Abstract
Objective
Anti-carbamylated protein (anti-CarP) antibodies could further elucidate early RA pathogenesis and predict clinical disease. We compared diagnostic accuracy of anti-CarP antibodies for future RA to other RA-related antibodies in military personnel.
Methods
Stored pre-RA diagnosis serum samples from 76 RA cases were tested for anti-CarP Fetal Calf Serum (FCS), anti-CarP Fibrinogen (Fib), anti-CCP2, RF-Neph, and RF-isotypes (IgM, IgG, and IgA). Positivity for all antibodies was determined as ≥2SD of log-transformed means from controls. Relationships between autoantibodies and future RA were assessed in prediagnosis serum for all RA cases compared to controls using sensitivity, specificity, and logistic regression. Differences in diagnostic accuracy between antibody combinations were assessed using comparisons of area under the curves (AUCs).
Results
Anti-CarP-FCS was 26% sensitive and 95% specific for future RA, where anti-CarP-Fib was 16% sensitive and 95% specific for future RA. Anti-CarP-FCS positivity was associated with future RA, while anti-CarP-Fib trended towards association. The antibody combination of anti-CCP2 and/or ≥2 RFs (RF-Neph and/or RF-isotypes) resulted in an AUC of 0.72 for future RA, where the AUC was 0.71 with the addition of anti-CarP-FCS to this prior combination.
Conclusion
Adding anti-CarP-FCS to antibody combinations did not improve AUC. However, anti-CarP-FCS was associated with future onset of RA, and was present in prediagnosis serum in ~10% of RA cases negative for anti-CCP2, but positive for RF.
Key Indexing Terms: Rheumatoid Arthritis, Autoantibodies, Epidemiology
INTRODUCTION
Discovery of antibodies to citrullinated protein antigens (ACPAs) has improved our understanding of the seropositive subset of rheumatoid arthritis (RA). ACPAs and rheumatoid factor (RF) are present in serum of RA patients years before clinical diagnosis of RA(1–6). While these autoantibodies, particularly anti-cyclic citrullinated peptide (anti-CCP), are highly specific (~95–99%) for RA, the sensitivity in those who later develop RA is notably lower (<70%)(1,3,5,6). Testing for RF and anti-CCP simultaneously can improve sensitivity by ~4–7%, while maintaining high specificity (~90–98%)(3,5). Yet, sensitivity of these combinations is still limited. Identifying additional autoantibodies that improve sensitivity for RA while maintaining high specificity would be a useful diagnostic and prediction tool(7).
Shi and colleagues reported the discovery of anti-carbamylated protein antibodies (anti-CarP) in patients with RA(8,9), including anti-CCP negative patients as well(8,9). Presence of anti-CarP in early RA was associated with increased disease severity, manifested by future joint destruction(8), and were detectable in some children with Juvenile Idiopathic Arthritis(10). Additionally, in subjects without current RA, but positive for anti-CCP2 and/or RF-IgM with a history of arthralgia, anti-CarP was 57% sensitive and 94% specific in identifying individuals who later develop classified RA (2010 ACR/EULAR criteria)(11). Furthermore, in stored samples collected prior to RA onset, anti-CarP was present prior to RA diagnosis in 5/79 subjects who were otherwise negative for anti-CCP2 and RF-IgM(12).
Anti-CarP alone or in combination with other clinically available RA-related autoantibodies could be useful in predicting future onset of RA. This study evaluated the timing of appearance and diagnostic accuracy for future RA of anti-CarP compared to other RA-related autoantibodies in U.S. Military Personnel.
MATERIALS AND METHODS
Study Population
The study population has been previously described(2–4). Subjects are military personnel, consisting of 83 RA cases and 82 controls with stored pre- and post-diagnosis serum samples. RA cases were identified at the Walter Reed Army Medical Center Rheumatology Clinic, and evaluated in clinic from 1989 to 2003. RA cases had date of RA diagnosis, age at diagnosis, and race determined by chart review. RA cases had both pre- and post-diagnosis serum samples (n=290, mean samples/subject=3.5) available through the Department of Defense Serum Repository (DoDSR). A subset of RA cases were determined as seropositive RA based on post-diagnosis RF positivity identified on chart review, or if either their pre- or post-diagnosis samples tested positive for RF (any assay) and/or anti-CCP2 within 1 year of their diagnosis as described previously(2,3). Controls were also derived from the DoDSR and matched to cases on age (case age at diagnosis), sex, race, number of samples available (n=290, mean samples/subject=3.5), duration of sample storage, and enlistment region.
Biomarker Analyses
Samples were tested in the Rheumatology Clinical Research Laboratory at the University of Colorado for clinically available RA-related autoantibodies, including RF by several methods and anti-cyclic citrullinated peptide version 2 (anti-CCP2). RF was measured by nephelometry (RF-Neph)(Dade Behring, Newark, Delaware, USA), and RF isotypes IgM, IgG, and IgA were measured using ELISA kits (INOVA Quanta Lite, San Diego, USA). Anti-CCP2 was measured by the Diastat kit (Axis-Shield, Dundee, UK).
Anti-CarP Testing
Generation of Carbamylated Antigens
Carbamylated proteins were generated as described by Shi et al.(8). Fetal Calf Serum (FCS)(Bodinco, Alkmaar, Netherlands) was carbamylated or left untreated. For generating carbamylated FCS (Ca-FCS), FCS was diluted in H2O to 4 mg/mL and potassium cyanate (Sigma, St. Louis, USA) was added to a concentration of 1M. Following incubation at 37°C for 12 hours, the sample was extensively dialyzed against H2O, using 10K MWCO SnakeSkin Dialysis Tubing (Thermo Scientific, Waltham, USA). Protein concentration was measured by both Nanodrop (Thermo Scientific) and BCA Protein Assay (Thermo Scientific). Carbamylated fibrinogen (Ca-Fib) was generated by incubating 5 mg/mL human fibrinogen (Fib)(Sigma) with 0.5M potassium cyanate at 4°C for 3 days, followed by dialysis against phosphate buffered saline (PBS).
