Abstract
Neurostimulation is one manifestation of neuromodulation of the gastrointestinal (GI) tract. This manuscript reviews the history of neurostimulation of the GI tract with emphasis on current methods of stimulation. Upper GI disorders can be modulated with both temporary (placed endoscopically or surgically) or permanent (placed surgically) gastric electrical stimulation (GES) devices. The current gastrointestinal (GI) neurostimulation of stomach (GES) devices have been used in both children and adults and some patients have been followed in excess of 15 years with good long-term results. Similar GES devices have also been used for a variety of lower GI disorders, including constipation and fecal incontinence, for a number of years. Based on these recent developments, the future uses of neurostimulation in the GI tract are discussed with an emphasis on new applications and innovations.
Keywords: gastric electrical stimulation, nausea and vomiting, gastroparesis, neuromodulation
Background
This manuscript had its origins in a symposium on GI Neurostimulation held at the International Neuromodulation Society (INS) meeting in Berlin on June 13, 2013. The purpose of the symposium and also this manuscript is to review the current status and recent developments concerning neurostimulation of the gastrointestinal (GI) tract with an emphasis on both test/temporary and implanted/permanent stimulation.
History
The possibility of neurostimulation in the gastrointestinal tract goes back at least 100 years. Nobel Prizes given in 1906, to Pavlov, for integrative physiology and 1908, to Golgi and Cajal, for their understanding of nervous structure and function, illustrated advances made in medical knowledge that allowed consideration of what today we call neurostimulation, as a type of neuromodulation. By the early 1910s a number of individuals had reported successful neurostimulation and the description of gastrointestinal physiology by Alvarez and others in the 1920s continued interest in this area.1, 2 By the 1980s, the current generation of pulse generators was developed, such as the Medtronic Itrel for neurostimulation for pain, and later used for trials of gastric and other stimulation. The current era of neurostimulation of the gastrointestinal tract occurred over the last 2 decades and has been summarized recently.3
Use of GI Stimulation for Upper Gut Disorders
Introduction
Gastrointestinal electrical stimulation was first applied clinically for use in the stomach about 25 years ago. Two main techniques emerged by about 1990: one utilizing near physiologic frequencies of the stomach with higher energies and another utilizing frequencies several times higher than physiologic with quite lower energies.4, 5 The technique which is commercially available utilizes higher frequencies and lower energies and is often described as neural stimulation; the majority of the clinical and clinical research activity has utilized this approach.
Placement of GES devices requires surgery, but due to the high cost of implantation as well as uncertainty about patient response, a number of approaches for temporary stimulation have recently been applied. Two of these newer approaches are reviewed below. The first approach involves endoscopic implantation of temporary electrode(s) in the gastric mucosa and is discussed for both adults and children. The second approach involves percutaneous placement of electrodes with endoscopic guidance and has been used primarily in adults but also in a few children.6
GI Tract Neural Innervation
