1031. doi: 10.1126/science.1082031. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez M, Darimont C, Drapeau V, Emady-Azar S, Lepage M, Rezzonico E, et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br J Nutr. 2014;111:1507–1519. doi: 10.1017/S0007114513003875. [DOI] [PubMed] [Google Scholar]
- 20.Thompson JR, Gerald PF, Willoughby ML, Armstrong BK. Maternal folate supplementation in pregnancy and protection against acute lymphoblastic leukemia in childhood: a case-control study. Lancet. 2001 Dec 8;358:1935–1940. doi: 10.1016/S0140-6736(01)06959-8. [DOI] [PubMed] [Google Scholar]
- 21.Supic G, Jagodic M, Magic Z. Epigenetics: a new link between nutrition and cancer. Nutr Cancer. 2013;65:781–792. doi: 10.1080/01635581.2013.805794. [DOI] [PubMed] [Google Scholar]
- 22.Vickers MH. Early life nutrition, epigenetics and programming of later life disease. Nutrients. 2014;6:2165–2178. doi: 10.3390/nu6062165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. Cell. 2014;157:95–109. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.