Detection of Anti-CarP-FCS by ELISA
Non-modified FCS and Ca-FCS were coated at 10 µg/mL in 50 µL (diluted in pH 9.6 0.1M carbonate-bicarbonate buffer)(CB) on Nunc Maxisorp plates (Thermo Scientific) overnight. After washing in PBS containing 0.05% tween (Sigma)(PT), plates were blocked by incubating 100 µL PBS/1% bovine serum albumin (BSA)(Sigma) for 6 hours at 4°C. Following additional washing, wells were incubated with 50 µL serum at a 1/50 dilution in PBS/0.05% tween/1% BSA buffer (PTB) on ice overnight. All subsequent incubations were performed in PTB. As a standard, serial dilutions of a pool of positive sera were used. Human IgG was detected using rabbit anti-human IgG antibody (DAKO, Heverlee, Belgium) incubated on ice for 3.5 hours. After washing, wells were incubated on ice for 3.5 hours with horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG antibody (DAKO). The final wash was followed by visualization of HRP enzyme activity using 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)(ABTS) as substrate. We transformed the absorbance on both Ca-FCS and FCS to aU/mL and subtracted the background signal (aU/mL) of FCS from the signal (aU/mL) of CarP-FCS to analyze the specific anti-CarP-FCS reactivity.
Detection of anti-CarP-Fib by ELISA
Non-modified Fib and Ca-Fib were coated at 20 µg/mL in 50 µL (diluted in pH 9.0 PBS) on NuncMaxisorp plates (Thermo Scientific) overnight. Following washing in PT, plates were blocked by incubating 200 µL pH 9.0 PBS/2% BSA for 2 hours at 4°C. Following additional washing, wells were incubated with 50 µL serum at a 1/50 dilution in radioimmunoassay (RIA) buffer (10 mM Tris pH 7.6; 350 mM NaCl; 1% TritonX; 0.5% Na-deoxycholate; 0.1% SDS)(Sigma) on ice for 3 hours. All subsequent incubations were performed in RIA buffer. As a standard, serial dilutions of a pool of positive sera were used. Human IgG was detected using HRP-labeled rabbit anti-human IgG antibody (DAKO) incubated on ice for 2 hours. Following the last washings HRP enzyme activity was visualized using ABTS.
Defining Positivity for Antibodies
As no established cutoff levels for positivity exist for anti-CarP, we determined cutoff values for anti-CarP in serum. Anti-CarP were measured in 82 healthy controls. Binary cutoffs were determined for anti-CarP by randomly splitting the 82 healthy controls into two groups containing 41 subjects. The first group was used to determine the binary cutoff values for anti-CarP-FCS and -Fib (cutoff controls), and the second group (controls) was reserved for comparison analyses with the cases.
As we had multiple serum samples per subject, we defined anti-CarP positivity using a single sample from each cutoff control to retain statistical independence. We selected the single serum sample from each of the 41 cutoff controls with measured anti-CarP-FCS and anti-CarP-Fib closest in time to their matched RA case’s diagnosis date. Anti-CarP measures were natural log-transformed, as they were not normally distributed. Positivity for anti-CarP-FCS and anti-CarP-Fib was defined as ≥2 standard deviations (SD) above the natural log-transformed mean. After back-transforming these values, positivity was determined to be >427.4 aU/mL for anti-CarP-FCS and >233.8 aU/mL for anti-CarP-Fib.
To allow for comparable results across antibodies, positivity for RF-Neph, RF isotypes, and anti-CCP2 were also defined as ≥2 SDs above the natural log-transformed mean, using the same single serum sample from the 41 cutoff controls. After back-transforming these values, positivity was determined as follows: RF-Neph >24.0 units/mL; RF-IgM >13.5 units/mL; RF-IgG >25.0 units/mL; RF-IgA >17.5 units/mL; anti-CCP2 >0.6 units/mL.
Additionally, we considered cutoffs based on clinical recommendations for anti-CCP2 and the RF antibodies. Clinical positivity for RF and RF isotypes were determined using ACR Classification Criteria for RA specificities as being present in <5% of 491 healthy blood bank donor controls(13). Clinical positivity for RF antibodies were defined as follows: RF >24.4 units/mL; RF-IgM >13.6 units/mL; RF-IgG >10.9 units/mL; RF-IgA >10.5 units/mL. Clinical positivity for anti-CCP2 was based on manufacturer specification of >5 units/mL.
Once we defined antibody positivity using the single serum sample in the cutoff controls, we applied these positivity cutoffs to all the prediagnosis serum of RA cases and to all available serum for the remaining 41 controls reserved for comparison against the RA cases.
Diagnostic Accuracy and Association of Antibodies for Future RA
Using 2×2 tables, we determined diagnostic accuracy, measured by sensitivity and specificity, of each antibody or various combinations of antibodies ever testing positive at any point in the prediagnosis period for our RA cases, and at any point for our controls. Cases (76 of 83 total) with sufficient prediagnosis serum sample volumes (n=210 samples) were tested for all autoantibodies (anti-CarP-FCS, -Fib, anti-CCP2, and RF assays), and comparator controls (n=41). We characterized the diagnostic accuracy of antibody positivity, first based on the ≥2 SD above the mean cutoff, and then using clinical test-based cutoffs for RF and anti-CCP2 defined as ever testing positive in any sample any time before RA diagnosis for RA cases and any time for controls. We then characterized diagnostic accuracy of antibody positivity in seropositive RA cases; however, we did not present these results in a table, as they were similar to results in all RA cases.
Diagnostic accuracy was assessed at the following time periods before RA diagnosis: ≥0 to ≤1 year, >1 to ≤5 years, and >5 years. Of note was the period ≥0 to ≤1 year, where for RA cases, serum was limited strictly to this time period, whereas for controls, we evaluated samples ≤1 year and any time after their matched RA case diagnosis date. All other time periods were as specified for both RA cases and controls.