The enteric nervous system (ENS), as a separate functional unit, has a complex organizational structure, the details of which are beyond the scope of this article. In a most basic explanation, the GI tract, which is composed of two distinct muscle layers, has neural circuitry embedded to control-coordinated peristalsis thought the whole tract. Peristaltic signals are relayed through interstitial cells of Cajal throughout the GI tract muscle layers and these signals are influenced by a number of neuro-hormonal factors. The enteric nervous system also includes connections with the autonomic nervous system, which can relay signals from autonomic, or more indirectly, central nervous system input. Recent work has revealed further interactions of the ENS with the stool microbiome, including inflammatory and other cells, such as specific macrophages that also play a role in normal, and abnormal, GI motility.7
Endoscopic Temporary GES in Adults
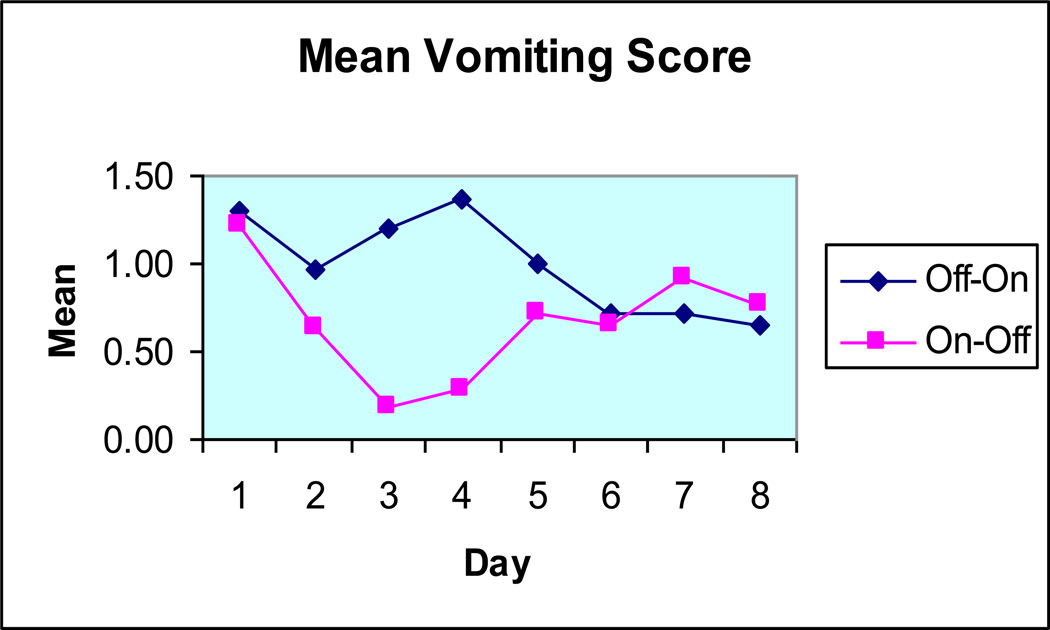
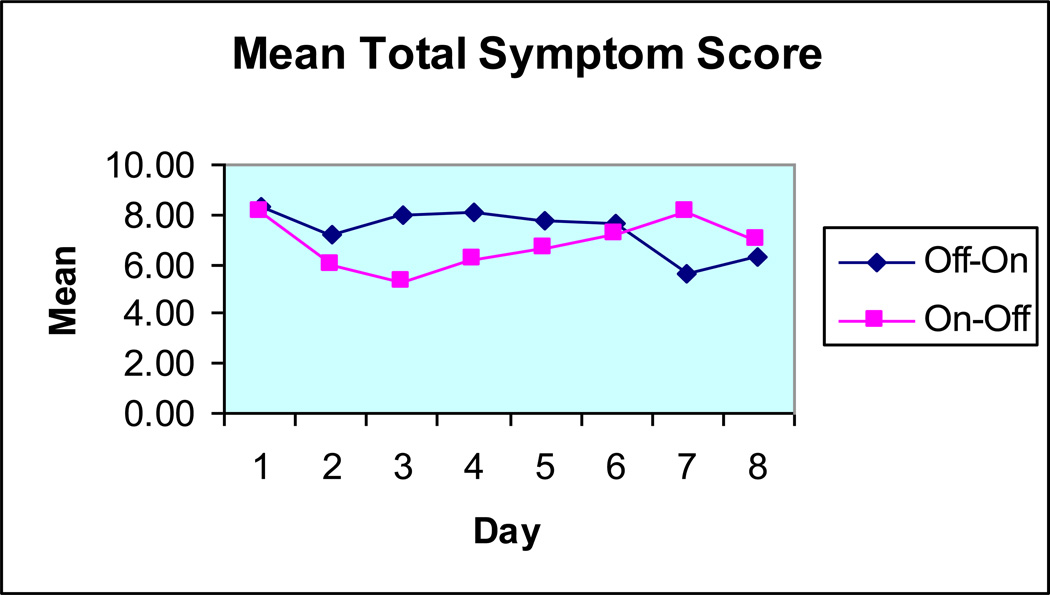
The original use of GES in the early to late 1990s involved the placement of a temporary stimulation lead, either through a percutaneous endoscopic gastrostomy (PEG) tube or via laparoscopy. In the early 2000s, centers in the Mid-South United States began using temporary endoscopic placement of modified cardiac stimulation leads. Figures 1 to 3 show the response to temporary endoscopic stimulation on GI symptoms in a series of 491 consecutive patients. Improvements were noted in vomiting, nausea and abdominal symptom scores over a period of up to a week in an open label trial. Tables 1 and 2 list the advantages and disadvantages of endoscopic temporary GES contrasted with PEG placed temporary GES, one of the original forms of temporary stimulation.
Figure 1.
The effect of temporary endoscopic GES on vomiting from 58 patients in randomized double-masked crossover trial. (Adapted from reference #15.)
Figure 3.
The effect of temporary endoscopic GES on Total GI Symptom score. (Adapted from reference #15.)
Table 1.
Advantages of temporary PEG placed stimulation:
|
Disadvantages of temporary endoscopic stimulation:
|
Table 2.
Advantages of temporary PEG placed stimulation:
|
Disadvantages of temporary PEG placed stimulation:
|
Currently, temporary endoscopic GES is often applied to patients whose outcome may not be clear and for non-traditional indications such as patients with previous complex surgeries, transplant patients and other patients where GES efficacy has not been shown. Some centers, however, use temporary endoscopic stimulation on all patients as a test phase, much like is done with sacral or spinal stimulation.8
Endoscopic Temporary GES in Pediatrics
Temporary endoscopic gastric electrical stimulation has also been applied to pediatric gastroenterology patients. Pediatric gastroparesis is a challenging medical problem with an unknown prevalence and having no consensus on diagnostic imaging; patients are often unresponsive to conventional therapies such as diet modification, medications or tube feeding.
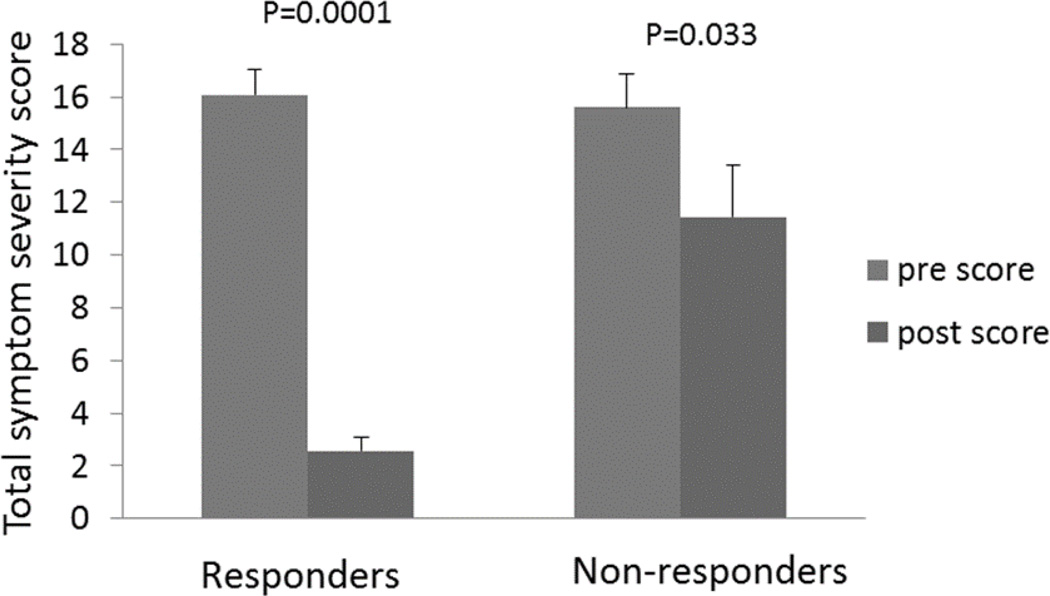
In a recent series from one center, forty-three children with documented gastroparesis who had failed conventional therapy were offered endoscopically placed temporary gastric electrical stimulation to determine if they would be candidates for permanent gastric electrical stimulation. Overall positive response rate, defined as the ability to tolerate regular diet with minimal or no symptoms, was 63%. Total symptom scores were obtained pre and post stimulator placement. Scores ranged from 0–5 for symptoms of nausea, bloating, vomiting, anorexia, and abdominal pain. Among responders, symptom scores decreased significantly. 93% of responders went on to permanent GES devices, at a rate very similar to the adult patients who had temporary endoscopic stimulation first as a test. 8 [See Figure 4]
Figure 4.
Comparison of total symptom score of responders vs. non-responders of temporary endoscopic GES in pediatric patients.
Percutaneous temporary GES
Another type of temporary GES utilizes a percutaneous approach (Figure 5). A temporary percutaneous GES (TPGES) test has been developed in Gothenburg, Sweden to select the patients who are responders to GES and thus candidates to receive a fully implantable device. Trials have been published showing its feasibility in patients not ordinarily given stimulation therapies.9, 10 This method has been shown to be well tolerated, safe, and allows for long periods of stimulation (months) if needed. Furthermore, it seems to be able to result in a high response rate in those selected for permanent GES.11
Figure 5.
Temporary GES electrodes inserted through the abdominal wall and connected to an external Enterra® device before being covered by protective adhesives.
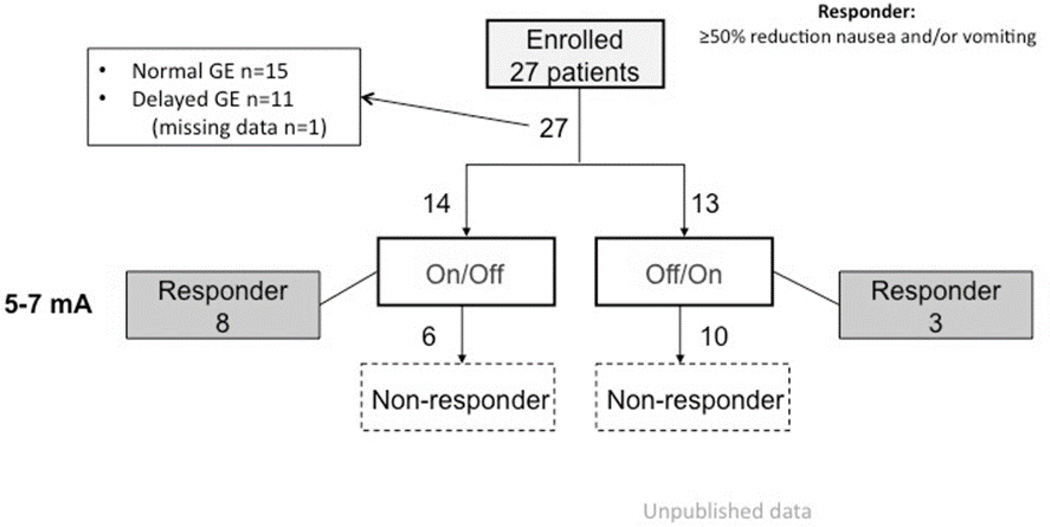
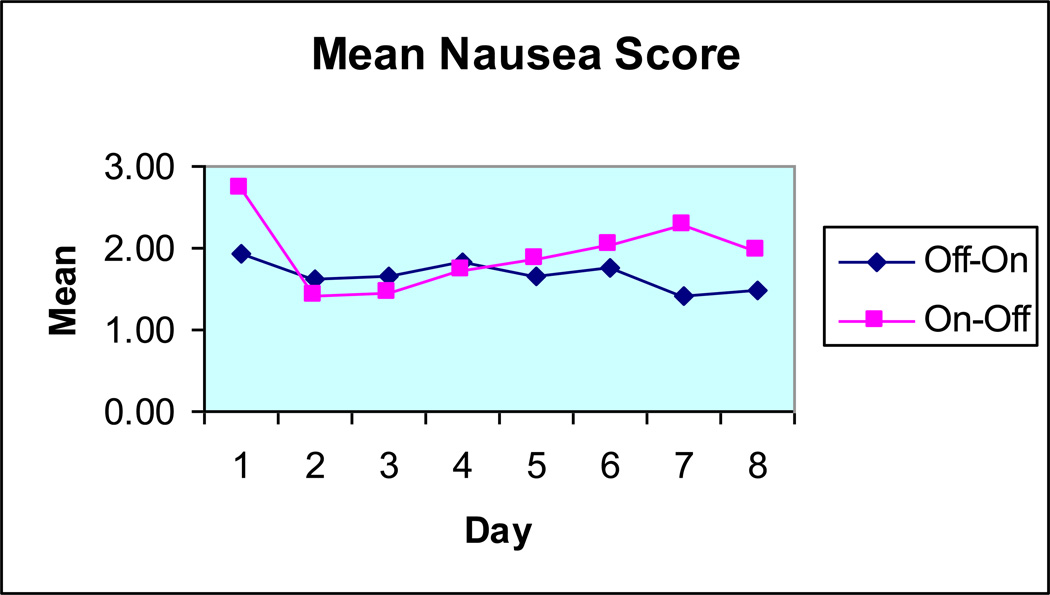
In TPGES, two percutaneous placed unipolar electrodes are inserted through a plastic cannula and anchored by flexible wing-like tines to the submucosal tissue. The uninsulated part of the electrode thereby contacts the muscularis propria of the gastric wall. The day after electrode insertion, the patient’s perception of impulses is checked and if it is not sensed at stimulation in the 5–7 mA range, randomization for two blinded test periods are performed: 2 weeks with stimulation ON followed by 2 weeks with stimulation OFF, or the opposite order. Results have been considered positive when a reduction of dominant symptom score of at least 50% is observed when comparing ON and OFF periods.9, 11 Still unpublished data extending the experience with double-blind stimulation shows that in non-diabetic gastroparesis patients with severe nausea and vomiting who do not respond to medical therapy, the response rates are not as high as for open GES stimulation (Fig. 6) Interestingly, neither clinical diagnosis nor gastric emptying status seems to be good predictors of response. TPGES may be effective in many patients with intractable nausea and vomiting, regardless of gastric emptying physiology; however, the indications for GES must be further explored where TPGES is used as a tool for patient selection.
Figure 6.
Study flow-chart showing a response rate for blinded TPGES comparing the on and off periods (not baseline symptoms) at 41%.
Mechanisms of Action of Gastrointestinal Neurostimulation
Many possible mechanisms of action have been proposed for GI neurostimulation of both the upper and lower gastrointestinal tracts.3, 12 Conceptually the mechanism of action (MOA) can be viewed as one of three possibilities: central, autonomic and/or enteric. The mechanism of action for upper gut stimulation, particularly gastric electrical stimulation (GES) has been studied in some detail. Central nervous system (CNS) effects for GES have conflicting data. Some studies have shown a central effect, especially parventricular excitability.13, 14 Other studies have shown no measureable effect, but those studies have speculated on the existence of visceral sensitivity and/or central excitability, which cannot be measured with current technology. Autonomic nervous system (ANS) mechanisms for GES and its MOA have focused on the two main ways to measure the ANS: 1) through direct classical measures of the autonomic nervous system cholinergic and adrenergic effects and 2) through indirect measures such as heart rate variability by power spectrum analysis. Both direct and indirect ANS measures have shown changes with GI neurostimulation.15, 16 Enteric effects for GES have been perhaps studied the most as possible MOA, with a number of studies showing effects of GI neurostimulation on the upper gut.17, 18 The mechanism of action for lower gut neurostimulation is discussed in that section, below.