The discriminative ability between antibodies, and combinations of antibodies between the 76 RA cases and 41 controls was assessed through comparisons of area under the curve (AUC) based on the binary cutoffs. Using a binary cutoff, the AUC is the average of sensitivity and specificity. This property allowed us to compare the combined improvement of diagnostic accuracy of both sensitivity and specificity. All AUC analyses accounted for comparisons of antibodies in the same individuals.
To complement diagnostic accuracy results, we used logistic regression analyses to characterize the relative association (odds ratio) between RA case status and presence of these autoantibodies in prediagnosis serum in both seropositive and seronegative RA cases compared to controls.
Assessing the Timing of Antibody Appearance
Timing of antibody appearance in prediagnosis serum was assessed in seropositive RA cases that were ever positive for more than one antibody during the prediagnosis period. We determined if anti-CCP2 was present in serum before appearance of anti-CarP-FCS or vice versa, and then if anti-CCP2 was present in serum before appearance of anti-CarP-Fib or vice versa. The appearance of RF-Neph in relation to anti-CarP-FCS and -Fib was also addressed. A small proportion of cases were already positive for these autoantibodies, representing left-censorship, likely underrepresenting the true mean duration of autoantibody positivity. Due to the small proportion of autoantibody positivity and left-censoring, the non-parametric signed-rank test was used instead of survival analysis to determine which autoantibody preceded the other based on the mean time of appearance for those with both antibodies.
Antibody Levels and Variability of Positivity in the Prediagnosis Period
Linear mixed models of natural log-transformed antibody levels characterized trends in mean anti-CarP-FCS, -Fib, RF-Neph, and anti-CCP2 for RA cases and controls up to 10 years before clinical diagnosis of RA. To characterize mean trends of anti-CarP-FCS and -Fib, we used all RA cases (n=76) and controls (n=76) who had measures of anti-CarP-FCS and -Fib, as these analyses did not depend on positivity cutoffs, giving an observation sample size of n=360. In our assessment of anti-CCP2 and RF-Neph trends, all RA cases (n=82) and controls (n=82) had complete measures of these antibodies, resulting in a larger observation sample size (n=441). We determined the best model fit for both linear and squared trends, and identified the time before RA diagnosis when mean levels of antibodies began to differ between RA cases and controls. To account for multiple comparisons at each time point within each model, we used a Scheffe p-value correction for comparisons in linear combinations(14).
Additionally, we characterized the variability of positivity in multiple samples over time for anti-CarP-FCS, -Fib, anti-CCP2, and RF-Neph in the prediagnosis period for seropositive RA cases and controls by determining the proportion of those who tested positive for an antibody, but had subsequent levels decreased below the cutoff.
All statistical analyses were performed using SAS version 9.3.
Ethical Considerations
The study protocol and analyses were approved by the respective Institutional Review Boards at the Walter Reed Army Medical Center and the University of Colorado.
RESULTS
Study Population Demographics
Demographic characteristics of RA cases, controls and cutoff controls were not statistically different (Table 1).
Table 1.
Descriptive statistics for RA cases, comparison control group, and the control group used to define the cutoff
| RA cases (n=76) (n samples=210) |
Controls (n=41) (n samples=136) |
Cutoff Controls† (n=41) (n samples=121) |
p* | |
|---|---|---|---|---|
| Number of Samples, Mean ± SD | 2.8 ± (1.1) | 3.3 ± (1.3) | 3.0 ± (1.2) | 0.07 |
| Age at Diagnosis, Mean ± SD years | 39.8 ± 9.9 | 40.6 ± 10.2 | 39.1 ± 9.6 | 0.79 |
| Male, n (%) | 45 (59.2) | 25 (61.0) | 23 (56.1) | 0.90 |
| Race, n (%) | 0.58 | |||
| White | 51 (67.1) | 31 (75.6) | 25 (61.0) | |
| Black | 21 (27.6) | 9 (22.0) | 12 (29.3) | |
| Other | 4 (5.3) | 1 (2.4) | 4 (9.7) |
Reported p-value is testing the difference across all three groups.
Control group used only to determine positivity cutoff for anti-CarP-FCS and -Fib.
Diagnostic Accuracy and Associations in RA Cases
The antibody systems anti-CCP2, RFs, and anti-CarP were all detected prior to RA diagnosis, as evidenced by a proportion of RA cases positive for these antibodies in the prediagnosis period. The sensitivity, specificity, and relative associations for future RA based on ever being antibody positive in serum at any time before clinical diagnosis of RA in our 76 seropositive and seronegative RA cases and 41 controls are presented in Table 2. Results are presented to allow the assessment of diagnostic accuracy with the addition of a positive test for anti-CarP-FCS compared to the more established RA-related autoantibodies, either as a single test or combination of results. As single tests, anti-CCP2 demonstrated the highest sensitivity (52%) for future RA, where RF-Neph, RF-IgG, RF-IgA, anti-CarP-FCS and -Fib had the highest specificities (~95–97%).
Table 2.