Most studies of GES have been open label or case studies; however, several have been controlled, and one, the WAVESS study, was the basis for the approval of GES by the US FDA as a humanitarian use device in 2000.19 A follow up study, the Enterra clinical trial(s), was not positive, but suffered from a major design flaw, as all patients were ON for 6 weeks before crossing over as continued ON or OFF. However, at one year, and beyond, the patients in the Enterra clinical studies did quite well.20, 21 Another study, the ENDOSTIM study, done with temporary gastric electrical stimulation, met all its endpoints, and also concluded that future studies should use parallel design, rather than cross over designs.22
GES for obesity
For GES to be clinically meaningful in the treatment for morbid obesity, it needs to be compared to outcomes from conventional bariatric operations and also newer endoscopic options. Weight loss by surgery is conventionally measured in terms of the percentage excess weight lost (%EWL=actual weight-ideal weight), and it is generally accepted that success is defined as a loss of >50% EWL.23 The mechanism of action of GES for treatment of obesity appears most likely to induce satiety by modulation of the gut-brain neural axis, gut peptide hormone release and gastric motor activity.24 As compared to conventional bariatric surgery, GES offers the specific advantages of facilitation of behavior modification with the potential to overcome issues of non-compliance, low surgical risk, and adjustability.
The first study of electrical stimulation of the stomach for treatment of obesity in humans occurred in 2002 and used a commercially-available implantable device called Transcend™.25 Between 2002 and 2006, there was an eight case-series of Transcend™ including two multi-center, open-label studies of over 300 patients. Follow-up with subjects >12 months reported that, on average, a loss of 20% of excess weight loss was achieved.26–32 These promising early data led to a randomized controlled trial (SHAPE; 2009; 194 patients), which disappointingly showed no significant difference in weight loss with stimulation vs. sham treatment at 12 months (%EWL: 11.8±17.6 vs. 11.7±16.9; respectively).33 This led to withdrawal of the device.
Another commercially available device is called Tantalus™, recently renamed Diamond™. The surgical procedure for implantation is more complex than Transcend and involves the placement of several electrodes on the stomach. The first study was reported in 200634 along with four subsequent studies.35–38 Data are reported on a total of about 100 patients. Outcome measures are variable; results indicate about 20% EWL, or actual weight loss of about 4.5 kg, at 12 months. Intriguingly, there is improvement in glycemic control in diabetic patients at levels that are unlikely to be explained solely by weight loss, implying that electrical stimulation exerts some, and hereto unexplained, metabolic control.
Another commercially available technology – termed VBLOC – involves surgical placement of cuff-like electrodes around the anterior and posterior vagal trunks at the level of the esophageal hiatus in the abdomen. There is only one published study on VBLOC: EMPOWER, a randomized controlled trial, comprising 294 patients from 15 USA centers. There was no difference in weight loss with treatment vs. sham at 12 months (%EWL, 17±2 vs. 16±2; respectively); however, it was speculated that there may have been significant confounding because of unplanned delivery of stimulus to the sham treatment group.39 A further trial - ReCharge - using a second-generation system, is currently ongoing in the US and has recently been reported.40
Data on weight loss by gastric neuromodulation are presently inconclusive but suggest the prospect of meaningful future treatment. Further ongoing trials are in progress and will need to be completed, analyzed and published before definitive conclusions about the efficacy of GI stimulation for obesity can be made. These ongoing studies include the EXILIS study (clinicaltrials.gov #NCT01823705), which is awaiting its one-year data.
GI Neurostimulation for Lower GI Disorders
The lower gastrointestinal tract is a logical location for the application of neurostimulation; however, attempts have been hampered by the challenges of accessibility of this area combined with uncertain knowledge of pathophysiology. Neurostimulation has been used for pelvic floor disorders, such as urinary retention and/or urinary incontinence and provides some insight into lower GI dysfunction. Since many patients with urinary dysfunction also have lower GI tract complaints, early reports of improvement in GI function in some patients has led to a number of investigations of neurostimulation on GI and specifically lower bowel dysfunction. This discussion concerns the effects of neurostimulation on the lower GI tract and specifically does not include effects on the urinary system which have been reviewed elsewhere.41
Fecal incontinence and constipation, two of the most common pelvic floor disorders, are associated with high levels of physical symptomatology and social disability. Fecal incontinence affects up to 10% of the general adult population42 and approximately twice as many adults suffer with constipation.43 First line medical and behavioral therapies are successful in the vast majority of patients, but there are large a number of individuals refractory to these conservative therapies; they can either live with their symptoms or consider more invasive surgical therapies.44
Lower GI stimulation, specifically sacral nerve stimulation (SNS), has emerged over the last two decades as a viable and increasingly available therapy for fecal incontinence.44 SNS is a minimally invasive technique that represents an in-between option as an alternative to surgery; it is associated with success in two-thirds of cases.45 46 A perceived advantage of SNS is that it allows a period of trial evaluation typically over two to three weeks: the so-called percutaneous nerve evaluation (PNE) phase. If there is subjective and objective evidence of improvement during the PNE phase, a permanent tined-lead implantation can be undertaken. With over 300 published papers in the last two decades, SNS has become the treatment standard to which other treatments are increasingly compared.47, 48 This is notwithstanding the lack of clarity as to the mechanism of action.
Use of SNS has widened to include patients with sphincter disruption, evacuation difficulty, neurogenic bowel dysfunction and recently even irritable bowel syndrome (IBS); however, the most common lower GI indication for SNS is constipation. Outcomes for this indication have been subject to a recent review.49 Ten studies, amounting to 125 permanent implants from a total of 225 PNE procedures (56%), revealed beneficial outcomes in approximately half the patients. To date, the longest follow-up period reported for this indication is 42 months in a small study of 13 female patients.50 The largest study (n=60) with medium-term follow up showed poorer results, with high rates of adverse effects mostly related to electrode displacement.51 Improvement in bowel frequency during PNE seemed to predict good response to permanent implantation.49 Optimising patient selection is critical to the use of SNS in treating constipation, although there is evidence of efficacy in both slow transit and rectal evacuation difficulty.52
Newer techniques of neuromodulation for lower GI symptomatology have emerged in the last few years: percutaneous posterior tibial nerve stimulation (PTNS) and transcutaneous tibial nerve stimulation (TTNS). To date there is almost no data on PTNS in constipation; however, there are published results in fecal incontinence. This study data is limited by the lack of long-term results, with the longest being 12 months.53 This study showed a response rate of 59% with median reduction of episodes of fecal incontinence from 7 to 3 per week. These results are comparable to the efficacy of SNS at this short-term time point; however, the effectiveness beyond one year is not known; the lack of evidence prevents meaningful discussion about TTNS.
Neuromodulation in the context of neurogenic bowel dysfunction is well studied but lacks clinical application. A range of techniques has been investigated in uncontrolled small studies. Nerve re-routing in patients with complete or incomplete injuries may improve defecation through mechanical stimulation of dermatomes. Sacral nerve stimulation has been shown to benefit some individuals with incomplete neurological lesions. Peripheral nerve stimulation (electrical or magnetic) represents a less invasive alternative.
Two recent reviews have evaluated sacral stimulation for both fecal incontinence 54 and constipation.55 For fecal incontinence, sacral stimulation appears to be efficacious and similar to other modalities, such as percutaneous or transcutaneous tibial nerve stimulation although long term data is still being acquired.54 For constipation, sacral nerve stimulation, in a smaller number of studies than fecal incontinence, appears to be effective, although the results need to be confirmed in longer term, prospective studies.55
Mechanisms of Action of Neurostimulation on the Lower GI Tract
As mentioned under the sections on upper GI stimulation, above, the mechanism of action could include central, autonomic, enteric and/or other pathways. A recent systematic review of sacral stimulation includes an analysis of sacral stimulation effects on the symptoms of constipation and incontinence.56 This review not only looked at central effects but also at enteric as well as anal-rectal function. Based on the data in this review, the authors concluded “the influence of SNS on anorectal function occurs at a pelvic afferent or central level” rather than a peripheral neurostimulation effect. Although pelvic nerves contain autonomic fibers, this review did not address the autonomic nervous system directly as had been examined in upper GI tract stimulation studies.
Discussion
A number of GI neurostimulation methods have been introduced with the emphasis on their clinical applications. With the exception of the Enterra® therapy, other GI neuromodulation methods have not received regulatory approval for clinical use. However, many lessons have been learned and newer and more advanced methodologies are on the horizon.
In the area of GES for treating gastric motility disorders, two distinctive methods have been introduced: the Enterra® therapy (high frequency/low energy) and the gastric pacing method (low frequency/high energy). The Enterra® therapy has been shown to be effective in treating nausea and vomiting in patients with gastroparesis as well as patients with other disorders. Although it is not completely understood, the anti-emetic effect of the Enterra® therapy is believed to be centrally mediated via the vagal pathway. One human clinical study showed an activation of brain functions. 16
The gastric pacing method aims at the pacing of intrinsic gastric slow waves. It has been shown to entrain gastric slow waves and improve gastric dysrhythmia as well as accelerate gastric emptying. The main difficulty in bringing it to bedside is the feasibility of developing an implantable pulse generator that is capable to deliver pulses within a span of a few hundred milliseconds. In addition to battery issues that could now be resolved with the use of remote charging, electronic charge balance seems to be a major issue. By synchronizing the delivery of pulses with intrinsic gastric slow waves (a newer method called synchronized GES), researchers have demonstrated enhancement of gastric contractions, acceleration of gastric emptying, and improved gastric accommodation.57 This synchronized method of GES seems more appropriate for treating patients with gastric motility disorders. However, a new generation of implantable pulse generator needs to be developed.
In the area of GES for treating obesity, a number of mechanistic studies have been published recently. Although no commercially available device is currently ready for clinical use, the clinical application of GES for obesity is promising. It has become clear now that the failure of previous clinical trials was attributed to the use of the wrong device in the case of IGS® and the development of a wrong hypothesis in the case of Tantalus™. In the IGS® method, a nerve stimulation device that was only capable of delivering pulses with width ≤0.6ms was used for treating obesity. A number of recent preclinical studies have demonstrated convincingly that for GES to be effective in treating obesity, the pulse width should be ≥ 2ms.24, 58 GES using such wide pulses has been shown to persistently and substantially reduce food intake and body weight via multiple mechanisms involving gastric motility, gastrointestinal hormones, and central neurons and hormones. 58 Tantalus™ is a powerful device that is able to alter gastric motility. Unfortunately, the device was used to enhance gastric emptying instead of therapeutically impairing gastric motility.34, 35
VLBOC is the first vagal nerve stimulation method that has been applied clinically for treating obesity. As stated earlier, the failure of its first clinical trial was probably attributed to the delivery of electrical stimulation at 40Hz and 1mA in the control group. Other issues are also believed to be involved in the lack of efficacy in its first clinical trial. One of the major problems with VBLOC is the selection of stimulation parameters and stimulation on-off configurations. VBLOC, as indicated by its name, was designed to block vagal nerve transmission. While it is constructive to block the vagal efferent activity so that the digestion process is delayed, it is counter-constructive to block the vagal afferent pathway. The blockage of satiety signaling from the gut to the brain may promote eating and consumption of more food. More studies are needed to optimize vagal nerve stimulation therapies for treating obesity.
Neuromodulation for treating lower GI diseases is an emerging and interesting area. Interestingly, SNS is clinically applied for treating both fecal incontinence and constipation, two diseases having different or even opposite pathophysiologies. It is important to explore its mechanisms of action that are little known. Other emerging therapies, such as PTNS and TTNS are attractive due to their noninvasiveness or minimal invasiveness. More studies are needed to explore their efficacies and mechanisms as well as optimization of therapies.
Summary and Conclusions
The effects, mechanisms and applications of GI recent neurostimulation methods appear closely related to available stimulation devices currently available. GI neurostimulation, as a type of neuromodulation, has been demonstrated to function at several locations in the GI tract for a variety of disorders. The future of neurostimulation in the GI tract will likely be influenced by a better understanding of pathophysiology as well as the development of new techniques and devices for neuromodulation.