Diagnostic accuracy† and odds ratio (OR) of RA-related antibodies in prediagnosis serum samples for future RA. Positivity defined as ≥2 SD above the mean in healthy controls reserved to define cutoff: RF-Neph >24.0 units/mL; RF-IgM >13.5 units/mL; RF-IgG >25.0 units/mL; RF-IgA >17.5 units/mL; anti-CCP2 >0.6 units/mL; anti-CarP-FCS >427.4 units/mL; anti-CarP-Fib >233.9 units/mL.
| Antibody | RA (+/76) |
Cont. (+/41) |
SN (%) |
SP (%) |
OR | 95% CI | p |
|---|---|---|---|---|---|---|---|
| Anti-CCP2 | 40/76 | 4/41 | 52.6 | 90.2 | 10.28 | 3.34–31.68 | <0.01 |
| RF-Neph | 27/76 | 1/41 | 35.5 | 97.6 | 22.04 | 2.87–169.36 | <0.01 |
| RF IgM | 32/76 | 5/41 | 42.1 | 87.8 | 5.24 | 1.85–14.82 | <0.01 |
| RF IgG | 8/76 | 2/41 | 10.5 | 95.1 | 2.29 | 0.46–11.35 | 0.31 |
| RF IgA | 24/76 | 2/41 | 31.6 | 95.1 | 9.00 | 2.01–40.38 | <0.01 |
| ≥1 RF* | 42/76 | 8/41 | 55.3 | 80.5 | 5.10 | 2.08–12.47 | <0.01 |
| ≥2 RF* | 24/76 | 2/41 | 31.4 | 95.1 | 9.00 | 2.01–40.38 | <0.01 |
| Anti-CarP-FCS | 20/76 | 2/41 | 26.3 | 95.1 | 6.96 | 1.54–31.52 | 0.01 |
| Anti-CarP-Fib | 12/76 | 2/41 | 15.8 | 95.1 | 3.66 | 0.78–17.21 | 0.10 |
| Anti-CCP2 and/or ≥1 RF* | 51/76 | 11/41 | 67.1 | 73.2 | 5.56 | 2.40–12.89 | <0.01 |
| Anti-CCP2 and/or ≥1 RF* and/or anti-CarP-FCS | 52/76 | 13/41 | 68.4 | 68.3 | 4.68 | 2.06–10.56 | <0.01 |
| Anti-CCP2 and/or ≥2 RF* | 44/76 | 6/41 | 57.9 | 85.4 | 8.02 | 3.02–21.34 | <0.01 |
| Anti-CCP2 and/or ≥2 RF* and/or anti-CarP-FCS | 46/76 | 8/41 | 60.5 | 80.5 | 6.32 | 2.57–15.54 | <0.01 |
RA cases sample n=210, avg. samples/subject=2.8; controls sample n=136, avg. sample/subject=3.3.
Contains the following statistics: sensitivity (SN), specificity (SP)
Count of RF by nephelometry and RF isotypes (IgM, IgG, IgA).
NA: OR, 95% CI, and p cannot be calculated because no controls met positivity criteria.
Diagnostic accuracy statistics and relative associations for future RA based on clinically relevant cutoffs for RF and anti-CCP2 are presented in Table 3, and were qualitatively similar to Table 2.
Table 3.
Diagnostic accuracy† and odds ratio (OR) of RA-related antibodies in prediagnosis serum samples for future RA. Positivity for RF defined on clinical recommendations as present in <5% of 491 healthy blood donors: RF >24.4 units/mL; RF-IgM >13.6 units/mL; RF-IgG >10.9 units/mL; RF-IgA >10.5 units/mL. Positivity for anti-CCP2 based on manufacturer specifications at >5 units/mL. Positivity for anti-CarP defined as ≥2 SD above the mean in healthy controls reserved to define cutoff: anti-CarP-FCS >427.4 units/mL; anti-CarP-Fib >233.9 units/mL.
| Antibody | RA (+/76) |
Cont. (+/41) |
SN (%) |
SP (%) |
OR | 95% CI | p |
|---|---|---|---|---|---|---|---|
| Anti-CCP2 | 34/76 | 0/41 | 44.7 | 100.0 | NA | NA | NA |
| RF | 27/76 | 1/41 | 35.5 | 97.6 | 22.04 | 2.87–169.36 | <0.01 |
| RF IgM | 32/76 | 5/41 | 42.1 | 87.8 | 5.24 | 1.85–14.82 | <0.01 |
| RF IgG | 11/76 | 3/41 | 14.5 | 92.7 | 2.14 | 0.56–8.17 | 0.26 |
| RF IgA | 30/76 | 3/41 | 39.5 | 92.7 | 8.26 | 2.34–29.19 | <0.01 |
| ≥1 RF* | 43/76 | 9/41 | 56.6 | 78.1 | 4.63 | 1.95–11.03 | <0.01 |
| ≥2 RF* | 29/76 | 3/41 | 38.2 | 92.7 | 7.82 | 2.21–27.64 | <0.01 |
| Anti-CarP-FCS | 20/76 | 2/41 | 26.3 | 95.1 | 6.96 | 1.54–31.50 | <0.01 |
| Anti-CarP-Fib | 12/76 | 2/41 | 15.8 | 95.1 | 3.66 | 0.78–17.23 | 0.08 |
| Anti-CCP2 and/or ≥1 RF* | 48/76 | 9/41 | 63.2 | 78.1 | 6.10 | 2.54–14.61 | <0.01 |
| Anti-CCP2 and/or ≥1 RF* and/or anti-CarP-FCS | 49/76 | 11/41 | 64.5 | 73.2 | 4.95 | 2.15–11.41 | <0.01 |
| Anti-CCP2 and/or ≥2 RF* | 41/76 | 3/41 | 53.9 | 92.7 | 14.84 | 4.21–52.25 | <0.01 |
| Anti-CCP2 and/or ≥2 RF* and/or anti-CarP-FCS | 43/76 | 5/41 | 56.6 | 87.8 | 9.38 | 3.32–26.53 | <0.01 |
RA cases positive for RF and/or CCP2 sample n=166, avg. sample/subject=2.5; controls sample n=135, avg. sample/subject=3.3.
Contains the following statistics: sensitivity (SN), specificity (SP).
Count of RF by nephelometry and RF isotypes (IgM, IgG, IgA).
NA: OR, 95% CI, and p cannot be calculated because no controls met positivity criteria.
Diagnostic accuracy statistics and relative associations for future RA at different time intervals in our 76 seropositive and seronegative RA cases and 41 controls are presented in Table 4. Similar trends in sensitivity and specificity were observed across time periods.
Table 4.
Diagnostic accuracy† and odds ratio (OR) of RA-related antibodies in serum samples at different time intervals during the prediagnosis period for future RA. Positivity defined as ≥2 SD above the mean in healthy controls reserved to define cutoff: RF-Neph >24.0 units/mL; RF-IgM >13.5 units/mL; RF-IgG >25.0 units/mL; RF-IgA >17.5 units/mL; anti-CCP2 >0.6 units/mL; anti-CarP-FCS >427.4 units/mL; anti-CarP-Fib >233.9 units/mL.
| Antibody | RA (+/n) |
Cont. (+/n) |
SN (%) |
SP (%) |
OR | 95% CI | p |
|---|---|---|---|---|---|---|---|
|
≥0 to ≤1 year before RA Diagnosis (RA cases sample n=23, avg. sample/subject=1; controls sample n=37, avg. sample/subject=1.3) | |||||||
| Anti-CCP2 | 14/23 | 2/28 | 60.9 | 92.9 | 20.22 | 3.83–106.81 | <0.01 |
| ≥1 RF* | 15/23 | 4/28 | 65.2 | 85.7 | 11.25 | 2.88–43.95 | <0.01 |
| ≥2 RF* | 8/23 | 2/28 | 34.8 | 92.9 | 6.93 | 1.30–37.01 | 0.02 |
| Anti-CarP-FCS | 7/23 | 2/28 | 30.4 | 92.9 | 5.69 | 1.05–30.85 | 0.04 |
| Anti-CarP-Fib | 5/23 | 1/28 | 21.7 | 96.4 | 7.49 | 0.81–69.62 | 0.08 |
| Anti-CCP2 and/or ≥1 RF* | 17/23 | 5/28 | 73.9 | 82.1 | 13.03 | 3.41–49.88 | <0.01 |
| Anti-CCP2 and/or ≥1 RF* and/or anti-CarP-FCS | 17/23 | 7/28 | 73.9 | 75.0 | 8.50 | 2.40–30.09 | <0.01 |
| Anti-CCP2 and/or ≥2 RF* | 16/23 | 4/28 | 69.6 | 85.7 | 13.71 | 3.44–54.61 | <0.01 |
| Anti-CCP2 and/or ≥2 RF* and/or anti-CarP-FCS | 16/23 | 6/28 | 69.6 | 78.6 | 8.38 | 2.36–29.74 | <0.01 |
|
>1 to ≤5 years before RA Diagnosis (RA cases sample n=81, avg. sample/subject=1.7; controls sample n=40, avg. sample/subject=1.4) | |||||||
| Anti-CCP2 | 25/49 | 1/29 | 51.0 | 96.6 | 29.17 | 3.67–231.56 | <0.01 |
| ≥1 RF* | 22/49 | 4/29 | 44.9 | 86.2 | 5.09 | 1.54–16.84 | <0.01 |
| ≥2 RF* | 13/49 | 1/29 | 26.5 | 96.6 | 10.11 | 1.25–81.99 | 0.03 |
| Anti-CarP-FCS | 9/49 | 0/29 | 18.4 | 100.0 | NA | NA | NA |
| Anti-CarP-Fib | 6/49 | 1/29 | 12.2 | 96.6 | 3.91 | 0.45–34.24 | 0.22 |
| Anti-CCP2 and/or ≥1 RF* | 32/49 | 5/29 | 65.3 | 82.8 | 9.04 | 2.92–27.94 | <0.01 |
| Anti-CCP2 and/or ≥1 RF* and/or anti-CarP-FCS | 32/49 | 5/29 | 65.3 | 82.8 | 9.04 | 2.92–27.94 | <0.01 |
| Anti-CCP2 and/or ≥2 RF* | 28/49 | 2/29 | 57.1 | 93.1 | 18.00 | 3.84–84.26 | <0.01 |
| Anti-CCP2 and/or ≥2 RF* and/or anti-CarP-FCS | 29/49 | 2/29 | 59.2 | 93.1 | 19.57 | 4.18–91.76 | <0.01 |
|
>5 years before RA Diagnosis (RA cases sample n=89, avg. sample/subject=1.9; controls sample n=57, avg. sample/subject=2.1) | |||||||
| Anti-CCP2 | 15/48 | 1/27 | 31.3 | 96.3 | 11.82 | 1.46–95.37 | 0.02 |
| ≥1 RF* | 20/48 | 3/27 | 41.7 | 88.9 | 5.71 | 1.51–21.60 | 0.01 |
| ≥2 RF* | 8/48 | 0/27 | 16.7 | 100.0 | NA | NA | NA |
| Anti-CarP-FCS | 8/48 | 1/27 | 16.7 | 96.3 | 5.20 | 0.61–44.05 | 0.14 |
| Anti-CarP-Fib | 5/48 | 1/27 | 10.4 | 96.3 | 3.02 | 0.33–27.33 | 0.32 |
| Anti-CCP2 and/or ≥1 RF* | 23/48 | 4/27 | 47.9 | 85.2 | 5.29 | 1.59–17.62 | <0.01 |
| Anti-CCP2 and/or ≥1 RF* and/or anti-CarP-FCS | 24/48 | 5/27 | 50.0 | 81.5 | 4.40 | 1.43–13.54 | <0.01 |
| Anti-CCP2 and/or ≥2 RF* | 16/48 | 1/27 | 33.3 | 96.3 | 13.00 | 1.62–104.58 | 0.02 |
| Anti-CCP2 and/or ≥2 RF* and/or anti-CarP-FCS | 17/48 | 2/27 | 35.4 | 92.6 | 6.86 | 1.45–32.52 | 0.02 |
Contains the following statistics: sensitivity (SN), specificity (SP).
Count of RF by nephelometry and RF isotypes (IgM, IgG, IgA); individual RF antibodies not presented to save space.
NA: OR, 95% CI, and p cannot be calculated because no controls met positivity criteria.