Figure 2.
The mean and median values for nausea in the same group as Figure 1.
Table 3.
| Comparison (with others above) for Direct serosal temp GES: |
Advantages:
|
Disadvantages:
|
Table 4.
| Total of 43 patients | Male | Female |
|---|---|---|
| Gender (%) | 19 | 81 |
| Mean age (years) | 13 | 14 |
| Range (years) | 5–18 | 3–19 |
| Abnormal scintigraphy (%) | 100 | 94 |
| Comorbidity (%) | 63 | 66 |
Acknowledgments
The authors would like to thank the International Neuromodulation Society for the opportunity to present some of this work in Berlin in June 2013. They would also like to thank Ed Miller and Catherine McBride at the University of Louisville for help in formatting the manuscript.
Grant support, (Dr. Chen): National Institutes of Health, NIH AT004489.
Footnotes
Author contributions:
Drs. Abell and Chen designed the study. All authors helped contribute to the manuscript content, writing and revision of the submitted manuscript.
Relevant Conflict of Interest Disclosures:
Dr. Abell is a consultant, licensor and investigator for Medtronic. Some of the techniques of neurostimulation discussed are part of IP from the University of MS, now assigned to ADEPT-GI.
Contributor Information
Thomas L. Abell, Division of Gastroenterology, Hepatology and Nutrition, Department of Internal Medicine, University of Louisville, Louisville, Kentucky, United States.
Jiande Chen, Division of Gastroenterology and Hepatology, Department of Medicine, Johns Hopkins University, Baltimore, Maryland and Ningbo Pace Translational Medical Research Center, Beilun, Ningbo, China.
Anton Emmanuel, Consultant Gastroenterologist, University College London Hospital and National Hospital for Neurology and Neurosurgery, London, United Kingdom.
Christopher Jolley, Department of Pediatrics, Division of Pediatric Gastroenterology, Heptatology and Nutrition, University of Florida, Gainesville, Florida, United States.
Abeezar I. Sarela, Department of Upper Gastrointestinal Surgery, St. James’s University Hospital, The University of Leeds School of Medicine, Leeds, United Kingdom.
Hans Törnblom, Department of Internal Medicine, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
References
- 1.Dieffenbach WH. Electric treatment of intestinal obstruction and postoperative paralysis of the bowel. JAMA. 1911;LVI(13):958–959. [Google Scholar]
- 2.Alvarez W. The electrogastrogram and what it shows. JAMA. 1922;78(15):1116–1119. [Google Scholar]
- 3.Soffer E, Abell T, Lin Z, et al. Review article: gastric electrical stimulation for gastroparesis--physiological foundations, technical aspects and clinical implications. Aliment Pharmacol Ther. 2009 Oct;30(7):681–694. doi: 10.1111/j.1365-2036.2009.04082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Familoni BO, Abell TL, Voeller G, Salem A, Gaber O. Electrical stimulation at a frequency higher than basal rate in human stomach. Dig Dis Sci. 1997 May;42(5):885–891. doi: 10.1023/a:1018852011857. [DOI] [PubMed] [Google Scholar]
- 5.McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1998 Mar;114(3):456–461. doi: 10.1016/s0016-5085(98)70528-1. [DOI] [PubMed] [Google Scholar]
- 6.Elfvin A, Gothberg G, Lonroth H, Saalman R, Abrahamsson H. Temporary percutaneous and permanent gastric electrical stimulation in children younger than 3 years with chronic vomiting. J Pediatr Surg. 2011 Apr;46(4):655–661. doi: 10.1016/j.jpedsurg.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Muller PA, Koscso B, Rajani GM, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014 Jul 17;158(2):300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayinala S, Batista O, Goyal A, et al. Temporary gastric electrical stimulation with orally or PEG-placed electrodes in patients with drug refractory gastroparesis. Gastrointest Endosc. 2005 Mar;61(3):455–461. doi: 10.1016/s0016-5107(05)00076-3. [DOI] [PubMed] [Google Scholar]
- 9.Elfvin A, Andersson S, Abrahamsson H, Edebo A, Simren M, Lonroth H. Percutaneous implantation of gastric electrodes - a novel technique applied in animals and in patients. Neurogastroenterol Motil. 2007 Feb;19(2):103–109. doi: 10.1111/j.1365-2982.2006.00858.x. [DOI] [PubMed] [Google Scholar]
- 10.Andersson S, Lonroth H, Simren M, Ringstrom G, Elfvin A, Abrahamsson H. Gastric electrical stimulation for intractable vomiting in patients with chronic intestinal pseudoobstruction. Neurogastroenterol Motil. 2006 Sep;18(9):823–830. doi: 10.1111/j.1365-2982.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- 11.Andersson S, Ringstrom G, Elfvin A, Simren M, Lonroth H, Abrahamsson H. Temporary percutaneous gastric electrical stimulation: a novel technique tested in patients with non-established indications for gastric electrical stimulation. Digestion. 2011;83(1–2):3–12. doi: 10.1159/000291905. [DOI] [PubMed] [Google Scholar]
- 12.Yin J, Abell TD, McCallum RW, Chen JD. Gastric neuromodulation with Enterra system for nausea and vomiting in patients with gastroparesis. Neuromodulation. 2012 May-Jun;15(3):224–231. doi: 10.1111/j.1525-1403.2012.00429.x. discussion 231. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Chen JD. Systematic review: applications and future of gastric electrical stimulation. Aliment Pharmacol Ther. 2006 Oct 1;24(7):991–1002. doi: 10.1111/j.1365-2036.2006.03087.x. [DOI] [PubMed] [Google Scholar]