Of the 76 RA cases with anti-CarP tested, 67 (88.1%) were defined as having seropositive RA (RF and/or anti-CCP2) as described in the Methods. Overall, the diagnostic accuracy statistics for future seropositive RA were similar to those found in all RA cases (data not shown). Among the 28 seropositive RA cases who were positive for ≥1 RF, but never positive for anti-CCP2 at any time prior to RA diagnosis, anti-CarP-FCS was present in 3 (10.7%) of these individuals. None of the 9 RA cases classified as seronegative RA for both RF and anti-CCP2 were positive for anti-CarP-FCS or anti-CarP-Fib based on our defined cutoffs.
The combination of antibodies, anti-CCP2 and/or ≥1 RF (nephelometry or isotypes) demonstrated 67% sensitivity and 73% specificity for future RA, with an AUC of 0.70; the addition of anti-CarP-FCS increased sensitivity to 68%, while decreasing specificity to 68%, resulting in an AUC of 0.68, which was not significantly different from the AUC for anti-CCP2 and/or ≥1 RF (p=0.33). The profile anti-CCP2 and/or ≥2 RFs (nephelometry or isotypes) demonstrated 58% sensitivity and 85% specificity, with an AUC of 0.72; the addition of anti-CarP-FCS increased sensitivity to 61%, while decreasing specificity to 81%, resulting in an AUC of 0.71, which was not significantly different from the AUC for anti-CCP2 and/or ≥2 RF (p=0.56).
Timing of Antibody Appearance
Table 5 presents the order of antibody appearance in seropositive RA cases testing positive in the prediagnosis period for both antibodies. Overall, when able to assess, anti-CCP2 was present prior to anti-CarP-FCS and -Fib, where RF-Neph was present after anti-CarP-FCS and -Fib. However these results were not significantly different.
Table 5.
Appearance of first antibody in seropositive RA cases where both antibodies of interest were present in prediagnosis serum.
| Comparing anti-CCP2 to anti-CarP-FCS | Mean Years of anti-CCP2 | p* | |
|---|---|---|---|
| Anti-CCP2 preceded Anti-CarP-FCS | 4/16 cases | Preceding anti-CarP-FCS | |
| Anti-CarP-FCS preceded Anti-CCP2 | 0/16 cases | 0.76 | 0.13 |
| First appearance in same sample | 12/16 cases | ||
| Comparing anti-CCP2 to anti-CarP-Fib | Mean Years of anti-CCP2 | p* | |
| Anti-CCP2 preceded Anti-CarP-Fib | 4/12 cases | Preceding anti-CarP-Fib | |
| Anti-CarP-Fib preceded Anti-CCP2 | 0/12 cases | 1.01 | 0.13 |
| First appearance in same the sample | 8/12 cases | ||
| Comparing RF-Neph to anti-CarP-FCS | Mean Years of anti-CarP-FCS | p* | |
| RF-Neph preceded Anti-CarP-FCS | 2/16 cases | Preceding RF-Neph | |
| Anti-CarP-FCS preceded RF-Neph | 3/16 cases | 0.08 | 0.81 |
| First appearance in the same sample | 11/16 cases | ||
| Comparing RF-Neph to anti-CarP-Fib | Mean Years of anti-CarP-Fib | p* | |
| RF-Neph preceded Anti-CarP-Fib | 2/7 cases | Preceding RF-Neph | |
| Anti-CarP-Fib preceded RF-Neph | 1/7 cases | 0.01 | 1.00 |
| First appearance in the same sample | 4/7 cases | ||
p-value for signed rank test
Trends in Antibody Levels during the Prediagnosis Period
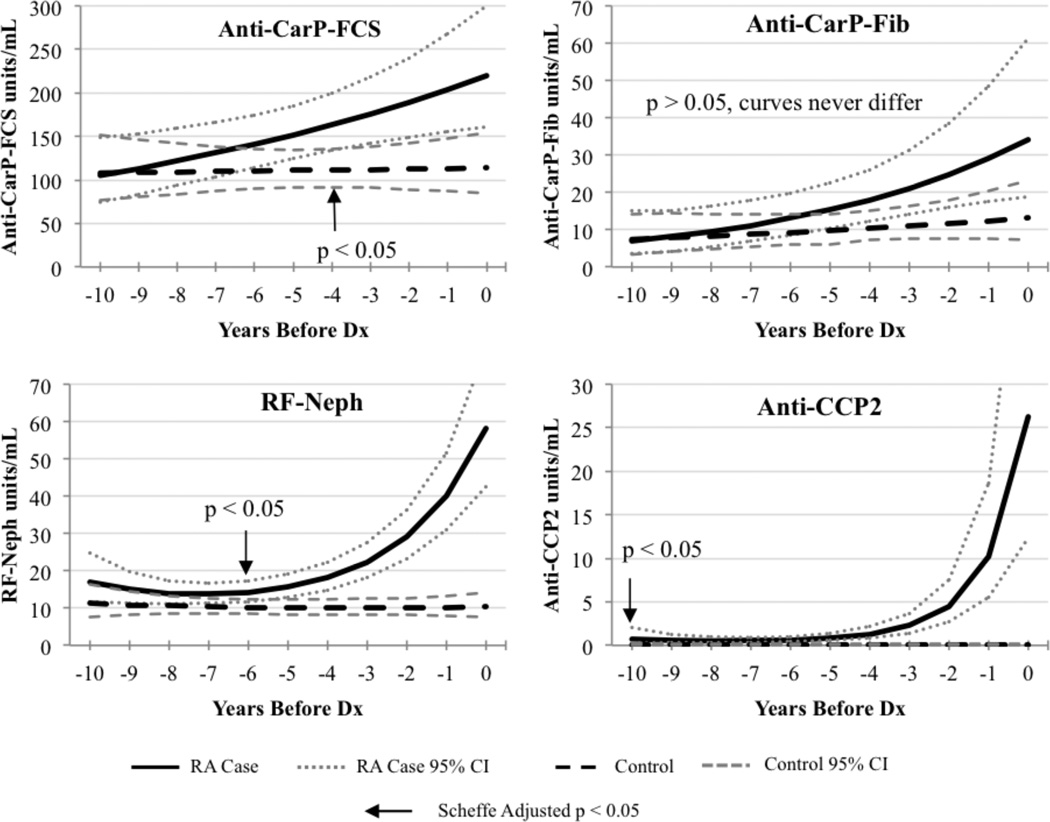
Figure 1 characterizes mean levels of antibodies, back-transformed from the log scale, over the ten year period preceding RA case diagnosis. There was a general trend for increasing mean levels of antibodies over time for anti-CarP-FCS, anti-CarP-Fib, anti-CCP2, and RF-Neph in RA cases, where the levels in controls remained stable. The mean levels of anti-CarP-FCS were significantly higher for RA cases compared to controls 4 years before RA diagnosis. Mean anti-CarP-Fib levels were higher in RA cases compared to controls, but this trend was not statistically significantly. For anti-CCP2 and RF-Neph antibodies, mean levels exponentially increased in RA cases shortly before RA diagnosis, and mean levels for controls remained consistently low. Anti-CCP2 levels were significantly higher in RA cases compared to controls 10 years before RA diagnosis. Mean levels of RF-Neph were significantly higher in RA cases compared to controls 6 years before RA diagnosis.