- 14.Frokjaer JB, Ejskjaer N, Rask P, et al. Central neuronal mechanisms of gastric electrical stimulation in diabetic gastroparesis. Scand J Gastroenterol. 2008;43(9):1066–1075. doi: 10.1080/00365520802028221. [DOI] [PubMed] [Google Scholar]
- 15.Lin Z, Sarosiek I, Forster J, McCallum RW. Symptom responses, long-term outcomes and adverse events beyond 3 years of high-frequency gastric electrical stimulation for gastroparesis. Neurogastroenterol Motil. 2006 Jan;18(1):18–27. doi: 10.1111/j.1365-2982.2005.00732.x. [DOI] [PubMed] [Google Scholar]
- 16.McCallum RW, Dusing RW, Sarosiek I, Cocjin J, Forster J, Lin Z. Mechanisms of symptomatic improvement after gastric electrical stimulation in gastroparetic patients. Neurogastroenterol Motil. 2010 Feb;22(2):161–167. e150–e161. doi: 10.1111/j.1365-2982.2009.01389.x. [DOI] [PubMed] [Google Scholar]
- 17.Anand C, Al-Juburi A, Familoni B, et al. Gastric electrical stimulation is safe and effective: a long-term study in patients with drug-refractory gastroparesis in three regional centers. Digestion. 2007;75(2–3):83–89. doi: 10.1159/000102961. [DOI] [PubMed] [Google Scholar]
- 18.Williams PA, Nikitina Y, Kedar A, Lahr CJ, Helling TS, Abell TL. Long-term effects of gastric stimulation on gastric electrical physiology. J Gastrointest Surg. 2013 Jan;17(1):50–55. doi: 10.1007/s11605-012-2020-5. discussion p.55-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003 Aug;125(2):421–428. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 20.McCallum RW, Snape W, Brody F, Wo J, Parkman HP, Nowak T. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010 Nov;8(11):947–954. doi: 10.1016/j.cgh.2010.05.020. quiz e116. [DOI] [PubMed] [Google Scholar]
- 21.McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013 Oct;25(10) doi: 10.1111/nmo.12185. 815-e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011 Sep;74(3):496–503. e493. doi: 10.1016/j.gie.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Brien PE, MacDonald L, Anderson M, Brennan L, Brown WA. Long-term outcomes after bariatric surgery: fifteen-year follow-up of adjustable gastric banding and a systematic review of the bariatric surgical literature. Ann Surg. 2013 Jan;257(1):87–94. doi: 10.1097/SLA.0b013e31827b6c02. [DOI] [PubMed] [Google Scholar]
- 24.Hasler WL. Methods of gastric electrical stimulation and pacing: a review of their benefits and mechanisms of action in gastroparesis and obesity. Neurogastroenterol Motil. 2009 Mar;21(3):229–243. doi: 10.1111/j.1365-2982.2009.01277.x. [DOI] [PubMed] [Google Scholar]
- 25.Cigaina V. Gastric pacing as therapy for morbid obesity: preliminary results. Obes Surg. 2002 Apr;12(Suppl 1):12S–16S. doi: 10.1007/BF03342141. [DOI] [PubMed] [Google Scholar]
- 26.Champion JK, Williams M, Champion S, Gianos J, Carrasquilla C. Implantable gastric stimulation to achieve weight loss in patients with a low body mass index: early clinical trial results. Surg Endosc. 2006 Mar;20(3):444–447. doi: 10.1007/s00464-005-0223-5. [DOI] [PubMed] [Google Scholar]
- 27.Favretti F, De Luca M, Segato G, et al. Treatment of morbid obesity with the Transcend Implantable Gastric Stimulator (IGS): a prospective survey. Obes Surg. 2004 May;14(5):666–670. doi: 10.1381/096089204323093462. [DOI] [PubMed] [Google Scholar]
- 28.De Luca M, Segato G, Busetto L, et al. Progress in implantable gastric stimulation: summary of results of the European multi-center study. Obes Surg. 2004 Sep;14(Suppl 1):S33–S39. doi: 10.1007/BF03342136. [DOI] [PubMed] [Google Scholar]
- 29.Cigaina V. Long-term follow-up of gastric stimulation for obesity: the Mestre 8-year experience. Obes Surg. 2004 Sep;14(Suppl 1):S14–S22. doi: 10.1007/BF03342133. [DOI] [PubMed] [Google Scholar]
- 30.Cigaina V, Hirschberg AL. Gastric pacing for morbid obesity: plasma levels of gastrointestinal peptides and leptin. Obes Res. 2003 Dec;11(12):1456–1462. doi: 10.1038/oby.2003.195. [DOI] [PubMed] [Google Scholar]
- 31.D'Argent J. Gastric electrical stimulation as therapy of morbid obesity: preliminary results from the French study. Obes Surg. 2002 Apr;12(Suppl 1):21S–25S. doi: 10.1381/096089202762552638. [DOI] [PubMed] [Google Scholar]
- 32.Shikora SA. "What are the yanks doing?" the U.S. experience with implantable gastric stimulation (IGS) for the treatment of obesity - update on the ongoing clinical trials. Obes Surg. 2004 Sep;14(Suppl 1):S40–S48. doi: 10.1007/BF03342137. [DOI] [PubMed] [Google Scholar]
- 33.Shikora SA, Bergenstal R, Bessler M, et al. Implantable gastric stimulation for the treatment of clinically severe obesity: results of the SHAPE trial. Surg Obes Relat Dis. 2009 Jan-Feb;5(1):31–37. doi: 10.1016/j.soard.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Bohdjalian A, Prager G, Aviv R, et al. One-year experience with Tantalus: a new surgical approach to treat morbid obesity. Obes Surg. 2006 May;16(5):627–634. doi: 10.1381/096089206776945101. [DOI] [PubMed] [Google Scholar]
- 35.Sanmiguel CP, Conklin JL, Cunneen SA, et al. Gastric electrical stimulation with the TANTALUS System in obese type 2 diabetes patients: effect on weight and glycemic control. J Diabetes Sci Technol. 2009 Jul;3(4):964–970. doi: 10.1177/193229680900300445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Policker S, Haddad W, Yaniv I. Treatment of type 2 diabetes using meal-triggered gastric electrical stimulation. Isr Med Assoc J. 2009 Apr;11(4):206–208. [PubMed] [Google Scholar]