Figure 1.
Trends in the mean levels of anti-CarP-FCS, anti-CarP-Fib, RF-Neph, and anti-CCP2 between RA cases and controls during the prediagnosis period. Arrows indicate where the mean levels between cases and controls are significantly different in the prediagnosis period.
Autoantibody Fluctuations in Positivity Over Time
In the 20 seropositive RA cases positive for anti-CarP-FCS, 6 (30%) had anti-CarP-FCS levels decrease below the cutoff in subsequent prediagnosis samples, and 1 of 2 controls that tested positive had levels decrease below the cutoff. Of the 12 anti-CarP-Fib positive cases, 2 (17%) had anti-CarP-Fib levels decrease below the cutoff, while the 2 controls that tested positive remained positive. Furthermore, among the 39 seropositive RA cases positive for anti-CCP2, 1 (3%) had anti-CCP2 levels decrease below the cutoff, while 2 of the 4 controls that tested positive for anti-CCP2 had levels decrease below the cutoff. Among the 27 seropositive RA cases positive for RF-Neph, 5 (19%) had RF-Neph levels decrease below the cutoff, and 4 of the 8 controls had RF-Neph levels decrease below the cutoff.
DISCUSSION
Our results indicate anti-CarP-FCS and anti-CarP-Fib are present in prediagnosis serum of RA cases. Both anti-CarP-FCS and -Fib exhibited lower sensitivity (<30%) than anti-CCP2 or RF, although the specificity for anti-CarP was comparatively high (>95%). Anti-CarP-FCS exhibited a greater sensitivity and the same specificity as anti-CarP-Fib. Furthermore, anti-CarP-FCS was significantly associated with future RA, while anti-CarP-Fib only trended towards a significant association, which influenced our decision to consider calculations of diagnostic accuracy for future RA using only anti-CarP-FCS in antibody combinations. While we did not observe significant differences in AUCs with the addition of anti-CarP-FCS to combinations of anti-CCP and/or RFs, we did observe a modestly increased sensitivity and decreased specificity for future RA. This could suggest utility of anti-CarP in assays that test for multiple antibodies at once, or for assessment of risk of future erosive disease in individuals who exhibit anti-CarP(8).
Notably, as no recommended cutoff for anti-CarP exists, we randomly split the controls to define cutoff levels, reserving one set of controls as an independent comparison group. This split may introduce bias because of unequal groups, although similar demographic characteristics between our control groups (Table 1) suggest such bias was minimal. Additionally, the smaller control groups may allow outlier values to influence cutoff values, decreasing our ability to detect significant associations. However the ≥2 SD above the mean cutoff for anti-CarP-FCS in our reduced sample size of 41 was >427, whereas if we used all 82 controls, our cutoff would have been >472; analysis of variance indicated that splitting the controls did not result in a statistically different cutoff levels (p=0.64). Both these cutoff levels are higher than Shi and colleagues in their initial work on the anti-CarP system, where the positivity cutoff level was >348(8). Therefore, our higher cutoff levels for anti-CarP may be less sensitive for future RA than previously reported studies.
Our higher cutoff levels, in addition to small case numbers, could explain why we did not observe anti-CarP present in any seronegative RA cases. However, anti-CarP-FCS was still present in prediagnosis serum samples in 10.7% of RA cases who never tested positive for anti-CCP2, which is supported by other studies that have found anti-CarP in 8%-16% of ACPA negative RA patients(8,9). Additionally, Shi and colleagues reported anti-CarP-FCS present in 27% of RA patients prior to diagnosis(12), which is similar to this study’s sensitivity for future RA of 26%.
As stated in the Methods, we used the same process for determining cutoff levels for anti-CCP2 and RF as was used for anti-CarP. This was done to allow for fairer comparisons of diagnostic accuracy across antibodies. Additionally, results based on the >2 SD cutoffs for RF and anti-CCP2 antibodies were qualitatively similar to results based on clinical test-based cutoffs. One issue regarding defining cutoffs could be our use of only healthy controls, as the reactivity of each autoantibody system could be lower, thereby resulting in a higher specificity than if other autoimmune disease groups were used. This issue of reactivity in healthy controls should be considered in future studies.
Several features of the antibodies tested herein are of interest in the pathophysiology of RA development. There was a non-significant trend for anti-CCP2 to appear prior to anti-CarP-FCS and Fib, and in some cases both anti-CCP2 and anti-CarP appeared prior to RFs. Given the relatively close temporal relationship between initial positivity of the anti-CCP2 and anti-CarP, it is possible the immune processes driving the break in tolerance to these structurally distinct autoantigens are similar in time and mechanism(15), or could suggest a degree of cross-reactivity between ACPA and anti-CarP in some of the patients at this early time point in the evolution of disease(16–18). The higher sensitivity for disease of anti-CCP could represent a dominant autoimmune response to citrullinated antigens; alternatively, differences in the assay sensitivity between a commercially developed, optimized and validated assay compared to a preclinical research-based method may underlie this difference.
In future studies, a larger number of RA cases would increase the ability to determine potential differences in biologic processes behind the RF, ACPA, and anti-CarP systems.
Acknowledgments
Sources of Support:
This work is supported by a Rheumatology Research Foundation Disease Targeted Innovative Research Grant, NIH T32 AR007534, the Walter S. and Lucienne Driskill Foundation, the Dutch Arthritis Foundation, the IMI JU funded project BeTheCure contract no 115142-2, The Netherlands Proteomics Center, and the Center for Medical Systems Biology as part of The Netherlands Genomics Initiative. L.T. is supported by a ZON-MW Vidi grant and by a fellowship from Janssen Biologics.
The material contained herein is made available for the purpose of peer review and discussion and does not necessarily reflect the views of the Department of the Army, Department of the Navy, or the Department of Defense.
References
- 1.Rantapää-Dahlqvist S, de Jong BAW, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 2.Majka DS, Deane KD, Parrish LA, Lazar AA, Barón AE, Walker CW, et al. Duration of preclinical rheumatoid arthritis-related autoantibody positivity increases in subjects with older age at time of disease diagnosis. Ann Rheum Dis. 2008;67:801–807. doi: 10.1136/ard.2007.076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deane KD, O’Donnell CI, Hueber W, Majka DS, Lazar AA, Derber LA, et al. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum. 2010;62:3161–3172. doi: 10.1002/art.27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolfenbach JR, Deane KD, Derber LA, O’Donnell CI, Gilliland WR, Edison JD, et al. Autoimmunity to peptidyl arginine deiminase type 4 precedes clinical onset of rheumatoid arthritis. Arthritis Rheum. 2010;62:2633–2639. doi: 10.1002/art.27570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielen MMJ, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MHMT, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: A study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 6.Kokkonen H, Mullazehi M, Berglin E, Hallmans G, Wadell G, Rönnelid J, et al. Antibodies of IgG, IgA and IgM isotypes against cyclic citrullinated peptide precede the development of rheumatoid arthritis. Arthritis Res Ther. 2011;13:R13. doi: 10.1186/ar3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trouw LA, Mahler M. Closing the serological gap: promising novel biomarkers for the early diagnosis of rheumatoid arthritis. Autoimmun Rev. 2012;12:318–322. doi: 10.1016/j.autrev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Shi J, Knevel R, Suwannalai P, van der Linden MP, Janssen GMC, van Veelen PA, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci. 2011;108:17372–17377. doi: 10.1073/pnas.1114465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang X, Trouw LA, van Wesemael TJ, Shi J, Bengtsson C, Källberg H, et al. Anti-CarP antibodies in two large cohorts of patients with rheumatoid arthritis and their relationship to genetic risk factors, cigarette smoking and other autoantibodies. Ann Rheum Dis. 2014;73:1761–1768. doi: 10.1136/annrheumdis-2013-205109. [DOI] [PubMed] [Google Scholar]
- 10.Muller PCEH, Anink J, Shi J, Levarht EWN, Reinards THCM, Otten MH, et al. Anticarbamylated protein (anti-CarP) antibodies are present in sera of juvenile idiopathic arthritis (JIA) patients. Ann Rheum Dis. 2013;72:2053–2055. doi: 10.1136/annrheumdis-2013-203650. [DOI] [PubMed] [Google Scholar]
- 11.Shi J, van de Stadt LA, Levarht EWN, Huizinga TWJ, Toes REM, Trouw LA, et al. Brief Report: Anti-carbamylated protein antibodies are present in arthralgia patients and predict the development of rheumatoid arthritis. Arthritis Rheum. 2013;65:911–915. doi: 10.1002/art.37830. [DOI] [PubMed] [Google Scholar]
- 12.Shi J, van de Stadt LA, Levarht EWN, Huizinga TWJ, Hamann D, van Schaardenburg D, et al. Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis. Ann Rheum Dis. 2014;73:780–783. doi: 10.1136/annrheumdis-2013-204154. [DOI] [PubMed] [Google Scholar]
- 13.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 14.Lazar AA, Zerbe GO. Solutions for determining the significance region using the Johnson-Neyman Type Procedure in generalized linear (mixed) models. J Educ Behav Stat. 2011;36:699–719. doi: 10.3102/1076998610396889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trouw LA, Huizinga TWJ, Toes REM. Autoimmunity in rheumatoid arthritis: different antigens--common principles. Ann Rheum Dis Suppl. 2012;(72 Suppl 2):ii132–ii136. doi: 10.1136/annrheumdis-2012-202349. [DOI] [PubMed] [Google Scholar]
- 16.Scinocca M, Bell DA, Racapé M, Joseph R, Shaw G, McCormick JK, et al. Antihomocitrullinated fibrinogen antibodies are specific to rheumatoid arthritis and frequently bind citrullinated proteins/peptides. J Rheumatol. 2014;41:270–279. doi: 10.3899/jrheum.130742. [DOI] [PubMed] [Google Scholar]
- 17.Turunen S, Koivula M-K, Melkko J, Alasaarela E, Lehenkari P, Risteli J. Different amounts of protein-bound citrulline and homocitrulline in foot joint tissues of a patient with anti-citrullinated protein antibody positive erosive rheumatoid arthritis. J Transl Med. 2013;11:224. doi: 10.1186/1479-5876-11-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi J, Willemze A, Janssen GMC, van Veelen PA, Drijfhout JW, Cerami A, et al. Recognition of citrullinated and carbamylated proteins by human antibodies: specificity, cross-reactivity and the “AMC-Senshu” method. Ann Rheum Dis. 2013;72:148–150. doi: 10.1136/annrheumdis-2012-201559. [DOI] [PubMed] [Google Scholar]