- 37.Bohdjalian A, Prager G, Rosak C, et al. Improvement in glycemic control in morbidly obese type 2 diabetic subjects by gastric stimulation. Obes Surg. 2009 Sep;19(9):1221–1227. doi: 10.1007/s11695-009-9901-z. [DOI] [PubMed] [Google Scholar]
- 38.Bohdjalian A, Ludvik B, Guerci B, et al. Improvement in glycemic control by gastric electrical stimulation (TANTALUS) in overweight subjects with type 2 diabetes. Surg Endosc. 2009 Sep;23(9):1955–1960. doi: 10.1007/s00464-008-0222-4. [DOI] [PubMed] [Google Scholar]
- 39.Sarr MG, Billington CJ, Brancatisano R, et al. The EMPOWER study: randomized, prospective, double-blind, multicenter trial of vagal blockade to induce weight loss in morbid obesity. Obes Surg. 2012 Nov;22(11):1771–1782. doi: 10.1007/s11695-012-0751-8. [DOI] [PubMed] [Google Scholar]
- 40.Ikramuddin S, Blackstone RP, Brancatisano A, et al. Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA. 2014 Sep 3;312(9):915–922. doi: 10.1001/jama.2014.10540. [DOI] [PubMed] [Google Scholar]
- 41.Brazzelli M, Murray A, Fraser C. Efficacy and safety of sacral nerve stimulation for urinary urge incontinence: a systematic review. J Urol. 2006 Mar;175(3 Pt 1):835–841. doi: 10.1016/S0022-5347(05)00326-5. [DOI] [PubMed] [Google Scholar]
- 42.Navarro JM, Arroyo Sebastian A, Perez Vicente F, et al. [Sacral root neuromodulation as treatment for fecal incontinence. Preliminary results] Rev Esp Enferm Dig. 2007 Nov;99(11):636–642. doi: 10.4321/s1130-01082007001100003. [DOI] [PubMed] [Google Scholar]
- 43.Pare P, Ferrazzi S, Thompson WG, Irvine EJ, Rance L. An epidemiological survey of constipation in canada: definitions, rates, demographics, and predictors of health care seeking. Am J Gastroenterol. 2001 Nov;96(11):3130–3137. doi: 10.1111/j.1572-0241.2001.05259.x. [DOI] [PubMed] [Google Scholar]
- 44.Chatoor DR, Taylor SJ, Cohen CR, Emmanuel AV. Faecal incontinence. Br J Surg. 2007 Feb;94(2):134–144. doi: 10.1002/bjs.5676. [DOI] [PubMed] [Google Scholar]
- 45.Wong MT, Meurette G, Rodat F, Regenet N, Wyart V, Lehur PA. Outcome and management of patients in whom sacral nerve stimulation for fecal incontinence failed. Dis Colon Rectum. 2011 Apr;54(4):425–432. doi: 10.1007/DCR.0b013e318200f866. [DOI] [PubMed] [Google Scholar]
- 46.Matzel KE. Sacral nerve stimulation for faecal incontinence: its role in the treatment algorithm. Colorectal Dis. 2011 Mar;13(Suppl 2):10–14. doi: 10.1111/j.1463-1318.2010.02519.x. [DOI] [PubMed] [Google Scholar]
- 47.Altomare DF, De Fazio M, Giuliani RT, Catalano G, Cuccia F. Sphincteroplasty for fecal incontinence in the era of sacral nerve modulation. World J Gastroenterol. 2010 Nov 14;16(42):5267–5271. doi: 10.3748/wjg.v16.i42.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meurette G, La Torre M, Regenet N, Robert-Yap J, Lehur PA. Value of sacral nerve stimulation in the treatment of severe faecal incontinence: a comparison to the artificial bowel sphincter. Colorectal Dis. 2009 Jul;11(6):631–635. doi: 10.1111/j.1463-1318.2008.01633.x. [DOI] [PubMed] [Google Scholar]
- 49.Sharma A, Bussen D, Herold A, Jayne D. Review of sacral neuromodulation for management of constipation. Surg Innov. 2013 Dec;20(6):614–624. doi: 10.1177/1553350613475882. [DOI] [PubMed] [Google Scholar]
- 50.Naldini G, Martellucci J, Moraldi L, Balestri R, Rossi M. Treatment of slow-transit constipation with sacral nerve modulation. Colorectal Dis. 2010 Nov;12(11):1149–1152. doi: 10.1111/j.1463-1318.2009.02067.x. [DOI] [PubMed] [Google Scholar]
- 51.Maeda Y, Lundby L, Buntzen S, Laurberg S. Sacral nerve stimulation for constipation: suboptimal outcome and adverse events. Dis Colon Rectum. 2010 Jul;53(7):995–999. doi: 10.1007/DCR.0b013e3181d64207. [DOI] [PubMed] [Google Scholar]
- 52.Kamm MA, Dudding TC, Melenhorst J, et al. Sacral nerve stimulation for intractable constipation. Gut. 2010 Mar;59(3):333–340. doi: 10.1136/gut.2009.187989. [DOI] [PubMed] [Google Scholar]
- 53.Govaert B, Pares D, Delgado-Aros S, La Torre F, Van Gemert WG, Baeten CG. A prospective multicentre study to investigate percutaneous tibial nerve stimulation for the treatment of faecal incontinence. Colorectal Dis. 2010 Dec;12(12):1236–1241. doi: 10.1111/j.1463-1318.2009.02020.x. [DOI] [PubMed] [Google Scholar]
- 54.Thin NN, Horrocks EJ, Hotouras A, et al. Systematic review of the clinical effectiveness of neuromodulation in the treatment of faecal incontinence. Br J Surg. 2013 Oct;100(11):1430–1447. doi: 10.1002/bjs.9226. [DOI] [PubMed] [Google Scholar]
- 55.Thomas GP, Dudding TC, Rahbour G, Nicholls RJ, Vaizey CJ. Sacral nerve stimulation for constipation. Br J Surg. 2013 Jan;100(2):174–181. doi: 10.1002/bjs.8944. [DOI] [PubMed] [Google Scholar]
- 56.Carrington EV, Evers J, Grossi U, et al. A systematic review of sacral nerve stimulation mechanisms in the treatment of fecal incontinence and constipation. Neurogastroenterol Motil. 2014 Sep;26(9):1222–1237. doi: 10.1111/nmo.12388. [DOI] [PubMed] [Google Scholar]
- 57.Lei Y, Chen JD. Effects of dual pulse gastric electrical stimulation on gastric tone and compliance in dogs. Dig Liver Dis. 2009 Apr;41(4):277–282. doi: 10.1016/j.dld.2008.07.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu X, Lei Y, Chen JD. Effects and mechanisms of electrical stimulation of the stomach, duodenum, ileum, and colon on gastric tone in dogs. Dig Dis Sci. 2010 Apr;55(4):895–901. doi: 10.1007/s10620-009-0830-4. [DOI] [PubMed] [Google Scholar]